Abstract
Background
Several Japanese studies have focused on identifying prognostic factors in patients with positive lymph nodes to predict recurrence rate and disease-free survival (DFS). However, different treatment protocol is followed in Japan compared with the European and American approach. This study was designed to investigate whether the number and/or location of lymph nodes predicts prognosis in patients with DTC treated with total thyroidectomy, lymph node dissection, and postoperative radioactive iodine ablation.
Methods
All 402 patients who were treated at the Department of Nuclear Medicine between 1998 and 2010 for DTC were reviewed. Patients were treated with (near) total thyroidectomy, lymph node dissection on indication, and postoperative I-131 ablation. Median follow-up was 49 (range, 10–240) months. Outcome measures were recurrence rate, disease-free survival, and mean time to recurrence.
Results
Ninety-seven patients had proven lymph node metastases. Recurrence rate was significantly higher in patients with positive lymph nodes in the lateral compartment vs. patients with lymph node metastasis in the central compartment (60 vs. 30%, p = 0.007). Disease-free survival and mean time to recurrence also were significantly shorter (30 vs. 52 months, p = 0.035 and 7 vs. 44 months, p = 0.004, respectively). The number of lymph nodes and extranodal growth were not significantly associated with the outcome measures used.
Conclusions
The location of positive lymph nodes was significantly correlated with the risk of recurrence and a shorter DFS. Hence, the TNM criteria are useful in subdividing patients based on risk of recurrence and DFS.
Introduction
Differentiated thyroid cancer (DTC) is the most common type of thyroid malignancies. The disease-related mortality for DTC is very low, but spread to the regional lymph nodes frequently occurs, especially in patients with papillary thyroid cancer [1, 2]. The TNM classification [3] describes the presence of positive cervical lymph nodes (CLN) as an independent risk factor for recurrence in all patients older than age 45 years with follicular thyroid cancer (FTC) and papillary thyroid cancer (PTC). The disease-related mortality, however, is not influenced by this parameter. The TNM classification further subdivides patients with positive lymph nodes into N1a, i.e., positive nodes in the central compartment (level VI), and N1b patients, i.e., with positive nodes in the lateral compartment (levels II-IV/V). N1b status in patients with DTC is thereby implied to result in worse disease-free survival (DFS).
Most of the research focused on DFS and recurrence rate related to the location and number of positive lymph nodes has been done by Ito et al. from Japan [4–7]. One of their most recent publications implicates that DFS of N1b patients is only significantly lower than that of N1a patients when: 1) lymph nodes are larger than 3 cm; 2) five or more positive lymph nodes are present in the lateral compartment; or 3) extranodal growth is present [5]. The main problem of applying results of these studies to the European and American population is the different treatment of patients with DTC in Japan, with a much lower rate of total thyroidectomies and the limited use of radioactive I-131 therapy. However, European and American guidelines are primarily based on the results of these Japanese studies [8, 9]. The purpose of this study was to investigate the impact of the location and the number of cervical lymph node metastases on recurrence rate and DFS in patients with DTC treated in accordance with European/American standards, with (near) total thyroidectomy and postoperative I-131 ablation.
Patients and methods
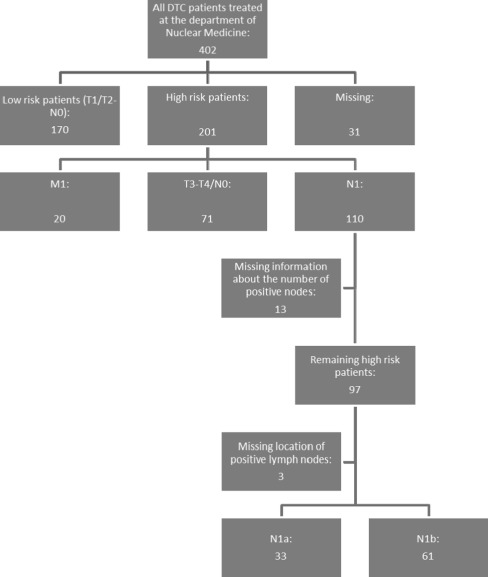
We performed a retrospective study in the University Medical Center Utrecht. All patients referred to our Nuclear Medicine department for I-131 ablation therapy after surgical treatment for well-DTC between January 1998 and April 2010 were reviewed (Fig. 1).
Fig. 1.
Flowchart of all patients with DTC
Low-risk patients were defined as patients with small tumors (T1–T2) without the presence of lymph node metastasis (N0). All other patients were defined as high-risk patients. This staging is in concordance with the seventh edition of the TNM AJCC staging criteria. Patients were staged after their initial surgery. Patients were excluded from analysis when distant metastasis were present or when the number of positive lymph nodes could not be determined.
Initial treatment
Patients were treated according to the known extent of disease at time of diagnosis. All patients were treated by (near) total thyroidectomy followed by I-131 thyroid remnant ablation. All patients received an ablative dosage varying from 3700 to 7400 mBq, 4–6 weeks after thyroid resection. Patients were not substituted with levothyroxine in the weeks ahead of ablation therapy to raise endogenous TSH production. Treatment traditionally consists of surgery followed by radioiodine ablation for every patient treated for DTC, regardless of size, extension or lymph node involvement in the Netherlands. Only dosages vary from 3700 MBq for low-risk patients to 7400 MBq for patients with distant metastasis.
Preoperative examination consisted of ultrasound of the neck and thorough physical examination. If enlarged lymph nodes were detected, a lymph node dissection was performed. Lymph node dissection also was performed based on operative findings suggestive of lymph node spread. All patients who were treated with lateral lymph node resection also had central neck dissection.
Laboratory analysis and diagnostic whole body scan
Blood samples were obtained at various moments during follow-up. On the day of administration of the ablative dosage, blood samples were obtained to measure the levels of thyroid stimulating hormone (TSH), thyroid hormone, thyroglobulin (Tg), and thyroglobulin antibodies (Tg-Ab).
Every 6 months, Tg level and Tg-Ab were measured without TSH stimulation and ultrasound of the neck was performed to screen for recurrent disease. TSH stimulated specimen (by LT4 withdrawal or after using intramuscular rhTSH injections) were drawn at least after 1.4 and 9 years with the simultaneous performance of a diagnostic whole body scan using 370 mBq I-131. Tg and TgAb levels were measured using the DYNOtest Tg-pluS (Brahms Diagnostica GmBH, Berlin, Germany). The functional sensitivity for this assay is 0.2 ng/mL.
Additional treatment
Based on findings during follow-up examination, some patients received additional treatment. Additional treatment included central (level VI) and/or lateral neck dissection (levels II–V), administration of therapeutic dosages of I-131, and external radiotherapy.
Measures of outcome
For this study, we defined three major measures of outcome: recurrence of disease, DFS, and mean time to recurrence. Patients were diagnosed with disease recurrence whenever one of the following features was present: cytological/histological evidence of newly developed disease, rising Tg or Tg-Ab levels in patients with previously undetectable levels or detectable Tg/Tg-Ab levels combined with positive ultrasound (cervical lymphadenopathy), diagnostic whole body or posttherapy scintigraphy, or PET-CT 9-12 months after initial therapy. Some of these patients already had detectable Tg level, neck ultrasound suspicious for lymph node recurrence, or uptake on postablation scan within 9–12 months after therapy and are therefore better referred to as patients with persistence disease.
Whenever one of these features remained positive during total follow-up, a patient was diagnosed as never free of disease. DFS was defined as the time in months without evidence of disease recurrence. Mean time to recurrence was calculated for all the patients diagnosed with recurrent disease.
Statistical analysis
Statistical analysis was performed by using SPSS® for Windows 15.0 (SPSS, Inc., Chicago, IL). For numeric variables, Pearson’s chi-squared test was used, whereas continuous variables were compared by using ANOVA. The Kaplan–Meier curve was used to compare DFS rates. p < 0.05 was considered to be statistically significant.
Results
As shown in the flowchart, a total of 402 patients with DTC were reviewed. A total of 97 patients (24%) had a known number of positive lymph nodes at time of initial diagnosis without the presence of distant disease and were available for analysis. Median follow-up was 49 (range, 10–240) range. Eighty-five percent of the patients with N1b status also had lymph node involvement of the central compartment. Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics of high-risk patients N1M0 (n = 97)
| Gender | |
| M | 38% (n = 37) |
| F | 62% (n = 60) |
| Mean age | 48 yr (S.D. ± 17) |
| Histology | Papillary: 96% |
| Follicular: 4% | |
| N stage | 1a: 34% (n = 33) |
| 1b: 63% (n = 61) | |
| No. of lymph nodes | 1–4: 66% (n = 66) |
| 5–9: 18% (n = 17) | |
| 10–14: 7% (n = 7) | |
| 15–19: 2% (n = 2) | |
| 20–24: 4% (n = 4) | |
| ≥25: 1% (n = 1) | |
| Mean preablative TSH (±SD) | 92 (±41) |
| Median follow-up (m) | 49 |
| Recurrence rate | 46% (n = 45) |
| Disease-related mortality | 4% (n = 7) |
Forty-five patients (46%) were diagnosed with recurrent disease. Most recurrences were of lymphatic origin with the involvement of regional and/or distant lymph nodes (83%) One third of the patients were treated with additional neck dissection, whereas the remaining patients were treated with one or more additional therapeutic dosages of I-131. Five patients were additionally treated with external radiotherapy.
The number of positive lymph nodes was not significantly related to the risk of recurrence, DFS, or time until recurrence for the whole group (Table 2). When analyzing only the group of patients with N1b status, the number of lymph nodes also did not significantly influence recurrence rate (p = 0.308). The only factor that significantly influenced recurrence rate and DFS in multivariate analysis was found to be the presence of positive lymph nodes in the lateral compartment. Thirty percent of the patients with positive central lymph nodes developed recurrent disease compared with 60% of the patients with positive lateral lymph nodes (p = 0.007).
Table 2.
Recurrence rate and DFS related to the number of positive lymph nodes
| No. of positive lymph nodes | Total patients | Recurrence rate | p value | Mean DFS (mo) | p value | Mean time to recurrence (mo) | p value |
|---|---|---|---|---|---|---|---|
| <5 | 66 | 50% | 0.879 | 37 | 0.85 | 16 | 0.428 |
| >5 | 31 | 51% | 36 | 7 | |||
| <10 | 83 | 47% | 0.145 | 37 | 0.764 | 15 | 0.494 |
| >10 | 14 | 69% | 33 | 6 | |||
| <15 | 90 | 49% | 0.414 | 38 | 0.306 | 14 | 0.711 |
| >15 | 7 | 67% | 18 | 7 |
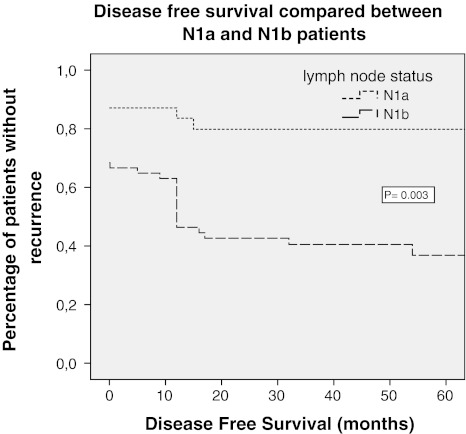
The mean DFS for N1a patients was 52 versus 30 months for N1b patients (p = 0.035). Also, the mean time to recurrence was significantly shorter in patients with positive nodes of the lateral compartment (7 vs. 44 months, p = 0.004, respectively). This difference also applied for patients with N1b status without the presence of central lymph nodes (p = 0.023).
All disease-related deaths (n = 4) were observed in the patients with N1b status; however, this was not statistically significant (p = 0.139), possibly due to the low number of patients. Results are shown in Table 3; Fig. 2.
Table 3.
Recurrence rate and DFS related to N stage
| N stage | Total patients | Recurrence rate | p value | Mean DFS (mo) | p value | Mean time to recurrence (mo) | p value |
|---|---|---|---|---|---|---|---|
| N1a | 33 | 30% | 0.007 | 52 | 0.035 | 44 | 0.004 |
| N1b | 61 | 60% | 30 | 7 |
Fig. 2.
Kaplan–Meier curve for disease-free survival
Discussion
The presence of lymph node spread at the time of diagnosis predicts a higher risk of recurrent disease for patients with well-DTC. We found that the location of the lymph nodes significantly influences recurrence rate, time to recurrence, and DFS. Patients with clinically evident lymph nodes of the lateral compartment have a significantly higher recurrence rate compared with patients with positive central lymph nodes.
Our research addresses the clinical implication of lymph node involvement in a European patient group. Most studies focusing on this subject are from Asia, especially Japan, where treatment strategy of patients is substantially different from the European method, whereas European and American treatment strategy is quite similar.
Results of our study can be used to further specify treatment and follow-up recommendations for the European and American population. Furthermore, results of Asian studies are similar, which might indicate that treatment can be performed adequately in different ways for patients with thyroid cancer.
We also showed that N1b status, in the large majority of cases, can be considered as more advanced disease compared with patients with only lymph node spread to the central compartment. Yet, at the same time our research shows that patients with the solitary involvement of lateral lymph nodes have a higher recurrence rate and shorter DFS.
Our results are in concordance with the findings of the Japanese studies, which found that the presence of clinically evident positive lymph nodes in the lateral compartment was a predictor of higher recurrence rate and shorter DFS [5, 10, 11].
The number of positive lymph nodes or the presence of extranodal growth did not contribute significantly to a worse DFS or higher recurrence rate in our study. This is in contradiction with the results of Ito et al., who found that the number (>5 positive nodes) and the presence of extranodal growth did significantly influence DFS. The study of Lee et al. [12] also investigated the prognostic significance of the number of positive nodes, but only in N1a patients; their preliminary results suggest that more than five positive lymph nodes in the central compartment may affect recurrence rate and DFS. Analysis of our N1a- and N1b-positive patients did not show a significant correlation between the number of lymph nodes and disease recurrence (p = 0.549, p = 0.308). Age and extranodal growth also did not show a significant correlation with recurrence rate (p = 0.165, p = 0.549). Size of the metastatic nodes also has been indicated as a prognostic factor by Ito et al. and Sugitani et al. [13]. Our data missed information about the size of positive lymph nodes and therefore could not be analyzed regarding this parameter.
Different explanations could exist for the different findings between our study and the studies performed in Asia. The main difference between our study population and the Japanese population is the nature of the treatment of thyroid cancer. Whereas routine ablation of the thyroid remnant is performed in Europe and the United States, it is not frequently used in Japan and other Asian countries. The Japanese guidelines for the treatment and follow-up of thyroid cancer also differ on various other points from the American and European guidelines [14]. Therefore, the results of Asian studies, especially about prognostic significance, follow-up, and treatment, are sometimes difficult to generalize to the European and American population.
The use of routine ablation therapy in western countries might be responsible for the different outcomes in respect to the prognostic significance of the number and growth type of positive lymph nodes between our study and the Japanese studies. The ablation therapy might compensate for a higher number of lymph nodes and the presence of extranodal growth in our patients, neutralizing it as a significant prognostic factor. The ablation dosages used in our study varied, but the current opinion in our institution is that low ablation dosages are just as effective higher dosages.
It is quite possible that the number of lymph nodes and the presence of extranodal growth do influence recurrence rate and DFS in the western population, but that our study population was too small to achieve statistical significance for these factors. Japanese treatment, especially lymph node resection, is performed more often and is usually more aggressive due to the less frequent use of radioactive iodine. More information about the lymph nodes is therefore collected and more variables can more easily be analyzed.
Limitations of our study include its retrospective nature. Randomized and prospective studies are, however, almost impossible to perform because of the large number of patients that should be included and the long follow-up period. In addition, a median follow-up of 49 months for high-risk patients may be considered relatively short, because recurrences can occur after 10 years. Even though most recurrences are identified in an early stage of disease (mean time of recurrence in our study was 14 months), studies with longer follow-up are necessary to support our findings. Our study, however, did analyze all patients treated in an academic institution during the course of more than 12 years.
With this study, we confirm the adequacy of the seventh edition of the AJCC Cancer Staging Manual concerning the subdivision of patients based on lymph node status (N1a vs. N1b). Other factors, such as the number of positive lymph nodes or the presence of extranodal growth, could not be confirmed as prognostic factors that significantly influence the recurrence rate and DFS in our study population.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Baek SK, Jung KY, Kang SM, et al. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid. 2010;20(2):147–152. doi: 10.1089/thy.2008.0243. [DOI] [PubMed] [Google Scholar]
- 2.Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16(12):1229–1242. doi: 10.1089/thy.2006.16.1229. [DOI] [PubMed] [Google Scholar]
- 3.Edge SB. AJCC cancer staging manual. 7. New York: Springer; 2010. [Google Scholar]
- 4.Ito Y, Tomoda C, Uruno T, et al. Ultrasonographically and anatomopathologically detectable node metastases in the lateral compartment as indicators of worse relapse-free survival in patients with papillary thyroid carcinoma. World J Surg. 2005;29(7):917–920. doi: 10.1007/s00268-005-7789-x. [DOI] [PubMed] [Google Scholar]
- 5.Ito Y, Miyauchi A. Lateral lymph node dissection guided by preoperative and intraoperative findings in differentiated thyroid carcinoma. World J Surg. 2008;32(5):729–739. doi: 10.1007/s00268-007-9315-9. [DOI] [PubMed] [Google Scholar]
- 6.Ito Y, Fukushima M, Tomoda C, et al. Prognosis of patients with papillary thyroid carcinoma having clinically apparent metastasis to the lateral compartment. Endocr J. 2009;56(6):759–766. doi: 10.1507/endocrj.K09E-025. [DOI] [PubMed] [Google Scholar]
- 7.Ito Y, Tomoda C, Uruno T, et al. Preoperative ultrasonographic examination for lymph node metastasis: usefulness when designing lymph node dissection for papillary microcarcinoma of the thyroid. World J Surg. 2004;28(5):498–501. doi: 10.1007/s00268-004-7192-z. [DOI] [PubMed] [Google Scholar]
- 8.Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154(6):787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 9.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 10.Ito Y, Higashiyama T, Takamura Y, et al. Risk factors for recurrence to the lymph node in papillary thyroid carcinoma patients without preoperatively detectable lateral node metastasis: validity of prophylactic modified radical neck dissection. World J Surg. 2007;31(11):2085–2091. doi: 10.1007/s00268-007-9224-y. [DOI] [PubMed] [Google Scholar]
- 11.Ito Y, Miyauchi A, Jikuzono T, et al. Risk factors contributing to a poor prognosis of papillary thyroid carcinoma: validity of UICC/AJCC TNM classification and stage grouping. World J Surg. 2007;31(4):838–848. doi: 10.1007/s00268-006-0455-0. [DOI] [PubMed] [Google Scholar]
- 12.Lee YS, Lim YS, Lee JC, et al. Clinical implication of the number of central lymph node metastasis in papillary thyroid carcinoma: preliminary report. World J Surg. 2010;34(11):2558–2563. doi: 10.1007/s00268-010-0749-0. [DOI] [PubMed] [Google Scholar]
- 13.Sugitani I, Kasai N, Fujimoto Y, et al. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004;135(2):139–148. doi: 10.1016/S0039-6060(03)00384-2. [DOI] [PubMed] [Google Scholar]
- 14.Takami H, Ito Y, Okamoto T, et al. Therapeutic strategy for differentiated thyroid carcinoma in Japan based on a newly established guideline managed by Japanese Society of Thyroid Surgeons and Japanese Association of Endocrine Surgeons. World J Surg. 2011;35(1):111–121. doi: 10.1007/s00268-010-0832-6. [DOI] [PubMed] [Google Scholar]