Abstract
Background
Alternatives to the available stavudine-containing paediatric fixed-dose combination (FDC) tablets are rapidly needed due to concerns regarding the cumulative toxicity of long-term stavudine exposure. We report the bioavailability and short-term safety of a novel paediatric FDC tablet of zidovudine (ZDV)/lamivudine (3TC)/nevirapine (NVP; 30/15/28 mg) in HIV-infected children.
Methods
In this Phase I/II open-label pharmacokinetic study, 42 children weighing 6–30 kg treated with NVP-based HAART for ≥4 weeks were randomized to receive the FDC tablets (GPO-VIR Z30) or the liquid formulations. Dosing was weight-based. Intensive 12-h blood sampling was performed after 2 weeks; subjects then crossed-over to the alternate formulation at equal doses and sampling repeated 2 weeks later. Pharmacokinetic parameters were determined by non-compartmental analysis. Buccal-swab samples were collected for cytochrome P450 (CYP)2B6 polymorphism analysis.
Results
With the FDC tablet, the geometric mean (90% CI) area under the curve (AUC) for ZDV, 3TC and NVP was 1.58 (1.49–1.68), 7.78 (7.38–8.19) and 68.88 (62.13–76.36) μg•h/ml, respectively. Rules for NVP therapeutic inadequacy were defined a priori, and despite lower NVP exposure with the tablet (P<0.001), the levels remained therapeutically adequate. ZDV AUC was similar between formulations. 3TC exposure was significantly higher with the tablet but comparable to historical data in adults and children taking branded tablets. While receiving the tablet, NVP AUC in children with CYP2B 516 GG (45%), GT (45%) and TT (10%) genotypes were 67.0, 74.5 and 106.4 μg•h/ml, respectively (P=0.04).
Conclusions
Disparities in drug exposure between formulations were observed; however, the FDC tablet delivered therapeutically adequate exposures of each drug and could well play an important role in simplifying antiretroviral treatment for children.
Introduction
In 2008, an estimated 2.1 million children <15 years old were living with HIV [1]. The introduction of affordable paediatric fixed-dose combination (FDC) tablets would expand antiretroviral treatment programs throughout the world. Indeed, the availability of antiretroviral FDCs for adults has simplified the treatment of HIV/AIDS and improved long-term drug adherence. Due to the absence of appropriate paediatric formulations, adult FDCs have been administered to children in many resource-limited settings. Although studies have demonstrated that cutting the adult FDC tablets resulted in acceptable drug levels and clinical response [2-4], effective and affordable paediatric FDCs are urgently needed [5].
As for adults, the first FDC tablets developed for children contained stavudine (d4T), lamivudine (3TC) and nevirapine (NVP). Several d4T/3TC/NVP paediatric FDC tablets have been developed [6], including a chewable tablet developed by the Thai Government Pharmaceutical Organization (GPO) [7]. In 2008, the World Health Organization (WHO) Paediatric Antiretroviral Working Group highlighted the need for affordable, safe and high-quality antiretroviral FDC formulations for paediatric use and proposed several priority FDC combinations to be developed [8]. In response, the Thai GPO developed a new paediatric FDC tablet, called GPO-VIR Z30, replacing d4T with zidovudine (ZDV), using a ratio of ZDV:3TC:NVP of 30:15:28 mg, in accordance with the initial ratio proposed by the WHO [5].
Access to paediatric FDC tablets that contain ZDV is timely as the WHO has recently recommended that countries phase out d4T and develop plans to move towards ZDV- or tenofovir-based first-line regimens [9]. In Thailand, antiretroviral treatment guidelines have already been modified to include ZDV in the preferred first-line non-nucleoside reverse transcriptase inhibitor-based HAART regimens for children [10].
We report the relative bioavailability and short-term safety of this novel paediatric FDC tablet in comparison to the individual branded liquid formulations in HIV-infected Thai children. Also, as accumulating evidence indicates that single nucleotide polymorphisms in the cytochrome P450 (CYP)2B6 gene affect NVP drug disposition [11] and clinical response in children [12], we assessed the influence of CYPB2B6 polymorphisms on NVP exposure.
Methods
The International Maternal Pediatric Adolescent AIDS Clinical Trial (IMPAACT) P1069 study (clinicaltrials. gov identifier NCT00672412) was a Phase I/II two-arm randomized open-label multiple dose pharmacokinetic (PK) cross-over study in HIV-infected Thai children ≥5 months to <13 years of age. HIV-infected children, clinically stable on NVP plus two nucleoside reverse transcriptase inhibitors (NRTIs) and receiving a maintenance dose of NVP for ≥4 weeks, were screened after the parent or legal guardian provided informed consent. Assent from the child was age-based and obtained following local ethical guidelines at each of the four clinical sites: ≥7 years old (two sites), ≥10 years old (one site) and exempt for all subjects (one site). Children who provided assent must have had their HIV status disclosed to them before the study was discussed with them. Children were excluded if, within 14 days prior to entry, there was a documented history of immunological failure or any of the following laboratory abnormalities: haemoglobin ≤8 g/dl (<2 years of age), haemoglobin ≤9 g/dl (≥2 years of age), platelets ≤75,000 mm3, aspartate aminotransferase or alanine aminotransferase or alkaline phosphatase >3× the upper limit of normal, creatinine >1 mg/dl, any other grade 3 or above laboratory toxicity, acute hepatitis due to any cause, pregnancy, chemotherapy, vomiting or diarrhoea above grade 2 within 30 days prior to entry, or active opportunistic infections. The study was approved by the ethics committees at the four participating hospitals, the Thai Ministry of Public Health and University of California (San Diego, La Jolla, CA, USA).
Study design
Children were stratified into four dosage groups based on their weight at study entry: group 1 included those ≥6–8 kg, group 2 >8–16 kg, group 3 >16–23 kg and group 4 >23–30 kg. At entry, children were randomized into one of two treatment arms: arm A received GPO-VIR Z30 paediatric tablets (ZDV/3TC/NVP; 30/15/28 mg/tablet) orally every 12 h and arm B received ZDV (Retrovir®), 3TC (Epivir®) and NVP (Viramune®) liquid formulations orally every 12 h. Weight band dosing was designed to achieve ZDV (360–480 mg/m2/day) and 3TC (4 mg/kg) dosing as close as possible to the recommended doses, while maintaining the NVP dose within the recommended range of 300–400 mg/m2/day (Table 1). For each child, the drug doses of the liquid formulations were equal to the individual drug components in the GPO-VIR Z30 tablets. At 2 weeks after randomization, intensive PK blood sampling was performed at the hospital. Prior to each PK blood sampling, the last six drug doses were closely monitored for drug adherence. During the 24 h immediately prior to each PK blood sampling, direct observed therapy was performed by a health care worker, while for the previous 48 h, drug adherence was confirmed via telephone by study staff. During the PK blood sampling, children were required to fast for ≥2 h before and 1 h after study drug administration. Blood samples (2 ml each) were collected at predose and 0.5, 1, 2, 4, 8 and 12 h post-dose. Immediately following the PK sampling, children were crossed-over to the alternate formulation at equal doses and identical blood sampling was performed 2 weeks later. Following the second PK study the children resumed the same antiretroviral drugs they received prior to enrolment and were followed for an additional 4 weeks to monitor safety. The Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (version 1.0) was used to grade all adverse events.
Table 1.
Administration of GPO-VIR Z30 tablets per dose by weight groups
| Group | Weight, kg | GPO-VIR Z30, tablets/dose | Children enrolled, n | ZDV dose, mg/m2/daya | 3TC dose, mg/kg/daya | NVP dose, mg/m2/daya |
|---|---|---|---|---|---|---|
| ≥6–8 kg | 6–6.9 | 2 | 4 | 343 (325–367) | 9.1 (8.7–9.9) | 320 (303–343) |
| 7–8 | 3 | 2 | 458 (427–489) | 11.9 (11.3–12.6) | 428 (398–457) | |
| >8–16 kg | >8–11.9 | 3 | 4 | 374 (337–423) | 9.0 (7.9–9.5) | 349 (315–395) |
| 12–16 | 4 | 8 | 391 (355–459) | 8.3 (7.6–9.8) | 365 (331–428) | |
| >16–23 kg | >16–16.9 | 4 | 1 | 334 (334–334) | 7.1 (7.1–7.1) | 312 (312–312) |
| 17–19.9 | 5 | 2 | 410 (393–426) | 8.3 (8.0–8.7) | 383 (367–398) | |
| 20–23 | 6 | 9 | 434 (404–449) | 8.7 (7.9–9.0) | 405 (377–419) | |
| >23–30 kg | >23–24.9 | 6 | 6 | 393 (377–400) | 7.6 (7.2–7.8) | 367 (352–373) |
| 25–30 | 7 | 6 | 419 (402–439) | 7.6 (7.1–8.2) | 391 (375–409) | |
| Overall | − | − | 42 | 403 (325–489) | 8.2 (7.1–12.6) | 376 (303–457) |
Values shown are median (range). NVP, nevirapine; ZDV, zidovudine; 3TC, lamivudine.
Antiretroviral drug level measurement and analysis
Blood samples were centrifuged and the plasma stored at -70°C. All plasma samples were sent to the PHPT-IRD Laboratory at the Faculty of Associated Medical Sciences, Chiang Mai University (Chiang Mai, Thailand) for drug level measurement using validated high performance liquid chromatography (HPLC) methods. NVP plasma levels were measured with a lower limit of quantification of 50 ng/ml [13]. A combined HPLC assay was used to measure ZDV and 3TC plasma levels and the lower limit of quantification was 25 ng/ml. The PHPT-IRD laboratory participates in the AIDS Clinical Trial Group, Pharmacology Quality Control programme [14]. PK analysis was performed with WinNonLin software (Version 5.2; Pharsight, Sunnyvale, CA, USA) using non-compartment methods. Individual area under the curve (AUC0–12; μg•h/ml) was determined by the linear trapezoidal rule and the maximum concentration (Cmax; μg/ml) and time to maximum concentration (h) were defined as the highest observed concentration and the time it occurred. The minimum concentration (Cmin; μg/ml) was defined as the lowest observed concentration within the intensive sampling period. Terminal elimination half-life (t½) was estimated for ZDV and 3TC using the equation t½ =Ln2/Kel (where Kel is the elimination rate constant), and oral clearance (CL/F) was calculated using the equation CL/F=AUC/dose. The NVP terminal t½ was not calculated due to the short duration of blood sampling (that is, <5 t½) and subsequently the apparent volume of distribution was not estimated.
Pharmacogenomic study
A buccal swab sample was collected from each child using a sterile cytobrush. DNA was extracted using a DNeasy kit (Qiagen, Valencia, CA, USA) and PCR amplifications were performed using two primer pairs corresponding to exon 9 and 4 of the CYP2B6 gene [15]. Restriction fragment length polymorphism RFLP analysis was performed using BglII for CYP2B6*6 (516G>T) and BsrI for CYP2B6*5 (1459C>T). Positive and negative controls were analysed in parallel. To verify the restriction fragment length polymorphism analysis, automated sequencing was performed.
Statistical methods
In determining therapeutic adequacy of the paediatric FDC tablet, a greater emphasis was placed on NVP, since the plasma concentrations of ZDV and 3TC are not precise surrogates for the active intracellular NRTI-triphosphate concentrations or virological response, and low NVP plasma concentrations are associated with poor virological suppression [16]. Sample size calculations were based on achieving therapeutically adequate exposures of NVP with the FDC tablet and it was required that the 90% CI for NVP AUC with the tablet lay completely within ±15% of the mean adult exposure (estimated by Boehringer Ingelheim at 63.6 μg•h/ml with a sd of 1.33). Accrual of 35 children would provide >80% power of affirming the tablets if the true NVP AUC was 63.6 μg•h/ml. To ensure that all weight ranges were represented, the enrolment was stratified by weight: group 1 ≥6–8 kg (n=5–8), group 2 >8–16 kg (n=9–12), group 3 >16–23 kg (n=9–12) and group 4 >23–30 kg (n=8–12).
Also, rules were defined a priori for NVP therapeutic inadequacy. If the 90% CI for the geometric mean NVP AUC lay either entirely below 70% of the reported adult NVP exposure of 63.6 μg•h/ml (that is, an AUC equivalent to 44.5 μg•h/ml) or entirely above the symmetrical upper boundary on a log10 scale (equivalent to 90.9 μg•h/ml) the NVP exposure delivered by the FDC tablet was declared therapeutically inadequate. The lower target of 70% was based on achieving a drug exposure equivalent to an average NVP concentration of 3.4 μg/ml, which is the recommended minimum trough level [17]. Given the absence of recommended minimum plasma NRTI concentrations, no a priori therapeutic inadequacy thresholds were defined for ZDV and 3TC. Historical drug exposure data in adult studies receiving branded tablets at the recommended dose were used as reference exposures for therapeutic adequacy. Wilcoxon signed rank tests were utilized to compare PK parameters of each drug between formulations. Among CYP2B6 genotype variants, NVP AUCs were compared using a general linear model analysis.
Results
A total of 42 children (18 males) were enrolled: 6 children in the lowest weight group (≥6–8 kg) and 12 children in each of the other three stratified weight groups. Within each weight group, equal numbers of children were randomized to each initial treatment arm. At baseline, the median (range) age was 6 years (0.5–12), weight 19 kg (6–29), CD4+ T-cell percentage was 32% (8–50), and absolute CD4+ T-cell count was 1,102 cells/mm3 (398–4,640). The median duration of NVP-based HAART prior to entry was 38.6 months (range 1.5–80). Drug dosing was by weight bands as shown in Table 1. Overall, 41 children completed both PK assessments (1 child discontinued the study drugs after the first PK study due to neutropaenia while taking GPO-VIR Z30). All children had 100% adherence in the 72 h prior to each of the PK blood samplings.
Plasma concentration–time curves for ZDV, 3TC and NVP administered using GPO-VIR Z30 tablets or the liquid formulations are shown in Figure 1. PK parameters for each formulation are presented in Table 2 alongside historical reference drug exposure data in adults using branded tablets. The AUC for ZDV was not significantly different between formulations and was within the range previously reported in adults. For 3TC, the AUC and Cmax were significantly higher with the GPO-VIR Z30 tablet compared to the liquid (Table 2); nevertheless, the 3TC exposure achieved with the tablet was within the range of values reported in adults using the branded 3TC tablets (Table 2).
Figure 1.
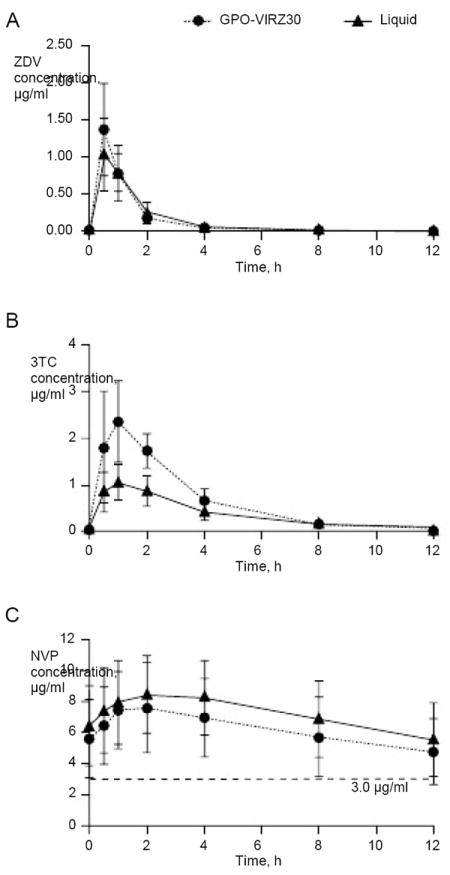
Concentration versus time curves of ZDV, 3TC and NVP for the GPO-VIR Z30 paediatric FDC tablet and the liquid formulations
Mean (sd) concentration versus time curves of (A) zidovudine (ZDV), (B) lamivudine (3TC) and (C) nevirapine (NVP) for the GPO-VIR Z30 paediatric fixed-dose combination tablet (n=41) compared to liquid formulation in the same subjects. The dashed horizontal line in (C) indicates the recommended minimum NVP concentration (3.0 μg/ml).
Table 2.
Pharmacokinetic parameters of the GPO-VIR Z30 tablet and individual branded liquid formulations
| Parameter | GPO-VIR Z30 (n=42)a | Liquid (n=41)a | P-valueb | Historical data for branded tablets in HIV-infected adultsc |
|---|---|---|---|---|
| ZDVd | ||||
| AUC0-12, μg•h/ml | 1.58 (1.49–1.68) | 1.62 (1.53–1.72) | 0.78 | 1.80 (1.52–2.14) |
| Cmax, μg/ml | 1.44 (1.32–1.56) | 1.07 (0.98–1.18) | <0.0001 | 1.43 (1.14–1.80) |
| 3TC | ||||
| AUC0–12, μg•h/ml | 7.78 (7.38–8.19) | 4.39 (4.05–4.75) | <0.0001 | 8.54 (3.2) |
| Cmax, μg/ml | 2.52 (2.33–2.73) | 1.09 (0.99–1.19) | <0.0001 | 2.07 (0.82) |
| Cmin, μg/ml | 0.07 (0.07–0.08) | 0.08 (0.07–0.09) | 0.04 | 0.33 (0.2) |
| NVP | ||||
| AUC0–12, μg•h/ml | 68.88 (62.13–76.36) | 81.88 (74.59–89.89) | <0.0001 | 54.5 (48.0–71.8) |
| Cmax, μg/ml | 7.67 (7.04–8.34) | 8.39 (7.75–9.08) | 0.004 | 5.7 (5.00–7.44) |
| Cmin, μg/ml | 4.19 (3.66–4.81) | 4.94 (4.32–5.65) | <0.0001 | 3.73 (3.20–5.08) |
Reported values are geometric mean (90% CI).
The P-values are from paired Student’s t-tests comparing the two formulations (n=41).
References are as follows: GSK data for zidovudine (ZDV) 300 mg, single dose (area under the curve [AUC]0-∞, geometric mean [95% CI]) [19]; lamivudine (3TC) 150 mg twice daily (mean AUC0-12 [sd]) [25]; and nevirapine (NVP) EMEA viramune summary (median AUC0-12 [range]) [26].
ZDV minimum plasma concentration (Cmin) was below lower limit of assay quantification (<0.025 μg/ml) over all weight groups. Cmax, maximum plasma concentration.
NVP PK parameters were significantly lower with the FDC tablet; however, the rules defined a priori for NVP therapeutic inadequacy were not met, that is, the 90% CI for the NVP AUC of 62.1–76.4 μg•h/ml lay entirely within the defined therapeutic target (44.5–90.9 μg•h/ml), and well within adult reference range. Also, although an apparent sequence effect for NVP PK parameters was found, with a higher NVP AUC (and Cmax) observed in the group who received the liquid formulations before receiving GPO-VIR Z30 tablets (P=0.01), the geometric mean NVP AUC remained significantly lower with GPO-VIR Z30 tablets after taking into account the sequence effect. With the FDC dosing strategy used, a regression analysis of log transformed NVP AUC (controlling for sequence effect) revealed a significant association between NVP exposure and age (P=0.04) characterized by a 3% increase per year of age. Eight children had an NVP Cmin<3.0 μg/ml while taking the FDC tablet (range 0.53–2.94 μg/ml); six of these children also had a low NVP Cmin while taking the liquid formulation (range 0.9–2.95 μg/ml). These children were distributed across all weight strata (one ≥6–8 kg, two >8–16 kg, three >16–23 kg and two >23–30 kg). Of these children with low Cmin, six had the CYP2B6 516 G/G genotype and two children had the G/T genotype. All eight children received an NVP dose >300 mg/m2/day (range 312–419). Of note, the child receiving 312 mg/m2/day had the lowest Cmin (0.53 μg/ml) while receiving the FDC and the liquid formulation (0.90 μg/ml). The child receiving the highest NVP dose (419 mg/m2/day) had a Cmin of 2.74 μg/ml with the FDC and 3.53 μg/ml with the liquid. Geometric mean tablet/liquid AUC ratios were 0.99 (0.92–1.06) for ZDV, 1.79 (1.68–1.90) for 3TC and 0.85 (0.81–0.88) for NVP. We found no relationship between the length of NVP treatment and NVP AUC (r=0.2; P=0.21).
CYP2B6 genotype results were available for all 42 children. The G/G, G/T and T/T alleles were found in 19 (45%), 19 (45%) and 4 (10%) children, respectively. For both the formulations, children with the CYP2B6 T/T genotype had significantly higher NVP AUC compared to the other genotypes. For the FDC tablet the NVP AUC for the G/G, G/T and T/T genotypes were 67.0, 74.5 and 106.4 μg•h/ml (P=0.04), respectively, and for the liquid formulation 75.4, 90.7 and 122.6 μg•h/ml (P=0.01), respectively (Figure 2).
Figure 2.
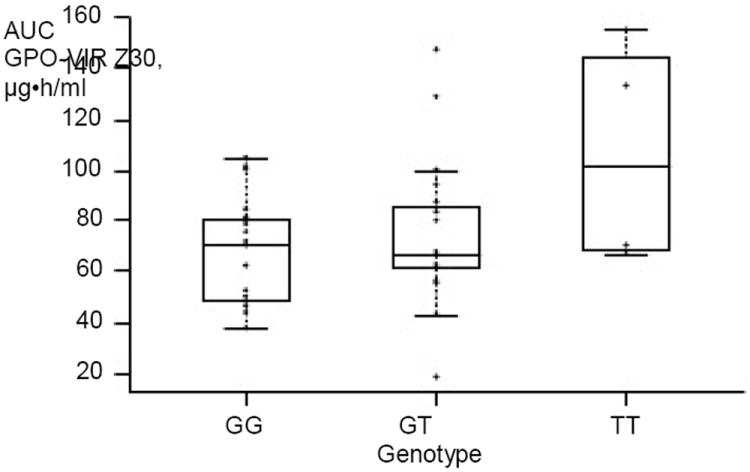
Nevirapine plasma exposure and CYP2B6 516 G>T polymorphisms after administration of GPO-VIR Z30 in HIV-infected Thai children
Comparison of GG versus GT versus TT genotypes, P=0.04. Each circle represents one child, the middle bar indicates the median, and the box represents the IQR. AUC, area under the curve.
Safety
Five children had grade 3 or 4 toxicities: two children had hyperamylasemia, one child had eyelid oedema, and two children had neutropaenia. None of these events were considered to be related to the study drugs, with the exception of a grade 4 neutropaenia that occurred in one child after receiving the FDC tablet. This child subsequently stopped GPO-VIR Z30 (at the time of the crossover into the liquid formulation) and did not start the liquid formulations; the neutropaenia resolved to grade 1 a week later. Mild gastrointestinal events were reported in five children after the dose administration (three children after administration of the liquid formulations, and two children after both formulations). Both formulations were well tolerated. The majority of subjects reported a preference for GPO-VIR Z30 over the liquid formulations.
Discussion
To date, paediatric FDCs have been limited to d4T/3TC/NVP, and although this combination has helped simplify and expand access to antiretroviral treatment, concerns have been raised regarding the cumulative toxicity of long-term d4T exposure and ZDV/3TC is now the preferred first-line NRTI backbone. In this study we compared the bioavailability and short-term safety of a novel paediatric FDC tablet of ZDV/3TC/NVP compared to the individual branded liquid formulations, and assessed if the FDC tablet delivered therapeutically adequate exposures of NVP in HIV-infected children. We found that the drug exposure differed between the liquids and FDC tablet for NVP and 3TC; however, based on historical data in adults receiving branded tablets, the FDC tablet delivered therapeutically adequate exposures of each drug in HIV-infected Thai children between 6 to 30 kg.
ZDV exposure was equivalent between the FDC tablet and liquid formulation. In the literature, ZDV exposure values reported in adults following a standard 300 mg dose twice daily varies significantly across studies, and include 1.46 [18], 1.80 [19] and 2.30 μg•h/ml [20]. Differences in the number and timing of blood sampling may explain part of the variation observed as peak ZDV concentrations can be difficult to determine; nevertheless, the exposure observed with the FDC tablet and liquid falls within the range of those observed in HIV-infected adults.
The higher bioavailability of 3TC with the FDC tablet compared to the liquid formulation found in the current study is consistent with our previous study of a d4T/3TC/NVP FDC (GPO-VIR S7) tablet, where significantly higher 3TC exposure with the tablet formulation was also observed [7]. In these two studies, the 3TC exposure in HIV-infected Thai children receiving the branded liquid formulation were similar (4.52 and 4.39 μg•h/ml) and within the expected range reported for children using the liquid formulation [21]. While bioanalytical errors or incorrect dose administration could explain the difference in the 3TC bioavailability observed, no bioanalytical or drug administration errors were identified. Our data are also supported by recent data from the ARROW study in African children weighing between 12 to 15 kg, which reported that 3TC exposures were approximately 55% higher in those receiving the branded tablet compared to those receiving the branded liquid formulation [22]. The 3TC exposure reported in children using the branded tablet was 8.2 μg•h/ml and the tablet/liquid geometric mean ratio (90% CI) was 1.58 (1.37–1.81), both similar to the GPO-VIR Z30 FDC tablet in this study. It is important to note that the 3TC exposure with the paediatric FDC in Thai and branded tablets in African children are comparable to adult exposures following standard 150 mg twice daily dosing, which have been reported as 6.5 [23], 7.0 [24] and 8.5 μg•h/ml [25], as well as in older children taking branded 3TC tablets (6.5 μg•h/ml) [21] and other generic FDC tablets (range 4.2–6.8 μg•h/ml) [6]. The clear explanation for the difference in 3TC bioavailability between branded liquid and tablet formulations in children remains to be found. Although the duration on the paediatric FDC tablet was relatively short in our study, the higher 3TC exposures observed did not seem to lead to any additional drug-associated toxicities.
In terms of therapeutic adequacy of the paediatric FDC tablet, a greater emphasis was placed on NVP than the other two drugs, as low plasma concentrations of NVP are associated with poor virological suppression [16], while plasma concentrations of ZDV and 3TC are not precise surrogates for the active intracellular NRTI-triphosphate concentrations and there are currently no defined Cmin associated with therapeutic efficacy. In this study, NVP exposure was significantly lower with the FDC tablet than with liquid; however, it met the therapeutic adequacy rules developed to ensure that the tablet would at least deliver a drug exposure equivalent to maintaining an average NVP concentration above the minimum recommended target. Indeed, the NVP exposure with the FDC tablet was between those observed in adults receiving branded tablets [26] and children receiving an FDC tablet [6]. In our study, eight children had an NVP Cmin<3.0 μg/mlm while on FDC and six of these children also had a low Cmin while taking the liquid formulation. The low NVP Cmin levels could have been due to poor drug adherence and/or a formulation effect; however, strict rules for drug adherence prior to PK sampling (direct observed therapy prior to the PK sampling) were set to ensure accurate NVP concentration data and similar concentrations were observed between formulations, therefore it is unlikely that the low levels can be explained by either poor drug adherence or formulation effects in these children. The NVP dose of 150 mg/m2 evaluated in the registration study (BI Trial 1100.1368) was predicted to produce an average steady state concentration of 5.3 μg/ml in children aged 3 months to 16 years, similar to 4–6 μg/ml reported in adults dosed at 200 mg twice daily [27]. Of note, in the registration trial the mean (sd) trough NVP concentration was 5.83 μg/ml (3.14) and this implies that approximately 17% of children will have trough levels <3.0 μg/ml, which is similar to the proportion of children observed in our study. Previous studies, including one using the paediatric FDC of d4T/3TC/NVP by the Thai GPO (GPO-VIR S7), have suggested that young infants, particularly those <5 months old, have low NVP exposure [7,28,29]. This could be explained by the faster NVP clearance in younger children [30]. In this study, we found that NVP exposure increased with age, using the dose range of 300–400 mg/m2/day across all weights bands.
During the design stages of the GPO-VIR Z30 tablet, the ratio of ZDV:3TC:NVP chosen was based on the WHO Paediatric Antiretroviral Working Group’s recommendations from October 2006, which proposed a ZDV/3TC/NVP tablet of 60/30/55 mg [5]. In order to avoid pill cutting and allow more accurate dosing in young children, the GPO developed a smaller tablet using half the proposed doses: 30:15:28 mg. Using this ratio, we developed a weight band dosing strategy designed to achieve ZDV (360–480 mg/m2/day) and 3TC (4 mg/kg) dosing as close as possible to the recommended dose, while maintaining the NVP dose within the recommended range of 300–400 mg/m2/day. The WHO subsequently released a revised recommendation in 2008, lowering the NVP dosage from 55 to 50 mg for the ZDV/3TC/NVP tablet [31]. A subsequent analysis of NPV exposure, following the proposed WHO paediatric weight band dosing scheme, with varying NVP tablet strengths, predicted that the frequency of subtherapeutic exposure would be 2% lower with a 55 mg NVP tablet versus a 50 mg tablet, but with increased supratherapeutic exposure (12% higher when defined as 2× the mean NVP AUC) [32]. Given the absence of a clearly defined NVP toxicity threshold, it is not yet possible to definitely state that one NVP tablet strength is better than another. However, the results of the present study confirm that a paediatric FDC tablet containing 28 mg of NVP, administered following the proposed weight band dosing, provides therapeutically adequate NVP exposure with no reported NVP-associated side effects.
Genetic polymorphisms in drug metabolizing enzymes and transporters have been shown to contribute towards antiretroviral inter-individual drug variability [33,34]. NVP is extensively metabolized via CYP enzymes, in particular the isoenzymes CYP2B6 and CYP3A. In 126 children from PACTG 366 and 377 cohorts, the CYP2B6 516G>T single nucleotide polymorphism was associated with slower NVP oral clearance and an improved immunological response [12]. In the current study, the frequency of the T/T alleles was 10%, which is consistent with other reports in the Thai population [35], and was associated with significantly higher NVP exposure; however, there was no evidence of increased toxicities among children with this genotype.
We found no major safety concerns of GPO-VIR Z30, although by enrolling children who were stable on treatment regimens with NVP, we may have excluded those who were unable to tolerate NVP. In addition, ZDV-related toxicity may not have been evident due to the short duration of this study. However, we expected that this FDC’s toxicity would be similar to that found in studies with individual separate drug therapy. Of note, many children and caretakers preferred the FDC tablet over liquid formulations. Although the number of pills was high in older children, the small pill size made it acceptable to most children.
In summary, we observed differences in the bioavailability of the paediatric ZDV/3TC/NVP FDC tablet compared to the individual liquids. The higher 3TC tablet-bioavailability has been reported in other studies and did not lead to toxicity. Importantly, the new FDC tablet delivers therapeutic exposures of each drug and allows easy dose titration across the paediatric age continuum included in this study. When available, this FDC could well play an important role as first-line treatment in Thai children, as well as in other resource-limited settings.
Acknowledgments
We would like to thank all the children and families who participated in the P1069 trial, and all members of the IMPAACT P1069 study team in Thailand and the US (Additional file 1).
This study was supported by the International Maternal Pediatric Adolescent AIDS Clinical Trial Group (IMPAACT) funded by the National Institute of Allergy and Infectious Disease, National Institutes of Health and the Thai GPO. Pharmaceutical support was also provided by GPO.
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH) [AI068632]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement number 5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and number 1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C).
Additional file
Additional file 1: A list of the members of the IMPAACT P1069 study team can be found at http://www.intmedpress.com/uploads/documents/AVT-11-OA-1984_Chokephaibulkit_Add_file1.pdf
Footnotes
Disclosure statement
AE is the director of Research and Development Institute of the Thai GPO. GPO is the manufacturer of the GPO-VIR Z30 study drug, as well as many generic antiretroviral drugs used within the Thai National Antiretroviral Treatment Program. The GPO sponsored the branded liquid drugs used in this study. EC is a consultant to GlaxoSmithKline, Bristol–Myers Squibb and Johnson & Johnson. All other authors declare no competing interests.
References
- 1.World Health Organization, UNAIDS. Estimated number of children (<15 years) newly infected with HIV, 2007. [23 September 2010]; Updated December 2007. Available at: http://data.unaids.org/pub/EPISlides/2007/071118_epicore2007_slides_en.pdf.
- 2.Chokephaibulkit K, Plipat N, Cressey TR, et al. Pharmacokinetics of nevirapine in HIV-infected children receiving an adult fixed-dose combination of stavudine, lamivudine and nevirapine. AIDS. 2005;19:1495–1499. doi: 10.1097/01.aids.0000183625.97170.59. [DOI] [PubMed] [Google Scholar]
- 3.Puthanakit T, Aurpibul L, Sirisanthana T, Sirisanthana V. Efficacy of non-nucleoside reverse transcriptase inhibitor-based highly active antiretroviral therapy in Thai HIV-infected children aged two years or less. Pediatr Infect Dis J. 2009;28:246–248. doi: 10.1097/INF.0b013e31818dd72b. [DOI] [PubMed] [Google Scholar]
- 4.Puthanakit T, Aurpibul L, Oberdorfer P, et al. Sustained immunologic and virologic efficacy after four years of highly active antiretroviral therapy in human immunodeficiency virus infected children in Thailand. Pediatr Infect Dis J. 2007;26:953–956. doi: 10.1097/INF.0b013e318125720a. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Summary statement on WHO recommendations on ARV medicines for treating and preventing HIV infections in younger children. [23 September 2010]; Updated 30 November 2006, Available from http://www.who.int/hiv/mediacentre/news63/en/
- 6.L’homme RF, Kabamba D, Ewings FM, et al. Nevirapine, stavudine and lamivudine pharmacokinetics in African children on paediatric fixed-dose combination tablets. AIDS. 2008;22:557–565. doi: 10.1097/QAD.0b013e3282f4a208. [DOI] [PubMed] [Google Scholar]
- 7.Vanprapar N, Cressey TR, Chokephaibulkit K, et al. A chewable pediatric fixed-dose combination tablet of stavudine, lamivudine, and nevirapine: pharmacokinetics and safety compared with the individual liquid formulations in human immunodeficiency virus-infected children in Thailand. Pediatr Infect Dis J. 2010;29:940–944. doi: 10.1097/INF.0b013e3181e2189d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Preferred antiretroviral medicines for treating and preventing HIV infection in younger children: report of the WHO Paediatric Antiretroviral Working Group. Geneva: World Health Organization; 2008. [Google Scholar]
- 9.World Health Organization. [23 September 2010];Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents: November 2009 version. 2009 Nov; Updated 23 February 2010. Available from http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf.
- 10.Puthanakit T, Tangsathapornpong A, Anaworanich J, et al. Thai national guidelines for the use of antiretroviral therapy in pediatric HIV infection in 2010. Asian Biomedicine. 2010;4:505–513. [Google Scholar]
- 11.Mahungu T, Smith C, Turner F, et al. Cytochrome P450 2B6 516G-->T is associated with plasma concentrations of nevirapine at both 200 mg twice daily and 400 mg once daily in an ethnically diverse population. HIV Med. 2009;10:310–317. doi: 10.1111/j.1468-1293.2008.00689.x. [DOI] [PubMed] [Google Scholar]
- 12.Saitoh A, Sarles E, Capparelli E, et al. CYP2B6 genetic variants are associated with nevirapine pharmacokinetics and clinical response in HIV-1-infected children. AIDS. 2007;21:2191–2199. doi: 10.1097/QAD.0b013e3282ef9695. [DOI] [PubMed] [Google Scholar]
- 13.Droste JA, Verweij-Van Wissen CP, Burger DM. Simultaneous determination of the HIV drugs indinavir, amprenavir, saquinavir, ritonavir, lopinavir, nelfinavir, the nelfinavir hydroxymetabolite M8, and nevirapine in human plasma by reversed-phase high-performance liquid chromatography. Ther Drug Monit. 2003;25:393–399. doi: 10.1097/00007691-200306000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Holland DT, DiFrancesco R, Connor JD, Morse GD. Quality assurance program for pharmacokinetic assay of antiretrovirals: ACTG proficiency testing for pediatric and adult pharmacology support laboratories, 2003 to 2004: a requirement for therapeutic drug monitoring. Ther Drug Monit. 2006;28:367–374. doi: 10.1097/01.ftd.0000211817.58052.b8. [DOI] [PubMed] [Google Scholar]
- 15.Jacob RM, Johnstone EC, Neville MJ, Walton RT. Identification of CYP2B6 sequence variants by use of multiplex PCR with allele-specific genotyping. Clin Chem. 2004;50:1372–1377. doi: 10.1373/clinchem.2004.031708. [DOI] [PubMed] [Google Scholar]
- 16.Veldkamp AI, Weverling GJ, Lange JM, et al. High exposure to nevirapine in plasma is associated with an improved virological response in HIV-1-infected individuals. AIDS. 2001;15:1089–1095. doi: 10.1097/00002030-200106150-00003. [DOI] [PubMed] [Google Scholar]
- 17.Acosta EP, Gerber JG. Position paper on therapeutic drug monitoring of antiretroviral agents. AIDS Res Hum Retroviruses. 2002;18:825–834. doi: 10.1089/08892220260190290. [DOI] [PubMed] [Google Scholar]
- 18.Crémieux AC, Katlama C, Gillotin C, Demarles D, Yuen GJ, Raffi F. A comparison of the steady-state pharmacokinetics and safety of abacavir, lamivudine, and zidovudine taken as a triple combination tablet and as abacavir plus a lamivudine-zidovudine double combination tablet by HIV-1-infected adults. Pharmacotherapy. 2001;21:424–430. doi: 10.1592/phco.21.5.424.34497. [DOI] [PubMed] [Google Scholar]
- 19.Wang LH, Chittick GE, McDowell JA. Single-dose pharmacokinetics and safety of abacavir (1592U89), zidovudine, and lamivudine administered alone and in combination in adults with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1999;43:1708–1715. doi: 10.1128/aac.43.7.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore KH, Barrett JE, Shaw S, et al. The pharmacokinetics of lamivudine phosphorylation in peripheral blood mononuclear cells from patients infected with HIV-1. AIDS. 1999;13:2239–2250. doi: 10.1097/00002030-199911120-00006. [DOI] [PubMed] [Google Scholar]
- 21.Burger DM, Verweel G, Rakhmanina N, et al. Age-dependent pharmacokinetics of lamivudine in HIV-infected children. Clin Pharmacol Ther. 2007;81:517–520. doi: 10.1038/sj.clpt.6100118. [DOI] [PubMed] [Google Scholar]
- 22.Gitta PK, Kendall L, Musiime V, et al. Pharmacokinetics of lamivudine, abacavir, and zidovudine administered twice daily as syrups versus scored tablets in HIV-infected Ugandan children. J Int AIDS Soc. 2010;13(Suppl 4):176. [Google Scholar]
- 23.Hosseinipour MC, Corbett AH, Kanyama C, et al. Pharmacokinetic comparison of generic and trade formulations of lamivudine, stavudine and nevirapine in HIV-infected Malawian adults. AIDS. 2007;21:59–64. doi: 10.1097/QAD.0b013e3280117ca0. [DOI] [PubMed] [Google Scholar]
- 24.Vezina HE, Henry K, Ravindran GD, et al. A randomized crossover study to determine bioequivalence of generic and brand name nevirapine, zidovudine, and lamivudine in HIV-negative women in India. J Acquir Immune Defic Syndr. 2006;41:131–136. doi: 10.1097/01.qai.0000199098.95967.ab. [DOI] [PubMed] [Google Scholar]
- 25.Bruno R, Regazzi MB, Ciappina V, et al. Comparison of the plasma pharmacokinetics of lamivudine during twice and once daily administration in patients with HIV. Clin Pharmacokinet. 2001;40:695–700. doi: 10.2165/00003088-200140090-00005. [DOI] [PubMed] [Google Scholar]
- 26.European Medicines Agency (EMEA) Annex I: summary of product characteristics. Viramune 200 mg tablets. [23 September 2010]; Updated 6 August 2010. Available from http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000183/WC500051481.pdf.
- 27. [24 February 2011];Clinical pharmacology review: NDA: 20-636 SE5 027 & 20-933 SE5 017. Updated 8 September 2008, Available from http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm072778.pdf.
- 28.Mulenga V, Fillekes Q, Kabamba D, et al. Pharmacokinetics of nevirapine in 3- to 6-kg, HIV-infected infants taking pediatric fixed-dose combination tablets. 16th Conference on Retroviruses and Opportunistic Infections; 8–10 February 2009; Montreal, QC, Canada. Abstract 881. [Google Scholar]
- 29.Mosha LB, Musoke P, Parsons T, et al. Nevirapine concentrations in HIV-infected Ugandan children on adult fixed-dose combination tablet ART, with and without rifampicin-based treatment for active M. Tuberculosis infection. 16th Conference on Retroviruses and Opportunistic Infections; 8–10 February 2009; Montreal, QC, Canada. Abstract 909. [Google Scholar]
- 30.Luzuriaga K, Bryson Y, McSherry G, et al. Pharmacokinetics, safety, and activity of nevirapine in human immunodeficiency virus type 1-infected children. J Infect Dis. 1996;174:713–721. doi: 10.1093/infdis/174.4.713. [DOI] [PubMed] [Google Scholar]
- 31.WHO paediatric antiretroviral working group. Preferred antiretroviral medicines for treating and preventing HIV infection in younger children. [23 September 2010]; Updated 20 January 2008. Available from http://www.who.int/hiv/pub/paediatric/antiretroviral/en/index.html.
- 32.Capparelli E, Kabamba D, Cressey TR, Burger DM. Nevirapine exposure with WHO pediatric weight band dosing – enhanced therapeutic concentrations predicted based on extensive international pharmacokinetic experience. 15th Conference on Retroviruses and Opportunistic Infections; 3–6 February 2008; Boston, MA, USA. Abstract 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fellay J, Marzolini C, Meaden ER, et al. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet. 2002;359:30–36. doi: 10.1016/S0140-6736(02)07276-8. [DOI] [PubMed] [Google Scholar]
- 34.Haas DW, Ribaudo HJ, Kim RB, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18:2391–2400. [PubMed] [Google Scholar]
- 35.Puthanakit T, Tanpaiboon P, Aurpibul L, Cressey TR, Sirisanthana V. Plasma efavirenz concentrations and the association with CYP2B6-516G >T polymorphism in HIV-infected Thai children. Antivir Ther. 2009;14:315–320. [PubMed] [Google Scholar]


