Abstract
In-situ crosslinkable polyethylene glycol (PEG)-based polymers play an increasing role in surgical practice as sealants that provide a barrier to fluid/gas leakage and adhesion formation. This study investigated the gelation behavior and physical properties of hydrogels formed from homogeneous and blended solutions of two acrylated poloxamines (Tetronics® T1107 and T904) of varying molecular weight and hydrophilic/lipophilic balance relative to a PEG control. Hydrogels were formed by reverse thermal gelation at physiological temperature (T1107-containing formulations) and covalent crosslinking by Michael-type addition with dithiothreitol. All poloxamine-based hydrogels exhibited thermosensitive behavior and achieved significantly reduced swelling, increased tensile properties, and increased tissue bond strength relative to the PEG hydrogel at physiological temperature. Swelling and tensile properties of all poloxamine-based hydrogels were significantly greater at 37 °C relative to 4 °C, suggesting that their improved physical properties derive from cooperative crosslinking by both noncovalent and covalent mechanisms. Poloxamine-based hydrogels were cytocompatible and underwent hydrolytic degradation over 2 to 5 weeks depending on their T1107/T904 composition. In conclusion, select poloxamine-based hydrogels possess a number of properties potentially beneficial to tissue sealant applications including substantial increase in viscosity between room/physiological temperatures, resistance to cell adhesion, and maintenance of stable volume during equilibration.
1. Introduction
Sutures and surgical staples are the most widely used methods of wound closure, despite limitations including the requirement for anesthesia; risk of infection; time and skill required; and difficulty in application/retention in soft tissues such as internal organs.[1, 2] This has generated considerable interest in the development of in-situ curable polymeric materials that may be used as tissue adhesives/sealants either alone or as an adjunct to sutures. One major group of these materials are tissue glues based on naturally-derived materials such as fibrin glue, gelatin-resorcinol-formaldehyde glue, and more recently BioGlue® (CryoLife) composed of bovine serum albumin crosslinked by glutaraldehyde. Fibrin and BioGlue® are FDA approved and have been used successfully in many clinical applications.[3-5] Although advantageous because of their intrinsic biodegradability, naturally-derived materials involve risks of possible viral transmission, hypersensitive reactions to bovine proteins, and relatively low mechanical properties. In addition, cytoxicity has been reported with the use of low molecular weight aldehyde-based crosslinkers.[6] To overcome these limitations, recent studies have focused on the development of fully synthetic alternatives.
Alkyl-2-cyanoacrylates were the first materials investigated as synthetic tissue adhesives. These low molecular weight monomers undergo rapid polymerization upon exposure to weak bases present in tissue. Cyanoacrylates with short side chains (methyl/ethyl) were first studied, but deemed unsuitable due to high stiffness and evidence of cytotoxicity and tissue necrosis.[7, 8] Butyl (Indermil®, Covidien and Histoacryl®, Tissueseal) and octyl (Dermabond®, Closure Medical) derivatives have been approved for topical wound closure and as bacterial barriers.[9-11] However, internal use of cyanoacrylates remains limited due to their relatively slow degradation and concerns about toxicity of the degradation products. Recent developments in materials for internal applications have focused on various polyethylene glycol (PEG)-based hydrogels such as FocalSeal® (Genzyme BioSurgery), CoSeal® (Baxter) and DuraSeal™ (Confluent Surgical). FocalSeal® consists of PEG diols modified with hydrolytically degradable ester linkages and terminal acrylates for photoinitiated polymerization.[12] It has been successfully used for sealing lung air leaks, but its widespread adoption has been hindered by relatively slow curing and the additional surgical equipment required.[13, 14] CoSeal® and DuraSeal™ are both crosslinked by step-growth polymerizations of 4-arm PEG-based macromonomers with degradable linkages and terminal reactive groups. CoSeal® and Duraseal™ are FDA-approved for vascular and dural sealing, respectively, and have shown efficacy in other experimental models as well.[15-17] A common challenge to PEG-based sealants is significant post-polymerization swelling resulting in increases in volume ranging from 50 to 400% relative to the initial volume dispensed.[18-20] Two recent reports have described serious clinical complications believed to be attributable to swelling-induced compression of neighboring tissues.[21, 22]
The objective of this study was to develop and characterize hydrogels based on acrylated poloxamine (Tetronic®) block copolymers. Poloxamines are a family of 4-arm polypropylene oxide (PPO)-polyethylene oxide (PEO) block copolymers that spontaneously form micelles in aqueous solution consisting of a hydrophobic PPO core domain surrounded by a hydrophilic PEO shell.[23] They have recently been investigated for applications in drug delivery, gene therapy, and tissue engineering. [24-29] Cellesi and co-workers first described the fabrication of poloxamine microspheres for cell encapsulation based on a “tandem gelation” process combining reverse thermal gelation and covalent crosslinking.[30, 31] We hypothesized that the combination of noncovalent and covalent crosslinking available with these materials would provide improved physical properties for sealant applications relative to conventional PEG-based hydrogels prepared solely by covalent crosslinking. In this study, hydrogels were crosslinked from homogeneous and blended solutions of two poloxamines (T1107 and T904) of different molecular weight and hydrophilic/lipophilic balance. All poloxamine-based hydrogels exhibited thermosensitive behavior and at physiological temperature achieved significantly lower swelling and significantly higher tensile properties and tissue bond strength relative to the PEG hydrogel control.
2. Materials and methods
2.1 Materials
Tetronics® T1107 (T1107, MW:15 kDa, HLB:18-23) and T904 (T904, MW:6.7 kDa, HLB:12-18) were kindly donated by BASF corporation (USA). Acrylated 4-arm polyethylene glycol (PEG; MW: 10 kDa) prepared as previously described [32] was a gift from Dr. Andrew Metters (Clemson University). Acryloyl chloride, celite fine 500, and 4-methoxyphenol were purchased from Sigma-Aldrich (St. Louis, MO, USA). Toluene (HPLC grade), ethyl ether (anhydrous, BHT stabilized), hexanes (HPLC grade), and anhydrous sodium sulfate were purchased from Fisher scientific (NJ, USA). Dichloromethane (HPLC grade), triethylamine (TEA), dithiothreitol (DTT), sodium bicarbonate, calcium hydride, and CDCl3 were obtained from Acros Organics (NJ, USA). Dichloromethane was dried with calcium hydride and stored over molecular sieves (Grade 514, Type 4A). All other chemicals were used as received.
2.2 Preparation and characterization of acrylated poloxamines
Preparation of Acrylated T1107(T1107 ACR)
T1107 ACR was prepared by reaction of the T1107 terminal hydroxyl groups with acryloyl chloride. All glassware was dried in a vacuum oven and fully purged with argon before use. 15g (1 mmol) T1107 was dehydrated by azeotropic distillation with toluene for two hours and the toluene was then removed by rotary evaporation (Buchi Rotavapor®, Switzerland). After cooling to room temperature, dried T1107 was dissolved in 120 ml dry dichloromethane in a 250 ml round bottom flask under an argon atmosphere. Once the T1107 was completely dissolved, TEA (4 mmol) was added and the reaction flask cooled on an ice bath. Acryloyl chloride (8 mmol) in 30 ml dry dichloromethane was added dropwise with an addition funnel over two hours. The ice bath was removed after 2 hours and the reaction was allowed to continue at room temperature for 24 hours. The reactant was filtered through celite to remove TEA-HCl salt, and then concentrated by rotary evaporation to reduce the solvent to 1/10th of its initial volume. The residue was precipitated in 500 ml cold ethyl ether, recovered by filtration, and dried under vacuum for a few hours. The product was redissolved in 150 ml dichloromethane, washed with 15 ml 10% w/v sodium bicarbonate solution until the pH of the solution was neutral, followed by water washes (15 ml each) until the pH of the water was neutral, and then dried with anhydrous sodium sulfate. The solution was concentrated by rotary evaporation, the residue precipitated in ethyl ether (-20°C), and washed 3 times with cold ethyl ether. The final product was recovered by filtration, dried 48 hours in a vacuum desiccator, and stored at 4 °C.
Preparation of acrylated T904 (T904 ACR)
T904 was dried and reacted with acryloyl chloride in the presence of TEA as described above for 24 hours at 4 °C. The purification procedure was adjusted to account for the reduced thermal stability and increased ether solubility of acrylated T904. Briefly, after filtration and solvent reduction (in the presence of a small amount of 4-methoxyphenol), the residue was precipitated in 50:50 ethyl ether:hexane overnight at -20 °C. After decantation of the supernatant, the product was redissolved in dichloromethane, washed as described above, concentrated and precipitated once in 50:50 ethyl ether:hexane and recrystallized twice from ice cold ethyl ether after 48 hours at 4 °C. The product was dried in a vacuum dessicator at 4 °C and stored at -20 °C.
1H-NMR (CDCl3) characterization
T1107 ACR and T904 ACR: δ= 1.1 (m, PPO CH3), 3.4 (m, PPO CH), 3.54 (m, PPO CH2), 3.65 (m, PEO CH2), 4.32 (t, -CCH2OC(=O)-), 5.8 and 6.4 (d, 2H, acrylic −CH2), 6.15 (q, 1H, acrylic −CH) ppm. A representative NMR spectra of acrylated T1107 is shown in Figure 1. Percent acrylation efficiency was calculated based on the ratio of the integrals of the PEG back bone (δ=3.5~3.7) and the acrylate peaks (δ=6.15-6.4). Acrylation efficiencies of 94-100% (T1107 ACR) and 84-90% (T904 ACR) were consistently obtained. The ratio of acrylate to activated PEG terminal methylene (δ=4.32) peaks closely approximated the 3:2 theoretical value, confirming efficient removal of unreacted acryloyl chloride.
Figure 1.
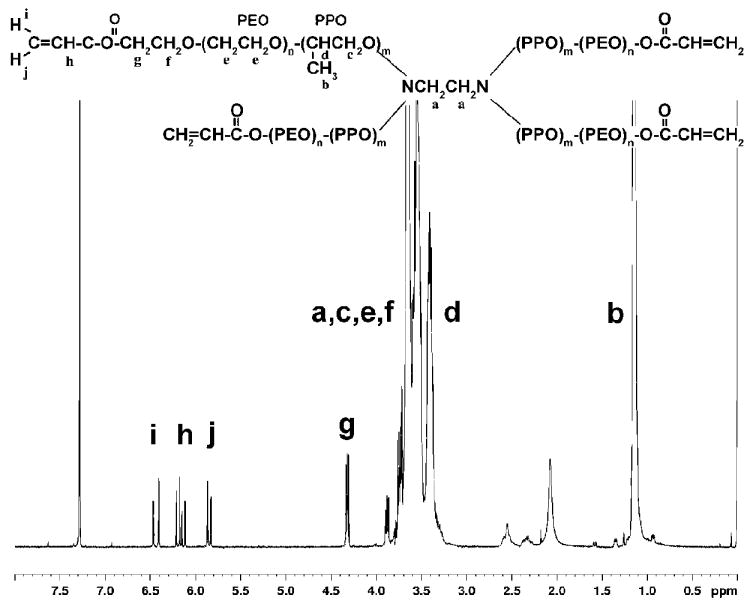
Poloxamine (Tetronic®) structure (T1107: n=60, m=19; T904: n=15, m=17) and representative 1H-NMR spectrum of acrylated T1107.
2.3 Gelation Analysis
Unmodified (reverse thermal gelation) or acrylated (tandem crosslinking by reverse thermal gelation and Michael-type addition) poloxamine macromers were dissolved at varying T1107/T904 mass ratios (100/0, 75/25, 50/50, 0/100) in PBS (pH7.4, 50mM; 5.75g/l Na2HPO4, 0.88g/l NaH2PO4, and 4.8g/l NaCl).[33] A 4-arm PEG macromer was included as a control. All samples were prepared at a final macromer concentration of 30% w/v (Table I).
Table I.
Formulation of Poloxamine and DTT Solutions
| Tetronic® / Tetronic® ACR Solution | DTT/Buffer Solution | ||||
|---|---|---|---|---|---|
| T1107/T904 composition | T1107 (mg) | T904 (mg) | PBS (μl) | DTT (mg) | PBS (μl) |
| 100/0 | 30 | 0 | 60 | 0.61 | 10 |
| 75/25 | 22.5 | 7.5 | 0.79 | ||
| 50/50 | 15 | 15 | 0.97 | ||
| 0/100 | 0 | 30 | 1.35 | ||
PEG control formulation: 30 mg 4-arm 10 kDa PEG in 60 μl PBS and 0.93 mg DTT in 10 μl PBS. Buffer alone without added DTT was used for reverse thermal gelation analysis.
Reverse thermal gelation
Thermally-induced noncovalent gelation was assessed using both the tube inversion method and rheometry. For the tube inversion test, 2 ml macromer solutions in 5 ml vials were placed in a temperature-controlled water bath at 4 °C. The bath temperature was increased 1 °C / 5 minutes and at each time point the samples were removed and inverted. The gel point was recorded as the temperature at which the solution did not flow.
Rheological analysis was performed using a Physica MCR300 modular compact rheometer (Anton Paar, USA) with Physica universal software 200 and parallel plate geometry (50 mm diameter). Macromer solutions (4 °C) were pipetted onto the bottom plate (pre-cooled to 4 °C) and the top plate was lowered to create a 0.2 mm gap. A temperature-sweep (1.2 °C/min) from 4 – 40 °C was performed at constant frequency (0.1 Hz) and strain (0.01). The gel point was determined as the crossing point of the storage (G’) and loss (G”) moduli. The viscosity of the various poloxamine solutions was characterized at 4°C, 24°C and 37°C. Samples were loaded and allowed 15 minutes to achieve uniform temperature. Viscosity was measured as a function of shear rate ranging from 0.01 to 1 s-1.
Tandem crosslinking
Acrylated macromers were covalently crosslinked using DTT as a model thiol-containing compound. Crosslinking kinetics were analyzed by parallel plate rheology. Solutions of T1107/T904 ACR and DTT prepared at 4 °C were mixed at a 1:1 thiol:acrylate molar ratio (30% w/v final Tetronic® concentration), quickly vortexed, centrifuged to remove air bubbles, and pipetted on the center of the bottom plate pre-warmed to 37 °C to model the physiological application temperature. The top plate was lowered to create a 0.2 mm gap and oscillatory shear immediately applied at constant frequency (0.1 Hz) and strain (0.01). Storage and loss moduli were recorded as a function of time. The gelation time was determined as the temperature at which the storage modulus exceeded the loss modulus.
The efficiency of covalent crosslinking was also characterized using Ellman’s reagent to measure the content of free thiols. Acrylated poloxamines and PEG were mixed with DTT at 1:1 acrylate:thiol ratio (25 μl volume), allowed to react for 10 minutes, and then added to 2.725 ml reaction buffer (0.1 M sodium phosphate buffer, pH 8.0, with 1 mM EDTA) with 50 μl Ellman’s reagent (4 mg/ml). Comparable aliquots of DTT used for crosslinking were diluted 1:5 and assayed to determine the initial thiol concentration. Standards were prepared from a dilution series of cysteine hydrochloride monohydrate. Samples and standards were incubated 15 minutes and absorbance values measured at 412 nm.
2.4 Degree of gelation and swelling behavior
Various T1107/T904 ACR formulations (30% w/v) were covalently crosslinked with DTT in 100 μl sample volumes between glass coverslips separated by 1 mm Teflon spacers. After overnight crosslinking at 37 °C in humidified Petri dishes, the initial wet weight (Wwi) was measured. Samples were lyophilized (FreeZone® 4.5 Benchtop freeze dry systems of ©Labconco Corp.; RV5 rotary vane pump of BOC Edwards), weighed (Wd1), and then allowed to swell in 37 °C PBS (pH 7.4) for 24 hours to remove any uncrosslinked residual macromer. The discs were lyophilized and weighed (Wd2). The gel content was calculated as
To determine the equilibrium swelling ratio, dry hydrogel discs were placed in PBS (pH 7.4) for 24 hours at 4 or 37 °C. Excess buffer solution on the surface of gels was gently blotted with a piece of Kimwipe and the respective wet weights (Ww4, Ww37) were measured. The volumetric swelling ratio (Q) was calculated as:
where ρp is the polymer density (1.04 g/ml for all macromers), ρs the solvent density (1g/ml for PBS), and Ww one of the various wet weights measured (Wwi, Ww4, or Ww37). In order to investigate reversible thermosensitive swelling properties, hydrogel discs in PBS were repeatedly transferred between 4 and 37 °C every 24 hours for a total of 96 hours.
2.5 Hydrogel degradation
Hydrogel discs covalently crosslinked as described above were incubated in 37°C PBS (pH7.4) overnight to remove any unreacted macromer, freeze-dried, weighed (Wd0), and incubated in 4ml of PBS (pH 7.4) at 37 °C on a shaker plate. At specified time points, gel discs (n=4 / time point) were collected, weighed wet, rinsed with water, and then freeze-dried to determine their final mass (Wdt). Hydrogel degradation was assessed by volumetric swelling and percent mass loss determined using the following equation:
2.6 Mechanical analysis
Tensile properties
Covalently crosslinked hydrogels were cut into dog-bone shaped specimens (initial dimensions-20 mm gauge length × 5 mm width × 1 mm thickness), equilibrated overnight in PBS (pH7.4) at 4 or 37°C, and their final dimensions measured using digital calipers. Five to six specimens of each group were subjected to uniaxial tensile testing at a constant crosshead speed of 10 mm/min using an MTS Synergie 100 equipped with 10N load cell. Because the instrument did not have a temperature-controlled environment, each sample was transferred individually from the equilibration condition and immediately tested at room temperature. In order to facilitate gripping of the specimens, pieces of Kimwipe were applied to the grip portions of the dog bones. All samples were subjected to two cycles of 20% strain and relaxation to ensure sample slippage did not occur and then strained to failure. Young’s modulus was calculated as the tangential slope of the initial linear region of the stress-strain curve.
Tissue Bond strength
The tissue bond strength was investigated using rat skin according to ASTM 2255. Humanely euthanized rats were donated from other investigator’s IACUC-approved protocols. Rat skin samples were collected using sterile surgical tools, stored in 150mM sterile NaCl, and cut into 4cm×1cm strips. Various hydrogel solutions mixed with DTT were applied to a 1cm×1cm area of one skin strip and another skin strip was placed over it. The bonded tissue was incubated at 37°C overnight in a humidified atmosphere and then equilibrated in 37°C PBS (pH7.4) before testing. More than five specimens of each group were subjected to uniaxial strain at a constant crosshead speed of 10 mm/min. All samples were strained until the two skins were detached from each other. The bonding strength was calculated as the maximum load divided by the bonded area.
2.7 Cytotoxicity
Cytotoxicity tests were performed according to ISO 10993-5:2009 “Biological evaluation of medical devices-Part 5: tests for in vitro cytotoxicity” with the exception of the use of normal human dermal fibroblasts (NHDF, Lonza, Walkersville, MD) instead of the L929 fibroblast cell line.
Test on extracts / degradation products
Poloxamine solutions were mixed with DTT as described above (30% w/v polymer, 1:1 acrylate:thiol ratio, 50 μl final volume) and allowed to crosslink for 10 minutes at 37 °C. For extract testing, the samples were incubated for 24 hours at 37 °C in 0.5 ml culture medium [DMEM/F12 (Mediatech, Manassas, VA), 10% bovine growth serum (Hyclone, Logan, UT) and 1X penicillin/streptomycin (Mediatech)]. Degradation products were prepared by incubating gels for approximately 1 hour in 1 N NaOH until completely degraded. After pH neutralization with 1 N HCl, the resulting gel degradation products were lyophilized, reconstituted in 100 μl culture medium per 50 μl gel volume, and sterile filtered. NHDF (P3-P5) were trypsinized and seeded at 5 × 104 cells/well in 12 well plates. After 24 hours incubation, culture medium was removed and replaced with either extract solution diluted 1:1 with normal medium or gel degradation products (100 μl per ml culture medium). Positive control wells received only fresh medium. Pluronic® F127 prepared at comparable concentration was included in the gel degradation products assay as an additional positive control. Cells were cultured an additional 24 hours and then transferred to serum-free medium containing 240 μl MTT reagent (2 mg/ml). After 4 hours, the formazan crystals were dissolved in DMSO and optical density measured at 570 nm. Cell viability was expressed as a percentage of the positive control.
Test by direct contact
Two different direct contact assays were performed. In the first, poloxamine solutions were mixed with DTT as described above, 10 μl drops applied to the center of 12 well plates, and allowed to crosslink for 10 minutes at room temperature. In the second assay, acrylate-PEG-GRGDS (1 mM) and fibronectin (100 μg/ml) were incorporated during gelation. 50 μl samples were crosslinked for 10 minutes between glass coverslips in order to obtain flat surfaces and then transferred to 12 well plates. Both types of samples were seeded with 5 × 104 NHDF per well and cultured for 24 hours. Cells were stained with 2 μM calcein AM and 2.5mM propidium iodide (PI), rinsed with PBS, visualized by fluorescence microscopy (Zeiss Axiovert 200), and digitally imaged.
2.8 Statistical analysis
Quantitative data for gel content, volumetric swelling ratio, and mechanical properties were compared by ANOVA using Tukey’s method for post-hoc comparisons. P values <0.05 were considered to be statistically significant.
3. Results
3.1 Reverse thermal gelation
We first investigated the thermosensitive properties of homogeneous and blended solutions of two different poloxamine copolymers with varying molecular weight and HLB. The viscosity of all poloxamine solutions exhibited a shear-thinning response as a function of shear rate ranging between 0.01 and 1 s-1 (data not shown). At constant shear rate (0.1 s-1), viscosity dramatically increased with increasing T1107 content and temperature (Figure 2). Sol-gel transition temperatures determined by the tube inversion method and rheological analysis are shown in Table II. All groups containing T1107 gelled below physiological temperature, while homogenous T904 and the PEG control did not gel within the temperature range tested (4-40 °C). Sol-gel transition temperatures measured by both tube inversion and rheology decreased with increasing T1107 content, although differences in the absolute values measured by the two methods were noted in the 75/25 and 50/50 groups. For all T1107-containing samples, the storage modulus increased with temperature above the sol-gel transition temperature, eventually reaching peak levels between 30-35 °C. The peak storage moduli significantly varied among the three groups and increased with increasing T1107 content (Table II).
Figure 2.
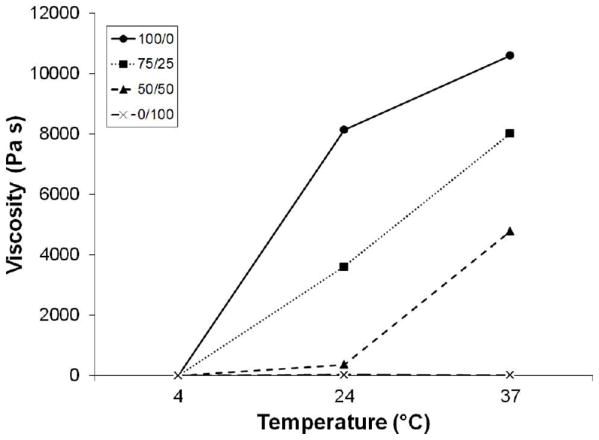
The effect of temperature on viscosity of samples with varying T1107/T904 composition (100/0 (●), 75/25 (■), 50/50 (▲), and 0/100 (×); 30% w/v) at fixed shear rate (0.1 s-1).
Table II.
Crosslinking of Poloxamine Solutions
| T1107/T904 composition (total 100%) | Reverse Thermal Gelation | Tandem crosslinking via reverse thermal gelation and Michael-type addition | |||
|---|---|---|---|---|---|
| Tube inversion | Rheometry | ||||
| Gelation point (°C) | Gelation point (°C) | Peak storage modulus (kPa) | Gelation time (sec) | Peak storage modulus (kPa) | |
| 100/0 | 22 | 20.73±0.23 | 36.25±4.88 | 23.5±2.12 | 52.61±11.26 |
| 75/25 | 26 | 21.40±0.40 | 26.60±4.17 | 24.17±5.58 | 56.34±6.77 |
| 50/50 | 31 | 23.67±1.22 | 15.13±2.65 | 88.33±12.58 | 56.57±6.50 |
| 0/100 | ND | ND | - | 153.33±5.77 | 45.97±5.38 |
| 4-arm PEG | ND | ND | - | - | - |
Reverse thermal gelation was monitored in the range of temperature between 4°C and 40°C. ND=not detectable.
3.2 Tandem crosslinking via reverse thermal gelation and Michael-type addition
Rheology was used to measure the gelation times of solutions composed of varying T1107 ACR / T904 ACR ratios (final concentration of all samples = 30% w/v) during tandem crosslinking at 37 °C by both reverse thermal gelation and Michael-type addition with DTT. All samples gelled in less than 3 minutes, with gelation time increasing as the T1107 content decreased (Table II). The storage modulus of all samples reached a peak level within approximately 15 minutes. Storage moduli values did not significantly vary among the different formulations but were all significantly higher than corresponding samples formed by reverse thermal gelation alone. Analysis of thiol content using Ellman’s reagent demonstrated that less than 3% of the original thiols remained in all formulations after 10 minutes reaction time (data not shown).
3.3 Gel Content and Swelling behavior
Gel content was consistently high (around 97%) for all poloxamine formulations and PEG controls with no significant differences among the various groups (data not shown). Hydrogel swelling was first evaluated between initial (immediately following crosslinking) and equilibrium conditions at constant temperature (37 °C). Because of the uniformly high gel contents, polymer mass fraction was essentially constant and the difference between initial and equilibrium swelling ratios was expressed as a percent change in gel volume (Figure 3) resulting from changes in water content during equilibration. While the volume of 4-arm PEG based hydrogels increased nearly 300% between initial and equilibrium conditions, the change in volume of all poloxamine-based hydrogels was less than 100% and significantly lower than the PEG control. Among the various poloxamine-based hydrogels, the percent change in gel volume significantly decreased with increasing T904 ACR content, with the 0/100 group (T904 ACR alone) showing essentially no change in volume during equilibration with excess buffer.
Figure 3.
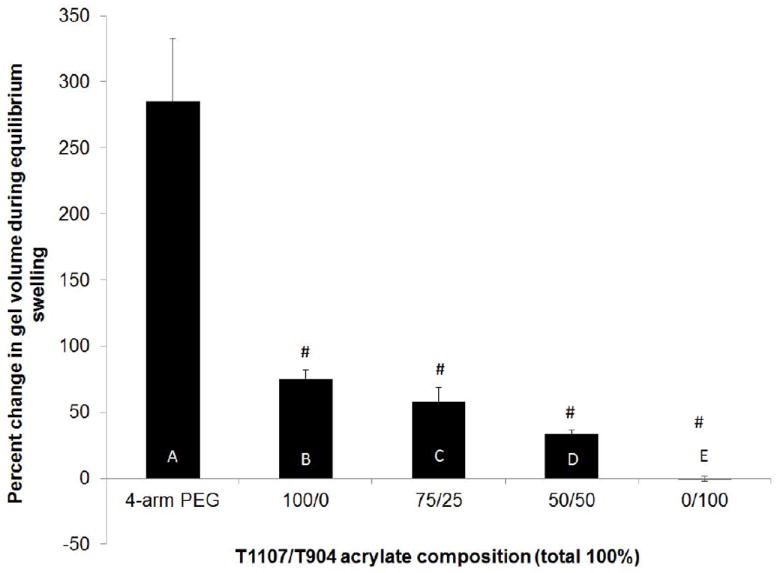
Percent change in hydrogel volume between crosslinking and equilibrium swelling. (# indicates the significant difference between initial and equilibrium, letters indicate significant differences between groups).
In order to investigate thermosensitive swelling properties, the equilibrium volumetric swelling ratios were measured after swelling at 4 and 37 °C (Figure 4). All poloxamine-based gels exhibited significantly higher swelling at 4 relative to 37 °C, while swelling of the PEG control was not significantly affected by temperature. Among the various poloxamine formulations, swelling decreased with increasing T904 ACR content. The poloxamine-based gels were then transferred several times between the two temperature conditions to demonstrate the thermoreversible nature of their swelling (Figure 5).
Figure 4.
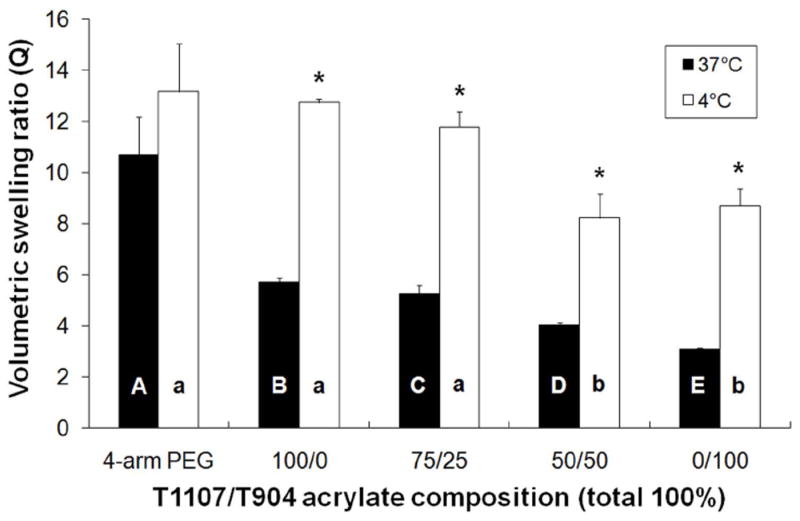
The effect of temperature on the volumetric swelling ratio of various poloxamine-based hydrogels (* indicates significant difference between two temperatures, letters indicate significant differences between groups).
Figure 5.
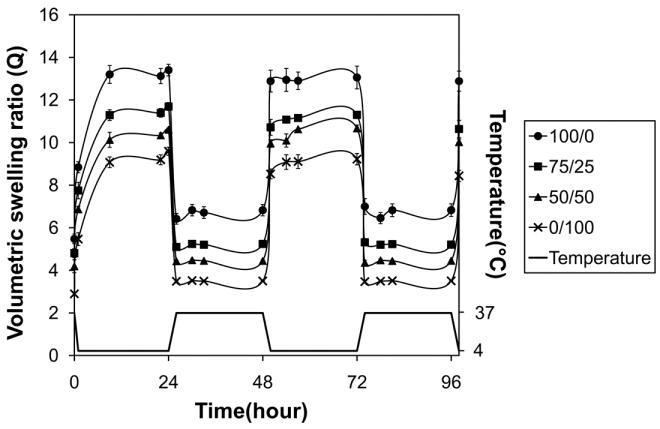
Thermoreversible swelling behavior of various poloxamine-based hydrogels; 100/0 (●), 75/25 (■), 50/50 (▲), and 0/100 (×).
3.4 Hydrogel degradation
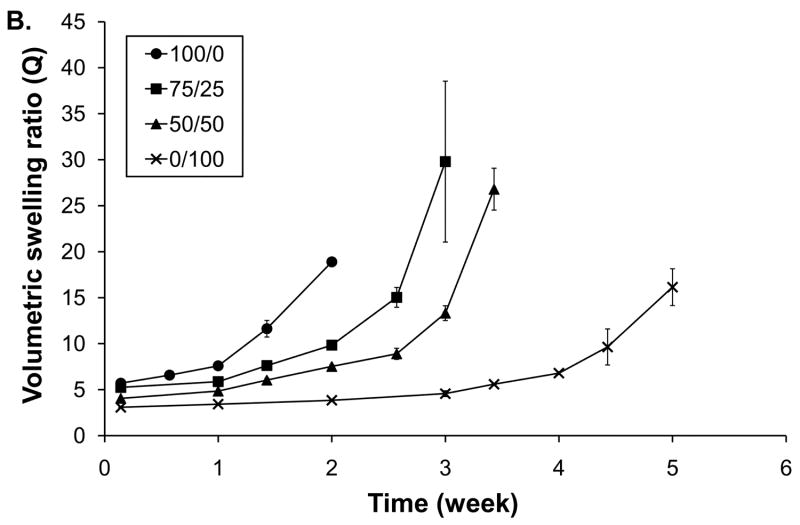
The hydrolytic degradation of hydrogels with varying T1107 / T904 ACR composition was analyzed by swelling and mass loss (Figure 6). All of the hydrogels exhibited an initial period of limited mass loss and stable swelling ranging from 2-4 weeks in length. Once the gels reached approximately 20% mass loss, swelling and mass loss accelerated and reverse gelation occurred within one week. The time required for complete degradation increased with increasing T904 ACR content. Homogeneous T1107 ACR hydrogels (100/0) completely degraded by 18 days, followed thereafter by 75/25, 50/50 and 0/100 gels, which exhibited total mass loss after 24 days, 28 days, and 38 days, respectively.
Figure 6.
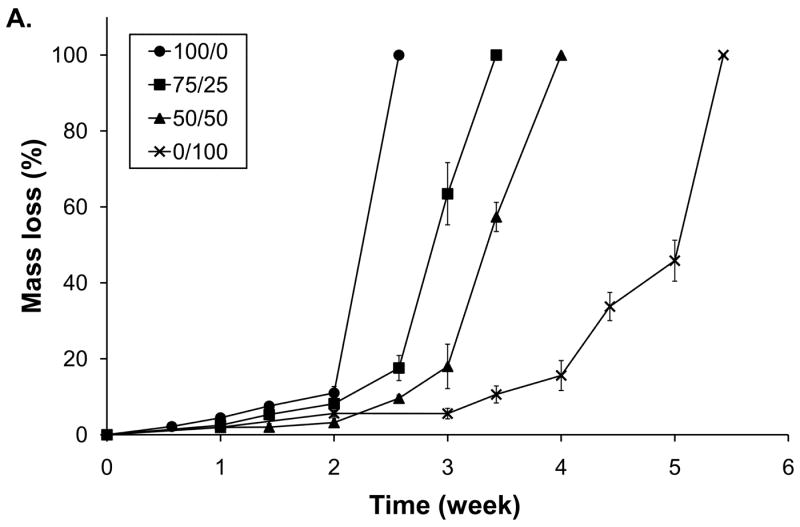
Degradation (A) and swelling (B) of various poloxamine-based hydrogels; 100/0 (●), 75/25 (■), 50/50 (▲), and 0/100 (×).
3.5 Tensile properties
The tensile properties of poloxamine-based hydrogels were tested immediately following overnight equilibration at 4 and 37 °C (Figure 7). All poloxamine-based hydrogels exhibited significantly increased Young’s modulus and strain at failure at 37 °C relative to 4 °C. In addition, the tensile properties of all poloxamine-based hydrogels were significantly greater than the PEG control at 37 °C; with 0/100 (T904 only) hydrogels reaching over 2 fold increase. Among the various poloxamine formulations, Young’s modulus increased with increasing T904 ACR content at both 4 and 37 °C. Elongation to failure increased in a similar manner for samples equilibrated at 37 °C, while those equilibrated at 4 °C did not exhibit any significant differences.
Figure 7.
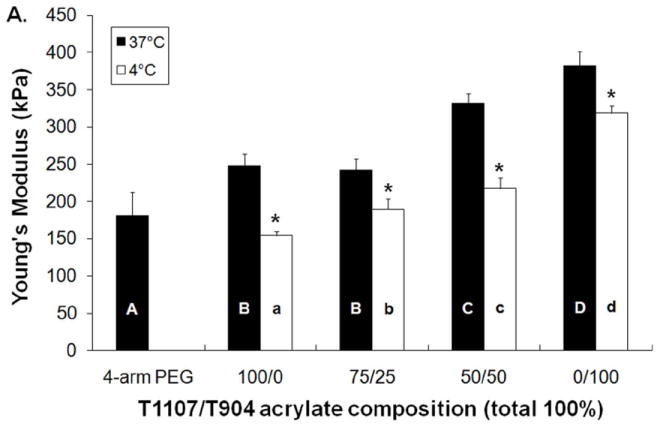
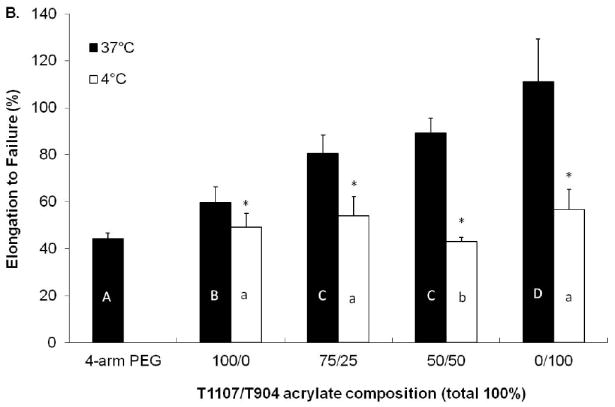
Young’s modulus (A) and elongation to failure (B) of various poloxamine-based hydrogels equilibrated at 4 and °37 C. (* indicates significant difference between two temperatures, letters indicate significant differences between groups).
3.6 Bonding strength
The tissue bonding strength of all poloxamine-based hydrogels ranged between approximately 10-25 kPa (Figure 8). Average bonding strength increased with increasing T904 content; however, the differences were not statistically significant. All poloxamine formulations displayed significantly greater bonding strength than the PEG hydrogel.
Figure 8.
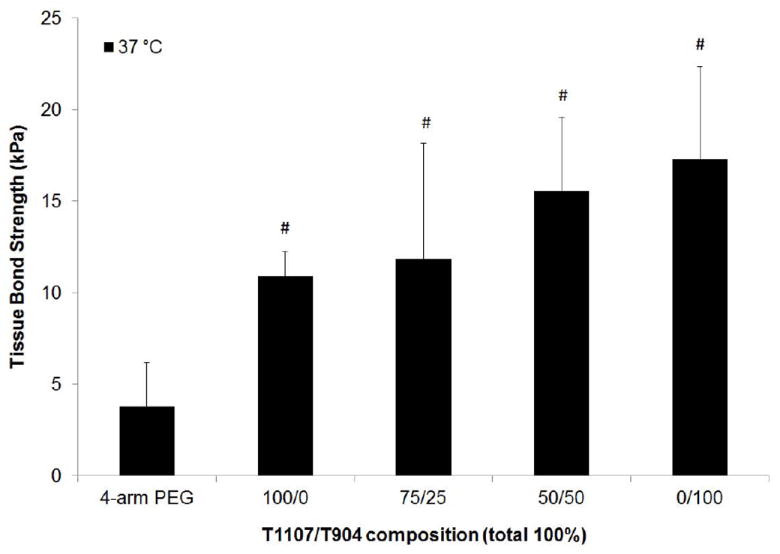
Tissue bond strength of various poloxamine and PEG hydrogels after overnight bonding at 37 °C. # indicates significant difference relative to PEG control.
3.7 Cytotoxicity
Cytotoxicity was first evaluated by culturing cell in the presence of extracts released from gels after 10 minutes crosslinking. Cell viability measured by MTT assay was greater than 90% of the positive control (no extracts) for all poloxamine-based hydrogel groups and not significantly different from the positive control (Figure 9A). When cultured in the presence of gel degradation products, cell number significantly increased for the 100/0 (T1107 only) and significantly decreased for the 0/100 (T904 only) groups relative to the untreated control (Figure 9B). Only the increase in the 100/0 group was significantly different than the F127 control.
Figure 9.
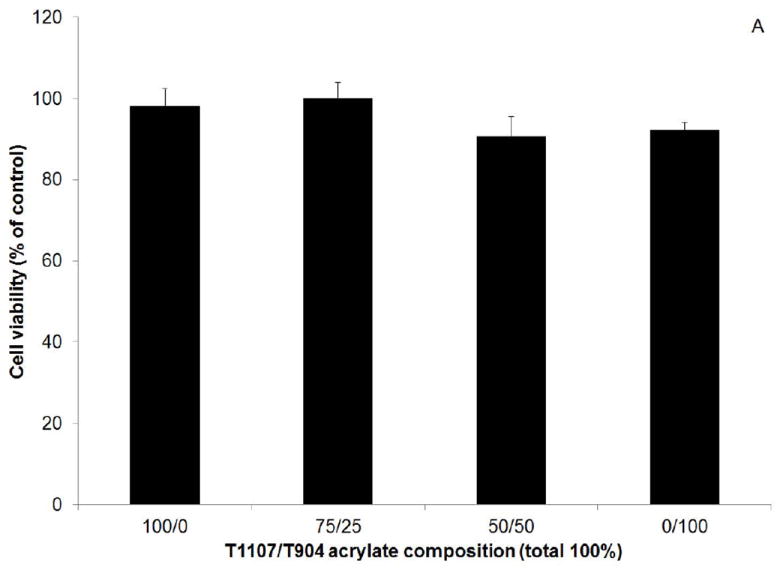
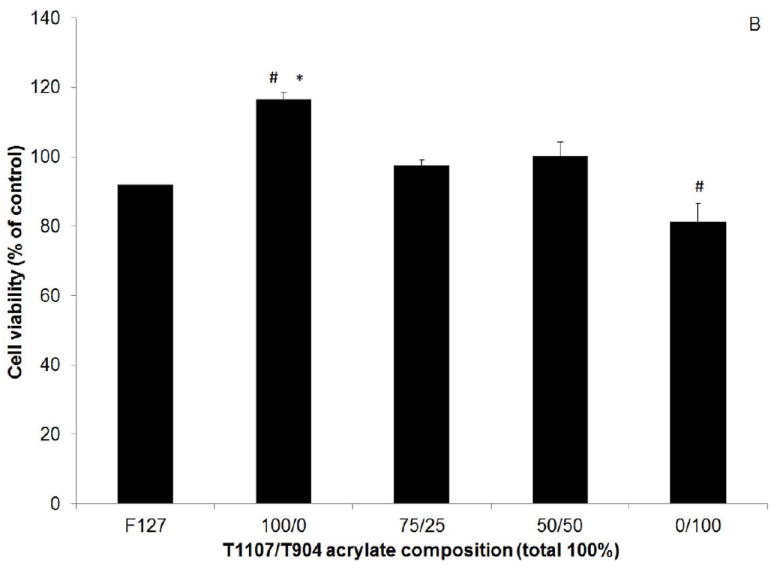
Viability of normal human dermal fibroblasts cultured in the presence of poloxamine-based hydrogel extracts (A) and degradation products (B) as a percent of control (media only). # indicates significant difference relative to control, * indicates significant difference relative to F127.
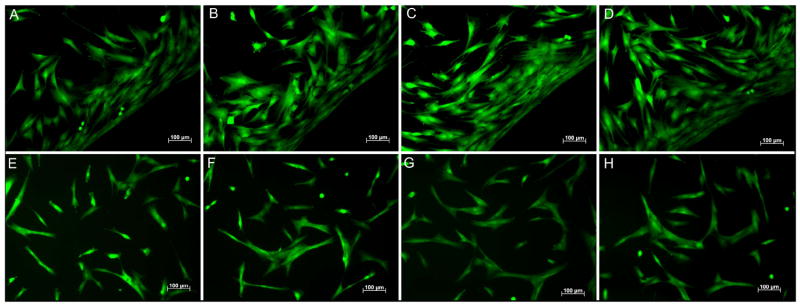
Two direct contact cytotoxicity assays were also performed. Fibroblasts seeded in culture wells containing gel samples adhered and spread on the plates around the samples. All hydrogels were completely resistant to cell adhesion, resulting in increased cell density at the gel edges (Figure 10 A-D, the gel sample is located in the lower right hand corner of all images). In the second direct contact assay, gels were crosslinked with incorporation of fibronectin and acrylated RGD peptides. Fibroblasts adhered and spread on the surface of all poloxamine-based hydrogels (Figure 10 E-H). In both direct contact assays, cell viability was uniformly high with essentially no dead cells detected.
Figure 10.
(A-D)-Representative images of normal human dermal fibroblasts adherent to culture plates in contact with poloxamine-based hydrogels. (E-H) Representative images of normal human dermal fibroblasts adherent to the surface of poloxamine-based hydrogels containing acrylated RGD peptides and fibronectin. T1107/T904 ratios: 100/0 (A,E); 75/25 (B,F); 50/50 (C,G); 0/100 (D,H). Cell viability was assessed by staining with calcein AM (green) and propdidium iodide (red). All scale bars = 100μm.
4. Discussion
Many PEG-based hydrogels in clinical use experience substantial swelling during post-crosslinking equilibration resulting in up to 50% (DuraSeal™), 300% (FocalSeal®), and 400% (CoSeal®) increases in gel volume.[18-20] Two recent reports have described serious clinical complications arising from swelling-induced compression of surrounding neural tissue.[21, 22] The goal of this study was to investigate the chemical and physical properties of hydrogels formed from amphiphilic poloxamine (Tetronic®) macromers. Amphiphilic block copolymers have been widely used in biomedical applications, particularly drug delivery, because of their capabilities for undergoing sol-gel transition near physiological temperature and hydrophobic drug solubilization.[34] Poloxamers (Pluronics®) in the form of linear triblock PEO-PPO-PEO copolymers have been one of the most widely studied systems. The reverse thermal gelation behavior of poloxamers is dependent on PEO/PPO composition and has been attributed to temperature-dependent changes in water structure around the PPO blocks, promoting micellization and eventual micelle aggregation into lattice structures[35, 36] Although less widely studied, poloxamines composed of four PPO-PEO arms synthesized from ethylene diamine have been shown to display similar capabilities for micellization/gelation and drug solubilization.[23, 28, 37]
Despite their potential, the successful application of poloxamer-based drug delivery systems has been hindered by their limited mechanical properties, short residence time, and high permeability to solutes.[36, 38] Two approaches to overcome these challenges have been the use of more hydrophobic polyester blocks and the incorporation of terminal reactive groups for covalent crosslinking.[34, 36] Cellesi and co-workers first described the “tandem gelation” concept using first poloxamers and later poloxamines modified with acrylate and thiol groups.[30, 31, 39] As a synthetic alternative to alginate for cell microencapsulation, these materials allowed rapid reverse thermal gelation when microspheres contacted a 37 °C collection bath combined with subsequent Michael-type addition crosslinking for covalent stabilization. Several other groups have adopted similar strategies for improved poloxamer-based drug delivery systems.[40-42] Such dual crosslinking characteristics are also potentially advantageous for sealant/adhesive applications, where near instantaneous reverse thermal gelation can prevent runoff and allow more precise application, while slower covalent crosslinking governs the final physical properties.
In these studies, we evaluated hydrogels formed by two poloxamines of different molecular weight and HLB. We consider the blended formulations, particularly the 50/50 ratio, to be the most promising candidates. In relatively concentrated solution (30% w/v), both the pure T1107 and 75/25 T1107/T904 groups are close to their gel point and relatively viscous at room temperature, making them difficult to handle. In contrast, pure T904 as well as a 25/75 blend (tested only in preliminary studies) lack reverse thermal gelation behavior within the physiological range. The 50/50 formulation maintains relatively low viscosity at room temperature for easy handling and reverse thermal gelation behavior with a substantial increase in viscosity between room and physiological temperatures (Figure 2). These characteristics may be particularly useful in a sealant application, allowing easy dispensing in the liquid state and near instantaneous gelation upon contact with tissue for highly controlled application. Blended formulations also exhibit an increase in gel point and decrease in storage modulus with increasing T904 content, suggesting that T904 moderately interferes with the noncovalent gelation efficiency of T1107. The difference in the ability of T1107 and T904 to undergo reverse thermal gelation is most likely attributable to differences in the PEO/PPO ratio and molecular weight.[35] One limitation of our studies is that all reverse thermal gelation analysis was performed with unmodified polymers, however, we replicated the tube inversion test with acrylated derivatives and all gel points were within 1-2 °C of the native polymers. This is consistent with previous studies demonstrating measurable but modest effects of end group modification of reverse thermal gelation behavior of other amphiphilic polymers.[43] A second limitation is that the rheometer used lacked a humidified chamber and although most tests were relatively brief, the possibility of some evaporation cannot be eliminated and this may have contributed to the different gel point values measured for the 75/25 and 50/50 groups by rheology and tube inversion.
For tandem crosslinking by both reverse thermal gelation and covalent bonding, poloxamines were modified with terminal acrylate groups and mixed with stoichiometric amounts of dithiothreitol (DTT). All formulations gelled within approximately 30 seconds to 2 ½ minutes under physiological conditions (37 °C). The increase in gelation time with increasing T904 content reflects the diminishing (75/25 and 50/50) and eventual loss (0/100) of the contribution of reverse thermal gelation provided by the T1107 component. Because the deprotonated thiolate is the reactive species in such Michael-type addition reactions[44], the reaction rate and gelation time for covalent crosslinking can be readily tuned for specific applications by changing the solution pH of the reactants.
We next examined the swelling behavior of poloxamine hydrogels relative to a PEG hydrogel control. Because 4-arm PEGs are not readily available with molecular weights similar to T1107 and T904, we used a 10 kDa PEG that is approximately equivalent to the 50/50 blend formulation (average molecular weight = 10,850 kDa). PEG-based hydrogels exhibit considerable water uptake after crosslinking during initial equilibration with excess solvent resulting in increased gel volume.[33] For the PEG control used in these studies, this increase in volume was approximately 300%, reasonably close to values reported in the literature for CoSeal® and FocalSeal®.[18, 19] This effect was significantly reduced for all poloxamine-based hydrogels (<100%) and effectively eliminated in the homogeneous T904 (0/100) formulation (Figure 3). In addition, the change in volume for the PEG control (~300%) is within the range reported for commercially available PEG-based sealants. All poloxamine-based hydrogels showed thermoresponsive swelling behavior (Figures 4 and 5), indicating that noncovalent, hydrophobic interactions play an important role in restraining swelling at physiological temperature. In the context of sealants applied to internal tissues/organs, the improved ability of poloxamine-based hydrogels to maintain stable volume during equilibration suggests these materials may be able to reduce pressure exerted on neighboring tissues and associated clinical complications.[21, 22] Swelling also decreased with increasing T904 content at both 4 and 37 °C, consistent with the increasing number of acrylate groups and resulting crosslink density at constant polymer mass concentration, as well as increased macromer hydrophobicity. Several groups have reported similar temperature-dependent swelling that decreases at physiological temperature for other tandem gelling materials such as N-isopropylacrylamide (NIPPAm) copolymers and F127-modified fibrinogen.[45-47] To our knowledge, this report is the first to describe hydrogels capable of maintaining stable volume and swelling during equilibration. Due to their protonable central nitrogen atoms, poloxamine micellization has also been shown to be pH-dependent.[23] Although not explored in these studies, it is likely that poloxamine-based hydrogels will also exhibit pH-dependent swelling.
Michael-type addition reactions between acrylates and thiols form thioether-ester bonds that are susceptible to hydrolysis.[48] All poloxamine-based hydrogels exhibited swelling and degradation profiles consistent with bulk hydrolysis of hydrogel networks (Figure 6).[49] The time required for degradation increased with increasing T904 content, consistent with increased crosslink density and hydrophobicity. Although the T904 gels exhibit swelling during degradation, no significant change is observed until after 3 weeks when the mechanical properties of the gel and its ability to exert compression on surrounding tissues are expected to be substantially reduced. Hydrogel mechanical properties also increased with increasing T904 content, with the homogeneous T904 (0/100) formulation achieving approximately two times the elastic modulus and elongation to failure of the PEG control (Figure 7). The mechanical properties of all poloxamine-based hydrogels were temperature-dependent, demonstrating that at physiological temperature covalent and noncovalent interactions cooperatively contribute to the material properties. These results are consistent with several previous studies of covalently crosslinked, thermosensitive materials; including NIPAAm-based copolymers[46, 47, 50], poly(N-2-hydroxypropyl) methacrylamide lactate-b-PEG[51], PEG methyl ether methacrylate-poly(propylene glycol) methacrylate-ethylene glycol dimethacrylate[52], and dextran-Pluronic® hydrogels[53].
The tissue bond strength of all poloxamine-based hydrogels was significantly greater than the PEG control and comparable to or modestly exceeding values reported for commercial fibrin glue in the literature.[54, 55] Poloxamine hydrogels are expected to bond to tissue through mechanical interdigitation with tissue microarchitecture, as well as through covalent crosslinking to free thiols present in ECM proteins, the number of which may be increased by brief exposure to DTT during gelation. Elisseeff and co-workers recently described a strategy to improve the interfacial bonding between polysaccharide hydrogels and cartilage by functionalizing chondroitin sulfate with both methacrylate groups for photocrosslinking and aldehyde groups for tissue adhesion.[56] We expect that the tissue bond strength of our materials can be further improved in the future by a similar principle through copolymerization with an acrylate/aldehyde difuctional poloxamer.
In our final studies, we investigated the in vitro cytocompatibility of poloxamine-based hydrogels. When crosslinked at 1:1 acrylate:thiol ratio, all gels demonstrated good cytocompatibility. Consistent with the high gelation efficiency measured by Ellman’s assay, gel extracts did not significantly reduce cell viability. Gel degradation products were generally cytocompatible with a moderate, but significant decrease in cell viability (81% of control) observed in the 0/100 (T904 only) group. This is likely attributable to the relatively higher molar concentration of macromer in this group, since at constant 30% w/v, the molar concentration increases with increasing content of the lower molecular weight T904 component. The final concentration of degradation products in this assay was relatively high (1.5 mg/ml) and amphiphilic copolymers are known to interact with the plasma membrane and affect its permeability.[57] In direct contact assays, cells were unable to attach to the crosslinked gels without modification with adhesive ligands. This indicates that even pure T904 gels with approximately 50% PPO ciontent still retain the resistance to cell adhesion characteristic of PEG-based hydrogels. In the context of sealant applications, this will likely be very beneficial in providing resistance to adhesion formation. Similar to PEG-based hydrogels[58], when modified with adhesive ligands, fibroblasts were able to adhere and spread on all poloxamine-based hydrogels. A particular benefit of poloxamines is their structural and compositional similarity to poloxamers that have a well-established record of safety in clinical practice and have been used as pharmaceutical excipients in FDA-approved applications.[59,60]
5. Conclusion
Poloxamine-based hydrogels are attractive new candidates for tissue sealant/adhesive applications. By combining their intrinsic amphiphilic nature with modification for chemical reactivity, very rapid reverse thermal gelation upon application at physiological temperature combined with covalent crosslinking for improved stability can be achieved. Poloxamine-based hydrogels achieved significantly reduced swelling and increased tensile and tissue bonding properties relative to a PEG-based control hydrogel that derive from this combination of noncovalent and covalent crosslinking. Their improved ability to maintain stable volume during equilibration may reduce the risk of compression of surrounding tissue and extend their utility to closed cavities, which are contraindicated for existing PEG-based sealants. Future studies will examine their utility for bladder repair where high compliance is required and as delivery vehicles for hydrophobic drugs and bioactive molecules.
Acknowledgments
The authors gratefully acknowledge funding from NIH grant #P20RR021949 through the SC COBRE Center of Biomaterials for Tissue Regeneration and NIH grant # R21EB008785.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quinn JV. Overview of tissue adhesives. In: Quinn JV, editor. Tissue Adhesives in Clinical Medicine. 2. Hamilton: BC Decker; 2005. pp. 1–13. [Google Scholar]
- 2.Webster I, West PJ. Adhesives for medical applications. In: Dumitriu S, editor. Polymeric biomaterials. 2. New York: Marcel Dekker, Inc.; 2002. rev. and expanded ed. [Google Scholar]
- 3.Rousou J, Levitsky S, Gonzalez-Lavin L, Cosgrove D, Magilligan D, Weldon C, et al. Randomized clinical trial of fibrin sealant in patients undergoing resternotomy or reoperation after cardiac operations. A multicenter study. J Thorac Cardiovasc Surg. 1989;97:194–203. [PubMed] [Google Scholar]
- 4.Coselli JS, Bavaria JE, Fehrenbacher J, Stowe CL, Macheers SK, Gundry SR. Prospective randomized study of a protein-based tissue adhesive used as a hemostatic and structural adjunct in cardiac and vascular anastomotic repair procedures. J Am Coll Surg. 2003;197:243–52. doi: 10.1016/S1072-7515(03)00376-4. discussion 52-3. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz M, Madariaga J, Hirose R, Shaver TR, Sher L, Chari R, et al. Comparison of a new fibrin sealant with standard topical hemostatic agents. Arch Surg. 2004;139:1148–54. doi: 10.1001/archsurg.139.11.1148. [DOI] [PubMed] [Google Scholar]
- 6.Furst W, Banerjee A. Release of glutaraldehyde from an albumin-glutaraldehyde tissue adhesive causes significant in vitro and in vivo toxicity. Ann Thorac Surg. 2005;79:1522–8. doi: 10.1016/j.athoracsur.2004.11.054. discussion 9. [DOI] [PubMed] [Google Scholar]
- 7.Woodward SC, Herrmann JB, Cameron JL, Brandes G, Pulaski EJ, Leonard F. Histotoxicity of Cyanoacrylate Tissue Adhesive in the Rat. Ann Surg. 1965;162:113–22. doi: 10.1097/00000658-196507000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houston S, Hodge JW, Jr, Ousterhout DK, Leonard F. The effect of alpha-cyanoacrylates on wound healing. J Biomed Mater Res. 1969;3:281–9. doi: 10.1002/jbm.820030208. [DOI] [PubMed] [Google Scholar]
- 9.Howell JM, Bresnahan KA, Stair TO, Dhindsa HS, Edwards BA. Comparison of effects of suture and cyanoacrylate tissue adhesive on bacterial counts in contaminated lacerations. Antimicrob Agents Chemother. 1995;39:559–60. doi: 10.1128/aac.39.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinn J, Wells G, Sutcliffe T, Jarmuske M, Maw J, Stiell I, et al. A randomized trial comparing octylcyanoacrylate tissue adhesive and sutures in the management of lacerations. JAMA. 1997;277:1527–30. [PubMed] [Google Scholar]
- 11.Sinha S, Naik M, Wright V, Timmons J, Campbell AC. A single blind, prospective, randomized trial comparing n-butyl 2-cyanoacrylate tissue adhesive (Indermil) and sutures for skin closure in hand surgery. J Hand Surg Br. 2001;26:264–5. doi: 10.1054/jhsb.2000.0572. [DOI] [PubMed] [Google Scholar]
- 12.Sawhney AS, Pathak CP, Hubbell JA. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(alpha-hydroxy acid) diacrylate macromers. Macromolecules. 1993;26:581–7. [Google Scholar]
- 13.Gillinov AM, Lytle BW. A novel synthetic sealant to treat air leaks at cardiac reoperation. J Card Surg. 2001;16:255–7. doi: 10.1111/j.1540-8191.2001.tb00517.x. [DOI] [PubMed] [Google Scholar]
- 14.Reece TB, Maxey TS, Kron IL. A prospectus on tissue adhesives. Am J Surg. 2001;182:40S–4S. doi: 10.1016/s0002-9610(01)00742-5. [DOI] [PubMed] [Google Scholar]
- 15.Glickman M, Gheissari A, Money S, Martin J, Ballard JL. A polymeric sealant inhibits anastomotic suture hole bleeding more rapidly than gelfoam/thrombin: results of a randomized controlled trial. Arch Surg. 2002;137:326–31. doi: 10.1001/archsurg.137.3.326. discussion 32. [DOI] [PubMed] [Google Scholar]
- 16.Boogaarts JD, Grotenhuis JA, Bartels RH, Beems T. Use of a novel absorbable hydrogel for augmentation of dural repair: results of a preliminary clinical study. Neurosurgery. 2005;57:146–51. doi: 10.1227/01.neu.0000164384.05351.59. discussion -51. [DOI] [PubMed] [Google Scholar]
- 17.Tan C, Utley M, Paschalides C, Pilling J, Robb JD, Harrison-Phipps KM, et al. A prospective randomized controlled study to assess the effectiveness of CoSeal((R)) to seal air leaks in lung surgery. Eur J Cardiothorac Surg. 2011;40:304–8. doi: 10.1016/j.ejcts.2010.11.079. [DOI] [PubMed] [Google Scholar]
- 18.Torchiana DF. Polyethylene glycol based synthetic sealants: potential uses in cardiac surgery. J Card Surg. 2003;18:504–6. doi: 10.1046/j.0886-0440.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- 19.Instructions for use. CoSeal, Baxter rev. 2009 [Google Scholar]
- 20.Instuctions for use. DuraSeal, Confluent Surgical [Google Scholar]
- 21.Blackburn SL, Smyth MD. Hydrogel-induced cervicomedullary compression after posterior fossa decompression for Chiari malformation. Case report. J Neurosurg. 2007;106:302–4. doi: 10.3171/ped.2007.106.4.302. [DOI] [PubMed] [Google Scholar]
- 22.Thavarajah D, De Lacy P, Hussain R, Redfern RM. Postoperative cervical cord compression induced by hydrogel (DuraSeal): a possible complication. Spine. 2010;35:E25–6. doi: 10.1097/BRS.0b013e3181b9fc45. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Lorenzo C, Gonzalez-Lopez J, Fernandez-Tarrio M, Sandez-Macho I, Concheiro A. Tetronic micellization, gelation and drug solubilization: Influence of pH and ionic strength. Eur J Pharm Biopharm. 2007;66:244–52. doi: 10.1016/j.ejpb.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Prokop A, Kozlov E, Moore W, Davidson JM. Maximizing the in vivo efficiency of gene transfer by means of nonviral polymeric gene delivery vehicles. J Pharm Sci. 2002;91:67–76. doi: 10.1002/jps.1171. [DOI] [PubMed] [Google Scholar]
- 25.Pitard B, Bello-Roufai M, Lambert O, Richard P, Desigaux L, Fernandes S, et al. Negatively charged self-assembling DNA/poloxamine nanospheres for in vivo gene transfer. Nucleic Acids Res. 2004;32:e159. doi: 10.1093/nar/gnh153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sosnik A, Sefton MV. Semi-synthetic collagen/poloxamine matrices for tissue engineering. Biomaterials. 2005;26:7425–35. doi: 10.1016/j.biomaterials.2005.05.086. [DOI] [PubMed] [Google Scholar]
- 27.Go DH, Joung YK, Lee SY, Lee MC, Park KD. Tetronic-oligolactide-heparin hydrogel as a multi-functional scaffold for tissue regeneration. Macromolecular Biosci. 2008;8:1152–60. doi: 10.1002/mabi.200800098. [DOI] [PubMed] [Google Scholar]
- 28.Lee JS, Bae JW, Joung YK, Lee SJ, Han DK, Park KD. Controlled dual release of basic fibroblast growth factor and indomethacin from heparin-conjugated polymeric micelle. Int J Pharm. 2008;346:57–63. doi: 10.1016/j.ijpharm.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Lopez J, Alvarez-Lorenzo C, Taboada P, Sosnik A, Sandez-Macho I, Concheiro A. Self-associative behavior and drug-solubilizing ability of poloxamine (tetronic) block copolymers. Langmuir. 2008;24:10688–97. doi: 10.1021/la8016563. [DOI] [PubMed] [Google Scholar]
- 30.Cellesi F, Tirelli N, Hubbell JA. Materials for cell encapsulation via a new tandem approach combining reverse thermal gelation and covalent crosslinking. Macromol Chem Phys. 2002;203:1466–72. [Google Scholar]
- 31.Cellesi F, Tirelli N, Hubbell JA. Towards a fully-synthetic substitute of alginate: development of a new process using thermal gelation and chemical cross-linking. Biomaterials. 2004;25:5115–24. doi: 10.1016/j.biomaterials.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 32.DuBose JW, Cutshall C, Metters AT. Controlled release of tethered molecules via engineered hydrogel degradation: model development and validation. J Biomed Mater Res A. 2005;74:104–16. doi: 10.1002/jbm.a.30307. [DOI] [PubMed] [Google Scholar]
- 33.Elbert DL, Pratt AB, Lutolf MP, Halstenberg S, Hubbell JA. Protein delivery from materials formed by self-selective conjugate addition reactions. J Control Release. 2001;76:11–25. doi: 10.1016/s0168-3659(01)00398-4. [DOI] [PubMed] [Google Scholar]
- 34.Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. European Journal of Pharmaceutics and Biopharmaceutics. 2008;68:34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fusco S, Borzacchiello A, Netti PA. Perspectives on: PEO-PPO-PEO triblock copolymers and their biomedical applications. J Bioact Compat Pol. 2006;21:149–64. [Google Scholar]
- 36.He CL, Kim SW, Lee DS. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J Control Release. 2008;127:189–207. doi: 10.1016/j.jconrel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Chiappetta DA, Degrossi J, Teves S, D’Aquino M, Bregni C, Sosnik A. Triclosan-loaded poloxamine micelles for enhanced topical antibacterial activity against biofilm. Eur J Pharm Biopharm. 2008;69:535–45. doi: 10.1016/j.ejpb.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 38.Ruel-Gariepy E, Leroux JC. In situ-forming hydrogels--review of temperature-sensitive systems. Eur J Pharm Biopharm. 2004;58:409–26. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 39.Cellesi F, Weber W, Fussenegger M, Hubbell JA, Tirelli N. Towards a fully synthetic substitute of alginate: Optimization of, a thermal gelation/chemical cross-linking scheme (“Tandem” gelation) for the production of beads and liquid-core capsules. Biotechnol Bioeng. 2004;88:740–9. doi: 10.1002/bit.20264. [DOI] [PubMed] [Google Scholar]
- 40.Sosnik A, Cohn D, San Roman J, Abraham GA. Crosslinkable PEO-PPO-PEO-based reverse thermo-responsive gels as potentially injectable materials. J Biomater Sci Polym Ed. 2003;14:227–39. doi: 10.1163/156856203763572680. [DOI] [PubMed] [Google Scholar]
- 41.Sosnik A, Cohn D. Ethoxysilane-capped PEO-PPO-PEO triblocks: a new family of reverse thermo-responsive polymers. Biomaterials. 2004;25:2851–8. doi: 10.1016/j.biomaterials.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 42.Niu G, Zhang H, Song L, Cui X, Cao H, Zheng Y, et al. Thiol/acrylate-modified PEO-PPO-PEO triblocks used as reactive and thermosensitive copolymers. Biomacromolecules. 2008;9:2621–8. doi: 10.1021/bm800573e. [DOI] [PubMed] [Google Scholar]
- 43.Niu GG, Du FY, Song L, Zhang HB, Yang J, Cao H, et al. Synthesis and characterization of reactive poloxamer 407s for biomedical applications. J Control Release. 2009;138:49–56. doi: 10.1016/j.jconrel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 44.Lutolf MP, Tirelli N, Cerritelli S, Cavalli L, Hubbell JA. Systematic modulation of Michael-type reactivity of thiols through the use of charged amino acids. Bioconjug Chem. 2001;12:1051–6. doi: 10.1021/bc015519e. [DOI] [PubMed] [Google Scholar]
- 45.Shachaf Y, Gonen-Wadmany M, Seliktar D. The biocompatibility of PluronicF127 fibrinogen-based hydrogels. Biomaterials. 2010;31:2836–47. doi: 10.1016/j.biomaterials.2009.12.050. [DOI] [PubMed] [Google Scholar]
- 46.Lee BH, West B, McLemore R, Pauken C, Vernon BL. In-situ injectable physically and chemically gelling NIPAAm-based copolymer system for embolization. Biomacromolecules. 2006;7:2059–64. doi: 10.1021/bm060211h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robb SA, Lee BH, McLemore R, Vernon BL. Simultaneously physically and chemically gelling polymer system utilizing a poly(NIPAAm-co-cysteamine)-based copolymer. Biomacromolecules. 2007;8:2294–300. doi: 10.1021/bm070267r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Metters A, Hubbell J. Network formation and degradation behavior of hydrogels formed by Michael-type addition reactions. Biomacromolecules. 2005;6:290–301. doi: 10.1021/bm049607o. [DOI] [PubMed] [Google Scholar]
- 49.Metters AT, Lin C. Biodegradable hydrogels: tailoring properties and function through chemistry and structure. In: Wong JY, Bronzino JD, editors. Biomaterials. Boca Raton: CRC Press; 2007. pp. 5–1.pp. 5–44. [Google Scholar]
- 50.Hacker MC, Klouda L, Ma BB, Kretlow JD, Mikos AG. Synthesis and characterization of injectable, thermally and chemically gelable, amphiphilic poly(N-isopropylacrylamide)-based macromers. Biomacromolecules. 2008;9:1558–70. doi: 10.1021/bm8000414. [DOI] [PubMed] [Google Scholar]
- 51.Vermonden T, Fedorovich NE, van Geemen D, Alblas J, van Nostrum CF, Dhert WJ, et al. Photopolymerized thermosensitive hydrogels: synthesis, degradation, and cytocompatibility. Biomacromolecules. 2008;9:919–26. doi: 10.1021/bm7013075. [DOI] [PubMed] [Google Scholar]
- 52.Tai H, Wang W, Vermonden T, Heath F, Hennink WE, Alexander C, et al. Thermoresponsive and photocrosslinkable PEGMEMA-PPGMA-EGDMA copolymers from a one-step ATRP synthesis. Biomacromolecules. 2009;10:822–8. doi: 10.1021/bm801308q. [DOI] [PubMed] [Google Scholar]
- 53.Lin C, Zhao P, Li F, Guo FF, Li ZQ, Wen XJ. Thermosensitive in situ-forming dextran-pluronic hydrogels through Michael addition. Mater Sci Eng, C. 2010;30:1236–44. [Google Scholar]
- 54.Burke SA, Ritter-Jones M, Lee BP, Messersmith PB. Thermal gelation and tissue adhesion of biomimetic hydrogels. Biomed Mater. 2007;2:203–10. doi: 10.1088/1748-6041/2/4/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mo X, Iwata H, Ikada Y. A tissue adhesives evaluated in vitro and in vivo analysis. J Biomed Mater Res A. 2010;94:326–32. doi: 10.1002/jbm.a.32788. [DOI] [PubMed] [Google Scholar]
- 56.Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, et al. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6:385–92. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yi X, Batrakova E, Banks WA, Vinogradov S, Kabanov AV. Protein conjugation with amphiphilic block copolyers for enhanced cellular delivery. Bioconj Chem. 2008;19:1071–1077. doi: 10.1021/bc700443k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266–76. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 59.Bromberg LE, Ron ES. Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Mater Sci Eng, C. 1998;31:197–221. doi: 10.1016/s0169-409x(97)00121-x. [DOI] [PubMed] [Google Scholar]
- 60.Kabanov AV, Alakhov VY. Pluronic block copolymers in drug delivery: from micellar nanocontainers to biological response modifiers. Crit Rev Ther Drug Carrier Syst. 2002;19:1–72. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]