Abstract
Mole-rat of the genus Fukomys are mammals whose life span is strongly influenced by reproductive status with breeders far outliving nonbreeders. This raises the important question of whether increased longevity of the breeders is reflected in atypical expression of biochemical markers of aging. Here, we measured markers of glycation and advanced glycation end-products formed in insoluble skin collagen of Ansell’s mole-rat Fukomys anselli as a function of age and breeding status. Glucosepane, pentosidine, and total advanced glycation end-product content significantly increased with age after correction for breeder status and sex. Unexpectedly, total advanced glycation end-products, glucosepane, and carboxymethyl-lysine (CML) were significantly higher in breeders versus nonbreeders suggesting that breeders have evolved powerful defenses against combined oxidant and carbonyl stress compared with nonbreeders. Most interestingly, when compared with other mammals, pentosidine formation rate was lower in mole-rat compared with other short-lived rodents confirming previous observations of an inverse relationship between longevity and pentosidine formation rates in skin collagen.
Keywords: Longevity, Collagen, Glycemia, Markers, Oxidative stress
AFRICAN mole-rat (family Bathyergidae) are a rodent family endemic to sub-Saharan Africa comprising of six genera: Heterocephalus, Heliophobius, Bathyergus, Georhychus, Cryptomys, and Fukomys (1). For several reasons, this family is especially suited for aging studies. First, all bathyergid species are strictly subterranean and thus presumably suffer a lower risk of dying from predators, parasites, or climatic extremes than surface-dwelling rodents. This may explain the extraordinary longevity exhibited by some species (2,3), which have documented maximum life spans of more than 16 years (Fukomys mechowii [4]), approximately 20 years (Fukomys anselli [5]), or even 30 years (Heterocephalus glaber [6]). Second, the Bathyergidae are one of a very few mammalian families to contain species with a eusocial organization, namely in the naked mole-rat Heterocephalus and several species of the genus Fukomys (7–11). This means that just like hymenopterans and termites, societies of these mole-rat are characterized by monopolization of reproduction by very few individuals (often just a single breeding pair), reproductive altruism of their offspring which involves nonbreeding and cooperative alloparental brood care, and long-lasting philopatry resulting in an overlap of several adult generations (7–9,11). Evolution of eusociality has led to a peculiar aging phenotype in eusocial insects, namely extreme longevity (some ant queens can live up to 30 years [12]), caste-specific aging rates within species, and a reversal of the trade-off between reproductive investment and longevity (12), which is characteristic of most other organisms (13–15).
In the genus Fukomys sp., but apparently not in H glaber (16,17), it is observed in the laboratory setting that breeders far outlive nonreproductive “helpers.” For example, breeders of both sexes live approximately 1.5–2 times longer than their nonbreeding counterparts, both in terms of mean and maximum life span (4,5). The remarkable benefit of breeding in this species is greater than most experimental interventions that have extended longevity in other vertebrate model organisms. These include caloric restriction (18) and diets containing resveratrol, as tested in the short-lived fish Nothobranchius furzeri (19), or rapamycin, which was tested in mice (20). Thus, Fukomys mole-rat present themselves as unique subjects for the study of divergent aging rates within a single mammalian genotype, therefore avoiding the inevitable limitations of interspecies comparisons (21). The mechanisms for life prolongation in breeders versus nonbreeders are currently not understood (4,5).
In the present study, we measured skin glycation and advanced glycosylation (or glycoxidation) end products, termed “advanced glycation end-products (AGEs),” which were previously found to increase with individual age in long-lived tissue proteins of many vertebrates (22–25) and have been shown to correlate with overall longevity across several mammal species (26). AGEs are irreversible bonds between proteins and carbohydrates formed as the stable end products of the Maillard reaction. This term collectively describes unprogrammed nonenzymatic reactions between proteins and carbohydrates, ultimately modifying both reaction partners (27).
In skin, the extent of glycation of collagen is dependent upon several factors, one of which is glycemic status. Glycation of collagen is in equilibrium with ambient blood glucose concentration, thus the higher the glycemia, the greater the extent of collagen glycation (28). The reaction of glucose with collagen occurs readily by an amino-carbonyl reaction between a lysyl residue and a glucose molecule forming an initial Schiff base followed by an Amadori product. With increasing age, the advanced Maillard reaction converts the Amadori products into irreversible AGEs (29,30). It has been hypothesized that AGE formation might contribute to the aging process as heavy loads of AGEs might have the potential to impair protein folding, stability, and function (31) and subsequently tissue integrity (29,32–34). However, whether the role of AGEs in aging is causative or just correlative is still under debate (27).
Here, we have quantified a number of markers of the Maillard reaction (ie, glycation and AGE formation) in skin collagen of individual Ansell's mole-rat (F anselli) and determined whether there is a relationship between the accumulation rates of these products with chronological age and breeder status. Assays comprise the Amadori product (fructose-lysine), which nonoxidatively gives rise to the irreversible AGE cross-link glucosepane (Figure 1 [33]). Additionally, we have determined the methylglyoxal-derived AGEs, that is, carboxyethyl-lysine (CEL) and methylglyoxal hydroimidazolone isomer 1 (MG-H1); the oxidative fragmentation product of fructose-lysine, that is, carboxymethyl-lysine (CML) and pentosidine; and the product of glyoxal reaction with arginine residues, that is, glyoxal hydroimidazolone 1 (G-H1). These may also originate from non-Amadori pathways, including glycolysis and the metabolism of threonine and aminoacetone (methylglyoxal), or from lipid peroxidation and inflammation (glyoxal [35–37]). Except for fructose-lysine, all these AGE markers have previously been shown to significantly increase with age in human skin collagen (34).
Figure 1.
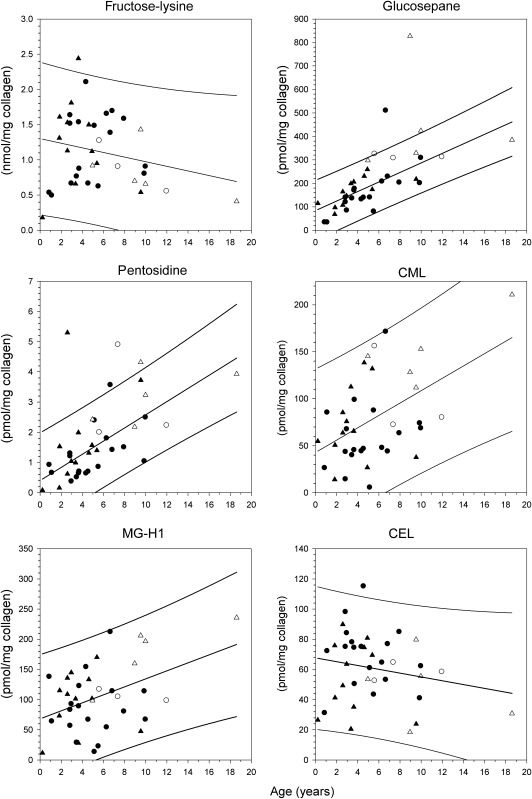
Levels of fructose-lysine, glucosepane, pentosidine, carboxymethyl-lysine (CML), methylglyoxal hydroimidazolone isomer 1 (MG-H1), and carboxyethyl-lysine (CEL) quantified in insoluble collagen skin from Ansell’s mole-rat as a function of age, gender, and breeder status. Levels are expressed as picomoles per milligram collagen except for fructose-lysine (nanomoles per milligram collagen). For each graph, the regression line and 95% confidence intervals are shown as determined for all mole-rat in the study. The equations for these regression lines are shown in Table 1. For glucosepane and pentosidine, there were two outliers each, which were not included in these analyses (see Methods). •, male nonbreeder; ▴, female nonbreeder; ○, male breeder; Δ, female breeder.
The purpose of this study was to establish the potential of these markers as possible biomarkers of aging in F anselli in exploring the mechanism for life extension in this species based upon the Maillard reaction. In particular, two hypotheses were tested: (a) AGEs should accumulate in skin collagen with chronological age and (b) breeders, being longer lived, should accumulate AGEs at a slower rate than the shorter-lived nonbreeders, as would be predicted if the accumulation of AGEs was part of/causal for the aging process itself.
MATERIALS AND METHODS
Chemicals
Unless indicated, chemicals were purchased from either Sigma–Aldrich or Thermo Fisher Scientific. The internal standards used for mass spectrometry were synthesized as described (36). The pentosidine standard used in the high-performance liquid chromatography (HPLC) assays was obtained from the International Maillard Reaction Society (www.imars.org/online/).
Mole-Rat and Husbandry
The Ansell’s mole-rat, F anselli, was used in this study. Colonies have been maintained in captivity in Frankfurt a. Main, Germany, since 1984, and in Essen, Germany, since 1995, under conditions previously detailed elsewhere (5,38). In brief, animals are kept as pairs or families in terraria with horticultural peat, whereas food (mainly carrots and potatoes, regularly supplemented with apple, grain feed, and lettuce) was provided ad libitum. Light regime followed the natural local rhythms. Room temperature was maintained between ca. 20–24°C throughout all seasons. Systemic breeding started using a stock of 29 wild-caught animals. In order to maintain sufficiently high levels of genetic diversity in our breeding stock, we supplemented this initial population with several dozens of additional wild-caught founder animals from various field trips in subsequent years. Over the whole study period, breeders (founders of laboratory colonies) were recruited randomly and retained breeding status until they or their partner died with most of their offspring remaining in their natal colonies. Due to a strict inbreeding avoidance naturally inherited by these social animals, adult offspring remain reproductively quiescent unless removed from their colonies and paired to an unfamiliar mate (38).
Breeders and Nonbreeders
The breeders used in this study consisted of animals with an age of 5 to >18 years (mean: 9.61 years) that had been reproductively active for at least 12 months and successfully used to establish a new colony. Note that male and female breeders have entered the study in different numbers because we only used laboratory-raised individuals whose age was exactly known. In many of our current laboratory colonies, the reproductive pair consists of one wild-caught and one laboratory-raised animal, and in such cases, only the latter could be incorporated into our study.
Nonbreeders consisted of laboratory-born females and males from 3 months up to >9 years of age (mean: 4.33 years) that never had sexual intercourse with conspecifics before or after reaching sexual maturity.
Procurement of Skin Biopsies
Skin biopsies were obtained surgically from individual mole-rat. Upon anesthesia with isoflurane, a 4- to 6-mm diameter skin biopsy was taken from the dorsal region after shaving with surgical clippers and prepping with iodine solution. The incision was closed with surgical suture. Each biopsy was washed in saline and stored at −80°C. Upon completion of procurement, all biopsies were shipped from Germany to Cleveland using express courier service. Biopsies were stored at −80°C until processing. All surgical procedures were approved by the university’s animal welfare officer and the responsible authorities of North Rhine Westphalia, Germany.
Processing of Skin Biopsies
Biopsies were extracted for 24 hours at 4°C with 2:1 chloroform:methanol in glass tubes followed by washing with methanol and then water. Samples were placed in 2-mL polypropylene micro tubes (SARSTEDT, Newton, NC) and homogenized by hand with a glass rod in 0.7 mL Chelex-treated phosphate-buffered saline. The insoluble material was recovered with a high-speed microcentrifuge (TOMY MRX-151; Tomy Seiko Co., Tokyo, Japan) run at maximum speed (15,223 rpm ∼10,363g). The insoluble material was sequentially extracted for 24 hours at 4°C with 1 mL each of 1 M NaCl, 0.5 M acetic acid, and 0.005% porcine pepsin (P-6887; Sigma) in 0.5 M acetic acid as previously described (39). The insoluble collagen was recovered with the microcentrifuge and freeze dried. The average yield of insoluble collagen from all 38 skin biopsies was 3.13 ± 1.1 mg (SD).
Enzymatic Digestion of Skin Collagen
One milligram of each insoluble collagen preparation was weighed-out and placed in a 0.5-mL polypropylene micro tube (SARSTEDT). Each sample was washed twice with 250 μL of buffer consisting of 0.1 M CaCl2 and 0.02 M HEPES, pH 7.5. The pellet was digested for 24 hours at 37°C with 100 μL (28 U) collagenase (C0773; Sigma). The supernatant was removed and the residual pellet was redigested for 24 hours with 40 μL (11 U) collagenase. The supernatants were combined and, in turn, sequentially digested at 37°C at 24-hour intervals with 5 μL (0.024 U) peptidase (P7500; Sigma), 10 μL (0.2 U) pronase (Roche), and 2 μL (0.04 U) aminopeptidase M (Roche) as described (33). Collagen was determined in the digests by the hydroxyproline assay assuming a content of 14% hydroxyproline by weight (26). The digestion efficiency averaged 53% ± 14% based on this assay.
Preparation of Acid Hydrolysates
One milligram of each insoluble collagen sample was weighed and placed in a 13 × 100–mm glass tube with a Teflon-lined screw cap (40). To each tube was pipetted 1 mL of 6 N HCl, which was degassed and purged with nitrogen beforehand. Each tube was purged with nitrogen before sealing with the cap. All tubes were placed in a 110°C oven for 18 hours followed by evaporating the acid with a SpeedVac Concentrator (Savant; Thermo Fisher). Each sample was reconstituted with 300 μL water and filtered using a Spin-X centrifuge tube filter (Costar; Corning Inc., Corning, NY). The collagen content of each sample was determined by the hydroxyproline assay (26).
Analytical Measurements
Levels of fructose-lysine (FL), glucosepane, carboxymethyl-lysine (CML), carboxyethyl-lysine (CEL), methylglyoxal hydroimidazolone isomer 1 (MG-H1), glyoxal hydroimidazolone isomer 1 (G-H1), and methionine sulfoxide (MetSox) were determined in the digests by electrospray positive ionization–mass spectrometric multiple reaction monitoring (ESI + MRM) as described (36).
The liquid chromatography-tandem mass spectrometry system consisted of a Waters Alliance 2695 Separations Module HPLC coupled to a Micromass Quattro Ultima triple quadropole mass spectrometer, both operated with a computer using MassLynx Software V4.1 (Waters Corp., Milford, MA). Products were separated by using two Hypercarb HPLC columns connected in series: first, 50 × 2.1 mm, 5 μM Hypercarb; second, 250 × 2.1 mm, 5 μM Hypercarb (Thermo Fisher).
The solvents used were (a) 0.1% formic acid in water buffered to pH 3.6 with ammonia, (b) 90% acetonitrile (Burdick & Jackson) in water, and (c) water with 50% tetrahydrofuran and 0.1% formic acid. The gradient program consisted as follows: 0–17 minutes, 100% A; 17–25 minutes, 0%–34.4% B (linear); 25–30 minutes, 34.4% B; 31–41 minutes, 100% C; and 42–70 minutes, 100% A.
For each sample, 30 μL of digest was mixed with 70 μL solvent A, of which 40 μL of this mixture (≈350 ± 90 μg total collagen) was injected onto the HPLC at flow rate 200 μL/min. The order of elution from the second column was MetSox (6.81 minutes), CML (7 minutes), CEL (8.71 minutes), and FL (8.81 minutes). This was followed by detaching the second column from the system at 18 minutes using the three-column selection valve in the Alliance after which MG-H1 (24 minutes), G-H1 (24.47 minutes), and glucosepane (25.7 minutes) eluted from the first column.
The following transitions were monitored by the MRM program (m/z molecular ion→m/z product ion): MetSox (166→102), CML (205→130), CEL (219→130), FL (309→84), MG-H1 (229→114), G-H1 (215→152), and glucosepane (429→384); internal standards, d4-MetSox (170→106), d4-CML (209→134), d3-CEL (222→85), 13C-FL (315→84), d3-MG-H1 (232→115), d2-G-H1 (217→102), and 13C-glucosepane (435→390).
Pentosidine was quantified by HPLC autofluorescence (41). The Waters HPLC system consisted of a model 680 gradient controller, two 515 HPLC pumps, and a 717 Autosampler. Fluorescence was monitored at excitation/emission 335/385 nm by a JASCO 821-FP detector (JASCO, Easton, MD). Separations were made with a 150 × 2.1 mm, 3 μM Discovery HS C18 column using a 20 × 2.1 mm, 3 μM, 120 A Discovery HS C18 guard column (Sigma–Aldrich) at flow rate 200 μL/min. The solvents were (a) 98% water—2% acetonitrile with 0.1 M heptafluorobutyric acid (HFBA) and (b), 60% acetonitrile—40% water. The gradient program was 0–35 minutes, 15%–23% B (linear); 35–37 minutes, 23%–100% B (linear); 37–49 minutes, 100% B; 50–53 minutes, 100% A; and 54–60 minutes, 15% B. For each sample, 64 μg of collagen in a 10 μL volume was injected onto the HPLC. Pentosidine eluted at ≈36 minutes.
Statistical Analyses
The main questions of this study were whether the levels of markers for protein glycation (FL), protein oxidation (MetSox), single endpoints of the Maillard reaction (“AGEs”), or total AGE content (defined as the sum of all AGEs, ie, CML, CEL, pentosidine, glucosepane, G-H1, and MG-H1, as expressed in picomoles per milligram collagen) are associated with chronological age and whether their accumulation reflects the divergent aging rates of breeders versus nonbreeders. We therefore computed a general linear model with age as the continuous predictor variable and breeding status and sex as fixed covariates. We examined all three hierarchically nested regression models (Model 1: age; Model 2: age + status; Model 3: age + status + sex), reporting two-sided p values and β estimators of the effect with 95% confidence intervals. For correlative analysis between marker levels, partial correlations controlling for age, sex, and status were conducted. Here, two-sided p values and Pearson’s r correlation coefficients are being reported.
The statistical test for outliers was made according to the method of Snedecor and Cochran (42). Upon testing, both glucosepane and pentosidine were found to have two outliers above the 95% confidence intervals of the entire cohort (Figure 1): glucosepane at (x, y) coordinates (6.62, 512), (8.94, 827), both p < .0001; pentosidine (2.59, 5.3), (7.34, 4.92), p = .0001 and p < .0001, respectively. These outliers were omitted from the statistical analyses.
Because pentosidine formation rate in skin collagen had previously been shown to correlate with maximum life span across different animal species (26), a follow-up analysis was made to determine pentosidine formation rate of Ansell’s mole-rat and relate it to the respective values of other animal species (Table 3). In this analysis, the plot of pentosidine level versus age for each animal was modeled by a quadratic function, whereas the coefficient of x2 for each equation represented the accelerated rate component of pentosidine formation in skin (Table 3). For mole-rat, this meant remodeling the pentosidine data shown in Figure 1 from a linear to a quadratic equation (Table 3). Because it was previously found that pentosidine accumulation with age in skin collagen was best modeled overall by a quadratic function in individual animal species (26), this uniformity in the use of the same statistical model allowed the comparison of pentosidine formation rates across these diverse species. In Table 3, rates for shrew, dog, cow, pig, and human represent an updated modification from that previously published (26) using pentosidine data corrected for differences in pentosidine standard now and then. More recent data have also been added including that for the Fischer 344 rat (43), C57BL/6 mouse (41), DBA mouse (DBA/2NNia; D. R. Sell, Ph.D, unpublished results, 2000), and ad libitum–fed rhesus and squirrel monkeys (22).
Table 3.
The Comparison of Pentosidine Formation Rate in Skin Collagen of Ansell's Mole-Rats With Other Animal Species*
| Correlation With Age |
||||||
| Species | Maximum Life Span (y)† | Level vs Age Regression Line Model (quadratic equation) | n | r | p | Pentosidine Formation Rate (coefficient of x2) |
| Fischer 344 rat | 3.0 | y = 0.182x2 + 0.066x + 0.006 | 26 | .93 | <.0001 | 0.1823 |
| DBA mouse | 2.5 | y = 0.059x2 + 0.068x + 0.020 | 50 | .94 | <.0001 | 0.0593 |
| C57 mouse | 3.0 | y = 0.036x2 + 0.072x + 0.036 | 31 | .64 | .0001 | 0.0357 |
| Least shrew | 3.5 | y = 0.081x2 + 0.195x + 0.118 | 41 | .96 | <.0001 | 0.0807 |
| Dog | 20 | y = 0.044x2 + 0.334x + 0.634 | 44 | .93 | <.0001 | 0.0441 |
| Cow | 20(30)† | y = 0.042x2 + 0.235x + 0.326 | 17 | .92 | <.0001 | 0.0424 |
| Pig | 27 | y = 0.020x2 + 0.083x + 0.087 | 37 | .98 | <.0001 | 0.0196 |
| Ansell’s mole-rat | 20‡ | y = 0.007x2 + 0.123x + 0.517 | 36 | .74§ | <.0001 | 0.0073 |
| Squirrel monkey | 30 | y = 0.002x2 + 0.003x + 0.746 | 42 | .40 | .008 | 0.0017 |
| Rhesus monkey | 40 | y = 0.001x2 + 0.070x + 0.990 | 41 | .75 | <.0001 | 0.0012 |
| Human | 120 | y = 0.002x2 + 0.088x + 0.927 | 44 | .93 | <.0001 | 0.0020 |
Notes: Plot of pentosidine level (y, picomoles per milligram collagen) versus age (x, years) was modeled by a quadratic equation for each animal species (26).
Pentosidine formation rate was determined by the coefficient of x2 (26).
Maximum life spans were from Sell and colleagues (26) and updated using the following sources: de Magalhaes and Costa (44); that is, An Age/The Animal Ageing & Longevity Database (http://genomics.senescence.info/species/); F344 rat, DBA, and C57 mouse were from Turturro and colleagues (45); domestic cow is estimated to be either 20 (46) or 30 years (47).
The maximum reported life span for Ansell's mole-rat is ≈20 years (5). However, this is an underestimation due to the original mole-rat had been caught in the wild as an adult of unknown age and survived in the laboratory for more than 19 years.
To allow comparisons with the other results, this value was derived from r2 in Model 1 (Table 2) without correction for status or sex.
Slope comparisons between breeders and nonbreeders (Table 1) were done by the methods of Snedecor and Cochran (42). Regression analysis, analysis of covariance, Pearson and partial correlations, and Levene’s test of equality of variances were computed by SPSS v11.5 or v17.0 (IBM Corp., Somers, NY). For data plotting (Figures 1 and 2), the 95% confidence intervals of prediction were determined by SigmaPlot v11 (Systat Software Inc., Chicago, IL). A significance level α of 5% was applied in all tests.
Table 1.
Individual Rates of Age-Related Accumulation of Maillard Reaction Products in Insoluble Skin Collagen of Ansell's Mole-Rat Categorized into Nonbreeders Versus Breeders
| Correlation With Age |
Slope Comparison Probability (nonbreeder vs breeder) | |||||||
| Maillard Reaction Product | Cohort | n | M ± SD* (pmol or nmol/mg collagen) | Regression Equation x = Age (years) y = Marker Level | r | p | Accumulation Rate† (slope) | p |
| Carboxymethyl-lysine (CML) | All | 38 | 79 ± 48 | y = 43 + 7x | .50 | .001 | 6.6 | >.60 |
| Nonbreeders | 30 | 65 ± 38 | y = 55 + 2x | .15 | .42 | 2.3 | ||
| Breeders | 8 | 132 ± 45 | y = 90 + 4x | .43 | .29 | 4.4 | ||
| Carboxyethyl-lysine (CEL) | All | 38 | 61 ± 23 | y = 68 − 1.3x | −.20 | .22 | −1.27 | >.60 |
| Nonbreeders | 30 | 63 ± 23 | y = 64 − 0.3x | −.03 | .87 | −0.28 | ||
| Breeders | 8 | 52 ± 19 | y = 66 − 1.5x | −.34 | .42 | −1.49 | ||
| Glyoxal hydroimidazolone (G-H1) | All | 38 | 28 ± 17 | y = 31 − 0.5x | −.11 | .51 | −0.51 | >.70 |
| Nonbreeders | 30 | 29 ± 17 | y = 29 − 0.1x | −.02 | .93 | −0.11 | ||
| Breeders | 8 | 25 ± 20 | y = 33 − 0.8x | −.17 | .68 | −0.80 | ||
| Methylglyoxal hydroimidazolone (MG-H1) | All | 38 | 105 ± 55 | y = 68 + 7x | .44 | .006 | 6.6 | .16 |
| Nonbreeders | 30 | 92 ± 49 | y = 89 + 0.7x | .04 | .85 | 0.7 | ||
| Breeders | 8 | 152 ± 55 | y = 69 + 9x | .68 | .06 | 8.7 | ||
| Pentosidine | All | 36‡ | 1.60 ± 1.09 | y = 0.4 + 0.2x | .74 | <.0001 | 0.22 | .062 |
| Nonbreeders | 29 | 1.29 ± 0.88 | y = 0.3 + 0.2x | .64 | <.0001 | 0.22 | ||
| Breeders | 7 | 2.90 ± 0.93 | y = 1.8 + 0.1x | .55 | .20 | 0.11 | ||
| Glucosepane | All | 36‡ | 193 ± 96 | y = 84 + 20x | .78 | <.0001 | 20.3 | .016 |
| Nonbreeders | 29 | 157 ± 65 | y = 77 + 19x | .73 | <.0001 | 18.5a | ||
| Breeders | 7 | 341 ± 46 | y =289 + 5x | .54 | .21 | 5.4b | ||
| Total AGEs§ | All | 34‡ | 454 ± 168 | y = 284 + 32x | .71 | <.0001 | 31.7 | .659 |
| Nonbreeders | 28 | 395 ± 102 | y = 310 + 19x | .49 | <.01 | 19.3 | ||
| Breeders | 6 | 731 ± 139 | y = 587 + 14x | .51 | .153 | 14.6 | ||
| Total bound glucose¶ | All | 36‡ | 1320 ± 529 | y = 1378 − 11x | .007 | .329 | 11.0 | .243 |
| Nonbreeders | 29 | 1343 ± 564 | y = 1235 + 25x | .11 | .277 | 25.2 | ||
| Breeders | 7 | 1223 ± 355 | y = 1691 − 48x | .63 | .065 | −48.2 | ||
| Fructose-lysine | All | 38 | 1.12 ± 0.52 | y = 1.3 − 0.03x | −.23 | .17 | −0.03 | .10 |
| Nonbreeders | 30 | 1.19 ± 0.54 | y = 1.2 + 0.01x | .044 | .82 | 0.009 | ||
| Breeders | 8 | 0.86 ± 0.35 | y = 1.4 − 0.05x | −.646 | .08 | −0.053 | ||
| Methionine sulfoxide (MetSox) | All | 37‡ | 14 ± 7 | y = 13 − 0.04x | −.03 | .86 | −0.038 | >.80 |
| Nonbreeders | 29 | 14 ± 8 | y = 13 − 0.1x | −.06 | .75 | −0.12 | ||
| Breeders | 8 | 13 ± 3 | y = 15 − 0.2x | −.32 | .44 | −0.19 | ||
Notes: Products listed are contributable to glycation (fructose-lysine), AGEs (glucosepane, pentosidine, CML, MG-H1, CEL, G-H1), and oxidation (MetSox). AGEs = advanced glycation end-products.
Levels for fructose-lysine and methionine sulfoxide are expressed as nanomoles per milligram collagen, all others picomoles per milligram collagen.
Formation rate is equivalent to the slope of the linear regression line equation. Values (ie, means or accumulation rates) within a parameter with different superscript letters are significantly different (p < .05).
Two outliers are not included in the analyses of glucosepane and pentosidine, one outlier is not included in the analysis of MetSox (see Figure 1).
Define as sum of all AGEs, that is, glucosepane + pentosidine + CML + CEL + MG-H1 + G-H1.
Fructose-lysine levels were converted to picomoles per milligram collagen and added to glucosepane.
Figure 2.
Levels of glyoxal hydroimidazolone isomer 1 (G-H1) and methionine sulfoxide (MetSox) determined in insoluble collagen skin from Ansell's mole-rat. Levels for G-H1 and MetSox are expressed as picomoles and nanomoles per milligram collagen, respectively (see Figure 1).
RESULTS
Figures 1 and 2 show insoluble skin collagen levels of products due to glycation (fructose-lysine), AGE (glucosepane, pentosidine, CML, MG-H1, CEL, and G-H1), and oxidation (MetSox) plotted versus chronological age for Ansell’s mole-rat differentiated as to breeder status and sex. Mean values, regression analyses, and slope comparisons for marker levels in breeding and nonbreeding Ansell’s mole-rat are summarized in Table 1.
When chronological age was used as the only predictor variable across all animals (Model 1, Table 2), significant increases were found for the following markers: total AGEs, glucosepane, pentosidine, CML, and MG-H1 (all p ≤ .01). After including breeding status (Model 2, Table 2), the age effect remained significant only for total AGEs, glucosepane, and pentosidine (p ≤ .05); breeding status itself influenced marker levels significantly in total AGEs, glucosepane, and CML. Surprisingly, in all these markers, the values were higher for breeders than for nonbreeders after controlling for age and sex. All age and status effects remained significant after including sex as a third variable (Model 3, Table 2). A significant sex effect was detectable for one AGE (CEL: p = .049), and this variable affected total AGEs borderline nonsignificantly (p = .070, Table 2). Of note, in both of these markers and also in other markers with nonsignificant but relatively small p values (CML, MG-H1), the respective levels were higher in females than in males. Still, in all markers that were significantly affected by breeding status, the values were higher for breeders than for nonbreeders after controlling for age and sex (see Tables 1 and 2). Markers of protein glycation (FL) or oxidation (MetSox) did not accumulate with age and were not affected by breeding status or sex (Table 2). Overall, these results would support the first hypothesis (see Introduction) but reject the second one.
Table 2.
Levels of Advanced Glycation End-Products (AGEs), Fructose-lysine and Methionine Sulfoxide (MetSox) as a Function of Chronological Age, Breeder Status, and Sex
| Model 1: Age |
Model 2: Age + Status |
Model 3: Age + Status + Sex |
||||||||||
| β | 95% Confidence | p | % Variance Explained* | β | 95% Confidence | p | % Variance Explained* | β | 95% Confidence | p | % Variance Explained* | |
| CML | ||||||||||||
| Age (y) | 6.247 | 2.35 to 10.14 | .003 | 0.250 | 3.139 | −1.26 to 7.54 | .157 | 0.057 | 3.47 | −0.85 to 7.80 | .112 | 0.073 |
| Status | — | — | — | — | 51.31 | 12.45 to 90.18 | .011 | 0.170 | 44.94 | 6.10 to 83.77 | .025 | 0.140 |
| Sex | — | — | — | — | — | — | — | — | 20.62 | −5.06 to 46.30 | .112 | 0.073 |
| All | 0.250 | 0.379 | 0.424 | |||||||||
| CEL | ||||||||||||
| Age (y) | −0.834 | −0.29 to 1.26 | .424 | 0.020 | −0.77 | −3.33 to 1.795 | .512 | 0.010 | −1.01 | −3.48 to 1.46 | .413 | 0.020 |
| Status | — | — | — | — | −7.40 | −30.04 to 15.24 | .546 | 0.012 | −2.83 | −25.0 to 19.34 | .797 | 0.002 |
| Sex | — | — | — | — | — | — | — | — | −14.74 | −29.4 to 0.77 | .049 | 0.109 |
| All | 0.020 | 0.054 | 0.157 | |||||||||
| G-H1 | ||||||||||||
| Age (y) | −0.58 | 19.87 to 40.47 | .458 | 0.017 | −0.392 | −2.372 to 1.589 | .691 | 0.005 | −0.398 | −2.42 to 1.62 | .692 | 0.005 |
| Status | — | — | — | — | −1.775 | −19.27 to 15.72 | .838 | 0.001 | −1.66 | −19.8 to 16.49 | .854 | 0.001 |
| Sex | — | — | — | — | — | — | — | — | −0.382 | −12.4 to 11.62 | .949 | 0.000 |
| All | 0.017 | 0.013 | 0.013 | |||||||||
| MG-H1 | ||||||||||||
| Age (y) | 6.46 | 1.86 to 11.06 | .007 | 0.203 | 3.956 | −1.62 to 9.532 | .159 | 0.056 | 4.360 | −1.14 to 9.86 | .116 | 0.071 |
| Status | — | — | — | — | 39.83 | −9.41 to 89.07 | .109 | 0.072 | 32.102 | −17.3 to 81.47 | .195 | 0.049 |
| Sex | — | — | — | — | — | — | — | — | 24.973 | −7.67 to 57.62 | .129 | 0.066 |
| All | 0.203 | 0.250 | 0.300 | |||||||||
| Pentosidine | ||||||||||||
| Age (y) | 0.218 | 0.149 to 0.287 | .000 | 0.547 | 0.176 | 0.093 to 0.260 | .000 | 0.357 | 0.183 | 0.099 to 0.266 | .000 | 0.384 |
| Status | — | — | — | — | 0.649 | 0.124 to 1.422 | .097 | 0.081 | 0.499 | −0.296 to 1.30 | .210 | 0.049 |
| Sex | — | — | — | — | — | — | — | — | 0.344 | 0.167 to 0.855 | .180 | 0.055 |
| All | 0.547 | 0.584 | 0.607 | |||||||||
| Glucosepane | ||||||||||||
| Age (y) | 20.31 | 14.65 to 25.97 | .000 | 0.610 | 13.11 | 7.507 to 18.71 | .000 | 0.407 | 13.440 | 7.95 to 18.93 | .000 | 0.437 |
| Status | — | — | — | — | 113.2 | 61.63 to 164.9 | .000 | 0.376 | 107.35 | 56.3 to 158.36 | .000 | 0.365 |
| Sex | — | — | — | — | — | — | — | — | 26.104 | −6.82 to 59.03 | .166 | 0.075 |
| All | 0.610 | 0.757 | 0.775 | |||||||||
| Total AGEs | ||||||||||||
| Age (y) | 31.66 | 20.27 to 43.05 | .000 | 0.501 | 17.275 | 6.065 to 28.49 | .004 | 0.242 | 18.185 | 7.35 to 29.02 | .002 | 0.281 |
| Status | — | — | — | — | 237.32 | 128.3 to 346.3 | .000 | 0.389 | 215.25 | 107.6 to 322.9 | .000 | 0.357 |
| Sex | — | — | — | — | — | — | — | — | 61.565 | −5.28 to 128.4 | .070 | 0.105 |
| All | 0.501 | 0.695 | 0.727 | |||||||||
| FL | ||||||||||||
| Age (y) | −0.031 | −0.082 to 0.02 | .221 | 0.046 | −0.016 | −0.07 to 0.042 | .583 | 0.009 | −0.016 | −0.075 to 0.04 | .598 | 0.008 |
| Status | — | — | — | — | −0.251 | −0.77 to 0.263 | .328 | 0.027 | −0.258 | −0.79 to 0.28 | .332 | 0.028 |
| Sex | — | — | — | — | — | — | — | — | 0.023 | −0.33 to 0.38 | .897 | 0.001 |
| All | 0.046 | 0.079 | 0.079 | |||||||||
| MetSox | ||||||||||||
| Age (y) | −0.24 | −0.931 to 0.44 | .475 | 0.016 | −0.297 | −1.12 to 0.523 | .468 | 0.015 | −0.256 | −1.08 to 0.567 | .531 | 0.012 |
| Status | — | — | — | — | 1.279 | −5.96 to 8.517 | .722 | 0.004 | 0.511 | −6.88 to 7.91 | .889 | 0.001 |
| Sex | — | — | — | — | — | — | — | — | 2.481 | −2.41 to 7.37 | .310 | 0.030 |
| All | 0.016 | 0.015 | 0.045 | |||||||||
| Total bound glucose† | ||||||||||||
| Age (y) | −10.92 | −60.59 to 38.75 | .658 | 0.006 | −5.002 | −67.18 to 57.18 | .871 | 0.001 | −4.136 | −67.4 to 59.13 | .895 | 0.001 |
| Status | — | — | — | — | −93.07 | −665.9 to 479.7 | .743 | 0.003 | −108.6 | −696 to 478.89 | .709 | 0.004 |
| Sex | — | — | — | — | — | — | — | — | 68.61 | −310 to 447.82 | .715 | 0.004 |
| All | 0.006 | 0.009 | 0.013 | |||||||||
Notes: Significance (p < .05) is indicated by bold font.
Variance explained: η2 for single factors, combined r2 for the whole models.
Total glucose bound to collagen expressed as the sum of individual levels of FL + glucosepane (Table 1).
Most animals in the study were adults whose age ranged between 1 and 12 years, but two subadult animals (<1 year) and one very old individual (nearly 19 years) also belonged to the data set. As these “outliers” could have flawed the analysis due to developmental or senescence effects, we repeated Model 3 (Table 2) without them. Excluding these individuals from the model did not change the results significantly (not shown).
After controlling for age, breeding status, and sex, the correlation between fructose-lysine and glucosepane (a collagen cross-link derived from fructose-lysine) for the complete cohort failed to reach significance, although only marginally (p = .097, r = .303). Also, fructose-lysine correlated marginally, but nonsignificantly, with G-H1 (p = .099, r = .283). Among the AGEs, MG-H1 correlated significantly with CML (p = .002, r = .503) and G-H1 (p = .027, r = .373). MG-H1 also correlated significantly with MetSox, a marker of protein oxidation (p = .016, r = .403; see Supplementary Figure 1 for scatterplots of all significant and borderline nonsignificant partial correlations).
Pentosidine formation rate in skin collagen of Ansell’s mole-rat (defined as the coefficient of x2 for the species’ equation which represents the accelerated rate component of pentosidine formation in skin) was 0.0073, which is much slower than that of shorter-lived rodents (laboratory mice: 0.0357–0.0593; laboratory rats: 0.1823) and even slightly slower than that of pigs (0.0196), dogs (0.0441), or cows (0.0424). The latter data are from Sell and colleagues (26). Only nonhuman primates and humans had lower values than mole-rat (squirrel monkeys: 0.0017; rhesus monkeys: 0.0012; humans: 0.0020; Table 3). Overall, this result is consistent with the fact that pentosidine in part reflects lower damage accumulation rate and an inverse relationship with aging.
DISCUSSION
The Ansell’s mole-rat F anselli is a social rodent species with strongly divergent survival rates. Reproductive status is the main determinant for fast (nonbreeders) or slow (breeders) aging (5). The mechanisms behind this unusual scheme are not yet understood. As previously explained, two hypotheses under evaluation in this investigation were that AGEs would accumulate with age in skin collagen of these mole-rat and that this accumulation rate would be slower in the breeders versus the nonbreeders.
In support of the first hypothesis, total AGE levels significantly increased with age in skin collagen of nonbreeding mole-rat (Figure 1; Table 2). When measured individually, the best markers of chronological age were glucosepane and pentosidine, whereas the other AGEs turned out to be less indicative in this respect. Glucosepane levels were by far highest among all AGEs accounting on average for ∼44% of total AGE content. This is in good agreement with studies in humans where glucosepane is also the major protein cross-link (33). For example, in skin samples of nondiabetic humans, levels increased with age rising up to ∼2,000 pmol/mg collagen (33). This compares well with the finding of 484 pmol/mol collagen (mean 193 pmol/mg collagen; Table 1) in mole-rat at the end of their lives at ∼20 years. Pentosidine, on the other hand, is present in much lower levels in mole-rat skin (maximum 4.32, mean 1.60 pmol/mg collagen; Table 1). Its strong correlation with chronological age is not surprising as pentosidine is a well-established generalized marker of tissue modification by the advanced glycosylation/Maillard reaction in other species, including primates, rodents, and birds (22,23,25). In this regard, pentosidine formation rates have been shown to be inversely correlated with maximum life span across different animal species (26). Rates were highly accelerated in short-lived species like the rat (Rattus norvegicus) or the shrew (Cryptotis parva) in comparison to longer-lived species like monkeys or humans. In further validation of this relationship, we tested whether the rate found in mole-rat skin reflects its long life span. Indeed, the rate of pentosidine formation in mole-rat was by orders of magnitudes slower than that for shorter-lived rodents like laboratory mice or rats and even slightly slower than that of pigs, dogs, or cows (Table 3). The correlation of pentosidine levels with chronological age was lower than in humans but similar to (or even higher than) the respective values in other model species, including C57BL/6 mice or monkeys (Table 3). Taken together, our results further confirm the general applicability of pentosidine as a biomarker of chronological age in mammals by extending it to Fukomys mole-rat. However, the concept of the existence of biomarkers of aging is controversial with many differences in opinion about the criteria and paradigm of “good” candidate markers. A thorough discussion of this issue would go beyond the scope of this paper. Readers interested in this topic are therefore referred to other sources (44–49).
As to the second hypothesis, levels of total AGEs, glucosepane, and CML were unexpectedly higher in breeders versus nonbreeders after controlling for age and gender (Tables 1 and 2). The opposite effect—higher AGE levels in nonbreeders than in breeders—was never detected. This is in sharp contrast to studies on caloric restriction in rats (23) and chickens (25) all of which were shown to have reduced levels of various AGEs, although less clear results were obtained in calorie-restricted nonhuman primates (22) and rats (24). Assigning breeder status to individual Fukomys mole-rat in the laboratory can be regarded as a taxon-specific model to extend longevity similar to caloric restriction, feeding with resveratrol or treatment with rapamycin (20). Our observation that AGE levels were not decreased in the longer-lived mole-rat cohorts, but even enhanced their levels in some cases, seems to reject the hypothesis that AGE accumulation is always predictive for, or even causal to, the aging process itself (ie, at the organismic level) and thus merits a deeper discussion.
As to glycation, higher levels of glucosepane or other AGEs in skin collagen of breeders could be representative of increased glycemia and/or faster conversion of fructose-lysine to glucosepane (or other AGEs) in this cohort. Interestingly, total glucose bound to collagen, as defined as the sum of fructose-lysine + glucosepane levels, was identical between these two cohorts (Table 2). Also, the slopes of the regression lines indicated that fructose-lysine levels did not change with age in nonbreeders and even tended to decrease in breeders (not depicted). This suggests that breeders and nonbreeders do not differ in glycemic status but that there is rather an accelerated conversion of fructose-lysine to glucosepane or other AGEs in breeders. One possible explanation for this might be that breeding requires changes in phosphate metabolism. Being herbivorous rodents without exposure to sunlight, mole-rat are most probably in a natural state of vitamin D deficiency (see respective findings in naked mole-rat H glaber (54) and Damaraland mole-rat Fukomys damarensis [55]). Vitamin D deficiency is associated with hypocalcemia and/or hypophosphatemia causing impaired fertility in female rodents (56). Consequentially, vitamin D–deficient rats need to be fed a high-calcium and phosphate diet in order to restore full reproductive capacity (56). It therefore seems possible that in the absence of sunlight or direct nutritional sources of vitamin D, onset of reproduction may trigger changes in calcium and phosphorus absorption in order to assure reproductive success. This might lead to higher circulating levels of these elements in reproductive mole-rat and thus enhanced AGE formation in this cohort. Indeed, recent in vitro experiments by Nemet and colleagues (57) have shown that metal-free phosphate ions can catalyze glucosepane formation from fructose-lysine in glucose-incubated bovine serum albumin. If true in vivo, this may help explain why breeders have higher AGE levels than nonreproductive animals and why females tend to have higher AGE levels than males. It is important to note, though, that increased phosphorus levels can also improve reproduction in male mammals (58).
In female F anselli, sexual activity is associated with higher mean levels of estradiol and progesterone as well as estradiol levels increase after mating (59). In males, the issue has not yet been thoroughly investigated, but it is reasonable to expect that testosterone levels are on average higher in breeders than in nonbreeders. However, it is unlikely that higher sex steroid hormone levels in breeding mole-rat could help to explain why breeders have higher AGE levels than nonbreeders. If anything, higher sex steroid levels are expected to slow down glucose-associated AGE formation, not vice versa, because low testosterone levels lead to insulin resistance and hence higher blood glucose levels (60).
One intriguing aspect of the elevated AGE levels in breeders is that AGE formation (ie, carbonyl stress) is partly linked to oxidative processes (27,34). Reactive oxygen species catalyze chemical modifications of proteins by the Maillard reaction in vivo (27), and the Maillard reaction, in turn, also produces reactive oxygen species (61). Thus, AGE accumulation such as CML and pentosidine can be regarded as an indicator of continued exposure to reactive oxygen species, and the results obtained here would challenge oxidative stress theories of aging. Interestingly, Buffenstein and colleagues (62) have reported higher accrued levels of oxidized protein as reflected by isoprostanes, malondialdehyde, and reactive carbonyl species in the homogenates of liver, muscle, and kidney of the long-lived naked mole-rat, H. glaber, versus the much shorter-lived CB6F1 hybrid mouse strain when age matched either physiologically or chronologically. Because they found no correlation between oxidative stress and life span in naked mole-rat exhibiting a prolonged healthy life span, they concluded that reactive oxygen species were not the key determinants of aging, but instead, protein stability and their resistance to oxidative stress were the primary factors explaining the longevity in this species (63). In light of this, one explanation for our unexpected result may be that breeding in Fukomys activates mechanisms that stabilize the proteome as a protection against the consequences of metabolic changes at other levels; that is, oxidative and carbonyl stress formation (see above). If true, this would help to explain the divergent aging rates between the cohorts. Under this scenario, AGE levels as high as in breeders simply would not have been detected in nonbreeders because most of them would have died earlier. Our present longitudinal study design does not allow us to answer this question thoroughly. However, studies are currently in progress in which oxidative challenge, AGE formation, and protein stability are being followed simultaneously in age-matched breeder and nonbreeder pairs.
FUNDING
This work was supported by the Zentrum für Wasser- und Umweltforschung of the University of Duisburg-Essen (P.D. and S.B.), a research grant of the German Society of Laboratory Animal Science GV SOLAS (P.D.), a German Academic Exchange Service travel grant (P.D. and S.B.), the National Institute of Diabetes and Digestive and Kidney Diseases grant number DK079432 (D.R.S.), and National Institute on Aging grant AG18629 (V.M.M.).
SUPPLEMENTARY MATERIAL
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/.
Acknowledgments
We thank Hynek Burda for providing the mole-rat and Stephan Koeppen for assistance with obtaining skin biopsies. We are also very grateful for statistical help provided by André Scherag and valuable comments on the manuscript given by Brian Henry.
References
- 1.Kock D, Ingram CM, Frabotta LJ, Honeycutt RL, Burda H. On the nomenclature of Bathyergidae and Fukomys n. (Mammalia: Rodentia) Zootaxa. 2006;1142:51–55. [Google Scholar]
- 2.Dammann P, Burda H. Senescence patterns in African mole-rats (Bathyergidae, Rodentia) In: Begall S, Burda H, Schleich CE, editors. Subterranean Rodents—News From Underground. Heidelberg, Germany: Springer; 2007. pp. 253–265. [Google Scholar]
- 3.Gorbunova V, Bozzella MJ, Seluanov A. Rodents for comparative aging studies: from mice to beavers. Age (Dordr) 2008;30:111–119. doi: 10.1007/s11357-008-9053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dammann P, Sumbera R, Massmann C, Scherag A, Burda H. Extended longevity of reproductives appears to be common in Fukomys mole-rats (Rodentia, Bathyergidae) PLoS One. 2011;6:e18757. doi: 10.1371/journal.pone.0018757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dammann P, Burda H. Sexual activity and reproduction delay ageing in a mammal. Curr Biol. 2006;16:R117–R118. doi: 10.1016/j.cub.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Liang S, Mele J, Wu Y, Buffenstein R, Hornsby PJ. Resistance to experimental tumorigenesis in cells of a long-lived mammal, the naked mole-rat (Heterocephalus glaber) Aging Cell. 2010;9:626–635. doi: 10.1111/j.1474-9726.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarvis JU. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science. 1981;212:571–573. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- 8.Jarvis JUM, Bennett NC. Eusociality has evolved independently in two genera of bathyergid mole-rats—but occurs in no other subterranean mammal. Behav Ecol Sociobiol. 1993;33:253–260. [Google Scholar]
- 9.Burda H, Honeycutt RL, Begall S, Locker-Grütjen O, Scharff A. Are naked and common mole-rats eusocial, and if so why? Behav Ecol Sociobiol. 2000;47:293–303. [Google Scholar]
- 10.Scantlebury M, Speakman JR, Oosthuizen MK, Roper TJ, Bennett NC. Energetics reveals physiologically distinct castes in a eusocial mammal. Nature. 2006;440:795–797. doi: 10.1038/nature04578. [DOI] [PubMed] [Google Scholar]
- 11.Sherman PW, Lacey EA, Reeve HK, Keller L. The eusociality continuum. Behav Ecol. 1995;6:102–108. [Google Scholar]
- 12.Keller L, Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of aging. Nature. 1997;389:958–960. [Google Scholar]
- 13.Bell G. The costs of reproduction and their consequences. Am Nat. 1980;116:45–76. [Google Scholar]
- 14.Stearns SC. The Evolution of Life Histories. Oxford, UK: Oxford University Press; 1992. [Google Scholar]
- 15.Flatt T. Survival costs of reproduction in Drosophila. Exp Gerontol. 2010;46:369–375. doi: 10.1016/j.exger.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Sherman PW, Jarvis JUM. Extraordinary life spans of naked mole-rats (Heterocephalus glaber) J Zool. 2002;258:307–311. [Google Scholar]
- 17.Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci. 2005;60:1369–1377. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- 18.Mattson MP. Energy intake, meal frequency, and health: a neurobiological perspective. Annu Rev Nutr. 2005;25:237–260. doi: 10.1146/annurev.nutr.25.050304.092526. [DOI] [PubMed] [Google Scholar]
- 19.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 20.Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austad SN. Comparative biology of aging. J Gerontol A Biol Sci Med Sci. 2009;64:199–201. doi: 10.1093/gerona/gln060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sell DR, Lane MA, Obrenovich ME, et al. The effect of caloric restriction on glycation and glycoxidation in skin collagen of nonhuman primates. J Gerontol A Biol Sci Med Sci. 2003;58:508–516. doi: 10.1093/gerona/58.6.b508. [DOI] [PubMed] [Google Scholar]
- 23.Cefalu WT, Bell-Farrow AD, Wang ZQ, et al. Caloric restriction decreases age-dependent accumulation of the glycoxidation products, N epsilon-(carboxymethyl)lysine and pentosidine, in rat skin collagen. J Gerontol Biol Sci. 1995;50:B337–B341. doi: 10.1093/gerona/50a.6.b337. [DOI] [PubMed] [Google Scholar]
- 24.Novelli M, Masiello P, Bombara M, Bergamini E. Protein glycation in the aging male Sprague-Dawley rat: effects of antiaging diet restrictions. J Gerontol A Biol Sci. 1998;53:B94–B101. doi: 10.1093/gerona/53a.2.b94. [DOI] [PubMed] [Google Scholar]
- 25.Iqbal M, Probert LL, Alhumadi NH, Klandorf H. Protein glycosylation and advanced glycosylated endproducts (AGEs) accumulation: an avian solution? J Gerontol Biol Sci. 1999;54:B171–B176. doi: 10.1093/gerona/54.4.b171. [DOI] [PubMed] [Google Scholar]
- 26.Sell DR, Lane MA, Johnson WA, et al. Longevity and the genetic determination of collagen glycoxidation kinetics in mammalian senescence. Proc Natl Acad Sci U S A. 1996;93:485–490. doi: 10.1073/pnas.93.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baynes JW. The role of AGEs in aging: causation or correlation. Exp Gerontol. 2001;36:1527–1537. doi: 10.1016/s0531-5565(01)00138-3. [DOI] [PubMed] [Google Scholar]
- 28.Dunn JA, McCance DR, Thorpe SR, Lyons TJ, Baynes JW. Age-dependent accumulation of Nϵ-(carboxymethyl)lysine and Nϵ-(carboxymethyl) hydroxylysine in human skin collagen. Biochemistry. 1991;30:1205–1210. doi: 10.1021/bi00219a007. [DOI] [PubMed] [Google Scholar]
- 29.Monnier VM. Toward a Maillard reaction theory of aging. Prog Clin Biol Res. 1989;304:1–22. [PubMed] [Google Scholar]
- 30.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 31.Morimoto RI, Cuervo AM. Protein homeostasis and aging: taking care of proteins from the cradle to the grave. J Gerontol A Biol Sci Med Sci. 2009;64:167–170. doi: 10.1093/gerona/gln071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerami A. Hypothesis: glucose as mediator of aging? J Am Geriatr Soc. 1985;33:626–634. doi: 10.1111/j.1532-5415.1985.tb06319.x. [DOI] [PubMed] [Google Scholar]
- 33.Sell DR, Biemel KM, Reihl O, Lederer MO, Strauch CM, Monnier VM. Glucosepane is a major protein cross-link of the senescent human extracellular matrix. Relationship with diabetes. J Biol Chem. 2005;280:12310–12315. doi: 10.1074/jbc.M500733200. [DOI] [PubMed] [Google Scholar]
- 34.Kristal BS, Yu BP. An emerging hypothesis: synergistic induction of aging by free radicals and Maillard reactions. J Gerontol. 1992;47:B107–B114. doi: 10.1093/geronj/47.4.b107. [DOI] [PubMed] [Google Scholar]
- 35.Monnier VM. Intervention against the Maillard reaction in vivo. Arch Biochem Biophys. 2003;419:1–15. doi: 10.1016/j.abb.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Fan X, Sell DR, Zhang J, et al. Anaerobic vs aerobic pathways of carbonyl and oxidant stress in human lens and skin during aging and in diabetes: a comparative analysis. Free Radic Biol Med. 2010;49:847–856. doi: 10.1016/j.freeradbiomed.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rabbani N, Thornalley PJ. Glycation research in amino acids: a place to call home. Amino Acids. 2010 doi: 10.1007/s00726-010-0782-1. doi: 10.1007/s00726-010-0782-1. [DOI] [PubMed] [Google Scholar]
- 38.Burda H. Individual recognition and incest avoidance in eusocial common mole-rats rather than reproductive suppression by parents. Experientia. 1995;51:411–413. doi: 10.1007/BF01928906. [DOI] [PubMed] [Google Scholar]
- 39.Sell DR, Monnier VM. Age-related association of tail tendon break time with tissue pentosidine in DBA/2 vs C57BL/6 mice: the effect of dietary restriction. J Gerontol Biol Sci. 1997;52:B277–B284. doi: 10.1093/gerona/52a.5.b277. [DOI] [PubMed] [Google Scholar]
- 40.Sell DR, Carlson EC, Monnier VM. Differential effects of type 2 (non-insulin-dependent) diabetes mellitus on pentosidine formation in skin and glomerular basement membrane. Diabetologia. 1993;36:936–941. doi: 10.1007/BF02374476. [DOI] [PubMed] [Google Scholar]
- 41.Sell DR, Kleinman NR, Monnier VM. Longitudinal determination of skin collagen glycation and glycoxidation rates predicts early death in C57BL/6NNIA mice. FASEB J. 2000;14:145–156. doi: 10.1096/fasebj.14.1.145. [DOI] [PubMed] [Google Scholar]
- 42.Snedecor GW, Cochran WG. Statistical Methods. Ames, IA: The Iowa State University Press; 1980. [Google Scholar]
- 43.Sell DR, Nelson JF, Monnier VM. Effect of chronic aminoguanidine treatment on age-related glycation, glycoxidation, and collagen cross-linking in the Fischer 344 rat. J Gerontol Biol Sci. 2001;56:B405–B411. doi: 10.1093/gerona/56.9.b405. [DOI] [PubMed] [Google Scholar]
- 44.de Magalhaes JP, Costa J. A database of vertebrate longevity records and their relation to other life-history traits. J Evol Biol. 2009;22:1770–1774. doi: 10.1111/j.1420-9101.2009.01783.x. [DOI] [PubMed] [Google Scholar]
- 45.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol Biol Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 46.Spector IM. Animal longevity and protein turnover rate. Nature. 1974;249:66. doi: 10.1038/249066a0. [DOI] [PubMed] [Google Scholar]
- 47.Rockstein M, Chesky JA, Sussman ML. The comparative biology and evolution of aging. In: Finch CE, Hayflick L, editors. Handbook of the Biology of Aging. New York, NY: Van Nostrand Reinhold; 1977. pp. 3–34. [Google Scholar]
- 48.Simm A, Nass N, Bartling B, Hofmann B, Silber RE, Navarrete SA. Potential biomarkers of ageing. Biol Chem. 2008;389:257–265. doi: 10.1515/BC.2008.034. [DOI] [PubMed] [Google Scholar]
- 49.Miller RA, Bookstein F, Van der Meulen J, et al. Candidate biomarkers of aging: age-sensitive indices of immune and muscle function covary in genetically heterogeneous mice. J Gerontol Biol Sci. 1997;52:B39–B47. doi: 10.1093/gerona/52A.1.B39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker GT, 3rd, Sprott RL. Biomarkers of aging. Exp Gerontol. 1988;23:223–239. doi: 10.1016/0531-5565(88)90025-3. [DOI] [PubMed] [Google Scholar]
- 51.McClearn GG. Biomarkers of age and aging. Exp Gerontol. 1997;32:87–94. doi: 10.1016/s0531-5565(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 52.Mooradian AD. Biomarkers of aging: do we know what to look for? J Gerontol. 1990;45:B183–B186. doi: 10.1093/geronj/45.6.b183. [DOI] [PubMed] [Google Scholar]
- 53.Johnson TE. Recent results: biomarkers of aging. Exp Gerontol. 2006;41:1243–1246. doi: 10.1016/j.exger.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 54.Buffenstein R, Sergeev IN, Pettifor JM. Vitamin D hydroxylase and their regulation in a naturally vitamin D-deficient subterranean mammal, the naked mole rat (Heterocephalus glaber) J Endocrinol. 1993;138:59–64. doi: 10.1677/joe.0.1380059. [DOI] [PubMed] [Google Scholar]
- 55.Pitcher T, Pettifor JM, Buffenstein R. The effect of dietary calcium content and oral vitamin D3 supplementation on mineral homeostasis in a subterranean mole-rat Cryptomys damarensis. Bone Miner. 1994;27:145–157. doi: 10.1016/s0169-6009(08)80216-4. [DOI] [PubMed] [Google Scholar]
- 56.Johnson LE, DeLuca HF. Reproductive defects are corrected in vitamin D-deficient female rats fed a high calcium, phosphorus and lactose diet. J Nutr. 2002;132:2270–2273. doi: 10.1093/jn/132.8.2270. [DOI] [PubMed] [Google Scholar]
- 57.Nemet I, Strauch CM, Monnier VM. Favored and disfavored pathways of protein crosslinking by glucose: glucose lysine dimer (GLUCOLD) and crossline versus glucosepane. Amino Acids. 2010;40:167–181. doi: 10.1007/s00726-010-0631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arbeiter K. Effects of nutrition upon the quality of bovine semen. Andrologia. 1973;5:175–178. [PubMed] [Google Scholar]
- 59.Hagemeyer P, Lange S, Broecker-Preuss M, Burda H. The influence of olfactory stimulus and sexual activity on gonadal steroids in eusocial mole-rats. Folia Zool. 2009;58:65–74. [Google Scholar]
- 60.Traish AM, Saad F, Guay A. The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance. J Androl. 2009;30:23–32. doi: 10.2164/jandrol.108.005751. [DOI] [PubMed] [Google Scholar]
- 61.Lee C, Yim MB, Chock PB, Yim HS, Kang SO. Oxidation-reduction properties of methylglyoxal-modified protein in relation to free radical generation. J Biol Chem. 1998;273:25272–25278. doi: 10.1074/jbc.273.39.25272. [DOI] [PubMed] [Google Scholar]
- 62.Andziak B, O’Connor TP, Qi W, et al. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5:463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- 63.Pérez VI, Buffenstein R, Masamsetti V, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci U S A. 2009;106:3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.