Abstract
C57BL/6J are the most commonly used strain of mouse for stroke experiments but vascular anatomy of the Circle of Willis within this strain is extremely variable and the cortex has extensive collateralization. This causes large variability in stroke models that target the middle cerebral artery proximally and confers resistance to ischemia in those that target it distally. We tested the hypothesis that by combining distal middle cerebral artery occlusion with 1 hour of hypoxia, we could generate a large lesion that causes a behavioral deficit with low variability. We found that this new distal hypoxic (DH) model of stroke generates a lesion with a volume of 25% of the ipsilateral hemisphere, extends to the motor cortex and causes a behavioral deficit. It also has a very clear border, exceptionally low variability, and can be performed by a single surgeon on up to 30 animals a day. Moreover, survivability is 100% in young adult animals, the model can be performed on old animals, and therapeutic intervention can reduce infarct volume. Therefore DH stroke is an excellent complement to existing stroke models and could be used for preclinical studies in C57BL/6J mice.
Keywords: New Mouse Stroke Model, Cerebral Ischemia, Hypoxia, Circle of Willis Variability
1. Introduction
The successful translation of therapeutic strategies for the treatment of stroke is dependent on the reproducibility and reliability of animal stroke models. C57BL/6J (B6) mice are the most commonly used strain of mouse for stroke experiments, due to their ease of genetic manipulation, but B6 mice exhibit large variability in the anatomy of the Circle of Willis and numerous studies have shown that this affects lesion volume in multiple models of cerebral ischemia (Barone et al., 1993; Fujii et al., 1997; Kitagawa et al., 1998; Sheng et al., 1999; Wellons et al., 2000; Yonekura et al., 2004).
The Circle of Willis is a network of blood vessels at the base of the brain that connects the vertebral and carotid circulations and prevents the brain from being starved of blood when one system is occluded. Despite all B6 mice being genetically identical, they do not all have a complete Circle of Willis. Instead, in some individuals the posterior communicating artery (PComA) is missing on one or both sides of the brain (Barone et al., 1993; Kitagawa et al., 1998). In a study by McColl et al only 10% of B6 mice were found to have a complete Circle of Willis, 60% were missing a single PComA and 30% were missing both the left and the right PComA (McColl et al., 2004). Many of the stroke models currently used rely on using one or both common carotid arteries to target downstream circulation and so they are susceptible to variability in Circle of Willis anatomy. Our aim has been to develop a model of stroke that creates a large lesion in B6 mice independent of variability in Circle of Willis anatomy.
Currently, it is possible to bypass the problem of variability in Circle of Willis anatomy by ligating the middle cerebral artery distally (dMCAO). This generates a lesion of consistent size and location, however the lesion is small, typically comprising ~10% of the ipsilateral hemisphere. This may be due to another feature of the cerebral vasculature of B6 mice, which is that they have extensive collateralization between the anterior cerebral artery (ACA) and middle cerebral artery (MCA) (Wang et al., 2010). On average B6 mice have 9 collaterals per hemisphere in this location (Wang et al., 2010), which means that when the MCA is distally occluded, much of the territory fed by the MCA escapes ischemia due to blood flow from ACA collaterals.
The small size of the lesion produced by dMCAO in B6 mice is not an inherent disadvantage. However, the location of the lesion, the whisker barrel cortex, prevents this model from being more widely used. This location prevents dMCAO stroked B6 mice from exhibiting an easily discernible behavioral impairment, making the assessment of recovery, a key goal of stroke research, difficult. The size of the lesion after dMCAO can be increased by simultaneously ligating one or both carotid arteries, but these strategies substantially increase mortality and increase variability by now making the model dependent on Circle of Willis anatomy.
To circumvent these anatomical idiosyncrasies of B6 mice we tested the effect of combining dMCAO with 1 hour of hypoxia (DH stroke). We found that the application of hypoxia expands the lesion generated by dMCAO to incorporate more of the somatosensory cortex and some of the motor cortex, and thereby cause a more easily detectible behavioral deficit. Furthermore, because dMCAO circumvents the problem of inconsistency in Circle of Willis anatomy, the volume of the resulting lesion has exceptionally low variability.
2. Materials and Methods
2.1. Mice
Mice were male BALB/c or C57BL/6J. Adult mice were 5–6 months old. Aged mice were 18 months old. All procedures met NIH guidelines with the approval of the Stanford University Institutional Animal Care and Use Committee.
2.2. India ink Perfusion
To visualize the anatomy of the Circle of Willis 14 male B6 mice (5 months old) were perfused with 0.9% NaCl followed by 25% India ink in 0.9% NaCl/7.5% gelatin at 37°C.
2.3. Surgery
Intraluminal Filament Model of Middle Cerebral Artery Occlusion (MCAO)
Performed by one of the early developers of MCAO in the mouse; Dr Nikola Lessov as he describes in (Clark et al., 1997) (all other surgeries performed by Dr Kristian Doyle). Briefly, mice were anesthetized by 2% isoflurane in 100% oxygen, an incision was made in the neck to expose the common carotid arteries and a silicone-coated 7-0 monofilament nylon surgical suture was inserted into the right carotid. The suture was threaded through the external carotid to the internal carotid and further advanced until it blocked the bifurcation point of the MCA and anterior cerebral artery. The filament was kept in the vessel lumen for 60 min, thereby occluding the MCA. After 60 min the filament was removed, restoring blood flow. Cerebral blood flow (CBF) was monitored throughout surgery by laser Doppler flowmetry (Periflow 5000; Perimed, Sweden). Body temperature was monitored throughout surgery using a rectal thermometer (Hi-Lo Temp 8200 Patient Temperature Monitor, Sensortek Inc, Lake forest, CA, USA). Following surgery, mice were kept on a thermal barrier pad with free access to soft food and water and were sacrificed after 24 hours (N = 14).
Hypoxic/Ischemic Stroke
Mice were anesthetized by 2% isoflurane in 100% oxygen. An incision was made in the neck to expose the common carotid arteries and the right common carotid artery was permanently ligated (Levine, 1960). After a recovery period of approximately 1 hour mice were placed in a plastic chamber containing 8% oxygen and 92% nitrogen for 20 minutes. The chamber, similar in size to a standard mouse cage, was submerged in a 37°C water bath to maintain normothermia during hypoxia. Fresh gas flowed continuously into the chamber throughout hypoxia through vents in the chamber lid. Following hypoxia mice were kept in a warm cage to maintain normothermia and had free access to soft food and water. Mice were sacrificed after 24 hours (N = 14).
Distal Middle Cerebral Artery Occlusion
dMCAO was induced as described previously (Tamura et al., 1981). Briefly, mice were anesthetized by isoflurane inhalation (2% isoflurane in 100% oxygen) and the skin over the right temple was shaved. Skin was swabbed with chlorhexidine and an incision was made to expose the temporalis muscle. A pocket was created in the temporalis muscle and the right middle cerebral artery (MCA) was identified through the skull underneath. A microdrill was used to penetrate the skull and expose the underlying MCA. The meninges were cut and the vessel was cauterized using a small vessel cauterizer. After complete transection of the MCA was visually confirmed, the temporalis muscle was replaced and the wound closed using surgical glue. Throughout surgery body temperature was maintained at 37°C using a feedback controlled heating blanket. Following surgery mice were kept in a warm cage to maintain normothermia and had free access to soft food and water. Sham mice were treated identically except their MCA was not cauterized. Mice were sacrificed after 24 hours for infarct evaluation (N = 8 per group), or after 22 days for the ladder test (N = 10 per group).
Distal Hypoxic (DH) Model of Stroke
dMCAO was performed as described above. Immediately following surgery mice were placed in a large chamber (Coy vinyl anaerobic chamber; Coy Laboratory Products) containing 8% oxygen and 92% nitrogen for 1 hour (Figure 2A). Throughout hypoxia air temperature was held at 37°C by use of a feedback controlled heating fan. During hypoxia the temperature of a sentinel mouse was monitored every 15 minutes and found to hold constant at 37°C. Fresh gas flowed continuously into the chamber throughout hypoxia. Following hypoxia mice were kept in a warm cage to maintain normothermia and had free access to soft food and water. Sham mice underwent sham dMCAO as described above and 1 hour of hypoxia. For the hypothermia treated group the heating fan was turned off during hypoxia, resulting in an ambient temperature of 22°C. Mouse rectal temperature was recorded at the time of entry and exit from the chamber to confirm hypothermia and normothermia in the respective treatment groups (N = 8 adult and 8 aged for infarct evaluation, and N = 10 for each behavior test). Mice were sacrificed after 24 hours for infarct evaluation and at later time points for experiments evaluating recovery.
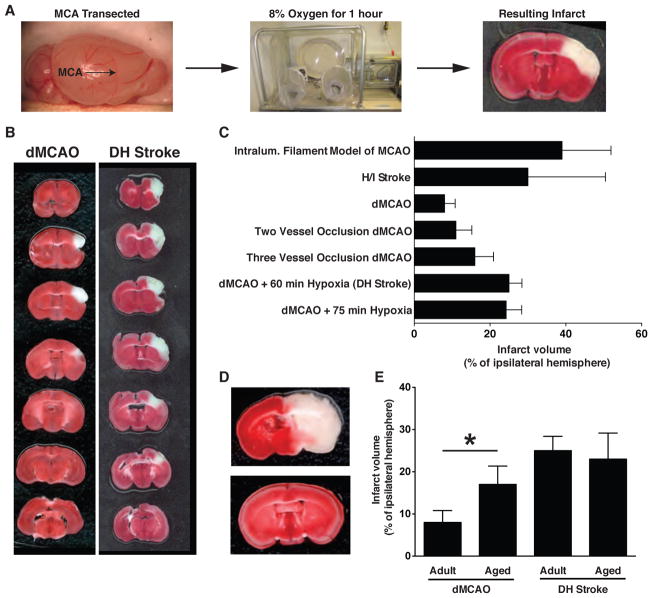
Figure 2.
A) Schematic to show the location of the middle cerebral artery and the sequence of events for performing DH stroke. B) Representative TTC stain to show the entire lesion after dMCAO and DH stroke in B6 mice. C) Comparison of infarct volume and variability of DH stroke with alterative common models of focal stroke. Error bars are SD. D) TTC stains from two mice given H/I stroke with 20 minutes of hypoxia to highlight the extensive variability that this model can produce in B6 mice. E) DH stroke is well tolerated by old mice and infarct volume is unchanged. Conversely, infarct volume is increased after dMCAO in aged mice. *p < 0.05 by Student’s t-test compared to adult mice that underwent dMCAO. Error bars are SD.
Two Vessel Occlusion Model of dMCAO
Mice were anesthetized by 2% isoflurane in 100% oxygen. An incision was made in the neck to expose the common carotid arteries and the right common carotid artery was permanently ligated. Immediately afterwards the neck wound was closed with surgical glue, the animal was turned onto its side and dMCAO was performed as described above. Mice were sacrificed after 24 hours (N = 5).
Three Vessel Occlusion Model of dMCAO
Mice were anesthetized by 2% isoflurane in 100% oxygen. An incision was made in the neck to expose the common carotid arteries and a suture was tied around each carotid artery to occlude blood flow. A timer was started and the animal was turned onto its side to perform dMCAO as described above. After 30 minutes untying the sutures terminated occlusion of the common carotid arteries. Afterwards the neck wound was closed using surgical glue. Mice were sacrificed after 24 hours (N = 5).
2.4. Analgesia/Antibiotics
Immediately following each surgery mice received 10mg/kg of Baytril (enrofloxacin) and 0.1mg/kg of Buprenorphine for infection prophylaxis and pain management.
2.5. Behavior
Handling
In the week prior to behavior training, each mouse was handled every other day for a duration of 5 minutes each session.
Y Maze
Mice were tested in a Y-shaped maze with three white, opaque plastic arms set at a 120° angle from each other. After introduction to the center of the maze, mice were allowed to freely explore the three arms for 8 minutes. Over the course of multiple arm entries, normal mice show a tendency to enter a less recently visited arm. An entry was recorded when all four limbs were within an arm. The number of arm entries and the number of triads were recorded in order to calculate % spontaneous alternation. 20 mice were used for this experiment, 10 received DH stroke and 10 sham dMCAO + 1 hr hypoxia.
Ladder Test
A plexiglass ladder measuring 30 inches across with rungs 0.25 inches apart was supported on clean mouse cages and filmed from underneath (Metz and Whishaw, 2002). To run each trial, mice were removed from their home cage and filmed as they walked across the ladder back towards their home cage. Mice underwent four training sessions on the ladder in the two weeks prior to stroke. For the first session mice were sent across the ladder 3 times. For the second training session mice were sent across the ladder twice. For the third and fourth training sessions mice only went across the ladder once. Each run was filmed and scored for missteps. Post stroke testing was performed in the same way on days 1, 4, 8, 15, and 22 post stroke, with one run per mouse. Data was obtained from a total of 31 mice divided into the following groups: dMCAO (N = 8), sham dMCAO (N = 7), DH stroke (N = 10), sham dMCAO + 1hr hypoxia (N = 6).
Neurological Evaluation
Assessment of neurological outcome was made by an observer blinded to the identity of the mice, and began the day before surgery and continued until post-surgery day 35. The neurological scoring system used was a modification of a well-established sensorimotor assessment system (Garcia et al., 1995). Briefly, the modified system comprised of 5 tests with scores of 0 to 3 for each test (maximum score = 15) (Table 1).
Table 1.
Scoring criteria for neurological evaluation after DH stroke in mice. In the body proprioception and vibrissae touch tests, a fine-tipped paintbrush was swiped against each side of the trunk or vibrissae, respectively. Mice were held by the tail 5cm above the table top for assessment of forelimb and hindlimb symmetry. Lateral turning abilities were observed as the mouse was suspended by the tail 10 cm above the tabletop. In the forelimb walking test, mice were held by the tail and made to walk on forelimbs while the hindlimbs were kept in the air.
| TEST | 3 | 2 | 1 | 0 |
|---|---|---|---|---|
| Body Proprioception | Bilateral brisk response | Bilateral weak response or brisk ipsi and weak contra response | Unilateral response | No response |
| Vibrissae Touch | Bilateral brisk response | Bilateral weak response or brisk ipsi and weak contra response | Unilateral response | No response |
| Limb Symmetry | Forelimb and hindlimb extended | Mid flexion of forelimb and/or mid flexion of hindlimb | Contra forelimb flexed but hindlimb extended or forelimb extended and hindlimb flexed | Contra forelimb and hindlimb completely flexed |
| Lateral Turning | Bilateral turning equal and >45 degrees | Bilateral turning equal but <45 degrees | Unequal turning | No turning |
| Forelimb Walking | Briskly walks forward in symmetry | Moves to one side | Moves in circle | Cannot move on forelimbs |
2.6. Infarct Evaluation
To visualize the region of infarction animals were sacrificed at 24 hours post stroke and their brains processed for TTC staining. Brains were sectioned into 1mm thick slices and each slice was immersed in 1.5% TTC in PBS at 37°C for 15 minutes and then fixed in 10% formalin. The area of the ipsilateral hemisphere and the area of the infarct on each section were measured using NIH ImageJ. Measurements were multiplied by the distance between sections (1mm) and then summed over the entire brain to yield volume measurements. 40μm sections were also stained in 0.5% filtered cresyl violet solution to assess whether DH stroke causes damage to the hippocampus.
2.7. Caspase 3 Immunoflorescence
Immunoflorescence for anti-activated caspase 3 (1:1000; Cell Signaling), was performed on PFA fixed free-floating coronal brain sections (40μm) using standard techniques. Sections were mounted in a medium containing DAPI to enable identification of the CA1 region of the hippocampus.
2.8. Statistical Analysis
Data are expressed as mean +/− standard deviation (SD) or standard error of the mean (SEM). Statistical analyses were performed with Prism 5 software (GraphPad, San Diego, CA). Means between two groups were compared with two-tailed, unpaired Student’s t tests. Comparisons of means from multiple time points for the ladder test were analyzed with one-way ANOVA followed by Tukey’s multiple comparison test.
3. Results
3.1. Variability in Circle of Willis Anatomy in B6 mice
The patency of the ipsilateral PComA has repeatedly been shown to be a major determinant of infarct volume after proximal ligation of the MCA (Barone et al., 1993; Fujii et al., 1997; Kitagawa et al., 1998; Sheng et al., 1999; Wellons et al., 2000; Yonekura et al., 2004). McColl et al report that only 10% of B6 mice have a complete Circle of Willis, with 60% missing a single PComA and 30% missing both the left and right PComA (McColl et al., 2004). This variability in anatomy is corroborated by Majid et al who find that the PComA is unilaterally or bilaterally absent in 45% of B6 mice (Majid et al., 2000). To confirm these findings we perfused 5 month old male B6 mice with India ink and found that the PComA was unilaterally absent in 57% of mice (N = 8), bilaterally absent in 7% of mice (N = 1 ) and only fully formed in 36% of mice (N = 5). Figure 1 shows an example of a fully formed Circle of Willis (A) and a Circle of Willis that only has a PComA present on the right side (B).
Figure 1.
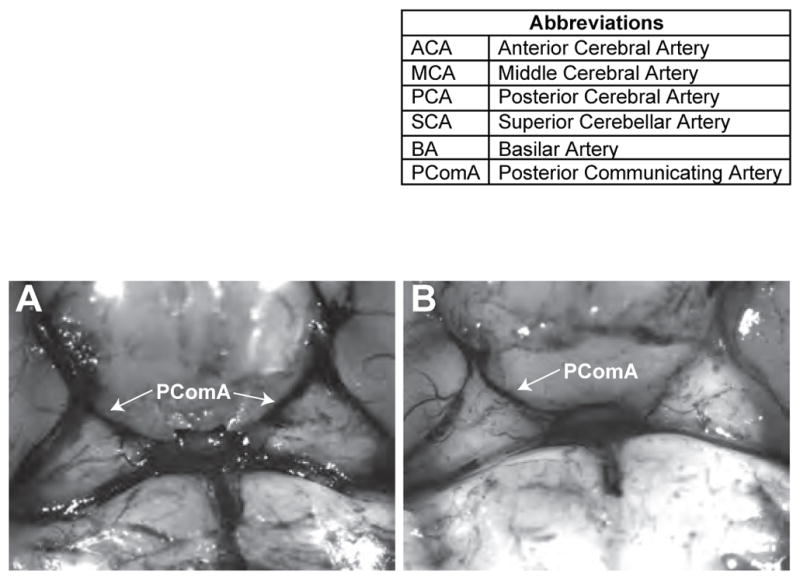
Representative images of Circle of Willis anatomy in B6 mice. In A both PComAs are present. In B the PComA is absent on the left side (PComA = posterior communicating artery).
3.2. The addition of 1 hour of hypoxia increases lesion size after dMCAO in B6 mice
Variability in Circle of Willis anatomy can be bypassed by ligating the middle cerebral artery distally (dMCAO) but this only results in a very small lesion in B6 mice. To test if combining dMCAO with 1 hour of hypoxia (DH stroke) increases lesion size in B6 mice, lesion volume generated by DH stroke was compared to lesion volume generated by dMCAO. At 24 hours post stroke, dMCAO yielded an infarct volume of 8% (SD+/−2.8%) of the ipsilateral hemisphere and DH stroke resulted in a lesion that was 25% (SD+/−3.4%) of the ipsilateral hemisphere, thus demonstrating that the addition of 1 hour of hypoxia causes a >300% increase in infarct volume (Figure 2 & Table 2). The lesion generated by dMCAO was predominantly restricted to the barrel cortex, while the lesion generated by DH stroke extended beyond the barrel cortex and somatosensory cortex and extended into the primary motor cortex. DH stroke did not cause any overt injury to deeper brain structures and the border between infarcted and non-infarcted tissue was sharp.
Table 2.
Comparison of infarct volume and variability between common stroke models. The sample size required for each stroke model to detect a 20% difference in infarct volume between two groups was calculated using a two group power analysis.
| Stroke model | Mean infarct volume (% of ipsilateral hemisphere) | SD | N | Variability ratio: SD/Mean | Sample size to detect 20% difference in infarct volume |
|---|---|---|---|---|---|
| Suture model of MCAO | 39 | 12.9 | 14 | 0.33 | 15 |
| H/I stroke | 30 | 20.42 | 14 | 0.68 | 63 |
| Two vessel occlusion | 11 | 4.2 | 5 | 0.38 | 20 |
| Three vessel occlusion | 16 | 4.9 | 5 | 0.31 | 13 |
| dMCAO (adult) | 8 | 2.8 | 8 | 0.35 | 17 |
| dMCAO (aged) | 17 | 4.34 | 8 | 0.26 | 9 |
| DH stroke (adult) | 25 | 3.4 | 8 | 0.14 | 4 |
| DH stroke (aged) | 23 | 6.18 | 6 (+2 died) | 0.27 | 10 |
SD = standard deviation. N = number of animals used per stroke model. Adult = 5 month old mice. Aged = 18 month old mice.
To test if increasing the duration of hypoxia increases lesion volume even further, B6 mice underwent dMCAO with 75 minutes of hypoxia. At 24 hours post stroke, this resulted in a lesion volume of 23% of the ipsilateral hemisphere, indicating that extending the duration of hypoxia does not cause further expansion of the lesion (Figure 2B).
3.3. DH Stroke has exceptionally low variability
We compared infarct volume and variability obtained with DH stroke to infarct volume and variability of several established models of stroke - two vessel occlusion dMCAO, three vessel occlusion dMCAO, intraluminal filament MCAO, and hypoxic ischemic (H/I) stroke. Two vessel occlusion dMCAO and three vessel occlusion dMCAO generated lesions of 11% (SD+/−4.2%) and 16%(SD+/−4.9%) of the ipsilateral hemisphere respectively (Figure 2B & Table 2). The intraluminal filament model of MCAO resulted in a lesion that encompassed 39%(SD+/−12.9%) of the ipsilateral hemisphere, while lesion volume after 20 minute H/I stroke was 30%(SD+/−20.4%) of the ipsliateral hemisphere (Figure 2B & Table 2).
These values were used to calculate the ratio of standard deviation/infarct volume for all of the stroke models tested (Table 2). By normalizing variability to infarct volume this calculation provides an empirical measure for comparing the uniformity of each stroke model. A value closer to 1 indicates more variability. The DH model of stroke gave the smallest ratio of all the models tested with a ratio of 0.14. The intraluminal filament model of stroke, the three vessel occlusion model and the two vessel occlusion model all had greater values, 0.33, 0.31 and 0.38 respectively. The greatest variability was seen with the H/I model of stroke, which yielded a ratio of 0.68. In this model, genetically identical B6 mice that had undergone the same procedure could present with either enormous infarcts or an almost complete absence of injury (Figure 2C). This stroke model relies upon ligation of the right carotid artery to generate a lesion in areas of the brain that are fed by vessels far downstream from the point of ligation. Therefore this high degree of variability is likely due to both differences in Circle of Willis anatomy as well as other downstream differences in the circulatory system between individual mice.
The small variability of the DH model of stroke means that fewer animals are required for a stroke study. For example, for a hypothetical neuroprotection study designed to detect a >20% difference in infarct volume, power analysis predicts that the intraluminal filament model of stroke would require a sample size of 15 mice per group, and the H/I model would require a sample size of 63 mice per group. In contrast, the sample size required for the DH model of stroke is only 4 per group, a significant reduction in animal use (Table 2).
3.4. DH stroke can be performed on aged mice
To determine if aged animals can tolerate 1 hour of hypoxia after dMCAO, and if infarct volume after DH stroke is affected by age, we performed dMCAO or DH stroke on 18 month old B6 mice. Six out of eight mice that underwent DH stroke survived the procedure and infarct volume was unaffected. The two mice that died did so during the 1 hour period of hypoxia. This indicates that although it is possible to perform DH stroke on aged mice, an allowance must be made for a mortality rate of 25%. Interestingly, although all of the aged mice that underwent dMCAO survived, infarct volume was significantly increased in the 18 month old cohort (Figure 2D).
3.5. The addition of 1 hour of hypoxia does not increase lesion size after dMCAO in BALB/c mice
B6 mice have extensive collateralization between the anterior cerebral artery (ACA) and middle cerebral artery (MCA), in contrast BALB/c mice do not have as many connections between these two circulatory systems and this anatomical difference is reported to be a major determinant of stroke severity (Zhang et al., 2010). To compare the effect of dMCAO and DH stroke in each of these mouse strains BALB/c mice underwent dMCAO or dMCAO plus 1 hour of hypoxia and were euthanized 24 hours later for determination of infarct size. In BALB/c mice, dMCAO generated an infarct that had a volume of 24% of the ipsilateral hemisphere and the addition of 1 hour of hypoxia (DH stroke) resulted in an equivalent infarct volume of 21% (Figure 3A & B).
Figure 3.
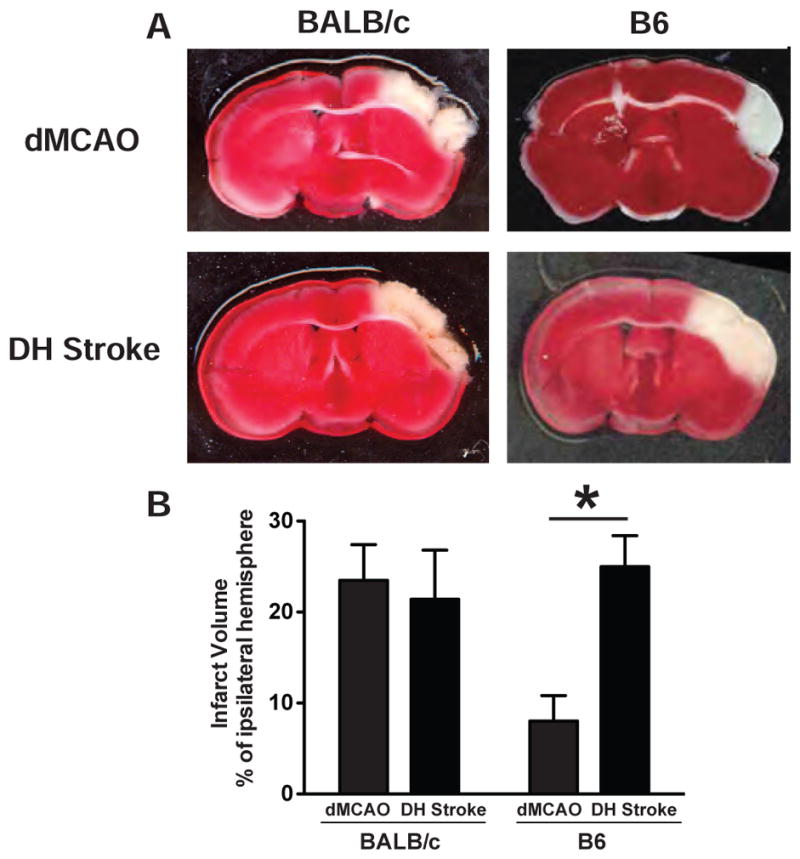
A) Representative TTC stains after dMCAO and DH stroke in BALB/c and B6 mice. B) Lesion volume after dMCAO and DH stroke in BALB/c and B6 mice. The addition of 1 hour of hypoxia significantly increased lesion volume in B6 but not BALB/c mice. *p<0.05 by Student’s t-test compared to B6 mice that underwent dMCAO. Error bars are SD.
3.6. DH stroke does not injure the hippocampus
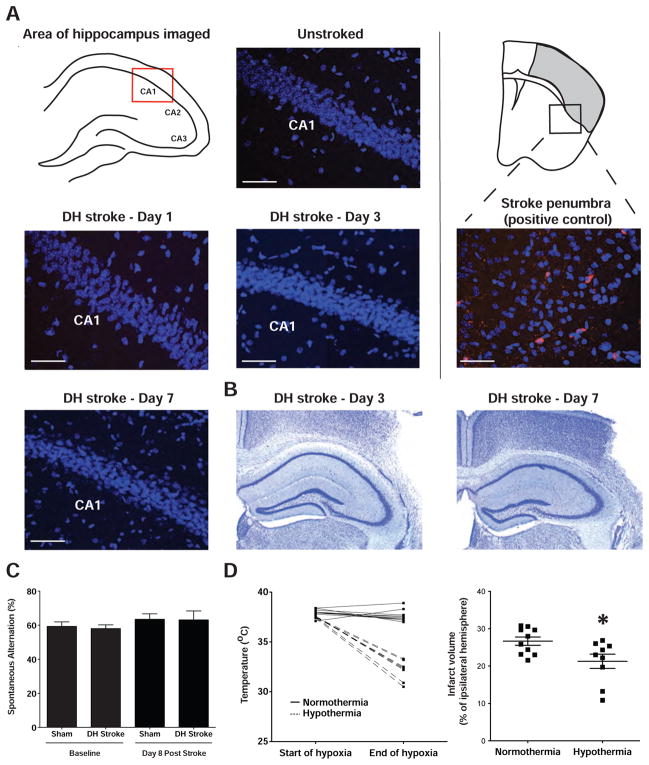
Pyramidal neurons in the CA1 sector of the hippocampus are particularly vulnerable to hypoxia and ischemia and selective loss of CA1 neurons can take 2–3 days to develop (Kirino, 2000; Liu et al., 2009). Therefore, we examined the hippocampus for evidence of apoptosis by activated caspase 3 immunostaining at days 1, 3 and 7 after ischemia. We did not observe any cell death in any part of the hippocampus at any time point (Figure 4A). To confirm these findings we also performed a cresyl violet stain of the hippocampus and did not observe neuronal loss or the presence of pyknotic neurons (Figure 4B). To further verify that DH stroke does not cause damage to the hippocampus, a separate group of mice were tested on the Y maze, a test of spontaneous alternation that requires intact short-term memory. No deficit was observed on this test on day 8 after stroke (Figure 4C).
Figure 4.
The DH model of stroke does not cause overt damage to the hippocampus and the model can be used for studies of neuroprotection. A) The CA1 region of the hippocampus, which contains the neurons most sensitive to ischemia, did not show any evidence of activated caspase 3 immunoreactivity (marker of apoptosis) on day 1, 3 or 7 post DH stroke. The stroke penumbra on day 1 is shown as a positive control for activated caspase 3 staining. Blue = DAPI, Red = activated caspase 3. Scale bar = 50μm. B) Cresyl Violet Staining. The hippocampus is intact and does not show evidence of neuronal loss or pyknotic neurons on day 3 and day 7 after DH stroke. C) Y maze test of spontaneous alternation pre-stroke and at 8 days (Hsiao et al., 1996). Normal mice should have an alternation rate of ~60% and cognitively impaired mice randomly enter each arm, resulting in an alternation rate of 50%. There is no impairment before stroke or 8 days later. Error bars are SEM. D) Hypothermia during hypoxia significantly reduces infarct volume in the DH model of stroke suggesting that there is a salvageable penumbra for studies of neuroprotection. *p<0.05 by Student’s t-test compared to normothermia control group. Error bars are SEM.
3.7. DH stroke can be used for studies of neuroprotection
To determine if the penumbra generated by DH stroke is salvageable by neuroprotective intervention we tested whether hypothermia during hypoxia would reduce infarct volume. We induced hypothermia by carrying out hypoxia in a chamber maintained at room temperature instead of 37°C. This modification of our procedure reduced mouse temperature over the course of the 1 hour period of hypoxia to 32°C. This level of hypothermia significantly reduced infarct volume by 22% and thus demonstrates that the DH model of stroke could be used to test stroke therapies (Figure 4D).
3.8. DH stroke causes a motor deficit
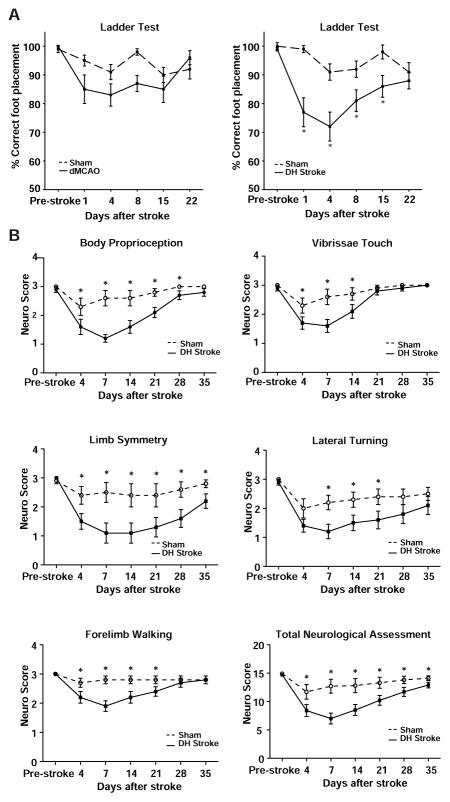
To determine if the DH model of stroke causes a behavioral deficit, a separate group of B6 mice were tested for their ability to traverse a horizontal ladder without making errors in foot placement. This test revealed that DH stroke causes significant hemiparesis to the contralateral front paw, when compared to mice that have undergone sham DH stroke. There was a significant deficit in correct foot placement by one-way ANOVA on days 1, 4, 8 and 15 after stroke. During this time there was progressive recovery and the deficit was no longer apparent on day 22 (Figure 5A). dMCAO or sham dMCAO (craniectomy but no ligation of the MCA) did not cause a deficit (Figure 5A). To confirm that DH stroke causes a behavioral deficit, mice also underwent neurological evaluation for 5 weeks after stroke. Mice had a sensory deficit in body proprioception and vibrissae sensitivity that lasted for 2–4 weeks (Figure 5B), and a motor deficit that showed progressive recovery but was still present at 5 weeks (Figure 5B).
Figure 5.
The DH model of stroke causes a behavioral deficit. A) Mice that underwent right side DH stroke showed significant hemiparesis in the left front paw for 15 days following stroke. Mice that underwent sham DH stroke (hypoxia + craniectomy, but no ligation of the MCA), sham dMCAO (craniectomy but no ligation of the MCA) or dMCAO did not have significant ladder deficits. Groups have been separated onto two graphs for clarity. * p<0.05 compared to sham by one way ANOVA followed by Tukey’s multiple comparison test. B) Neurological evaluation reveals a sensory and motor deficit after DH stroke. Mice that underwent right side DH stroke showed significant sensory deficits in body proprioception and vibrissae touch. They also had significant motor deficits in limb symmetry, lateral turning ability and forelimb walking ability. * P<0.05 by Student’s t-test compared to mice that underwent sham DH stroke. Error bars are SEM.
4. Discussion
We have developed a new model of stroke, which we call the DH model of stroke for distal MCAO occlusion + hypoxia. Our goal was to develop a model of stroke for B6 mice that causes a robust behavioral deficit for the assessment of recovery but with low variability. The DH model of stroke consists of performing a craniectomy and ligating the right middle cerebral artery using a small vessel cauterizer. Immediately following surgery mice are given hypoxia by placing them in a chamber containing 8% oxygen for 1 hour. The resulting infarct has a well-defined boundary for easy discrimination of infarcted tissue, a penumbra so that brain tissue can be salvaged via neuroprotective strategies, and less variability per volume of infarcted tissue than any of the preexisting models that we tested.
The DH model of stroke causes a behavioral deficit that is discernable by the ladder test. The deficit was predominantly localized to the contralateral front paw and lasted for more than 2 weeks. The ladder test has several advantages over alternative motor tests; it is fast to perform, with mice taking less than 25 seconds to traverse the ladder, and the recording of missed steps is extremely objective. Mice also had a sensorimotor deficit on a commonly used method of neurological evaluation (Garcia et al., 1995). This test revealed that mice have a significant sensory deficit that is detectable for 4 weeks and a significant motor impairment that is detectable for 5 weeks. The fact that there was substantial recovery in all of these tests shows that DH stroke is a useful model for investigating mechanisms of recovery from stroke.
The DH model of stroke does not cause mortality in adult B6 mice and is tolerated by 18 month old mice. There is a 75% survival rate in aged mice and infarct volume is not increased. This is in stark contrast to the intraluminal filament model of stroke, where in adult mice 60 minutes of occlusion can cause >80% mortality in the first week after stroke; mortality in aged animals is likely to be even higher (Kitagawa et al., 1998). This means that the intraluminal filament model of MCAO cannot easily be applied to studies that look at recovery or aging. The DH model of stroke presents an attractive alternative.
There are drawbacks to using hypoxia as part of a stroke model. Hypoxia does not reduce blood flow in the same way as an occluded artery and hypoxia likely causes transcriptional changes throughout the brain and other organs (Ran et al., 2005). However, the application of hypoxia did not cause any overt injury to any location outside of the stroke lesion and the ischemia sensitive hippocampus was functionally and histologically intact 1 week after stroke. Moreover, sham and DH stroked animals survive for over 5 weeks following hypoxia with no obvious adverse affects, with both groups continuing to groom and gain weight normally. Other investigators have also treated rodents with 3 hours of 8% oxygen with no apparent adverse effects (Ran et al., 2005). Therefore 1 hour of 8% oxygen does not appear to be injurious per se.
An experienced surgeon can perform the surgery required for the DH model of stroke in 15 minutes. This is significantly less time than is required for the intraluminal filament model of MCAO or for the two and three vessel occlusion models of stroke. This means that studies can be performed with a high throughput approach and the surgery required for a large cohort does not need to be split over multiple days, which streamlines behavioral testing. This coupled with the low variability associated with DH stroke should be weighed against the disadvantage of using hypoxia.
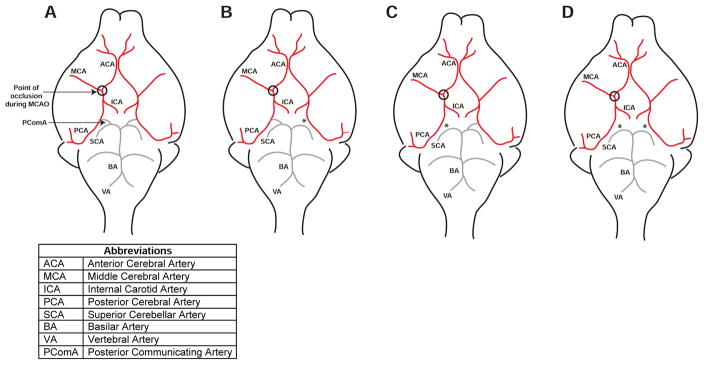
The commonly used intraluminal filament model of MCAO with 60 minutes of occlusion, causes a large infarct and a behavioral deficit; however in B6 mice variability of up to 40% is considered acceptable, as described in a recently published set of standard operating procedures (Dirnagl and MCAO-SOP, 2009). Much of this variability is caused by variation in the anatomy of the Circle of Willis and the patency of the ipsilateral PComA (Barone et al., 1993; Fujii et al., 1997; Kitagawa et al., 1998; Sheng et al., 1999; Wellons et al., 2000; Yonekura et al., 2004). When the ipsilateral PComA is present only the territory fed by the MCA becomes ischemic during MCAO, but when the PComA is absent the territory fed by the posterior cerebral artery also becomes ischemic (Figure 6). Altering the duration of MCAO will not bypass this fundamental difference in anatomy.
Figure 6.
Impact of variation in Circle of Willis anatomy on blood flow after MCAO. A–D) B6 mice present with at least 4 different PComA anatomical variations. In A & B the ipsilateral PComA is present, therefore the superior cerebellar artery (SCA) can feed blood into the ipsilateral posterior cerebral artery (PCA), resulting in a smaller infarct when the origin of the middle cerebral artery (MCA) is occluded. In C & D the ipsilateral PComA is absent, therefore there is no collateral flow from the SCA into the PCA, resulting in a larger infarct when the origin of the MCA is occluded. The location of the end of the suture used to occlude the MCA during MCAO is circled. * Denotes missing PComA.
Variability in Circle of Willis anatomy is a problem that does not just affect the intraluminal filament model of MCAO. It likely has an equivalent impact in all other models of stroke that rely on using one or both common carotid arteries to target downstream circulation such as the three vessel, two vessel, and hypoxic/ischemic models of stroke.
Circle of Willis anatomy is not the only complication specific to B6 mice that makes them problematic for stroke studies. The distal model of middle cerebral artery occlusion creates a much smaller lesion in B6 mice than in BALB/c mice, and does not extend to the primary motor cortex to cause a motor deficit (Majid et al., 2000). This variability in lesion size is independent of PComA anatomy (Majid et al., 2000). Instead, it may be due to the existence of many more cross connections between the ACA and the MCA in the pial circulation of B6 mice as shown by Wang et al (Wang et al., 2010). B6 mice have a well developed pial collateral network of blood vessels averaging 9 pial collaterals per hemisphere, in contrast to BALB/c mice which average less than one (Wang et al., 2010). Performing the dMCAO model of stroke in BALB/c mice is a simple way of generating a large, highly reproducible lesion with a motor deficit, however in practice this is rarely done since BALB/c mice have poor vision, high emotional reactivity, are worse than B6 mice at learning behavior, and most transgenic mice are created on a B6 background (Brown and Wong, 2007; Crawley et al., 1997).
There is no reperfusion after ligation of the MCA with the DH model of stroke and so it cannot be used to model reperfusion injury. However, it is possible to tie a suture around the MCA at the point of ligation and so this could be a means of modifying the model to study reperfusion. We have not performed DH stroke in female mice, and so there is a possibility that infarct size may be different in females. However, we do not expect that DH stroke will have more variability in females, and so it is likely to be just as useful for studies using female mice.
In summary we present here a mouse model of stroke that is especially useful for B6 mice. This model creates a lesion in the primary motor cortex with low variability, and causes hemiparesis to the contralateral front paw and a sensorimotor deficit from which there is recovery in the weeks after stroke. Surgery can be performed in a high throughput manner and using therapeutic hypothermia we have shown that the model can be used to test potential neuroprotectants. Importantly, the model can be performed on aged mice. Together these data demonstrate that the DH model of stroke is a useful new tool for stroke research.
Highlights.
C57BL/6 mice are the most commonly used strain of mouse for stroke experiments.
The Circle of Willis in this strain is extremely variable.
This causes large variability in most commonly used stroke models.
We introduce a stroke model tailored for C57BL/6 mice that bypasses Circle of Willis variability.
Acknowledgments
This work was supported by an Ellison Medical Foundation/AFAR Postdoctoral Fellowship to KPD and NINDS KO8 NS050304 and American Heart Association 10GRNT4140073 to MSB. The authors have no conflict of interest. We would like to thank Dr. Mary Stenzel-Poore for technical support, Dr. Nikola Lessov for performing the intraluminal filament model of MCAO, and Geoffrey Stanley and Dr. Kereshmeh Taravosh-Lahn for their assistance with pilot experiments.
Footnotes
Conflict of Interest Disclosure
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barone FC, Knudsen DJ, Nelson AH, Feuerstein GZ, Willette RN. Mouse strain differences in susceptibility to cerebral ischemia are related to cerebral vascular anatomy. J Cereb Blood Flow Metab. 1993;13:683–92. doi: 10.1038/jcbfm.1993.87. [DOI] [PubMed] [Google Scholar]
- Brown RE, Wong AA. The influence of visual ability on learning and memory performance in 13 strains of mice. Learn Mem. 2007;14:134–44. doi: 10.1101/lm.473907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WM, Lessov NS, Dixon MP, Eckenstein F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol Res. 1997;19:641–8. doi: 10.1080/01616412.1997.11740874. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–24. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Dirnagl U MCAO-SOP Mot. Standard operating procedures (SOP) in experimental stroke research: SOP for middle cerebral artery occlusion in the mouse. Nature Precedings. 2009 [Google Scholar]
- Fujii M, Hara H, Meng W, Vonsattel JP, Huang Z, Moskowitz MA. Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57black/6 mice. Stroke. 1997;28:1805–10. doi: 10.1161/01.str.28.9.1805. discussion 11. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–34. doi: 10.1161/01.str.26.4.627. discussion 35. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death. Neuropathology. 2000;20(Suppl):S95–7. doi: 10.1046/j.1440-1789.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, Yanagihara T. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 1998;18:570–9. doi: 10.1097/00004647-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Levine S. Anoxic-ischemic encephalopathy in rats. Am J Pathol. 1960;36:1–17. [PMC free article] [PubMed] [Google Scholar]
- Liu F, Schafer DP, McCullough LD. TTC, fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid A, He YY, Gidday JM, Kaplan SS, Gonzales ER, Park TS, Fenstermacher JD, Wei L, Choi DW, Hsu CY. Differences in vulnerability to permanent focal cerebral ischemia among 3 common mouse strains. Stroke. 2000;31:2707–14. doi: 10.1161/01.str.31.11.2707. [DOI] [PubMed] [Google Scholar]
- McColl BW, Carswell HV, McCulloch J, Horsburgh K. Extension of cerebral hypoperfusion and ischaemic pathology beyond MCA territory after intraluminal filament occlusion in C57Bl/6J mice. Brain Res. 2004;997:15–23. doi: 10.1016/j.brainres.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and coordination. J Neurosci Methods. 2002;115:169–79. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Ran R, Xu H, Lu A, Bernaudin M, Sharp FR. Hypoxia preconditioning in the brain. Dev Neurosci. 2005;27:87–92. doi: 10.1159/000085979. [DOI] [PubMed] [Google Scholar]
- Sheng H, Laskowitz DT, Pearlstein RD, Warner DS. Characterization of a recovery global cerebral ischemia model in the mouse. J Neurosci Methods. 1999;88:103–9. doi: 10.1016/s0165-0270(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang H, Dai X, Sealock R, Faber JE. Genetic architecture underlying variation in extent and remodeling of the collateral circulation. Circ Res. 2010;107:558–68. doi: 10.1161/CIRCRESAHA.110.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellons JC, 3rd, Sheng H, Laskowitz DT, Burkhard Mackensen G, Pearlstein RD, Warner DS. A comparison of strain-related susceptibility in two murine recovery models of global cerebral ischemia. Brain Res. 2000;868:14–21. doi: 10.1016/s0006-8993(00)02216-2. [DOI] [PubMed] [Google Scholar]
- Yonekura I, Kawahara N, Nakatomi H, Furuya K, Kirino T. A model of global cerebral ischemia in C57 BL/6 mice. J Cereb Blood Flow Metab. 2004;24:151–8. doi: 10.1097/01.WCB.0000096063.84070.C1. [DOI] [PubMed] [Google Scholar]
- Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. 2010;30:923–34. doi: 10.1038/jcbfm.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]