Abstract
How a drug distributes within highly compartmentalized mammalian cells can affect both the activity and pharmacokinetic behavior. Many commercially available drugs are considered to be lysosomotropic, meaning they are extensively sequestered in lysosomes by an ion trapping-type mechanism. Lysosomotropic drugs typically have a very large apparent volume of distribution and a prolonged half-life in vivo, despite minimal association with adipose tissue. In this report we tested the prediction that the accumulation of one drug (perpetrator) in lysosomes could influence the accumulation of a secondarily administered one (victim), resulting in an intracellular distribution-based drug interaction. To test this hypothesis cells were exposed to nine different hydrophobic amine-containing drugs, which included imipramine, chlorpromazine and amiodarone, at a 10 µM concentration for 24 to 48 hours. After exposure to the perpetrators the cellular accumulation of LysoTracker Red (LTR), a model lysosomotropic probe, was evaluated both quantitatively and microscopically. We found that all of the tested perpetrators caused a significant increase in the cellular accumulation of LTR. Exposure of cells to imipramine caused an increase in the cellular accumulation of other lysosomotropic probes and drugs including LyosTracker Green, daunorubicin, propranolol and methylamine; however, imipramine did not alter the cellular accumulation of non-lysosomotropic amine-containing molecules including MitoTracker Red and sulforhodamine 101. In studies using ionophores to abolish intracellular pH gradients we were able to resolve ion trapping-based cellular accumulation from residual pH-gradient independent accumulation. Results from these evaluations in conjunction with lysosomal pH measurements enabled us to estimate the relative aqueous volume of lysosomes of cells before and after imipramine treatment. Our results suggest that imipramine exposure caused a 4-fold expansion in the lysosomal volume, which provides the basis for the observed drug interaction. The imipramine-induced lysosomal volume expansion was shown to be both time- and temperature-dependent and reversed by exposing cells to hydroxypropyl-β-cyclodextrin, which reduced lysosomal cholesterol burden. This suggests that the expansion of lysosomal volume occurs secondary to perpetrator-induced elevations in lysosomal cholesterol content. In support of this claim, the cellular accumulation of LTR was shown to be higher in cells isolated from patients with Niemann-Pick Type C disease, which are known to hyper-accumulate cholesterol in lysosomes.
Keywords: lysosome, cationic amphiphillic drug, drug interaction, lysosomotropic, phospholipidosis, lysosomal volume
INTRODUCTION
A common feature among the majority of currently marketed small molecular weight therapeutics is the presence of a weakly basic amine functional group. The presence of this group often results in intracellular accumulation of the drug within discrete acidic subcellular compartments, most notably lysosomes. These compounds are often referred to as lysosomotropic amines and accumulate in lysosomes by a mechanism known as pH partitioning or ion trapping.1 At physiological pH these compounds are significantly unionized and passively diffuse across the lipid bilayers of cellular organelles. Upon entering the acidic environment of the lysosome these amines become predominately ionized and therefore less able to efficiently diffuse out, resulting in pronounced accumulation. Reaching concentrations within the lysosome several orders of magnitude greater than that seen in the extracellular space, these agents are capable of causing various physiological and morphological perturbations of the lysosomal apparatus.2
Drugs that are extensively sequestered in lysosomes have been reported to alter the organelles structure and function in many ways.3–6 One effect that has garnered great interest is the capacity of these drugs to inhibit lysosomal lipid metabolism.7 This effect is most closely associated with a subgroup of lysosomotropic amine drugs known as cationic amphiphilic drugs (CADs). These compounds are typically described as containing both a hydrophobic and a hydrophilic domain (i.e., amphiphilic) with the hydrophobic domain containing an aromatic ring or ring system and the hydrophilic domain containing an ionizable amine functional group.8 At therapeutically relevant concentrations CADs have been shown to cause the lysosomal accumulation of various lipid species, including sphingomyelin, phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, lysobisphosphatidic acid and cholesterol.9–12 In fact, CADs are often used in cell culture models to induce a lysosomal lipid storage disease phenotype similar to that seen in cells isolated from patients with Niemann-Pick disease (NPD).13, 14 Interestingly, work in our lab and in others have shown that in addition to the lysosomal accumulation of lipids in NPD cells, these cells also hyperaccumulate amine-containing compounds, presumably in a lysosomal system that has seen a vast expansion in its volume.15, 16 These observations along with previous work that has related drug-induced lipidosis to the hyperaccumulation of amine-containing drugs17, 18 led us to hypothesize that CADs, because of their ability to mimic the NPD lipidosis phenotype, would also cause an increase in the cellular accumulation of other amine-containing drugs in the lysosomal apparatus. Such an ability of CADs to alter the cellular retention of a second drug further led us to speculate the potential for a novel mechanism through which drug interactions of clinical relevance can occur at the level of intracellular drug distribution.
Drug interactions typically result from the ability of one drug to alter the pharmacokinetic properties of a second drug; that is the absorption, distribution, metabolism or excretion (ADME) properties.19 The ability of these interactions to cause clinically relevant changes in drug efficacy and/or toxicity has yielded a large body of research that seeks to elucidate the various pathways through which drugs can interact within the complex biological matrix of the human body. Although the majority of this work involves drug interactions occurring through modulation of drug metabolizing enzymes20 or membrane drug transporters,21 there has been an effort to describe how the propensity of amine-containing drugs to accumulate in lysosomes can result in distribution-based drug interactions.22, 23 Because of their tendency to accumulate in lysosomes and in lysosome-rich tissues,24 amine-containing drugs often have a high apparent volume of distribution.25 Therefore, if factors, such as drug co-administration, can influence the deposition of these amine-containing drugs into lysosome-rich tissues then the distribution profile of these drugs could be significantly altered. Such deviations in the distribution profile of the drugs are expected to have implications in drug efficacy or toxicity.
In this report we show that exposure to various CADs results in an increase in the cellular accumulation of secondarily administered amine-containing drugs. This effect was found to be specific to amine-containing compounds that are substrates for ion trapping-based accumulation in lysosomes. Mechanistic studies reveal that CADs cause an increase in the apparent lysosomal volume of cells in a time- and temperature-dependent manner, consistent with an energy-dependent cellular remodeling process. Treatments known to reverse the CAD-induced lipidosis prevent the apparent increase in lysosomal volume. Our results are consistent with the hypothesis that CADs, through their ability to inhibit cellular lipid metabolism, cause an expansion of the lysosomal compartment of cells resulting in the increased cellular uptake of lysosomotropic amine compounds, thus revealing a novel pathway for intracellular distribution-based pharmacokinetic drug interactions. Our results suggest that the observed interaction is associated with the physicochemical properties of the drugs involved and does not appear to be related to therapeutic classification.
EXPERIMENTAL SECTION
Cell Lines and Reagents
LysoTracker Red DND-99 (LTR), LysoTracker Green DND-26 (LTG), MitoTracker Red FM, sulforhodamine 101, anionic 70,000 mol. wt. Oregon Green Dextran, Dulbecco’s phosphate buffered saline (D-PBS) and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Invitrogen (Carlsbad, CA). Daunorubicin was purchased from Oakwood Products (West Columbia, SC). Nigericin, monensin, hydroxypropyl-β-cyclodextrin (HPCD), propranolol, imipramine, concanamycin A, ammonium chloride, haloperidol, risperidone, chlorpromazine, lidocaine, bupivacaine, amiodarone, and verapamil were purchased from Sigma-Aldrich (St. Louis, MO). [3H]propranolol was purchased from GE Healthcare (Waukesha, WI). Lipoprotein-depleted serum (LPDS) was purchased from Millipore (Billerica, MA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA). Wild type (WT) human fibroblasts (catalogue # CRL-2076) were purchased from ATCC (Manassas, VA). NPC1−/− (catalogue # GM03123) and NPC2−/− (catalogue # GM18455) human fibroblasts were purchased from Coriell Cell Repository (Camden, NJ). MDA-1986 squamous cell carcinoma cells from the laboratory of Dr. M.S. Cohen at the University of Kansas Medical Center. All cell cultures were grown in DMEM supplemented with 10% FBS and maintained at 37 °C and 5% CO2, all experiments were carried out under these conditions unless otherwise stated. Cells were routinely subcultured to maintain 60 to 80% confluency and experiments were carried out within 10 passages following removal from cryopreservation.
Drug Treatments
Cells were seeded onto multi-well polystyrene plates and allowed to attach overnight. Imipramine and the other CADs were added at a concentration of 10 µM for 24 to 48 h, unless otherwise specified. Lysosomal alkalinizing treatments, including; 30 µM chloroquine, 10 mM ammonium chloride and 200 nM concanamycin A were applied for a total drug treatment time of 2 h. Lysosomal pH knockdown experiments were conducted using a 2 h treatment with the ionophores, 10 µM nigericin and 20 µM monensin. In the pH dependent binding studies, cell growth medium was exchanged with a pH 5.0 buffered solution containing 150 mM sodium chloride, 20 mM Mes, 5 mM potassium chloride and 1 mM magnesium sulfate prior to the addition of the ionophores. In the temperature dependency study cells were either maintained in 37°C media or switched to 4°C media followed by the addition of imipramine or vehicle alone (D-PBS). After 4 h at either 4°C or 37°C, cells were washed three times with 37°C D-PBS and replaced with fresh 37°C growth media containing 200 nM LTR without imipramine. In lipidosis-reversing treatments, growth media was removed and replaced with either fresh growth media, fresh growth media supplemented with 0.1% HPCD or growth media without FBS but supplemented with 3 mg/ml LPDS. After 24 h, either imipramine or vehicle alone was added to each treatment group for an additional 24 h.
Cell Imaging Studies
Human fibroblasts were viewed using a Nikon Eclipse 80i epifluorescence microscope equipped with a 40× (1.30 NA) oil immersion objective and the corresponding filter cube that best matched the spectral properties of each fluorophore. Images were acquired using an ORCA ER camera (Hamamatsu). Images were analyzed using Metamorph version 7.0 (Universal Imaging) and ImageJ (free online at rsbweb.nih.gov) software. Human fibroblasts were grown on glass coverslips and following a 46 h treatment with 10 µM imipramine or vehicle alone the indicated fluorophore was added for 2 h. Concentrations of 100 nM LTR, 1 µM LTG, 1 µM daunorubicin, 5 µM sulforhodamine 101 and 100 nM MitoTracker Red were used. At the end of the 48 h treatment period cells were washed twice with 37°C D-PBS rapidly and immediately mounted on glass microscopy slides for live-cell imaging. Images of intact cells not exposed to fluorescent probe were acquired to account for potential autofluorescence and used for background correction. Images were captured under identical instrument settings (i.e., detector gain, illumination intensity, exposure time and magnification) and were scaled identically to allow for direct comparison of fluorophore accumulation and distribution.
Drug Accumulation Assays
Cells were exposed to the indicated compound for 2 h under normal growth conditions, except in the temperature dependency study where a 30 min incubation was used to avoid potential reversion of the CAD-induced phenotype. Fluorescent compound concentrations used were identical to those used in the Cell Imaging Studies with the exception of LTR which was used at 200 nM in most experiments. Propranolol was added at a concentration of 100 nM containing 10 nM [3H]propranolol and [14C]methylamine was added at a concentration of 10 µM. Cells were then washed twice with 37°C D-PBS rapidly to prevent loss of cell-associated compound by diffusion. Cells were lysed in either 0.1 M NaOH or lysis buffer (50 mM tris base, 150 mM NaCl, 1% NP40 adjusted to pH 7.4). Lysed samples were immediately measured using a Bio-Tek FL600 microplate fluorescence reader or a Beckman LS 60001C liquid scintillation counter. Background signal contribution from non-specific binding to the plate surface was subtracted from each measurement. Cell protein content was measured using the BCA method, and fluorescence (RFU) or radioactivity (DPM) counts were normalized to cellular protein and overall cellular accumulation was expressed in counts per microgram of protein or as a percentage of vehicle-treated control cells.
Drug Uptake and Normalized Release Studies
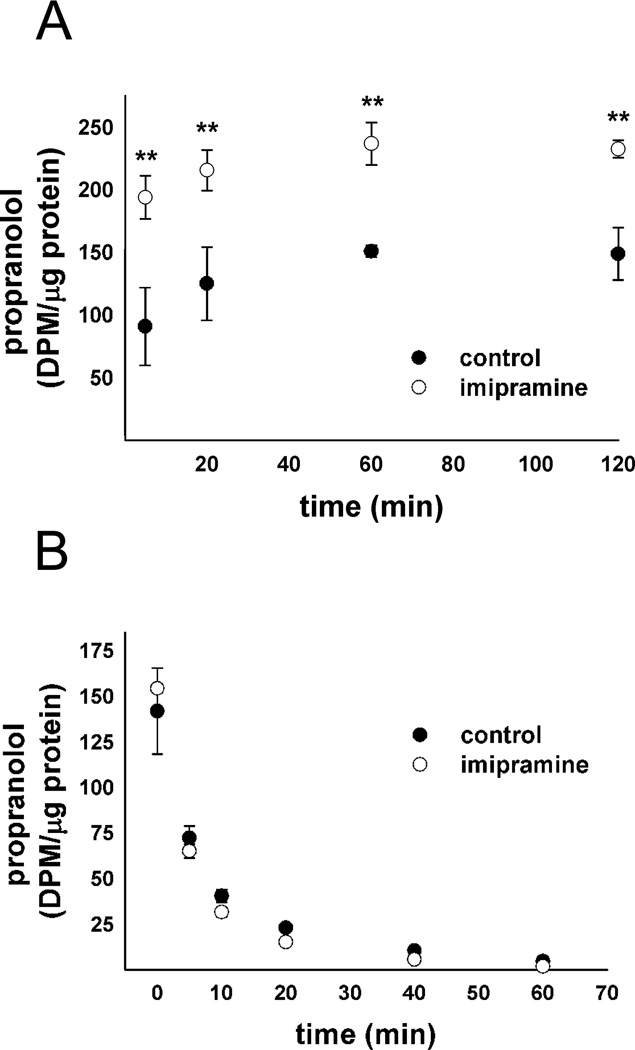
Cells pretreated with 10 µM imipramine or vehicle alone for 48 h were exposed to 100 nM propranolol, containing 10 nM [3H]propranolol for various times up to 2 h. At the end of the uptake period, drug containing media was removed and cells were washed twice rapidly with 37°C D-PBS. Lysis buffer (pH 7.4, 50 mM tris base, 150 mM NaCl, 1% NP40) was added to each well and incubated at 37°C for 5 min. Cell lysate was transferred to ScintiVerse BD Cocktail (Fisher) for scintillation counting and measured for protein content using the BCA method. In addition, a dose-titration experiment was conducted in imipramine treated cells to establish a dose of propranolol necessary to give equivalent cellular propranolol levels in vehicle-treated control and impramine treated cells for the normalized release study (data not shown). A dose of 74 nM propranolol was established and the ratio of labeled to unlabeled propranolol was maintained.
Following a 46 h treatment with imipramine or vehicle alone, 100 nM propranolol was added to vehicle-treated control cells and 74 nM propranolol was added to imipramine treated cells, containing 10 nM [3H]propranolol and 7.4 nM [3H]propranolol, respectively. Following a 2 h incubation, the media was removed and cells were washed once with 37°C D-PBS. Fresh media without propranolol was added to the cells. Imipramine or vehicle alone was maintained in the media through the entirety of the experiment. At set timepoints, media was removed and transferred to scintillation fluid. Fresh media was added at each timepoint up to 60 min. After the last timepoint, lysis buffer (pH 7.4, 50 mM tris base, 150 mM NaCl, 1% NP40) was added and incubated at 37°C for 5 min. Cell lysate was transferred to scintillation fluid and the remaining cell associated [3H]propranolol was measured along with cell protein content using the BCA method. [3H]propranolol in each sample was measured using a Beckman LS 60001C liquid scintillation counter. Radioactivity counts (DPM) were normalized to cellular protein content and plotted in counts per microgram of protein as a function of time.
Lysosomal pH Measurements
The influence of imipramine treatment on lysosomal pH was measured using a previously published protocol with slight modifications.26 Briefly, MDA-1986 cells were plated onto 8-chamber glass microscope slides and allowed to adhere overnight. Following a 24 h exposure to 10 µM imipramine or vehicle alone, growth media was replaced with phenol red-free growth media containing 1 mg/ml anionic 70,000 mol. wt. Oregon Green Dextran. Imipramine or vehicle alone was maintained in the media through the entirety of the experiment. Cells were incubated for 2 h under normal growth conditions followed by removal of the fluorescent dextran conjugate containing media. Cells were washed twice with 37°C sterile D-PBS. Pre-warmed phenol red free growth media was added and cells were incubated under normal growth conditions for 6 h to chase the dextran conjugated probe into lysosomes. Cells were then washed twice with and then maintained in a pH 7.4 buffer containing 150 mM NaCl, 20 mM MES, 5 mM KCl and 1 mM MgSO4. Fluorescence intensity was measured while exciting at wavelengths of 451 nm and 495 nm on a microscope equipped with a Photon Technology International (Birmingham, NJ) Ratiomaster excitation spectrofluoromoter, a D/F/TR multiple bandpass dichroic mirror, a 525/10 nm emission filter and a photomultiplier tube detector. Ratios of fluorescence intensity emissions at 525/10 nm (RFU495 nm/ RFU451 nm) were used to calculate lysosomal pH according to lysosomal pH calibration curves constructed in both imipramine treated and vehicle-treated control cells (data not shown). Calibration curves were created by measuring the fluorescence emission ratio of the dextran conjugated probe localized to lysosomes in cells exposed to pH 4.0, 5.0, 5.5 and 6.0 buffer supplemented with 10 µM nigericin and 20 µM monensin, as established previously.27 A linear fit of the calibration curves allowed calculation of the lysosomal pH from the resulting best fit line equation.
Lysosomal Volume Calculations
The amount of LTR accumulated in cells by ion trapping-based accumulation in lysosomes was determined in vehicle-treated control cells and cells pretreated with 10 µM imipramine for 24h using eq. 1 where Dlys represents the amount of LTR accumulated in cells by ion trapping, Dtotal represents the amount of LTR that accumulates under normal growth conditions and Dnig/mon represents the amount of LTR that accumulates in cells following disruption of intracellular pH gradients with the ionphores nigericin and monensin.
| (1) |
In eq. 2 the fold change in ion trapping-based accumulation of LTR following treatment with imipramine (ΔDlys) is directly related to changes in both lysosomal volume (ΔVlys) and lysosomal concentrations of LTR (Δ[D]lys).
| (2) |
Eq.3 was derived by de Duve (1974) in his original work detailing the ion trapping theory for weak base accumulation in lysosomes. The equation simply states that for a weakly basic compound, such as LTR, the concentration in lysosomes ([D]lys) relative to the extracellular space ([D]ext) is approximately equal to the ratio of the hydrogen ion concentration in the lysosome ([H+]lys) compared to the extracellular space ([H+]ext).
| (3) |
Rearrangement of eq. 3 yields eq. 4. Assuming that in our experimental model the concentration of LTR in the extracellular matrix and the extracellular pH are held constant, the relationship between changes in lysosomal LTR concentrations and lysosomal pH can be described by eq. 5. It can therefore be stated that changes in the lysosomal concentration of LTR in lysosomes is directly proportional to changes in the concentration of hydrogen ions in the lysosome.
| (4) |
| (5) |
Substituting eq. 5 into eq. 2 yields eq.6, which relates changes in LTR ion trapping in lysosomes to changes in lysosomal pH and/or lysosomal volume.
| (6) |
Direct measurements of lysosomal pH and ion trapping-based accumulation of LTR in vehicle-treated control and imipramine treated cells allowed for an approximation of the relative lysosomal volume change resulting from imipramine treatment.
RESULTS
The ability of CADs to induce a generalized lipidosis in cultured cells and in vivo has been extensively studied yet there remains little consensus regarding the therapeutic and/or toxicological ramifications of it. In this report we examine how the CAD-induced lipidosis influences the intracellular accumulation of amine-containing drugs that are substrates for ion trapping-based accumulation. The observation that substrates for ion trapping hyperaccumulate in cells with functional mutations in the Niemann Pick C1 protein led us to hypothesize that exposure of cells to therapeutic agents that are known to induce a Niemann Pick C1 mutation phenotype would also cause the hyperaccumulation of amines. If correct, this hypothesis would provide the foundation for a therapeutically important consequence of CAD-induced lipidosis in the form of a distribution-based drug interaction pathway.
CAD Exposure and the Accumulation of Lysosomotropic Amine-Containing Compounds
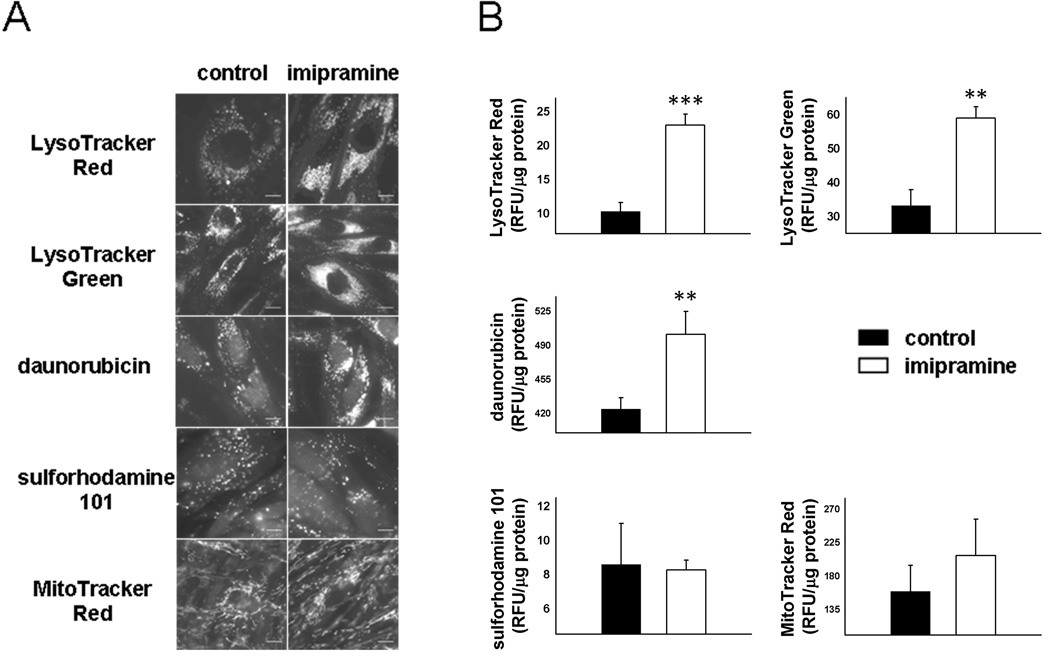
We first set out to test if CADs could increase the lysosomal and cellular accumulation of drugs or drug-like small molecules that are substrates for ion trapping-based accumulation in lysosomes. Imipramine, a prototypical CAD that belongs to the tricyclic antidepressant class of therapeutics, was exposed to human fibroblasts at a concentration of 10 µM for 48 h. Cells were subsequently exposed to two well-documented substrates for ion trapping-based accumulation in lysosomes, namely LysoTracker Red (LTR) and LysoTracker Green (LTG). As previously reported28, 29 both probes have a punctate intracellular distribution indicative of lysosomal sequestration (Figure 1A). Interestingly, cells that were pretreated with imipramine consistently appeared to accumulate significantly more LTR and LTG relative to vehicle-treated control cells. Quantitative measurements of total cellular accumulation of these amine-containing compounds also showed an increase following imipramine treatment (Figure 1B), thus supporting the microscopic observations. This CAD-induced phenotype was also observed with the weakly basic anticancer agent daunorubicin (Figure 1) and the β-adrenergic receptor antagonist propranolol (data not shown), both of which have been shown to accumulate in lysosomes through an ion trapping-based mechanism.30, 31
Figure 1.
Cellular accumulation of amine-containing compounds in human fibroblasts monitored by, (A) epifluorescence microscopy or (B) quantification of total cellular accumulation, following a 48 h treatment with vehicle alone or 10 µM imipramine. Scale bars represent 10 µm. Cellular accumulation of the compounds was measured in fluorescence units (RFU) normalized to protein content and is represented as mean ± S.D from three independent experimental evaluations (**, p < 0.01; ***, p < 0.001 by Student’s t test).
It is possible that imipramine could enhance the cellular accumulation of the previously tested amines according to a mechanism that is unrelated to their ion trapping-based accumulation. Specifically, CAD treatment could theoretically expand the number of lipid binding sites associated with the cell and therefore increase total cellular uptake of drugs. In addition, the CADs could alter the intrinsic permeability of the plasma membrane of cells to drugs in such a way that the total cellular accumulation of the drug is increased in the presence of imipramine. In an effort to rule out these possibilities we tested if imipramine pretreatment could increase the cellular accumulation of amines that are not substrates for ion trapping in lysosomes. Specifically, sulforhodamine 101 and MitoTracker Red are low-molecular weight, amine-containing compounds that are not substrates for ion trapping in lysosomes.30, 32 We found that both of these non-lysosomotropic amines had no apparent differences in cellular accumulation and distribution regardless of imipramine pretreatment (Figure 1). Visual observations made using fluorescence microscopy (Figure 1A) were supported by quantitative measurements (Figure 1B).
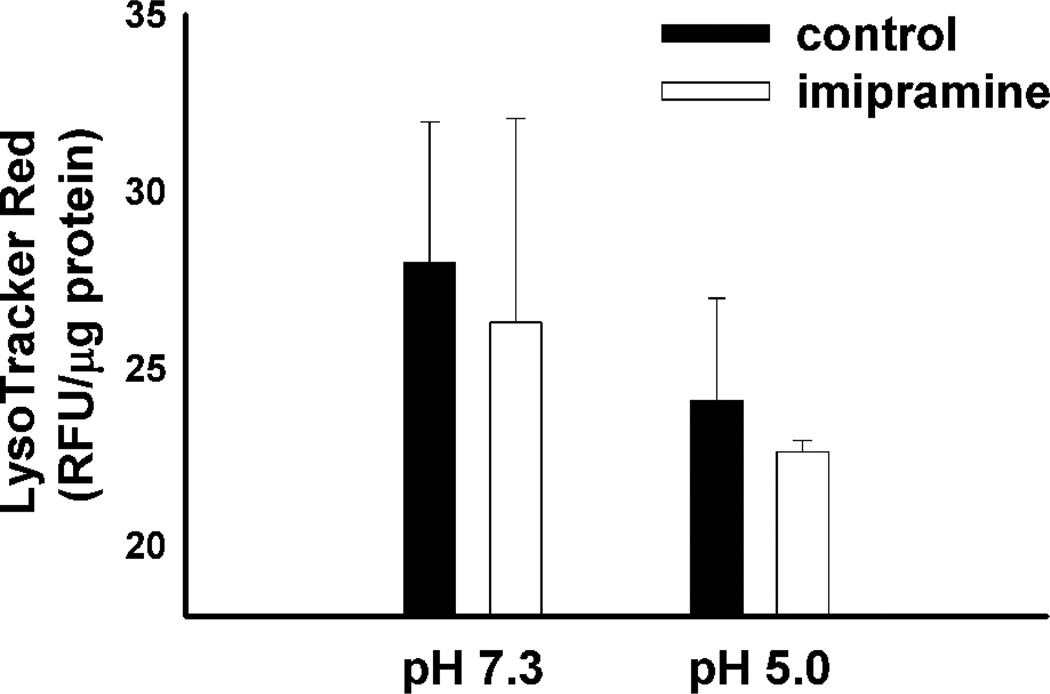
To further confirm that the CAD-induced enhancement in amine accumulation was indeed reliant on intracellular pH gradients (i.e., lysosome-to-cytosol) we evaluated the cellular accumulation of LTR in cells that were pretreated with the ionophores nigericin and monensin. When treated with ionophores the pH of all cellular compartments are equal to that of the cell culture medium. Under these conditions no intracellular pH gradients exist and therefore no ion trapping-based accumulation can theoretically occur. We treated both vehicle-treated control cells and cells pretreated with imipramine with the ionophores and evaluated differences in LTR uptake. Interestingly, treatment of cells with ionophores under normal pH conditions (i.e., pH 7.3) negated any CAD-induced enhancements in LTR accumulation (Figure 2). These results suggest that the CAD-induced enhancement in LTR accumulation is dependent upon ion trapping and cannot simply be attributed to enhanced binding sites or changes in membrane permeability in CAD treated cells. In addition, to address the possibility that amine-containing compounds accumulate through pH dependent binding, which may be predicted to occur in the acidic lysosome, we measured the cellular accumulation of LTR in vehicle-treated and imipramine-treated cells that were exposed to ionophores in a pH 5.0 buffered solution (Figure 2). The failure of imipramine to increase the cellular accumulation of LTR suggests that the observed hyperaccumulation does not occur through the accumulation of pH-dependent binding sites that may be predicted with the accumulation of lysosomal phospholipids. The CAD-induced hyperaccumulation of propranolol was also found to be reversed by the disruption of intracellular pH gradients (data not shown). Further studies found that imipramine causes a dramatic increase in the accumulation of the hydrophilic marker of aqueous lysosomal volume, methylamine (Supplemental Figure 1), which would not be predicted to be a substrate for phospholipid binding based on its hydrophilicity33 and has been previously used to indirectly measure the aqueous lysosomal storage volume of cells.16, 34 Together, these results suggest that accumulation of the amine-containing compounds isn’t caused by the binding to accumulated drug-binding sites, such as phospholipids, but rather suggests that it results from enhanced ion trapping in an expanded lysosomal compartment.
Figure 2.
LTR accumulation in MDA-1986 cells treated with vehicle alone or 10 µM imipramine following disruption of the lysosome-to-cytosol pH gradient with ionophore treatment at either, pH 7.3 or pH 5.0. Cellular accumulation of LTR was measured in fluorescence units (RFU) normalized to protein content and is represented as the mean ± S.D from three independent experimental evaluations.
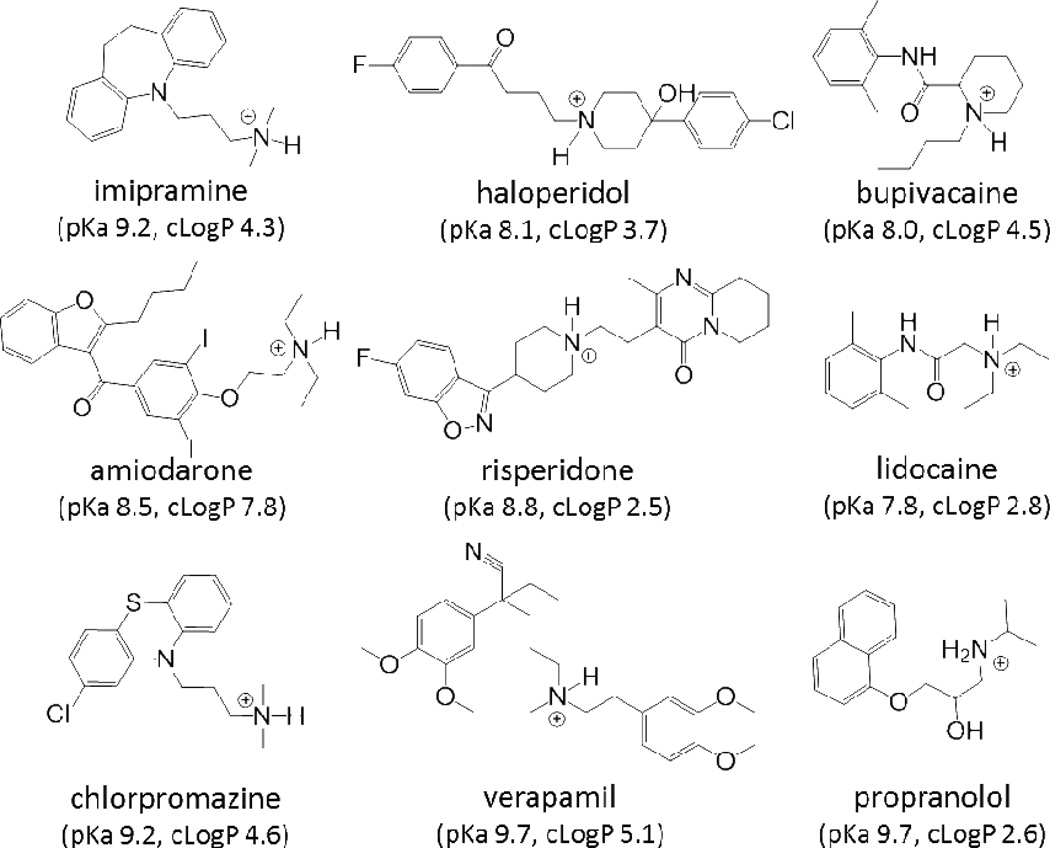
Imipramine is proposed to work by inhibiting the transporter-mediated uptake of neurotransmitters in neuronal cells.35 Considering that the cells used for these evaluations are non-neuronal, it is unlikely that the effect imipramine is having on lysosomes is directly related to its therapeutic activity. Instead, we hypothesize that it is the physicochemical properties of the drug that determine whether or not it perpetrates an interaction. To test this, we examined the propensity of a series of eight drugs with variable therapeutic classification but with similar cationic amphiphilic properties to imipramine to affect LTR accumulation. The cationic amphiphilic properties are defined by the presence of a weakly basic, ionizable amine (i.e., pKa) and the hydrophobicity of the compound (i.e., logP).36 The test drugs have amine functional groups with predicted pKa values between 7.8 and 9.7 and are relatively lipophilic with predicted logP values between 2.5 and 7.8. The structure and predicted physichochemical properties (i.e., pKa and logP) of each drug is provided in Figure 3. Structurally, all of the tested drugs can be described as containing a hydrophobic aromatic ring or ring system spatially separated from an ionizable secondary or tertiary amine.
Figure 3.
Structures of weakly basic drugs shown to cause a significant expansion in lysosomal volume. The predicted pKa and LogP values for each of the drugs are listed (predictions obtained using ChemAxon software available at http://www.chemicalize.org).
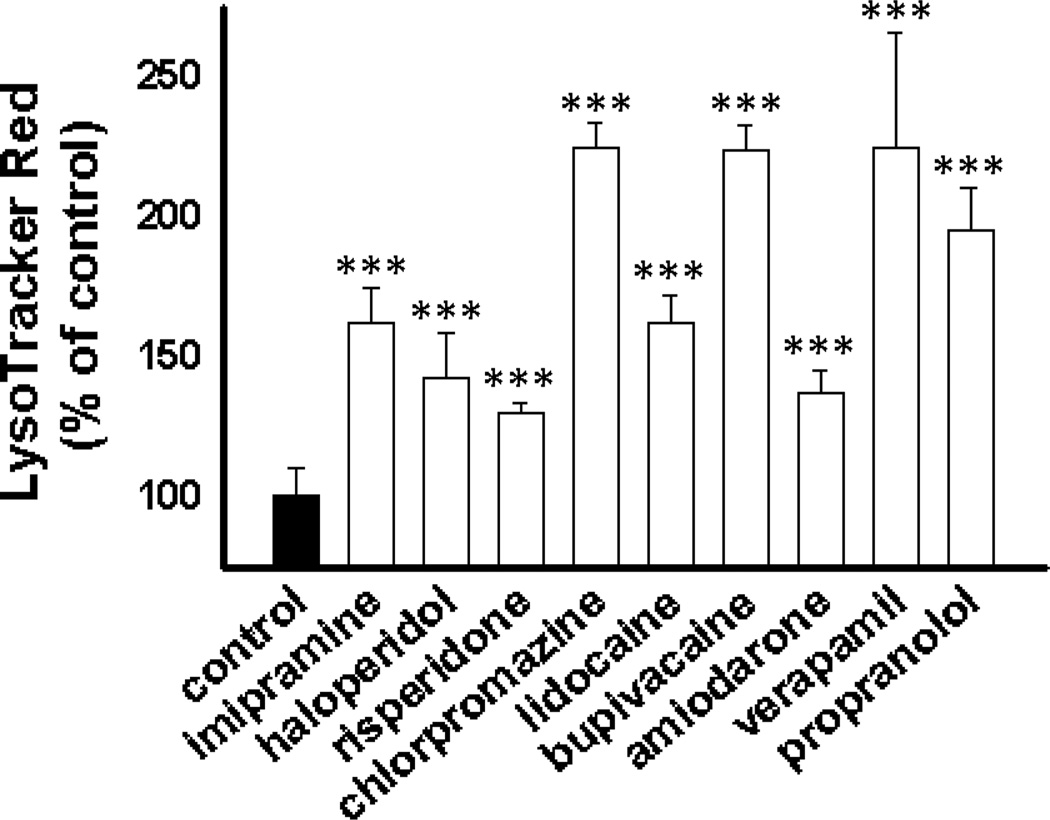
Interestingly, all of the CADs regardless of their therapeutic application caused a significant increase in the cellular accumulation of LTR (Figure 4). These results confirm that the physicochemical properties of the drug, not the therapeutic classification, drive the propensity for this observed interaction.
Figure 4.
LTR accumulation in MDA-1986 cells following a 24 h treatment with vehicle alone or various CADs at a concentration of 10 µM. Cellular accumulation of LTR as a percentage of vehicle-treated control cells is represented as mean ± S.D from three independent experimental evaluations (***, p < 0.001 by Student’s t test).
Studies on the Mechanism of Enhanced Accumulation of Amine-Containing Compounds
Having established that CADs cause an increase in ion trapping-based drug accumulation we further examined the potential basis for this. To narrow down the possible mechanisms responsible we sought to determine whether CADs increased drug accumulation through an increase in uptake or a decrease in release. Lysosomtropic compounds have a complicated release mechanism, including the potential for vesicle-mediated efflux,37, 38 transporter mediated efflux39 and simple passive diffusion40. We reasoned that increases in drug uptake following CAD exposure would be indicative of an increase in the capacity of the cell to ion trap the drug in lysosomes, either through a change in the pH or volume of the lysosomal system. Decreases in drug release following CAD exposure would be indicative of either inhibition of transporter-mediated or vesicle-mediated drug efflux. To display the therapeutic relevance of this interaction pathway we chose to use the commonly prescribed β-adrenergic antagonist propranolol, because it is a well-established substrate for ion trapping-based accumulation.31 We found that imipramine treatment causes a profound increase in propranolol uptake in fibroblasts (Figure 5A), but shows no effect on the normalized propranolol release (Figure 5B). A normalized release profile was constructed to allow direct comparison in the release profiles and was performed by reducing the propranolol dose in the imipramine-treated cells. An uptake and normalized release study was also conducted using LTG in place of propranolol in MDA-1986 cells and yielded similar results (Supplemental Figure 2). These results indicate that CADs increase the uptake of drugs that accumulate by ion trapping without significantly influencing release.
Figure 5.
Propranolol (A) uptake and (B) normalized release profile in human fibroblasts following a 48 h treatment with vehicle alone or 10 µM imipramine. Cellular [3H]propranolol levels were measured in radioactivity units (DPM) normalized to cellular protein content and is represented as mean ± S.D from three independent experimental evaluations (**, p < 0.01 by Student’s t test).
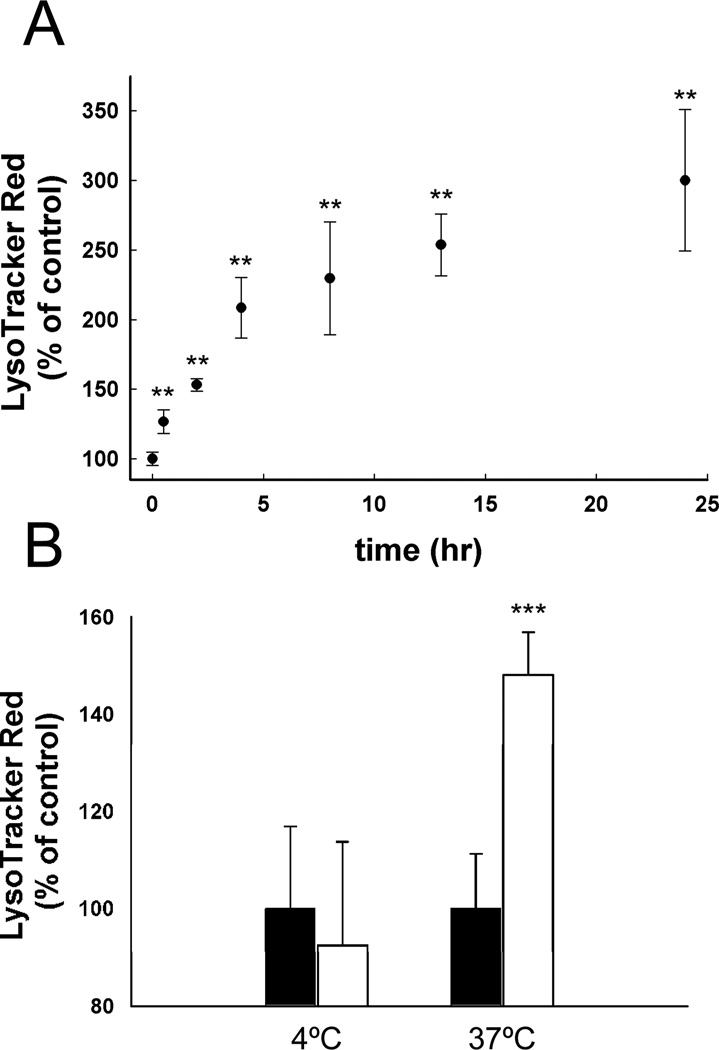
Based on the theory of ion trapping described by de Duve, the extent of lysosomal accumulation depends on the physicochemical properties of the drug (i.e., pKa and membrane permeability properties), as well as, the properties of the lysosomal system, such as pH and volume.1 Since the inherent physicochemical properties of the drug are not influenced by CAD pretreatment we must assume that the properties of the lysosomes are. The increased ion trapping capacity of cells would presumably result from a decrease in lysosomal pH, an increase in the lysosomal volume or a mixture of the two effects. Therefore we measured the effect of imipramine treatment on lysosomal pH and found that exposure to 10 µM imipramine for 24 h resulted in no significant change in lysosomal pH (pH 5.0 ± 0.9), as compared to vehicle-treated control cells (pH 4.9 ± 0.2) (Supplemental Figure 3). The lack of a decrease in lysosomal pH following CAD treatment suggests that CADs are causing an expansion of the volume of the lysosomal compartment. The steady-state volume of lysosomes is a function of membrane fusion and fission events that facilitate the biogenesis and depletion of lysosomes, respectively. Assuming that CADs interfere with one or both of these processes, the effect of CADs should be both time- and temperature-dependent. To test this possibility we incubated cells with 10 µM imipramine for varying amounts of time and measured the impact on LTR accumulation. We found that the CAD-induced enhancement in LTR accumulation was a progressive process with enhanced accumulation minimal at early incubation times and maximal after approximately 10 to 15 h of exposure (Figure 6A). Moreover, we found that incubation of cells with 10 µM imipramine for 4 h failed to enhance the accumulation of LTR unless the temperature of the incubation was maintained at 37°C. Cells incubated with or without CAD at 4°C for 4 h showed no differences in LTR accumulation at 37°C as compared to cells maintained at 37°C throughout the treatment period (Figure 6B). These results are important since they are consistent with the notion that CADs interfere with normal fusion and/or fission events that dictate the steady-state volume of lysosomes.
Figure 6.
(A) Time- and (B) temperature-dependent accumulation of LTR in MDA-1986 cells following treatment with vehicle alone (black bars) or 10 µM imipramine (white bars). Cellular accumulation of LTR as a percentage of the vehicle-treated control cells is represented as mean ± S.D from six independent experimental evaluations (**, p < 0.01; ***, p < .001 by Student’s t test).
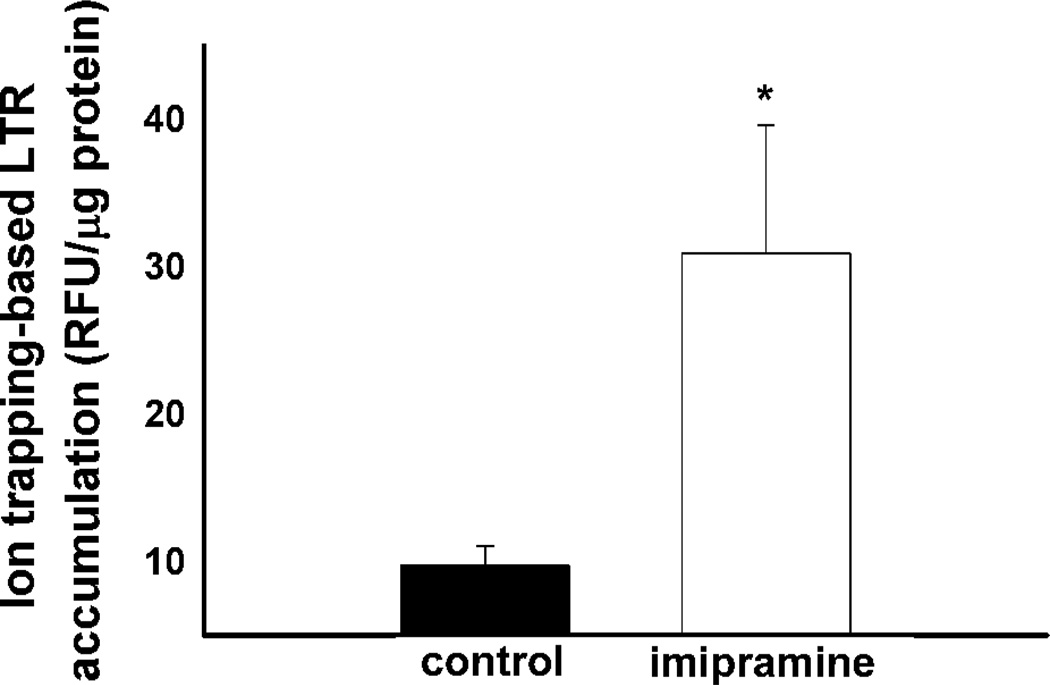
As previously stated the cellular accumulation of LTR and other weakly basic amine-containing compounds in lysosomes by ion trapping depends on both the pH and volume of the lysosomal compartment. Therefore, the change in the volume of the lysosomal compartment following imipramine treatment can be approximated if both lysosomal pH and the amount of amine accumulated by ion trapping are known. Having measured lysosomal pH changes after a 24 h exposure to imipramine (Supplemental Figure 3) we then sought to measure the amount of LTR retained in cells by ion trapping in lysosomes under the same treatment conditions. The amount of LTR accumulated in vehicle and imipramine treated MDA-1986 cells by ion trapping-based accumulation in lysosomes was measured. The ion trapping-based accumulation of LTR was determined by measuring the amount of LTR accumulated in cells with a normal lysosome-to-cytosol pH gradient and those where the pH gradient was eliminated by ionophore treatment. The difference in these values is expected to represent the amount of LTR that accumulates by ion trapping in lysosomes. It was found that cells treated with imipramine had a significantly elevated capacity to accumulate LTR by ion trapping (Figure 7). Using the equations provided in the Experimental section along with the lysosomal pH measurements and amount of LTR accumulated by ion trapping for vehicle and imipramine treated cells an approximately 4-fold increase in the lysosomal volume was determined to occur following a 24 h exposure of MDA-1986 cells to 10 µM imipramine.
Figure 7.
Ion trapping-based accumulation of LTR in MDA-1986 cells following a 24 h treatment with vehicle alone or 10 µM imipramine. Following imipramine treatment, LTR accumulation was measured with or without disruption of the lysosome-to-cytosol pH gradient by ionophore treatment. The difference in the amount of LTR accumulated in cells with and without an intact lysosome-to-cytosol pH gradient was determined to be the amount of LTR accumulated by ion trapping. This value represents the amount of LTR accumulated in cells through ion trapping in lysosomes. Cellular accumulation of LTR was measured in fluorescence units (RFU) normalized to cellular protein content and expressed as the mean ± S.D from three independent experimental evaluations (*, p < 0.05 by Student’s t test).
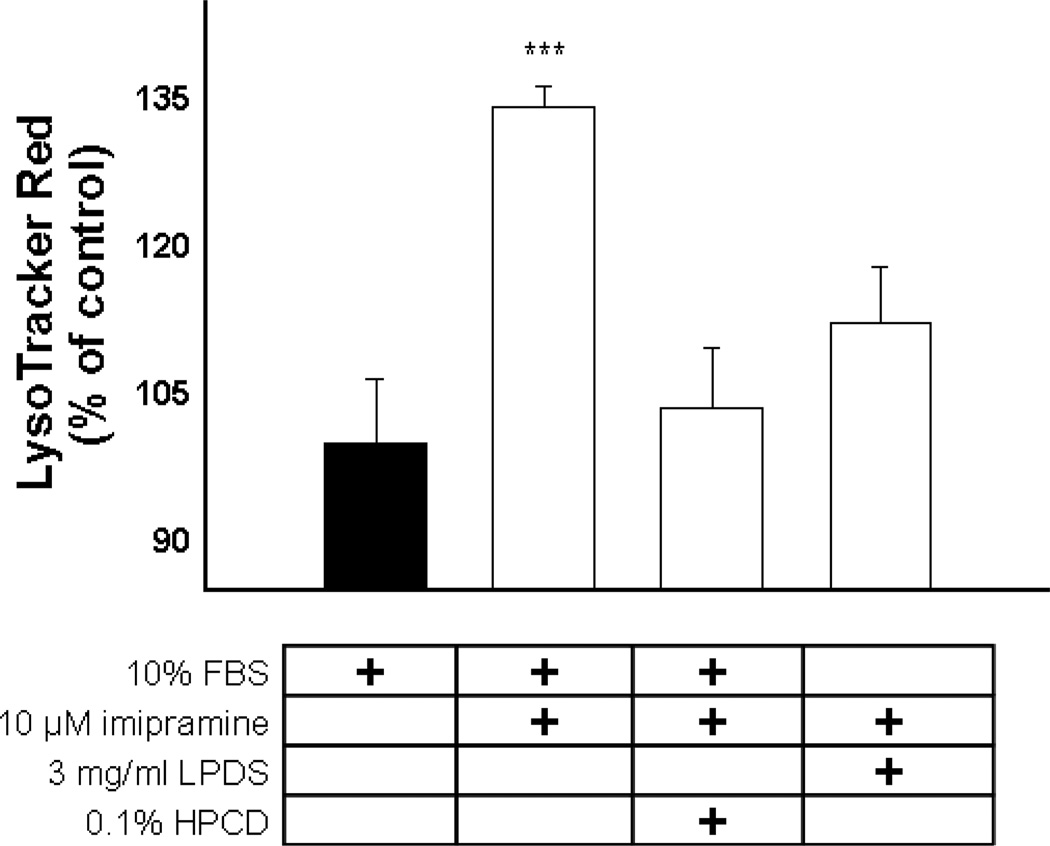
A comparison of LTR accumulation in wild type (WT) human fibroblasts with or without imipramine treatment with human fibroblasts from NPD patients with loss-of-function mutations in either the Niemann-Pick type C1 (NPC1) or Niemann-Pick type C2 (NPC2) protein revealed that imipramine causes an increase in LTR accumulation similar to that seen in cells isolated from NPD patients (Supplemental Figure 4). The possibility that the cellular accumulation of cholesterol was contributing to the observed expansion of the lysosomal compartment was suggested by studies that found a reduction in LTR accumulation in fibroblasts with mutations in NPC1 or NPC2 that were grown in lipoprotein-depleted media (Supplemental Figure 5). Both treatment with 0.1% HPCD41 and growth in lipoprotein-depleted media42 have been shown to at least partially reverse the lipidosis observed in NPD fibroblasts. Therefore, we evaluated that ability of these treatments to prevent the enhanced cellular accumulation of LTR observed following imipramine treatment. We found that both lipidosis reversing treatments significantly reduced LTR accumulation in imipramine treated cells (Figure 8). Therefore, it appears that the increase in lysosomotropic amine retention following imipramine treatment is directly related to the ability of imipramine to inhibit normal cholesterol clearance from late endocytic compartments.
Figure 8.
LTR accumulation in MDA-1986 cells following treatment with 10 µM imipramine (white bars) with or without lipidosis-reversing treatments, as compared to vehicle-treated control cells (black bar). Cellular accumulation of LTR as a percentage of the vehicle-treated control cells is represented as mean ± S.D from three independent experimental evaluations (***, p < .001 by Student’s t test).
DISCUSSION
Similar to previous observations in lysosomal lipid storage disease cells, prolonged exposure to lipidosis-inducing CADs caused a pronounced hyperaccumulation of amine-containing compounds that are substrates for lysosomal ion trapping.15, 16, 37 Imipramine was found to specifically increase the punctate vesicular staining and cellular accumulation of amine-containing compounds that are substrates for ion trapping-based accumulation in lysosomes but had no impact on the cellular accumulation of molecules that are not substrates (Figure 1). These results are suggestive of an increase in the ability of cells to accumulate amine-containing drugs in lysosomal structures following imipramine exposure and argue against a non-specific increase in cellular amine accumulation by increasing membrane permeability or by increasing the non-specific cellular binding sites for any amine-containing compounds.
Initial studies were conducted using a human fibroblast cell line. Their outstretched cellular morphology is conducive to microscopic evaluation, thus allowing greater visualization of intracellular fluorescent probe distribution. In addition, it allowed for comparisons between the CAD-induced phenotype and that observed in fibroblasts obtained from NPC disease patients. The similarity between the distribution and accumulation of LTR in NPC disease and CAD-treated fibroblasts was notable as can be seen by the TOC Figure and Supplemental Figure 4. A 48 h CAD-treatment was initially chosen based on previous studies showing a significant time-dependent induction of the lipidosis in human fibroblasts that is most pronounced at this time point.43 Subsequent studies sought to illustrate that our findings are not specific to one cell type, but instead are representative of cells regardless of their source. The squamous cell carcinoma cell line MDA-1986 was chosen for this purpose. The high proliferation rate of these cells allowed for rapid growth in culture which made them preferable for high throughput analysis. Therefore, multiple evaluations were undertaken using this cell line including the time-dependency of the CAD-induced increase in LTR accumulation. It was found that maximal response was achieved within 24 hours of drug exposure (Figure 6A). Therefore, subsequent experiments conducted in this cell line were carried out using the optimized 24 h drug treatment, which was also found to be adequate in the fibroblast cell line (Supplemental Figure 4). A 24 h imipramine treatment was used in the determination of lysosomal ion trapping-based accumulation of LTR (Figure 7) and changes in lysosomal pH (Supplemental Figure 3) that were used to calculate changes in apparent lysosomal volume.
In these studies, a variety of lysosomotropic amine compounds were used to probe for this drug interaction pathway, including: LTR, LTG, daunorubicin, propranolol and methylamine. The diversity of compounds used was to ultimately illustrate the broad implications of this observed phenomenon. Not only did this work illustrate the capacity of CAD treatment to affect the cellular accumulation of model lysosomotropic probes, like LTR and LTG, but it also showed that it had implications for therapeutic compounds, such as daunorubicin and propranolol.
Because imipramine served as a model CAD, we wanted to be sure that its effect was reflective of its cationic amphiphilic properties and not some other innate pharmacological activity. Therefore, we tested eight other known CADs from a variety of pharmacological classes for their ability to influence cellular accumulation of LTR and found that they all significantly enhance LTR accumulation (Figure 4). The ability of CADs to increase the cellular accumulation of lysosomotropic amine drugs illustrates a novel pathway through which drug co-administration can lead to a distribution-based pharmacokinetic drug interaction. Although a comprehensive structure activity relationship was not evaluated, the series of tested CADs that were capable of causing a significant expansion of the lysosomal volume were characterized as cationic, with predicted pKa values between 7.8 and 9.7, as well as, lipophilic with a predicted logP between 2.5 and 7.8 (Figure 3).
The observed increase in amine-containing drug accumulation following CAD treatment would at first glance appear to directly contradict earlier work that has illustrated the capacity of lysosomotropic amine drugs to inhibit the cellular uptake of each other by competing for uptake into lysosomes.23, 24, 44–49 This competition was presumed to occur through the ability of lysosomotropic amine drugs to buffer the lysosomal pH and inhibit the lysosomal uptake of secondarily applied lysosomotropic amine drugs. We believe these apparent discrepancies originate from differences in drug dosing and duration of exposure leading to differential physiological effects on cells and the lysosomal system. These earlier studies relied on short-term, high-dose drug treatments which are known to cause acute lysosomal alkalinization and therefore reduced ion trapping of amines in lysosomes.49 These experiments were also conducted in purified lysosomes where it is not clear if the V-ATPase was fully active under the experimental conditions. Our studies relied on longer durations of drug exposure in intact cells with relatively lower treatment doses which is more closely associated with the induction of a cellular lipidosis.50–52 In fact, we carried out additional experiments with treatments known to cause lysosomal alkalinization6, 53 by exposing cells to either 30 µM chloroquine, 10 mM ammonium chloride or 200 nM of the vacuolar-type H+-ATPase inhibitor concanamycin A, concurrent with the 2 h LTR accumulation assay (Supplemental Figure 6). Ammonium chloride is a weak base that requires millimolar drug concentrations to cause a significant alkalinization of lysosomes, whereas chloroquine is a dibasic amine that is capable of causing a significant buffering of the lysosome at concentrations in the micromolar range.4, 6, 54 All lysosomal alkalinizing treatments were found to drastically decrease the cellular accumulation of LTR under these conditions. Thus, our results don’t argue against previous observations of potential drug interactions occurring through drug-induced lysosomal alkalinization, but offers an additional pathway through which amine-containing drugs that accumulate in lysosomes can influence the pharmacokinetic properties of each other depending on their physiological effect on the lysosomal apparatus. Since the therapeutic concentrations of most CADs are well below the concentrations necessary to cause lysosomal alkalinization and since most of the typical CADs are used as chronic therapies we would argue that CAD-induced lipidosis is a more likely clinical scenario than lysosomal alkalinization.
By enhancing the cellular retention of this secondarily applied drug, one would expect changes in the drug’s pharmacokinetic profile, such as an increase in the drug’s volume of distribution and half-life. Following the initial characterization of this novel drug interaction pathway we set out to understand the mechanism through which CADs cause the increased cellular accumulation of compounds that are substrates for ion trapping in lysosomes. We found that imipramine’s ability to increase LTR accumulation was strongly dependent on imipramine exposure time (Figure 6A). Long exposure times were found to be needed for the maximum observed effect (>15 hrs). In addition, we found at low temperature (4°C) imipramine was unable to induce the cellular changes needed for the increased LTR accumulation observed at the physiological temperature of 37°C (Figure 6B). Our results are unable to rule out the possibility that a reduction in the cellular accumulation of imipramine at 4°C is responsible for the observed temperature-dependency, but we believe that together these results argue against the possibility that imipramine is in some way inhibiting an acute cellular efflux pathway for LTR and is consistent with imipramine inducing an energy-dependent cellular remodeling process that results in enhanced LTR accumulation.
The predominant mechanism for uptake of lysosomotropic amines, such as LTR, is thought to be through ion trapping,1 but realizing that work in our lab and others have shown that amines often accumulate in cells to a degree greater than that predicted by ion trapping alone suggests the potential for other mechanisms of intracellular and intralysosomal retention.31, 49, 55, 56 The observation that elimination of the lysosome-to-cytosol pH gradient reversed the ability of imipramine to influence LTR accumulation suggested that this remodeling process resulted in an increase in the lysosomal ion trapping capacity of cells (Figure 2). A comparison of the amount of LTR accumulated in cells by ion trapping in lysosomes in control cells and cells treated with imipramine showed a significant increase in lysosomal ion trapping-based accumulation of LTR in imipramine treated cells (Figure 7).
The assumption that lysosomal pH dependent accumulation of LTR is solely mediated by the ion trapping mechanism led us to speculate that imipramine can cause one of two effects, 1) increased lysosomal volume or 2) decreased lysosomal pH, either of which would increase the amount of LTR accumulating in the cell by ion trapping in lysosomes. To distinguish the two possibilities, measurements of lysosomal pH were undertaken and showed a slight but insignificant increase in lysosomal pH following imipramine treatment (Supplemental Figure 3). Therefore we concluded that imipramine causes the increased cellular accumulation of LTR by causing an expansion of the lysosomal volume, but because definitive lysosomal volume measurements were not undertaken we have denoted this change as an increase in the apparent lysosomal volume. These observations were consistent with our microscopy studies that suggested an increase in the vesicular volume that stained with the various lysosomotropic dyes (Figure 1).
A rather simple relationship is predicted to exist between the amount of drugs in lysosomes sequestered by ion trapping, lysosomal volume and lysosomal pH. Therefore, measurement of the effect of imipramine on the ion trapping-based accumulation of an LTR (Figure 7) and lysosomal pH (Supplemental Figure 3) allows for an approximation of the imipramine-induced expansion of the lysosomal volume. The equations for these simple relationships are detailed in the Experimental section. Using the data collected in MDA-1986 cells following a 24 h exposure to 10 µM imipramine we found an approximately 3-fold increase in the steady state accumulation of LTR by ion trapping in lysosomes and a 0.1 unit increase in the lysosomal pH. Together, these results would predict an approximately 4-fold increase in lysosomal volume in the imipramine treated cells.
In order to assess if imipramine’s mechanism of increasing amine-drug accumulation was through a decrease in lysosomotropic amine efflux kinetic measurements of both lysosomotropic amine uptake and release were undertaken. Using two model lysosomotropic amine compounds, LTG (Supplemental Figure 2) and propranolol (Figure 5), we found that imipramine treatment doesn’t inhibit lysosomotropic amine efflux but rather increases uptake. These results suggest that it is the enhanced steady-state volume of lysosomes associated with imipramine exposure that contributes to increased accumulation.
Based on our hypothesis that imipramine’s capacity to induce a cellular lipidosis is related to its ability to cause an expansion of the apparent lysosomal volume and since the time-dependency of this effect follows quite well to previous reports on the time dependency of CAD induced lipid trafficking defects50, 51, 57 we tested the ability of lipidosis-correcting treatments to prevent lysosomal volume expansion. We found that both, growth in lipoprotein-depleted media and treatment with HPCD, as lipidosis reversing therapies, prevented imipramine from increasing the cellular accumulation of our model lysosomotropic amine probe, LTR (Figure 8). These results suggest that the lysosomal volume expansion is secondary to the capacity of the perpetrator to inhibit the normal trafficking of LDL-derived cholesterol out of lysosomes.
Our results are in agreement with recent work that has shown a progressive, non-steady state accumulation profile for the phospholipidosis-inducing drug chloroquine.17 Similar to our results these authors found that chloroquine at concentrations between 25 and 200 µM induced the formation of an expanded acidic vesicular compartment that resulted in enhanced cellular accumulation of the lipidosis-inducing drug. Their data shows a 10- to 30-fold increase in vesicular volume with up to a 1 unit upward shift in the vesicular pH. Although our CAD treatments showed a less pronounced increase in vesicular volume and no significant increase in vesicular pH, we believe that such effects would be observed with increasing drug concentrations.
Drug-drug interactions involving lysosomes examined in this work are projected to have clinical significance. On a cellular level, we predict that drug-induced changes in the extent of lysosomal sequestration can directly influence activity by altering the availability of the drug to bind with intended extralysosomal targets. We also predict that this drug-interaction could cause significant changes in the pharmacokinetic behavior of drugs. In an intact animal the extensive sequestration of a drug in lysosomes is the cause for an extremely large apparent volume of distribution and a prolonged elimination half-life. Accordingly, we anticipate that pre-administration of a perpetrator in an animal could cause a significant increase in the apparent volume of distribution and half-life of a subsequently administered victim drug. This could have a significant impact on therapeutic outcomes, particularly for those drugs that have a narrow therapeutic index.
It is interesting to entertain the likely possibility that many of the drugs that we have shown to act as perpetrators in the interaction would also fall victim to it. In a clinical setting this could result in volume of distribution and half-life parameters that appear to increase with the time of administration. In support of this notion, kinetic studies of amiodarone in humans indicated that half-life was 10–17 hours after a single i.v. dose and 8–21 days after chronic administration.58
Collectively, this work illustrates an intracellular drug interaction pathway resulting from the ability of CADs to cause an expansion of the apparent lysosomal volume of cells. The perpetrator drug in such an interaction is the CAD, which causes an increase in the volume of the lysosomal compartment. The victim drug would include any drug that accumulates in lysosomes by the ion trapping-based mechanism, where lysosomal volume is an important factor in determining total cellular drug accumulation. Therefore, victim drugs can include both CAD and non-CAD lysosomotropic drugs. This lysosomal volume expansion was found to be directly related to the CAD-induced cellular lipidosis and to result in the increased cellular uptake of drugs that are substrates for ion trapping-based accumulation in lysosomes. Our evaluations in this manuscript were performed using two different cell lines from separate lineages. The fact that our results were consistently observed in these different cell lines suggests that this drug-interaction could be universal and apply to any cells chronically exposed to CADs at relatively low concentrations. Further in vivo studies will help reveal if this is the case.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Dr. Damon T. Jacobs and Randall L. Logan for their thoughtful insight in reviewing this manuscript. This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant T32-GM008359].
ABBREVIATIONS
- CAD
cationic amphiphilic drug
- DMEM
Dulbecco’s modified Eagle’s medium
- D-PBS
Dulbecco’s phosphate buffered saline
- FBS
fetal bovine serum
- HPCD
hydroxypropyl-β-cyclodextrin
- LPDS
lipoprotein-depleted serum
- BCA
bicinchoninic acid
- LTR
LysoTracker Red DND-99
- LTG
LysoTracker Green DND-26
- WT
wild type
- NPD
Niemann-Pick disease
- NPC1
Niemann-Pick type C1
- NPC2
Niemann-Pick type C2
REFERENCES
- 1.de Duve C, de Barsy T, Poole B, Trouet A, Tulkens P, Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- 2.Dean RT, Jessup W, Roberts CR. Effects of exogenous amines on mammalian cells, with particular reference to membrane flow. Biochem J. 1984;217(1):27–40. doi: 10.1042/bj2170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hein L, Lullmann-Rauch R, Mohr K. Human accumulation potential of xenobiotics: potential of catamphiphilic drugs to promote their accumulation via inducing lipidosis or mucopolysaccharidosis. Xenobiotica. 1990;20(11):1259–1267. doi: 10.3109/00498259009046842. [DOI] [PubMed] [Google Scholar]
- 4.Poole B, Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. The Journal of cell biology. 1981;90(3):665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohkuma S, Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. The Journal of cell biology. 1981;90(3):656–664. doi: 10.1083/jcb.90.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lullmann H, Lullmann-Rauch R, Wassermann O. Drug-induced phospholipidoses. II. Tissue distribution of the amphiphilic drug chlorphentermine. CRC Crit Rev Toxicol. 1975;4(2):185–218. doi: 10.1080/10408447509164014. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell WH. Cationic amphiphilic drug-induced phospholipidosis. Toxicol Pathol. 1997;25(1):53–60. doi: 10.1177/019262339702500111. [DOI] [PubMed] [Google Scholar]
- 9.Reasor MJ. Phospholipidosis in the alveolar macrophage induced by cationic amphiphilic drugs. Fed Proc. 1984;43(11):2578–2581. [PubMed] [Google Scholar]
- 10.Yamamoto A, Adachi S, Kitani T, Shinji Y, Seki K. Drug-induced lipidosis in human cases and in animal experiments. Accumulation of an acidic glycerophospholipid. Journal of biochemistry. 1971;69(3):613–615. [PubMed] [Google Scholar]
- 11.Yamamoto A, Adachi S, Ishikawa K, Yokomura T, Kitani T. Studies on drug-induced lipidosis. 3. Lipid composition of the liver and some other tissues in clinical cases of "Niemann-Pick-like syndrome" induced by 4,4'-diethylaminoethoxyhexestrol. Journal of biochemistry. 1971;70(5):775–784. doi: 10.1093/oxfordjournals.jbchem.a129695. [DOI] [PubMed] [Google Scholar]
- 12.Yoshikawa H. Effects of drugs on cholesterol esterification in normal and Niemann-Pick type C fibroblasts: AY-9944, other cationic amphiphilic drugs and DMSO. Brain Dev. 1991;13(2):115–120. doi: 10.1016/s0387-7604(12)80118-5. [DOI] [PubMed] [Google Scholar]
- 13.Roff CF, Goldin E, Comly ME, Cooney A, Brown A, Vanier MT, Miller SP, Brady RO, Pentchev PG. Type C Niemann-Pick disease: use of hydrophobic amines to study defective cholesterol transport. Dev Neurosci. 1991;13(4–5):315–319. doi: 10.1159/000112179. [DOI] [PubMed] [Google Scholar]
- 14.Sakuragawa N, Sakuragaw M, Kuwabara T, Pentchev PG, Barranger JA, Brady RO. Niemann-Pick disease experimental model: sphingomyelinase reduction induced by AY-9944. Science. 1977;196(4287):317–319. doi: 10.1126/science.66749. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann AM, Krise JP. Niemann-Pick C1 functions in regulating lysosomal amine content. J Biol Chem. 2008;283(36):24584–24593. doi: 10.1074/jbc.M803715200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopitz J, Harzer K, Kohlschutter A, Zoller B, Blenck N, Cantz M. Methylamine accumulation in cultured cells as a measure of the aqueous storage compartment in the laboratory diagnosis of genetic lysosomal diseases. Am J Med Genet. 1996;63(1):198–202. doi: 10.1002/(SICI)1096-8628(19960503)63:1<198::AID-AJMG35>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 17.Zheng N, Zhang X, Rosania GR. Effect of phospholipidosis on the cellular pharmacokinetics of chloroquine. The Journal of pharmacology and experimental therapeutics. 2011;336(3):661–671. doi: 10.1124/jpet.110.175679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reasor MJ. Influence of a pre-existing phospholipidosis on the accumulation of amiodarone and desethylamiodarone in rat alveolar macrophages. Res Commun Chem Pathol Pharmacol. 1991;72(2):169–181. [PubMed] [Google Scholar]
- 19.Singh SS. Preclinical pharmacokinetics: an approach towards safer and efficacious drugs. Curr Drug Metab. 2006;7(2):165–182. doi: 10.2174/138920006775541552. [DOI] [PubMed] [Google Scholar]
- 20.Ogu CC, Maxa JL. Drug interactions due to cytochrome P450. Proc (Bayl Univ Med Cent) 2000;13(4):421–423. doi: 10.1080/08998280.2000.11927719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giacomini KM. Membrane transporters in drug disposition. J Pharmacokinet Biopharm. 1997;25(6):731–741. doi: 10.1023/a:1025733918160. [DOI] [PubMed] [Google Scholar]
- 22.Kornhuber J, Henkel AW, Groemer TW, Stadtler S, Welzel O, Tripal P, Rotter A, Bleich S, Trapp S. Lipophilic cationic drugs increase the permeability of lysosomal membranes in a cell culture system. J Cell Physiol. 2010;224(1):152–164. doi: 10.1002/jcp.22112. [DOI] [PubMed] [Google Scholar]
- 23.Daniel WA. Mechanisms of cellular distribution of psychotropic drugs. Significance for drug action and interactions. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(1):65–73. doi: 10.1016/s0278-5846(02)00317-2. [DOI] [PubMed] [Google Scholar]
- 24.Daniel WA, Wojcikowski J. The role of lysosomes in the cellular distribution of thioridazine and potential drug interactions. Toxicology and applied pharmacology. 1999;158(2):115–124. doi: 10.1006/taap.1999.8688. [DOI] [PubMed] [Google Scholar]
- 25.Bickel MH, Graber BE, Moor M. Distribution of chlorpromazine and imipramine in adipose and other tissues of rats. Life Sci. 1983;33(20):2025–2031. doi: 10.1016/0024-3205(83)90742-7. [DOI] [PubMed] [Google Scholar]
- 26.Ndolo RA, Forrest ML, Krise JP. The role of lysosomes in limiting drug toxicity in mice. J Pharmacol Exp Ther. 2010;333(1):120–128. doi: 10.1124/jpet.109.160226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altan N, Chen Y, Schindler M, Simon SM. Defective acidification in human breast tumor cells and implications for chemotherapy. J Exp Med. 1998;187(10):1583–1598. doi: 10.1084/jem.187.10.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neun BW, Stern ST. Monitoring lysosomal activity in nanoparticle-treated cells. Methods Mol Biol. 2011;697:207–212. doi: 10.1007/978-1-60327-198-1_22. [DOI] [PubMed] [Google Scholar]
- 29.Shi H, He X, Yuan Y, Wang K, Liu D. Nanoparticle-based biocompatible and long-life marker for lysosome labeling and tracking. Anal Chem. 2010;82(6):2213–2220. doi: 10.1021/ac902417s. [DOI] [PubMed] [Google Scholar]
- 30.Gong Y, Duvvuri M, Krise JP. Separate roles for the Golgi apparatus and lysosomes in the sequestration of drugs in the multidrug-resistant human leukemic cell line HL-60. The Journal of biological chemistry. 2003;278(50):50234–50239. doi: 10.1074/jbc.M306606200. [DOI] [PubMed] [Google Scholar]
- 31.Cramb G. Selective lysosomal uptake and accumulation of the beta-adrenergic antagonist propranolol in cultured and isolated cell systems. Biochem Pharmacol. 1986;35(8):1365–1372. doi: 10.1016/0006-2952(86)90283-2. [DOI] [PubMed] [Google Scholar]
- 32.Duvvuri M, Gong Y, Chatterji D, Krise JP. Weak base permeability characteristics influence the intracellular sequestration site in the multidrug-resistant human leukemic cell line HL-60. The Journal of biological chemistry. 2004;279(31):32367–32372. doi: 10.1074/jbc.M400735200. [DOI] [PubMed] [Google Scholar]
- 33.Seydel JK, Wassermann O. NMR-studies on the molecular basis of drug-induced phospholipidosis--II. Interaction between several amphiphilic drugs and phospholipids. Biochemical pharmacology. 1976;25(21):2357–2364. doi: 10.1016/0006-2952(76)90028-9. [DOI] [PubMed] [Google Scholar]
- 34.Kopitz J, Gerhard C, Hofler P, Cantz M. [14C]Methylamine accumulation in cultured human skin fibroblasts--a biochemical test for lysosomal storage and lysosomal diseases. Clinica chimica acta; international journal of clinical chemistry. 1994;227(1–2):121–133. doi: 10.1016/0009-8981(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 35.Langer SZ, Moret C, Raisman R, Dubocovich ML, Briley M. High-affinity [3H]imipramine binding in rat hypothalamus: association with uptake of serotonin but not of norepinephrine. Science. 1980;210(4474):1133–1135. doi: 10.1126/science.7444441. [DOI] [PubMed] [Google Scholar]
- 36.Ploemen JP, Kelder J, Hafmans T, van de Sandt H, van Burgsteden JA, Saleminki PJ, van Esch E. Use of physicochemical calculation of pKa and CLogP to predict phospholipidosis-inducing potential: a case study with structurally related piperazines. Exp Toxicol Pathol. 2004;55(5):347–355. doi: 10.1078/0940-2993-00338. [DOI] [PubMed] [Google Scholar]
- 37.Gong Y, Duvvuri M, Duncan MB, Liu J, Krise JP. Niemann-Pick C1 protein facilitates the efflux of the anticancer drug daunorubicin from cells according to a novel vesicle-mediated pathway. J Pharmacol Exp Ther. 2006;316(1):242–247. doi: 10.1124/jpet.105.089482. [DOI] [PubMed] [Google Scholar]
- 38.Michalik M, Pierzchalska M, Pabianczyk-Kulka A, Korohoda W. Procaine-induced enhancement of fluid-phase endocytosis and inhibition of exocytosis in human skin fibroblasts. Eur J Pharmacol. 2003;475(1–3):1–10. doi: 10.1016/s0014-2999(03)02000-4. [DOI] [PubMed] [Google Scholar]
- 39.Willingham MC, Cornwell MM, Cardarelli CO, Gottesman MM, Pastan I. Single cell analysis of daunomycin uptake and efflux in multidrug-resistant and -sensitive KB cells: effects of verapamil and other drugs. Cancer Res. 1986;46(11):5941–5946. [PubMed] [Google Scholar]
- 40.Dudley AJ, Brown CD. pH-dependent transport of procainamide in cultured renal epithelial monolayers of OK cells: consistent with nonionic diffusion. Br J Pharmacol. 1995;116(1):1685–1691. doi: 10.1111/j.1476-5381.1995.tb16392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abi-Mosleh L, Infante RE, Radhakrishnan A, Goldstein JL, Brown MS. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. Proc Natl Acad Sci U S A. 2009;106(46):19316–19321. doi: 10.1073/pnas.0910916106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas GH, Tuck-Muller CM, Miller CS, Reynolds LW. Correction of sphingomyelinase deficiency in Niemann-Pick type C fibroblasts by removal of lipoprotein fraction from culture media. J Inherit Metab Dis. 1989;12(2):139–151. doi: 10.1007/BF01800716. [DOI] [PubMed] [Google Scholar]
- 43.Appelqvist H, Nilsson C, Garner B, Brown AJ, Kagedal K, Ollinger K. Attenuation of the lysosomal death pathway by lysosomal cholesterol accumulation. Am J Pathol. 2011;178(2):629–639. doi: 10.1016/j.ajpath.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wojcikowski J, Daniel WA. Thioridazine-fluoxetine interaction at the level of the distribution process in vivo. Pol J Pharmacol. 2002;54(6):647–654. [PubMed] [Google Scholar]
- 45.Daniel WA, Wojcikowski J, Palucha A. Intracellular distribution of psychotropic drugs in the grey and white matter of the brain: the role of lysosomal trapping. Br J Pharmacol. 2001;134(4):807–814. doi: 10.1038/sj.bjp.0704319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wojcikowski J, Daniel WA. Distribution interactions between perazine and antidepressant drugs. In vivo studies. Pol J Pharmacol. 2000;52(6):449–457. [PubMed] [Google Scholar]
- 47.Daniel WA, Wojcikowski J. Lysosomal trapping as an important mechanism involved in the cellular distribution of perazine and in pharmacokinetic interaction with antidepressants. Eur Neuropsychopharmacol. 1999;9(6):483–491. doi: 10.1016/s0924-977x(99)00034-6. [DOI] [PubMed] [Google Scholar]
- 48.Daniel WA, Wojcikowski J. Interactions between promazine and antidepressants at the level of cellular distribution. Pharmacol Toxicol. 1997;81(6):259–264. [PubMed] [Google Scholar]
- 49.Ishizaki J, Yokogawa K, Ichimura F, Ohkuma S. Uptake of imipramine in rat liver lysosomes in vitro and its inhibition by basic drugs. J Pharmacol Exp Ther. 2000;294(3):1088–1098. [PubMed] [Google Scholar]
- 50.Martin WJ, 2nd, Kachel DL, Vilen T, Natarajan V. Mechanism of phospholipidosis in amiodarone pulmonary toxicity. J Pharmacol Exp Ther. 1989;251(1):272–278. [PubMed] [Google Scholar]
- 51.Liscum L, Faust JR. The intracellular transport of low density lipoprotein-derived cholesterol is inhibited in Chinese hamster ovary cells cultured with 3-beta-[2-(diethylamino)ethoxy]androst-5-en-17-one. J Biol Chem. 1989;264(20):11796–11806. [PubMed] [Google Scholar]
- 52.Yoshida Y, Arimoto K, Sato M, Sakuragawa N, Arima M, Satoyoshi E. Reduction of acid sphingomyelinase activity in human fibroblasts induced by AY-9944 and other cationic amphiphilic drugs. J Biochem. 1985;98(6):1669–1679. doi: 10.1093/oxfordjournals.jbchem.a135438. [DOI] [PubMed] [Google Scholar]
- 53.Woo JT, Shinohara C, Sakai K, Hasumi K, Endo A. Isolation, characterization and biological activities of concanamycins as inhibitors of lysosomal acidification. J Antibiot (Tokyo) 1992;45(7):1108–1116. doi: 10.7164/antibiotics.45.1108. [DOI] [PubMed] [Google Scholar]
- 54.Hollemans M, Elferink RO, De Groot PG, Strijland A, Tager JM. Accumulation of weak bases in relation to intralysosomal pH in cultured human skin fibroblasts. Biochim Biophys Acta. 1981;643(1):140–151. doi: 10.1016/0005-2736(81)90226-1. [DOI] [PubMed] [Google Scholar]
- 55.Duvvuri M, Krise JP. A novel assay reveals that weakly basic model compounds concentrate in lysosomes to an extent greater than pH-partitioning theory would predict. Mol Pharm. 2005;2(6):440–448. doi: 10.1021/mp050043s. [DOI] [PubMed] [Google Scholar]
- 56.Vestal RE, Kornhauser DM, Shand DG. Active uptake of propranolol by isolated rabbit alveolar macrophages and its inhibition by other basic amines. J Pharmacol Exp Ther. 1980;214(1):106–111. [PubMed] [Google Scholar]
- 57.Xia Z, Ying G, Hansson AL, Karlsson H, Xie Y, Bergstrand A, DePierre JW, Nassberger L. Antidepressant-induced lipidosis with special reference to tricyclic compounds. Prog Neurobiol. 2000;60(6):501–512. doi: 10.1016/s0301-0082(99)00036-2. [DOI] [PubMed] [Google Scholar]
- 58.Riva E, Aarons L, Latini R, Neyroz P, Urso R. Amiodarone kinetics after single i.v. bolus and multiple dosing in healthy volunteers. Eur J Clin Pharmacol. 1984;27(4):491–494. doi: 10.1007/BF00549600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.