Abstract
The transverse carpal ligament (TCL) forms the palmar boundary of the carpal tunnel and plays an important role in carpal tunnel mechanics. TCL hypertrophy has been observed for individuals with carpal tunnel syndrome (CTS) and postulated as a potential etiological factor. Ultrasound is particularly advantageous for TCL imaging because of its capability of detecting the interfaces between the TCL and other tissues. The purposes of this study were to develop an ultrasound based method to measure the TCL thickness and to test the validity and reliability of this method. Three operators conducted two sessions of ultrasound examination on 8 cadaveric specimens and 8 healthy volunteers. A custom script was used to calculate TCL thickness along the TCL length from the ultrasound images. The ultrasound based TCL thickness of the cadaveric specimens was compared to the dissection based TCL thickness for validation. The results showed Pearson’s correlation coefficients of 0.867–0.928, intraclass correlation coefficient (ICC) values of 0.726–0.865, a standard error of measurement of 0.02–0.07 mm, and a minimal detectable difference of 0.05–0.15 mm. The high correlation coefficients and small errors indicate that the ultrasound based method is valid for measuring TCL thickness. Furthermore, ultrasound measurements showed excellent intra-operator and inter-operator reliability with ICC values as 0.826–0.933 and 0.840–0.882, respectively. The ultrasound based TCL thickness was in the range of 0.93–2.34 (1.54 ± 0.33) mm and agreed well with previous studies. The ultrasound method developed in this study is a valuable tool to examine morphological properties of healthy and pathological TCLs.
Keywords: Ultrasound, transverse carpal ligament, validity, reliability
INTRODUCTION
Carpal tunnel syndrome (CTS) is one of the most common hand disorders and is caused by median nerve compression within the carpal tunnel (Papanicolaou et al. 2001). Anatomically, the carpal tunnel is formed by the transverse carpal ligament (TCL) at its volar border and the interconnected carpal bones at its medial, lateral, and dorsal borders. The TCL prevents volar migration of the median nerve and flexor tendons, and plays an important role in carpal tunnel mechanics (Brooks et al. 2003; Guo et al. 2009; Li et al. 2009; Xiu et al. 2010; Li et al. 2011). The TCL is a mechanical constraint of the carpal tunnel associated with median nerve compression neuropathy. This constraint is alleviated by the surgical release of the TCL, which results in an increased cross-sectional area of the carpal tunnel (Kato et al. 1994) and a reduced carpal tunnel pressure (Gelberman et al. 1981). In particular, TCL hypertrophy has been observed in CTS patients (John et al. 1983; Yamagami et al. 1994; Ferrari et al. 1997), suggesting that TCL thickening may contribute to pathomechanics of the carpal tunnel and eventually cause CTS.
Attempts have been made to examine TCL morphology using different techniques (Tanzer 1959; Merhar et al. 1986; Cobb et al. 1993; Tanabe and Okutsu 1997; Pacek et al. 2010; Stecco et al. 2010). Various TCL thicknesses were reported, mainly in the range of 1–3 mm. Though the previous studies provided information regarding the TCL thickness, they had limitations. The cadaveric studies that used calipers (Tanzer 1959), micrometers (Tanabe and Okutsu 1997), photography (Cobb et al. 1993), and silicon casting (Pacek et al. 2010) to measure the TCL thickness required dissection and therefore were not applicable to clinical use. Imaging techniques used in the live subject studies, e.g. computed tomography (CT) (Merhar et al. 1986) and magnetic resonance imaging (MRI) (Stecco et al. 2010), were expensive, time consuming, and insufficient to distinguish the TCL from the surrounding soft tissues. Compared to the previously used techniques, ultrasound is particularly advantageous for TCL imaging because of its capability of detecting the interfaces between TCL and other tissues. Furthermore, ultrasound is inexpensive, quick, portable, non-invasive, and suitable for routine clinical assessment.
A few studies investigated the validity and reliability of ultrasound assessment of soft tissue thicknesses, including studies for tendons (Richards et al. 2001; Ying et al. 2003; O’Connor et al. 2004; Brushoj et al. 2006; Collinger et al. 2009), ligaments (Balint and Sturrock 2001), and muscles (Bunce et al. 2002; Kellis et al. 2009; Bentman et al. 2010). However, the validity and reliability of ultrasound assessment of TCL thickness has not been studied. The purposes of this study were to develop an ultrasound based method to measure the TCL thickness and to test the validity and reliability of this method.
MATERIALS AND METHODS
Study design
This study used two subject groups including cadaveric specimens and healthy volunteers. First, ultrasound examination was performed on eight cadaveric hands followed by the direct measurement of the dissected TCL. Validity was analyzed based on the TCL thicknesses measured using the ultrasound and dissection methods. Second, ultrasound imaging was conducted on the right hands of eight healthy volunteers. Three operators carried out two sessions of ultrasound imaging on each specimen or volunteer, allowing analysis of the intra-operator and inter-operator reliability.
Cadaveric specimens and healthy volunteers
Eight fresh-frozen cadaveric hands (6 males and 2 females) were used in this study. These cadaveric specimens had an age of 51 ± 9 years and a body mass index (BMI) of 24 ± 2 kg/m2. The specimens were stored at −20°C and thawed at room temperature overnight the day before the experiment. Eight healthy, male volunteers (age: 29 ± 7 years, BMI: 25 ± 4 kg/m2) were recruited for this study with approval of the local institutional review board. Informed consent was obtained from all human subjects prior to the study. Ultrasound imaging was performed on the right hands only. Exclusion criteria for specimens and volunteers were traumatic or degenerative disorders to the hand and wrist.
Ultrasound examination
Each hand of the cadaver specimens and healthy volunteers was secured in a custom-made thermoplastic splint using Velcro straps with the wrist in a supine, anatomically neutral position (Fig. 1a). The fingers were at full extension and the thumb was at 0° palmar abduction and 45° radial abduction. An ultrasound system (Acuson S2000, Siemens Medical Solutions USA Inc., Mountain View, CA, USA) with a linear array transducer (18L6 HD) was used for this study.
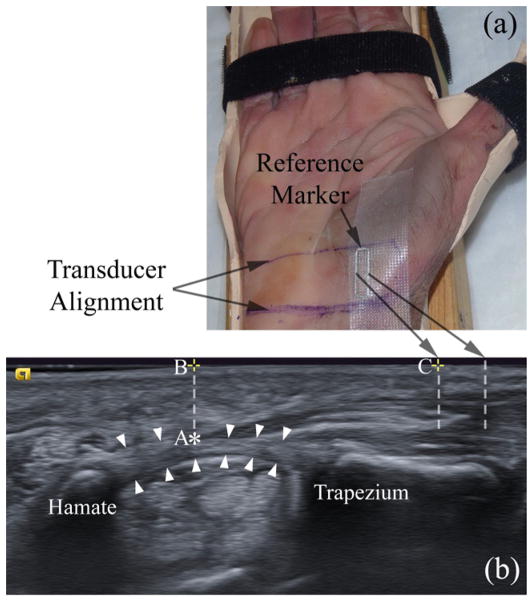
Figure 1.
A cadaveric hand is secured in a custom-made splint (a) for ultrasound imaging (b). White arrowheads indicate the TCL. Points A (‘*’), B (‘+’ on the left), and C (‘+’ on the right) indicate the thenar muscle’s ulnar point (TUP), the surface projection of the TUP, and the surface intersection of the left interference line generated by the reference marker, respectively.
To compare the ultrasound and dissection measurements of TCL thickness at identical locations, ultrasound imaging procedures for cadaveric specimens were standardized. First, a desired plane of view was chosen so that the ridge of the trapezium and the hook of the hamate were identified in the image. Second, the ultrasound transducer was held in place while the outline of the transducer was traced on the palmar wrist with a permanent marker. Third, a steel rectangular-shaped reference marker was affixed on the radial aspect of the wrist using Transpore™ surgical tape (Fig. 1a). The long bars of the marker created interference patterns visible on the ultrasound image (Fig. 1b), providing reference positions on the image (Collinger et al. 2009). Finally, the transducer was aligned with the traced outline for ultrasound imaging. All three operators were present to set up the cadaveric specimens. They performed the ultrasound examinations one after the other without removing the reference marker or the transducer alignment marks because it was important to leave the reference marker in the same location for dissection. For healthy volunteers, a desired plane of view where the trapezium and hamate could be identified was chosen and ultrasound images were recorded. The surface reference marker was not used for volunteers because there was no need to compare with dissection measurements.
Three operators, who were specifically trained for imaging the carpal tunnel at the hamate level, conducted ultrasound examinations on each specimen and volunteer in two sessions. Three carpal tunnel images were collected by each operator during each session. Care was taken to maintain the transducer at the same location, avoid exerting undue skin pressure by the transducer, and keep the ultrasound machine settings constant. The ultrasound machine was operated at the 2D mode with tissue harmonic imaging and tissue equalization, imaging frequency was set at 12 MHz, and image field depth was 20 mm.
TCL thickness measured from the ultrasound images
The volar and dorsal boundaries of the TCL were traced using the multi-point selection tool in ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA). All images were processed using a custom MATLAB (The MathWorks, Natick, MA, USA) script to measure the TCL thickness at various locations (Fig. 2). The script contained the following six steps: (1) read the X and Y coordinates of the volar and dorsal boundaries of the TCL discretely selected in ImageJ and fill in missing pixels along the TCL boundaries by linear interpolation, (2) calculate the coordinates of the middle line of the TCL based on the interpolated volar and dorsal boundaries, (3) fit each of the interpolated volar, middle, and dorsal curves with a fourth-order polynomial, (4) determine a line perpendicular to the fitted middle curve at a given location, (5) find the intersection points between this perpendicular line and the volar and dorsal curves, and (6) calculate the distance between the two intersection points which corresponds to the TCL thickness at this location.
Figure 2.
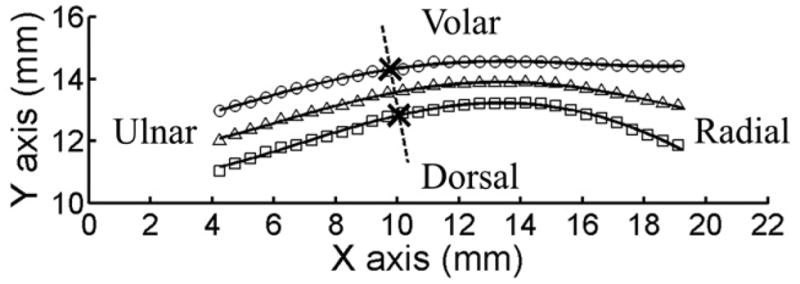
A representative, digitalized outline of the TCL for thickness calculation. The interpolated volar and dorsal boundaries of the TCL are indicated by symbols ○, and □, respectively. Symbol △ indicates the middle points derived from corresponding volar and dorsal data. For clarity, the symbols are shown every 7 pixels. The solid lines represent the fitted fourth-order polynomials. The dotted line is perpendicular to the fitted middle line of the TCL. The intersection points between the dotted line and the fitted volar and dorsal curves of the TCL are indicated by ‘×’. The TCL thickness at this location is 1.51 mm measured as the distance between the two intersection points.
Multiple locations on the TCL were examined in order to find the most reliable location for TCL thickness measurement since previous studies showed that TCL thickness varies along the length (Pacek et al. 2010). The thenar muscle’s ulnar point (TUP), which is the most ulnar aspect of the thenar muscle attachment to the TCL, was chosen as the first location because TUP is a unique anatomical feature that can be easily and consistently distinguished on the ultrasound image. Two more locations, 3 mm ulnar to TUP and 3 mm radial to TUP, were also investigated. In addition, the middle third of the TCL was used as a fourth location to see whether an average TCL thickness is more reliable than a single-point measurement.
For later reference of corresponding locations on a dissected TCL, several landmarks were identified in the ultrasound image (Fig. 1b), including the TUP (Point ‘A’, indicated by ‘*’), the surface projection of the TUP (Point ‘B’, indicated by ‘+’ on the left), and the surface intersection of the left interference line (Point ‘C’, indicated by ‘+’ on the right). The distance between Points ‘B’ and ‘C’ was obtained by three operators on their own images using the measurement tool built in the ultrasound software with a precision of 0.1 mm. The average distance was then used to determine the TUP on the dissected TCL.
Thickness measured from the dissected TCL
The cadaveric hand was dissected to obtain the TCL after ultrasound imaging. First, the physical location of Point ‘B’ (defined in the ultrasound image) was marked on the skin based on the distance between Points ‘B’ and ‘C’. A caliper with a precision of 0.01 mm was used to measure this distance on the cadaveric hand. A cross centered at Point ‘B’ was drawn on the hand with a permanent marker. A 20 mm (radial-ulnar) × 30 mm (proximal-distal) rectangular window centered at Point ‘B’ was drawn (Fig. 3a). Skin, fat, muscles, and fascia within the window were removed to expose the TCL. Two threads were aligned with the cross and the intersection point was marked with a fine point tip and tissue ink to indicate the location of the TUP. The locations 3 mm ulnar and radial to the TUP were also marked. The TCL was dissected out of the cadaveric specimen (Fig. 3b). Finally, the thickness of the dissected TCL was measured by a linear variable differential transducer (LVDT) at the marked locations 1, 2, and 3 (Fig. 3b). Three measurements were made at each location on each TCL.
Figure 3.
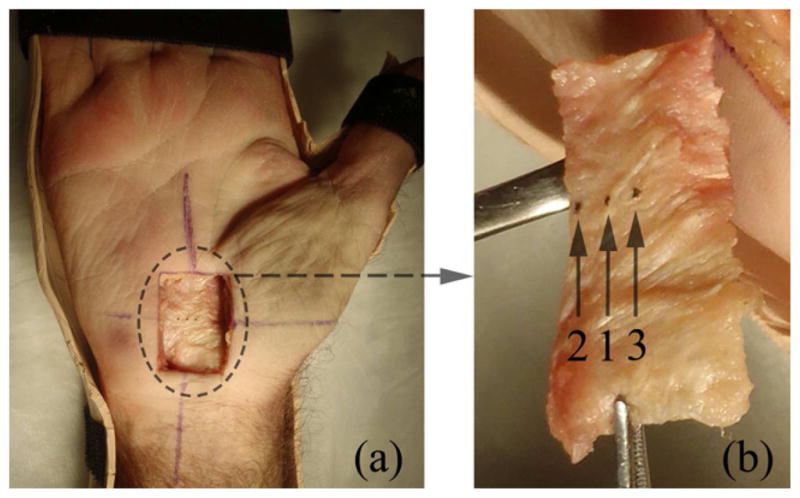
Dissection of the TCL. (a) A cadaveric hand with a 20 mm (radial-ulnar) × 30 mm (proximal-distal) dissection window. The exposed TCL (inside of the dashed circle) was later dissected out of the hand. (b) A dissected TCL. The three locations for TCL thickness measurements were marked by tissue ink and indicated by arrows and numbers. Location 1, 2, and 3 correspond to the thenar muscle’s ulnar point (TUP), 3 mm ulnar to TUP, and 3 mm radial to TUP, respectively.
Statistical analysis
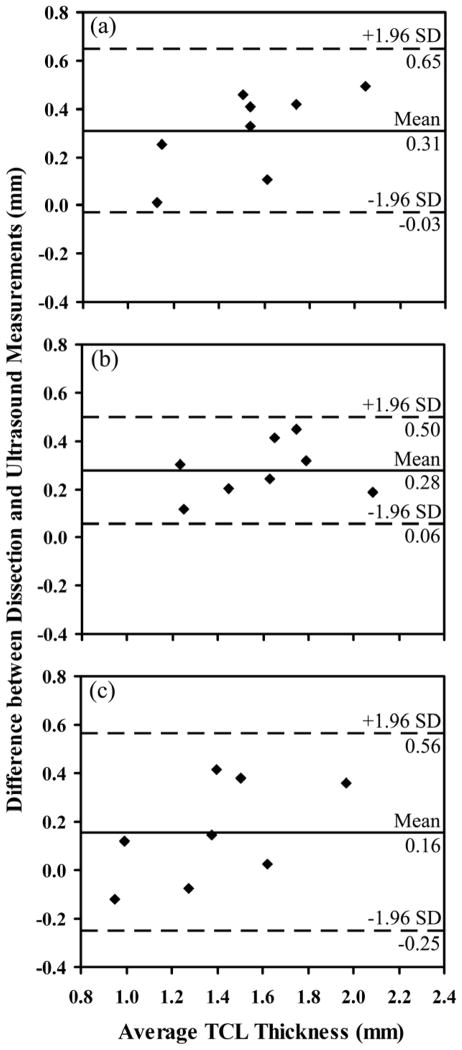
Statistical analysis was performed using SPSS for Windows (IBM SPSS Inc., Chicago, IL, USA). The validity of ultrasound based TCL thicknesses against dissection based thicknesses was analyzed with Pearson’s correlation coefficient and intraclass correlation coefficient (ICC). The former was used to investigate the linear relationship between the two measures, while the latter provided the level of agreement between the two. For validity, an ICC2,2 model was used, with the first number indicating a two-way random effect model and the second number representing two measures (ultrasound vs. dissection). The standard deviation (SD) of the differences between the two measures was used to calculate the standard error of measurement ( , where r is the Cronbach’s alpha reliability) and the minimal detectable difference ( for a confidence interval of 90%). A Bland-Altman plot was used to provide a visual representation of the degree of agreement and to identify bias and outliers.
The reliability of ultrasound measurement of TCL thickness was examined using ICC and SEM. A 95% confidence interval (CI) was calculated for each ICC value. For intra-operator reliability, an ICC3,2 model was used, based on a two-way mixed effect model (i.e. ‘3’) and two sessions (i.e. ‘2’). For inter-operator reliability, an ICC2,3 model was used, based on a two-way random effect model (i.e. ‘2’) and three operators (i.e. ‘3’). For biostatistics, reliability is considered excellent if the ICC value is greater than or equal to 0.75, fair to good if it is between 0.40 and 0.75, and poor if it is less than 0.40 (Rosner 2006).
RESULTS
Validity based on cadaveric specimens
The ultrasound measurement displayed Pearson’s correlation coefficients of 0.867–0.928 and ICC values of 0.726–0.865 (Table 1) compared with the dissection measurements. The SEM was less than 0.07 mm, and MDD90 was less than 0.15 mm (Table 1). Fig. 4 shows the Bland-Altman plots of the difference in TCL thickness measured by dissection and ultrasound imaging vs. the average TCL thickness measured by the two methods. The mean difference between the dissection measurements and the ultrasound measurements was in the range of 0.16–0.31 mm.
Table 1.
Validity of ultrasound assessment of TCL thickness: A comparison between ultrasound and dissection measurements (N = 8)
| Location | TCL thickness (mm) mean ± SD (range)
|
Pearson’s correlation coefficient | ICC2,2 (95% CI) | SEM (mm) | MDD90 (mm) | |
|---|---|---|---|---|---|---|
| Ultrasound | Dissection | |||||
| 1 (TUP) | 1.38 ± 0.25 (1.02–1.80) | 1.69 ± 0.36 (1.13–2.30) | 0.904 | 0.726 (−0.232–0.950) | 0.05 | 0.12 |
| 2 (3 mm ulnar to TUP) | 1.46 ± 0.28 (1.08–1.99) | 1.74 ± 0.30 (1.31–2.18) | 0.928 | 0.779 (−0.137–0.963) | 0.02 | 0.05 |
| 3 (3 mm radial to TUP) | 1.31 ± 0.28 (0.93–1.79) | 1.46 ± 0.40 (0.89–2.15) | 0.867 | 0.865 (0.300–0.973) | 0.07 | 0.15 |
Figure 4.
Bland-Altman plots of the difference in TCL thickness measured by dissection and ultrasound imaging vs. the average TCL thickness measured by the two methods. (a), (b) and (c) correspond to location 1 (TUP), location 2 (3 mm ulnar to TUP), and location 3 (3 mm radial to TUP), respectively.
Reliability of ultrasound imaging
Statistical analyses performed separately on cadaveric specimens and healthy volunteers showed comparable reliability values. Therefore, ultrasound measurements of both cadavers and volunteers were combined for the overall reliability analysis. All three operators had similar intra-operator reliability with ICC3,2 values in the range of 0.826–0.933 and SEM values in the range of 0.09–0.16 mm. Representative intra-operator reliability values for one operator are shown in Table 2. The inter-operator reliability showed ICC2,3 values of 0.840–0.882 and SEM of 0.11–0.14 mm (Table 3).
Table 2.
Representative intra-operator reliability of ultrasound assessment of TCL thickness (N = 16)
| Location | TCL thickness (mm) mean ± SD (range)
|
ICC3,2 (95% CI) | SEM (mm) | |
|---|---|---|---|---|
| Session I | Session II | |||
| 1 (TUP) | 1.58 ± 0.41 (0.98–2.62) | 1.67 ± 0.47 (1.08–2.57) | 0.909 (0.739–0.968) | 0.13 |
| 2 (3 mm ulnar to TUP) | 1.67 ± 0.41 (0.97–2.62) | 1.78 ± 0.46 (1.12–2.82) | 0.869 (0.626–0.954) | 0.16 |
| 3 (3 mm radial to TUP) | 1.51 ± 0.39 (0.98–2.47) | 1.62 ± 0.50 (1.01–2.54) | 0.894 (0.698–0.963) | 0.14 |
| 4 (middle third) | 1.54 ± 0.40 (1.03–2.59) | 1.62 ± 0.45 (1.06–2.49) | 0.895 (0.700–0.963) | 0.14 |
Table 3.
Inter-operator reliability of ultrasound assessment of TCL thickness (N = 16)
| Location | TCL thickness (mm) mean ± SD (range)
|
ICC2,3 (95% CI) | SEM (mm) | ||
|---|---|---|---|---|---|
| Operator A | Operator B | Operator C | |||
| 1 (TUP) | 1.62 ± 0.42 (1.03–2.49) | 1.42 ± 0.25 (0.94–2.02) | 1.57 ± 0.39 (1.07–2.51) | 0.859 (0.663–0.947) | 0.13 |
| 2 (3 mm ulnar to TUP) | 1.72 ± 0.41 (1.04–2.49) | 1.53 ± 0.24 (1.18–1.94) | 1.61 ± 0.35 (0.99–2.48) | 0.840 (0.633–0.939) | 0.14 |
| 3 (3 mm radial to TUP) | 1.56 ± 0.42 (1.04–2.35) | 1.36 ± 0.29 (0.84–1.98) | 1.55 ± 0.51 (0.87–2.95) | 0.871 (0.695–0.951) | 0.14 |
| 4 (middle third) | 1.58 ± 0.41 (1.09–2.45) | 1.38 ± 0.24 (0.90–1.92) | 1.52 ± 0.35 (1.03–2.45) | 0.882 (0.703–0.957) | 0.11 |
TCL thickness measured from the ultrasound images
All ultrasound images taken by the three operators during the two sessions were combined to calculate an average TCL thickness for each specimen and volunteer because of the excellent intra-operator and inter-operator reliability. The TCL thicknesses were 1.54 ± 0.32 mm, 1.62 ± 0.30 mm, 1.49 ± 0.38 mm, and 1.49 ± 0.31 mm, for TUP, 3 mm ulnar to TUP, 3 mm radial to TUP, and the middle third of the TCL, respectively. There were no significant differences in the thicknesses measured at the four locations as determined by one-way ANOVA (p = 0.64). The mean TCL thickness was 1.54 ± 0.33 mm when combining all measurements made at all four locations.
DISCUSSION
In general, ultrasound imaging of soft tissues has been validated with cadaveric dissection (Kellis et al. 2009). Therefore, dissection based TCL thickness was chosen as the gold standard for ultrasound validation in this study. The high ICC values (> 0.7) and small errors (< 0.15 mm) indicated that ultrasound was a valid method to measure TCL thickness. If one specimen was thicker than another determined by dissection, the same trend was observed by ultrasound. However, dissection based TCL thicknesses were consistently larger (0.16–0.31 mm) than ultrasound measurements, suggesting that there was a systematic bias. Two factors might have contributed to this bias, including the assumption of speed of sound and incomplete tissue removal. First, a commercial ultrasound system always sets a default sound speed of 1540 m/s, which may be different from the actual speed of sound traversing in the TCL. Indeed, it was shown that the speed of sound in collagenous tissues was ~1650 m/s (Miles 1996; Garcia et al. 2003). The ultrasound measured TCL thickness can be corrected using the following formula: tc = tm·1650/1540 ≈ 1.07·tm, where tc and tm are the corrected and measured TCL thicknesses, respectively. This means that the ultrasound measured TCL thickness was underestimated by ~7% due to the assumption of speed of sound at 1540 m/s. Second, incomplete removal of soft tissues surrounding the TCL resulted in an overestimated dissection based TCL thickness. Previous histological analysis showed that there were two different fibrous structures in the flexor retinaculum, a superficial layer of fascia and a deep layer of ligamentous tissue (i.e. TCL) (Stecco et al. 2010). It is difficult to distinguish these two layers during dissection and inclusion of non-ligamentous tissues on the dissected TCL tends to overestimate TCL thickness. In contrast, ultrasound has the capability of identifying the true TCL because different ultrasonic patterns are formed at the interfaces between different tissues. Nonetheless, the dissection measurements provide useful validation for ultrasound measurement.
Ultrasound assessment of TCL thickness on both the cadaveric specimens and healthy volunteers showed excellent intra-operator and inter-operator reliability (ICCs > 0.8). The 95% CIs for both intra-operator and inter-operator reliability were narrow, indicating that this study had enough statistical power with a sample size of 16 when combining cadaveric specimens and healthy volunteers. The SEM was relatively small (≤ 0.16 mm) compared with the TCL thickness (0.93–2.34 mm), suggesting that ultrasound is a valuable imaging modality to monitor subtle morphological changes of the TCL. A clinical study (Yamagami et al. 1994) showed that the TCL thickness in 61 CTS patients was in the range of 2–10 mm with a mean of 4 mm, which was much greater than normal TCL thickness. Therefore, our ultrasound imaging method can be potentially used to identify TCL hypertrophy associated with CTS.
For cadaveric specimens, TCL thicknesses have been reported to be 1.5–6.0 mm, 2.0–2.8 mm, 0.8–2.5 mm, and 1.3–3.0 mm when measured using calipers (Tanzer 1959), micrometers (Tanabe and Okutsu 1997), photography (Cobb et al. 1993), and silicon casting (Pacek et al. 2010), respectively. For live subjects, TCL thicknesses were 1.09–1.18 mm, and 1.26 ± 0.32 mm when measured by CT (Merhar et al. 1986) and MRI (Stecco et al. 2010), respectively. In this study, the mean TCL thickness was 0.93–2.34 (1.54 ± 0.33) mm, which agreed well with the TCL thicknesses reported in the literature. Furthermore, previous studies showed that the TCL thicknesses measured directly from cadaveric specimens (Tanzer 1959; Cobb et al. 1993; Tanabe and Okutsu 1997; Pacek et al. 2010) were generally larger than those measured by imaging techniques (Merhar et al. 1986; Stecco et al. 2010). Our finding that the dissection based TCL thicknesses were greater than the ultrasound based measurements also agreed with this trend. As explained before, this was likely due to mismatched speed of sound and the difficulty in distinguishing and removing non-ligamentous tissues during TCL dissection. Furthermore, no significant differences in the TCL thicknesses were found among the four locations. Since the thenar muscle’s ulnar point (TUP) is a unique anatomical landmark that can be easily and consistently identified on the ultrasound image, it is recommended that clinical assessment of TCL thickness be made at this point.
This study has a few limitations. First, the speed of sound in the TCL (~1650 m/s) is different from the default value of 1540 m/s in commercial ultrasound systems. As mentioned previously, the assumed speed of sound results in an underestimation of TCL thickness by ~7%. It is recommended that a correction factor be applied to the TCL thickness measured by the ultrasound system to obtain the absolute value of thickness data. However, this systematic error will not affect hypothesis testing in studies that investigate relative changes, such as whether CTS patients have thicker TCLs than healthy control subjects or whether TCL gets thicker over time with repetitive use. Second, most of the cadaveric specimens and all of the healthy volunteers in this study were males. This is due to limited number of specimens and subjects. However, the gender effect should play little role in this study as within-subject comparisons were used for validity and reliability analysis. Third, a custom MATLAB script was used to calculate the TCL thickness at various locations which may be difficult to replicate in clinical practice. However, the results showed that TCL thickness was reliably determined at the TUP, which allows obtaining thickness data directly using the measurement tools built in the ultrasound systems.
In summary, the ultrasound imaging method developed in this study was demonstrated to be valid and reliable for measuring TCL thickness. This method is valuable for future studies to detect subtle morphological changes of the TCL thickness in longitudinal and comparative studies between CTS patients and healthy control subjects.
Acknowledgments
This study was supported by a National Institutes of Health grant R03AR054510. The authors acknowledge Joshua L. Gordon and John Cheng for their assistance of ultrasound imaging and cadaveric dissection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balint PV, Sturrock RD. Intraobserver repeatability and interobserver reproducibility in musculoskeletal ultrasound imaging measurements. Clin Exp Rheumatol. 2001;19:89–92. [PubMed] [Google Scholar]
- Bentman S, O’Sullivan C, Stokes M. Thickness of the middle trapezius muscle measured by rehabilitative ultrasound imaging: description of the technique and reliability study. Clin Physiol Funct Imaging. 2010;30:426–31. doi: 10.1111/j.1475-097X.2010.00960.x. [DOI] [PubMed] [Google Scholar]
- Brooks JJ, Schiller JR, Allen SD, Akelman E. Biomechanical and anatomical consequences of carpal tunnel release. Clin Biomech (Bristol, Avon) 2003;18:685–93. doi: 10.1016/s0268-0033(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Brushoj C, Henriksen BM, Albrecht-Beste E, Holmich P, Larsen K, Bachmann Nielsen M. Reproducibility of ultrasound and magnetic resonance imaging measurements of tendon size. Acta Radiol. 2006;47:954–9. doi: 10.1080/02841850600854936. [DOI] [PubMed] [Google Scholar]
- Bunce SM, Moore AP, Hough AD. M-mode ultrasound: a reliable measure of transversus abdominis thickness? Clin Biomech (Bristol, Avon) 2002;17:315–7. doi: 10.1016/s0268-0033(02)00011-6. [DOI] [PubMed] [Google Scholar]
- Cobb TK, Dalley BK, Posteraro RH, Lewis RC. Anatomy of the flexor retinaculum. J Hand Surg [Am] 1993;18:91–9. doi: 10.1016/0363-5023(93)90251-W. [DOI] [PubMed] [Google Scholar]
- Collinger JL, Gagnon D, Jacobson J, Impink BG, Boninger ML. Reliability of quantitative ultrasound measures of the biceps and supraspinatus tendons. Acad Radiol. 2009;16:1424–32. doi: 10.1016/j.acra.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari FS, Della Sala L, Cozza S, Guazzi G, Belcapo L, Mariottini A, Bolognini A, Stefani P. High-resolution ultrasonography in the study of carpal tunnel syndrome. Radiol Med (Torino) 1997;93:336–41. [PubMed] [Google Scholar]
- Garcia T, Hornof WJ, Insana MF. On the ultrasonic properties of tendon. Ultrasound Med Biol. 2003;29:1787–97. doi: 10.1016/s0301-5629(03)01069-x. [DOI] [PubMed] [Google Scholar]
- Gelberman RH, Hergenroeder PT, Hargens AR, Lundborg GN, Akeson WH. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63:380–3. [PubMed] [Google Scholar]
- Guo X, Fan Y, Li ZM. Effects of dividing the transverse carpal ligament on the mechanical behavior of the carpal bones under axial compressive load: a finite element study. Med Eng Phys. 2009;31:188–94. doi: 10.1016/j.medengphy.2008.08.001. [DOI] [PubMed] [Google Scholar]
- John V, Nau HE, Nahser HC, Reinhardt V, Venjakob K. CT of carpal tunnel syndrome. AJNR Am J Neuroradiol. 1983;4:770–2. [PMC free article] [PubMed] [Google Scholar]
- Kato T, Kuroshima N, Okutsu I, Ninomiya S. Effects of endoscopic release of the transverse carpal ligament on carpal canal volume. J Hand Surg [Am] 1994;19:416–9. doi: 10.1016/0363-5023(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Kellis E, Galanis N, Natsis K, Kapetanos G. Validity of architectural properties of the hamstring muscles: correlation of ultrasound findings with cadaveric dissection. J Biomech. 2009;42:2549–54. doi: 10.1016/j.jbiomech.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Li ZM, Masters TL, Mondello TA. Area and shape changes of the carpal tunnel in response to tunnel pressure. J Orthop Res. 2011;29:1951–6. doi: 10.1002/jor.21468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZM, Tang J, Chakan M, Kaz R. Carpal tunnel expansion by palmarly directed forces to the transverse carpal ligament. J Biomech Eng. 2009;131:081011. doi: 10.1115/1.3148469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merhar GL, Clark RA, Schneider HJ, Stern PJ. High-resolution computed tomography of the wrist in patients with carpal tunnel syndrome. Skeletal Radiol. 1986;15:549–52. doi: 10.1007/BF00361053. [DOI] [PubMed] [Google Scholar]
- Miles CA. Ultrasonic properties of tendon: velocity, attenuation, and backscattering in equine digital flexor tendons. J Acoust Soc Am. 1996;99:3225–32. doi: 10.1121/1.414866. [DOI] [PubMed] [Google Scholar]
- O’Connor PJ, Grainger AJ, Morgan SR, Smith KL, Waterton JC, Nash AF. Ultrasound assessment of tendons in asymptomatic volunteers: a study of reproducibility. Eur Radiol. 2004;14:1968–73. doi: 10.1007/s00330-004-2448-4. [DOI] [PubMed] [Google Scholar]
- Pacek CA, Chakan M, Goitz RJ, Kaufmann RA, Li ZM. Morphological Analysis of the Transverse Carpal Ligament. Hand (N Y) 2010;5:77–81. doi: 10.1007/s11552-009-9219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou GD, McCabe SJ, Firrell J. The prevalence and characteristics of nerve compression symptoms in the general population. J Hand Surg [Am] 2001;26:460–6. doi: 10.1053/jhsu.2001.24972. [DOI] [PubMed] [Google Scholar]
- Richards PJ, Dheer AK, McCall IM. Achilles tendon (TA) size and power Doppler ultrasound (PD) changes compared to MRI: a preliminary observational study. Clin Radiol. 2001;56:843–50. doi: 10.1053/crad.2001.0784. [DOI] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of biostatistics. Belmont, CA: Duxbury Press; 2006. [Google Scholar]
- Stecco C, Macchi V, Lancerotto L, Tiengo C, Porzionato A, De Caro R. Comparison of transverse carpal ligament and flexor retinaculum terminology for the wrist. J Hand Surg Am. 2010;35:746–53. doi: 10.1016/j.jhsa.2010.01.031. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Okutsu I. An anatomical study of the palmar ligamentous structures of the carpal canal. J Hand Surg Br. 1997;22:754–7. doi: 10.1016/s0266-7681(97)80441-x. [DOI] [PubMed] [Google Scholar]
- Tanzer RC. The carpal-tunnel syndrome; a clinical and anatomical study. J Bone Joint Surg Am. 1959;41-A:626–34. [PubMed] [Google Scholar]
- Xiu KH, Kim JH, Li ZM. Biomechanics of the transverse carpal arch under carpal bone loading. Clin Biomech (Bristol, Avon) 2010;25:776–80. doi: 10.1016/j.clinbiomech.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami T, Higashi K, Handa H, Minouchi K, Fujii M, Nishihara K, Kaji R. Carpal tunnel syndrome: clinical experience of 61 cases. No Shinkei Geka. 1994;22:617–20. [PubMed] [Google Scholar]
- Ying M, Yeung E, Li B, Li W, Lui M, Tsoi CW. Sonographic evaluation of the size of Achilles tendon: the effect of exercise and dominance of the ankle. Ultrasound Med Biol. 2003;29:637–42. doi: 10.1016/s0301-5629(03)00008-5. [DOI] [PubMed] [Google Scholar]