Abstract
Previous studies suggest that the age-related decline in circulating growth hormone (GH) and insulin-like growth factor-1 (IGF-1) levels significantly contribute to vascular dysfunction in aging by impairing cellular oxidative stress resistance pathways. Obesity in elderly individuals is increasing at alarming rates, and there is evidence suggesting that elderly individuals are more vulnerable to the deleterious cardiovascular effects of obesity than younger individuals. However, the specific mechanisms through which aging, GH/IGF-1 deficiency, and obesity interact to promote the development of cardiovascular disease remain unclear. To test the hypothesis that low circulating GH/IGF-1 levels exacerbate the pro-oxidant and proinflammatory vascular effects of obesity, GH/IGF-1–deficient Lewis dwarf rats and heterozygous control rats were fed either a standard diet or a high-fat diet (HFD) for 7 months. Feeding an HFD resulted in similar relative weight gains and increases in body fat content in Lewis dwarf rats and control rats. HFD-fed Lewis dwarf rats exhibited a relative increase in blood glucose levels, lower insulin, and impaired glucose tolerance as compared with HFD-fed control rats. Analysis of serum cytokine expression signatures indicated that chronic GH/IGF-1 deficiency exacerbates HFD-induced inflammation. GH/IGF-1 deficiency also exacerbated HFD-induced endothelial dysfunction, oxidative stress, and expression of inflammatory markers (tumor necrosis factor-α, ICAM-1) in aortas of Lewis dwarf rats. Overall, our results are consistent with the available clinical and experimental evidence suggesting that GH/IGF-1 deficiency renders the cardiovascular system more vulnerable to the deleterious effects of obesity.
Keywords: Accelerated aging, Endothelial dysfunction, IGF-1, Obesity, Vascular pathophysiology
OBESITY is increasing at alarming rates worldwide, and there is overwhelming evidence indicating that increased body weight is associated with accelerated atherosclerosis and increased rates of cardiovascular mortality (1). Epidemiological studies provide strong evidence that aging exacerbates the deleterious cardiovascular effects of obesity (2,3). Currently, 37.4% of adults aged 65 years and older are obese, yet there are only a few studies addressing the specific mechanisms through which aging and obesity interact to promote the development of vascular pathologies (including myocardial infarction, stroke, vascular dementia; for a recent review, see [4]). Previous studies in laboratory rodents and nonhuman primates provide evidence that increasing age renders the vasculature more prone to oxidative insult elicited by obesity and related metabolic conditions (including diabetes mellitus and the metabolic syndrome) likely by impairing cellular oxidative stress resistance (5–7). However, the mechanisms by which aging impairs vascular resistance to metabolic stress associated with obesity are not well understood.
In recent years, considerable evidence has accumulated that in addition to cell-autonomous changes in the gene expression signature in vascular cells, non-cell autonomous endocrine mechanisms also have an important role in age-related vascular impairment. In particular, circulating levels of growth hormone (GH) and, consequently, hepatic production of insulin-like growth factor-1 (IGF-1) significantly decline with age both in humans and laboratory animals (8–10), and the available evidence suggests that reduced GH and IGF-1 levels are causally linked to vascular impairment in aging (11–13). There is strong clinical and experimental evidence that GH and IGF-1 exert beneficial effects on cardiovascular function and cardiovascular mortality (12,14–22) and are atheroprotective (23,24) by regulating pathways involved in prevention of macromolecular damage. Importantly, previous studies in IGF-1–deficient Lewis dwarf rats (25) and Ames dwarf mice (26) demonstrated that low circulating IGF-1 levels are associated with impaired expression/activity of antioxidant enzymes in the vasculature, leading to increased vascular oxidative stress and endothelial dysfunction (26). Furthermore, our recent studies show that in mice, adult-onset endocrine IGF-1 deficiency (induced by adeno-associated viral knockdown of IGF-1, specifically in the liver of postpubertal mice using Cre-lox technology [27,28]) impairs the ability of vascular cells to mount an effective NF-E2-related factor 2 (Nrf2)-dependent antioxidant defense in response to oxidative stressors administered ex vivo (27). We demonstrated that the impairment of cellular oxidative stress resistance induced by IGF-1 deficiency renders cultured aorta segments vulnerable to the metabolic stress of high glucose treatment in vitro, resulting in an exacerbation of hyperglycemia-induced endothelial dysfunction, oxidative stress, and an increased rate of endothelial apoptosis (27).
The present study used Lewis dwarf rats as model system to test the hypothesis that low circulating IGF-1 levels impair vascular resistance to metabolic stress in vivo. The advantages of this experimental design are that the decline in plasma levels of GH and IGF-1 in Lewis dwarf rats mimic the endocrine changes associated with aging (25,29–31), and consumption of a high-fat diet (HFD) is known to result in endothelial dysfunction, vascular oxidative stress, and inflammation in young rodents (32–34), which is exacerbated in aged rodents (Z. Ungvari, M.D., Ph.D., A. Csiszar, M.D., Ph.D, manuscript in preparation, 2011). Using isolated aorta preparations, we assessed whether GH/IGF-1 deficiency in Lewis dwarf rats exacerbates endothelial dysfunction, oxidative stress, and vascular inflammation induced by consumption of a HFD.
METHODS
Animals
In the present study, we used male Lewis rats that are heterozygous or homozygous for the spontaneous autosomal recessive dw-4 mutation that results in a decrease in GH secretion from the pituitary gland and consequential low circulating IGF-1 levels (35–37). Because Lewis dwarf (dw-4/dw-4) rats exhibit chronically low levels of GH and IGF-1, which mimic age-related endocrine alterations, they are often used as models of accelerated vascular aging (25,36). To generate the cohort used in the present study, female heterozygous (dw-4/–) Lewis rats were bred with male homozygous Lewis dwarf rats (dw-4/dw-4) to generate heterozygous (dw-4/–) offspring with a normal phenotype (“control”) or homozygous rats (dw-4/dw-4) with a dwarf phenotype (“dwarf”). Classification as control or dwarf was based on their body weight as well as the serum IGF-1 levels at 33 days of age. At 6 weeks of age, dwarf and control animals were divided into four groups and placed on either a standard diet (SD) or HFD. The four groups were (a) control animals fed an SD, (b) control animals fed a HFD, (c) dwarf animals fed an SD diet, and (d) dwarf animals fed a HFD. The high-fat chow, commonly used to induce obesity, delivers 60% kcal from fat, whereas the SD provides only 10% kcal from fat (D12492, D12450B, respectively; Research Diets Inc., New Brunswick, NJ). The animals continued on the specified diets (with water and food ad libitum) for 7 months. Animals were housed in pairs in the Rodent Barrier Facility at University of Oklahoma Health Sciences Center on a 12-hour light/dark cycle. All procedures were approved by the Institutional Animal Care and Use Committee of University of Oklahoma Health Sciences Center.
Serum Biochemical Profile and Analysis of Circulating Levels of Metabolic Hormones and Inflammatory Cytokines
Whole blood was collected at sacrifice and was centrifuged at 2,500g for 20 minutes at 4°C; serum was collected, aliquoted, and stored at −80°C. Total IGF-1 levels (nanograms per milliliter) in serum were determined by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) as previously described (30,36,38,39). The serum biochemical profile was assessed by Vance Veterinary Laboratories (Oklahoma City, OK). Circulating levels of metabolic hormones (insulin, adiponectin, resistin, leptin), cytokines, chemokines, and other inflammatory markers relevant for aging research (40,41) (including tumor necrosis factor-alpha [TNF-α], interleukin [IL]-6, IL-7, IL-1α, leukemia inhibitory factor, IL-1β, interferon gamma—induced protein-10 [IP-10], IL-18, eotaxin, interferon gamma, IL-2, IL-17A, IL-3, monocyte chemotactic protein 3 [MCP-3], lymphotactin, vascular endothelial growth factor A [VEGF-A], monocyte chemotactic protein-1 [MCP-1], macrophage inflammatory protein-1 beta [MIP-1β], C-reactive protein, macrophage colony–stimulating factor-1 [M-CSF-1], IL-5, IL-11, oncostatin-M (OSM), macrophage inflammatory protein-2 [MIP-2], monocyte chemotactic protein-5 (MCP-5), fibroblast growth factor (FGF-basic), macrophage inflammatory protein-1 alpha [MIP-1α], macrophage inflammatory protein-3 beta [MIP-3β], macrophage inflammatory protein-1 gamma [MIP-1γ], granulocyte chemotactic protein-2 [GCP-2], stem cell factor, IL-4, RANTES, haptoglobin, IL-10, macrophage-derived chemokine [MDC]) were analyzed using a multiplex protein array system (Rules Based Medicine, Austin, TX) according to the manufacturer’s protocol.
Weight Gain and Body Composition
Body mass of each animal was recorded every 4 weeks. Body fat content was determined using magnetic resonance technology (MiniSpec LF90; Bruker Instruments, Billerica, MA) and reported as a percentage of total body mass.
Oral Glucose Tolerance Test
An oral glucose tolerance test was performed after fasting rats for 18 hours. d-Glucose (25% solution in water) was administered orally at a dosage of 1 g/kg body weight. Blood glucose was measured immediately prior to gavage of the solution and every 30 minutes thereafter up to 2 hours. Tail vein blood samples were taken using a sterile lancet (Medipoint, Mineola, NY), and glucose was measured with a OneTouch UltraMini glucose meter (LifeScan, Milpitas, CA).
Vessel Isolation and Functional Studies
The animals were fasted overnight and euthanized by decapitation 7 months after starting the HFD or SD. The aortas were isolated, cleaned, and sectioned. Then endothelial endothelial function was assessed by measuring relaxation of the aortic rings in response to acetylcholine and the calcium ionophore A23187, as previously described (25,27,33). Endothelium-independent vasorelaxation was assessed using S-nitroso-N-acetylpenicillamine (SNAP), a nitric oxide donor. In brief, an aorta ring segment (2 mm in length) was isolated from each animal and mounted on 40-μm stainless steel wires in myograph chambers (Danish Myo Technology A/S, Inc., Denmark) for measurement of isometric tension. The vessels were superfused with Krebs buffer solution (118 mM NaCl, 4.7 mM KCl, 1.5 mM CaCl2, 25 mM NaHCO3, 1.1 mM MgSO4, 1.2 mM KH2PO4, and 5.6 mM glucose; at 37°C; gassed with 95% air and 5% CO2). Optimal passive tension (as determined from the vascular length–tension relationship) was applied for 1 hour (equilibration period) and then relaxation of precontracted (by 10−6 mol/L phenylephrine) vessels to acetylcholine (from 10−9 to 10−6 mol/L), A23187 (from 10−9 to 10−7 mol/L), or S-nitroso-N-acetylpenicillamine (from 10−9 to 10−6 mol/L) was obtained.
Measurement of Vascular Production
content in the aorta was determined using dihydroethidium (DHE), an oxidative fluorescent dye, as previously reported (42,43). Freshly harvested vessels were incubated with DHE (3×10−6 mol/L; at 37°C for 30 minutes). After three washes with phosphate-buffered saline, the vessels were embedded in OCT medium and cryosectioned. Images of -specific red fluorescence were captured at 20× magnification and analyzed using Metamorph software, as previously reported (26,27). Three entire fields per vessel were analyzed with one image per field. The mean fluorescence intensities of DHE-stained nuclei in the endothelium and medial layer were calculated for each vessel. Thereafter, the intensity values for each animal in the group were averaged.
Quantitative Real-Time Reverse Transcription–Polymerase Chain Reaction
Quantitative real-time reverse transcription–polymerase chain reaction was used to analyze messenger RNA expression of TNF-α, IL-6, ICAM-1, and Nox1 in the aorta as previously reported (44–47). Total RNA was isolated using a Mini RNA Isolation Kit (Zymo Research, Orange, CA). The messenger RNA was then reverse transcribed using Superscript III RT (Invitrogen), and expression was analyzed using a Strategen MX3000 machine (44,48). To determine the primer efficiencies, a dilution series of a standard vascular sample was quantified for each plate. Quantification was performed using an efficiency-corrected ΔΔCq method. The reference genes Hprt, YWHAZ,B2M, and β-actin levels were quantified, and a normalization factor was calculated based on the geometric mean for internal normalization. The oligonucleotide sequences for quantitative real-time reverse transcription–polymerase chain reaction are listed in Table 1. Fidelity of the polymerase chain reaction was determined by melting temperature analysis and visualization of the product on a 2% agarose gel.
Table 1.
Oligonucleotides for Real-Time Reverse–Transcription Polymerase Chain Reaction
| Messenger RNA Targets | Description | Sense | Antisense |
| Nox1 | NADPH oxidase homolog 1 | TGAATCTTGCTGGTTGACACTTGC | GAGGGACAGGTGGGAGGGAAG |
| TNF-α | Tumor necrosis factor-alpha | AACCACCAAGCAGAGGAG | CTTGATGGCGGAGAGGAG. |
| IL-6 | Interleukin-6 | CTTCCAGCCAGTTGCCTTCTTG | TGGTCTGTTGTGGGTGGTATCC |
| ICAM-1 | Intercellular adhesion molecule-1 | CACAGCCTGGAGTCTC | CCCTTCTAAGTGGTTGGAA |
| HPRT | Hypoxanthine phosphoribosyltransferase 1 | AAGACAGCGGCAAGTTGAATC | AAGGGACGCAGCAACAGAC |
| ActB | Beta-actin | GAAGTGTGACGTTGACAT | ACATCTGCTGGAAGGTG |
| B2m | Beta-2-microglobin | ATTCACACCCACCGAGAC | GGATCTGGAGTTAAACTGGTC |
| Ywhaz | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide 1 | AACTGCCTACATATTGGT | CACACAGACTACACTCAT |
Data Analysis
Gene expression data were normalized to the respective control mean values. Statistical analyses of data were performed by one-way analysis of variance or by two-way analysis of variance followed by the Tukey post hoc test, as appropriate (49–58). A p < .05 was considered statistically significant. Data are expressed as means ± SEM.
RESULTS
Effects of Chronic HFD and GH/IGF-1 Deficiency on Body Mass and Body Composition
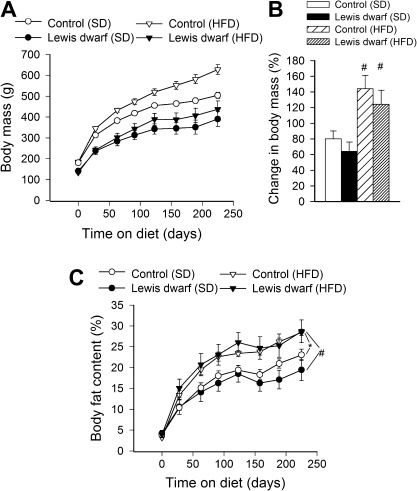
At the end of the experimental period, both dwarf rats and control rats consuming a HFD showed significantly increased body mass as compared with SD-fed animals (Figure 1A and B). The relative increase in body mass (calculated as a percentage of body mass at the beginning of treatment) of HFD-fed controls and Lewis dwarf rats did not differ significantly (Figure 1B). Feeding a HFD led to a similar increase in relative body fat content in control rats and Lewis dwarf rats (Figure 1C).
Figure 1.
(A) Changes in body mass of Lewis dwarf rats and control rats fed a high-fat diet (HFD) or standard diet (SD). Each time point represents the average body mass of each group. (B) Shows percentage change in body mass. Data are means ± SEM (n = 5–7); #p < .05 versus SD. (C) Body composition expressed as percent fat mass in Lewis dwarf rats and control rats fed a HFD or SD. HFD-fed rats of each genotype had significantly more body fat than those fed an SD. Data are means ± SEM (n = 5–7). *p < .05 versus control (SD); #p < .05 versus Lewis dwarf (SD).
Effects of Chronic HFD and GH/IGF-1 Deficiency on Blood Glucose Levels, Glucose Tolerance, and Circulating Levels of Metabolic Hormones
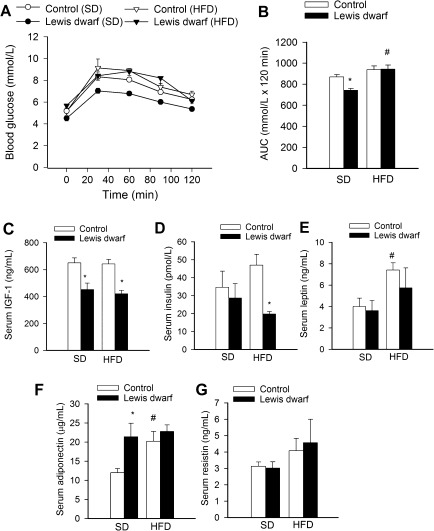
Fasting blood glucose levels were significantly lower in SD-fed Lewis dwarf rats than in SD-fed control rats (Figure 2A). Feeding a HFD did not change blood glucose levels in control rats, whereas HFD resulted in a significant increase in fasting blood glucose levels in Lewis dwarf rats (Figure 2A).
Figure 2.
(A) High-fat diet (HFD) impairs glucose tolerance in Lewis dwarf rats. Shown are changes in blood glucose levels after 1 g/kg oral glucose dose in Lewis dwarf rats and control rats fed a HFD or standard diet (SD). (B) Areas under the curves (AUC) were significantly reduced in Lewis dwarf rats fed an SD. HFD significantly increased AUC in Lewis dwarf rats eliminating the difference between the two genotypes. Data are means ± SEM; *p < .05 versus control; #p < .05 versus SD. (C–G) Serum levels of IGF-1 (C), insulin (D), leptin (E), adiponectin (F), and resistin (G). Data are means ± SEM; *p < .05 versus respective control; #p < .05 versus SD (n = 5–7).
To determine the effect of HFD on glucose tolerance, an oral glucose tolerance test was performed (Figure 2A). During the oral glucose tolerance test, there was a significant increase in plasma glucose levels in each group. Although control rats fed a HFD administration of glucose tended to lead to a more rapid increase of blood glucose levels and a higher peak value than in SD-fed control rats, these differences did not reach statistical significance. Comparison of the time course of relative changes in plasma glucose levels did not reveal a significant difference between SD-fed control rats and SD-fed Lewis dwarf rats. In contrast, in HFD-fed Lewis dwarf rats, administration of glucose led to more sustained increases in plasma glucose levels than in SD-fed Lewis dwarf rats (Figure 2A), indicating impaired glucose tolerance. This was also illustrated by the measurement of the calculated area under the curve (Figure 2B). The area under the curve was significantly reduced in SD-fed Lewis dwarf rats as compared with SD-fed control rats, whereas feeding a HFD resulted in a significant increase in area under the curve in Lewis dwarf rats eliminating the difference between the two genotypes (Figure 2B). In HFD-fed Lewis dwarf rats, glucose intolerance and the relative increase in fasting blood glucose levels were not associated with increases in serum cholesterol or triglyceride levels (Table 2).
Table 2.
Effects of a High-Fat Diet (HFD) on Various Serum Biomarkers in Control Rats and Insulin-Like Growth Factor-1/Growth Hormone–Deficient Lewis Dwarf Rats
| Parameter | Control (SD) | Lewis Dwarf (SD) | Control (HFD) | Lewis Dwarf (HFD) | Units |
| Fast triglycerides | 249.3 ± 55.8 | 137.16 ± 37.6 | 88.33 ± 10.6* | 73.4 ± 12.1* | mg/dL |
| Fast cholesterol | 122.5 ± 8.7 | 110.67 ± 11.0 | 96 ± 3.69 | 92 ± 1.97 | mg/dL |
| Total protein cc | 6.3 ± 0.17 | 6.1 ± 0.14 | 5.6 ± 0.06* | 5.66 ± 0.09* | g/dL |
| Albumin | 3.8 ± 0.09 | 3.71 ± 0.08 | 3.45 ± 0.03* | 3.48 ± 0.07* | g/dL |
| ALP | 95 ± 11.24 | 109 ± 12.20 | 116.3 ± 5.07 | 103.8 ± 13.9 | U/L |
| SGOT/AST | 129.6 ± 12.5 | 188.5 ± 26.21 | 182.16 ± 19.0 | 230 ± 51.19 | U/L |
| SGPT/ALT | 55.6 ± 4.7 | 69.67 ± 6.7 | 107.33 ± 17.6 | 96.6 ± 21.23 | U/L |
| Bilirubin | <0.1 | <0.1 | <0.1 | <0.1 | mg/dL |
| BUN | 15.1 ± 0.95 | 17.67 ± 0.77 | 12.83 ± 0.48 | 11.4 ± 0.68† | mg/dL |
| Creatinine | 0.45 ± 0.03 | 0.45 ± 0.03 | 0.43 ± 0.02 | 0.40 ± 0.03 | mg/dL |
| Cl | 105.3 ± 1.1 | 103.3 ± 0.4 | 105.8 ± 1.2 | 107.6 ± 1.2† | mmol/L |
| Na | 142 ± 1 | 140 ± 0.5 | 141.1 ± 1.4 | 143.4 ± 1.4 | mmol/L |
| K | 6.1 ± 0.7 | 6.5 ± 0.3 | 5.9 ± 0.3 | 6.3 ± 0.6 | mmol/L |
| Ca | 9.56 ± 0.2 | 9.15 ± 0.12 | 8.91 ± 0.09* | 8.9 ± 0.221 | mmol/L |
| PO4 | 4.06 ± 0.04 | 4.1 ± 0.03 | 3.95 ± 0.04 | 4.06 ± 0.02 | mmol/L |
| C-reactive protein | 377.8 ± 20.3 | 410.4 ± 32.1 | 429.4 ± 10.8 | 392.8 ± 22.8 | μg/mL |
| sVCAM-1 | 169.4 ± 4.8 | 195 ± 7.6* | 181.4 ± 5.3 | 183.8 ± 6.2 | ng/mL |
| VEGF | 252.0 ± 8.6 | 255.0 ± 34.5 | 284.2 ± 10.5 | 247.5 ± 34.9 | pg/mL |
| von Willebrand factor (vWF) | 83.74 ± 20 | 121.5 ± 21 | 91.24 ± 17.6 | 105.7 ± 14.7 | ng/mL |
| Tissue factor | 8.92 ± 0.64 | 6.58 ± 1.21 | 9.05 ± 1.03 | 4.22 ± 1.01 | ng/mL |
| Haptoglobin | 805.4 ± 80.6 | 603.6 ± 175 | 721 ± 58.4 | 657.8 ± 157.2 | μg/mL |
| ACE | 45.1 ± 2.6 | 51.5 ± 5.4 | 38.7 ± 3.2 | 42.6 ± 6.4 | ng/mL |
| Angiotensinogen | 232.6 ± 8.2 | 238.2 ± 6.4 | 229.4 ± 11.5 | 237.4 ± 5.2 | μg/mL |
Notes: Data are mean ± SEM (n = 5–7 for each data point). VEGF = vascular endothelial growth factor.
*p < .05 versus SD-fed control; †p < .05 versus SD-fed Lewis dwarf.
Lewis dwarf rats fed an SD had low serum IGF-1 levels, and they maintained low IGF-1 levels when fed a HFD (Figure 2C). Consumption of a HFD tended to increase insulin levels in control rats but not in Lewis dwarf rats (Figure 2D). As a result, serum insulin levels were significantly elevated in HFD-fed control rats as compared with HFD-fed Lewis dwarf rats (Figure 2D), indicating that insufficient insulin secretion was a contributing factor in impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR) was used to assess insulin resistance. It was calculated from fasting insulin and glucose concentration using the formula of Matthews and colleagues (59): HOMA-IR = fasting glucose (mmol/L) ×fasting insulin (mU/L)/22.5. There was no significant difference in HOMA-IR between the SD-fed groups (control [SD]: 1.18 ± 0.34; dwarf [SD]: 0.80 ± 0.24), whereas HOMA-IR was significantly lower in HFD-fed dwarf rats than in HFD-fed control rats (control [HFD]: 1.59 ± 0.21; dwarf [HFD]: 0.73 ± 0.04, p < .05). The quantitative insulin sensitivity check index (QUICKI) was calculated as an additional parameter to assess insulin sensitivity using the inverse of the sum of the logarithms of the fasting insulin and fasting glucose: 1/(log [fasting insulin in μU/mL] + log [fasting glucose in mg/dL]). There was no significant difference in QUICKI between the SD-fed groups (control [SD]: 0.38 ± 0.01; dwarf [SD]: 0.41 ± 0.02), whereas QUICKI was significantly greater in HFD-fed dwarf rats than in HFD-fed control rats (control [HFD]: 0.358 ± 0.008; dwarf [HFD]: 0.404 ± 0.004, p < .05).
In control rats fed a HFD, there was a significant increase in serum levels of leptin and adiponectin (Figure 2E and F, respectively). In contrast, baseline levels of adiponectin were significantly elevated in the Lewis dwarf rats, but the HFD was not associated with significant increases in serum levels of leptin (Figure 2E) or adiponectin (Figure 2F). Serum resistin levels in Lewis dwarf rats fed an SD were not significantly different from the levels measured in SD-fed control animals (Figure 2G). Consumption of a HFD did not increase significantly serum resistin levels in either group (Figure 2G).
Effects of Chronic HFD and GH/IGF-1 Deficiency on Circulating Levels of Inflammatory Cytokines and Chemokines
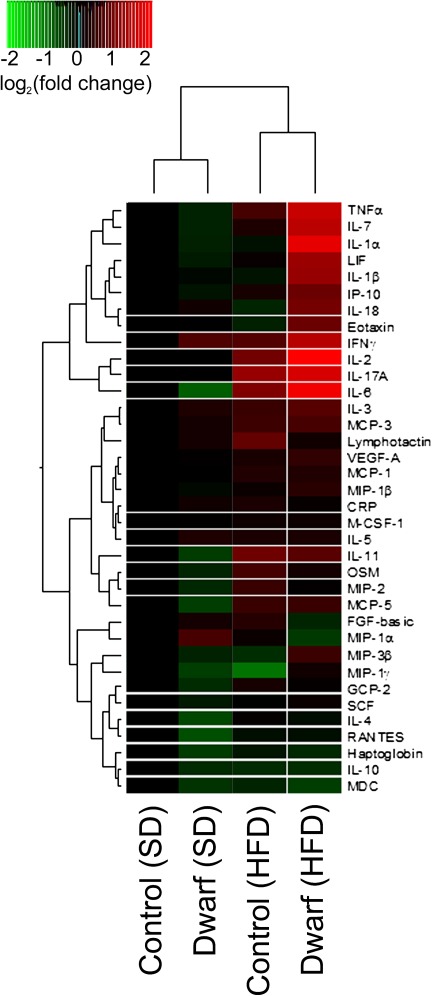
We examined the effects of GH/IGF-1 deficiency and HFD on inflammatory protein expression signature pattern in rat serum using a multiplex protein array. GH/IGF-1 deficiency in SD-fed Lewis dwarf rats was not associated with marked increases in serum levels of inflammatory markers (Figure 3). In contrast, several inflammatory markers had higher expression in HFD-fed Lewis dwarf rats compared with HFD-fed control rats (Figure 3). These markers included several cytokines (eg, TNF-α, IL-6, IL-1β, interferon gamma) and various chemokines (eg, eotaxin, MIP-3β, leukemia inhibitory factor).
Figure 3.
Proteomic profiles of serum inflammatory markers in Lewis dwarf rats and control rats fed a high-fat diet (HFD) or standard diet (SD). The heat map is a graphic representation of normalized cytokine serum concentration values depicted by color intensity, from highest (bright red) to lowest (bright green) expression. Values represent average serum protein expression levels (log2 [fold change, normalized to the respective control mean value]) of replicate SD-fed control rats (n = 5), HFD-fed control rats (n = 5), SD-fed Lewis dwarf rats (n = 5), and HFD-fed Lewis dwarf rats (n = 5). Lewis dwarf rats on HFD have the highest levels of inflammatory markers.
Effects of Chronic HFD and GH/IGF-1 Deficiency on Endothelial Function and Vascular Reactive Oxygen Species Production
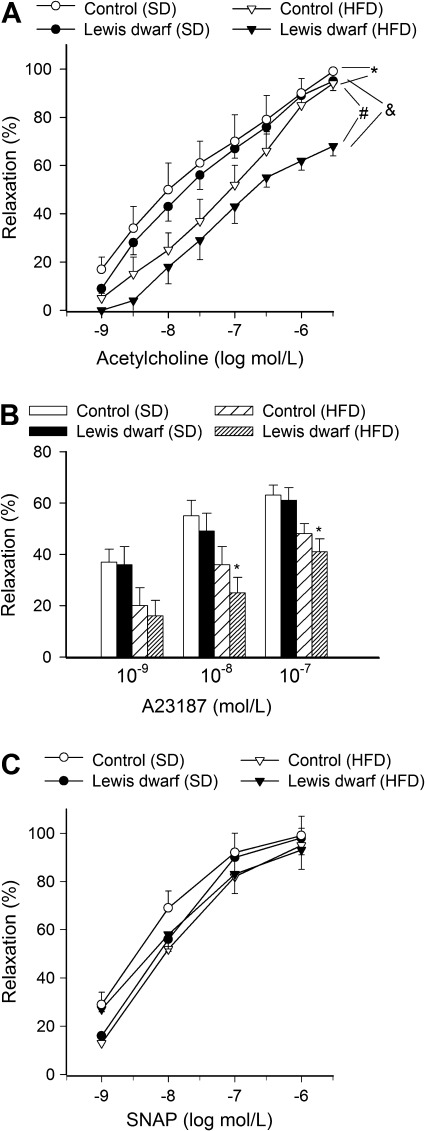
Feeding a HFD elicited significant endothelial dysfunction in aortas of control rats, as shown by the impaired relaxation responses to acetylcholine (Figure 4A) and the calcium ionophore, A23187 (Figure 4B). HFD-induced endothelial dysfunction was more severe in Lewis dwarf rats, as shown by the significantly diminished acetylcholine-induced (Figure 4A) and A23187-induced (Figure 4B) relaxations of aortas of these animals compared with responses obtained in vessels from HFD-fed control rats. In contrast, aorta relaxations in response to the endothelium-independent vasodilator SNAP were unaffected by either GH/IGF-1 deficiency or HFD (Figure 4C).
Figure 4.
Relaxation of aorta rings isolated from Lewis dwarf rats and control rats fed a high-fat diet (HFD) or standard diet (SD). Vasomotor responses were induced by the endothelium-dependent agents acetylcholine (A) and A23187 (B) and S-nitroso-N-acetylpenicillamine (SNAP; C), an endothelium-independent vasodilator. Data are means ± SEM; *p < .05 versus SD-fed control; #p < .05 versus SD-fed Lewis dwarf; &p < .05 versus HFD-fed control (n = 5–7).
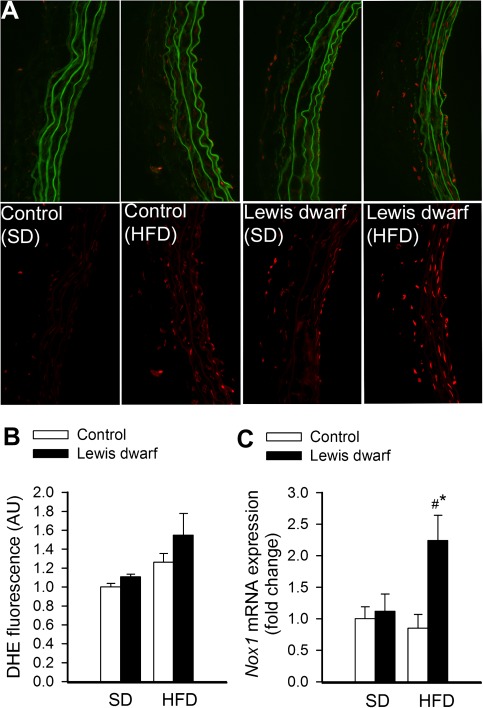
Representative fluorescent images of cross sections of DHE-stained aortas isolated from control and Lewis dwarf rats are shown in Figure 5A. Analysis of nuclear DHE fluorescent intensities indicated that the HFD tended to increase production more in vessels of Lewis dwarf rats than in vessels of control rats, although the difference did not reach statistical significance (Figure 5B). We also found that messenger RNA expression of the Nox1 subunit of the vascular NADPH oxidase was significantly upregulated in the aorta of HFD-fed Lewis dwarf rats as compared with both SD-fed Lewis dwarf rats and HFD-fed control rats (Figure 5C).
Figure 5.
Representative micrographs showing red nuclear dihydroethidium (DHE) fluorescence, representing cellular production, in sections of cultured aortas isolated from Lewis dwarf rats and control rats fed a high-fat diet (HFD) or standard diet (SD). For orientation purposes, overlay of DHE signal and green autofluorescence of elastic laminae is also shown (upper panels). Original magnification: 20×. (B) Summary data for nuclear DHE fluorescence intensities. Data are mean ± SEM. (C) Quantitative real-time reverse transcription–polymerase chain reaction data showing messenger RNA expression of Nox1 in the aortas of HFD-fed and SD-fed Lewis dwarf rats and control rats. Data are mean ± SEM (n = 5–7). *p < . 05 versus SD-fed Lewis dwarf; #p < . 05 versus HFD-fed controls.
Effects of Chronic HFD and GH/IGF-1 Deficiency on Vascular Inflammatory Gene Expression
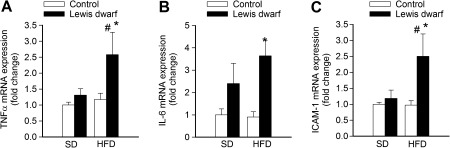
Under basal conditions, inflammatory gene expression did not differ significantly between control rats and Lewis dwarf rats (Figure 6). In contrast, GH/IGF-1 deficiency in Lewis dwarf rats exacerbated vascular inflammation induced by consumption of a HFD, as indicated by the significantly increased messenger RNA expression of TNF-α (Figure 6A), IL-6 (Figure 6B), and ICAM-1 (Figure 6C) in aortas of Lewis dwarf rats.
Figure 6.
Quantitative real-time reverse transcription–polymerase chain reaction data showing messenger RNA expression of tumor necrosis factor-α (TNF-α; A), interleukin-6 (IL-6; B), and ICAM-1 (C) in the aortas of control rats and Lewis dwarf rats fed a high-fat diet (HFD) or standard diet (SD). Data are mean ± SEM (n = 5–7). *p < . 05 versus HFD-fed controls; #p < . 05 versus SD-fed Lewis dwarf.
DISCUSSION
In the present study, the effects of HFD-induced obesity on vascular function and metabolic alterations in a rat model of GH/IGF-1 deficiency were investigated. Interestingly, Lewis dwarf rats share many characteristics with aged animals, including a moderate decline in circulating GH and IGF-1 levels, increased incidence of vascular oxidative stress (25), stroke (60), and neurocognitive decline (29,38,61). Our results provide compelling information that GH/IGF-1 deficiency not only impairs metabolic function but also exacerbates vascular dysfunction and increases the inflammatory response in response to a HFD. Our results are consistent with the hypothesis that the decrease in GH and IGF-1 contributes to vascular dysfunction and an increased inflammatory response with age.
We found that both control rats and Lewis dwarf rats gained significant weight and became obese when consuming a HFD. It is well known that GH and IGF-1 regulate fat metabolism and adipocyte function (62). However, contrary to our prediction, GH/IGF-1 deficiency in Lewis dwarf rats did not affect either relative weight gain or changes in body composition in response to the HFD challenge. Both Lewis dwarf rats that have a specific deficiency in GH and IGF-1 and Ames dwarf mice that have low levels of GH, IGF-1, thyroid-stimulating hormone, and prolactin exhibit low plasma insulin levels and maintain lower fasting glucose concentrations compared with controls, a phenomenon that has been attributed to increased insulin sensitivity in GH/IGF-1–deficient animals (63). It is well accepted that GH administration decreases insulin sensitivity (64), which underlies its diabetogenic effects (65). In addition to alterations in GH levels, previous studies in Ames dwarf mice suggest that increases in insulin sensitivity may be due, at least in part, to increased release of adiponectin and/or altered release of other adipokines (66) as well as changes in the insulin signaling pathway (63,67,68). Interestingly, a recent study on Ecuadorian individuals, who carry mutations in the GH receptor (GHR) gene, indicates that dysfunctional GH signaling and severe IGF-1 deficiency in humans are not associated with a relative hypoglycemia (69). Presently, the underlying mechanism responsible for the discrepancy among the metabolic phenotypes of the Lewis dwarf rats and Ames dwarf mice and GHR-deficient human dwarfs is not well understood.
Importantly, we found that when challenged with a HFD, Lewis dwarf rats exhibit a marked intolerance to glucose coupled with a significant decline in insulin levels as compared with the respective controls. Previous studies also showed that GH/IGF-1–deficient Ames dwarf mice have significantly reduced amounts of insulin and exhibit intolerance to glucose (63). Human dwarfs with GHR deficiency also exhibit an ∼68% decline in insulin (69). We posit that the lower than normal insulin levels in Ames dwarf mice and HFD-fed Lewis dwarf rats may be due to compromised β-cell numbers or function and impaired β-cell compensation in response to metabolic challenge. Indeed, recent studies demonstrate that GH and IGF-1 have key roles in the regulation of β-cell growth and insulin secretion (70). Importantly, when challenged with a HFD, mice with pancreatic β-cell–specific knockdown of the GH receptor (βGHRKO) exhibit impaired glucose tolerance and blunted glucose-stimulated insulin secretion (70).
Both aging and GH/IGF-1 deficiency were reported to be associated with complex alterations in the secretory phenotype of adipocytes. GH-deficient patients have significantly higher adiponectin and leptin levels than controls (71), and treatment with GH was reported to decrease the levels of both adipokines (72). Similar to findings in GH-deficient humans, we found that GH/IGF-1 deficiency in Lewis dwarf rats was associated with increased adiponectin levels but serum levels of leptin were within the normal range. Interestingly, Ames dwarf mice and other mouse models of GH deficiency also have higher levels of adiponectin but exhibit lower levels of leptin (66,73). We found that in control rats, the HFD increased circulating levels of adiponectin, extending the results of previous studies in mice (74,75) and rats (76) fed a similar diet. In healthy animals, high adiponectin levels may confer cardiovascular protection. Because cells of HFD-fed rodents develop adiponectin resistance (77), the vasoprotective action of adiponectin is likely blunted in these animals. Recent studies using adiponectin-deficient mice suggest that the increases in leptin and insulin in response to a HFD are dependent on increases in adiponectin levels (78). Consequently, the lack of significant changes in leptin and insulin in GH/IGF-1–deficient Lewis dwarfs may be the result of the absence of a rise in adiponectin when fed a HFD.
In control animals, as expected, the HFD stimulated an inflammatory response (76) increasing circulating levels of multiple inflammatory cytokines, including IL-6 and TNF-α. Importantly, analysis of serum cytokine expression signatures suggests that chronic GH/IGF-1 deficiency leads to a profound increase in HFD-induced inflammation. This effect is likely a contributing factor in the higher incidence of atherosclerosis and perhaps other chronic inflammatory diseases in IGF-1–deficient individuals. There are multiple sources of inflammatory cytokines and chemokines present in the circulation of HFD-fed animals, including the liver, vasculature, and adipose tissue. Deficiency for IGF-1 in mice has previously been shown to exacerbate HFD-induced inflammatory cytokine expression in the liver (79), but further studies are needed to characterize metabolic stress–induced changes in the secretome of adipocytes and other cell types in chronic GH/IGF-1 deficiency.
Changes in circulating factors and cellular metabolism induced by obesity and consumption of a HFD are known to impair endothelial function by upregulating reactive oxygen species (ROS) production in the vascular wall (33,80), an effect that appears to be exacerbated in aging (7,31,33,57). Our results suggest that GH/IGF-1 deficiency in Lewis dwarf rats exacerbates endothelial dysfunction associated with obesity, likely by increasing vascular oxidative stress. Previously, we found that mitochondrial ROS generation is increased and antioxidant defenses are impaired in arteries of GH/IGF-deficient Ames dwarf mice fed an SD (26). Moreover, isolated hepatic IGF-1 deficiency in mice exacerbates hyperglycemia-induced endothelial dysfunction in vitro (27), but further studies are required to determine whether obesity also exacerbates vascular oxidative stress and endothelial dysfunction in IGF-1–deficient mice. Taken together, the available experimental findings support the view that GH/IGF-1 deficiency in rodents mimics aspects of the vascular aging phenotype (33). Accordingly, consumption of a HFD results in a significantly more severe endothelial impairment in arteries of aged mice as compared with that in vessels from young mice (Z. Ungvari, M.D., Ph.D, manuscript in preparation, 2011).
In previous studies, aging was reported to exacerbate high glucose-induced ROS production in cultured arteries (6), likely by impairing cellular Nrf2-dependent pathways. Importantly, we found that low circulating IGF-1 levels in mice also impair the ability of vascular cells to mount an effective Nrf2-driven antioxidant defense necessary to withstand diverse oxidative stress challenges (27). Of note, IGF-1 was shown to increase Nrf2 activity in endothelial cells via the PI3 kinase–Akt pathway (27). Nrf2 dysfunction induced by endocrine IGF-1 deficiency directly mimics the vascular aging phenotype in that isolated arteries, and cultured endothelial and smooth muscle cells derived from aged animals exhibit a dysfunctional Nrf2-driven response under oxidative stress conditions (5,6). Previously, we demonstrated that genetic lack of a functional Nrf2/ARE pathway also results in significant increases in vascular ROS levels and exacerbation of endothelial dysfunction in arteries of HFD-fed Nrf2−/− mice (34). In addition, IGF-1 can also upregulate eNOS in endothelial cells (26). Most human data also support the concept that normal levels of GH and IGF-1 confer endothelial protection in young individuals. For example, in GH-deficient subjects, flow-mediated endothelium-dependent dilation of peripheral arteries is impaired even at young ages (11,81,82). Plasma markers of oxidative stress are also elevated in GH-deficient subjects and are lowered by GH replacement (82). Furthermore, in vitro IGF-1 and GH treatment reduces ROS production in cultured human endothelial cells (26). Thus, further studies are warranted to test the interaction of GH/IGF-1 deficiency and obesity in aged humans as well.
The deleterious effects of GH/IGF-1 deficiency are likely organ and disease specific. For example, both human GH/IGF-1–deficient dwarfs (69) and Ames dwarf mice (83) exhibit a significant decrease in cancer incidence. The incidence of liver disease also appears to be lower in human GH/IGF-1–deficient dwarfs (69). In that regard, it is interesting that the liver of HFD-fed Lewis dwarf rats also appears to be protected from damage inflicted by HFD (Z. Ungvari, M.D., Ph.D, unpublished observation, 2011), although the mechanism for this effect remains unknown.
There is strong evidence demonstrating that cellular oxidative stress associated with metabolic diseases and aging is causally linked to vascular inflammation (4). We found that obese GH/IGF-1–deficient Lewis dwarf rats exhibit an increased expression of inflammatory mediators and markers of endothelial activation as compared with arteries of obese control animals. These findings are consistent with the results of previous studies demonstrating that IGF-1 confers anti-inflammatory vascular effects (24) and that short-term GH treatment attenuates age-related inflammation, including upregulation of TNF-α, in the cardiovascular system of mouse models of accelerated aging (84). GH/IGF-1 deficiency in aging is also associated with upregulation of TNF-α, IL-6, and other proinflammatory cytokines (44,45,85,86). Importantly, incubation of cultured cells with sera from human GH/IGF-1–deficient dwarfs significantly impairs cellular resistance to H2O2 and upregulates cellular pathways that regulate inflammatory processes (including regulators of NF-κB and activators of IL-6 production [69]). It is likely that chronic oxidative stress and low-grade vascular inflammation associated with GH/IGF-1 deficiency in obese individuals promote the development of atherosclerosis. Indeed, in ApoE knockout mice which were fed a Western diet, even a 20% decline in circulating IGF-1 was shown to significantly increase atherosclerosis progression (87). In contrast, treatment of ApoE-null mice with IGF-1 significantly attenuates HFD-induced vascular oxidative stress, atherosclerotic plaque progression and vascular inflammation (24). Interestingly, a recent study on human dwarfs with GHR mutations demonstrated that severe IGF-1 deficiency in humans is associated with a shorter than expected life span (69), and analysis of the known causes of mortality (independent of accidents and death related to substance abuse) in this population reveals that almost half died as a result of stroke and cardiovascular disease (69). Because in the aforementioned human dwarf population IGF-1 deficiency is associated with an ∼69% increase in the rate of obesity (a cardiovascular risk factor per se), further studies are warranted to study the interaction among GH/IGF-1 deficiency, metabolic status, and parameters relevant for cardiovascular health in the general elderly population (88).
FUNDING
This work was supported by grants from the American Diabetes Association (to Z.U.), American Federation for Aging Research (to A.C. and W.E.S.), the Oklahoma Center for the Advancement of Science and Technology (to A.C., Z.U., and W.E.S.), the University of Oklahoma College of Medicine Alumni Association (to A.C.), the American Heart Association (A.C.), and the National Institutes of Health (AG031085 to A.C.; AT006526 to Z.U.; AG038747, NS056218, and P01 AG11370 to W.E.S.).
Acknowledgments
The authors would like to express their gratitude for the support of the Donald W. Reynolds Foundation, which funds aging research at the University of Oklahoma Health Sciences Center under its Aging and Quality of Life Program.
References
- 1.Fantuzzi G, Mazzone T. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol. 2007;27:996–1003. doi: 10.1161/ATVBAHA.106.131755. [DOI] [PubMed] [Google Scholar]
- 2.Osher E, Stern N. Obesity in elderly subjects: in sheep's clothing perhaps, but still a wolf! Diabetes Care. 2009;32(suppl 2):S398–S402. doi: 10.2337/dc09-S347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rimm EB, Stampfer MJ, Giovannucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older us men. Am J Epidemiol. 1995;141:1117–1127. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 4.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ungvari Z, Bailey-Downs L, Gautam T, et al. Age-associated vascular oxidative stress, nrf2 dysfunction and nf-kb activation in the non-human primate macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ungvari Z, Bailey-Downs L, Sosnowska D, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of nrf2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301(2):H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins AR, Lyon CJ, Xia X, et al. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- 8.Breese CR, Ingram RL, Sonntag WE. Influence of age and long-term dietary restriction on plasma insulin-like growth factor-1 (IGF-1), IGF-1 gene expression, and IGF-1 binding proteins. J Gerontol. 1991;46:B180–B187. doi: 10.1093/geronj/46.5.b180. [DOI] [PubMed] [Google Scholar]
- 9.D’Costa AP, Ingram RL, Lenham JE, Sonntag WE. The regulation and mechanisms of action of growth hormone and insulin-like growth factor 1 during normal ageing. J Reprod Fertil (Suppl.) 1993;46:87–98. [PubMed] [Google Scholar]
- 10.Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res. 2002;54:25–35. doi: 10.1016/s0008-6363(01)00533-8. [DOI] [PubMed] [Google Scholar]
- 11.Evans LM, Davies JS, Goodfellow J, Rees JA, Scanlon MF. Endothelial dysfunction in hypopituitary adults with growth hormone deficiency. Clin Endocrinol (Oxf) 1999;50:457–464. doi: 10.1046/j.1365-2265.1999.00671.x. [DOI] [PubMed] [Google Scholar]
- 12.Denti L, Annoni V, Cattadori E, et al. Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. Am J Med. 2004;117:312–317. doi: 10.1016/j.amjmed.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 13.Oflaz H, Sen F, Elitok A, et al. Coronary flow reserve is impaired in patients with adult growth hormone (GH) deficiency. Clin Endocrinol (Oxf) 2007;66:524–529. doi: 10.1111/j.1365-2265.2007.02767.x. [DOI] [PubMed] [Google Scholar]
- 14.Vasan RS, Sullivan LM, D’Agostino RB, et al. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann Intern Med. 2003;139:642–648. doi: 10.7326/0003-4819-139-8-200310210-00007. [DOI] [PubMed] [Google Scholar]
- 15.Roubenoff R, Parise H, Payette HA, et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89:114–120. doi: 10.1210/jc.2003-030967. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan RC, McGinn AP, Pollak MN, et al. High insulinlike growth factor binding protein 1 level predicts incident congestive heart failure in the elderly. Am Heart J. 2008;155:1006–1012. doi: 10.1016/j.ahj.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen T, Bengtsson BA. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet. 1990;336:285–288. doi: 10.1016/0140-6736(90)91812-o. [DOI] [PubMed] [Google Scholar]
- 19.Bates AS, Van’t Hoff W, Jones PJ, Clayton RN. The effect of hypopituitarism on life expectancy. J Clin Endocrinol Metab. 1996;81:1169–1172. doi: 10.1210/jcem.81.3.8772595. [DOI] [PubMed] [Google Scholar]
- 20.Bulow B, Hagmar L, Mikoczy Z, Nordstrom CH, Erfurth EM. Increased cerebrovascular mortality in patients with hypopituitarism. Clin Endocrinol. 1997;46:75–81. doi: 10.1046/j.1365-2265.1997.d01-1749.x. [DOI] [PubMed] [Google Scholar]
- 21.Tomlinson JW, Holden N, Hills RK, et al. Association between premature mortality and hypopituitarism. West midlands prospective hypopituitary study group. Lancet. 2001;357:425–431. doi: 10.1016/s0140-6736(00)04006-x. [DOI] [PubMed] [Google Scholar]
- 22.Forman K, Vara E, Garcia C, et al. Effect of a combined treatment with growth hormone and melatonin in the cardiological aging on male samp8 mice. J Gerontol A Biol Sci Med Sci. 2001;66A(8):823–834. doi: 10.1093/gerona/glr083. [DOI] [PubMed] [Google Scholar]
- 23.Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. IGF-1, oxidative stress and atheroprotection. Trends Endocrinol Metab. 2010;21:245–254. doi: 10.1016/j.tem.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sukhanov S, Higashi Y, Shai SY, et al. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2684–2690. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- 25.Ungvari Z, Gautam T, Koncz P, et al. Vasoprotective effects of life span-extending peripubertal GH replacement in Lewis dwarf rats. J Gerontol A Biol Sci Med Sci. 2010;65:1145–1156. doi: 10.1093/gerona/glq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csiszar A, Labinskyy N, Perez V, et al. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–H1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey-Downs LC, Mitschelen M, Sosnowska D, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr164. doi:10.1093/gerona/GLR164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitschelen M, Yan H, Farley JA, et al. Long-term deficiency of circulating and hippocampal insulin-like growth factor I induces depressive behavior in adult mice: a potential model of geriatric depression. Neuroscience. 2011;185:50–60. doi: 10.1016/j.neuroscience.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lichtenwalner RJ, Forbes ME, Sonntag WE, Riddle DR. Adult-onset deficiency in growth hormone and insulin-like growth factor-I decreases survival of dentate granule neurons: insights into the regulation of adult hippocampal neurogenesis. J Neurosci Res. 2006;83:199–210. doi: 10.1002/jnr.20719. [DOI] [PubMed] [Google Scholar]
- 30.Ramsey MM, Ingram RL, Cashion AB, et al. Growth hormone-deficient dwarf animals are resistant to dimethylbenzanthracine (dmba)-induced mammary carcinogenesis. Endocrinology. 2002;143:4139–4142. doi: 10.1210/en.2002-220717. [DOI] [PubMed] [Google Scholar]
- 31.Ungvari Z, Sosnowska D, Podlutsky A, Koncz P, Sonntag WE, Csiszar A. Free radical production, antioxidant capacity, and oxidative stress response signatures in fibroblasts from Lewis dwarf rats: effects of life span-extending peripubertal GH treatment. J Gerontol A Biol Sci Med Sci. 2011;66A(5):501–510. doi: 10.1093/gerona/glr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ungvari ZI, Bailey-Downs L, Gautam T, et al. Adaptive induction of nf-e2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133–H1140. doi: 10.1152/ajpheart.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charlton HM, Clark RG, Robinson IC, et al. Growth hormone-deficient dwarfism in the rat: a new mutation. J Endocrinol. 1988;119:51–58. doi: 10.1677/joe.0.1190051. [DOI] [PubMed] [Google Scholar]
- 36.Carter CS, Ramsey MM, Ingram RL, et al. Models of growth hormone and IGF-1 deficiency: applications to studies of aging processes and life-span determination. J Gerontol A Biol Sci Med Sci. 2002;57:B177–B188. doi: 10.1093/gerona/57.5.b177. [DOI] [PubMed] [Google Scholar]
- 37.Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and IGF-1 in aging and lifespan. Trends Genet. 2002;18:295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]
- 38.Hua K, Forbes ME, Lichtenwalner RJ, Sonntag WE, Riddle DR. Adult-onset deficiency in growth hormone and insulin-like growth factor-I alters oligodendrocyte turnover in the corpus callosum. Glia. 2009;57(10):1062–1071. doi: 10.1002/glia.20829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieves-Martinez E, Sonntag WE, Wilson A, et al. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. J Endocrinol. 2010;204:31–36. doi: 10.1677/JOE-09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kizer JR, Arnold AM, Jenny NS, et al. Longitudinal changes in adiponectin and inflammatory markers and relation to survival in the oldest old: the cardiovascular health study all stars study. J Gerontol A Biol Sci Med Sci. 2011;66A(10):1100–1107. doi: 10.1093/gerona/glr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kizer JR, Arnold AM, Strotmeyer ES, et al. Change in circulating adiponectin in advanced old age: determinants and impact on physical function and mortality. The cardiovascular health study all stars study. J Gerontol A Biol Sci Med Sci. 2010;65:1208–1214. doi: 10.1093/gerona/glq122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol. 2007;293:H919–H927. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- 43.Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase c-dependent activation of nad(p)h oxidase. Circulation. 2003;108:1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- 44.Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 45.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tnfalfa treatment in aging. Am J Pathol. 2007;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121–H2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- 47.Ungvari ZI, Orosz Z, Labinskyy N, et al. Increased mitochondrial h2o2 production promotes endothelial nf-kb activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 48.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates tnf-{alpha}-induced activation of coronary arterial endothelial cells: role of nf-{kappa}b inhibition. Am J Physiol. 2006;291:H1694–H1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- 49.Behrens MI, Silva M, Schmied A, et al. Age-dependent increases in apoptosis/necrosis ratios in human lymphocytes exposed to oxidative stress. J Gerontol A Biol Sci Med Sci. 2011;66:732–740. doi: 10.1093/gerona/glr039. [DOI] [PubMed] [Google Scholar]
- 50.Chandrashekara KT, Shakarad MN. Aloe vera or resveratrol supplementation in larval diet delays adult aging in the fruit fly, Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2011;66:965–971. doi: 10.1093/gerona/glr103. [DOI] [PubMed] [Google Scholar]
- 51.Chung E, Diffee GM. Effect of aging on power output properties in rat skinned cardiac myocytes. J Gerontol A Biol Sci Med Sci. 2011;66A(12):1267–1273. doi: 10.1093/gerona/glr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dekker P, de Lange MJ, Dirks RW, et al. Relation between maximum replicative capacity and oxidative stress-induced responses in human skin fibroblasts in vitro. J Gerontol A Biol Sci Med Sci. 2011;66:45–50. doi: 10.1093/gerona/glq159. [DOI] [PubMed] [Google Scholar]
- 53.Endt H, Sprung CN, Keller U, Gaipl U, Fietkau R, Distel LV. Detailed analysis of DNA repair and senescence marker kinetics over the life span of a human fibroblast cell line. J Gerontol A Biol Sci Med Sci. 2011;66:367–375. doi: 10.1093/gerona/glq197. [DOI] [PubMed] [Google Scholar]
- 54.Gunin AG, Kornilova NK, Vasilieva OV, Petrov VV. Age-related changes in proliferation, the numbers of mast cells, eosinophils, and cd45-positive cells in human dermis. J Gerontol A Biol Sci Med Sci. 2011;66:385–392. doi: 10.1093/gerona/glq205. [DOI] [PubMed] [Google Scholar]
- 55.McFarlane D, Wolf RF, McDaniel KA, White GL. Age-associated alteration in innate immune response in captive baboons. J Gerontol A Biol Sci Med Sci. 2011;66A(12):1309–1317. doi: 10.1093/gerona/glr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sloane LB, Stout JT, Vandenbergh DJ, Vogler GP, Gerhard GS, McClearn GE. Quantitative trait loci analysis of tail tendon break time in mice of c57bl/6j and dba/2j lineage. J Gerontol A Biol Sci Med Sci. 2011;66:170–178. doi: 10.1093/gerona/glq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith DL, Jr, Mattison JA, Desmond RA, et al. Telomere dynamics in rhesus monkeys: no apparent effect of caloric restriction. J Gerontol A Biol Sci Med Sci. 2011;66A(11):1163–1168. doi: 10.1093/gerona/glr136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu M, Hu J, Perez E, et al. Effects of long-term cranberry supplementation on endocrine pancreas in aging rats. J Gerontol A Biol Sci Med Sci. 2011;66A(11):1139–1151. doi: 10.1093/gerona/glr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 60.Sonntag WE, Carter CS, Ikeno Y, et al. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- 61.Grill JD, Sonntag WE, Riddle DR. Dendritic stability in a model of adult-onset IGF-I deficiency. Growth Horm IGF Res. 2005;15:337–348. doi: 10.1016/j.ghir.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Berryman DE, List EO, Sackmann-Sala L, Lubbers E, Munn R, Kopchick JJ. Growth hormone and adipose tissue: Beyond the adipocyte. Growth Horm IGF Res. 2011;21:113–123. doi: 10.1016/j.ghir.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dominici FP, Hauck S, Argentino DP, Bartke A, Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (irs)-1 and irs-2 in liver of Ames dwarf mice. J Endocrinol. 2002;173:81–94. doi: 10.1677/joe.0.1730081. [DOI] [PubMed] [Google Scholar]
- 64.Davidson MB. Effect of growth hormone on carbohydrate and lipid metabolism. Endocr Rev. 1987;8:115–131. doi: 10.1210/edrv-8-2-115. [DOI] [PubMed] [Google Scholar]
- 65.Borg KE, Brown-Borg HM, Bartke A. Assessment of the primary adrenal cortical and pancreatic hormone basal levels in relation to plasma glucose and age in the unstressed Ames dwarf mouse. Proc Soc Exp Biol Med. 1995;210:126–133. doi: 10.3181/00379727-210-43931. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z, Al-Regaiey KA, Masternak MM, Bartke A. Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. J Gerontol A Biol Sci Med Sci. 2006;61:323–331. doi: 10.1093/gerona/61.4.323. [DOI] [PubMed] [Google Scholar]
- 67.Louis A, Bartke A, Masternak MM. Effects of growth hormone and thyroxine replacement therapy on insulin signaling in Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2010;65:344–352. doi: 10.1093/gerona/glq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masternak MM, Panici JA, Wang F, Wang Z, Spong A. The effects of growth hormone (GH) treatment on GH and insulin/IGF-1 signaling in long-lived Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2010;65:24–30. doi: 10.1093/gerona/glp172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu Y, Liu C, Sun H, et al. Growth hormone receptor regulates beta cell hyperplasia and glucose-stimulated insulin secretion in obese mice. J Clin Invest. 2011;121:2422–2426. doi: 10.1172/JCI45027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lopez-Siguero JP, Lopez-Canti LF, Espino R, et al. Effect of recombinant growth hormone on leptin, adiponectin, resistin, interleukin-6, tumor necrosis factor-alpha and ghrelin levels in growth hormone-deficient children. J Endocrinol Invest. 2011;34:300–306. doi: 10.1007/BF03347090. [DOI] [PubMed] [Google Scholar]
- 72.Andersson B, Carlsson LM, Carlsson B, Albertsson-Wikland K, Bjarnason R. Decrease in adiponectin levels correlates to growth response in growth hormone-treated children. Horm Res. 2009;71:213–218. doi: 10.1159/000201110. [DOI] [PubMed] [Google Scholar]
- 73.Arumugam R, Fleenor D, Freemark M. Effects of lactogen resistance and GH deficiency on mouse metabolism: pancreatic hormones, adipocytokines, and expression of adiponectin and insulin receptors. Endocrine. 2007;32:182–191. doi: 10.1007/s12020-007-9017-y. [DOI] [PubMed] [Google Scholar]
- 74.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee JH, Bullen JW, Jr., Stoyneva VL, Mantzoros CS. Circulating resistin in lean, obese, and insulin-resistant mouse models: lack of association with insulinemia and glycemia. Am J Physiol Endocrinol Metab. 2005;288:E625–E632. doi: 10.1152/ajpendo.00184.2004. [DOI] [PubMed] [Google Scholar]
- 76.Cano P, Cardinali DP, Rios-Lugo MJ, Fernandez-Mateos MP, Reyes Toso CF, Esquifino AI. Effect of a high-fat diet on 24-hour pattern of circulating adipocytokines in rats. Obesity (Silver Spring) 2009;17:1866–1871. doi: 10.1038/oby.2009.200. [DOI] [PubMed] [Google Scholar]
- 77.Mullen KL, Pritchard J, Ritchie I, et al. Adiponectin resistance precedes the accumulation of skeletal muscle lipids and insulin resistance in high-fat-fed rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R243–R251. doi: 10.1152/ajpregu.90774.2008. [DOI] [PubMed] [Google Scholar]
- 78.Hecker PA, O’Shea KM, Galvao TF, Brown BH, Stanley WC. Role of adiponectin in the development of high fat diet-induced metabolic abnormalities in mice. Horm Metab Res. 2011;43:100–105. doi: 10.1055/s-0030-1269898. [DOI] [PubMed] [Google Scholar]
- 79.Wu Y, Brodt P, Sun H, et al. Insulin-like growth factor-I regulates the liver microenvironment in obese mice and promotes liver metastasis. Cancer Res. 2010;70:57–67. doi: 10.1158/0008-5472.CAN-09-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Molnar J, Yu S, Mzhavia N, Pau C, Chereshnev I, Dansky HM. Diabetes induces endothelial dysfunction but does not increase neointimal formation in high-fat diet fed c57bl/6j mice. Circ Res. 2005;96:1178–1184. doi: 10.1161/01.RES.0000168634.74330.ed. [DOI] [PubMed] [Google Scholar]
- 81.Smith JC, Evans LM, Wilkinson I, et al. Effects of GH replacement on endothelial function and large-artery stiffness in GH-deficient adults: a randomized, double-blind, placebo-controlled study. Clin Endocrinol (Oxf) 2002;56:493–501. doi: 10.1046/j.1365-2265.2002.01514.x. [DOI] [PubMed] [Google Scholar]
- 82.Evans LM, Davies JS, Anderson RA, et al. The effect of GH replacement therapy on endothelial function and oxidative stress in adult growth hormone deficiency. Eur J Endocrinol. 2000;142:254–262. doi: 10.1530/eje.0.1420254. [DOI] [PubMed] [Google Scholar]
- 83.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 84.Forman K, Vara E, Garcia C, Ariznavarreta C, Escames G, Tresguerres JA. Cardiological aging in sam model: effect of chronic treatment with growth hormone. Biogerontology. 2010;11(3):275–286. doi: 10.1007/s10522-009-9245-z. [DOI] [PubMed] [Google Scholar]
- 85.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in rat coronary arteries. FASEB J. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 86.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 87.Shai SY, Sukhanov S, Higashi Y, Vaughn C, Rosen CJ, Delafontaine P. Low circulating insulin-like growth factor I increases atherosclerosis in ApoE-deficient mice. Am J Physiol Heart Circ Physiol. 2011;300(5):H1898–H1906. doi: 10.1152/ajpheart.01081.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thorner MO. Statement by the growth hormone research society on the GH/IGF-I axis in extending health span. J Gerontol A Biol Sci Med Sci. 2009;64:1039–1044. doi: 10.1093/gerona/glp091. [DOI] [PubMed] [Google Scholar]