Abstract
Context
Although numerous studies of the efficacy of exercise are reported, few studies have evaluated changes in characteristics of exercise dose in women with cancer both during and after cancer treatment.
Objectives
To describe the characteristics of exercise dose (i.e., frequency, duration, and intensity), and to evaluate for differences in symptom severity (i.e., fatigue, sleep disturbance, depression, pain) between women who did and did not exercise during and after cancer treatment.
Methods
In a sample of 119 women, two groups were classified: exercisers and nonexercisers. Exercisers were defined as women who met specific criteria for frequency (three times per week), duration (20 minutes per session), intensity (moderate), and mode (aerobic). Nonexercisers were defined as women who did not meet all of these criteria. Evaluation of exercise dose was completed at baseline (T1: the week before chemotherapy cycle two), at the end of cancer treatment (T2), and at the end of the study (T3: approximately one year after the T1 assessment) using self-report exercise questionnaires.
Results
Approximately 50% of the participants exercised during and 70% exercised after treatment. At T1, exercisers had lower total fatigue, lower behavioral and sensory subscale fatigue scores, and lower depression scores (P =0.038) than nonexercisers. No significant differences in sleep disturbance or pain were found between groups. At T2, exercisers had lower cognitive/mood subscale fatigue and depression scores than nonexercisers (P =0.047). At T3, no significant differences were found between groups in any symptom severity scores.
Conclusion
Both during and after cancer treatment, achieving or maintaining exercise guideline levels were met by most patients. Further study is needed to examine the link between exercise dose and symptom severity.
Keywords: Exercise dose, cancer, fatigue, sleep disturbance, pain, depression, cancer treatment
Introduction
Based on a series of systematic reviews and meta-analyses,1–7 exercise interventions are known to improve physiologic and psychological outcomes in patients during and after cancer treatment.8–15 In addition, the most current systemic review and meta analysis16 reviewed 82 studies and showed a large range of effects in upper and lower body strength from physical activity interventions during or after cancer treatment. However, a range of challenges are noted for individuals who are initiating and sustaining exercise doses, and for investigators who are conducting exercise intervention studies. The challenges for the individual include a need to change behavior and initiate exercise activities, which is especially difficult for a person who is sedentary.17 Once the exercise begins, the challenge shifts to sustaining this new behavior.4, 17–20 Both of these challenges are made more difficult when individuals experience distressing physical and psychological effects from cancer and its treatment.4, 21–23 Challenges for investigators include not being able to control for participants’ previous exercise dose, specifically for those who exercised regularly before the start of the study, and not knowing participants’ exercise capabilities. Dealing with the wide range of exercise interventions and adherence with the exercise intervention are additional challenges.
An evaluation of cancer patients’ level of adherence with an exercise intervention is important because long-term maintenance of exercise activities is essential to maintaining the benefits of exercise over the course of the disease and its treatment.24–29 Many beneficial effects of regular physical activity fade within two weeks if physical activity is not maintained and the effects will disappear within two to eight months without a return to regular exercise.30 Whereas most of the published exercise interventions include details about frequency, duration, and intensity, they differ widely (i.e., ranging from two to three times per week, from ten to sixty minutes, and from 40% to 75% of maximum heart rate). Because of these variations, it is difficult to evaluate adherence across previous studies.11–15
The American Cancer Society’s (ACS) guidelines for cancer patients state that they should exercise three to five times per week, for 20 to 60 minutes per session, at moderate intensity, and use aerobic exercise.31 However, no studies were found that evaluated the same cohort of cancer patients’ adherence with these recommended guidelines both during and after cancer treatment. Therefore, the aims of this study were to describe the characteristics of exercise dose (i.e., frequency, duration, intensity) in a sample of women both during and after cancer treatment and to evaluate for differences in symptom severity (i.e., fatigue, sleep disturbance, depression, pain) in exercisers versus nonexercisers, at three time points.
Methods
Study Design
This study was part of a longitudinal, randomized controlled trial (RCT) that tested the effectiveness of a systematic exercise intervention on fatigue (i.e., PRO-SELF®: Fatigue Control Program).32 In this RCT, participants were randomly assigned to one of three groups: group 1 (EE) received their exercise prescription throughout the study period (both during and after cancer treatment); group 2 (CE) received their exercise prescription after having completed cancer treatment; and group 3 (CC) received usual care throughout the study period (during and after cancer treatment). No differences were found among the three groups for changes in fatigue over time. However, fatigue severity increased and decreased over time regardless of group assignment.18 Therefore, for the current analysis, all patients were combined to examine their exercise doses. In addition, no significant differences were obtained among groups in symptoms of sleep disturbance, depression, or pain. Therefore, for the current analysis, all patients were combined to examine their exercise doses. This analysis evaluated three exercise dose characteristics (i.e., frequency, duration, and intensity) at three time points: baseline (the week before the second chemotherapy [CTX] treatment [T1]), at the completion of cancer treatment (T2), and at the end of the study (T3: approximately one year after T1), and compared fatigue, sleep disturbance, depression, and pain between exercisers and nonexercisers.
Settings and Participants
Participants were recruited from six outpatient oncology clinics in the San Francisco Bay area. All participants were females who were ≥ 18 years of age with a confirmed diagnosis of breast, colorectal, or ovarian cancer; beginning their first cycle of CTX; able to read, write, and understand English; had a Karnofsky Performance Status (KPS) score of ≥ 60; and were able to provide written informed consent. Participants were excluded from the study if they were receiving concurrent radiation therapy (RT) or bone marrow transplantation or had uncontrolled hypertension or diabetes mellitus, a pain intensity rating of ≥ 3on a 0 to10 numeric rating scale (NRS), a lytic bone lesion or orthopedic limitations, a history of major depression or sleep disorders, CTX within the past year, a diagnosis of AIDS-related malignancies or leukemia, or absolute contradictions to exercise testing as established by American College of Sports Medicine (ACSM) guidelines.33
Procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco, and at each recruitment site. Participants who met the study criteria and were scheduled to begin CTX were told about the study by the referring oncologists and nurses at each recruitment site, and then approached by the research staff. After written informed consent was obtained, participants completed a packet of questionnaires in the clinic or in their home. Questionnaires were returned when patients came in for exercise testing. Participants were classified as exercisers if their actual exercise doses measured at each time point corresponded to the minimum criteria recommended by the ACS guidelines for frequency (three times per week), duration (20 minutes per session), intensity (moderate), and mode (aerobic). Aerobic exercise included activities such as walking, jogging/running, swimming, and cycling. Nonexercisers were defined as individuals who did not fulfill these criteria.
Measures
A Demographic Profile Form, completed by the participants at T1, obtained information on age, income, ethnicity, and gender. In addition, at T1, T2, and T3, participants provided information on occupational status, KPS score, menopausal status, and symptoms.34–36 Three items from the Demographic Profile Form were used to evaluate exercise dose at the three time points. To measure frequency, participants were asked, "How often do you exercise per week?" Possible answers ranged from once a week to more than five times per week. To measure duration, participants were asked, "On average, how long do you exercise at each session?" Possible answers for duration ranged from five minutes to more than 45 minutes per session. To measure intensity, participants were asked, "On average, how hard do you exercise at each session?" and the choices ranged from easy to very hard.
The KPS scale measures the physical abilities of the patient based on the definitions provided on a 0 to 100 scale. Since its development, the scale has been used extensively in oncology to evaluate performance status. A score of 100% indicates that the individual is able to carry on normal activities and that there is no decrease in performance status. A score of 30 indicates that the individual is severely disabled and needs to be hospitalized. The KPS has well-established inter-rater reliability, concurrent validity, and criterion validity.37–40
The Medical Record Review Form was used to obtain medical and treatment-related data. Information was obtained on diagnosis, CTX protocol, treatment goals, and response to CTX and/or RT.34, 36
The Piper Fatigue Scale (PFS) is a 22-item instrument41 that evaluates subjective fatigue (i.e., behavioral, affective, sensory, cognitive/mood). Each item is rated on a 0 (none) to 10 (a great deal) NRS. Total and subscale scores are calculated, which can range from 0 to 10. It was originally developed to measure fatigue in persons with cancer and has excellent reliability and validity estimates.42–46 Fatigue scores are categorized by mild (1–3), moderate (4–6), and severe (7–10).41 In the current study, the Cronbach’s alpha for the PFS ranged from 0.96 to 0.97.
The General Sleep Disturbance Scale (GSDS) consists of 21 items that evaluate various aspects of sleep disturbance (i.e., quality, quantity, sleep latency, waking up during sleep, daytime sleepiness, medication use).47 Items are rated on a 0 (never) to 7 (every day) NRS. The 21 items are summed to yield a total score that ranges from 0 (no disturbance) to 147 (extreme disturbance). A score ≥ 43 reflects sleep disturbance.48 The GSDS has well-established reliability and validity in cancer patients.49–51 In the current study, Cronbach’s alpha ranged from 0.83 to 0.86.
The Center for Epidemiological Studies Depression Inventory (CES-D) is a 20-item self-report instrument that measures the clinical syndrome of depression.52 Each item is rated on a four-point scale (0–3). Scores can range from 0 to 60, with higher scores reflecting more depressive symptoms. A score ≥16 indicates the need for a clinical evaluation.52 The CES-D has well-established reliability and validity across samples of persons with cancer receiving surgery or RT.46, 49, 51, 53 In the current study, the Cronbach’s alpha for the CES-D ranged from 0.80 to 0.89.
The Worst Pain Intensity Scale is a single-item, 0 (no pain) to 10 (worst pain imaginable) NRS that patients used to rate their worst pain in the past 24 hours. A descriptive NRS is a valid and reliable measure of pain intensity.54
Data Analyses
Data were analyzed using SPSS version 15 (SPSS Inc., Chicago, IL). Prior to the analysis of the specific study aims, appropriate descriptive statistics were calculated for all variables at each point in time. Descriptive statistics were used to characterize the sample demographically and clinically. Chi-square tests were used for categorical variables. Independent sample t-tests were used to compare continuous variables between exercisers and nonexercisers at each time point. In addition, Mann-Whitney U tests were used to evaluate for differences in exercise doses (i.e., frequency, duration, intensity) between exercisers and nonexercisers because of the expectation of skewness in their distributions. Two-tailed tests were used at an alpha of 0.05. Comparisons were made between patients who dropped out to those who did not to determine if differential bias existed on the outcomes because of dropout.
Results
Demographic Characteristics
Of the 252 women initially approached and screened, 119 women were eligible to participate. Detailed information of individuals who did not participate is described elsewhere.18 Major reasons for refusal included not interested, not eligible, or too busy. No significant differences were found in any demographic variables between participants who completed and dropped out of the study.18
As shown in Table 1, 44.1% of the participants were classified as exercisers at T1. No differences were found at any time point in demographic and clinical/treatment characteristics between exercisers and nonexercisers. At T1, participants were asked if they were involved in a regular exercise program. A significantly higher number of participants in the exerciser group responded “yes” to this question (P<0.005). This pattern continued at T2 and T3. No difference was found in the proportion of exercisers and nonexercisers who received RT after their initial course of CTX.
Table 1.
Differences in Demographic and Clinical Characteristics across Time between Exercisers and Nonexercisers
| All patients (N=119) |
T 1c | T2d | T 3e | ||||
|---|---|---|---|---|---|---|---|
| M (SD) | Nonexerciser n=65 |
Exerciser n=52 |
Nonexerciser n=56 |
Exerciser n=46 |
Nonexerciser n=32 |
Exerciser n=70 |
|
| Age. year | 50.5 (9.4) | 50.54 (9.51) | 50.63 (9.34) | 50.53 (10.24) | 49.76 (8.81) | 51.63 (11.13) | 49.66 (8.89) |
| Education, year | 16.2 (2.8) | 16.2 (2.9) | 16.2 (2.8) | 16.4 (2.8) | 16.2 (2.9) | 16.3 (3.1) | 16.3 (2.7) |
| Study period | |||||||
| Days | |||||||
| T1–T2 | 169 (64.6) | 165.3(64.7) | 172.8 (65.2) | 164.4(61.8) | 173.2 (66.2) | 166.0 (48.7) | 153.93 (43.87) |
| T2–T3 | 164.8 (60.8) | 159 (61.3) | 171.63 (60) | 155.6 (54.3) | 174.6 (66.7) | 167.1 (68.4) | 169.9 (65.7) |
| Karnofsky Performance | 87.63 (9.4) | 85.5 (9.9) | 90.5 (7.9)a | 84.4 (9.2) | 85.4 (9.4) | 87.8 (11.6) | 89.1 (9.6) |
| n (%) | |||||||
| Marital Status (married) | 80 (68.4) | 43 (66.2) | 36 (70.6) | 36 (65.5) | 34 (73.9) | 20 (62.5) | 51 (73.9) |
| Ethnicity | |||||||
| White | 88 (75.2) | 45 (69.2) | 42 (82.4) | 41 (74.5) | 35 (76.1) | 24 (75) | 55 (79.7) |
| Employed (full or part time) | 50 (44.2) | 25 (39.1) | 24 (50) | 39 (69.6) | 31 (37.4) | 26 (83.9) | 52 (75.4) |
| Income (≥40,000) | 94 (83.2) | 48 (77.4) | 45 (90) | 43 (82.7) | 38 (84.4) | 24 (80) | 56 (82.4) |
| Menopausal Status | |||||||
| Premenopausal | 40 (36.7) | 23 (37.1) | 17 (37) | 5 (9.4) | 4(9.5) | 2 (7.1) | 7 (10.8) |
| Perimenopausal | 19 (17.4) | 13 (21) | 6 (13) | 23 (43.4) | 20 (47.6) | 6 (21.4) | 16 (24.6) |
| Postmenopausal | 50 (45.9) | 26 (41..9) | 23 (50) | 25 (47.2) | 18 (42.9) | 20 (71.4) | 42 (64.6) |
| Sequential chemotherapy then radiation therapy | 59 (49.6) | 27 (40.9) | 32 (61.5) | 30 (53.6) | 26 (56.5) | 17 (53.1) | 38 (54.3) |
| Participate regular exercise program | 75 (64.7) | 28 (43.8) | 47 (92.2)b | 27 (49.1) | 39 (84.8)b | 11(35.5) | 52 (75.4) b |
| Cancer Stage | |||||||
| I | 40 (36) | 27 (43.5) | 13 (27.1) | 22 (40.7) | 17 (41.5) | 11 (36.7) | 12 (42.2) |
| II | 52 (46.8) | 24 (38.7) | 27 (56.3) | 26 (48.1) | 16 (39) | 13 (42.3) | 27 (42.2) |
| III | 19 (17.1) | 11 (17.7) | 8 (16.7) | 6 (11.1) | 8 (19.5) | 6 (20) | 10 (15.6) |
P <0.05;
P < 0.01
T1: The week before 2nd chemotherapy treatment,
T2: 4–6 months after T1
T3: Approximately 1 year after T1
Frequency, Duration, and Intensity of Exercise Dose at Three Time Points
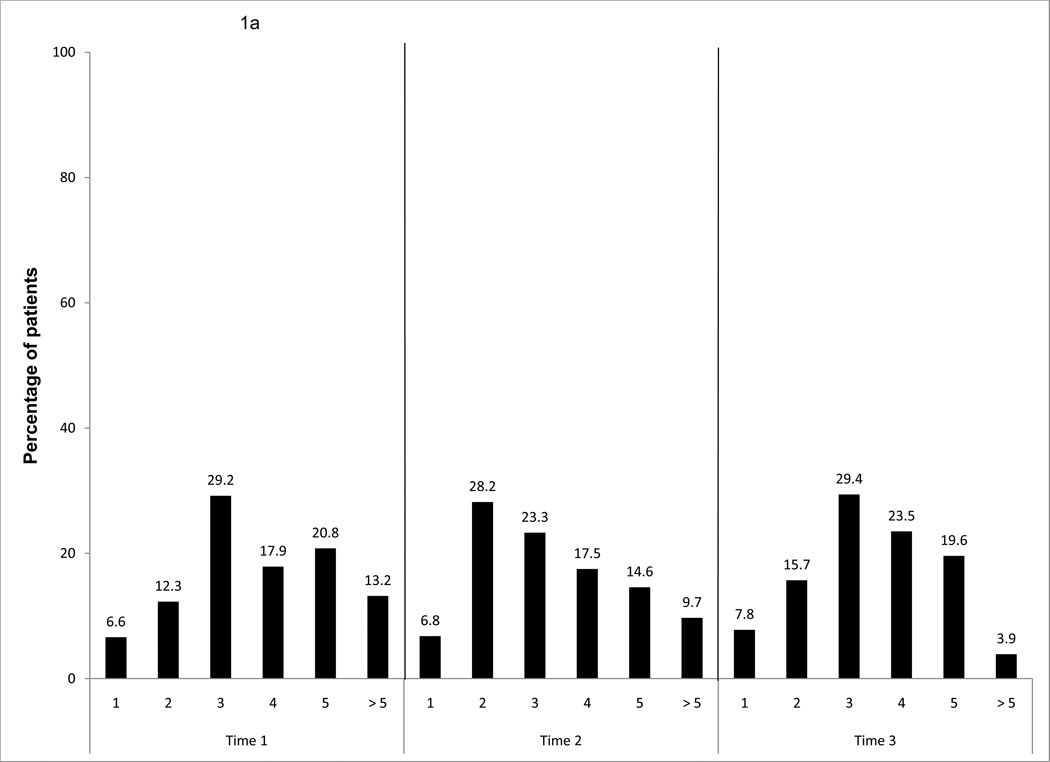
In the total sample, in terms of frequency of exercise dose, more than 80% of the sample exercised more than three times a week. The frequency of exercise decreased to twice a week during cancer treatment (T2), and then it increased to three times a week after completion of cancer treatment (T3) (Fig. 1a).
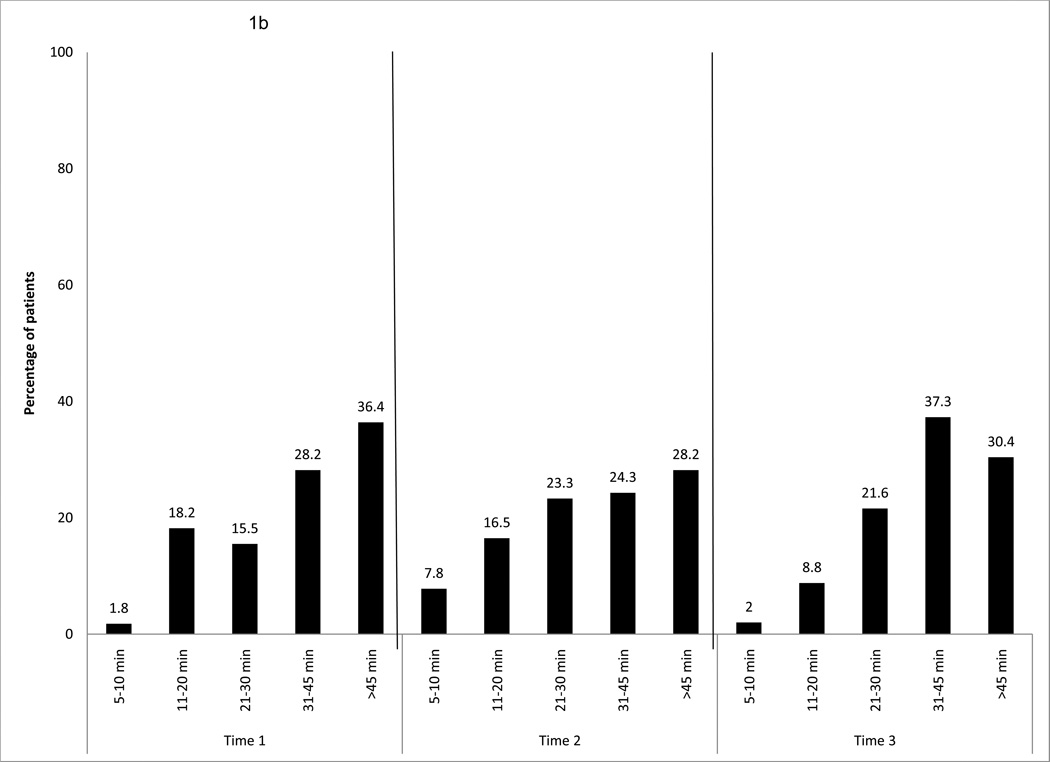
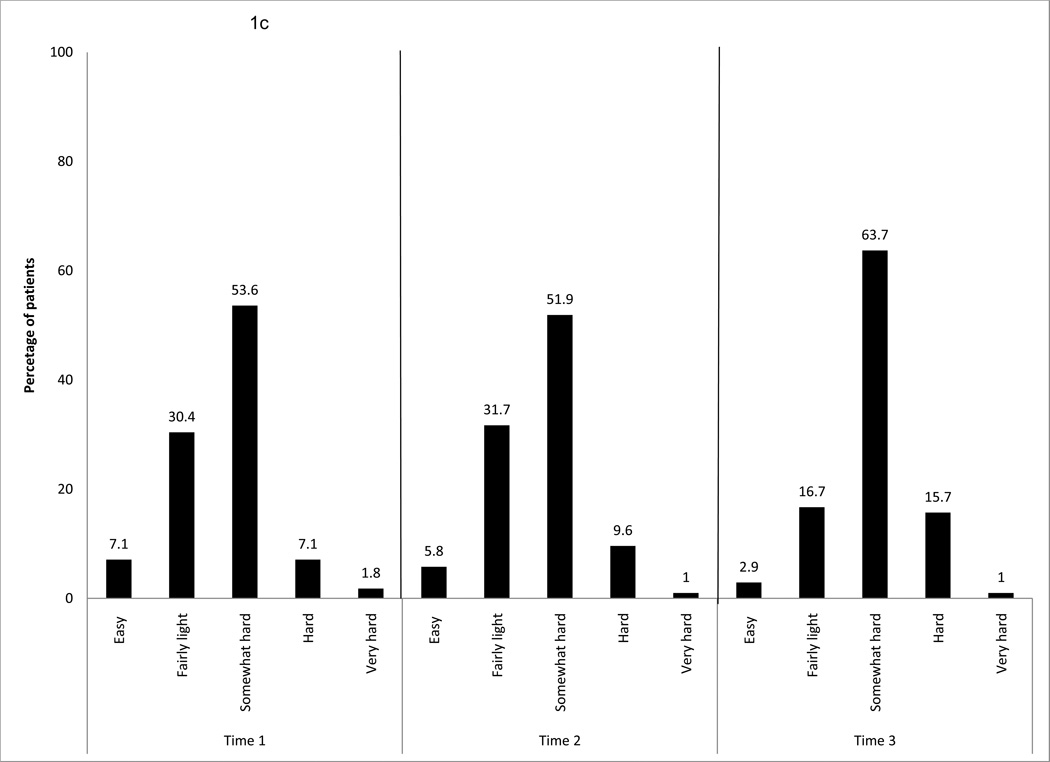
Figure 1.
a. Frequency of exercise behavior per week, over time for the entire sample
b. Duration of exercise behavior per week, over time for the entire sample
c. Intensity of exercise behavior per week, over time for the entire sample
In terms of duration of exercise per session at T1, about 80% of the sample exercised for more than 20 minutes per session and approximately 36% did more than 45 minutes per session. At T2, similar percentages were reported for duration, and more than 45 minutes per session was still the highest percentage. At T3, about 90% of the sample exercised more than 20 minutes per session, and 37% exercised 31 to 45 minutes per session (Fig. 1b).
The intensity rating of “somewhat hard” had the highest percentage among all of the participants at all three time points (54% to 64%). At T1 and T2, a similar pattern was found, with 30% of the sample at the “fairly light” level and 52% at the “somewhat hard” level of intensity. At T3, the rate increased to 64% of the sample exercising at the “somewhat hard” intensity level (Fig. 1c).
As to the mode or type of aerobic exercise, various types of aerobic exercise were reported: walking (72%–88%), bicycling (14%–26%), jogging (8.5%–16.5%), and swimming (6%–16%). Walking was the most popular mode of aerobic exercise in the total sample at all three time points. Approximately 50% of the participants at each time point (44% to 57%), were involved in additional “other” types of exercise on a regular basis during the study period. These various “other” exercises included aerobic exercises, the elliptical trainer, the Stairmaster®, or a combination of aerobic exercise with yoga.
As a total sample, a one group t-test was used to evaluate each of the exercise dose characteristics against the minimum criteria of ACS recommendations (three times per week, 20 minutes per session, and moderate intensity). The study participants exercised significantly more frequently (P ≤0.019) and longer (P < 0.0005) than the recommended levels (more than three times per week per week, 20 minutes per session), but less than the recommended level regarding intensity (“moderate”). They exercised significantly less hard at T1 and T2 (P <0.0005). No significant differences in intensity were found at T3 (P = 0.48).
A description of exercise doses of the exercisers and nonexercisers at each time point is shown in Table 2. The majority of the exercisers exercised more than three times a week, for more than 30 minutes per session, at a moderate intensity level at each time point. The majority of nonexercisers exercised twice a week, less than 20 minutes per session, and at a mild intensity level at each time point.
Table 2.
Differences over time in exercise behaviors between exercisers and nonexercisers
| Nonexerciser | Exerciser | ||||||
|---|---|---|---|---|---|---|---|
| T1 (n=66) | T2(n=56) | T3(n=32) | T1(n=52 | T2(n=46) | T3(n=70) | ||
| % | % | ||||||
| Frequency (How often/week) | 1 | 13 | 12.5 | 25 | 0 | 0 | 0 |
| 2 | 24.1 | 51.8 | 50 | 0 | 0 | 0 | |
| 3 | 27.8 | 21.4 | 18.8 | 30.8 | 26.1 | 31.4 | |
| 4 | 7.4 | 1.8 | 0 | 28.8 | 37 | 34.3 | |
| 5 | 9.8 | 8.9 | 3.1 | 32.7 | 21.7 | 27.1 | |
| >5 | 18.5 | 3.6 | 3.1 | 7.7 | 15.7 | 43 | |
| Duration (minutes/session) | 1–10 | 3.4 | 14.3 | 6.3 | 0 | 0 | 0 |
| 11–20 | 34.5 | 30.4 | 28.1 | 0 | 0 | 0 | |
| 21–30 | 19 | 25 | 37.5 | 11.5 | 21.7 | 14.3 | |
| 31–45 | 25.9 | 14.3 | 21.9 | 30.8 | 34.8 | 44.3 | |
| >45 | 17.2 | 16.1 | 6.3 | 57.7 | 43.5 | 41.4 | |
| Intensity (how hard/per session) | Easy | 13.3 | 10.7 | 9.4 | 0 | 0 | 0 |
| Mild | 56.7 | 58.9 | 53.1 | 0 | 0 | 0 | |
| Moderate | 30 | 26.8 | 37.5 | 80.8 | 80.4 | 75.7 | |
| High | 0 | 3.6 | 0 | 15.4 | 17.4 | 22.9 | |
| Very high | 0 | 0 | 0 | 3.8 | 2.2 | 1.4 | |
Comparison of Symptom Severity Between Exercisers and Nonexercisers
At T1, the exercisers had significantly lower total fatigue scores and lower behavioral and sensory fatigue subscale scores than the nonexercisers (P = 0.037, 0.018, 0.04; respectively) (Table 3). The exercisers reported significantly lower depression scores than the nonexercisers (P =0.038). However, the depression scores for both groups were lower than the cutoff score of 16 for being at risk for depression.52 No significant statistical differences in sleep disturbance or pain (P = 0.49, 0.88, respectively) were found between exercisers and nonexercisers. In both groups, the mean sleep disturbance score was above the cutoff of ≥ 43 for a clinically significant level of sleep disturbance.48
Table 3.
Differences over time in fatigue, depression, sleep disturbance and pain between exercisers and nonexercisers
| T1 | T2 | T3 | |||||
|---|---|---|---|---|---|---|---|
| Nonexerciser | Exerciser | Nonexerciser | Exerciser | Nonexerciser | Exerciser | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Total | 3.58 (2.14) | 2.77 (0.037)a | 3.74 (1.99) | 3.1 (2.15) | 2.8 (2.29) | 3.04 (1.86) | |
| Behavioral | 3.03 (2.53) | 1.99 (0.18) a | 3.17 (2.53) | 2.65 (2.37) | 2.09 (2.49) | 2.01(2.07) | |
| Fatigue | Affective | 3.74 (2.63) | 3.05 (2.32) | 3.99 (2.46) | 3.43 (2.84) | 2.96 (2.84) | 3.38 (2.3) |
| Sensory | 4.02 (2.62) | 3.07 (0.16) a | 3.99 (2.42) | 3.25 (2.56) | 3.2 (2.31) | 3.37 (2.23) | |
| Cognitive/Mood | 3.33 (2.24) | 2.62 (0.07) | 3.68 (2.04) | 2.87 (0.047)a | 2.92 (2.18) | 2.97 (1.82) | |
| Depression | 13.10 (8.16) | 10.08 (0.038) a | 13.95 (9.32) | 10.11 (0.047)a | 10.59 (9.84) | 11.58 (9.84) | |
| Sleep Disturbance | 47.49 (20.53) | 44.95 (17.93) | 49.55 (20.08) | 45.08 (21.09) | 43.42 (19.42) | 44.84 (20.07) | |
| Pain | 1.61 (2.5) | 1.54 (2.45) | 2.38 (2.64) | 2.43 (2.73) | 2.34 (2.47) | 2.17 2.36) | |
P < 0.05
At T2, the exercisers reported significantly lower cognitive/mood fatigue subscale scores than the nonexercisers (P = 0.047). The exercisers had significantly lower depression scores than the nonexercisers (P =0.047) and both groups’ depression scores were lower than the cutoff score of 16. No significant differences were found in sleep disturbance (P = 0.28) and pain (P = 0.91) scores between the exercisers and nonexercisers.
At T3, no significant differences were found in any symptom severity scores between the two groups. Participants in both groups reported mild fatigue, pain, and sleep disturbance at each time point but were not at risk for depression at any of the three time points.
Discussion
Few studies have examined the characteristics of exercise dose longitudinally in the same women both during and after cancer treatment. Of note, at each time point, the women who were categorized as exercisers exceeded ACS exercise guideline levels. In addition, the average fatigue score at the beginning of cancer treatment (T1) in this group was significantly lower as were the behavioral and sensory fatigue subscale scores. However, this difference was not maintained at the completion of cancer treatment (T2) or six months after completion of cancer treatment (T3). In addition, the difference in fatigue scores between the exercisers and nonexercisers at T1 may not represent a clinically meaningful difference. Piper et al. suggested that a one unit change in fatigue score (e.g., from 3 to 4, on a 0 to 10 scale) represented a clinically significant change in fatigue.41
Past Exercise Experience
One of the study findings of past exercise experience is that women who participated in a regular exercise program before the beginning of CTX were likely to continue their exercise activities during and after cancer treatment. At T1, approximately 65% of participants were engaged in a regular exercise program. As would be expected, a significantly higher percentage of women who were participating in a regular exercise program were classified in the exerciser group at all three time points (P <0.0005). This finding is consistent with work by Courneya and colleagues who found that one of the strongest independent predictors of exercise contamination in a wait-list control group was past exercise experience.25 In addition, in another study, baseline physical activity predicted mean number of steps per week over the 12 weeks of a home-based exercise program in 43 breast cancer survivors.19 Taken together, these findings suggest that patients who are engaged in physical activity prior to cancer treatment are more likely to continue to exercise both during and after cancer treatment. Additional research is warranted to determine how and why previous exercise experience facilitates behavior changes, especially during a stressful time such as receiving cancer treatment.
Exercise Doses and Adherence to Exercise Interventions
The use of various definitions of exercise adherence, as well as different operational definitions of exercise adherence, makes direct comparisons across studies difficult. However, the exercise adherence rates during or after cancer treatment in this study are similar to previous reports for both home-based and supervised exercise that ranged from approximately 60% to 98%.21, 25, 26, 55–62 Using the ACS exercise guidelines, 50% of the patients in this study adhered to these guidelines during cancer treatment. Of note, 70% of these patients adhered to these guidelines after cancer treatment, similar to percentages reported in previous studies that followed patients for a much shorter period of time.21, 60, 63 While the nonexericsers did not meet all three minimum exercise dose characteristics, most of them performed some physical activities.
Exercise Doses and Symptom Severity
Several studies have demonstrated that increased levels of physical activity during and after cancer treatment are associated with decreased fatigue severity, increased physical performance, and improved quality of life.1, 10, 64–66 In this study, improvements in fatigue and depression at the beginning (T1) and completion of the cancer treatment (T2) were found in the exercisers. It is still not entirely clear why the exercisers who had exercised more than the ACS recommendations both during and after cancer treatment did not differ significantly from the nonexercisers. In all patients, fatigue severity remained in the mild range throughout the study. One explanation may be the “floor effect” of low fatigue and other symptom severity levels at the beginning of the study. Namely, a limited opportunity existed for the exercise dose to demonstrate a significant reduction in fatigue or other symptoms in this sample.18
According to McNeely’s meta analysis,5 the pooled results of the six studies that examined the effect of exercise on fatigue showed statistically significant improvements in this symptom in only two studies.5, 65, 67 Both studies evaluated the effects of exercise following primary cancer treatment. During adjuvant cancer treatment, exercise had no effect on fatigue severity. The evidence suggests that exercise does not decrease fatigue in women undergoing adjuvant cancer treatment. Although the effects were nonsignificant in four studies,21, 60, 68–69 all point estimates were in favor of exercise, which suggests the need for more research on the optimal dose and timing of exercise before this approach to reducing fatigue severity is rejected. Since fatigue is reported to be associated with sleep, depression, and pain, one can intuitively consider that improving fatigue might result in reductions in other symptoms.70 Several studies found improvements in fatigue, depression, and sleep, but not in pain, with regular exercise.14, 71–75
Several study limitations are worth noting: self-report of exercise activities; demographic characteristics; and data collection time point. This study data were obtained through self-report, without a supplemental objective measure of exercise (e.g., pedometer). Several demographic characteristics were reported as the determinants of physical exercise in cancer patients, such as higher education level, lower body mass index76 and women with breast cancer.16 These characteristics in our sample limit the generalizability of the study findings. Furthermore, the exercise data were collected at only three time points over 12 months and not continuously.
The use of a self-report exercise dose supplemented with an objective measure was reported recently. Swenson et al.77 described a physical activity protocol for 36 patients with breast cancer receiving CTX. This study used a pedometer that captured home-based exercise and daily chores. The participants were asked to complete 10,000 steps daily. The mean (standard deviation) number of steps per day for the initial six-week period was 7,363±2,421, and increased after each CTX cycle. The adherence rate with this protocol was 74%. As might be expected, participants who reported more fatigue and hours spent sleeping/reclining at baseline were less adherent with the study protocol.77
Although a considerable amount of research on exercise in cancer patients exists, several issues remain to be investigated including: a need to incorporate more frequent and thorough measures of physiological and psychological outcomes; analysis of changes in exercise dose and their causes during and after cancer treatment; the relationship between duration of regular exercise dose and symptoms; and optimal dose and timing of exercise during and after cancer treatment. Additional research is warranted on the barriers to exercise not only during but also following cancer treatment.
Lastly, since participation in a regular exercise program at baseline and all three time points was almost universal in the exercise group, it is important to consider a number of issues related to an exercise prescription. These issues include: initial assessment of individual patients, monitoring and ensuring the safety and efficacy of the exercise program, and encouraging maintenance of the exercise program during and after cancer treatment. Different intervention strategies may be needed for the previous exercisers to maintain their activities during cancer treatment, and for the previous nonexercisers to initiate and sustain their activities during cancer treatment.
Acknowledgments
This work was funded by NIH NCI grant CA83316, and supported by NIH/NCRR UCSF-GCRC (currently named Clinical and Translational Science Institute-CTSI) grant number MO1 RR00079. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
The authors thank Ambar Munoz for assisting in preparation of the paper and to the patients who gave so generously of their time.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflicts of interest.
References
- 1.Galvao DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005;23(4):899–909. doi: 10.1200/JCO.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007;26(6):660–667. doi: 10.1037/0278-6133.26.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005;23(16):3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- 4.Markes M, Brockow T, Resch KL. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2006;(4):CD005001. doi: 10.1002/14651858.CD005001.pub2. [DOI] [PubMed] [Google Scholar]
- 5.McNeely ML, Campbell KL, Rowe BH, et al. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz KH, Holtzman J, Courneya KS, et al. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 7.Thorsen L, Courneya KS, Stevinson C, Fossa SD. A systematic review of physical activity in prostate cancer survivors: outcomes, prevalence, and determinants. Support Care Cancer. 2008;16(9):987–997. doi: 10.1007/s00520-008-0411-7. [DOI] [PubMed] [Google Scholar]
- 8.MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patients' functional capacity. Nurs Res. 1989;38(6):348–351. [PubMed] [Google Scholar]
- 9.Schwartz AL, Mori M, Gao R, Nail LM, King ME. Exercise reduces daily fatigue in women with breast cancer receiving chemotherapy. Med Sci Sports Exerc. 2001;33(5):718–723. doi: 10.1097/00005768-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21(9):1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 11.Courneya KS, Vallance JKH, McNeely ML, et al. Exercise issues in older cancer survivors. Crit Rev Oncol Hematol. 2004;51:249–261. doi: 10.1016/j.critrevonc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Holtzman J, Schmitz K, Babes G, et al. Effectiveness of behavioral interventions to modify physical activity behaviors in general populations and cancer patients and survivors. Evid Rep Technol Assess (Summ) 2004;(102):1–8. doi: 10.1037/e439832005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingram C, Courneya KS, Kingston D. The effects of exercise on body weight and composition in breast cancer survivors: an integrative systematic review. Oncol Nurs Forum. 2006;33(5):937–947. doi: 10.1188/06.ONF.937-950. quiz 948–950. [DOI] [PubMed] [Google Scholar]
- 14.Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Med Sci Sports Exerc. 2002;34(12):1863–1867. doi: 10.1097/00005768-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Hacker E. Exercise and quality of life: strengthening the connections. Clin J Oncol Nurs. 2009;13(1):31–39. doi: 10.1188/09.CJON.31-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 17.Rogers LQ, Hopkins-Price P, Vicari S, et al. Physical activity and health outcomes three months after completing a physical activity behavior change intervention: persistent and delayed effects. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1410–1418. doi: 10.1158/1055-9965.EPI-08-1045. [DOI] [PubMed] [Google Scholar]
- 18.Dodd MJ, Cho MH, Miaskowski C, et al. A randomized controlled trial of home-based exercise for cancer-related fatigue in women during and after chemotherapy with or without radiation therapy. Cancer Nurs. 2010;33(4):245–257. doi: 10.1097/NCC.0b013e3181ddc58c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinto BM, Rabin C, Dunsiger S. Home-based exercise among cancer survivors: adherence and its predictors. Psychooncology. 2009;18(4):369–376. doi: 10.1002/pon.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 21.Mock V, Frangakis C, Davidson N, et al. Exercise manages fatigue during breast cancer treatment: a randomized controlled trial. Psychooncology. 2005;14(6):464–477. doi: 10.1002/pon.863. [DOI] [PubMed] [Google Scholar]
- 22.Griffith K, Wenzel J, Shang J, et al. Impact of a walking intervention on cardiorespiratory fitness, self-reported physical function, and pain in patients undergoing treatment for solid tumors. Cancer. 2009;115(20):4874–4884. doi: 10.1002/cncr.24551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin ML, Crumley D, McTiernan A, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97(7):1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santacroce SJ, Maccarelli LM, Grey M. Intervention fidelity. Nurs Res. 2004;53(1):63–66. doi: 10.1097/00006199-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Courneya KS, Friedenreich CM, Sela RA, et al. Exercise motivation and adherence in cancer survivors after participation in a randomized controlled trial: an attribution theory perspective. Int J Behav Med. 2004;11(1):8–17. doi: 10.1207/s15327558ijbm1101_2. [DOI] [PubMed] [Google Scholar]
- 26.Kim CJ, Kang DH, Smith BA, Landers KA. Cardiopulmonary responses and adherence to exercise in women newly diagnosed with breast cancer undergoing adjuvant therapy. Cancer Nurs. 2006;29(2):156–165. doi: 10.1097/00002820-200603000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Glass JM, Lyden AK, Petzke F, et al. The effect of brief exercise cessation on pain, fatigue, and mood symptom development in healthy, fit individuals. J Psychosom Res. 2004;57(4):391–398. doi: 10.1016/j.jpsychores.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Lennon SL, Quindry J, Hamilton KL, et al. Loss of exercise-induced cardioprotection after cessation of exercise. J Appl Physiol. 2004;96(4):1299–1305. doi: 10.1152/japplphysiol.00920.2003. [DOI] [PubMed] [Google Scholar]
- 29.Friedman LM, Furberg CD, DeMets DL. Fundamentals of clinical trials. 3rd ed. New York, NY: Springer; 1998. [Google Scholar]
- 30.Office of the U.S. Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 1996. Physical activity and health: a report of the surgeon general. [Google Scholar]
- 31.Humpel N, Iverson DC. Review and critique of the quality of exercise recommendations for cancer patients and survivors. Support Care Cancer. 2005;13(7):493–502. doi: 10.1007/s00520-005-0811-x. [DOI] [PubMed] [Google Scholar]
- 32.Dodd MJ, Miaskowski C. The PRO-SELF Program: a self-care intervention program for patients receiving cancer treatment. Semin Oncol Nurs. 2000;16(4):300–308. doi: 10.1053/sonu.2000.16586. discussion 308–316. [DOI] [PubMed] [Google Scholar]
- 33.American College of Sports Medicine. Guidelines for exercise testing and prescription. 5th ed. Philadelphia: Williams & Wilkins; 1995. [Google Scholar]
- 34.Dodd MJ, Painter P, Miaskowski C, et al. Exercise: a feasibility study of an intervention for fatigue in cancer patients: P30 feasibility study. San Francisco: Research Center for Symptom Management, University of California; 1996–1997. [Google Scholar]
- 35.Painter P, Stewart A, Dibble S, et al. Exercise intervention after kidney transplant RO1 NR 02880. Bethesda, MD: National Institutes of Health, National Institute of Nursing Research; 1993–1996. [Google Scholar]
- 36.Dodd MJ, Painter P, Miaskowski C, et al. Exercise: an intervention for fatigue in cancer patients. P30 Pilot Study NR 03927. Bethesda, MD: National Institutes of Health, National Institute of Nursing Research; 1997–1998. [Google Scholar]
- 37.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2(3):187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 38.Hyde LK, Wolf J, McCracken S, Yesner M. Natural course of inoperable lung cancer. Chest. 1973;64(3):309–312. doi: 10.1378/chest.64.3.309. [DOI] [PubMed] [Google Scholar]
- 39.Karnofsky D. Performance scale. In: Kennealey GT, Mitchell MS, editors. Factors that influence the therapeutic response in cancer: a comprehensive treatise. New York: Plenum Press; 1977. [Google Scholar]
- 40.Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53(9):2002–2007. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 41.Piper BF, Dibble SL, Dodd MJ, et al. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25(4):677–684. [PubMed] [Google Scholar]
- 42.Winningham M, MacVicar M. The effects of aerobic exercise on patient reports of nausea. Oncol Nurs Forum. 1998;15:447–450. [PubMed] [Google Scholar]
- 43.Dimeo FC, Tilmann MH, Bertz H, et al. Aerobic exercise in the rehabilitation of cancer patients after high dose chemotherapy and autologous peripheral stem cell transplantation. Cancer. 1997;79(9):1717–1722. [PubMed] [Google Scholar]
- 44.Young-McCaughan S, Sexton DL. A retrospective investigation of the relationship between aerobic exercise and quality of life in women with breast cancer. Oncol Nurs Forum. 1991;18(4):751–757. [PubMed] [Google Scholar]
- 45.Piper BF, Lindsey AM, Dodd MJ. Fatigue mechanisms in cancer patients: developing nursing theory. Oncol Nurs Forum. 1987;14(6):17–23. [PubMed] [Google Scholar]
- 46.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28(3):465–470. [PubMed] [Google Scholar]
- 47.Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992;15:493–498. doi: 10.1093/sleep/15.6.493. [DOI] [PubMed] [Google Scholar]
- 48.Lee KA, Gay CL. Sleep in late pregnancy predicts length of labor and type of delivery. Am J Obstet Gynecol. 2004;191(6):2041–2046. doi: 10.1016/j.ajog.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 49.Miaskowski C, Cooper BA, Paul SM, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33(5):E79–E89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 50.Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999;17(5):320–332. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 51.Pud D, Ben Ami S, Cooper BA, et al. The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. J Pain Symptom Manage. 2008;35(2):162–170. doi: 10.1016/j.jpainsymman.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 53.Dodd M, Cho MH, Cooper B, Miaskowski C. The effects of the symptom cluster of fatigue, sleep disturbance, depression, and pain on clinical outcomes in women during and after adjuvant cancer treatment for breast cancer. Eur J Oncol Nurs. 2010;14:101–110. doi: 10.1016/j.ejon.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4(1):2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 55.Mock V, Dow KH, Meares CJ, et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol Nurs Forum. 1997;24(6):991–1000. [PubMed] [Google Scholar]
- 56.Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657–665. doi: 10.1200/JCO.2001.19.3.657. [DOI] [PubMed] [Google Scholar]
- 57.Courneya KS, Mackey JR, Bell GJ, et al. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 58.Courneya KS, McKenzie DC, Reid RD, et al. Barriers to supervised exercise training in a randomized controlled trial of breast cancer patients receiving chemotherapy. Ann Behav Med. 2008;35:116–122. doi: 10.1007/s12160-007-9009-4. [DOI] [PubMed] [Google Scholar]
- 59.Courneya KS, Segal RJ, Gelmon K, et al. Six-month follow-up of patient-rated outcomes in a randomized controlled trial of exercise training during breast cancer chemotherapy. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2572–2578. doi: 10.1158/1055-9965.EPI-07-0413. [DOI] [PubMed] [Google Scholar]
- 60.Campbell A, Mutrie N, White F, McGuire F, Kearney N. A pilot study of a supervised group exercise program as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. Eur J Oncol Nurs. 2005;9:56–63. doi: 10.1016/j.ejon.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Wilson RW, Taliaferro LA, Jacobsen PB. Pilot study of a self-administered stress management and exercise intervention during chemotherapy for cancer. Support Care Cancer. 2006;14(9):928–935. doi: 10.1007/s00520-006-0021-1. [DOI] [PubMed] [Google Scholar]
- 62.Haykowsky MJ, Mackey JR, Thompson RB, Jones LW, Paterson DI. Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin Cancer Res. 2009;15(15):4963–4967. doi: 10.1158/1078-0432.CCR-09-0628. [DOI] [PubMed] [Google Scholar]
- 63.Courneya KS, Friedenreich CM, Sela RA, et al. The group psychotherapy and home-based physical exercise (group-hope) trial in cancer survivors: physical fitness and quality of life outcomes. Psychooncology. 2003;12(4):357–374. doi: 10.1002/pon.658. [DOI] [PubMed] [Google Scholar]
- 64.Mock V, Pickett M, Ropka M, et al. Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Pract. 2001;9(3):119–127. doi: 10.1046/j.1523-5394.2001.009003119.x. [DOI] [PubMed] [Google Scholar]
- 65.Courneya KS. Exercise in cancer survivors: an overview of research. Med Sci Sports Exerc. 2003;35(11):1846–1852. doi: 10.1249/01.MSS.0000093622.41587.B6. [DOI] [PubMed] [Google Scholar]
- 66.Courneya KS, Friedenreich CM, Quinney HA, et al. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care (Engl) 2003;12(4):347–357. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 67.Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;23(15):3577–3587. doi: 10.1200/JCO.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 68.Battagliani C. A randomized study on the effects of a prescribed exercise intervention on lean mass and fatigue changes in breast cancer patients during treatment. Greeley, CO: University of Northern Colorado; 2004. [Google Scholar]
- 69.Drouin J. Aerobic exercise training effects on physical function, fatigue and mood, immune status, and oxidative stress in subjects undergoing radiation treatment for breast cancer. Detroit: Wayne State University; 2002. [Google Scholar]
- 70.de Jong N, Candel MJ, Schouten HC, Abu-Saad HH, Courtens AM. Prevalence and course of fatigue in breast cancer patients receiving adjuvant chemotherapy. Ann Oncol. 2004;15(6):896–905. doi: 10.1093/annonc/mdh229. [DOI] [PubMed] [Google Scholar]
- 71.Pinto BM, Trunzo JJ, Reiss P, Shiu SY. Exercise participation after diagnosis of breast cancer: trends and effects on mood and quality of life. Psychooncology. 2002;11(5):389–400. doi: 10.1002/pon.594. [DOI] [PubMed] [Google Scholar]
- 72.Kolden GG, Strauman TJ, Ward A, et al. A pilot study of group exercise training (GET) for women with primary breast cancer: feasibility and health benefits. Psychooncology. 2002;11(5):447–456. doi: 10.1002/pon.591. [DOI] [PubMed] [Google Scholar]
- 73.Segar ML, Katch VL, Roth RS, et al. The effect of aerobic exercise on self-esteem and depressive and anxiety symptoms among breast cancer survivors. Oncol Nurs Forum. 1998;25(1):107–113. [PubMed] [Google Scholar]
- 74.Pinto BM, Maruyama NC. Exercise in the rehabilitation of breast cancer survivors. Psychooncology. 1999;8(3):191–206. doi: 10.1002/(SICI)1099-1611(199905/06)8:3<191::AID-PON355>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 75.Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Ann Behav Med. 1999;21(2):171–179. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- 76.Pickett M, Mock V, Ropka ME, et al. Adherence to moderate-intensity exercise during breast cancer therapy. Cancer Pract. 2002;10(6):284–292. doi: 10.1046/j.1523-5394.2002.106006.x. [DOI] [PubMed] [Google Scholar]
- 77.Swenson KK, Nissen MJ, Henly SJ. Physical activity in women receiving chemotherapy for breast cancer: adherence to a walking intervention. Oncol Nurs Forum. 2010;37(3):321–330. doi: 10.1188/10.ONF.321-330. [DOI] [PubMed] [Google Scholar]