Abstract
Src is a known regulator of focal adhesion turnover in migrating cells; but, in contrast, Src is generally assumed to play little role in differentiated, contractile vascular smooth muscle (dVSM). The goal of the present study was to determine if Src-family kinases regulate focal adhesion proteins and how this might affect contractility of non-proliferative vascular smooth muscle. We demonstrate here, through the use of phosphotyrosine screening, deconvolution microscopy imaging, and differential centrifugation, that the activity of Src family kinases in aorta is regulated by the alpha agonist and vasoconstrictor phenylephrine, and leads to focal adhesion protein phosphorylation and remodeling in dVSM. Furthermore, Src inhibition via morpholino knockdown of Src or by the small molecule inhibitor PP2 prevents phenylephrine-induced adhesion protein phosphorylation, markedly slows the tissue’s ability to contract and decreases steady state contractile force amplitude. Significant vasoconstrictor-induced and Src-dependent phosphorylation of Cas pY-165, FAK pY-925, paxillin pY-118, and Erk1/2 were observed. However, increases in FAK 397 phosphorylation were not seen, demonstrating differences between cells in tissue versus migrating, proliferating cells. We show here that Src, in a cause-and effect manner, regulates focal adhesion protein function and consequently, modulates contractility during the action of a vasoconstrictor. These data point to the possibility that vascular focal adhesion proteins may be useful drug discovery targets for novel therapeutic approaches to cardiovascular disease.
Keywords: Src, focal adhesion, morpholino antisense, ERK, differentiated vascular smooth muscle
INTRODUCTION
Focal adhesions (FAs) are sites of connection of the extracellular matrix, via transmembrane integrins, to the actin cytoskeleton. Turnover of FAs is well known to be regulated by growth factors and chemokines during cell migration and proliferation (Burridge and Chrzanowska-Wodnicka, 1996; Geiger et al., 2001; Taniyama et al., 2003). Differentiated vascular smooth muscle (dVSM) cells regulate the contractile tone of blood vessels and hence are a major regulator of blood pressure. These cells also rely on adhesions of the cells to the blood vessel matrix in order to transmit forces throughout the blood vessel wall, but it is not clear whether these adhesions are significantly regulated.
FAs of cultured VSM cells have been shown to be regulated during angiotensin signaling (Taniyama et al., 2003); however, the FAs (also called dense plaques) of non-proliferating dVSM cells of the blood vessel wall have generally been considered to play a largely structural role, despite recent evidence of dVSMC cytoskeletal plasticity (Kim et al., 2008; Kim et al., 2010). AFM studies have demonstrated that matrix proteins strongly attach to cultured VSMCs and induce myogenic-like, force generating reactions in response to externally applied forces (Sun et al., 2008) but whether this occurs in dVSM cells is not known.
A few studies, however, have pointed to the possibility of FA remodeling in dVSM. A GPCR agonist-induced increase tyrosine phosphorylation of paxillin, a scaffolding protein of FAs (Ward et al., 2002), has been reported in dVSM. Src activation has also been implicated in hypoxic pulmonary constriction of airway smooth muscle (Knock et al., 2008) and BK channel activation in microvessels (Wu et al., 2008); but, whether these findings are linked to FA dynamics and the way in which those dynamics impact vascular tone is not known.
The Src family of tyrosine kinases was originally discovered by Bishop and Varmus (Bishop et al., 1978). Src family kinases have been shown to play a major role as regulators of FAs in non-muscle cells and to transmit integrin-dependent signals central to cell migration and proliferation (Arias-Salgado et al., 2003). During cell migration, Src family kinases are known to cycle in and out of FAs and regulate rearrangement of actin structures. Conversely, kinase-inactive Src or Src inhibitors reduce the rate of VSM cell spreading (Ishida et al., 1999; Yamboliev et al., 2001). Src activation in cultured cells leads to phosphorylation of several FA proteins and downstream ERK activation, leading to turnover of FAs (Berk and Corson, 1997; Fincham et al., 2000; Kim et al., 2009). In contrast, it is generally assumed that FAs of non-proliferating, non-migrating dVSM are fixed structures, but this has not been clearly shown.
The purpose of the present study was to investigate the degree to which Src-dependent signaling is activated during contraction of blood vessels and to determine its effect on the function of dVSM. We report here that vasoconstrictor stimulation leads to Src-dependent FA activation and loss of Cas, a Src substrate, from the FA complex. Furthermore, we show, in a cause-and effect manner, that Src regulates contractility of vascular smooth muscle by altering the rate and amplitude of development of alpha agonist-induced tone in vascular tissue.
MATERIALS AND METHODS
Tissue preparation and contractility measurements
We confirm that all procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Boston University. The animals were maintained in a manner consistent with the NIH Guide for the Care and Use of Laboratory Animals and were obtained and used in compliance with federal, state, and local laws.
Ferrets (Marshall Farms, North Rose, NY) were euthanized by an overdose (15ml) of isoflurane by inhalation, as confirmed by cessation of heart beat, and the aorta was quickly removed from animals and placed in an oxygenated physiological salt solution (PSS) consisting (in mM) of 120 NaCl, 5.9 KCl, 1.2 NaH2PO4, 25 NaHCO3, 11.5 dextrose, 1 CaCl2, and 1.4 MgCl2, and dissected under a dissecting microscope in oxygenated (95% O2 – 5% CO2) PSS to remove connective tissue and the endothelium. Tissues were allowed to equilibrate at 37 °C for 1 hour in oxygenated PSS and then were stimulated for 10 min with 51 mM KCl PSS (51mM of NaCl replaced with KCl in PSS) to test their viability and for normalization. At the end of the experiment the tissues were quick-frozen by immersion into dry ice-acetone slurry containing 10 mM dithiothreitol (DTT) for further biochemical study. Isometric force was recorded at 37°C as previously described (Li et al., 2009).
Western blot analysis and measurement of LC20 phosphorylation
Quick-frozen tissues were homogenized at 4°C as previously described (Marganski et al., 2005). Densitometry was performed with an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE) allowing staining of individual blots on two separate channels of different color. Where phosphoproteins were normalized for lane loading by comparison to another protein, the two signals were recorded for the same lanes of the same blot on different channels. Glycerol-urea gel electrophoresis was used to separate nonphosphorylated, monophosphorylated, and diphosphorylated light chains as described previously (Kim et al., 2000). LC20 phosphorylation (in mole phosphate per mole LC20) was calculated from densitometric analyses as following: area (phosphorylated) / [area (unphosphorylated) + area (phosphorylated)].
Immunoprecipitation and Mass Spectrometry
Muscle strips were frozen in a dry ice/acetone slurry containing DTT and homogenized as previously described (Li et al., 2009). The supernatant was precleared with protein A agarose and then incubated with anti-phosphotyrosine conjugated agarose beads overnight at 4°C as previously described (Li et al., 2009). The immune complex beads were washed, resuspended in sample buffer (25mM Tris (pH 6.8), 4% SDS, 20% glycerol, 5% β-mercaptoethanol, 1mM EDTA and 0.001% bromophenol blue), and boiled at 100°C for 5 min.
The protein band of interest was excised from Coomassie blue stained PAGE gels. Mass spectrometry for protein identification was performed by the Tufts University Core Facility (Boston, Massachusetts) as previously described (Li et al., 2009). Excised bands were subjected to in-gel reduction, alkylation, and enzymatic digestion with trypsin (Roche Applied Science, Indianapolis, IN) and LC/MS/MS analysis.
Cell Isolation and imaging
Cells were enzymatically dissociated from aorta tissue by a previously published method (Kim et al., 2008). The wash buffer containing the dissociated cells was poured over glass coverslips on ice under an atmosphere of 100% oxygen.
dVSMCs were fixed with 4% paraformaldehyde, permeabilized using 0.1% Triton-X 100, blocked with 10% goat serum as previously described (Kim et al., 2010). For deconvolution microscopy, 3-D image stacks were acquired with a Nikon Eclipse TE 2000-E inverted microscope equipped with a Nikon Plan Apochromat 60XA (NA 1.4) oil immersion objective. Images were recorded by a high-resolution fluorescence CCD camera (CoolSNAP™ HQ2, Photometrics®) with NIS-Elements Advanced Research (Nikon) software and out-of-focus fluorescent blur was removed by deconvolution of Z-stacks (Richardson-Lucy algorithm, constrained iterative-maximum likelihood estimation algorithm) as previously described (Kim et al., 2010).
Differential centrifugation for Tissue fractionation
Methods have been previously described (Kim et al., 2010). Briefly, tissues were quick frozen and homogenized at 4°C. with 0.5 ml buffer I (Tris-HCl 20 mM pH 7.5, NaCl 50 mM, sucrose 250 mM, dithiothreitol 10 mM, EGTA 3 mM, MgCl2 5 mM, ATP 1 mM and protease inhibitors). Homogenized tissue was centrifuged at 100,000 g for 1 hour and the supernatant collected as the cytosolic fraction. The pellet was resuspended in 0.5 ml buffer II (Tris-HCl 20 mM pH 7.5, sucrose 250 mM, Triton X-100 0.5%, DTT 10 mM, EGTA 3 mM, MgCl2 5mM, ATP 1 mM and protease inhibitors), extracted at 4°C for 1hr and centrifuged at 100,000 g for 1 hour. This supernatant was collected as the Triton X-soluble or membrane fraction. The pellet was resuspended in 0.5 ml buffer III (Tris-HCl 20 mM pH 7.5, sucrose 250 mM, Triton X-100 0.5%, sodium dodecyl sulfate (SDS) 1.2%, DTT 10 mM, EGTA 3 mM, MgCl2 5 mM, ATP 1 mM and protease inhibitors), extracted at 4°C for 1 hour and briefly centrifuged. This final supernatant was collected as the Triton-resistant or cytoskeletal fraction.
Introduction of antisense morpholinos and organ culture
Morpholino oligonucleotides were purchased from Gene Tools, LLC (Philomath, OR). Src expression was suppressed using the antisense morpholino oligonucleotide 5′-TGGGCTTGCTCTTGTTGCTACCCAT-3′. A scrambled morpholino oligonucleotide 5′-AGGGTGTTCTATCTCTTGCTCCCTG-3′ or sham loading served as control. No differences were seen between the effect of scrambled and sham controls so the data were merged. Aorta rings were loaded with the morpholino oligonucleotides at 50 μmol/L using a previously described protocol (Kim et al., 2010). Tissues were kept overnight in serum-free organ culture at room temperature. On the fourth day of organ culture, the viability of the preparation and contractile function were tested by measuring the response to 51mM KCl PSS. At the end of the experiment, the muscle strips were quick-frozen for biochemical analysis.
Materials
PP2 was purchased from EMD Biosciences (La Jolla, CA). Antibodies used were: p44/42 MAP kinase1/2 (rabbit, 1:1000), p130Cas (mouse, 1:500), paxillin (mouse clone 5H11 1:2000) and phospho-tyrosine (mouse clone 4G10, 1:500) were from Millipore (Billerica, MA). Phospho-FAK Y925 (rabbit, 1:200), Tyr118 paxillin (rabbit 1:500), phosphoY165Cas (rabbit, 1:500), Fgr (rabbit,1:500), Hck (rabbit, 1:500), a-Tubulin (mouse, 1:1000) and phospho-ERK (mouse, 1:1500) were purchased from Cell Signaling Technology (Danvers, MA). Phospho-FAK Y397 (mouse,1:1000) was purchased from BD Transduction Laboratories (San Jose, CA). FAK (rabbit,1:300), c-Src polyclonal antibody (rabbit, 1:1000), and PKC delta polyclonal antibody (rabbit, 1:500) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Myosin light chain (mouse clone MY-21, 1:1000) was from Sigma-Aldrich (St. Louis, MO).
Statistics
Data are expressed as mean± SE in all graphs. Significance of difference between two individual sets of means was taken at p<0.05 by an unpaired Student’s t test unless indicated otherwise.
RESULTS
Screening of Src-dependent phosphotyrosine signaling shows 4 major PE-dependent signals at masses of 130–135, 120–125, 68–75, and 60kDa in aorta
Most of the well-characterized downstream signaling events in dVSM involve Ser/Thr phosphorylation events. Thus, as an initial screen for focal adhesion signaling events in response to stretch or GPCR-stimulation in aorta, we probed quick-frozen aorta strips for phosphotyrosine in immunoblots (Fig. 1A). Four conditions were studied: (1) slack, unstimulated strips, (2) strips equilibrated at optimal length for force production, previously determined to occur at 2g resting tension (unpublished data), (3) strips at optimal length and contracted with 10−5 M phenylephrine (PE) for 10 minutes and (4) strips pre-treated with the Src small molecule inhibitor PP2 then stretched to optimal length and contracted with PE. As is shown in Fig. 1B, on average, no significant increase in phosphorylation with stretch was detected; however, with PE-activation, at least 4 prominent groups of bands (at 130–135kDa, 120–125kDa, 68–75kDa, and 60kDa) showed statistically significant increases in phosphotyrosine staining.
Figure 1. Phosphotyrosine screening of aorta samples demonstrates PE-dependent increases and PP2-dependent decreases.
(A) Typical blot, phosphotyrosine staining of homogenized samples is shown for samples frozen at slack length, stretched to optimal length (2g stretch), stretched strips exposed to 10−5 M PE for 10 min and 10 min 10−5 M PE stimulated strips pretreated with 10 mM PP2, a Src inhibitor. (B) Average densitometry of phosphotyrosine staining of bands of indicated molecular mass, normalized to staining for total PKC used as a loading control. n=3. *p<0.05, **p<0.01, ***p<0.001 compared to unstimulated slack strip; +p<0.05, ++p<0.01 compared to PE+PP2 strip.
When aortic tissues were pretreated with the small molecule Src inhibitor, PP2, at the start of the experiment, the PE-induced increases in tyrosine phosphorylation were all decreased in a statistically significant manner (Fig. 1B). Based on molecular mass, we speculated that these bands could represent stimulus-induced tyrosine phosphorylation of the well-known FA proteins Cas (130kDa), FAK (125kDa), paxillin (68–75kDa) and Src family kinases (60kDa). Thus we probed the samples with phosphoprotein-specific antibodies.
Cas and Paxillin are tyrosine phosphorylated in a PP2-sensitive manner, in response to PE
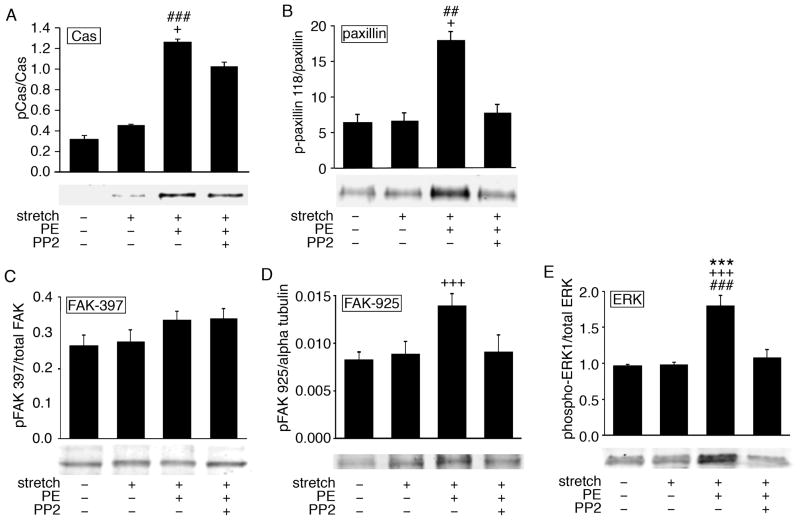
We pursued the possibility, based on phosphotyrosine screening, that the 130kDa tyrosine phosphorylated band includes Cas, a 130kDa, well-known, Src substrate tyrosine-phosphorylated in focal adhesions of cultured cells (Fonseca et al., 2004). As is shown in Fig. 2A, when we probed with a Cas-specific phosphoantibody, a pronounced increase in Cas phosphorylation was seen in response to PE but not stretch. The response was inhibited by PP2 pretreatment. Densitometry of the phospho-Cas bands, normalized to total Cas, confirmed that the changes are significant.
Figure 2. Tyrosine phosphorylation of FA proteins.
The graphs show the densitometric analysis of normalized tyrosine phosphorylation of (A) Cas, (B) paxillin, (C) FAK tyrosine 397, (D) FAK tyrosine 925 and (E) ERK in response to PE. Typical western blots with samples quick frozen at the indicated conditions are shown below the graphs. Note that tyrosine phosphorylation of Cas, paxillin, ERK and FAK tyrosine 925, but not FAK tyrosine 397, are sensitive to the Src inhibitor PP2. n=3–4. ## p<0.01, ### p<0.001 compared to stretch only, *** p<0.001 compared to unstimulated slack, + p<0.05, +++ p<0.001 compared PP2.
Paxillin, a protein that runs at 68–70 kDa is another known Src substrate in FAs of migrating and proliferating cells. When we probed with a phosphoantibody specific for phospho-paxillin Y118, a pronounced increase in signal was seen with the addition of PE but not with stretch and was inhibited by PP2 pretreatment. (Fig. 2B).
FAK Y925, but not Y397, is phosphorylated in a PP2-sensitive manner in response to PE
We tested the possibility that FAK, a component of most FAs in cultured cells, was contained in the 125kDa tyrosine-phosphorylated band by probing with FAK site-specific phosphoantibodies. First, we probed phosphorylation at Y397, a site known to be linked to integrin engagement (Mitra et al., 2006), but no significant, PP2-dependent change in the phospho-397 signal was detected in response to either a stretch stimulus or PE (Fig. 2C). In contrast, when we probed with a phospho-Y925 antibody, a prominent increase in the signal was seen in response to PE and this effect was also inhibited by PP2 pretreatment (Fig. 2D). Stretch to optimal length was not associated with any detectable change in the signal.
ERK activation in response to PE is PP2-sensitive
Phosphorylation at Y925 has been linked to downstream ERK signaling. Interestingly, PE has previously been shown to trigger an increase in ERK phosphorylation in this tissue (Khalil et al., 1995; Khalil and Morgan, 1993); however, ERK activation in this tissue has not been previously linked to FA signaling. We tested the idea that the ERK activation might be sensitive to FA signaling by pretreating strips with PP2 and, as shown in Fig. 2E, PP2 inhibits PE-induced ERK activation.
Src phosphorylation is not detectably increased at either Y416 or Y529 by stretch or PE
A prominent PE-dependent 60 kDa band is seen on phosphotyrosine screening (Fig. 1). Src isoforms run at 58–60 kDa so we used two color immunoblot to co-stain phosphotyrosine bands with a pan Src antibody. As is shown in figure 3A, Src exactly overlaps the prominent 60kDa phosphotyrosine band. Src is known to have two tyrosine phosphorylation sites, Y416 (an activating site) and Y529 (an inhibitory site). Signals were seen with phosphoantibodies to these sites, but no statistically significant change in the signal was detectable with stretch or PE (Fig. 3, B and C) suggesting that SRC may be constitutively activated by the connection of integrins to the matrix within the vascular tissue.
Figure 3. Src phosphorylation in response to PE and effect of Src inhibitor PP2 on force.
(A) p60 overlaps exactly with Src in a 2-color LiCor blot. (B and C) Src phosphorylation is not detected at either Y416 (B) or at Y529 (C) after stretch or PE. The graphs represent average densitometry data of normalized Src phosphorylation at the indicated sites (n=3–5). (D–F) Src inhibition with PP2 slows the time course and decreases the maximum force production in the presence of PE (n=3–6). (D) Chart recording to illustrate how measurements were made. (E) Time to 50% maximal force production is increased after the addition of PE in the presence of pretreatment with PP2. (F) Maximal amplitude of contraction to PE is decreased by pretreatment with PP2. * p<0.05 compared to PE. (G) Pretreatment with PP2 does not affect myosin light chain phosphorylation.
The identity of the 60kDa band whose tyrosine phosphorylation increases in the presence of PE is thus uncertain. The possibility that another protein may be present in the 60kDa band was pursued by cutting the 60kDa band from gels of phosphotyrosine immunoprecipates of PE-stimulated vascular strip homogenates and subjecting the band to HPLC MS/MS. Matches included a 60 kDa chaperonin subunit and ribulose-1,5-bisphosphate carboxylase/oxygenase, but no FA proteins were identified.
PP2 slows the time course and decreases the amplitude of the PE contraction with no change in myosin light chain phosphorylation
The question arises as to what the functional effect of adhesion-plaque signaling is in dVSM, a non-migrating tissue that would be expected to have mature, relatively non-dynamic FAs and whose main function is to regulate vascular diameter, blood flow and blood pressure. Thus, we probed for effects of Src-dependent signaling on contractile function by measuring whether PP2 could inhibit contractile amplitude or time-course. Fig. 3D shows a chart recording to illustrate how measurements were made. The results show that PP2 significantly increases the time to 50% maximal force development (Fig. 3E) and significantly decreases the steady state force amplitude (Fig. 3F) in response to PE. To confirm that these inhibitory actions of PP2 on contractility are not due to non-specific actions of PP2 on other pathways known to regulate smooth muscle contractility, such as those that involve MLCK, we also measured myosin light chain phosphorylation with glycerol/urea gels (Fig. 3G). The effects of PE on myosin light chain phosphorylation are shown at 2 minutes, when phosphorylation is maximal. As can be seen, pretreatment with PP2 had no detectable effect on the PE-induced increase in myosin light chain phosphorylation.
Three members of the Src family are expressed in dVSM
To further test the involvement of Src in dVSM FA signaling, we performed Src knock-down experiments with aortic tissue strips. As a first step, we determined if multiple isoforms of the Src family were present in this tissue and, if so, which are present. A set of unique PCR primers (Table 1) was designed for the known Src family members, using human sequences, given the high homology between human and ferret sequences. As is shown in Fig. 4A, expression at the message level for 3 isoforms, c-Src, Fgr, and Hck was detected by PCR. These results were pursued at the protein level with isoform specific immunoblots. As is shown in Fig. 4B, the same isoforms were found to be present at the protein level. From this information we designed a pan-Src sequence near the initiation codon that is common to these three isoforms and used this sequence for knockdown experiments.
Table 1.
Primers Used to Perform PCR of Src Family Isoforms
| Isoform | Primer Fwd | Primer Rev |
|---|---|---|
| c-Src | 5′ gcaacaagagcaagcccaagg 3′ | 5′ ctcagcctggatggagtcg 3′ |
| Fgr | 5′ gcgagaagttccacatcctgaac 3′ | 5′ agctgcaccagcttgtcgtg 3′ |
| Fyn | 5′ actacaacaacttccacgcagcc 3′ | 5′ accagcttgtcgtgcttcagc 3′ |
| Yes | 5′ gcctgcagattccattcaggc 3′ | 5′ cagccatatcaaccagctgtgg 3′ |
| Blk | 5′ cacaggaagagaaggctatgtgc 3′ | 5′ ttcatcacgttggcctcaccc 3′ |
| Hck | 5′ aagacctcagcttccagaaggg 3′ | 5′ atctcccaggcatctttctccc 3′ |
| Lck | 5′ caacggtggcttctacatctcc 3′ | 5′ ccacttaatgggaaacttggccc 3′ |
| Lyn | 5′ caacgtccaataaacagcaaaggcc 3′ | 5′ catctttatcccatggcttctgtgg 3′ |
| Frk | 5′ gatagaccgcaactccatacagc 3′ | 5′ gaatttatta(cg)tacgattggcttcggg 3′ |
| Yrk | 5′ actgcaaggagaagatatccggc 3′ | 5′ cctttcgtcgtctcactctcc 3′ |
Figure 4. Effects of Src antisense morpholino knockdown on FA protein / ERK phosphorylation and force.
(A) PCRs with specific primers show that at least three members of the Src family, Src, Fgr and Hck, are expressed in dVSM (agarose gel image inverted for optimal display). (B) Morpholino Src family knockdown downregulates Src and Fgr expression but upregulates Hck expression. In each case the lanes are from the same blot but an intervening blank lane was spliced out of the Hck blot. (C) Morpholino Src knockdown decreases tyrosine phosphorylation of FA proteins Cas, FAK and paxillin, as well as ERK phosphorylation. Phosphotyrosine screening demonstrates an inhibition of PE-stimulated increases in phosphotyrosine at 135 kDa (Cas), 125 kDa (FAK) and 75kDa (paxillin). ERK phosphorylation was detected with a phospho-specific ERK antibody. Graph shows average densitometry values (n=3–8). (D) Time to 50% force generation is increased by Src knockdown (graph on left) and PE-stimulated increase in maximal force is inhibited by Src knockdown (graph on right). n=3–8. * p<0.05 compared to control, + p< 0.05 compared to morpholino.
Morpholino antisense knockdown of Src causes a decrease in tyrosine phosphorylation and a slowing of vascular tone development
Because of slow protein turn-over and poor transfectibility of dVSM, knockdown experiments are challenging. Using morpholino antisense loaded into aortic rings in serum-free organ culture over 4 days, we were able to see a significant knockdown of both the c-Src and Fgr isoforms compared to controls of scrambled sequence-loaded or sham-loaded rings (Fig. 4B). Interestingly, a, presumably compensatory, upregulation of Hck was also consistently seen. This compensatory upregulation may have somewhat blunted the functional effects of Src knockdown.
We then assayed for an effect in phosphotyrosine screens and found that pan-Src knockdown causes a statistically significant decrease in PE-induced increases in tyrosine phosphorylation (quantitated for p135, p125 and p68–75). Similarly, morpholino knockdown significantly decreased the PE-induced increase in ERK phosphorylation (Fig. 4C).
We also monitored the time to 50% tone development and the steady state increase in maximal force in response to PE (Fig. 4D) in the control and knockdown tissues and, similar to the findings with PP2, Src knockdown led to a statistically significant slowing in the rate of tone development and decrease in maximal force.
PE activation leads to Src translocation and FA protein redistribution
FA phosphorylation is associated with FA remodeling in cultured cells (Fincham et al., 2000; Kim et al., 2009) and with Src cycling between FAs, cell membranes and cytosol (Thomas and Brugge, 1997). ERK activation is also known, in cultured migrating cells, to be associated with FA signaling (Fincham et al., 2000). How these processes relate to a possible FA turnover in nonmigrating, nonproliferating dVSM cells is not clear and it is possible that in the dVSM cell the tyrosine phosphorylations measured here do not translate into recycling of FA proteins.
Therefore, we imaged Src distribution in freshly dissociated dVSM cells with deconvolution microscopy. Imaging of total Src indicates that, in unstimulated dVSM cells, Src is located in a patchy distribution primarily at the cell cortex. In the presence of PE, however, Src displays a more pronounced central distribution, possibly in vesicles, as described for migrating cells (Seong et al., 2009) (Fig. 5A).
Figure 5. PE causes cellular redistribution of Src and the FA protein Cas.
(A.) Deconvolution microscopy (center sections) of Src/Vinculin co-stained unstimulated cell shows adjacency of the 2 proteins in unstimulated cells and an increase in central vesicular-like staining in the presence of PE. (B) Differential centrifugation analysis of Src (left graph) and Cas (right graph) in unstimulated (light gray bars) and PE (dark gray bars) stimulated tissues. Cytosolic, membrane, and cytoskeletal fractions normalized as a percentage of the total (n = 5–6). * p<0.05 compared to resting values.
We also performed differential centrifugation experiments, probing the localization of Src and also of Cas, a typical FA protein and Src substrate. As is shown in Fig. 5B (left-hand graph), Src shows a marked redistribution from the cytoskeleton, presumably in FAs, to the membrane fraction, possibly corresponding to the vesicular distribution seen in the imaging experiments. Similarly, Cas is decreased in the cytoskeletal fraction, and increased in the membrane fraction, in response to PE stimulation (Fig. 5B, right-hand graph).
Thus, activation of FA signaling with the vasoconstrictor PE leads to Src-dependent phosphorylation of substrates, resulting in dynamic changes in FA protein composition.
DISCUSSION
The vascular smooth muscle cell can exist in two qualitatively different phenotypes, the contractile, non-migratory, non-proliferative dVSM cell and the migratory, proliferative VSM cell. The main finding of the present study is that a vasoconstrictor alpha agonist can induce significant Src-dependent remodeling of FAs in non-migrating dVSM cells, reflected by biochemical modulations of FA proteins, redistribution of the FA protein Cas between cellular compartments, and changes in contractility.
It is well known that growth factors and other agonists can induce FA remodeling associated with migration of proliferative VSM cells, but it is not generally expected that Src family kinases play a pronounced role in contractile dVSM. Biochemically, most well-studied signaling pathways in the dVSM cell (such as MLCK, PKC, PKA, PKG, CaMKII, and ILK pathways) involve Ser/Thr phosphorylations. In the present study, phosphotyrosine screening and phosphoprotein-specific immunoblots identified tyrosine phosphorylation during the action of the vasoconstrictor PE of at least 3 FA proteins: Cas, FAK and paxillin. Previously, Ohanian et al. have reported tyrosine phosphorylation of paxillin in mesenteric vessels in response to noradrenaline, but attributed this to PYK2 signaling and did not actually demonstrate remodeling of FA molecules (Ohanian et al., 2005). In contrast, Flavahan et al. have reported that alpha agonists do not cause cytoskeletal remodeling of dVSM in mouse tail arterioles (Flavahan et al., 2005). In other types of contractile smooth muscle, notably airway and myometrial smooth muscle, there is strong evidence for tyrosine phosphorylation of FA proteins in contractile smooth muscle in response to contractile agonists (Huang et al., 2011; Li et al., 2009; Tang et al., 2002), but even there, whether Src family kinases play a role to regulate smooth muscle contractility has not been clear.
In the present study, Src inhibition by the small molecule inhibitor PP2 and knockdown by morpholino antisense oligonucleotides modulated not only the phosphorylation status of dVSM FAs but also significantly modulated contractile function of the aortic dVSM preparations. In both cases, Src inhibition produced a consistent and statistically significant inhibition of maximal steady state force in response to PE and a slowing of the rate of development of the PE-induced vascular tone. Conversely, Src activation is expected to increase contractility. An increase in maximal agonist-induced force amplitude is expected to lead to an increase in blood pressure and possible decrease in blood flow. Notably, FAK activation has recently been associated with hypertension in a rat aortic banding model of hypertension (Sugimura et al., 2010). Additionally, it has recently become clear that aortic stiffening is an early and independent predictor of adverse cardiovascular outcomes and also that the dVSM cell plays a role in age dependent vascular stiffening (Mitchell, 2009; Qiu et al., 2010). Thus, the finding reported here that the FA and hence its connections to the extracellular matrix and the intracellular cytoskeleton can be remodeled, suggests that such changes may play a role in total aortic stiffness and is of considerable relevance for the development of possible therapeutic strategies for treatment of age-dependent aortic stiffening. The effect of Src inhibition/knockdown to cause a significant slowing of the contraction is consistent with an altered FA compliance.
Of interest is our finding that although alpha agonist activation led to Src-dependent tyrosine phosphorylations of FA proteins, no significant change could be detected in phosphorylation of Src itself. Similarly, no change in FAK Y397 phosphorylation was seen in response to PE even though there was a clear PE-induced and Src-dependent phosphorylation of FAK Y925. In migrating proliferative cells, both FAK 397 and Src 416 have been attributed to auto-phosphorylation in response to integrin engagement (Clark and Brugge, 1993; Lietha et al., 2007; Salazar and Rozengurt, 2001). Even though we assayed for stretch-dependent changes in phosphorylation at these sites and saw none, it is possible that the pre-stress present in the 3-dimensional tissue due to tensed chronic cell-to-cell and cell-to-matrix connections leads to a constitutive phosphorylation of these sites. Assuming this is the case, a constitutively activated Src could be held in check in the absence of agonist activation by lack of access to its substrates until the development of active contractile force.
Although no PE-induced increase in FAK Y397 was seen, PE activation did lead to a pronounced increase in FAK Y925 phosphorylation. This site has been linked, in proliferative cells, to downstream ERK activation (Mitra and Schlaepfer, 2006). The present study, for the first time, has shown that the previously described alpha agonist-induced ERK activation (Khalil et al., 1995) is Src-dependent in dVSM. ERK inhibition in aortic tissue was previously shown to lead to an inhibition of contractile force (Dessy et al., 1998). Thus the possibility arises that the site of action of ERK in contractile dVSM may reside in the FA.
In summary, we show here, for the first time, that the alpha agonist and vasoconstrictor PE activates, in contractile dVSM, Src-dependent regulation of FAs and Src-dependent changes in contractility. These results may have major implications for potential novel drug discovery targets aimed at the treatment of hypertension and age-dependent aortic stiffening.
Acknowledgments
Contract grant sponsor: NHLBI; Contract grant number: HL31704; HL080003; HL086655
We thank Sabah Malek and Beth Columbo who performed preliminary experiments in the early parts of this study.
Footnotes
The authors have no conflict of interest to declare.
References
- Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci U S A. 2003;100(23):13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk BC, Corson MA. Angiotensin II signal transduction in vascular smooth muscle: role of tyrosine kinases. Circ Res. 1997;80(5):607–616. doi: 10.1161/01.res.80.5.607. [DOI] [PubMed] [Google Scholar]
- Bishop JM, Baker B, Fujita D, McCombe P, Sheiness D, Smith K, Spector DH, Stehelin D, Varmus HE. Genesis of a virus-transforming gene. Natl Cancer Inst Monogr. 1978;(48):219–223. [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Redistribution of activated pp60c-src to integrin-dependent cytoskeletal complexes in thrombin-stimulated platelets. Mol Cell Biol. 1993;13(3):1863–1871. doi: 10.1128/mcb.13.3.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessy C, Kim I, Sougnez CL, Laporte R, Morgan KG. A role for MAP kinase in differentiated smooth muscle contraction evoked by alpha-adrenoceptor stimulation. Am J Physiol Cell Physiol. 1998;275:C1081–C1086. doi: 10.1152/ajpcell.1998.275.4.C1081. [DOI] [PubMed] [Google Scholar]
- Fincham VJ, James M, Frame MC, Winder SJ. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. Embo J. 2000;19(12):2911–2923. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavahan NA, Bailey SR, Flavahan WA, Mitra S, Flavahan S. Imaging remodeling of the actin cytoskeleton in vascular smooth muscle cells after mechanosensitive arteriolar constriction. Am J Physiol Heart Circ Physiol. 2005;288(2):H660–669. doi: 10.1152/ajpheart.00608.2004. [DOI] [PubMed] [Google Scholar]
- Fonseca PM, Shin NY, Brabek J, Ryzhova L, Wu J, Hanks SK. Regulation and localization of CAS substrate domain tyrosine phosphorylation. Cell Signal. 2004;16(5):621–629. doi: 10.1016/j.cellsig.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2(11):793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhang W, Gunst SJ. Activation of vinculin induced by cholinergic stimulation regulates contraction of tracheal smooth muscle tissue. J Biol Chem. 2011;286(5):3630–3644. doi: 10.1074/jbc.M110.139923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Ishida M, Suero J, Takahashi M, Berk BC. Agonist-stimulated cytoskeletal reorganization and signal transduction at focal adhesions in vascular smooth muscle cells require c-Src. J Clin Invest. 1999;103(6):789–797. doi: 10.1172/JCI4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil RA, Menice CB, Wang C-L, Morgan KG. Phosphotyrosine-dependent targeting of mitogen-activated protein kinase in differentiated contractile vascular cells. Circ Res. 1995;76:1101–1108. doi: 10.1161/01.res.76.6.1101. [DOI] [PubMed] [Google Scholar]
- Khalil RA, Morgan KG. PKC-mediated redistribution of mitogen-activated protein kinase during smooth muscle activation. Am J Physiol. 1993;265:C406–C411. doi: 10.1152/ajpcell.1993.265.2.C406. [DOI] [PubMed] [Google Scholar]
- Kim HR, Gallant C, Leavis PC, Gunst SJ, Morgan KG. Cytoskeletal Remodeling in Differentiated Vascular Smooth Muscle is Actin Isoform-Dependent and Stimulus-Dependent. Am J Physiol Cell Physiol. 2008;295:C768–778. doi: 10.1152/ajpcell.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Graceffa P, Ferron F, Gallant C, Boczkowska M, Dominguez R, Morgan KG. Actin polymerization in differentiated vascular smooth muscle cells requires vasodilator-stimulated phosphoprotein. Am J Physiol Cell Physiol. 2010;298(3):C559–571. doi: 10.1152/ajpcell.00431.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Je HD, Gallant C, Zhan Q, Riper DV, Badwey JA, Singer HA, Morgan KG. Ca2+-calmodulin-dependent protein kinase II-dependent activation of contractility in ferret aorta. J Physiol. 2000;526(Pt 2):367–374. doi: 10.1111/j.1469-7793.2000.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6(10):587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- Knock GA, Snetkov VA, Shaifta Y, Drndarski S, Ward JP, Aaronson PI. Role of src-family kinases in hypoxic vasoconstriction of rat pulmonary artery. Cardiovasc Res. 2008;80(3):453–462. doi: 10.1093/cvr/cvn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Reznichenko M, Tribe RM, Hess PE, Taggart M, Kim H, DeGnore JP, Gangopadhyay S, Morgan KG. Stretch activates human myometrium via ERK, caldesmon and focal adhesion signaling. PLoS One. 2009;4(10):e7489. doi: 10.1371/journal.pone.0007489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietha D, Cai X, Ceccarelli DF, Li Y, Schaller MD, Eck MJ. Structural basis for the autoinhibition of focal adhesion kinase. Cell. 2007;129(6):1177–1187. doi: 10.1016/j.cell.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marganski WA, Gangopadhyay SS, Je H-D, Gallant C, Morgan KG. Targeting of a Novel Ca+2/Calmodulin-Dependent Protein Kinase II Is Essential for Extracellular Signal-Regulated Kinase-Mediated Signaling in Differentiated Smooth Muscle Cells. Circ Res. 2005;97(6):541–549. doi: 10.1161/01.RES.0000182630.29093.0d. [DOI] [PubMed] [Google Scholar]
- Mitchell GF. Arterial Stiffness and Wave Reflection: Biomarkers of Cardiovascular Risk. Artery Res. 2009;3(2):56–64. doi: 10.1016/j.artres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, Mikolon D, Molina JE, Hsia DA, Hanson DA, Chi A, Lim ST, Bernard-Trifilo JA, Ilic D, Stupack DG, Cheresh DA, Schlaepfer DD. Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene. 2006;25(44):5969–5984. doi: 10.1038/sj.onc.1209588. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18(5):516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Ohanian V, Gatfield K, Ohanian J. Role of the Actin Cytoskeleton in G-Protein-Coupled Receptor Activation of PYK2 and Paxillin in Vascular Smooth Muscle. Hypertension. 2005;46(1):93–99. doi: 10.1161/01.HYP.0000167990.82235.3c. [DOI] [PubMed] [Google Scholar]
- Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Vascular Smooth Muscle Cell Stiffness As a Mechanism for Increased Aortic Stiffness With Aging. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar EP, Rozengurt E. Src family kinases are required for integrin-mediated but not for G protein-coupled receptor stimulation of focal adhesion kinase autophosphorylation at Tyr-397. J Biol Chem. 2001;276(21):17788–17795. doi: 10.1074/jbc.M100984200. [DOI] [PubMed] [Google Scholar]
- Seong J, Lu S, Ouyang M, Huang H, Zhang J, Frame MC, Wang Y. Visualization of Src activity at different compartments of the plasma membrane by FRET imaging. Chem Biol. 2009;16(1):48–57. doi: 10.1016/j.chembiol.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura K, Fukumoto Y, Nawata J, Wang H, Onoue N, Tada T, Shirato K, Shimokawa H. Hypertension promotes phosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 in rats: implication for the pathogenesis of hypertensive vascular disease. Tohoku J Exp Med. 2010;222(3):201–210. doi: 10.1620/tjem.222.201. [DOI] [PubMed] [Google Scholar]
- Sun Z, Martinez-Lemus LA, Hill MA, Meininger GA. Extracellular matrix-specific focal adhesions in vascular smooth muscle produce mechanically active adhesion sites. Am J Physiol Cell Physiol. 2008;295(1):C268–278. doi: 10.1152/ajpcell.00516.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DD, Wu MF, Opazo Saez AM, Gunst SJ. The focal adhesion protein paxillin regulates contraction in canine tracheal smooth muscle. J Physiol. 2002;542(Pt 2):501–513. doi: 10.1113/jphysiol.2002.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniyama Y, Weber DS, Rocic P, Hilenski L, Akers ML, Park J, Hemmings BA, Alexander RW, Griendling KK. Pyk2- and Src-dependent tyrosine phosphorylation of PDK1 regulates focal adhesions. Mol Cell Biol. 2003;23(22):8019–8029. doi: 10.1128/MCB.23.22.8019-8029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Ward DT, Alder AC, Ohanian J, Ohanian V. Noradrenaline-induced paxillin phosphorylation, ERK activation and MEK-regulated contraction in intact rat mesenteric arteries. J Vasc Res. 2002;39(1):1–11. doi: 10.1159/000048988. [DOI] [PubMed] [Google Scholar]
- Wu X, Yang Y, Gui P, Sohma Y, Meininger GA, Davis GE, Braun AP, Davis MJ. Potentiation of large conductance, Ca2+-activated K+ (BK) channels by alpha5beta1 integrin activation in arteriolar smooth muscle. J Physiol. 2008;586(6):1699–1713. doi: 10.1113/jphysiol.2007.149500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamboliev IA, Chen J, Gerthoffer WT. PI 3-kinases and Src kinases regulate spreading and migration of cultured VSMCs. Am J Physiol Cell Physiol. 2001;281(2):C709–718. doi: 10.1152/ajpcell.2001.281.2.C709. [DOI] [PubMed] [Google Scholar]