Abstract
During the formation of neuronal circuits, neurons respond to diffusible cues secreted by target tissues. Often, target-derived signals act on nerve terminals to influence local growth events; in other cases, they are transported long-distance back to neuronal cell bodies to influence transcriptional changes necessary for neuronal survival and differentiation. Neurotrophins provide one of the best examples of target-derived cues that elicit an astonishingly diverse array of neuronal responses. Endocytic trafficking of neurotrophins and their receptors is a fundamental feature of neurotrophin signaling, allowing neurotrophins to control neuronal survival by retrograde transport of signaling endosomes containing ligand-receptor complexes. In this review, we summarize recent findings that provide new insight into the interplay between neurotrophin signaling and trafficking.
Keywords: neurotrophins, Trk receptors, signaling endosome, axon growth, sympathetic neurons, synapse assembly
Neurotrophin signaling: from nerve tip to cell body
The formation and refinement of neuronal circuits during development relies on interactions between neurons and their postsynaptic target tissues, often located millimeters or even meters away from the neuronal cell bodies. Diffusible cues secreted by target tissues impinge on nerve terminals to influence diverse aspects of neural development, including survival, axon growth and guidance, target innervation and synapse formation. The family of neurotrophins provides one of the best examples of these target-derived instructive cues. Nerve growth factor (NGF) is the founding member of the neurotrophin family of growth factors, which consists of NGF, brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5). Target-derived neurotrophins have been best-characterized in the peripheral nervous system where they are secreted from tissues innervated by sympathetic and sensory neurons, such as the salivary glands, muscles, visceral organs, and skin [1, 2]. As developing axons project into these target tissues, secreted neurotrophins bind their cognate receptor tyrosine kinase, TrkA (NGF), TrkB (BDNF and NT4/5), or TrkC (NT-3), present on the axon tips. In addition, all neurotrophins bind to a structurally unrelated p75 receptor, which can function together with Trk receptors or on its own to modulate neuronal responses to target-derived neurotrophins.
Neurons are highly polarized cells that pose a formidable challenge for growth factor signaling. A unique aspect of neurotrophin-Trk signaling is that although neurotrophins bind their cognate Trk receptor located on axons, they must control transcription and other cellular processes that take place long distances away in the cell body. In some cases, this distance may be 1000-fold greater than the diameter of the cell body. Thus, a fundamental cell biological question is to understand how target-derived neurotrophins acting locally on nerve terminals effect changes in gene expression in remote neuronal cell bodies. Currently, numerous lines of evidence support a model in which neurotrophins and their Trk receptors are internalized as ligand-receptor complexes in distal axons, into an endocytic signaling platform termed the ‘signaling endosome’ [3]. Signaling endosomes containing neurotrophin and Trk molecules are retrogradely transported along microtubules back to neuronal cell bodies where they signal to control transcriptional events [4]. Endocytic trafficking of neurotrophin-Trk complexes has been mostly studied in the context of supporting neuronal survival in the peripheral nervous system [5, 6]. This topic has been covered recently in several excellent reviews [3, 4, 7] (also see Box 1), and will not be discussed in detail here. The role of trafficking in controlling many other neurotrophin-mediated functions, such as axon growth, dendritogenesis and synaptogenesis, is less well understood (Box 2). In this review, we discuss a growing body of evidence that suggests that endocytic trafficking is a common mechanism that enables target-derived neurotrophic factors to exert global effects on neuronal development. A long-standing question in the field of neurotrophin-dependent trafficking concerns whether and how neurotrophin signaling influences its own trafficking. Here, we will summarize new exciting findings that provide evidence that neurotrophin signaling actively modulates both the core endocytic machinery and axonal cytoskeleton to influence its own trafficking.
Box 1. Alternative models of retrograde signaling.
The mechanism by which neurotrophins transmit signals retrogradely from distal axons to the neuronal soma has been a major focus of NGF research over the last decade. The evidence from many studies supports the hypothesis that NGF and TrkA are endocytosed as a ligand-receptor complex in distal axons and retrogradely transported to the cell bodies as a ‘signaling endosome’. The signaling endosome hypothesis has been reviewed in detail elsewhere and will not be discussed here [3, 4, 7].
It is important to note that other models of retrograde signaling have been proposed and may exist in parallel with the transport of signaling endosomes [6, 58, 59]. In support of alternative models, neurotrophins immobilized to beads can signal retrogradely and promote neuronal survival in the absence of internalization [6, 58]. TrkA endosomes may signal retrogradely without internalization of NGF. This model is considered plausible because highly concentrated TrkA can become autophosphorylated in the absence of NGF [60], and high concentrations of TrkA may be present in retrogradely transported vesicles. Another model states that downstream effectors of NGF-TrkA signaling may be transported, independent of the signaling endosome. However, inhibition of TrkA kinase activity or downstream effectors in the proximal axon segment does not prevent effector activation in the cell bodies [5], indicating that the NGF-TrkA signal is self-regenerative. Thus, retrogradely transported NGF-TrkA endosomes have all the necessary components to locally activate downstream signaling pathways in the cell bodies, which is inconsistent with the signaling effector model as a major contributor to retrograde signaling. Finally, it has been suggested that propagating waves of TrkA phosphorylation on the plasma membrane may convey retrograde signals within 1 minute after NGF application on distal axons [58, 61]. One would predict that a propagating Trk activation wave would be a bidirectional process. Yet, application of NGF to cell bodies and proximal axons does not result in the appearance of activated TrkA in distal axons [5]. These findings do not rule out the existence of alternative retrograde mechanisms, but they suggest that retrograde signaling endosomes represent the primary mode of NGF retrograde signaling.
Box 2. Regulation of sympathetic nervous system development by target innervation.
Postganglionic sympathetic neurons innervate a variety of peripheral targets to control tissue homeostasis. Sympathetic neurons have long-served as an amenable model system to study the developmental actions of target-derived signals. Sympathetic neurons arise from neural crest precursors that migrate ventrally from the dorsal neural tube to the vicinity of the dorsal aorta to coalesce into primordial ganglia. Proliferation of sympathetic neuroblasts overlaps temporally with differentiative changes including acquisition of noradrenergic features and projection of axons [62]. These early stages of neurogenesis and differentiation are influenced by intrinsic transcriptional determinants, and extrinsic signals including bone morphogenetic protein (BMP), Wnts and hepatocyte growth factor [63–65]. Sympathetic axons extend along intermediate targets such as the arterial vasculature in response to vasculature-derived cues including artemin, NT-3, and endothelin to reach final peripheral targets, where they undergo terminal extension and branching in response to target-derived NGF [8, 66]. For postganglionic sympathetic neurons, the period of target innervation (embryonic days E16.5 to the second postnatal week, in mice) coincides with the elaboration of dendrites, formation of synaptic connections with preganglionic sympathetic neurons and a pruning of cell number by apoptosis [8, 67, 68]. Approximately 50% of the total numbers of post-mitotic sympathetic neurons are eliminated by apoptosis around the time when their axons innervate target tissues. NGF has been shown to influence multiple steps of post-ganglionic sympathetic neuronal circuit assembly including the regulation of neuronal survival, axon innervation of targets, dendritic patterning and synaptogenesis [8]. Some target tissues, notably the sweat glands, also induce a switch in neurotransmitter phenotype of a subset of sympathetic neurons from adrenergic to cholinergic upon target innervation [8]. The identity of the responsible target-derived signal(s) remains to be clearly defined, although several candidate factors have been proposed.
Endocytic trafficking mechanisms in the regulation of axon growth
During neural development, the two best-characterized functions of target-derived neurotrophins are the regulation of neuronal survival and axon growth [2]. Developing neurons have been shown to respond to both intermediate and final target-derived neurotrophic cues to navigate along the intermediate vasculature and reach and innervate final target fields [8]. While significant attention has been focused on endocytic mechanisms regulating neurotrophin-dependent survival, much less is known about the contribution of endocytic trafficking to neurotrophin-mediated axon growth.
Terminal arborization of peripheral neurons is dependent on NGF [9–11]. NGF is expressed in peripheral targets innervated by sympathetic and sensory neurons. In NGF/Bax double knockout mice, elimination of the pro-apoptotic factor Bax concurrently with NGF allowed the assessment of NGF functions in axon growth in vivo, without the apoptosis that would be elicited by NGF deprivation [12]. A reduction of sympathetic and sensory innervation of several target tissues was observed in NGF/Bax mutant mice [9–11]. Given that NGF is a target derived growth factor, it may support axon growth either by local signaling in the axons or by initiating a retrograde signal to the cell bodies where it controls transcription (Figure 1). Several transcription factors that promote the expression of genes important for axon growth and branching have been identified as key targets of NGF-TrkA retrograde signaling (Figure 1A). cAMP-responsive element-binding protein (CREB), a transcription factor that is both necessary and sufficient to support the survival of neurotrophin-dependent neurons [13], also mediates axon growth in response to NGF [14]. The retrograde signaling triggered by NGF leads to CREB phosphorylation on Ser-133 in cell bodies [15]. However, in CREB/Bax mutant mice, severe deficits in peripheral sensory innervation were observed, without detectable loss of neurons, indicating that CREB also regulates the expression of genes necessary for axon growth [14]. More recent studies have implicated several transcription factors, including Nuclear Factor of Activated T cells (NFAT) [16], Serum Response Factor (SRF) [17], and Early Growth Response 3 (Egr3) [18], that are exclusively required for neurotrophin-dependent axon growth. Genes regulated by NGF-dependent signaling that promote axon growth and innervation of final target tissues, include the NGF receptor, TrkA, itself, [19, 20], cytoskeletal components, including α-tubulin, [21], β-actin and γ-actin [17] and regulators such as the Rho-specific guanine nucleotide exchange factor (GEF), Trio [22]. In developing sympathetic neurons, a member of the Wnt family of secreted ligands, Wnt5a, is up-regulated by target-derived NGF, revealing an unexpected hierarchical pathway of growth factor signaling that underlies target innervation [23]. Retrograde NGF signaling promotes Wnt5a expression in sympathetic neurons, and secretion of Wnt5a, in turn, exerts an autocrine effect on sympathetic axon branching. These findings suggest a regulatory mechanism whereby in-growing axons themselves produce diffusible cues dependent on signals derived from the target, to instruct target innervation. Thus, retrograde signaling, and possibly TrkA-signaling endosomes, is essential for target-derived NGF to activate transcriptional programs essential for final stages of target innervation.
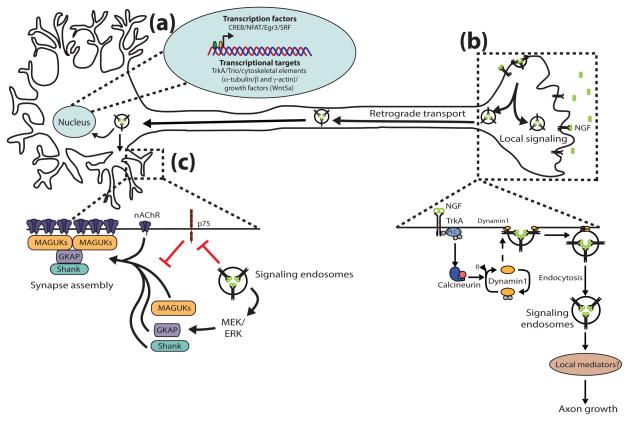
Figure 1.
Target-derived NGF mediates axon growth and synapse assembly in sympathetic neurons via endocytic signaling mechanisms. (a) Retrograde NGF signaling, likely via axonal transport of TrkA-signaling endosomes, activates transcription factors and the expression of downstream target genes essential for long-term axon growth and target innervation. (b) Local NGF-TrkA signaling from an endocytic platform in axons promotes axon growth. NGF-TrkA complexes are endocytosed via a signaling pathway that includes Phospholipase C-γ, calcineurin and dynamin1. Internalization of TrkA in distal axons is required for short-term axon growth, in a manner independent of transcriptional responses. (c) NGF-TrkA signaling endosomes are retrogradely transported long-distance from axon terminals to the distal dendrites of sympathetic neurons. In dendrites, NGF-TrkA endosomal complexes signal via the MEK/MAPK pathway to regulate the clustering of acetylcholine receptors (nAChRs) and pre-existing postsynaptic density components including MAGUK, GKAP and Shank. Endosomal TrkA signaling modulates the assembly of postsynaptic components, in part, by restricting the anti-synaptic actions of p75 signaling in dendrites.
In addition to retrograde activation of transcriptional responses, local endocytic signaling in axons is also critical for NGF-mediated growth. The first evidence that TrkA internalization is critical for neurite outgrowth was observed when a temperature-sensitive dynamin mutant was found to significantly attenuate NGF-induced neurite outgrowth in PC12 cells [24]. In sympathetic neurons, target-derived NGF regulates dynamin through a local calcineurin-mediated signaling pathway in axons (Figure 1B), [25]. Calcineurin is a calcium-responsive phosphatase that is highly enriched in the nervous system. NGF-TrkA signaling in distal axons induces calcineurin-dependent dephosphorylation of dynamin1, driving receptor internalization. Interestingly, endocytosis of TrkA is required for short-term axon growth and final target innervation in the sympathetic nervous system, although the precise mechanisms remain unclear.
How might local endocytosis in axons regulate NGF-dependent axon growth? One possibility is that endocytosis allows for efficient NGF-TrkA signaling. There is emerging experimental evidence highlighting a critical role for endocytosis of receptor tyrosine kinases in ensuring localized signaling responses to extracellular guidance/trophic cues. This is relevant for several developmental processes including directional migration [26], and filopodial sprouting by endothelial tip cells during angiogenesis [27]. Internalization might enhance downstream signaling by altering the complement of signaling effectors associated with the receptors or prolonging activation of receptors and downstream signaling effectors. Alternatively, internalization of Trk may promote the recycling of receptors back to the membrane to allow repeated interaction with the ligand. Blockade of endocytosis attenuated TrkA phosphorylation at the activation loop sites and reduced its catalytic activity in PC12 cells [24]. These findings support the idea that endocytosis enhances TrkA signaling responses, perhaps, by concentrating activated TrkA receptors in endocytic vesicles. Internalization might also regulate the kinetics of activation of downstream signaling pathways. Signaling by internalized TrkA receptors resulted in sustained Erk activation [28]. The persistence of NGF-mediated activation of Erk signaling may be dictated, in part, by the ability of TrkA receptors to sustain activity within the acidic environment of endosomes [29, 30], and also by a delay in the transition from early to late endosomes/lysosomes [28]. The ability of neurotrophins to sustain signaling following internalization may also be facilitated by the dynamic organization of the endocytic organelles in axons; the endosomes in distal axons are predominantly of neutral pH with the proportion of acidic vesicles increasing with proximity to neuronal soma [31]. Removal from the cell-surface may also protect TrkA receptors from plasma membrane-associated tyrosine phosphatases, with a mechanism resembling internalization of vascular endothelial growth factor receptor in endothelial cells [32]. A challenging goal for the future will be to characterize the precise differences in axonal NGF-TrkA signaling from an endocytic versus plasma membrane platform and how this relates to neurotrophin functions.
Retrograde neurotrophin signaling regulates dendritic growth and synapse assembly
The formation of dendrites and establishment of connections with presynaptic partners is an integral step in the normal development of most neurons. For postganglionic sympathetic neurons, the dendritic arbors increase in complexity upon innervation of peripheral target tissues, but are not affected by efferent fibers from the preganglionic neurons. Additionally, the complexity of dendritic arbors positively correlates with the size of the target tissue [33]. Treatment with exogenous NGF enhances the complexity of dendrites in sympathetic ganglia, increasing the number of primary dendrites, dendritic length, and branches [34, 35]. Conversely, NGF neutralizing antiserum had opposite effects [36]. Taken together, these results suggest that retrograde NGF signaling promotes the growth, arborization, and maintenance of dendrites in vivo. The precise signaling mechanisms and transcriptional targets necessary for this process remain to be determined.
Recently, a synaptogenic signal by which retrograde signaling endosomes contribute to the formation of synaptic connections has been identified in the sympathetic nervous system. NGF knockout mice display a dramatic loss of pre- and post-ganglionic synaptic specializations, without a loss of preganglionic axon innervation [37]. While exploring this mechanism, it was found that NGF-TrkA signaling endosomes are retrogradely transported from distal axons all the way to the distal dendrites of sympathetic neurons in vitro. In dendrites, NGF-TrkA endosomal complexes act through the MEK/MAPK pathway to mediate the assembly of post-synaptic density components, such as Membrane-Associated Guanylate Kinases (MAGUK), Shank, Guanylate Kinase-Associated Protein (GKAP), and nicotinic acetylcholine receptors (nAChRs) (Figure 1C). Astonishingly, NGF-mediated clustering of synaptic components is independent of new protein synthesis, indicating that NGF-TrkA signaling endosomes regulate the clustering of pre-existing synaptic components. These results support the hypothesis that endosomal NGF-TrkA complexes propagate intracellular signaling pathways throughout the entire neuron and exert control over many steps during the development of sympathetic circuitry, including dendritic arborization and the formation of connections with preganglionic sympathetic neurons.
Receptor trafficking modulates positive feedback loops during neuronal competition
During embryonic development, neurons are produced in vast excess and their survival is controlled by limiting amounts of target-derived growth factors. The final number of neurons is dependent on the balance between signals promoting cell survival and cell death. New evidence indicates that NGF signaling triggers positive feedback loops that help to determine the neurons that survive from those that undergo apoptosis. The feedback mechanisms may amplify subtle differences in the initial strength of NGF-TrkA signaling in certain neurons, thereby enhancing the ability of those neurons to gain access to limited amounts of NGF. Retrograde trafficking of NGF-TrkA complexes may play a prominent role in maintaining these feedback mechanisms (Figure 2).
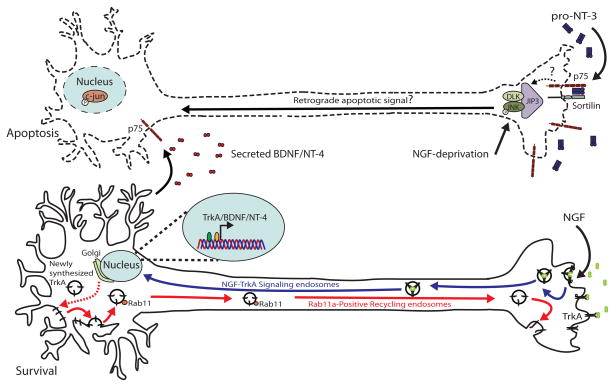
Figure 2.
Axonal trafficking of survival and apoptotic signals underlie a developmental competition for target-derived NGF. In competing neurons, retrograde NGF signaling regulates a positive feedback loop by which NGF enhances sensitivity to itself by activating the transcription of its receptor, TrkA. Increased responsiveness to ligand may also result from anterograde transcytosis of TrkA receptors from soma surfaces to axon terminals via Rab11-positive recycling endosomes. In winning neurons, retrograde NGF signaling regulates the transcription of BDNF and NT-4 that then exert a paracrine effect on punishing/killing neighboring neurons that do not gain sufficient access to NGF. BDNF/NT-4-dependent apoptosis is mediated by p75 receptors expressed on the “losing” neurons. DLK, JNK and JIP3 may be part of an apoptotic complex that is activated locally in axons upon NGF deprivation and retrogradely transported to mediate c-jun phosphorylation in cell bodies and neuronal cell death. Developmental apoptosis may also be elicited by target-derived pro-neurotrophins, such as pro-NT-3, that act on axon terminals to initiate a retrograde death signal, likely via activation of a p75-sortilin receptor complex. It remains to be determined if similar or distinct retrograde signals are activated upon NGF deprivation and by pro-neurotrophins.
In sympathetic neurons, retrograde NGF signaling regulates the transcription of factors that enhance their own access to NGF and weaken neighboring neurons that do not gain sufficient access to NGF. TrkA was identified as a potential competition-promoting factor that is induced in response to target-derived NGF [19, 20]. Increased levels of TrkA form a positive feedback loop by which NGF enhances sensitivity to itself. Thus, both the magnitude and duration of NGF signaling increase in a concentration-dependent manner following an initial exposure to NGF. Relatively small differences in the initial levels of NGF-TrkA signaling are amplified via increased TrkA transcription to establish differential sensitivities in competing neurons to target-derived NGF. Aside from regulating TrkA levels, NGF also regulates the expression of p75, BDNF, and NT4 [20, 38]. The ability of NGF to promote expression of these factors has a more sinister function. In an interesting model of paracrine inhibition, retrograde NGF signaling promotes the secretion of BDNF and NT4 to actively kill competing neurons that have low levels of NGF signaling, through a p75-mediated mechanism. A similar BDNF/p75-mediated paracrine signal also underlies the competitive elimination of sympathetic axons during developmental axon pruning in the sympathetic nervous system, which occurs long after the period of naturally occurring cell death [39].
Retrograde neurotrophin signaling may regulate Trk levels in developing axons at multiple levels, from transcriptional regulation to the anterograde transport of mature receptors. In an example of a positive feedback loop that does not depend on new protein synthesis, localized BDNF signaling enhanced the anterograde transport of TrkB receptors during axonal specification in hippocampal neurons [40]. In developing sympathetic neurons, it was reported that TrkA receptors on the surface of sympathetic neuron cell bodies are endocytosed and anterogradely transported to the axon terminals, by a transcytotic process [41]. NGF applied to distal axons enhanced the transcytosis of TrkA receptors from cell bodies to distal axons. Anterograde trafficking of Trk receptors were shown to be necessary for full signaling and growth responses to target-derived neurotrophins [41, 42]. Taken together, these findings suggest that retrograde neurotrophin signaling regulates transcriptional and trafficking-based feedback loops that are necessary for neuronal development.
Retrograde trafficking of apoptotic signals during development
In addition to pro-survival signals, retrograde transport of apoptotic signals that are initiated locally in nerve terminals may be part of a developmental competition for target-derived trophic support (Figure 2). In an in vitro NGF withdrawal paradigm that mimics the developmental competition for target-derived trophic factors, a signal originating locally in axons mediates phosphorylation of the pro-apoptotic transcription factor, c-jun, in cell bodies of sympathetic neurons [43]. In axons, local activation of the stress-induced kinases, Dual Leucine Zipper Kinase (DLK) and c-Jun-N-terminal kinase (JNK), might be responsible for initiating the apoptotic signal that culminates with c-jun phosphorylation and neuronal apoptosis [44]. Retrograde transport of the DLK-JNK-containing complexes along the microtubules could be mediated by the JIP3 scaffolding protein, which interacts with DLK-JNK and the p150-Glued subunit of dynactin complex [44, 45]. Retrograde trafficking of apoptotic signals during the developmental competition period may also be initiated by target-derived pro-neurotrophins. Pro-neurotrophins are the precursors of the family of neurotrophins and were shown recently to have biological effects of their own. Pro-neurotrophins act through the p75 receptor and its co-receptor, sortilin, to mediate apoptosis of select neuronal populations [46–48]. Pro-neurotrophin-3 (pro-NT-3) is normally expressed in peripheral tissues innervated by sympathetic neurons, and when added to distal axons of compartmentalized sympathetic cultures, promotes apoptotic cell death [49]. These findings provide intriguing evidence that signals activated locally in axons by deprivation of mature neurotrophins or secretion of pro-neurotrophins are retrogradely propagated to neuronal cell bodies to initiate pro-death transcriptional programs. Whether the apoptotic signals are transported in endosomes, their molecular composition and the nature of their interactions with survival signaling endosomes, remain to be elucidated.
Signaling endosomes in neuronal migration
Endocytic trafficking of neurotrophin receptors is not only a feature of differentiated neurons that have already innervated target tissues, but is also used during early stages of neural development to control the migration of immature neurons. In many non-neuronal cells, polarized trafficking regulates cell migration [50]. A series of elegant studies provided evidence of an endocytic trafficking pathway that regulates neuronal precursor cell migration; in particular, the sharpening of an extracellular neurotrophin gradient and polarizing of signaling endosomes to the leading edge of the migrating cells for signal amplification [51, 52]. In the developing cerebellum, cerebellar granule precursors migrate from a neurogenic niche in the external granule layer (EGL) toward their final destination in the internal granule layer (IGL). Recent findings indicate that endocytic trafficking of the TrkB receptor is required for directed cell migration in response to a BDNF gradient. TrkB-containing endosomes are localized to the leading edge of the migrating neuroblasts, and endocytic blockade, either via pharmacological or dominant negative strategies, reduced directional migration in culture and cerebellar slices [51]. TrkB endocytosis and cell polarization is mediated by the endocytic adaptor protein, Numb, which recruits the polarity protein, atypical protein kinase C (aPKC) to the leading edge of the migrating cells [52]. Phosphorylation of Numb by aPKC triggers a positive feedback loop, which enhances Numb binding to the TrkB receptor and facilitation of receptor endocytosis. How do the TrkB-signaling endosomes bring about cytoskeletal changes necessary for movement? One possibility may be the activation of Rac by the Rac-specific GEF, Tiam1, which is localized to the TrkB endosomes [52]. Together with earlier studies done in Drosophila border cells [26, 53], these studies add to emerging evidence of receptor tyrosine kinase signaling at the leading edge of migrating cells and a role for endocytic trafficking in modulating this process.
Neurotrophin signaling drives its own trafficking
Although it is established that endocytosis plays a critical role in regulating Trk signaling, less is known regarding how neurotrophin-mediated signaling affects its own transport. A possible model entails that receptors are trafficked as ‘passengers’ associated with a constitutively operating endocytic machinery that is not influenced by receptor signaling. Conversely, according to a ‘driver’ model, signals initiated from the cargo actively modulate the transport machinery [54]. Early evidence supporting the regulation of clathrin-mediated endocytosis by neurotrophin signaling was obtained from studies performed using PC12 cells and DRG neurons. NGF signaling increased the density of clathrin-coated pits at the cell surface, and led to the assembly of complexes containing phosphorylated TrkA receptors, clathrin and the adaptor protein, AP2 [55]. Post-translational modifications of endocytic proteins downstream of NGF signaling might mediate their recruitment to the plasma membrane. For example, NGF signaling induces the tyrosine phosphorylation of the clathrin heavy chain and its subsequent re-localization to the plasma membrane [55]. In sympathetic neurons, NGF-dependent activation of the phosphatase, calcineurin, results in dephosphorylation of dynamin1 [25], which might be responsible for its membrane localization. Monomeric Rab GTPases coordinate distinct stages of intracellular transport, including vesicle formation, motility, and targeting of vesicles to their proper sub-cellular locations. In motor neurons, Rab5 and Rab7 control retrograde axonal trafficking of vesicles containing neurotrophins and their receptors [56]. Rab27 and Rab11 mediate the anterograde delivery of Trk receptors to axons via the secretory [57] or transcytotic pathways [41], respectively. Modulation of Rab GTPase activity by Trk signaling might present another mechanism by which receptor signaling enhances its own trafficking, although this remains to be tested. It also remains unknown if neurotrophin signaling might modulate the processivity or directionality of axonal motors to influence their own transport. An intriguing question is whether the effects are specific for Trk receptors or if neurotrophin signaling has more global effects on stimulating endocytic trafficking of additional receptors.
Active transport of TrkA-containing vesicles in axons is dependent on the actin and microtubule cytoskeletal networks. A recent study suggested that two neurotrophins differentially control receptor trafficking through specific regulation of actin cytoskeletal dynamics [30]. In sympathetic neurons, both the intermediate target-derived neurotrophin, NT-3, and the final target-derived factor, NGF, activate the TrkA receptor to promote axon growth. However, only NGF is capable of signaling retrogradely to promote neuronal survival [10]. A key difference in the mechanisms of action of NGF and NT-3 lies in the control of retrograde receptor trafficking. While NGF promotes retrograde transport of TrkA signaling endosomes to activate pro-survival signaling pathways in neuronal soma, NT-3 does not [10]. Recently, it was found that only NGF/TrkA-containing endosomes are associated with the actin-modulatory proteins, Rac and cofillin, facilitating actin severing, which allows the signaling endosomes to overcome a dense actin meshwork to travel retrogradely [30]. Conversely, NT-3 is incapable of recruiting these actin modulators to the TrkA-containing endosomes, and is therefore unable to promote retrograde transport and neuronal survival.
Concluding remarks
From a classical view of endocytosis and retrograde trafficking of neurotrophin receptors mediating neuronal survival, the scenario has broadened to incorporate many other neurotrophin-mediated functions that depend on endocytosis. We now know that neurotrophin-mediated endocytic trafficking regulates various developmental events including axon growth, synaptogenesis and even degenerative changes. Many questions, however, remain to be addressed regarding the endocytic mechanisms underlying the distinct functions. Are there specialized signaling endosomes for each function? How does local endocytic signaling in axon terminals mediate axon growth? How are signaling endosomes transported within dendrites, and how do they communicate with postsynaptic components? The new findings that Trk receptors travel anterogradely from the soma surfaces to axonal membranes and retrogradely from axon surfaces to dendrites suggest that endocytic trafficking is used as a means of communication between two spatially distinct membrane domains, the axonal and somatodendritic membranes. Are different modes of receptor internalization employed for retrogradely trafficked versus anterogradely transcytosing Trk receptors? The identification of specific signaling pathways and the endocytic machinery associated with the retrograde and anterograde transport will clarify how target-derived neurotrophins regulate this long-distance communication between the axonal and somatodendritic surfaces. Answers to some of the remaining questions may come from live-cell imaging to visualize, in real-time, neurotrophin-mediated endocytic events in distinct sub-cellular compartments in neurons (see Box 3). In addition, biochemical approaches in neuronal culture systems will help identify additional endocytic proteins and signaling effectors that underlie the distinct neurotrophin-dependent functions. Finally, the use of mouse genetics or knockdown approaches will be invaluable in confirming the relevance of the identified proteins in neurotrophin-mediated development in vivo.
Box 3. Techniques that facilitate the study of neurotrophin-mediated trafficking in neurons.
Compartmentalized neuronal cultures have proved to be an essential tool for studying the local and retrograde actions of target-derived neurotrophins, and the mechanisms underlying their directional trafficking in polarized neurons. Compartmentalized cultures allow the separation of neuronal cell bodies and distal axons into distinct fluid environments and target-derived neurotrophins or other factors can be added exclusively to distal axons, thus recapitulating the in vivo environment. This culture system, where a three-chambered teflon divider is sealed with silicone grease onto the bottom of a collagen-coated tissue-culture dish was pioneered by Robert Campenot in 1970’s [69]. Although the teflon-grease barrier is fluid impermeable, neurons plated in the central compartments are able to extend axonal projections through the barrier. A limitation to the traditional Campenot chambers is that their use is restricted to certain neuronal populations (peripheral sympathetic and sensory neurons) that project axons long enough (often, several millimeters in culture) to penetrate through the grease barrier. Recently, the advent of microfluidic devices imprinted from lithography-generated epoxy molds has revolutionized the use of compartmentalized chambers for neurobiology. Microfluidic chambers rely on the presence of microgrooves to separate the soma and axonal compartments, with a small hydrostatic pressure difference preventing diffusion between the two compartments [70]. Microfluidic chambers are better suited to the compartmentalized cultures of central nervous system (CNS) neurons that project shorter axons in culture. Since the microfluidic chambers permit plating of neurons on a glass substratum, they are also compatible with live imaging and confocal microscopy.
In the past, investigations of retrograde neurotrophin trafficking in compartmentalized cultures have relied on the immunocytochemical or biochemical detection of radiolabeled neurotrophin or phosphorylated Trk receptors that appear in neuronal cell bodies, after ligand stimulation of distal axons. Recently, more sensitive fluorescence-based techniques have been developed in order to visualize retrograde transport neurotrophin and their receptors, in real-time. Quantum-dot-labeled NGF (QD-NGF) was used to track the movements of NGF in real time in compartmentalized cultures of sensory neurons [71]. Quantum dots are crystalline semiconductors whose size and shape dictate their fluorescent characteristics. The high intensity and photoblinking properties of quantum dots enabled a quantitative analysis of the number of NGF molecules per endosome, revealing that most endosomes contained a single dimer of QD-NGF, which is probably sufficient to maintain signaling during retrograde transport [71]. Antibody feeding assays are a well established immunocytochemical method for following the trafficking of cell surface receptors [72]. This assay has been recently adapted to follow the endocytosis, recycling, anterograde and retrograde transport of Trk receptors [37, 41, 73]. Compartmentalized cultures expressing epitope-tagged Trk (FLAG-Trk) receptors are fed with fluorescent anti-FLAG antibodies, followed by microscopy to visualize the subcellular distribution of the internalized antibody-receptor complexes. In a recent study, the generation of a knock-in mouse line expressing FLAG-TrkA enabled the visualization of the trafficking of endogenous TrkA-containing endosomes in compartmentalized cultures of sympathetic neurons [37].
Acknowledgments
We thank Antonella Riccio, Chris Deppmann and Haiqing Zhao for insightful comments. We apologize to authors whose work could not be cited due to space limitations. The authors’ work is supported by NIH grants R01NS073751, R01MH080738 and R21DK090624 (R.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Snider WD. Functions of the Neurotrophins during Nervous System Development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 2.Huang E, Reichardt L. Neurotrophins: Roles in neuronal development and function. Annu Rev Neurosci. 2001;24:667–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howe CL, Mobley WC. Long-distance retrograde neurotrophic signaling. Current opinion in neurobiology. 2005;15:40–48. doi: 10.1016/j.conb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Zweifel LS, et al. Functions and mechanisms of retrograde neurotrophin signalling. Nature reviews Neuroscience. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
- 5.Ye H, et al. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron. 2003;39:57–68. doi: 10.1016/s0896-6273(03)00266-6. [DOI] [PubMed] [Google Scholar]
- 6.Heerssen HM, et al. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nature neuroscience. 2004;7:596–604. doi: 10.1038/nn1242. [DOI] [PubMed] [Google Scholar]
- 7.Ibanez CF. Message in a bottle: long-range retrograde signaling in the nervous system. Trends in cell biology. 2007;17:519–528. doi: 10.1016/j.tcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annual review of neuroscience. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- 9.Glebova NO, Ginty DD. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:743–751. doi: 10.1523/JNEUROSCI.4523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuruvilla R, et al. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Patel TD, et al. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo [see comments] Neuron. 2000;25:345–357. doi: 10.1016/s0896-6273(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 12.Deckwerth TL, et al. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron. 1996;17:401–411. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 13.Riccio A, et al. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286:2358–2361. doi: 10.1126/science.286.5448.2358. [DOI] [PubMed] [Google Scholar]
- 14.Lonze BE, et al. Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron. 2002;34:371–385. doi: 10.1016/s0896-6273(02)00686-4. [DOI] [PubMed] [Google Scholar]
- 15.Riccio A, et al. An NGF-TrkA-Mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997;227:1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- 16.Graef IA, et al. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell. 2003;113:657–670. doi: 10.1016/s0092-8674(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 17.Wickramasinghe SR, et al. Serum response factor mediates NGF-dependent target innervation by embryonic DRG sensory neurons. Neuron. 2008;58:532–545. doi: 10.1016/j.neuron.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eldredge LC, et al. Abnormal sympathetic nervous system development and physiological dysautonomia in Egr3-deficient mice. Development. 2008;135:2949–2957. doi: 10.1242/dev.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller FD, et al. Regulation of nerve growth factor receptor gene expression by nerve growth factor in the developing peripheral nervous system. The Journal of cell biology. 1991;112:303–312. doi: 10.1083/jcb.112.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deppmann CD, et al. A model for neuronal competition during development. Science. 2008;320:369–373. doi: 10.1126/science.1152677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathew TC, Miller FD. Increased expression of T alpha 1 alpha-tubulin mRNA during collateral and NGF-induced sprouting of sympathetic neurons. Developmental biology. 1990;141:84–92. doi: 10.1016/0012-1606(90)90103-p. [DOI] [PubMed] [Google Scholar]
- 22.Estrach S, et al. The Human Rho-GEF trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Current biology : CB. 2002;12:307–312. doi: 10.1016/s0960-9822(02)00658-9. [DOI] [PubMed] [Google Scholar]
- 23.Bodmer D, et al. Wnt5a mediates nerve growth factor-dependent axonal branching and growth in developing sympathetic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:7569–7581. doi: 10.1523/JNEUROSCI.1445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, et al. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:5671–5678. doi: 10.1523/JNEUROSCI.20-15-05671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bodmer D, et al. Isoform-specific dephosphorylation of dynamin1 by calcineurin couples neurotrophin receptor endocytosis to axonal growth. Neuron. 2011;70:1085–1099. doi: 10.1016/j.neuron.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jekely G, et al. Regulators of endocytosis maintain localized receptor tyrosine kinase signaling in guided migration. Developmental cell. 2005;9:197–207. doi: 10.1016/j.devcel.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Sawamiphak S, et al. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 28.Valdez G, et al. Trk-signaling endosomes are generated by Rac-dependent macroendocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12270–12275. doi: 10.1073/pnas.0702819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zapf-Colby A, Olefsky JM. Nerve growth factor processing and trafficking events following TrkA-mediated endocytosis. Endocrinology. 1998;139:3232–3240. doi: 10.1210/endo.139.7.6122. [DOI] [PubMed] [Google Scholar]
- 30.Harrington AW, et al. Recruitment of actin modifiers to TrkA endosomes governs retrograde NGF signaling and survival. Cell. 2011;146:421–434. doi: 10.1016/j.cell.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Overly CC, Hollenbeck PJ. Dynamic organization of endocytic pathways in axons of cultured sympathetic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:6056–6064. doi: 10.1523/JNEUROSCI.16-19-06056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanahan AA, et al. VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Developmental cell. 2010;18:713–724. doi: 10.1016/j.devcel.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voyvodic JT. Development and regulation of dendrites in the rat superior cervical ganglion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1987;7:904–912. doi: 10.1523/JNEUROSCI.07-03-00904.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snider WD. Nerve growth factor enhances dendritic arborization of sympathetic ganglion cells in developing mammals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8:2628–2634. doi: 10.1523/JNEUROSCI.08-07-02628.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruit KG, et al. Nerve growth factor regulates sympathetic ganglion cell morphology and survival in the adult mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10:2412–2419. doi: 10.1523/JNEUROSCI.10-07-02412.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruit KG, Snider WD. Administration or deprivation of nerve growth factor during development permanently alters neuronal geometry. The Journal of comparative neurology. 1991;314:106–113. doi: 10.1002/cne.903140110. [DOI] [PubMed] [Google Scholar]
- 37.Sharma N, et al. Long-distance control of synapse assembly by target-derived NGF. Neuron. 2010;67:422–434. doi: 10.1016/j.neuron.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller FD, et al. Nerve growth factor derived from terminals selectively increases the ratio of p75 to trkA NGF receptors on mature sympathetic neurons. Developmental biology. 1994;161:206–217. doi: 10.1006/dbio.1994.1021. [DOI] [PubMed] [Google Scholar]
- 39.Singh KK, et al. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nature neuroscience. 2008;11:649–658. doi: 10.1038/nn.2114. [DOI] [PubMed] [Google Scholar]
- 40.Cheng PL, et al. Self-amplifying autocrine actions of BDNF in axon development. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18430–18435. doi: 10.1073/pnas.1115907108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ascano M, et al. Axonal targeting of Trk receptors via transcytosis regulates sensitivity to neurotrophin responses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11674–11685. doi: 10.1523/JNEUROSCI.1542-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang SH, et al. JIP3 mediates TrkB axonal anterograde transport and enhances BDNF signaling by directly bridging TrkB with kinesin-1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:10602–10614. doi: 10.1523/JNEUROSCI.0436-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mok SA, et al. A retrograde apoptotic signal originating in NGF-deprived distal axons of rat sympathetic neurons in compartmented cultures. Cell research. 2009;19:546–560. doi: 10.1038/cr.2009.11. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh AS, et al. DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. The Journal of cell biology. 2011;194:751–764. doi: 10.1083/jcb.201103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavalli V, et al. Sunday Driver links axonal transport to damage signaling. The Journal of cell biology. 2005;168:775–787. doi: 10.1083/jcb.200410136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee R, et al. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 47.Nykjaer A, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 48.Volosin M, et al. Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:7756–7766. doi: 10.1523/JNEUROSCI.1560-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yano H, et al. Proneurotrophin-3 is a neuronal apoptotic ligand: evidence for retrograde-directed cell killing. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:14790–14802. doi: 10.1523/JNEUROSCI.2059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fletcher SJ, Rappoport JZ. Moving forward: polarised trafficking in cell migration. Trends in cell biology. 2010;20:71–78. doi: 10.1016/j.tcb.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Zhou P, et al. Polarized signaling endosomes coordinate BDNF-induced chemotaxis of cerebellar precursors. Neuron. 2007;55:53–68. doi: 10.1016/j.neuron.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou P, et al. Numb links extracellular cues to intracellular polarity machinery to promote chemotaxis. Developmental cell. 2011;20:610–622. doi: 10.1016/j.devcel.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Assaker G, et al. Spatial restriction of receptor tyrosine kinase activity through a polarized endocytic cycle controls border cell migration. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22558–22563. doi: 10.1073/pnas.1010795108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nature reviews Molecular cell biology. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 55.Beattie EC, et al. NGF signals through TrkA to increase clathrin at the plasma membrane and enhance clathrin-mediated membrane trafficking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:7325–7333. doi: 10.1523/JNEUROSCI.20-19-07325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deinhardt K, et al. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 57.Arimura N, et al. Anterograde transport of TrkB in axons is mediated by direct interaction with Slp1 and Rab27. Developmental cell. 2009;16:675–686. doi: 10.1016/j.devcel.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 58.MacInnis BL, Campenot RB. Retrograde support of neuronal survival without retrograde transport of nerve growth factor. Science. 2002;295:1536–1539. doi: 10.1126/science.1064913. [DOI] [PubMed] [Google Scholar]
- 59.Campenot RB, MacInnis BL. Retrograde transport of neurotrophins: fact and function. Journal of neurobiology. 2004;58:217–229. doi: 10.1002/neu.10322. [DOI] [PubMed] [Google Scholar]
- 60.Hempstead BL, et al. Overexpression of the trk tyrosine kinase rapidly accelerates nerve growth factor-induced differentiation. Neuron. 1992;9:883–896. doi: 10.1016/0896-6273(92)90241-5. [DOI] [PubMed] [Google Scholar]
- 61.Senger DL, Campenot RB. Rapid Retrograde Tyrosine Phosphorylation of TrkA and Other Proteins in Rat Sympathetic Neurons in Compartmented Cultures. J Cell Bioi. 1997;138:411–421. doi: 10.1083/jcb.138.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rohrer H. Transcriptional control of differentiation and neurogenesis in autonomic ganglia. The European journal of neuroscience. 2011;34:1563–1573. doi: 10.1111/j.1460-9568.2011.07860.x. [DOI] [PubMed] [Google Scholar]
- 63.Maina F, et al. Multiple roles for hepatocyte growth factor in sympathetic neuron development. Neuron. 1998;20:835–846. doi: 10.1016/s0896-6273(00)80466-3. [DOI] [PubMed] [Google Scholar]
- 64.Howard MJ. Mechanisms and perspectives on differentiation of autonomic neurons. Developmental biology. 2005;277:271–286. doi: 10.1016/j.ydbio.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 65.Armstrong A, et al. Frizzled3 is required for neurogenesis and target innervation during sympathetic nervous system development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:2371–2381. doi: 10.1523/JNEUROSCI.4243-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Makita T, et al. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature. 2008;452:759–763. doi: 10.1038/nature06859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rubin E. Development of the rat superior cervical ganglion: initial stages of synapse formation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1985;5:697–704. doi: 10.1523/JNEUROSCI.05-03-00697.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rubin E. Development of the rat superior cervical ganglion: ingrowth of preganglionic axons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1985;5:685–696. doi: 10.1523/JNEUROSCI.05-03-00685.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campenot RB. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park JW, et al. Microfluidic culture platform for neuroscience research. Nature protocols. 2006;1:2128–2136. doi: 10.1038/nprot.2006.316. [DOI] [PubMed] [Google Scholar]
- 71.Cui B, et al. One at a time, live tracking of NGF axonal transport using quantum dots. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13666–13671. doi: 10.1073/pnas.0706192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao TT, et al. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- 73.Chen ZY, et al. A novel endocytic recycling signal distinguishes biological responses of Trk neurotrophin receptors. Molecular biology of the cell. 2005;16:5761–5772. doi: 10.1091/mbc.E05-07-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]