Abstract
This review focuses on cardiovascular protective effects of insulin-like growth factor (IGF)-1, provides a landscape of molecular mechanisms involved in cardiovascular alterations in patients and animal models with congenital and adult-onset IGF-1 deficiency, and explores the link between age-related IGF-1 deficiency and the molecular, cellular, and functional changes that occur in the cardiovascular system during aging. Microvascular protection conferred by endocrine and paracrine IGF-1 signaling, its implications for the pathophysiology of cardiac failure and vascular cognitive impairment, and the role of impaired cellular stress resistance in cardiovascular aging considered here are based on emerging knowledge of the effects of IGF-1 on Nrf2-driven antioxidant response.
Keywords: Vascular aging, Myocardial infarction, Vascular dementia, Atherosclerosis, Endothelial dysfunction
DISRUPTION of the insulin/insulin-like growth factor (IGF)-1 pathway increases life span in invertebrates. In mammals, the loss of insulin signaling is lethal, whereas the effects of decreased IGF-1 signaling remain controversial. The current issue of the Journal of Gerontology: Biological Sciences and Medical Sciences is devoted to understanding the role of growth hormone (GH) and IGF-1 in the aging process in humans, resolving controversies existing in the field (1–3). The cardiovascular system is an important target organ for GH and IGF-1. There is evidence that cardiac myocytes, vascular endothelial and smooth muscle cells abundantly express IGF1R and that they are more sensitive to IGF-1 than to insulin (4–6). Diseases of the cardiovascular system (including heart failure, myocardial infarction, stroke, vascular dementia, high blood pressure and its complications, aortic aneurysm, peripheral artery disease) are the most common cause of morbidity and mortality among the elderly humans in the Western world, affecting over 80 million Americans (7). Because of the importance of age-related impairments of the heart and tissue blood supply in organismal senescence in humans, in this review, the effects of the GH/IGF-1 axis on the cardiovascular system are considered in terms of potential mechanisms involved in microvascular protection and cardiac protection in aging.
CARDIOVASCULAR PROTECTIVE EFFECTS OF IGF-1: LESSONS LEARNED FROM CONGENITAL AND ADULT-ONSET IGF-1 DEFICIENCIES
Secretion of GH and, consequently, hepatic production of IGF-1 significantly decline with age both in humans and laboratory animals (8–10). Here, we review the evidence obtained in human patients with endocrine IGF-1 deficiencies and animal models that mimic these conditions, which support the concept that IGF-1 exerts protective effects in the cardiovascular system (11–13).
Cardiovascular Dysfunction in Patients With IGF-1 Deficiency
Low circulating IGF-1 levels increase the risk for cardiovascular and cerebrovascular diseases in humans.—
It is well documented that in human patients, GH deficiency and low circulating levels of IGF-1 significantly increase the risk for cardiovascular and cerebrovascular diseases (14–20). In cross-sectional studies, low circulating IGF-1 was found to be associated with angiographically documented coronary artery disease (21). A prospective nested case–control study of over 600 initially healthy participants who were followed for 15 years demonstrated that lower than normal circulating IGF-1 levels increase the risk of coronary heart disease (22). This conclusion is supported by another prospective study of 1,185 men and women who were followed up for over a decade, showing that circulating IGF-1 levels predict fatal ischemic heart disease (19). Moreover, in patients, in the early phase of acute myocardial infarction, low circulating IGF-1 levels predict a worse prognosis (23). A cross-sectional study of 400 elderly men also documented an inverse correlation between circulating IGF-1 levels and carotid arterial intima–media thickness (24). Most human data also support the concept that normal levels of GH and IGF-1 are important for the maintenance of a healthy endothelial function. In patients with GH-deficiency, flow-mediated endothelium-dependent dilation of peripheral arteries is impaired (11,25,26). There is also a positive correlation between circulating IGF-1 levels and coronary flow reserve (27).
A significantly increased risk of ischemic stroke was also demonstrated in patients with low circulating IGF-1 levels (28). Studies on patients with ischemic stroke suggest that high circulating IGF-1 levels are associated with neurological recovery and a better functional outcome (29,30). These findings are significant as IGF-1 is known to exert neuroprotective and proangiogenic effects in animal models of stroke when given shortly after the insult (31). Taken together, the available human data uniformly support the concept that IGF-1 deficiency promotes the development of atherothrombotic diseases (for an excellent overview on the molecular mechanisms underlying the proatherogenic effects of endocrine IGF-1 deficiency, please refer to the review by Higashi and colleagues [1]).
Cardiovascular alterations in patients with congenital IGF-1 deficiency (Laron syndrome).—
Classic Laron syndrome is congenital IGF-1 deficiency caused by primary GH insensitivity (eg, due to GHR mutations), which is clinically characterized by dwarfism, micropenis, hypoglycemia, convulsions, osteoporosis, craniofacial abnormalities, cognitive impairment, and marked obesity (32). Anecdotal data suggest that dwarfism may increase risk of cardiovascular diseases. For example, the most famous person with dwarfism, Charles Sherwood Stratton (who performed with the Barnum Circus in the XIX. century under the stage name General Tom Thumb and met both President Lincoln and Queen Victoria), died of stroke at the age of 45. An important recent longitudinal study that followed human Laron dwarfs in Ecuador demonstrated that severe congenital IGF-1 deficiency in humans is associated with a shorter than expected life span (32) and analysis of the known causes of mortality (independent of death related to substance abuse and accidents) in this cohort reveals that almost half died as a result of stroke and cardiovascular disease (32). The available evidence suggest that untreated Laron dwarf patients have reduced cardiac dimensions and output (but normal left ventricular ejection fraction) at rest and reduced left ventricular contractile reserve following stress, alterations that can be improved by IGF-1 supplementation (33–35). It was suggested that low cardiac output contributes to the decreased exercise tolerance observed in patients with Laron syndrome (36). IGF-1 treatment was also shown to decrease circulating level of lipoprotein(a), a well-established risk factor for coronary artery disease, in patients with Larons syndrome (37). Interestingly, a recent pilot study on a relatively low number of patients found that flow-mediated dilation in the brachial artery is within normal limits in non–IGF-1-treated adult patients with Laron syndrome (38).
Diabetes mellitus is major risk factor for microvascular pathologies, and previous studies suggested that excessive GH secretion associated with acromegaly induces diabetes and promotes the microvascular complications of diabetes (39–41). It is well documented that GH deficiency in laboratory animals improves insulin sensitivity (42), and recent studies clearly demonstrate that the incidence of diabetes mellitus is very low in Ecuadorian Laron dwarf patients (43). Nevertheless, a recent study by Dr. Laron (44) reports two cases on type 2 diabetes in never-treated Israeli Laron dwarfs, which are associated with proliferative diabetic retinopathy, nephropathy, and subacute ischemic heart disease. These findings suggest that congenital IGF-1 deficiency, similar to GH excess in acromegaly, may also promote microvascular complications of diabetes mellitus.
Cardiovascular Dysfunction in Animal Models of IGF-1 Deficiency
Cardiovascular alterations in GH/IGF-1–deficient Lewis dwarf rats.—
The genetically GH/IGF-1–deficient strain of Lewis rats is a useful model of human GH/IGF-1 deficiency, as Lewis dwarf rats have normal pituitary function except for a selective genetic GH deficiency and they mimic many of the pathophysiological alterations present in human GH-deficient patients (including mild cognitive impairment (45) and resistance to development of cancer [46,47]). Also, similar to GH/IGF-1–deficient humans, Lewis dwarf rats do not exhibit a longevity phenotype (47). Importantly, GH/IGF-1 deficiency in Lewis rats significantly increases the incidence of late-life strokes (47), similar to the effects of GH/IGF-1 deficiency in elderly humans. Arteries of Lewis dwarf rats exhibit reduced glutathione and ascorbate content and increased oxidative stress (48). Fibroblasts derived from Lewis dwarf rats do not exhibit increased reactive oxygen species (ROS) production demonstrating the importance of circulating factors in generation of this phenotype (49). Vascular inflammation is upregulated and endothelial dysfunction is more pronounced in arteries of Lewis dwarf rats as compared with vessels of control littermates in response to metabolic stress associated with high-fat diet-induced obesity (50). Lewis dwarf rats exhibit low GH and IGF-1 levels throughout their entire developmental period. It is significant that short-term peripubertal treatment of GH-deficient Lewis dwarf rats with GH decreases the incidence and delays the occurrence of late-life stroke, which results in a significant (∼14%) extension of life span (47). Lewis dwarf rats exhibit decreased myocyte size, cardiac atrophy, impaired cardiac contractility, impaired diastolic function, and reduced calcium responsiveness of the myofilaments (51–54), which extend previous findings of cardiac atrophy and impaired cardiac performance in humans with GH/IGF-1 deficiency. After induction of myocardial ischemia, Lewis dwarf rats fail to develop compensatory hypertrophy of the noninfarcted posterior wall, which exacerbates the loss of myocardial function (55). These findings are congruent with the results of early studies on hypophysectomized rats, showing that GH was necessary for a normal compensatory hypertrophic response following experimental aortic constriction (56–58).
Cardiovascular alterations in GH/IGF-1–deficient Ames dwarf mice and Little mice.—
In Ames dwarf mice (Prop1df/df, which exhibit multiplex endocrine defects, including prolactin and TSH deficiency), low circulating GH and IGF-1 levels are associated with impaired expression of antioxidant enzymes and eNOS in the vasculature, leading to increased vascular oxidative stress and endothelial dysfunction (59). IGF-1 has been repeatedly shown to regulate angiogenic processes, and, as expected, GH/IGF-1 deficiency was shown to be associated with abnormal microvascular development in the tibial proximal epiphysis in Snell dwarf mice (Pit1dw/dw, which are phenotypically identical to Ames dwarf mice [60]).
Similar to Lewis dwarf rats, Ames dwarf mice also exhibit decreased myocyte size and cardiac atrophy (61) and impaired excitation–contraction coupling of the cardiac myocytes (62). However, there is a paucity of data on cardiac function in Ames dwarf mice in vivo. One important study demonstrated a loss of inotropic and lusitropic response in hearts of young Ames dwarf mice upon administration of dobutamine, indicating an impaired contractile and relaxation reserve (63). Mitochondrial ROS production is also increased both in the hearts and the vasculature of Ames dwarf mice (59). Furthermore, Ames dwarf mice also exhibit a faster rate of age-related decline in complex IV activity in cardiac mitochondria than wild-type control mice (64).
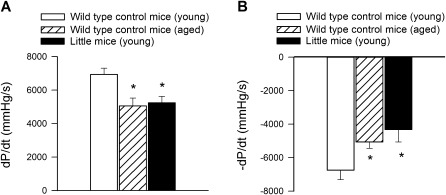
The Little mouse is generated by a missense mutation in the GH-releasing hormone receptor (Ghrhr), but the rest of its pituitary is intact, and, unlike the Ames dwarf mice, it has normal thyroid function. The Little mice have significantly reduced levels of both circulating IGF-1 and GH. Importantly, the cardiac phenotype of the Little mice is similar to that of Ames dwarf mice. Little mice exhibit cardiac atrophy, impaired systolic, and diastolic function (Figure 1) and impaired inotropic response upon administration of dobutamine (65).
Figure 1.
Endocrine insulin-like growth factor (IGF)-1 and growth hormone deficiency (due to a missense mutation in Ghrhr) impairs contractile properties of the heart of Little mice, mimicking the cardiac aging phenotype. Shown are (A) maximal rate of left ventricle contractility (+dP/dtmax; mmHg/s) and (B) maximal rate of left ventricle relaxation (−dP/dtmax; mmHg/s) in young Little mice and young (4-month-old) and aged (30-month-old) control wild-type mice. *p < .05. Data are mean ± SEM. Data points are replotted from (65).
Cardiovascular alterations in mouse models of isolated endocrine IGF-1 deficiency.—
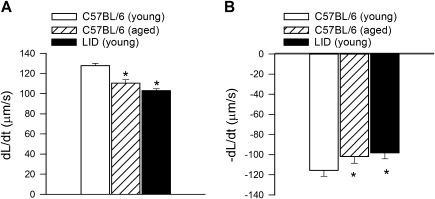
Ames dwarf and Snell dwarf mice exhibit complex endocrine defects (ie, chronic GH, TSH, and/or PRL deficiency), and in most animal models, IGF-1 deficiency is present during development, which results in dwarfism, altered metabolism, and/or complex epigenetic changes. Because of the inherent limitations of the aforementioned animal models, it has been realized that the role of IGF-1 deficiency in development of the cardiovascular aging phenotype has to be tested in animals that exhibit isolated endocrine IGF-1 deficiency in a temporarily controlled manner. Liver-specific IGF-1-deficient (LID) mice demonstrate a 75% reduction in circulating IGF-1 levels, but the IGF-1 deficiency is present during development. More recently, a novel mouse model of adult-onset isolated endocrine IGF-1 deficiency has been developed, using adeno-associated viral knockdown of IGF-1 specifically in the liver of postpubertal mice using Cre-lox technology (Igf1f/f + MUP-iCre-AAV8; [66,67]). This model results in a significant isolated decrease in circulating IGF-1 levels (∼50%). The available data are consistent with the hypothesis that isolated endocrine IGF-1 deficiency per se can promote the development of an accelerated cardiovascular aging phenotype. Specifically, contractility of cardiac myocytes isolated from liver-specific IGF-1-deficient mice is significantly impaired ([68]; Figure 2). Liver-specific IGF-1–deficient mice also exhibit deficient compensatory hypertrophic response following experimental aortic constriction, which impairs functional adaptation to pressure overload (69). It should be noted that LID mice exhibit marked glucose intolerance and accelerated development of diabetes, which may also contribute to the deterioration of cardiovascular dysfunction (70). Isolated IGF-1 deficiency in Igf1f/f + MUP-iCre-AAV8 mice is associated with dysregulation of Nrf2-dependent antioxidant responses in the vasculature, which promotes the development of marked endothelial dysfunction and endothelial apoptosis in the presence of increased oxidative stress (66), mimicking the aging phenotype. Disruption of IGF-1 signaling in mice by whole-body knockdown of IGF1R, by endothelial cell-specific knockdown of IGF1R, or by overexpression of IGFBP-1 result in a different vascular phenotype: impaired contractions to phenylephrine, a trend for decreased basal eNOS activation and/or increased insulin-induced NO generation (71). At present, the mechanisms underlying these discrepancies are not well understood. Mice lacking the IGF1R do not develop a lethal cardiac phenotype, but mice with combined deficiency of the insulin receptor and IGF1R in cardiac muscle develop early-onset dilated cardiomyopathy and die from heart failure within the first month of life despite normal glucose homeostasis (72).
Figure 2.
Isolated endocrine insulin-like growth factor (IGF)-1 deficiency impairs contractile properties of cardiomyocytes, mimicking the aging phenotype. Shown are (A) maximal velocity of shortening (+dL/dt) and (B) maximal velocity of relengthening (−dL/dt) in cardiomyocytes isolated from young (2- to 4-month-old) and old (24- to 26-month-old) C57BL/6 and young LID mouse hearts. *p < .05. Data are mean ± SEM. Data points are replotted from (68).
ROLE OF IGF-1 DEFICIENCY IN DEVELOPMENT OF THE CARDIOVASCULAR AGING PHENOTYPE
The medical literature is replete with evidence that circulating levels of IGF-1 significantly decline with age both in elderly humans and laboratory animals (for a review, see [3]), likely due to the significant age-related decline in GH secretion. On the basis of the temporal pattern of changes in circulating IGF-1 levels and development of cardiovascular dysfunction during aging, it is hypothesized that the age-related decline in circulating IGF-1 is causally involved in the development of aging phenotype in the heart and the vasculature (10). Here, we review the existing clinical and experimental evidence on the cardiac and microvascular protective effects of IGF-1 in aging in support of this view.
Endocrine Versus Paracrine IGF-1 Deficiency in Aging
It is known that a paracrine IGF-1 system is present both in the vascular wall and the parenchymal tissues in which the vessels are embedded (including the myocardium and the brain), which includes locally produced IGF-1, IGF1R, and multiple binding proteins (41). There is increasing evidence that circulating (liver-derived endocrine) and locally derived (paracrine) IGF-1 have unique physiological roles (67,73–81). Recent studies demonstrate that the paracrine IGF-1 system confers vasoprotection and cardioprotection (82–84) and contributes to maintenance of the structural and functional integrity of the microvasculature. The findings that components of the local IGF-1 system are often unaltered in animal models of isolated endocrine IGF-1 deficiency, suggest that the vascular paracrine IGF-1 system cannot compensate for deficiency of circulating IGF-1 (48).
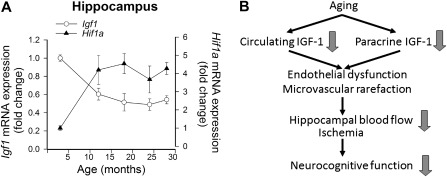
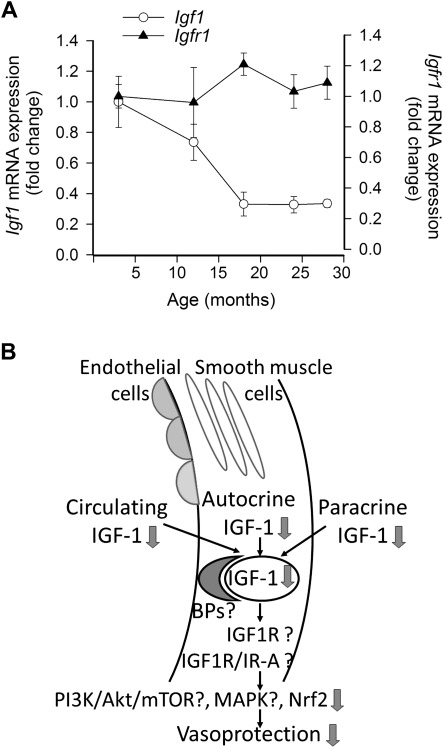
As noted earlier in this review, circulating IGF-1 levels decline with advancing age in humans as well as in laboratory animals. Previous studies reported that in the heart of F344 rats, there is an age-related downregulation of paracrine IGF-1 and IGF1R expression (85). Here, we provide evidence that there is a significant age-related decline in paracrine IGF-1 expression in the hippocampus of F344 × Brown Norway (BN) rats (Figure 3A), which is associated with hippocampal microvascular rarefaction. The inverse relationship between the age-related decline in expression of paracrine IGF-1 and increases in HIF-1α (a marker of hypoxia, Figure 3A) accords with the predictions based on the hypothesis that changes in IGF-1 signaling are causally related to hippocampal microvascular rarefaction in aging (Figure 3B; [86]). Moreover, expression of IGF-1 also significantly decreases with age in the cerebral vasculature itself (Figure 4A). We predict that alterations in the autocrine IGF-1 system, in addition to the age-related decline in endocrine and paracrine IGF-1, significantly contribute to the age-related phenotypic alterations in the cardiovascular system (Figure 4B). In that regard, it is interesting to note that caloric restriction, a prototypical antiaging dietary intervention, was shown to significantly increase cardiac expression of several components of the paracrine-autocrine IGF-1 system, including IGF1, IGF1R, and the insulin receptor (87). Similar effects of caloric restriction were also observed in rats (88). Interventional strategies that upregulate both endocrine and paracrine IGF-1 are expected to confer robust cardiovascular health benefits in aging.
Figure 3.
(A) Age-related decline in the expression of paracrine insulin-like growth factor (IGF)-1 in the hippocampi of F344 × BN rats is associated with induction of the hypoxia marker Hif1α. Previous studies demonstrate that old rats exhibit significant microvascular rarefaction. Messenger RNA expression of IGF-1 and Hif1α was assessed by quantitative real-time reverse transcription–polymerase chain reaction. Data are mean ± SEM. (B) Scheme illustrating the hypothesis that age-related decline in endocrine and paracrine IGF-1, hippocampal microvascular rarefaction, regional cerebral ischemia, and age-related cognitive decline are causally related.
Figure 4.
(A) Age-related decline in the expression of insulin-like growth factor (IGF)-1 in middle cerebral arteries of F344 × BN rats. Expression of IGF1R does not appear to change with age in the vascular wall. Messenger RNA expression of IGF-1 and IGF1R was assessed by quantitative real-time reverse transcription–polymerase chain reaction. Data are mean ± SEM. (B) Scheme showing the concept that aging results in a decline in circulating IGF-1 levels, decreases production of paracrine IGF-1 (in the brain and perhaps other organs), and downregulates expression of IGF-1 in cells of the vascular wall. Future studies should elucidate whether age-related IGF-1 deficiency is further exacerbated by age-related changes in vascular expression of IGF-1–binding proteins (BPs), IGF-1 receptors and alteration in signaling pathways activated by IGF-1 receptors.
Myocardial Protection by IGF-1
The available evidence suggests that IGF-1 signaling confers antiaging cardioprotective effects and that age-related loss of IGF-1 contributes to the development of the cardiac aging phenotype. The antiaging cardiac effects of endocrine and paracrine IGF-1 are likely multifaceted. In aged BN × F344 rats, restoration of IGF-1 levels by long-term GH replacement was associated with a significant improvement of diastolic function (89). Previous studies also demonstrated that in aged mice, cardiac overexpression of IGF-1 significantly improves cardiomyocyte contractile function (90), attenuates oxidative stress-mediated protein damage, normalizes Ca2+ homeostasis, reverses age-related alterations in the expression of pro- and antiapoptotic proteins, and decreases the rate of apoptosis (91). It is also likely that the beneficial effects of IGF-1 on myocardial perfusion also contribute to its cardioprotective effects in aging (see later). Interestingly, IGF-1 overexpression was also shown to rescue diabetes-induced cardiac dysfunction and attenuate oxidative stress (92) and protect from cardiomyocyte death after infarction. Data from the Framingham Heart Study support these experimental findings, showing an inverse correlation between circulating IGF-1 levels and the risk of congestive heart failure in elderly men and women (18). Successful cardiovascular aging in healthy centenarians is also associated with relatively high circulating IGF-1 levels (93).
Cardiac progenitor cells possess an autocrine/paracrine IGF-1/IGF1R system (94), which promotes their survival and proliferation (95). Beneficial effects of the IGF-1/IGF1R system of cardiac progenitor cells include its antioxidant effects, upregulation of telomerase activity, and a delay in replicative senescence (96). Senescence and death of cardiac progenitor cells are known to increase with age in mice impairing the growth and turnover of cells in the heart (96). Since myocyte death exceeds the rate of replacement of myocytes from cardiac stem cells, there is a gradual decline in the number of myocytes that eventually lead to heart failure in advanced age (96). Previous studies report that overexpression of IGF-1 in the mouse heart prevents the age-dependent loss of cardiac myocytes likely by protecting cardiac progenitor cells (96) and protecting the cardiac myocytes from apoptotic cell death (97–99). Recent studies reported that IGF-1 tethered to self-assembling peptide nanofibers also improves the recovery of myocardial structure and function in models of myocardial ischemia (94). These findings are consistent with previous observations that injection of IGF-1 in the damaged heart promotes the migration and homing of cardiac stem cells and facilitates neovascularization (95). Taken together, therapeutic targeting of IGF-1 signaling pathways combined with stem cell therapies may offer therapeutic opportunities for myocardial rejuvenation in aging. The recent identification of a subpopulation of human cardiac stem cells, which express IGF1R, produce autocrine IGF-1 and show superior therapeutic potential for myocardial regeneration (100) may be an important step toward that direction.
Recent studies demonstrate that expression of a locally acting IGF-1 propeptide (mIGF-1) promotes functional recovery of the heart after myocardial infarction, at least in part, by enhancing SIRT1 expression and increasing cellular oxidative stress resistance (83). The model used in these studies (cardiac-specific mIGF-1 transgenic mice in which SirT1 was depleted from adult cardiomyocytes in a tamoxifen-inducible and conditional fashion) will certainly be useful to test the role of mIGF-1 and SirT1 signaling in cardiac aging in future studies.
Microvascular Protection by IGF-1
Aging is associated with pathophysiologically relevant functional and phenotypic alterations in the microcirculation, including endothelial dysfunction, oxidative stress, chronic low-grade inflammation, and microvascular rarefaction (for a recent review, refer to [7]). Our current understanding is that both circulating IGF-1 levels and locally produced IGF-1 contribute to the maintenance of functional and structural integrity of the microcirculation, increasing NO bioavailability, decreasing ROS production, and exerting antiinflammatory, antiapoptotic, and proangiogenic effects. Thus, it is logical to assume that a combined endocrine and paracrine IGF-1 deficiency contributes to age-related microvascular alterations.
Delivery of nutrients, clearance of metabolites, and exchange of gases between the blood and tissues occur almost exclusively in the microcirculation, and adequate blood perfusion via the microcirculatory network is essential for the integrity of tissues and normal organ function. Age-related microvascular rarefaction (reduced number and combined length of small vessels in a given volume of tissue) has been observed in multiple organ systems, including the heart (101), kidney (102), and the skin (103). It is thought that the resulting inadequate perfusion contributes to tissue and organ dysfunction in aging. Importantly, as the nervous system ages, there is also a rarefaction of the microvasculature in the hippocampus and other regions of the brain, as well as alterations in the structure of the remaining vessels, which have been causally linked to cognitive dysfunction in the absence of or preceding neurodegeneration in the elderly humans (10,86,104,105). Age-related microvascular rarefaction contributes to a decline in regional cerebral blood flow that reduces metabolic support for neural signaling, especially when neuronal activity is high. In addition, aging reduces microvascular plasticity and the ability of the cerebral circulation to respond appropriately to changes in metabolic demand (104). Recent studies demonstrate that GH supplementation (which significantly elevates circulating levels of IGF-1) substantially increases cortical vascular density in older rats (86), which is accompanied by a significant improvement of cognitive function (10,106–110). Infusion of IGF-1 was also shown to elicit a significant (∼40%) increase in cerebromicrovascular density in the adult mouse brain (111). Previous studies also demonstrated that aging is associated with microvascular rarefaction in the rat heart (112). Treatment with GH was shown to increase regional myocardial blood flow and capillary density in aged rats (112).
The mechanisms by which IGF-1 reverses and/or prevents microvascular rarefaction and improves tissue blood supply are likely multifaceted. Apoptosis is an attractive hypothesis to account for age-related microvascular rarefaction. There is evidence that both in laboratory rodents and in nonhuman primates that the percentage of apoptotic endothelial cells significantly increases with age (113–116). The available data suggest that impaired bioavailability of NO, increased levels of tumor necrosis factor-α, and/or mitochondrial oxidative stress promote endothelial apoptosis in aging (113,114). IGF-1 is known the exert significant antiapoptotic effects in the heart, and it also inhibits oxidative stress-induced apoptosis in cultured endothelial cells, likely by preserving the functional integrity of the mitochondria (117). Further studies are warranted to test the hypothesis that restoration of endocrine and paracrine IGF-1 signaling in aging would also exert antiapoptotic effects at the level of the microcirculation, preventing and/or reversing microvascular rarefaction.
Another mechanism, which likely contributes to microvascular rarefaction, is an age-related impairment of angiogenesis (118). IGF-1 is known the exert significant proangiogenic effects, inducing proliferation of brain microvascular endothelial cells in culture through HIF-1α and VEGF, a canonical angiogenic pathway (111). Using a wide range of animal models of age-related cerebrovascular diseases, it has been confirmed that IGF-1, in addition to its direct neurotrophic effects, exerts angiogenic effects and that it protects the brain from experimental ischemic injury (119–125). These experimental findings are consistent with observations in stroke patients that low circulating levels of IGF-1 are associated with a poor outcome, suggesting that endocrine IGF-1 affects the evolution of cerebral infarction (12). Previous studies also showed that IGF-1 has a significant role in cerebral angiogenesis both during development and in adulthood (20,111). Importantly, physical exercise, which is known to increase cerebromicrovascular density in control mice, fails to do so in mice with low serum IGF-1 (111). Previous studies demonstrated that overexpression of IGF-1 either before or after induction of cerebral ischemia enhances neurovascular remodeling, increasing cerebromicrovascular density, and improves functional outcome in rodent models of ischemic stroke (31,126). In contrast, disruption of IGF-1 signaling by an anti-IGF-1 antibody abrogates perilesion microvascular growth in the brain (111). Further studies are needed to demonstrate that similar beneficial effects can be reached by GH or IGF-1 supplementation or by other pharmacological treatments targeting the microcirculation in elderly humans.
Age-related impairment of endothelial cell turnover due to decreased number and impaired function of endothelial progenitor cells may also negatively affect the microcirculation in aging. Importantly, age-dependent impairment of endothelial progenitor cells was reported to be corrected by the GH-mediated increase in circulating IGF-1 (127), which likely will exert beneficial effects on the regenerative capacity of the cardiovascular system in the elderly humans. Further support for the view that age-related alterations in endocrine factors adversely affect the function of endothelial progenitor cells comes from recent studies showing that the presence of sera from young rats (which have high IGF-1 levels) in the culture medium improves the function of endothelial progenitor cells isolated from aged rats (128).
Oxidative stress and decreased bioavailability of NO may be important in microvascular rarefaction. Previous studies have shown that in aging upregulation of NADPH oxidases and increased mitochondrial production of ROS result in significant oxidative stress in the microvascular endothelial and smooth muscle cells (reviewed in [7]). Age-related oxidative stress and downregulation of eNOS (129,130) impair the bioavailability of NO, which is likely to contribute to decreased microvascular density. This view is supported by the findings that rodents with genetically impaired NO signaling (131) or treated with NO synthesis inhibitors (132) develop microvascular rarefaction. Several lines of evidence suggest that age-related IGF-1 deficiency may promote vascular oxidative stress and decrease NO bioavailability. First, animal models of IGF-1 deficiency often exhibit increased ROS production and decreased NO bioavailability, mimicking the vascular aging phenotype (48,50,59). Second, treatment of aged rats with IGF-1 was shown to upregulate eNOS and improve bioavailability of NO (133,134). IGF-1 treatment has similar effects in mouse models of accelerated vascular aging (77). Furthermore, in vitro IGF-1 and GH treatment reduce ROS production in cultured human endothelial cells (59). In addition, IGF-1 can also upregulate eNOS in cultured endothelial cells (59).
It should be noted that the aforementioned cytoprotective effects of IGF-1 are also likely to contribute to its significant antiinflammatory and antiatherosclerotic action in aging, which is reviewed in detail by Higashi and co-workers (1).
Impaired Cellular Stress Resistance in Aging: Potential Role of IGF-1 Deficiency in Dysregulation of Nrf2
The stress-activated cap’n’collar transcription factor Nrf2 (nuclear factor [erythroid-derived 2]–like 2) is conserved in metazoans and has an important role in regulating the aging process by orchestrating the transcriptional response of cells to oxidative stress (135–137). In young animals, activation of the Nrf2 pathway has an essential role in cellular redox homeostasis in the heart and the vasculature, exerting cardioprotective and vasoprotective effects under pathophysiological conditions associated with oxidative stress (138,139). One of the hallmarks of aging is a marked age-related increase in the cellular generation of ROS in mammals (114,130). Yet, an adaptive Nrf2/ARE-driven antioxidant response fails to manifest in the aged vasculature (140,141). There is increasing evidence obtained both in laboratory rodents and nonhuman primates that aging is associated with Nrf2 dysfunction in endothelial and smooth muscle cells (141,142). The reduced capacity of cellular homeostatic mechanisms likely exacerbates age-related cellular oxidative stress and free radical–mediated cellular injury in aging.
Several lines of evidence suggest that age-related endocrine IGF-1 deficiency and vascular Nrf2 dysfunction in aging are causally linked. First, adult-onset endocrine IGF-1 deficiency in Igf1f/f + MUP-iCre-AAV8 mice results in significant impairment of the Nrf2-driven antioxidant response pathway, mimicking the aging phenotype (66). There are multiple mechanism by which IGF-1 deficiency may impair Nrf2 activity. In IGF-1–deficient mice, expression of Nrf2 and Nrf2 target genes tend to decrease (66), mimicking the changes in the expression of Nrf2 and Nrf2-driven genes observed aged vessels (141,143). Previous studies also documented decreased expression of Nrf2 in the blood vessels of Lewis dwarf rats (48) and downregulation of Nrf2 target genes in the aorta of Ames dwarf mice (59). Importantly, in the hippocampus of Igf1f/f + MUP-iCre-AAV8 mice, there is also a significant decline in expression of Nrf2 (W. Sonntag, PhD, unpublished data, 2011). Because Nrf2 expression and activity as well as expression of Nrf2 target genes also decrease in the brain (144) and other organs (145–147) of aged rodents, further studies are warranted to compare the gene-expression signatures induced by IGF-1 deficiency and aging in mice in multiple tissues and organs. Second, recent evidence suggest that IGF-1, in addition to regulating Nrf2 signaling at the level of Nrf2 expression, can also directly modulate the transcriptional activity of Nrf2 (66). On ligand binding, the IGF-1 receptor tyrosine kinase initiates multiple signaling cascades, including activation of the phosphatidylinositol 3-kinase (PI3K) pathway and its downstream effectors (including Akt1). Recent studies provide initial evidence that in endothelial cells, IGF-1 activates Nrf2 via an Akt1-dependent pathway (66). Previous studies also showed that activation of the IGF-1 receptor inhibits oxLDL-induced apoptosis in vascular smooth muscle cells through the PI3K/Akt signaling pathway (148). Importantly, dysregulation of Nrf2 in vessels of IGF-1–deficient mice is associated with exacerbation of vascular oxidative stress and endothelial dysfunction elicited by both hyperglycemia and the proatherogenic stressor oxLDL (66). Previous studies demonstrated that genetic absence of a functional Nrf2/ARE pathway also results in significant increases in vascular oxidative stress and exacerbation of endothelial dysfunction in arteries of high-fat diet-fed Nrf2−/− mice (139). Collectively, the aforementioned findings are consistent with the available clinical and experimental data suggesting that IGF-1 deficiency renders the cardiovascular system more vulnerable to oxidative stressors (149). This view is further supported by the findings that in mouse models of atherosclerosis, infusion of IGF-1 significantly decreases vascular oxidative stress and its pathological consequences (77). Because recent studies demonstrate that disruption of Nrf2 signaling impairs angiogenic capacity of microvascular endothelial cells in vitro (150), we cannot exclude the possibility that Nrf2 dysregulation contributes to microvascular rarefaction in IGF-1–deficient animals as well.
PERSPECTIVES
Although significant progress has been achieved in describing alterations in myocardial and vascular function and phenotype in human patients and laboratory animals with IGF-1 deficiency, the specific roles of paracrine versus endocrine IGF-1 signaling in cardiovascular aging need to be elucidated further. Future studies should focus on age-related changes in paracrine IGF-1 signaling both in the vascular wall and the supplied tissues. There is increasing evidence that IGF-1 regulates pathways involved in cellular stress resistance and ROS production, which have a key role in regulation of redox-sensitive proinflammatory pathways (eg, nuclear factor-κB signaling). These pathways are under intense investigation as the common denominators of the pathophysiology of several cardiovascular risk factors. Thus, research efforts should persist in these directions to fully elucidate the specific relationship between age-related alterations in endocrine and paracrine IGF-1 signaling, and the pathways involved in cellular oxidative stress resistance and their interaction with other risk factors that lead to the increased cardiovascular morbidity and mortality in the elderly humans. Further studies are also warranted to separate the cellular effects mediated by the insulin receptor and IGF1R in the cardiovascular system.
Future studies are also warranted to develop clinically relevant therapies to improve microvascular function and myocardial performance in the elderly humans. Despite the growing evidence that treatment with low doses of GH may exert beneficial effects in the cardiovascular system, the possible side effects of GH replacement (eg, its potential diabetogenic effect) should be better understood.
Recent landmark studies from the Bartke laboratory suggest that life-span extension in Ames dwarf mice depends on the presence of lower than normal GH and IGF-1 levels during a critical short time window during development (151). Thus, another important goal for future studies is to differentiate between early life effects versus late-life effects of IGF-1 on processes involved in cardiovascular aging and to explore the role of epigenetic regulation in the possible dichotomous actions of these hormones, as discussed in the overview in Sonntag and colleagues (3).
On the basis of the available evidence, it appears that cells of the cardiovascular system may be different from other cell types in regulation by and response to IGF-1. Further studies are evidently needed to investigate tissue specificity of the protective effects of the GH/IGF-1 pathway using primary endothelial cells, smooth muscle cells, and cardiac myocytes from multiple species in vitro and by comparing different tissues from animal models of GH/IGF-1 deficiency and animals treated with GH and/or IGF-1.
FUNDING
This work was supported by grants from the American Federation for Aging Research (to A.C.), the Oklahoma Center for the Advancement of Science and Technology (to A.C. and Z.U.), the American Heart Association (to A.C. and Z.U.), and the National Institutes of Health (AG031085 to A.C.; AT006526 to Z.U.).
Acknowledgments
The authors would like to express their gratitude for the support of the Donald W. Reynolds Foundation, which funds aging research at the University of Oklahoma Health Sciences Center under its Aging and Quality of Life Program.
References
- 1.Higashi Y, Sukhanov S, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls102. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novosyadlyy R, LeRoith D. Insulin-like growth factors and insulin: at the crossroad between tumor development and longevity. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls065. In press. [DOI] [PubMed] [Google Scholar]
- 3.Sonntag WE, Csiszar A, de Cabo R, Ferrucci L, Ungvari Z. IGF-1, growth hormone and mammalian aging: controversies and progress toward understanding the diverse actions of anabolic hormones during the lifespan. J Gerontol A Biol Sci Med Sci. 2012 In press. [Google Scholar]
- 4.Chisalita SI, Arnqvist HJ. Insulin-like growth factor I receptors are more abundant than insulin receptors in human micro- and macrovascular endothelial cells. Am J Physiol Endocrinol Metab. 2004;286:E896–E901. doi: 10.1152/ajpendo.00327.2003. [DOI] [PubMed] [Google Scholar]
- 5.Chisalita SI, Johansson GS, Liefvendahl E, Back K, Arnqvist HJ. Human aortic smooth muscle cells are insulin resistant at the receptor level but sensitive to IGF1 and IGF2. J Mol Endocrinol. 2009;43:231–239. doi: 10.1677/JME-09-0021. [DOI] [PubMed] [Google Scholar]
- 6.Johansson GS, Chisalita SI, Arnqvist HJ. Human microvascular endothelial cells are sensitive to IGF-I but resistant to insulin at the receptor level. Mol Cell Endocrinol. 2008;296:58–63. doi: 10.1016/j.mce.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breese CR, Ingram RL, Sonntag WE. Influence of age and long-term dietary restriction on plasma insulin-like growth factor-1 (IGF-1), IGF-1 gene expression, and IGF-1 binding proteins. J Gerontol. 1991;46:B180–B187. doi: 10.1093/geronj/46.5.b180. [DOI] [PubMed] [Google Scholar]
- 9.D’Costa AP, Ingram RL, Lenham JE, Sonntag WE. The regulation and mechanisms of action of growth hormone and insulin-like growth factor 1 during normal ageing. J Reprod Fertil. 1993;46:87–98. [PubMed] [Google Scholar]
- 10.Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res. 2002;54:25–35. doi: 10.1016/s0008-6363(01)00533-8. [DOI] [PubMed] [Google Scholar]
- 11.Evans LM, Davies JS, Goodfellow J, Rees JA, Scanlon MF. Endothelial dysfunction in hypopituitary adults with growth hormone deficiency. Clin Endocrinol. 1999;50:457–464. doi: 10.1046/j.1365-2265.1999.00671.x. [DOI] [PubMed] [Google Scholar]
- 12.Denti L, Annoni V, Cattadori E, et al. Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. Am J Med. 2004;117:312–317. doi: 10.1016/j.amjmed.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 13.Oflaz H, Sen F, Elitok A, et al. Coronary flow reserve is impaired in patients with adult growth hormone (GH) deficiency. Clin Endocrinol. 2007;66:524–529. doi: 10.1111/j.1365-2265.2007.02767.x. [DOI] [PubMed] [Google Scholar]
- 14.Rosen T, Bengtsson BA. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet. 1990;336:285–288. doi: 10.1016/0140-6736(90)91812-o. [DOI] [PubMed] [Google Scholar]
- 15.Bates AS, Van’t Hoff W, Jones PJ, Clayton RN. The effect of hypopituitarism on life expectancy. J Clin Endocrinol Metab. 1996;81:1169–1172. doi: 10.1210/jcem.81.3.8772595. [DOI] [PubMed] [Google Scholar]
- 16.Bulow B, Hagmar L, Mikoczy Z, Nordstrom CH, Erfurth EM. Increased cerebrovascular mortality in patients with hypopituitarism. Clin Endocrinol (Oxf) 1997;46:75–81. doi: 10.1046/j.1365-2265.1997.d01-1749.x. [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson JW, Holden N, Hills RK, et al. Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet. 2001;357:425–431. doi: 10.1016/s0140-6736(00)04006-x. [DOI] [PubMed] [Google Scholar]
- 18.Vasan RS, Sullivan LM, D’Agostino RB, et al. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann Intern Med. 2003;139:642–648. doi: 10.7326/0003-4819-139-8-200310210-00007. [DOI] [PubMed] [Google Scholar]
- 19.Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89:114–120. doi: 10.1210/jc.2003-030967. [DOI] [PubMed] [Google Scholar]
- 20.Conti E, Carrozza C, Capoluongo E, et al. Insulin-like growth factor-1 as a vascular protective factor. Circulation. 2004;110:2260–2265. doi: 10.1161/01.CIR.0000144309.87183.FB. [DOI] [PubMed] [Google Scholar]
- 21.Spallarossa P, Brunelli C, Minuto F, et al. Insulin-like growth factor-I and angiographically documented coronary artery disease. Am J Cardiol. 1996;77:200–202. doi: 10.1016/s0002-9149(96)90600-1. [DOI] [PubMed] [Google Scholar]
- 22.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106:939–944. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- 23.Conti E, Andreotti F, Sciahbasi A, et al. Markedly reduced insulin-like growth factor-1 in the acute phase of myocardial infarction. J Am Coll Cardiol. 2001;38:26–32. doi: 10.1016/s0735-1097(01)01367-5. [DOI] [PubMed] [Google Scholar]
- 24.van den Beld AW, Bots ML, Janssen JA, Pols HA, Lamberts SW, Grobbee DE. Endogenous hormones and carotid atherosclerosis in elderly men. Am J Epidemiol. 2003;157:25–31. doi: 10.1093/aje/kwf160. [DOI] [PubMed] [Google Scholar]
- 25.Smith JC, Evans LM, Wilkinson I, et al. Effects of GH replacement on endothelial function and large-artery stiffness in GH-deficient adults: a randomized, double-blind, placebo-controlled study. Clin Endocrinol. 2002;56:493–501. doi: 10.1046/j.1365-2265.2002.01514.x. [DOI] [PubMed] [Google Scholar]
- 26.Evans LM, Davies JS, Anderson RA, et al. The effect of GH replacement therapy on endothelial function and oxidative stress in adult growth hormone deficiency. Eur J Endocrinol. 2000;142:254–262. doi: 10.1530/eje.0.1420254. [DOI] [PubMed] [Google Scholar]
- 27.Galderisi M, Caso P, Cicala S, et al. Positive association between circulating free insulin-like growth factor-1 levels and coronary flow reserve in arterial systemic hypertension. Am J Hypertens. 2002;15:766–772. doi: 10.1016/s0895-7061(02)02967-9. [DOI] [PubMed] [Google Scholar]
- 28.Johnsen SP, Hundborg HH, Sorensen HT, et al. Insulin-like growth factor (IGF) I, -II, and IGF binding protein-3 and risk of ischemic stroke. J Clin Endocrinol Metab. 2005;90:5937–5941. doi: 10.1210/jc.2004-2088. [DOI] [PubMed] [Google Scholar]
- 29.De Smedt A, Brouns R, Uyttenboogaart M, et al. Insulin-like growth factor I serum levels influence ischemic stroke outcome. Stroke. 2011;42:2180–2185. doi: 10.1161/STROKEAHA.110.600783. [DOI] [PubMed] [Google Scholar]
- 30.Aberg D, Jood K, Blomstrand C, et al. Serum IGF-I levels correlate to improvement of functional outcome after ischemic stroke. J Clin Endocrinol Metab. 2011;96:E1055–E1064. doi: 10.1210/jc.2010-2802. [DOI] [PubMed] [Google Scholar]
- 31.Zhu W, Fan Y, Hao Q, et al. Postischemic IGF-1 gene transfer promotes neurovascular regeneration after experimental stroke. J Cereb Blood Flow Metab. 2009;29:1528–1537. doi: 10.1038/jcbfm.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laron Z, Klinger B, Silbergeld A. Patients with Laron syndrome have Osteopenia/Osteoporosis. J Bone Miner Res. 1999;14:156–157. doi: 10.1359/jbmr.1999.14.1.156. [DOI] [PubMed] [Google Scholar]
- 33.Feinberg MS, Scheinowitz M, Laron Z. Echocardiographic dimensions and function in adults with primary growth hormone resistance (Laron syndrome) Am J Cardiol. 2000;85:209–213. doi: 10.1016/s0002-9149(99)00642-6. [DOI] [PubMed] [Google Scholar]
- 34.Feinberg MS, Scheinowitz M, Laron Z. Cardiac dimension and function in patients with childhood onset growth hormone deficiency, before and after growth hormone retreatment in adult age. Am Heart J. 2003;145:549–553. doi: 10.1067/mhj.2003.175. [DOI] [PubMed] [Google Scholar]
- 35.Scheinowitz M, Feinberg MS, Laron Z. IGF-I replacement therapy in children with congenital IGF-I deficiency (Laron syndrome) maintains heart dimension and function. Growth Horm IGF Res. 2009;19:280–282. doi: 10.1016/j.ghir.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Ben-Dov I, Gaides M, Scheinowitz M, Wagner R, Laron Z. Reduced exercise capacity in untreated adults with primary growth hormone resistance (Laron syndrome) Clin Endocrinol (Oxf) 2003;59:763–767. doi: 10.1046/j.1365-2265.2003.01920.x. [DOI] [PubMed] [Google Scholar]
- 37.Laron Z, Wang XL, Klinger B, Silbergeld A, Wilcken DE. Insulin-like growth factor-I decreases serum lipoprotein (a) during long-term treatment of patients with Laron syndrome. Metabolism. 1996;45:1263–1266. doi: 10.1016/s0026-0495(96)90245-0. [DOI] [PubMed] [Google Scholar]
- 38.Shechter M, Ginsberg S, Scheinowitz M, Feinberg MS, Laron Z. Obese adults with primary growth hormone resistance (Laron syndrome) have normal endothelial function. Growth Horm IGF Res. 2007;17:165–170. doi: 10.1016/j.ghir.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Amemiya T, Toibana M, Hashimoto M, Oseko F, Imura H. Diabetic retinopathy in acromegaly. Ophthalmologica. 1978;176:74–80. doi: 10.1159/000308696. [DOI] [PubMed] [Google Scholar]
- 40.Inokuchi N, Ikeda T, Yasuda F, Shirai S, Uchinori Y. Severe proliferative diabetic retinopathy associated with acromegaly. Br J Ophthalmol. 1999;83:629–630. doi: 10.1136/bjo.83.5.628c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tran HA, Petrovsky N, Field AJ. Severe diabetic retinopathy: a rare complication of acromegaly. Intern Med J. 2002;32:52–54. [PubMed] [Google Scholar]
- 42.Dominici FP, Hauck S, Argentino DP, Bartke A, Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (IRS)-1 and IRS-2 in liver of Ames dwarf mice. J Endocrinol. 2002;173:81–94. doi: 10.1677/joe.0.1730081. [DOI] [PubMed] [Google Scholar]
- 43.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laron Z, Weinberger D. Diabetic retinopathy in two patients with congenital IGF-I deficiency (Laron syndrome) Eur J Endocrinol. 2004;151:103–106. doi: 10.1530/eje.0.1510103. [DOI] [PubMed] [Google Scholar]
- 45.Nieves-Martinez E, Sonntag WE, Wilson A, et al. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. J Endocrinol. 2010;204:31–36. doi: 10.1677/JOE-09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramsey MM, Ingram RL, Cashion AB, et al. Growth hormone-deficient dwarf animals are resistant to dimethylbenzanthracine (DMBA)-induced mammary carcinogenesis. Endocrinology. 2002;143:4139–4142. doi: 10.1210/en.2002-220717. [DOI] [PubMed] [Google Scholar]
- 47.Sonntag WE, Carter CS, Ikeno Y, et al. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- 48.Ungvari Z, Gautam T, Koncz P, et al. Vasoprotective effects of life span-extending peripubertal GH replacement in Lewis Dwarf rats. J Gerontol A Biol Sci Med Sci. 2010;65:1145–1156. doi: 10.1093/gerona/glq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ungvari Z, Sosnowska D, Podlutsky A, Koncz P, Sonntag WE, Csiszar A. Free radical production, antioxidant capacity, and oxidative stress response signatures in fibroblasts from Lewis dwarf rats: effects of life span-extending peripubertal GH treatment. J Gerontol A Biol Sci Med Sci. 2011;66:501–510. doi: 10.1093/gerona/glr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bailey-Downs LC, Sosnowska D, Toth P, et al. High-fat diet-induced endothelial impairment in obese Lewis Dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr197. doi:10.1093/gerona/glr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cittadini A, Stromer H, Vatner DE, et al. Consequences of growth hormone deficiency on cardiac structure, function, and beta-adrenergic pathway: studies in mutant dwarf rats. Endocrinology. 1997;138:5161–5169. doi: 10.1210/endo.138.12.5591. [DOI] [PubMed] [Google Scholar]
- 52.Longobardi S, Cittadini A, Stromer H, et al. Echocardiographic assessment of cardiac morphology and function in mutant dwarf rats. Growth Horm IGF Res. 2000;10:242–247. doi: 10.1054/ghir.2000.0160. [DOI] [PubMed] [Google Scholar]
- 53.Stromer H, Cittadini A, Grossman JD, Douglas PS, Morgan JP. Intrinsic cardiac muscle function, calcium handling and beta-adrenergic responsiveness is impaired in rats with growth hormone deficiency. Growth Horm IGF Res. 1999;9:262–271. doi: 10.1054/ghir.1999.0117. [DOI] [PubMed] [Google Scholar]
- 54.Groban L, Lin M, Kassik KA, Ingram RL, Sonntag WE. Early-onset growth hormone deficiency results in diastolic dysfunction in adult-life and is prevented by growth hormone supplementation. Growth Horm IGF Res. 2011;21:81–88. doi: 10.1016/j.ghir.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cittadini A, Grossman JD, Stromer H, Katz SE, Morgan JP, Douglas PS. Importance of an intact growth hormone/insulin-like growth factor 1 axis for normal post-infarction healing: studies in dwarf rats. Endocrinology. 2001;142:332–338. doi: 10.1210/endo.142.1.7913. [DOI] [PubMed] [Google Scholar]
- 56.Beznak M. The effect of adrenocortical hormones alone and in combination with growth hormone on cardiac hypertrophy and blood pressure of hypophysectomized rats. J Physiol. 1954;124:75–83. doi: 10.1113/jphysiol.1954.sp005086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beznak M. The restoration of cardiac hypertrophy and blood pressure in hypophysectomized rats by large doses of lyophilized anterior pituitary and growth hormone. J Physiol. 1954;124:64–74. doi: 10.1113/jphysiol.1954.sp005085. [DOI] [PubMed] [Google Scholar]
- 58.Beznak M. The effect of the pituitary and growth hormone on the blood pressure and on the ability of the heart to hypertrophy. J Physiol. 1952;116:74–83. doi: 10.1113/jphysiol.1952.sp004690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Csiszar A, Labinskyy N, Perez V, et al. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–H1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Buul-Offers S, Smeets T, Van den Brande JL. Effects of growth hormone and thyroxine on the relation between tibial length and the histological appearance of the proximal tibial epiphysis in Snell dwarf mice. Growth. 1984;48:166–175. [PubMed] [Google Scholar]
- 61.Helms SA, Azhar G, Zuo C, Theus SA, Bartke A, Wei JY. Smaller cardiac cell size and reduced extra-cellular collagen might be beneficial for hearts of Ames dwarf mice. Int J Biol Sci. 2010;6:475–490. doi: 10.7150/ijbs.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ren J, Brown-Borg HM. Impaired cardiac excitation-contraction coupling in ventricular myocytes from Ames dwarf mice with IGF-I deficiency. Growth Horm IGF Res. 2002;12:99–105. doi: 10.1054/ghir.2002.0267. [DOI] [PubMed] [Google Scholar]
- 63.Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived ames dwarf mice are resistant to chemical stressors. J Gerontol A Biol Sci Med Sci. 2009;64:819–827. doi: 10.1093/gerona/glp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choksi KB, Nuss JE, DeFord JH, Papaconstantinou J. Mitochondrial electron transport chain functions in long-lived Ames dwarf mice. Aging (Albany NY) 2011;3:754–767. doi: 10.18632/aging.100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reddy AK, Amador-Noguez D, Darlington GJ, et al. Cardiac function in young and old Little mice. J Gerontol A Biol Sci Med Sci. 2007;62:1319–1325. doi: 10.1093/gerona/62.12.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bailey-Downs LC, Mitschelen M, Sosnowska D, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr164. doi:10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitschelen M, Yan H, Farley JA, et al. Long-term deficiency of circulating and hippocampal insulin-like growth factor I induces depressive behavior in adult mice: a potential model of geriatric depression. Neuroscience. 2011;185:50–60. doi: 10.1016/j.neuroscience.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Q, Ceylan-Isik AF, Li J, Ren J. Deficiency of insulin-like growth factor 1 reduces sensitivity to aging-associated cardiomyocyte dysfunction. Rejuvenation Res. 2008;11:725–733. doi: 10.1089/rej.2008.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hua Y, Zhang Y, Ren J. Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Cell Mol Med. 2012;16:83–95. doi: 10.1093/gerona/glr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu R, Yakar S, Liu YL, et al. Liver-specific IGF-I gene deficient mice exhibit accelerated diabetes in response to streptozotocin, associated with early onset of insulin resistance. Mol Cell Endocrinol. 2003;204:31–42. doi: 10.1016/s0303-7207(03)00145-x. [DOI] [PubMed] [Google Scholar]
- 71.Abbas A, Imrie H, Viswambharan H, et al. The insulin-like growth factor-1 receptor is a negative regulator of nitric oxide bioavailability and insulin sensitivity in the endothelium. Diabetes. 2011;60:2169–2178. doi: 10.2337/db11-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laustsen PG, Russell SJ, Cui L, et al. Essential role of insulin and insulin-like growth factor 1 receptor signaling in cardiac development and function. Mol Cell Biol. 2007;27:1649–1664. doi: 10.1128/MCB.01110-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26:929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 74.Wang J, Niu W, Nikiforov Y, et al. Targeted overexpression of IGF-I evokes distinct patterns of organ remodeling in smooth muscle cell tissue beds of transgenic mice. J Clin Invest. 1997;100:1425–1439. doi: 10.1172/JCI119663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. IGF-1, oxidative stress and atheroprotection. Trends Endocrinol Metab. 2010;21:245–254. doi: 10.1016/j.tem.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shai SY, Sukhanov S, Higashi Y, Vaughn C, Kelly J, Delafontaine P. Smooth muscle cell-specific insulin-like growth factor-1 overexpression in Apoe-/- mice does not alter atherosclerotic plaque burden but increases features of plaque stability. Arterioscler Thromb Vasc Biol. 2010;30:1916–1924. doi: 10.1161/ATVBAHA.110.210831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sukhanov S, Higashi Y, Shai SY, et al. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2684–2690. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- 78.Conover CA, Bale LK, Mader JR, Mason MA, Keenan KP, Marler RJ. Longevity and age-related pathology of mice deficient in pregnancy-associated plasma protein-A. J Gerontol A Biol Sci Med Sci. 2010;65:590–599. doi: 10.1093/gerona/glq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Louis A, Bartke A, Masternak MM. Effects of growth hormone and thyroxine replacement therapy on insulin signaling in Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2010;65:344–352. doi: 10.1093/gerona/glq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masternak MM, Panici JA, Wang F, Wang Z, Spong A. The effects of growth hormone (GH) treatment on GH and insulin/IGF-1 signaling in long-lived Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2010;65:24–30. doi: 10.1093/gerona/glp172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Panici JA, Wang F, Bonkowski MS, et al. Is altered expression of hepatic insulin-related genes in growth hormone receptor knockout mice due to GH resistance or a difference in biological life spans? J Gerontol A Biol Sci Med Sci. 2009;64:1126–1133. doi: 10.1093/gerona/glp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Santini MP, Lexow J, Borsellino G, et al. IGF-1Ea induces vessel formation after injury and mediates bone marrow and heart cross-talk through the expression of specific cytokines. Biochem Biophys Res Commun. 2011;410:201–207. doi: 10.1016/j.bbrc.2011.05.081. [DOI] [PubMed] [Google Scholar]
- 83.Vinciguerra M, Santini MP, Claycomb WC, Ladurner AG, Rosenthal N. Local IGF-1 isoform protects cardiomyocytes from hypertrophic and oxidative stresses via SirT1 activity. Aging (Albany NY) 2010;2:43–62. doi: 10.18632/aging.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vinciguerra M, Santini MP, Martinez C, et al. mIGF-1/JNK1/SirT1 signaling confers protection against oxidative stress in the heart. Aging Cell. 2012;11:139–149. doi: 10.1111/j.1474-9726.2011.00766.x. [DOI] [PubMed] [Google Scholar]
- 85.Leri A, Kajstura J, Li B, et al. Cardiomyocyte aging is gender-dependent: the local IGF-1-IGF-1R system. Heart Dis. 2000;2:108–115. [PubMed] [Google Scholar]
- 86.Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- 87.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Caloric restriction and growth hormone receptor knockout: effects on expression of genes involved in insulin action in the heart. Exp Gerontol. 2006;41:417–429. doi: 10.1016/j.exger.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.D’Costa AP, Lenham JE, Ingram RL, Sonntag WE. Moderate caloric restriction increases type 1 IGF receptors and protein synthesis in aging rats. Mech Ageing Dev. 1993;71:59–71. doi: 10.1016/0047-6374(93)90035-p. [DOI] [PubMed] [Google Scholar]
- 89.Groban L, Pailes NA, Bennett CD, et al. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- 90.Li Q, Wu S, Li SY, et al. Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol. 2007;292:H1398–H1403. doi: 10.1152/ajpheart.01036.2006. [DOI] [PubMed] [Google Scholar]
- 91.Li Q, Ren J. Influence of cardiac-specific overexpression of insulin-like growth factor 1 on lifespan and aging-associated changes in cardiac intracellular Ca2+ homeostasis, protein damage and apoptotic protein expression. Aging Cell. 2007;6:799–806. doi: 10.1111/j.1474-9726.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 92.Ren J, Duan J, Thomas DP, et al. IGF-I alleviates diabetes-induced RhoA activation, eNOS uncoupling, and myocardial dysfunction. Am J Physiol Regul Integr Comp Physiol. 2008;294:R793–R802. doi: 10.1152/ajpregu.00713.2007. [DOI] [PubMed] [Google Scholar]
- 93.Paolisso G, Ammendola S, Del Buono A, et al. Serum levels of insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 in healthy centenarians: relationship with plasma leptin and lipid concentrations, insulin action, and cognitive function. J Clin Endocrinol Metab. 1997;82:2204–2209. doi: 10.1210/jcem.82.7.4087. [DOI] [PubMed] [Google Scholar]
- 94.Padin-Iruegas ME, Misao Y, Davis ME, et al. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120:876–887. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Urbanek K, Rota M, Cascapera S, et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 96.Torella D, Rota M, Nurzynska D, et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 97.Li Q, Li B, Wang X, et al. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997;100:1991–1999. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leri A, Liu Y, Wang X, et al. Overexpression of insulin-like growth factor-1 attenuates the myocyte renin-angiotensin system in transgenic mice. Circ Res. 1999;84:752–762. doi: 10.1161/01.res.84.7.752. [DOI] [PubMed] [Google Scholar]
- 99.Li B, Setoguchi M, Wang X, et al. Insulin-like growth factor-1 attenuates the detrimental impact of nonocclusive coronary artery constriction on the heart. Circ Res. 1999;84:1007–1019. doi: 10.1161/01.res.84.9.1007. [DOI] [PubMed] [Google Scholar]
- 100.D’Amario D, Cabral-Da-Silva MC, Zheng H, et al. Insulin-like growth factor-1 receptor identifies a pool of human cardiac stem cells with superior therapeutic potential for myocardial regeneration. Circ Res. 2011;108:1467–1481. doi: 10.1161/CIRCRESAHA.111.240648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Anversa P, Li P, Sonnenblick EH, Olivetti G. Effects of aging on quantitative structural properties of coronary vasculature and microvasculature in rats. Am J Physiol. 1994;267:H1062–H1073. doi: 10.1152/ajpheart.1994.267.3.H1062. [DOI] [PubMed] [Google Scholar]
- 102.Kang DH, Anderson S, Kim YG, et al. Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis. 2001;37:601–611. doi: 10.1053/ajkd.2001.22087. [DOI] [PubMed] [Google Scholar]
- 103.Montagna W, Carlisle K. Structural changes in aging human skin. J Invest Dermatol. 1979;73:47–53. doi: 10.1111/1523-1747.ep12532761. [DOI] [PubMed] [Google Scholar]
- 104.Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev. 2003;2:149–168. doi: 10.1016/s1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- 105.Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197(Pt 4):575–585. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hua K, Forbes ME, Lichtenwalner RJ, Sonntag WE, Riddle DR. Adult-onset deficiency in growth hormone and insulin-like growth factor-I alters oligodendrocyte turnover in the corpus callosum. Glia. 2008 doi: 10.1002/glia.20829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 108.Ramsey MM, Weiner JL, Moore TP, Carter CS, Sonntag WE. Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience. 2004;129:119–127. doi: 10.1016/j.neuroscience.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 109.Poe BH, Linville C, Riddle DR, Sonntag WE, Brunso-Bechtold JK. Effects of age and insulin-like growth factor-1 on neuron and synapse numbers in area CA3 of hippocampus. Neuroscience. 2001;107:231–238. doi: 10.1016/s0306-4522(01)00341-4. [DOI] [PubMed] [Google Scholar]
- 110.Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 111.Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khan AS, Lynch CD, Sane DC, Willingham MC, Sonntag WE. Growth hormone increases regional coronary blood flow and capillary density in aged rats. J Gerontol Biol Sci. 2001;56:B364–B371. doi: 10.1093/gerona/56.8.b364. [DOI] [PubMed] [Google Scholar]
- 113.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 114.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-TNF-alpha treatment in aging. Am J Pathol. 2007;170:388–398. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Asai K, Kudej RK, Shen YT, et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000;20:1493–1499. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- 117.Li Y, Wu H, Khardori R, Song YH, Lu YW, Geng YJ. Insulin-like growth factor-1 receptor activation prevents high glucose-induced mitochondrial dysfunction, cytochrome-c release and apoptosis. Biochem Biophys Res Commun. 2009;384:259–264. doi: 10.1016/j.bbrc.2009.04.113. [DOI] [PubMed] [Google Scholar]
- 118.Rivard A, Fabre JE, Silver M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 119.Guan J, Bennet L, George S, et al. Insulin-like growth factor-1 reduces postischemic white matter injury in fetal sheep. J Cereb Blood Flow Metab. 2001;21:493–502. doi: 10.1097/00004647-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 120.Guan J, Bennet TL, George S, et al. Selective neuroprotective effects with insulin-like growth factor-1 in phenotypic striatal neurons following ischemic brain injury in fetal sheep. Neuroscience. 2000;95:831–839. doi: 10.1016/s0306-4522(99)00456-x. [DOI] [PubMed] [Google Scholar]
- 121.Leinninger GM, Feldman EL. Insulin-like growth factors in the treatment of neurological disease. Endocr Dev. 2005;9:135–159. doi: 10.1159/000085763. [DOI] [PubMed] [Google Scholar]
- 122.Loddick SA, Liu XJ, Lu ZX, et al. Displacement of insulin-like growth factors from their binding proteins as a potential treatment for stroke. Proc Natl Acad Sci U S A. 1998;95:1894–1898. doi: 10.1073/pnas.95.4.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mackay KB, Loddick SA, Naeve GS, Vana AM, Verge GM, Foster AC. Neuroprotective effects of insulin-like growth factor-binding protein ligand inhibitors in vitro and in vivo. J Cereb Blood Flow Metab. 2003;23:1160–1167. doi: 10.1097/01.WCB.0000087091.01171.AE. [DOI] [PubMed] [Google Scholar]
- 124.Liu XF, Fawcett JR, Thorne RG, DeFor TA, Frey WH., 2nd Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J Neurol Sci. 2001;187:91–97. doi: 10.1016/s0022-510x(01)00532-9. [DOI] [PubMed] [Google Scholar]
- 125.Schabitz WR, Hoffmann TT, Heiland S, et al. Delayed neuroprotective effect of insulin-like growth factor-i after experimental transient focal cerebral ischemia monitored with mri. Stroke. 2001;32:1226–1233. doi: 10.1161/01.str.32.5.1226. [DOI] [PubMed] [Google Scholar]
- 126.Zhu W, Fan Y, Frenzel T, et al. Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke. 2008;39:1254–1261. doi: 10.1161/STROKEAHA.107.500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thum T, Hoeber S, Froese S, et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res. 2007;100:434–443. doi: 10.1161/01.RES.0000257912.78915.af. [DOI] [PubMed] [Google Scholar]
- 128.Zhu G, Song M, Wang H, et al. Young environment reverses the declined activity of aged rat-derived endothelial progenitor cells: involvement of the phosphatidylinositol 3-kinase/Akt signaling pathway. Ann Vasc Surg. 2009;23:519–534. doi: 10.1016/j.avsg.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 129.Forman K, Vara E, Garcia C, et al. Effect of a combined treatment with growth hormone and melatonin in the cardiological aging on male SAMP8 mice. J Gerontol A Biol Sci Med Sci. 2011;66:823–834. doi: 10.1093/gerona/glr083. [DOI] [PubMed] [Google Scholar]
- 130.Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 131.Kubis N, Besnard S, Silvestre JS, et al. Decreased arteriolar density in endothelial nitric oxide synthase knockout mice is due to hypertension, not to the constitutive defect in endothelial nitric oxide synthase enzyme. J Hypertens. 2002;20:273–280. doi: 10.1097/00004872-200202000-00017. [DOI] [PubMed] [Google Scholar]
- 132.Frisbee JC. Reduced nitric oxide bioavailability contributes to skeletal muscle microvessel rarefaction in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2005;289:R307–R316. doi: 10.1152/ajpregu.00114.2005. [DOI] [PubMed] [Google Scholar]
- 133.Pu XY, Wang XH, Gao WC, et al. Insulin-like growth factor-1 restores erectile function in aged rats: modulation the integrity of smooth muscle and nitric oxide-cyclic guanosine monophosphate signaling activity. J Sex Med. 2008;5:1345–1354. doi: 10.1111/j.1743-6109.2008.00817.x. [DOI] [PubMed] [Google Scholar]
- 134.Cittadini A, Monti MG, Castiello MC, et al. Insulin-like growth factor-1 protects from vascular stenosis and accelerates re-endothelialization in a rat model of carotid artery injury. J Thromb Haemost. 2009;7:1920–1928. doi: 10.1111/j.1538-7836.2009.03607.x. [DOI] [PubMed] [Google Scholar]
- 135.Pearson KJ, Lewis KN, Price NL, et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008;105:2325–2330. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Suh JH, Shenvi SV, Dixon BM, et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tullet JM, Hertweck M, An JH, et al. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ungvari ZI, Bailey-Downs L, Gautam T, et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133–H1140. doi: 10.1152/ajpheart.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ungvari Z, Bailey-Downs L, Gautam T, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-kB activation in the non-human primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Collins AR, Lyon CJ, Xia X, et al. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- 143.Ungvari Z, Bailey-Downs L, Sosnowska D, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am J Physiol. 2011;301:H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Morrison CD, Pistell PJ, Ingram DK, et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. 2010;114:1581–1589. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu J, Rong S, Xie B, et al. Procyanidins extracted from the lotus seedpod ameliorate age-related antioxidant deficit in aged rats. J Gerontol A Biol Sci Med Sci. 2010;65:236–241. doi: 10.1093/gerona/glp211. [DOI] [PubMed] [Google Scholar]
- 146.Reynolds SL, Haley WE, Kozlenko N. The impact of depressive symptoms and chronic diseases on active life expectancy in older Americans. Am J Geriatr Psychiatry. 2008;16:425–432. doi: 10.1097/JGP.0b013e31816ff32e. [DOI] [PubMed] [Google Scholar]
- 147.Chen CN, Brown-Borg HM, Rakoczy SG, Thompson LV. Muscle disuse: adaptation of antioxidant systems is age dependent. J Gerontol A Biol Sci Med Sci. 2008;63:461–466. doi: 10.1093/gerona/63.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Young FG. Permanent experimental diabetes produced by pituitary (anterior lobe) injections. Lancet. 1937;2:372–374. [Google Scholar]
- 149.Houssay BA. Carbohydrate metabolism. N Engl J Med. 1936;214:971–986. [Google Scholar]
- 150.Valcarcel-Ares MN, Gautam T, Warrington JP, et al. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr229. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24:5073–5079. doi: 10.1096/fj.10-163253. [DOI] [PMC free article] [PubMed] [Google Scholar]