Introduction
The pacemaker current, If, plays an important role in determining diastolic depolarization in sinoatrial node cells (SAN) and in modulating rhythm in response to regulatory inputs. If is generated by the Hyperpolarization-activated Cyclic Nucleotide-gated channels (HCN; isoforms 1, 2 and 4) and carried by sodium and potassium ions. Two important properties of this channel are activation upon hyperpolarization and direct interaction with cyclic nucleotides (especially, cAMP) via a Cyclic Nucleotide Binding Domain (CNBD).
If sensitivity to internal Ca2+ was first described by Hagiwara and Irisawa [1]. In rabbit SAN cells, reduction of intracellular Ca2+ from 100 nM to 0.1 nM induced downregulation of If and shifted activation to more negative voltages. Then, Zaza et al [2], using inside-out macro patches, demonstrated that Ca2+ did not directly modulate pacemaker channels. Further, Rigg et al. [3] found that If in guinea pig SAN is regulated by Ca2+ and calmodulin, but via a mechanism independent of CAMKII activation.
In guinea pig SAN myocytes Ca2+-chelation with BAPTA inhibits If [3,4]. In rabbit SAN, sarcoplasmic reticulum Ca2+ depletion by ryanodine does not affect basal If but reduces the shift in If activation induced by isoproterenol [5]. However, ryanodine does not affect the ability of If to be stimulated when the adrenergic cascade is bypassed by direct stimulation of adenylyl cyclase with forskolin [4] or by the introduction of permeable cAMP [5]. A similar observation was reported in thalamocortical cells, where Ca2+-mediated upregulation of Ih required adenylyl cyclase activity [6]. Disruption of normal Ca2+-homeostasis with ryanodine also influences sympathetic modulation of SAN cell rate in the same manner as it does If [5].
Nine adenylyl cyclase isoforms have been identified (AC 1–9), the features and tissue distribution of which differ significantly [7]. Some (e.g. AC5/6) are ubiquitously distributed throughout the body while others are largely limited to certain organs or tissues (AC1 in neuronal tissue, AC3 in olfactory epithelium, etc.). Three isoforms (AC1, AC3, AC8) are Ca2+-activated in the sub-micromolar range, and of these, AC1 and AC8 are calmodulin- (but not CAMKII-) dependent [7]. In contrast, AC5/6 is inhibited in the presence of micromolar Ca2+ concentrations and highly regulated by PKA and PKC phosphorylation [8]. The predominant cardiac AC isoforms are AC5/6 [9]. However, the cardiac pacemaker region also expresses AC1, AC2 and AC8 [4,10]. Thus, Ca2+-activated AC1 and AC8 in SAN may be responsible for the Ca2+-dependence of β-adrenergic modulation of If [4].
Although the data cited above are consistent with the hypothesis that the Ca2+ modulation of pacemaker current depends on the presence of Ca2+-stimulated AC isoforms, technical limitations such as the lack of reliable isoform-specific AC inhibitors complicate experiments to demonstrate a causal link within the SAN. Therefore we chose to over-express recombinant AC1 in neonatal rat ventricular myocytes (which do not have that isoform) and test whether this perturbation alters the Ca2+-dependence of β-adrenergic effects on HCN current.
Methods
Cell culture and infection
Neonatal rat ventricular myocytes (NRVM) were isolated as previously described [11]. Briefly, 1–2 days old Wistar rats were euthanized by decapitation according to protocols approved by the Institutional Animal Care and Use Committee of Columbia University. This protocol conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Hearts were quickly removed and ventricles were dissociated by a standard trypsinization procedure. After pre-plating for one hour to minimize fibroblast contamination, myocytes were harvested and plated in protamine-sulfate coated dishes or onto fibronectin-coated cover slips (for action potential recordings). Cells were incubated in MEM (CellGro) containing 10% fetal bovine serum for 24 hr, then switched to serum free medium (SFM; [12]). Adenoviral infection was performed on culture day 1, at multiplicity of infection (moi) 0.5 for each construct. Cells were exposed to the infectious mix for 2 hrs, rinsed twice and then incubated in SFM at 37°C, 5% CO2 for 3–4 days. For studies of pacemaker current, the cell monolayer was resuspended by brief exposure to 0.1% trypsin and the cells re-plated onto fibronectin-coated coverslips, then studied 2–6 hrs later.
Adenoviral vectors
AdGFP, AdmHCN2 and AdmHCN2R591E are described elsewhere [13]. Adenoviral constructs of FLAG-tagged AC1 and AC6 were a generous gift of Dr. Ross Feldman (University of West Ontario, London, Canada). All viruses were amplified in HEK 293 cells and titrated with anti-Adv Hexon antibodies. Final titers were 1×109 – 5×109 fluorescence forming units per ml (ffu/mL).
Immunoprecipitation and immunoblotting
72 hours after infection, cardiomyocytes were scraped into homogenization buffer (20 mM Tris-HCl (pH 7.5), 2 mM EDTA, 2 mM EGTA, 0.1 mM sodium orthovanadate, 0.042% b-mercaptoethanol, 1x protease inhibitor cocktail (Roche); 0.5mL per 100 mm dish), sonicated, and centrifuged at 185,000g for 1 hour at 4°C. The pellet was resuspended in solubilization buffer (25 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.5% NP-40, 0.1 mM sodium orthovanadate and 0.1 mM sodium fluoride) and cleared of insoluble materials. A protein assay (Bio-Rad DC Protein Assay) was performed on each set of samples, and all concentrations in the set were defined from the same calibration curve. Adenylyl cyclase was immunoprecipitated from 700 μg of particulate fraction with anti-FLAG antibodies (F1804; Sigma) according to manufacturer’s instructions. HCN2 was pulled down with 2μg of anti-HCN2 antibodies (APC-030; Alomone Labs, Israel) per 500 μg of soluble materials. Proteins were separated by 7.5% SDS-PAGE and transferred to nitrocellulose for immunoblotting with anti-HCN2 or anti-FLAG antibodies. Samples for detection of adenylyl cyclases were run unheated. Loading and transfer efficacy were controlled by ponceau staining. Epitope specific binding was visualized by enhanced chemiluminescence (Pierce). Results were replicated in four different culture preparations. Isoform-specific antibodies (Santa Cruz) were used for detection of endogenous AC1 (sc-586) and AC5/6 (sc-590) in rat brain membrane (a gift from Dr. Lori Isom) and neonatal rat ventricular myocytes.
cAMP measurements
For cAMP measurements, besides indicated experimental treatments, all groups were pre-treated with 100μM IBMX (Sigma) to inhibit phosphodiesterase activity, then harvested and processed according to manufacturers’ instructions. cAMP was determined using radioimmunoassay (Amersham Cyclic AMP (3H) Assay System, GE Healthcare, UK) or ELISA (Parameter™ Cyclic AMP Assay, R&D Systems, MN, USA).
Electrophysiology
Whole-cell HCN2 current and action potentials were recorded using patch electrodes. Cells were superfused with the following extracellular solution (mM): NaCl, 140; NaOH, 2.3; MgCl2, 1; KCl, 5.4; CaCl2, 1; HEPES, 5; glucose, 10; pH 7.4. Extracellular potassium was increased to 10mM and MnCl2 (2 mM) and BaCl2 (4 mM) (to eliminate ICa and IK1) were added while recording HCN2 current. Pipettes were filled with solution containing (mM): aspartic acid, 130; KOH, 146; NaCl, 10; CaCl2, 2; EGTA-KOH, 5; Mg-ATP, 2; HEPES-KOH, 10; pH 7.2. This formulation resulted in a calculated free intracellular Ca2+ concentration of ~100 nM (pCa~7). Calculations (MaxChelator) indicate that under these conditions, the addition of 5 μM of the cell-permeable Ca2+- chelating agent BAPTA-AM (Invitrogen) extracellularly will not reduce diastolic Ca2+ but will prevent free Ca2+ from rising from this value.
Currents were recorded at 35°C. An Axopatch-200B amplifier and pClamp9.2 software (Axon Instruments) were used for data acquisition and analysis. The protocol included test voltage steps from −25 to −125 mV with increments of 10 mV (from the holding potential −20 mV), followed by a voltage step to a negative voltage (−100 to −115 mV), where the tail currents were recorded. To completely deactivate HCN current, a pulse to −5 mV was applied between sweeps. The normalized plot of tail current versus test voltages was fitted with a Boltzmann function. The voltage of half maximal activation and slope factor were then defined from that fitting. Time constants of activation were obtained by fitting the early part of current traces with a monoexponential function; the initial delay and slow activation phase were ignored. Data are not corrected for liquid junction potential (9.8mV); doing so would shift measured voltages negative.
Rate study
For rate determination, myocytes were plated into 24 well dishes, pre-coated with protamine sulfate, to form a continuous monolayer. Beating rate was determined by counting spontaneous beats in each well under the light microscope for 15 sec.
Statistics
All data are presented as mean ± S.E.M. Statistical significance for multiple groups comparison was examined by One or Two Way ANOVA (followed by pairwise multiple comparisons (Bonferroni)) as appropriate. In some cases unpaired t test was used for comparison of two means. Values of p<0.05 were considered significant.
Results
AC1 and AC6 over-expression and interaction with mHCN2
First, we investigated the presence of endogenous AC1 and AC5/6 in our cultures using western blot analysis. Supplemental figure 1 demonstrates no detectable AC1 expression in NRVM cultures (left panel). The positive control, rat brain membranes, shows an epitope-specific band of expected molecular weight for rat AC1 (108kDa). On the other hand, probing neonatal myocyte membrane extracts with anti-AC5/6 antibodies reveals abundant reactivity at the expected molecular weight (suppl. fig. 1, right panel). This confirmed previously reported data regarding AC1 and AC5/6 expression in mammalian ventricle and validated the rationale for using NRVM as a model system for introducing a neuronal AC isoform.
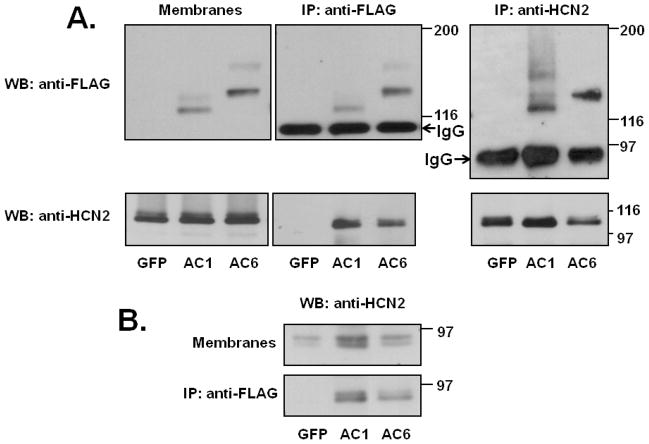
Cultures were exposed to AdmHCN2 along with AdGFP (control), AC1 or AC6. Over-expression of recombinant AC1 and AC6 was confirmed by western blot (figure 1A). Anti-FLAG antibodies detected bands of the expected molecular weight for both isoforms (left panel). No anti-FLAG specific signal was found in AdGFP-infected cultures. To explore the possibility of direct interaction with HCN2 channels, we immunoprecipitated AC with anti-FLAG antibodies, separated the obtained proteins by PAGE and probed with anti-HCN2 antibodies (figure 1A; middle panel). Both AC1 and AC6 pulled down HCN2 protein (figure 1A), suggesting possible interaction between these AC isoforms and HCN2. No protein was detected in the GFP lane. These results were replicated in four different culture preparations. To confirm specificity of the FLAG pull down, we also performed a reverse experiment, immunoprecipitating with anti-HCN2 antibodies and detecting FLAG-specific signal that was pulled down with the channel protein. As expected, a signal was found in AC1 and AC6 infected cultures, but not in GFP group (figure 1A; right panel). Further, both AC1 and AC6 isoforms also co-immunoprecipitated with endogenous HCN2 (figure 1B).
Figure 1.
A. Expression of recombinant adenylyl cyclase in NRVM. Cultures were co-infected with AdmHCN2 and one of the following: AdGFP, FLAG-tagged AdAC1 or FLAG-tagged AdAC6. 72 hours later cells were harvested, and the soluble membrane fraction was isolated. Soluble membranes from cultures infected with AdGFP, AdAC1 or AdAC6 were incubated with anti-FLAG or anti-HCN2 antibodies, separated by PAGE and probed with appropriate antibodies. B. Expression of endogenous HCN2 in NRVM and its co-immunoprecipitation with overexpressed adenylyl cyclases. The top panel shows detection of HCN2 in membrane fraction and the bottom panel – in anti-FLAG-immunoprecipitates.
Effect of AC overexpression on cAMP level
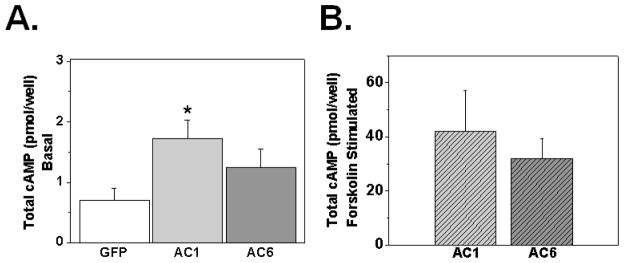
To investigate the functional effect of each AC isoform on basal cAMP, we measured total intracellular cAMP level in cultures infected with GFP, AC1 or AC6 (Figure 2A). Only AC1 induced a significant increase in total intracellular cAMP level (p<0.05 vs. GFP). In the absence of added exogenous agonist, the β-adrenoreceptor blocker propranolol (1μM) had no effect on cAMP accumulation induced by AC1-overexpression (suppl. fig. 2). In addition, the AC expression level was functionally evaluated by measuring forskolin-stimulated (10 μM) cAMP production in AC1 or AC6 infected cultures (Figure 2B). There was no significance difference between the two groups.
Figure 2.
Effect of AC1 and AC6 over-expression on basal and forskolin stimulated intracellular cAMP production (A). Cells were plated in 22-mm multiwell dishes, infected with AdGFP, AdAC1 or AdAC6 at moi 0.5 on day 1 and studied 72 hours later. n=6, *p<0.05 versus GFP by One Way ANOVA. B. Forskolin-stimulated (10μM) cAMP production was assessed by ELISA (n=4, p>0.05 by unpaired t-test).
The higher basal cAMP level with AC1 is reflected in a more positive position of the HCN2 activation relation
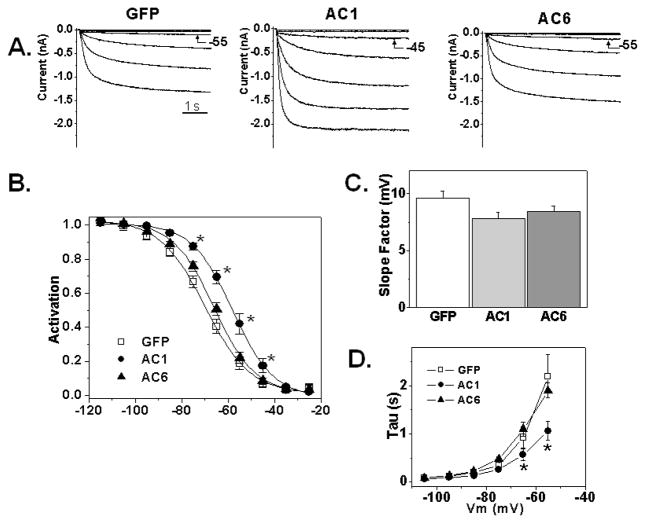
HCN2 current was recorded on days 3 or 4 post-infection with AdmHCN2 along with GFP, AC1 or AC6. No differences in cell capacitance were observed. The current density did not differ significantly between groups (88±18 pA/pF, 127±34 pA/pF and 81±17 pA/pF in GFP, AC1 and AC6 co-infected cultures respectively; p>0.05). However, the current in AC1 infected myocytes activated at significantly more positive voltages than in the GFP and AC6 groups (Figure 3A, B; p<0.001). This shift was accompanied by acceleration of kinetics and is consistent with the channel being activated by cAMP-binding [14]. Average midpoints of activation were −69±1.8 mV in GFP, −58±1.8 mV in AC1 and −65±1.2 mV in AC6 groups. Figure 3C shows corresponding values for slope factors (which did not differ) in the three groups and panel D provides data on mean activation kinetics.
Figure 3.
Effect of over-expression of AC isoforms on HCN2 current. A. Original recordings of HCN2. The current was evoked by applying hyperpolarizing voltages from −25 to −85 mV for 5 seconds. B. Average fractional activation of measured HCN2 current. The solid lines are the fits to the Boltzmann function. AC1 activation relation differs from GFP and AC6 (p<0.001) when tested by Two Way ANOVA with Bonferroni post test for pairwise comparison. (*- p<0.001 vs GFP and AC6). C. Slope factor of activation in AdGFP, AdAC1 and AdAC6 groups. D. Kinetics of the HCN2 activation. AC1 curve differs significantly from GFP and AC6 by Two Way ANOVA (p<0.001); the asterisks (*) indicate voltages that differ from GFP by pairwise multiple comparison (Bonferroni t-test; p<0.05)
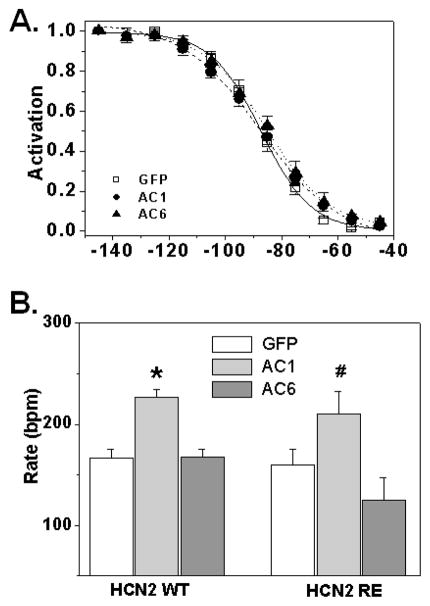
To investigate whether the observed effects of AC1 expression on HCN2 biophysics were due to direct cAMP activation of the channel, we co-expressed AC1 with HCN2R593E (HCN2RE), a mutant with markedly reduced (>1000 fold) affinity for cAMP [15,13]. Previously, we reported that in NRVM HCN2RE current activation is shifted about 12 mV negative compared to HCN2 [13]. AC1 did not change the voltage dependence of HCN2RE (figure 4A). V50 values were: GFP −87±2 mV; AC1: −87±2 mV; AC6: −84±2 mV (p>0.05); corresponding values for slope factors were: 8.9±0.6 mV, 11.5±0.5 mV and 11.3±1.0 mV (n=6–8) (p>0.05). The current density in the AC1 group did not differ from the GFP group (94.9±15.2 pA/pF vs 66.7±19.6 pA/pF in GFP, p>0.05). Together, these data are consistent with the effects of AC1 expression on HCN2 biophysical properties resulting from an elevation in basal cAMP.
Figure 4.
A. Effect of AC isoforms on HCN2RE fractional activation (n=6–8, p>0.05 by Two Way ANOVA). B. Effect of AC1 or AC6 co-expression with mHCN2 or mHCN2RE on the spontaneous beating rate of 4-day old NRVM cultures. *,# p<0.05 versus corresponding GFP-control (One Way ANOVA).
Acceleration of spontaneous beating rate in AC1-infected cultures
On day 4 after plating, neonatal rat ventricular myocytes in culture beat spontaneously with an average rate of 69±13 bpm (n=17). Over-expression of HCN2 significantly increased the beating rate to 167±8 bpm (n=34; p<0.05 vs. non infected by unpaired t-test). AC1 co-expression further accelerated beating rate, whereas cultures infected with AC6 (which do not have elevated cAMP) did not show increase in spontaneous activity (p>0.05). Average values are shown in figure 4B.
Surprisingly, the HCN2RE mutant was as effective in accelerating spontaneous beating rate in NRVM cultures in the absence or presence of the AC1 isoform as the wild type channel despite its more negative V50 (160±15 bpm; n=12). The observation that AC1 co-expression increases rate equally well with HCN2 or HCN2RE suggests additional cAMP dependent factors, besides the positive activation of expressed HCN2, contribute to the observed rate increase (see Discussion).
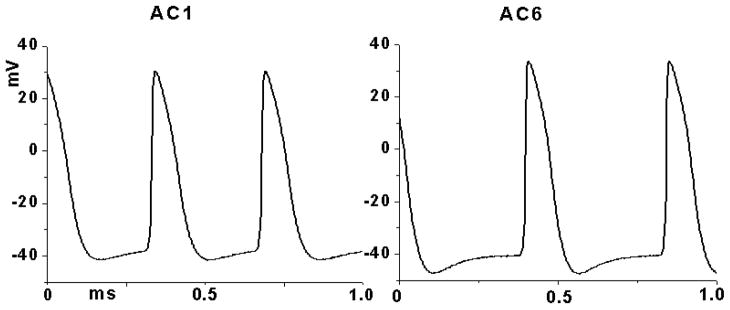
Representative spontaneous action potential recordings from cultures co-expressing WT HCN2 with AC1 or AC6 are shown in figure 5. Summary data of relevant action potential parameters are shown in Table 1 and indicate significant rate acceleration in the AC1 group and differences in the maximum diastolic potential (MDP) between the two groups. Maximum diastolic potential (MDP) was significantly more positive in AC1 cultures. In contrast, the rate of early diastolic depolarization (EDD), take off potential (TOP) and overshoot did not differ between AC1 and AC6 (figure 5; Table 1).
Figure 5.
Sample traces of spontaneous action potentials recorded from NRVM monolayers co-expressing HCN2 WT with AC1 (left) or AC6 (right).
Table 1.
Spontaneous action potential parameters from cultures co-expressing WT HCN2 with AC1 or AC6.
| Parameters | HCN2+AC1 | HCN2+AC6 |
|---|---|---|
| Rate (Hz) | 3.0±0.1* | 2.6±0.1 |
| MDP (mV) | −44.4±1.8* | −51.6±2.6 |
| EDD (mV/ms) | 0.042±0.005 | 0.043±0.01 |
| TOP (mV) | −39.8±2.8 | −45.1±2.6 |
| Overshoot (mV) | 30.4±1.2 | 35.4±2.3 |
| n | 7 | 5 |
p<0.05 vs AC6 (defined with unpaired t-test)
AC1 but not AC6 over-expression introduces Ca2+-sensitivity to the β-AR response of HCN2
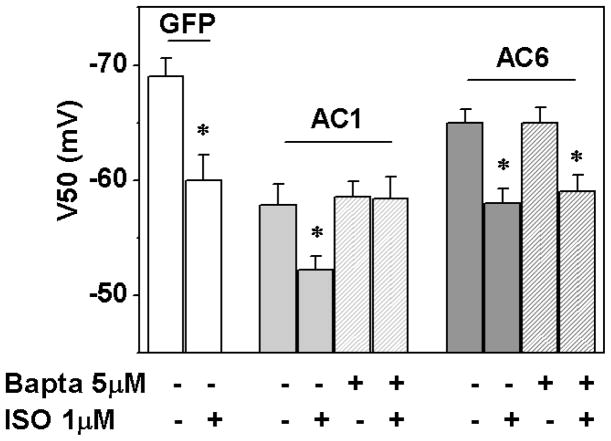
To stimulate β-adrenoreceptors we used a well known β-agonist isoproterenol (ISO) at a 1μM concentration, which produces a maximum effect in NRVM [16]. In GFP-expressing cultures, ISO induced the expected positive shift in the HCN2 activation relation (from −69.4±1.8 mV to −59.8±2.2 mV) (figure 6, p<0.05 by t-test). Compared to the GFP group, the HCN2 midpoint in the AC1 group was −58.7±1.5 mV. The introduction of 5μM BAPTA-AM did not significantly shift this value, consistent with the calculation that this concentration of BAPTA-AM would minimally affect basal [Ca2+]i and therefore basal cAMP level near the channels, unless more stringent chelating conditions were used (suppl. fig. 3). In the absence of BAPTA-AM, 1μM isoproterenol (ISO) induced a positive shift (to −52±1.4, n=5, p<0.05 vs. AC1 control), indicating that HCN2 was not fully saturated by cAMP under basal conditions. Importantly, this ISO effect was lost when cells were pre-incubated in BAPTA (−59±1.7 mV, n=8; figure 6), consistent with BAPTA preventing an ISO-induced increase in mean [Ca2+]i. In AC6-expressing myocytes, ISO shifted the HCN2 activation relation to a similar extent in the absence and presence of BAPTA-AM (−59±1.5 mV and −58±1.3 mV respectively; figure 6), confirming a lack of Ca2+-sensitivity in this setting. Taken together, these data suggest that the presence of AC1 can account for the Ca2+-sensitivity of HCN2 β-AR responsiveness.
Figure 6.
Midpoint of activation (V50) of mHCN2 and its responsiveness to β-adrenergic receptor agonist - isoproterenol (ISO, 1μM). Cells were pre-treated with 5 μM BAPTA-AM for 8 minutes; ISO perfusion was started 1 minute before recording. Two Way ANOVA was used for group comparison where ISO midpoint values were compared in relation to the presence/absence of BAPTA in AC1 or AC6 groups. *p<0.05 versus corresponding no ISO; n=5–9.
To complement the HCN2 current data, we evaluated ISO-induced intracellular cAMP production in cultures with or without BAPTA-AM pre-treatment. In the AC1 group, three out of four experiments showed a noticeable decrease in cAMP level after BAPTA, while in one experiment it was slightly higher after BAPTA-AM. In contrast, in the AC6 group, all four preparations had an increased level of cAMP after pretreatment compare to untreated. However, none of those changes were statistically significant.
Discussion
This study tested the hypothesis that the presence of a Ca2+-stimulated adenylyl cyclase isoform, as found in SAN [4,10], accounts for the reported Ca-dependence of the effect of adrenergic agonists to shift the If activation relation in SAN [5]. While this hypothesis might be addressed through methods such as siRNA to suppress AC1 in SAN, we chose a more direct approach to explore the impact of the presence of AC1, making use of NRVM cultures, which have certain similarities to, and advantages over, SAN cells in this context. Like SAN cells, NRVM cultures consist of small, spontaneously active cells lacking T-tubules. Both preparations appear to have a high basal cAMP level relative to adult ventricle [17,13] and automaticity that is decreased by If inhibition [18–20]. However, NRVM cells do not express AC1, providing a convenient model for testing the effect of adding a Ca2+-stimulated cyclase isoform. Further, such exogenous expression can readily be accomplished with high efficiency via adenovirus [14] within the typical 7 day period during which these cells are maintained in culture.
However, inference from studies in NRVM on the ionic basis of automaticity in SAN should be made with caution, since there are important differences between these two cell types. For example, native If is small in NRVM cells, which is why we co-expressed HCN2 in these studies. In addition, while both show some Ca2+-dependence to automaticity, SAN cells appear strongly dependent on the Na/Ca exchanger [21] while NRVM appear largely affected by altering Ca2+ influx [22]. Further, the NRVM action potential in culture has a Na dependent upstroke with maximal velocities on the order of 100 V/s [23], which is markedly higher than that of adult SAN cells [24]. It is because of these differences that we largely focused on the Ca2+-dependence of HCN current and its adrenergic responsiveness rather than rate.
Our key finding is that introducing AC1 into NRVM cells in culture results in the response of HCN2 to the adrenergic agonist isoproterenol becoming sensitive to disruption of intracellular Ca2+ homeostasis (figure 6). In conditions of normal intracellular Ca2+, ISO causes a comparable shift of HCN2 activation in the absence of added cyclase, or when either AC1 or AC6 is expressed. However, after BAPTA-AM pre-treatment, an ISO response persists in AC6 expressing cells but not in AC1 expressing cells. Prior studies by us and others reported that in SAN cells disruption of Ca2+ homeostasis results in loss of If responsiveness to ISO [5] and that SAN expresses Ca2+-stimulated cyclase isoforms AC1 and AC8 [4,10]. Our results, when considered along with these SAN studies, suggest that the presence of Ca2+-stimulated cyclase isoforms in the SAN accounts for the Ca2+-dependence of If adrenergic responsiveness in that tissue. The presence of Ca2+-dependent cyclase may also explain previous observations that If is modified by Ca2+ and calmodulin, but not CamKII [3], because AC1 and AC8 are Ca2+/calmodulin but not CaMKII dependent [7].
Several additional observations are of note in the present study. First, AC1 but not AC6 over-expression results in increased basal cAMP levels and a corresponding positive shift in the basal position of the HCN2 activation relation (figures 2 and 3), which is reversed by lowering basal intracellular Ca2+ (suppl. fig. 3). While the shift in basal HCN2 activation is consistent with a greater fraction of channels being cAMP-bound, this did not prevent ISO from causing a further shift in activation (under normal intracellular Ca2+) that was comparable to that with AC6 overexpression (5 mV vs 7 mV shift for AC1 vs AC6).
Virally delivered AC1 and AC6 were well expressed in this study and at functionally equivalent levels (figure 2B). This suggests the different effects of these two enzymes on cardiac myocytes might arise from distinct regulation [8] and/or ability to interact with other proteins [25]. For example, AC6 activity might be self limiting due to inhibition by PKA phosphorylation or elevation of intracellular Ca2+ [26]. Both AC1 and AC5/6 are known to reside in lipid rafts [27,10], but they interact with different anchoring proteins (Yotiao vs. AKAP79/150). Therefore, they may coordinate formation of different protein complexes [25] and lead to activation of different signaling pathways [28]. While our co-IP results (figure 1) indicate that HCN2 and AC1 are co-localized, whether they are in specialized membrane compartments requires further investigation.
Another interesting observation of this study relates to experiments on spontaneous rate. Our earlier action potentials measurements suggest that HCN2 expression in NRVM results in depolarization of the cells, with MDP settling near current activation threshold [14]. That is, HCN current limits the extent of membrane polarization. The more positive position of HCN2 is expected to result in even more depolarized MDP, and AC1-expressing NRVM support this hypotheses. However, other AP parameters such as EDD, take off potential and overshoot remain unaffected by AC1. Under such conditions (the less polarized MDP; identical EDD and TOP) the time to get from MDP to take-off potential is shortened, and so rate becomes faster. It is not surprising that EDD does not change since, for both AC6 and AC1 cells MDP is comparably near HCN2 threshold and thus the fraction of available current during diastole is similar.
Interestingly, HCN2RE, the mutated HCN2 with low cAMP affinity, resulted in a comparable spontaneous rate as HCN2 in the absence of added cyclase expression. This occurred despite the HCN2RE activation relation being more negative than that of HCN2, suggesting HCN2RE was still able to contribute significant depolarizing current during early diastole. The most likely explanation is our previous observation that in NRVM HCN2RE exhibits faster activation kinetics than HCN2 [13], which would result in more current earlier in diastole. We previously demonstrated with computer modeling that speeding HCN activation kinetics could result in faster spontaneous rates [29]. However, the fact that with AC1 co-expression there is an additional and comparable rate increase in HCN2 and HCN2RE cultures (figure 4B) despite the fact that HCN2RE voltage dependence is not affected by AC1 (figure 4A), suggests that AC1 may be increasing rate at least in part independently of any effect on the V50 of expressed pacemaker channels. It is unlikely this is occurring through endogenous HCN channels, since we do not observe any effect of AC1 expression on total pacemaker current in the HCN2RE cultures (figure 4A). Therefore, the effect of AC1 (but not AC6) to further increase rate in HCN2 or HCN2RE cultures likely reflects effects of the increased basal cAMP on other targets relevant to pacing. This could include L-type Ca2+ channels and other components of Ca2+ homeostasis. In preliminary studies, we find that AC1 expression increases peak L-type current density ~50%. Further, a separate preliminary study in a small set of cells found that AC1 expression alone (without HCN2 co-expression) increases rate ~40%.
The role of Ca2+-stimulated adenylyl cyclase in the SAN is not entirely clear. Younes et al, [10] proposed that AC1 and 8 are responsible for basal AC activity, while other isoforms (AC5/6) respond to agonist stimulation, a concept consistent with the above reasoning. However, their proposal was based on changes in total cAMP level in response to β-adrenergic stimulation of SAN cells under control and Ca2+-buffering conditions, and total cAMP might not reflect levels in submembrane domains. In our experiment, while having a clear BAPTA effect on ISO-stimulated current in AC1 cultures, we failed to observe a significant effect of BAPTA inhibition on total cAMP production. It has been reported that adrenergic stimulation of NRVM cultures results in localized cAMP elevations in regions on the order of 1 micron [30]. Thus, cAMP concentrations in the environment of If or other transmembrane channels may differ markedly from bulk measurements. The ability of ryanodine to interfere with the effect of β-adrenergic stimulation of If in SAN suggests Ca2+-stimulated adenylyl cyclases are among the effectors of β-adrenergic activation and contribute to alteration of at least sub-membrane intracellular cAMP content in response to sympathetic inputs [5].
In conclusion, Ca2+-activated adenylyl cyclases have emerged as new players in pacemaker function of the heart. They provide an important link between activity-dependent changes in intracellular Ca2+ content and production of cAMP, and thus a potential mechanism in SAN for coupling “membrane” and “Ca clock” mechanisms of automaticity [31]. How the presence of these isoforms in SAN impacts cardiac rhythm, including basal rate, stability of rhythm and response to autonomic stimulation, remains to be elucidated. Transgene expression in NRVM cultures provides a novel approach for dissecting these complex and interdependent mechanisms.
Supplementary Material
Highlights.
Ca-stimulated Adenylyl Cylase 1 (AC1) or AC6 was expressed with HCN2 in cardiac myocytes.
AC1, but not AC6 increased [cAMP]i, accelerated beating rate and shifted HCN2 activation positive.
In both AC1 and AC6 groups, isoproterenol induced a positive shift of HCN2 activation relation.
BAPTA-AM prevented the isoproterenol effect in the AC1 but not AC6 group.
AC1 plays a key role in sensitivity of β-adrenergic stimulation of HCN to [Ca]i.
Acknowledgments
We thank Haejung Chung for excellent technical support, G.J.J. Boink and M.R. Rosen for useful discussions and help with the manuscript. Supported by NIH HL-28958 and HL-094410.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hagiwara N, Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol. 1989;409:121–41. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaza A, Maccaferri G, Mangoni M, DiFrancesco D. Intracellular calcium does not directly modulate cardiac pacemaker (If) channels. Pflugers Arch. 1991;419(6):662–4. doi: 10.1007/BF00370312. [DOI] [PubMed] [Google Scholar]
- 3.Rigg L, Mattick PA, Heath BM, Terrar DA. Modulation of the hyperpolarization-activated current (I(f)) by calcium and calmodulin in the guinea-pig sino-atrial node. Cardiovasc Res. 2003;57(2):497–504. doi: 10.1016/s0008-6363(02)00668-5. [DOI] [PubMed] [Google Scholar]
- 4.Mattick P, Parrington J, Odia E, Simpson A, Collins T, Terrar D. Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the I(f) pacemaker current. J Physiol. 2007;582(Pt 3):1195–203. doi: 10.1113/jphysiol.2007.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucchi A, Baruscotti M, Robinson RB, DiFrancesco D. I(f)-dependent modulation of pacemaker rate mediated by cAMP in the presence of ryanodine in rabbit sino-atrial node cells. J Mol Cell Cardiol. 2003;35(8):905–13. doi: 10.1016/s0022-2828(03)00150-0. [DOI] [PubMed] [Google Scholar]
- 6.Lüthi A, McCormick DA. Modulation of a pacemaker current through Ca(2+)-induced stimulation of cAMP production. Nat Neurosci. 1999;2(7):634–41. doi: 10.1038/10189. [DOI] [PubMed] [Google Scholar]
- 7.Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol. 2000;279(3):F400–16. doi: 10.1152/ajprenal.2000.279.3.F400. [DOI] [PubMed] [Google Scholar]
- 8.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87(3):965–1010. doi: 10.1152/physrev.00049.2006. Review 9. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura M, Cooper DM. Cloning and expression of a Ca(2+)-inhibitable adenylyl cyclase from NCB-20 cells. Proc Natl Acad Sci U S A. 1992;89(15):6716–20. doi: 10.1073/pnas.89.15.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younes A, Lyashkov AE, Graham D, Sheydina A, Volkova MV, Mitsak M, et al. Ca(2+) -stimulated basal adenylyl cyclase activity localization in membrane lipid microdomains of cardiac sinoatrial nodal pacemaker cells. J Biol Chem. 2008;283(21):14461–8. doi: 10.1074/jbc.M707540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Protas L, Robinson RB. Neuropeptide Y contributes to innervation-dependent increase in ICa, L via ventricular Y2 receptors. Am J Physiol. 1999;277:H940–H946. doi: 10.1152/ajpheart.1999.277.3.H940. [DOI] [PubMed] [Google Scholar]
- 12.Mohamed SN, Holmes R, Hartzell CR. A serum-free, chemically-defined medium for function and growth of primary neonatal rat heart cell cultures. In Vitro. 1983;19:471–478. doi: 10.1007/BF02619594. [DOI] [PubMed] [Google Scholar]
- 13.Kryukova Y, Rybin VO, Qu J, Steinberg SF, Robinson RB. Age-dependent differences in the inhibition of HCN2 current in rat ventricular myocytes by the tyrosine kinase inhibitor erbstatin. Pflugers Arch. 2009;457(4):821–30. doi: 10.1007/s00424-008-0565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qu J, Barbuti A, Protas L, Santoro B, Cohen IS, Robinson RB. HCN2 overexpression in newborn and adult ventricular myocytes: distinct effects on gating and excitability. Circ Res. 2001;89:E8–14. doi: 10.1161/hh1301.094395. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol. 2001;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuznetsov V, Pak E, Robinson RB, Steinberg SF. Beta 2-adrenergic receptor actions in neonatal and adult rat ventricular myocytes. Circ Res. 1995 Jan;76(1):40–52. doi: 10.1161/01.res.76.1.40. [DOI] [PubMed] [Google Scholar]
- 17.Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98(4):505–14. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- 18.Er F, Larbig R, Ludwig A, Biel M, Hofmann F, Beuckelmann DJ, Hoppe UC. Dominant-negative suppression of HCN channels markedly reduces the native pacemaker current I(f) and undermines spontaneous beating of neonatal cardiomyocytes. Circulation. 2003;107(3):485–9. doi: 10.1161/01.cir.0000045672.32920.cb. [DOI] [PubMed] [Google Scholar]
- 19.DiFrancesco D, Camm JA. Heart rate lowering by specific and selective I(f) current inhibition with ivabradine: a new therapeutic perspective in cardiovascular disease. Drugs. 2004;64:1757–1765. doi: 10.2165/00003495-200464160-00003. [DOI] [PubMed] [Google Scholar]
- 20.Xiao J, Yang B, Lin H, Lu Y, Luo X, Wang Z. Novel approaches for gene-specific interference via manipulating actions of microRNAs: examination on the pacemaker channel genes HCN2 and HCN4. J Cell Physiol. 2007;212(2):285–92. doi: 10.1002/jcp.21062. [DOI] [PubMed] [Google Scholar]
- 21.Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na(+)-Ca(2+) exchanger: molecular partners in pacemaker regulation. Circ Res. 2001;88:1254–1258. doi: 10.1161/hh1201.092095. [DOI] [PubMed] [Google Scholar]
- 22.Schanne OF, Boutin L, Derosiers J. Effects of K-channel blockers, calcium, and verapamil suggest different pacemaker mechanisms in cultured neonatal rat and embryonic chick ventricle cells. Can J Physiol Pharmacol. 1989;67(7):795–800. doi: 10.1139/y89-126. [DOI] [PubMed] [Google Scholar]
- 23.Robinson RB, Legato MJ. Maintained differentiation in rat cardiac monolayer cultures: tetrodotoxin sensitivity and ultrastructure. J Mol Cell Cardiol. 1980;12(5):493–8. doi: 10.1016/0022-2828(80)90005-x. [DOI] [PubMed] [Google Scholar]
- 24.Baruscotti M, Westenbroek R, Catterall WA, DiFrancesco D, Robinson RB. The newborn rabbit sino-atrial node expresses a neuronal type I-like Na+ channel. J Physiol. 1997;498 ( Pt 3):641–8. doi: 10.1113/jphysiol.1997.sp021889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dessauer CW. Adenylyl cyclase--A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol. 2009;76(5):935–41. doi: 10.1124/mol.109.059345. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J. 2003;375(Pt 3):517–29. doi: 10.1042/BJ20031061. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houslay MD, Baillie GS, Maurice DH. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res. 2007;100(7):950–66. doi: 10.1161/01.RES.0000261934.56938.38. Review. [DOI] [PubMed] [Google Scholar]
- 28.Gros R, Ding Q, Chorazyczewski J, Pickering JG, Limbird LE, Feldman RD. Adenylyl cyclase isoform-selective regulation of vascular smooth muscle proliferation and cytoskeletal reorganization. Circ Res. 2006;99(8):845–52. doi: 10.1161/01.RES.0000245189.21703.c0. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X, Bucchi A, Oren RV, Kryukova Y, Dun W, Clancy CE, Robinson RB. In vitro characterization of HCN channel kinetics and frequency dependence in myocytes predicts biological pacemaker functionality. J Physiol. 2009;587(Pt 7):1513–25. doi: 10.1113/jphysiol.2008.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295(5560):1711–5. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 31.Lakatta EG, DiFrancesco D. What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol. 2009;47(2):157–70. doi: 10.1016/j.yjmcc.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.