Abstract
Because the initial reports demonstrating that circulating growth hormone and insulin-like growth factor-1 decrease with age in laboratory animals and humans, there have been numerous studies related to the importance of these hormones for healthy aging. Nevertheless, the role of these potent anabolic hormones in the genesis of the aging phenotype remains controversial. In this chapter, we review the studies demonstrating the beneficial and deleterious effects of growth hormone and insulin-like growth factor-1 deficiency and explore their effects on specific tissues and pathology as well as their potentially unique effects early during development. Based on this review, we conclude that the perceived contradictory roles of growth hormone and insulin-like growth factor-1 in the genesis of the aging phenotype should not be interpreted as a controversy on whether growth hormone or insulin-like growth factor-1 increases or decreases life span but rather as an opportunity to explore the complex roles of these hormones during specific stages of the life span.
Keywords: IGF-1, Longevity, Growth hormone
MORE than three decades have passed since the initial report demonstrating that circulating growth hormone (GH) decreases with age and almost as many years since the reports of the age-related decline in insulin-like growth factor-1 (IGF-1) both in laboratory animals and humans. Despite the 100s of primary studies and numerous reviews related to the importance of adequate levels of circulating GH and IGF-1 for healthy aging, the role of these potent anabolic hormones in the genesis of the aging phenotype remains highly controversial. Initially, the age-related decrease in GH and IGF-1 were considered to contribute to many aspects of aging including, but not limited to, accumulation of fat mass, cardiovascular dysfunction, as well as the decline in immune function, cellular protein synthesis, and muscle mass. Later studies indicated that reduction in levels of these hormones has an important role in the decline in cognitive function and increased risk for neurodegenerative diseases and stroke. Despite the clear evidence that GH/IGF-1 deficiency contributes to specific aspects of aging, subsequent studies revealed that some, but not all, rodent models with impaired GH/IGF-1 signaling exhibit an increased life span. Based on studies initially conducted in invertebrate organisms, such as Caenorhabditis elegans and Drosophila melanogaster, the corresponding data in mutant and transgenic mouse models supported the conclusion that IGF-1 signaling is part of a “conserved mechanism of aging” with decreased levels of GH/IGF-1 delaying, rather than accelerating, the aging process. Thus, two disparate concepts evolved and remain present in the literature (a) that the presence of normal levels of GH and IGF-1 accelerate aging and lack of these hormones or disruption of the signaling pathways governed by these hormones exert antiaging effects and (b) that the age-related decline in GH and IGF-1 contribute to the deterioration of physiological function and replacement of these hormones delay or reverse the aging phenotype.
The primary barriers to reconciling these two points of view are that they challenge our understanding of the relationship between pathology and aging, the nature of conserved mechanisms of aging, and the importance of changes in the levels of these hormones that occur throughout the life span. These conceptual differences have been exacerbated by numerous studies that draw conclusions about aging and life span based on a low number of experimental animals, suboptimal animal husbandry, and/or the absence of end-of-life pathology to corroborate the conclusions. This has resulted in a plethora of studies that provide varying levels of support for the hypothesis that IGF-1 deficiency increases life span. The purpose of this series of chapters in the current edition of The Journal of Gerontology: Biological Sciences and Medical Sciences is to bring clarity to the diverse views on GH and IGF-1, develop an initial model that encapsulates the known actions of these hormones throughout the life span, and propose their relationship to aging and age-related disease (1–5). We believe that by focusing on the apparent contradictory findings of these studies in the same issue, the varying points of view can begin to be reconciled and our understanding of aging and age-related disease can progress. We also propose new avenues for research targeting the primary mechanism(s) through which GH and IGF-1 signaling influence aging and the development of age-related diseases.
ARE GH AND/OR IGF-1 PART OF A CONSERVED MECHANISM OF AGING?
Neuronal Insulin-Like Signaling and Regulation of Longevity in Invertebrates
The insulin/IGF-1 pathway in mammals shares a common signaling pathway with invertebrates, and the discovery that DAF-2 signaling influences life span in this species is an important landmark in the biology of aging. The initial studies by Kenyon revealed that suppression of signaling in the DAF-2 pathway doubled life span and that the increased life span required DAF-16 (6). Subsequent studies indicated that the increase in life span could not only be induced during development (inducing dauer formation) but also could be induced in adulthood (7). The connection between the DAF-2 pathway and insulin/IGF-1 signaling was discovered in 1997 when it was reported that the DAF-2 receptor was a homolog of the human insulin/IGF-1 receptor (8). These studies provided the first evidence that hormones, normally important for growth and development and regulation of fuel homeostasis, had the potential to regulate life span. With the subsequent cloning of daf-16 and identification of this factor as part of the FOXO family of transcription factors (9,10), the relationship between the DAF-2 and DAF-16 pathway in C elegans, and the insulin/IGF-1 signaling, FOXO pathway in mammals was established.
In C elegans, expression and function of DAF-2 are highly organ specific: the abundance of DAF-2 in neuronal cells is consistent with the central role of the nervous system in DAF-2–dependent regulation of animal longevity (11,12). The available evidence suggests that in C elegans, regulation of life span is independent of the metabolic effects of insulin-like signaling in the gastrointestinal system and muscle tissue (11). Despite the exclusive role of neuronal insulin-like signaling in regulation of longevity in this model system, the link between IGF-1 signaling in the brain and mammalian longevity has not been investigated.
Studies on the arthropod D melanogaster provide further evidence that insulin/IGF-1 signaling in the central nervous is involved in regulation of life span in lower organisms (13). For example, ablation of insulin-like peptide-producing median neurosecretory cells in the brain of D melanogaster leads to a diabetes-like metabolic state, which is associated with an increased life span (13). Because the insulin/IGF-1 signaling the central nervous system can play both endocrine and cell-autonomous roles in extension of life span, Broughton and Partridge recently suggested that tissue-specific manipulation of insulin/IGF-1 signaling will be essential to better define the roles of paracrine and endocrine insulin/IGF-1 signaling in regulation of life span and organ function during aging in model organisms (14). Currently, it is unknown whether organ-specific insulin/IGF-1 action in arthropods is similar to that in mammals. For example, unlike in mammals, in which both insulin deficiency and low IGF-1 levels result in cardiovascular dysfunction, age-related alterations in cardiac function in flies were reported to be unaffected by disruption of insulin/IGF-1 signaling (15).
GH/IGF-1 Deficiency and Life Span in Rodents
The possibility that insulin/IGF-1 signaling is part of an evolutionarily conserved pathway that regulates life span received critical support when it was reported that genetic deficiencies in GH and IGF-1 in mice resulting from mutations in Pit-1 and prop-1 (Snell dwarf and Ames mice, respectively) are associated with a substantial increase in life span (16; also see the accompanying review by Brown-Borg and Bartke in the present issue). Pit-1 and prop-1 are transcription factors that regulate the development of specific cell types in the anterior pituitary gland, and mutations of these genes result in the absence of somatotropes, lactotropes, and thyrotropes that secrete GH, prolactin, and thyroid stimulating hormone, respectively. These animals exhibit altered secretion of glucocorticoids, insulin, and other important counter-regulatory hormones because circulating levels of GH and IGF-1 during early development have a role in maturation of other endocrine organs. In the early reviews of the area, it was suggested that it would be difficult to determine the specific role of GH and IGF-1 in aging in part due to the complex role of these hormones during development and the interactions between these hormones and other endocrine systems (17–19). Subsequently, the multiple endocrine deficiencies evident in these animals were addressed by developing a mouse model with a knockout of the GH receptor (ghr−/−). As expected, these animals are GH insensitive and demonstrate marked neonatal and postnatal reductions in IGF-1 levels and other hormones that are GH and IGF-1 dependent. Similar to the Ames and Snell dwarf mice, knockout of the GH receptor led to a substantial increase in life span. The concept that increased life span was the consequence of specific reductions in the GH and IGF-1 pathway was also supported by studies demonstrating that transgenic animals overexpressing GH throughout their life span (which exhibit levels of GH 100- to 1,000-fold higher than normal animals) are shorter lived (20). The aforementioned findings (and additional mouse models—see Table 1) gave important support for the interpretation that GH and IGF-1 deficiency delay mechanisms of aging. As a result, the hypothesis that GH/IGF-1 deficiency represents a conserved mechanism of aging developed widespread support, and it was predicted that disruption of GH/IGF-1 signaling would result in an extension of life span in other mammalian species, including man.
Table 1.
Summary of Lifespan Data from Mouse and Rat Strains with Mutations of the Growth Hormone/IGF-1 AXIS or Signaling Pathways
| Model | Strain | Wild-Type Life Span Days | Mutant Life Span Male % | Mutant Life Span Female % | N/Group | Pathology (P)/Health Span (H) | References |
| Ames | df/df | 832 | +49 | +67 | 13–17 | 16 | |
| Ames | df/df | 781 | +35 | 21–25 | P/H | 21 | |
| Snell | C3H/HeJxDW/J/F1 | 832 | +42 | 24–33 | 22 | ||
| ghr−/− | 129Ola/BalbC | 629–749 | +55 | +37 | 7–19 | 23 | |
| igf1r+/− | 129SV | 568 | +16 (ns) | +33 | 12–20 | 24 | |
| igf1r+/− | C57Bl/6 | 923–983 | −5 (ns) | +5–7 | 47–68 | P/H | 25 |
| ghrhr−/− | C57Bl/6J | 857–886 | +23 | +25 | 31–35 | 2 | |
| PAPPA−/− | C57Bl/6x129SV/E | 698 | +33 | +41 | 20–22 | 26 | |
| PAPPA−/− | C57Bl/6x129SV/E | 665 | +26 | 42–67 | P | 27 | |
| irs1+/− | C57Bl/6 | 738 | 0 (ns) | +14 (ns) | 21–79 | 28 | |
| irs1−/− | C57Bl/6 | 738 | +12 (ns) | +32 | 10–21 | 28 | |
| irs1−/− | C57Bl/6 | 782–786 | +14 | +7 | 12–37 | 29 | |
| irs2+/− | C57Bl/6 | 770 | 0 | 0 | 21–60 | 28 | |
| irs2+/− | C57Bl/6 | 789 | +17 | 13–17 | 30 | ||
| irs2−/− | C57Bl/6 | 770 | −85 | −22 | 14–30 | 28 | |
| Brain irs2+/− | C57Bl/6 | 791 | +18 | 31–50 | 30 | ||
| Brain irs2−/− | C57Bl/6 | 791 | +14 | 23–50 | 30 | ||
| Rat models | |||||||
| Antisense GH | Jcl:Wistar-TgN(tg/-) | 882 | +12.9 | nd | 30 | P | 31 |
| Antisense GH | Jcl:Wistar-TGN(tg/tg) | 882 | −5.6 | nd | 30 | P | 31 |
| dw/dw | Lewis | 899 | 0 | 0 | 16–25 | P/H | 32 |
Note: Data are included for several strains of animals with mutations in the growth hormone/IGF-1 axis. The background strain and lifespan (in days) is noted as well as the percent change in response to the germ-line mutation (compared to wild-type). Differences in lifespan between males and females are separated as detailed in the original manuscript or presented as combined data from both genders. The range of animal numbers/group are included. Analysis of pathology (P) or heathspan (H) is noted. nd = not determined; ns = not significant.
Despite the compelling data for enhanced life span in the presence of GH and IGF-1 deficiency in Ames dwarf and Snell dwarf mice, a review of the literature indicates that the effects of GH/IGF-1 deficiency on life span in many other rodent models are, in many cases, inconsistent. Increased life span in response to mutations in this pathway are, in many cases, gender specific with females demonstrating increased life span in response to some mutations and males in response to others. Similar findings have been reported for models that disrupt IGF-1 signaling. For example, initial studies by Holzenberger indicated that haploinsufficiency of igfr increased life span by 40% in females (no effect was observed in males) and decreased mortality in response to paraquat (24). However, the increase in life span in response to igfr haploinsufficiency was not confirmed when the study was repeated using optimal husbandry conditions (25). In the latter study, a modest 5% increase in life span was found in females and no increase in life span was evident in males. These diverse results are incompatible with the concept that reductions in IGF-1 signaling increase life span in mammals as was reported in invertebrate models. Additional discrepancies are evident when intracellular factors that are part of the insulin, IGF-1 signaling pathway are manipulated in mammals (eg, irs1 and irs2 heterozygous animals). Initially, it was reported that animals heterozygous for a null allele for irs2 exhibit increased life span (30)—however, this finding was not confirmed in another study (28). Initial analysis of irs1 knockout animals indicated that the effects were isolated to females (28), whereas another study indicated that both males and females demonstrated increased life span (29). The gender differences and inconsistency in reproducing key studies in support of a conserved mechanism of aging can in some instances be attributed to low number of animals included in the studies and atypical mortality data, but they also suggest that the effects of GH/IGF-1 deficiency are highly complex and may be strain, species, and/or gender specific. In fact, it has been suggested that there are only three (3) models of GH/IGF-1 deficiency that consistently show increased life span across multiple studies: Ames, Snell, and ghr knockout mice (33). Importantly, more modest reductions in GH and IGF-1 or induction in GH deficiency during adolescence has no effect on life span even though age-related pathology is modified (32). Thus, despite the general consensus that the GH/IGF-1 pathway is a conserved mechanism of aging, the data for increased life span in response to manipulation of this pathway in rodent models remain inconsistent and appear to be the result of studies in an important subset of animal models.
GH/IGF-1 Deficiency and Life Span in Humans
The critical question to be addressed in the GH/IGF-1 field is whether deficiency of these hormones increases life span in humans as has been reported in the murine models. Overall, the data in humans indicate that genetic disruption of GH/IGF-1 signaling does not result in increased life span and may, in fact, result in shorter lived individuals (Figure 1). Importantly, GH deficiency in humans is characterized by hypoglycemia, reduced muscle and bone mass, increased body fat, lipid abnormalities, insulin resistance, neurological deficits, and impaired energy metabolism, changes that are consistent with the known actions of these hormones. Anecdotal evidence in the medical literature has suggested that many human dwarfs are short lived (eg Charles Sherwood Stratton [1838–1883], who performed with the Barnum Circus in the 19th century under the stage name General Tom Thumb, died of stroke at the age of 45 and Nicolas Ferry [1741–1764], who was in the court of King Stanisław Leszczyński, was reported to develop signs of premature aging). Nevertheless, there have been documented examples of people with dwarfism who outlived their healthy peers (eg, the Polish-born proportional dwarf Józef Boruwłaski [1739–1837] who once entertained Queen Maria Theresa). Although the sample size is small, more rigorous studies of dwarf humans with a prop-1 deficiency from the Island of KrK in the Adriatic Sea indicate that these individuals exhibit a normal life span (35). Nevertheless, the population of individuals in this group does not exhibit an extended life span compared with the normal population as would be expected based on the data from the invertebrate models. Similar findings have been reported in studies of Laron dwarfs (mutation in the GH receptor resulting in deficient IGF-1 levels) and in an Ecuadorian population of dwarfs that exhibit a mutation similar to the Israeli Laron dwarfs (34). In this study, the survival curve for the IGF-1–deficient group did not indicate increased life span compared with the general population (Figure 1). The oldest documented living Ecuadorian IGF-1–deficient dwarf, a lady named Pastorita, is in her mid-80s, whereas the oldest documented “wild-type” human died at the age of 122 years. It is informative that over two thirds of the Ecuadorian dwarf population die by the age of 65. Importantly, analysis of cause of mortality indicated that Ecuadorian dwarfs not only exhibit a reduction in cancer risk but also appear to experience an increase in cardiovascular disease, as would be expected based on the important role of IGF-1 in these age-related pathologies (refer to references 1,2).
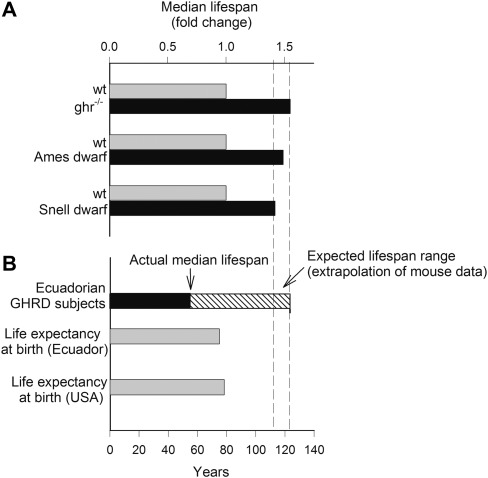
Figure 1.
Panel (A): GH/IGF-1 deficiency in ghr−/−, Ames, and Snell dwarf mice is associated with a substantial extension of life span compared with the respective wild-type controls. Relative increases in median life span, based on the data published in references (16,21–23), are shown. Panel (B): Human GHR-deficient (GHRD) Ecuadorian subjects do not exhibit a longevity phenotype. Median age at 50% survival for the GHRD subjects is indicated based on the survival curve for this population (34). The expected life span for GHRD subjects was calculated by extrapolation of the mouse data. For reference, the life expectancies at birth of the population in Ecuador and the United States are shown. Life-span expectancy data were taken from the Central Intelligence Agency World Factbook (https://www.cia.gov/library/publications/the-world-factbook/rankorder/2102rank.html).
A recent study analyzed the available data to determine the relationship between circulating IGF-1 levels and mortality in the general population using random-effects meta-analysis and dose–response meta-regression (36). Analysis of 12 studies with 14,906 participants clearly demonstrates that in humans, there is a U-shaped association between circulating IGF-1 levels and all-cause mortality. Important for the present discussion, low levels of IGF-1 translate into a significantly increased mortality risk in the general population, predominantly due to an increased incidence of cardiovascular diseases. In contrast, higher IGF-1 levels are associated with an increased cancer mortality (36,37).
Taken together, the human data do not reflect the increased life span found in Ames, Snell, and ghr knockout animals, suggesting that GH/IGF-1 signaling is not part of an evolutionarily conserved mechanism of life span regulation. Yet, the human data are consistent with the important role of GH/IGF-1 signaling in development and prevention of age-related organ-specific diseases.
Mutations in the igfr and foxo Genes
Although the available data to date do not support circulating GH or IGF-1 deficiency in humans as part of an evolutionarily conserved mechanism of longevity, there is evidence that mutations in the igfr gene and reduced signaling in the IGF receptor pathway are associated with longevity (38). igfr mutations that result in decreased IGF receptor function are enriched in centenarians (39). It is unknown whether these changes idicate that igfr receptor signaling is suppressed in multiple tissues throughout life, whether these changes directly contribute to extended life span, and/or whether these changes specifically alter the development of organ-specific diseases of aging. Thus, this area of research requires further investigation. To date, the nature of the specific genetic mutations and the association between IGF-1 signaling in diverse human populations and life span remains unknown.
Part of the cellular response to IGF-1 and insulin signaling is activation of intracellular AKT that regulates the FOXO pathway (a forkhead box DNA-binding protein homologous to daf-16 in C elegans). In mammals, there are four Fox-O isoforms (FOXO1, FOXO3a, FOXO4, and FOXO6) that exhibit functional redundancy although their physiological roles are diverse (40). FOXO proteins have an important role in regulation of apoptosis, gluconeogenesis, autophagy, cell proliferation, angiogenesis, and stress responses as well as stem cell maintenance (41–43). Insulin and IGF-1 through their intracellular pathways phosphorylate FOXO and facilitate their export from the nucleus (through binding to 14-3-3 proteins) leading to a decrease in function. In 2008, Willcox et al. (44) identified FOXO3a as an important susceptibility gene for human longevity, and this initial finding has been confirmed in numerous populations (eg, Germans, Danish, Chinese, Ashkenazi Jews, and Italians; 45–49). FOXO gene variants have been associated with longevity in numerous populations although the function of the variants and their relationship to FOXO activity has not been clearly established. Importantly, a recent study in Germans indicated that FOXO1, FOXO4, or FOXO6 were not associated with longevity (50; suggesting that increased life span is associated only with FOXO3a. Future research needs to be focused on how the multiple FOXO isoforms are regulated by insulin and IGF-1 peptides and the specific mechanisms through which the actions of FOXO gene variants influence life span.
Conclusions
Based on the current available studies, it appears that the definition of a conserved mechanism of aging requires clarification. If a “common” mechanism for increased life span does not relate to animals of different genders or more importantly to humans, it is difficult to conclude that it represents a conserved mechanism of aging. Importantly, the studies in humans on circulating GH and IGF-1 deficiency do not agree with the data from rodent models. GH/IGF-1–deficient dwarf humans can, under some circumstances, live as long as their non-GH/IGF-1–deficient peers, but in the majority of cases, they do not exhibit the increased life span that would be expected based on the relevant literature on laboratory animals. Nevertheless, there are convincing results demonstrating a role for GH/IGF-1 signaling both in development and prevention of organ-specific pathologies in aging.
THE INSULIN/IGF-1 PATHWAYS IN MAMMALS: SEPARATE BUT OVERLAPPING SYSTEMS
Certainly, part of the complexity and confusion in the insulin/IGF-1 field is the fact that there are distinct differences between the common insulin/IGF-1 pathway in invertebrates and the separate but interrelated systems that exist in mammals. For example, studies demonstrating that knockout of the insulin receptor in adipocytes increases life span (51) are generally interpreted as support for the conclusion that both insulin and IGF-1 signaling exhibit a negative effect on life span despite our understanding that these two hormones have independent roles in adipocytes (52). The interrelationship between insulin and IGF-1, however, is even more complex than originally proposed. Receptors for both insulin and IGF-1 are heterodimers with a high degree of homology. Recent data indicate that these receptors can, under some circumstances, exist as hybrid receptors (53–55). Nevertheless, the distribution and potentially unique function of these hybrid receptors remain unclear. If we are to resolve differences related to the effects of GH and IGF-1 on life span, we must be aware of the similarities and differences in IGF-1 and insulin signaling in mammals and avoid generalizations to the common pathway that exist in invertebrates.
THE PROLIFERATIVE EFFECTS OF GH/IGF-1 CONTRIBUTE TO INCREASED INCIDENCE OF NEOPLASTIC DISEASES: POSSIBLE ROLE IN DETERMINATION OF LIFE SPAN IN CANCER-PRONE STRAINS OF RODENTS
Although short-term administration of GH and IGF-1 has been reported to reverse specific functional aspects of aging in mammals, the close relationship between these hormones and cancer had been recognized for many years. Humans with excess GH and IGF-1 are at increased risk for neoplastic disease, and many tumors either express a high density of IGF receptors or produce their own IGF-1 through a paracrine mechanism that facilitates cellular proliferation. As a result, there is a valid concern, well documented in the literature, that increased levels of circulating GH and IGF-1 may increase cancer risk. Although there are studies in humans that support such a relationship, causality has not been clearly established and issues related to the specific levels and duration of GH and/or IGF-1 necessary to increase cancer risk remain unanswered.
In rodents, the relationship between GH/IGF-1 and cancer risk has more clarity. Studies in GH-deficient dwarf rats, for example, indicate that the potent carcinogen, 7,12–Dimethylbenz(a)anthracene, fails to induce mammary cancer when IGF-1 levels are reduced by approximately 50%. Furthermore, replacement of GH produces a dose-related increase in IGF-1, in the number of animals with mammary tumors, and the numbers of tumors per tumor-bearing animal (56). Some of the most intriguing studies that support a role for IGF-1 in cancer were conducted as either cross-sectional pathology or end-of-life pathology in models of GH and IGF-1 deficiency (32,56,57). Pathological analysis in Ames dwarf mice compared with control animals indicates a reduction in numbers of tumors and cancers of various types (21). The reduction in cancer incidence is at least one of the factors that contribute to increased life span especially with the profound reduction in circulating IGF-1 observed in these animals (16,58). Based on the well-documented effects of GH and IGF-1 on cancer and pathology and that most of the mouse models used for aging research were initially bred for cancer research, it would be reasonable to conclude that a modest increase in life span associated with GH/IGF-1 deficiency in cancer-prone strains of mice (eg, 10%–20%) can, in the absence of contradictory pathological data, be explained by the effects of GH and IGF-1 on cancer development rather than modifying an underlying mechanism of aging. It should be noted that genetic susceptibility to cancer and the relative contribution of neoplastic diseases to overall mortality differ among mouse strains and species. Thus, the effect of GH/IGF-1 deficiency on cancer mortality and median life span is likely strain and species dependent. The available data support this conclusion. GH/IGF-1–deficient Lewis dwarf rats, in which intracerebral hemorrhage is an important cause of death, do not exhibit a longevity phenotype in response to GH/IGF-1 deficiency. Rather, treatment with GH during adolescence is vasculoprotective and increases life span (32). Furthermore, in human GH/IGF-1–deficient dwarfs, a significant decrease in cancer risk is not associated with extension of life span (34). Given that subclinical and clinical symptoms exist at various time points prior to the frank manifestation of pathology in animal models, investigators need to be cognizant of the important interactions between pathology and measures of biological aging.
IGF-1 SPLICE VARIANTS, PARACRINE IGF-1, AND IGF-BINDING PROTEINS: UNEXPLORED AREAS OF RESEARCH
IGF-1 is a relatively complex gene spanning 90 kb with six exons. The primary transcript consists of alternative leader sequences and alternative splicing sites between exons 4,5, and 6. Translation is initiated at either exon 1 or 2 and then spliced to exon 3 and 4. Further splicing of exon 4 to exon 6 results in IGF-1Ea (the primary isoform), whereas splicing exons 4 to exons 5 and 6 results in IGF-1Eb. Although there is a controversy related to the in vivo importance of the products of this gene, it has been proposed that pro-IGF-1 is cleaved to the mature 70 amino acid IGF-1 peptide and variable E peptides. Ea is proposed to be 34 amino acids, whereas Eb is 10 amino acids. Interestingly, data indicate that IGF-1Ea or IGF-1Eb increases muscle mass in young animals (59–61), whereas mature IGF-1, Ea, or Eb peptides alone were not effective. These data potentially provide important evidence for a unique role for alternative splice variants of IGF-1. Although there is little information for the action of these peptides in tissues other than skeletal muscle, this is an important area for future investigation. For additional information on the importance of, and controversies in, the production of IGF-1 variants, please refer to excellent review by Matheny et al. (62).
As detailed earlier in this review, IGF-1 is produced locally by many tissues. Although the specific role of paracrine-regulated IGF-1 remains unclear, it has been implicated in the normalization of body growth in the liver-IGF-1–deficient mouse (63) and is likely a key factor in the tissue response to localized trauma, damage, or stress. Reductions in circulating IGF-1 in adults result in no compensatory rise in paracrine IGF-1 levels (at least in brain; 64), resulting in IGF-1 deficiency at the tissue level. However, in the long-lived Ames dwarf, paracrine IGF-1 levels in the brain are elevated despite the reduction in circulating IGF-1 (65). This is an important finding and suggests that early deficiency in IGF-1 may produce lifelong changes in local production of the hormone. If this finding were confirmed in other organs and tissues, it would challenge the concept that long-lived Ames dwarf animals are IGF-1 deficient.
Another important feature of IGF-1 regulation is the presence of at least six IGF-binding proteins (IGFBPs) that regulate IGF-1 activity. In the circulation, IGF-1 is carried by IGFBP-3 in a complex with an acid-labile subunit; numerous other IGFBPs sequester IGF-1 in the extracellular environment, effectively limiting access to the IGF receptor (66–68). Ultimately, IGF-1 is released from its binding proteins by IGFBP proteases that are the determinant of ligand availability (69). To date, there have been few studies that have manipulated these important aspects of the IGF-1 system and investigated effects on life span. Nevertheless, studies from the Conover laboratory demonstrated that knockout of pregnancy-associated plasma protein A, which is a metalloproteinase that degrades IGF-binding proteins and thus increases activity through the IGF receptor, increases life span by approximately 26% (26,27). The findings are consistent with an important effect of IGF-1 deficiency on aging and pathology because tumor development was delayed in these animals (70). Unfortunately, our understanding of the regulation of IGFBPs by proteases and their effect on health span is not complete and therefore further research in this area is essential.
GH/IGF-1 EXHIBITS DIFFERENTIAL EFFECTS ON SPECIFIC ORGANS/TISSUES
GH and IGF-1 have important trophic actions on many tissues. Both gene expression and levels of GH and IGF-1 decrease in the brain with age, and this decrease is closely associated with neuronal dysfunction. Certainly, it has been clear that administration of these hormones increases neuronal function and reverses age-related changes in several neurological measures when administered to aged individuals. IGF-1 has been shown to increase glucose metabolism, neurite outgrowth, neurogenesis, synaptic complexity, and reverse the age-related rise in oxidative stress. Based on the current literature, there is no disagreement that GH and IGF-1 are important for the support of cognitive function in healthy adults. The specific actions of these hormones on mammalian brain function and the effects of IGF-1 are reviewed in the chapter by Deak and Sonntag in this series.
Beneficial effects of GH and IGF-1 on vascular and cardiac function have been well established (refer to references 2 and 3). Furthermore, there are many studies that indicate an important role for IGF-1 or IGF-1–related factors (eg, mechano-growth factor) on muscle function. As previously noted, mechano-growth factor corresponds to IGF-1Eb in rodents (IGF-1Ec in humans) and is increased after mechanical strain or muscle damage. Synthetic mechano-growth factor-24, the C terminal peptide of IGF-1Eb, stimulates proliferation of myoblasts. Furthermore, an adenovirus that produces IGF-1Eb induces muscle hypertrophy (60). IGF-1Eb/mechano-growth factor increases proliferation and delays senescence of satellite cells in young animals (71). Finally, mature IGF-1 is important for excitation contraction coupling through its effects on the regulation of dihydropyridine and ryanodine receptors (72,73).
Despite beneficial actions of these hormones on numerous tissues, GH decreases insulin sensitivity and elevates blood levels of glucose. The complexity of the action of GH/IGF-1 pathway is illustrated by a recent study demonstrating that, in GH/IGF-1–deficient Lewis dwarf rats, high-fat diet–induced obesity results in impaired glucose tolerance, which is associated with exacerbated vascular dysfunction (74). Although the importance of insulin sensitivity for the increase in life span is unknown, this remains an important correlate of extended life span (75). As expected, extremely high levels of GH decrease insulin sensitivity and reduce life span. The relationship among GH, insulin sensitivity, and life span are reviewed by Brown-Borg and Bartke in this series (4).
In addition to increasing cancer risk, GH and IGF-1 have been implicated in kidney disease. Approximately 80% of Lewis rats exhibit a profound nephropathy as they age, and this is a major cause of death in a high percentage of these animals. A 50% reduction in GH/IGF-1 beginning at 5 months of age totally prevents the disease (32). Interestingly, replacement of GH for as little as 10 weeks (followed by GH/IGF-1 deficiency for the remainder of the life span) increases the incidence of nephropathy (W.E. Sonntag, M. Mitschelen, and Y. Ikeno, personal communications). Thus, in animal strains susceptible to kidney pathology, levels of circulating GH and IGF-1 are highly correlated with both incidence and disease progression that undoubtedly is a factor in limiting both quality of life and life span. Interestingly, GH/IGF-1 deficiency also protects Lewis dwarf rats against high-fat diet–induced hepatosteatosis (74), providing further evidence that strain-specific diseases are an important aspect of life span regulation by GH/IGF-1.
Based on the literature, GH and IGF-1 have both beneficial and deleterious effects on specific pathologies that undoubtedly influence life span. Therefore, in many cases, the consequences of GH and IGF-1 deficiency are dependent on the species, background strain, and pathologies that the species or strain is susceptible. Those animals that are at risk for cancer, liver, or kidney disease will likely exhibit a shortened life span in response to elevated levels of GH and IGF-1, and we expect that those animals with reduced risk for these diseases will likely not exhibit increased life span in response to this intervention. Similarly, those species at risk for specific cardiovascular diseases (stroke, myocardial infarction, heart failure, vascular cognitive impairment) may benefit from elevated levels of these hormones. These effects are consistent with the classical actions of GH and IGF-1 being important anabolic agents that stimulate cell growth, proliferation, and tissue repair. Because cardiovascular diseases, metabolic diseases, and cancer are all important health issues in the elderly population, the effects of GH/IGF-1 pathway on human health span and life span are predictably complex.
GH/IGF-1 REPLACEMENT LATE IN LIFE AFFECTS HEALTH SPAN
Studies in the early 1980s indicated that GH and IGF-1 decrease with age in rodents, nonhuman primates, and humans (19,76–78). Importantly, decreases in these potent anabolic hormones correlate quite well with decreases in muscle mass, immune function, and many other aspects of aging, and several groups of investigators concluded that at least part of the aging phenotype resulted from a deficiency of circulating GH and IGF-1. Using classical endocrine approaches, investigators reported that GH or IGF-1 replacement increased cellular protein synthesis and immune function and decreased fat mass—end points that are modified with age and all classic targets for the actions of GH and IGF-1 (19). Importantly, GH and IGF-1 replacement were observed to have beneficial actions on cardiac and vascular function as well as on brain function (79,80). These studies used doses of GH and IGF-1 that recapitulated the levels of these hormones in younger animals (and were markedly lower than levels of the hormone found in GH transgenic animals). Certainly, it is well known that very high levels of these hormones produce acromegaly and increase cancer risk. Investigations of GH/IGF-1 replacement resulted in a wide range of manuscripts indicating that these hormones prevented or reversed specific aspects of the aging phenotype. However, there were few studies conducted with sufficient statistical power to address the potential effects of GH replacement on life span, and the majority of the studies were specifically designed to investigate variables associated with health span.
Based on the decreases in GH and IGF-1 with age in both rodents and humans and their association with clinical conditions associated with aging, the concept of a “somatopause” (decreases in GH and IGF-1 with age) that leads to functional changes normally associated with age became prevalent in the clinical literature. Early studies by Rudman et al. (81) supported such a role for GH and IGF-1 by demonstrating that GH replacement increased lean body mass and vertebral bone density and reduced fat mass in older individuals. Unfortunately, many of the clinical studies with GH replacement have revealed significant side effects including insulin insensitivity and increased incidence of carpal tunnel syndrome. Despite these limitations, there remains a close association between GH deficiency and cardiac dysfunction, atherosclerosis and vascular rarefaction, cognitive dysfunction, and reduced exercise capacity.
The vast majority of research scientists who study the preclinical and clinical actions of GH and IGF-1 do not advocate its long-term use in humans. Unfortunately, research from many of these laboratories has been exploited by those promising a “cure” for aging and age-related diseases based on highly selective views of the literature while minimizing the risks. The majority of the scientifically rigorous studies that investigate the effects of GH and IGF-1 replacement recognize the potential for both beneficial and deleterious pathological effects and use the intervention to assess the underlying cellular pathways that contribute to aging and age-related disease. Therefore, replacement of GH and IGF-1 are not appropriate as a clinical intervention to treat symptoms of aging in humans.
EVIDENCE THAT DEVELOPMENTAL EFFECTS OF GH/IGF-1 AFFECT CELLULAR STRESS RESISTANCE AND LATE-LIFE MORTALITY: A POSSIBLE EXPLANATION FOR THE CONTROVERSY OVER THE RELATIONSHIP AMONG IGF-1 LEVELS, HEALTH SPAN, AND LIFE SPAN
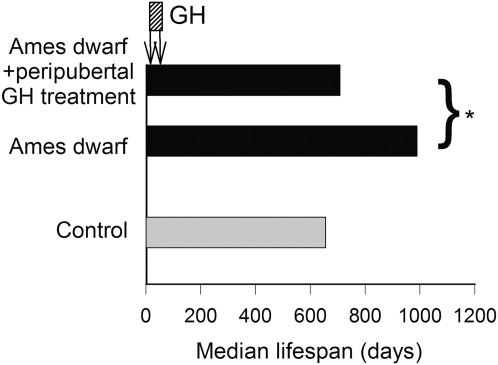
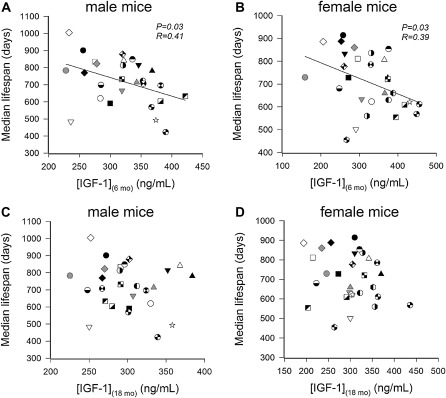
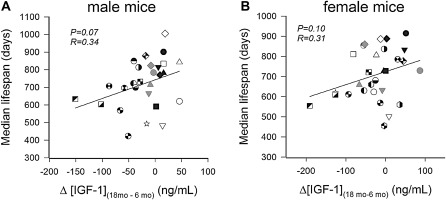
One common variable among all the mutant and transgenic animals that demonstrate increased life span is a reduction in circulating GH and/or IGF-1 levels early during the life span. Previous data indicate that GH and IGF-1 have important roles in the development in brain function as well as function of numerous tissues throughout the body. For example, pancreatic development is dependent on adequate levels of these hormones for the development of islets, and the absence of these hormones results in insulin insufficiency in adulthood. Therefore, the question arises whether the timing of hormonal deficiency during the life span as well as the extent of IGF-1 deficiency are relevant variables that contribute to life-span regulation. Studies from our own laboratory using the GH-deficient dwarf rat indicate that modest (40%–50%) reductions in IGF-1 levels in rodents throughout life have no effect on life span although the reduction does impair health span and reduces specific age-related pathologies (32,82). End-of-life pathological analysis indicated that the incidence of cancer and kidney disease was significantly reduced in early-onset GH/IGF-1–deficient animals. Interestingly, normalization of circulating GH/IGF-1 levels for only 10 weeks in dwarf animals (from 4 to 14 weeks of age) was able to increase life span by 15% compared with vehicle-treated dwarf and wild-type animals—an effect that was mediated by delaying a single pathology, intracerebral hemorrhage (32). This finding is consistent with the well-established actions of IGF-1 on vasculature that are reviewed by Ungvari and Csiszar in this series and further suggests that short periods of GH treatment during adolescence have effects that are manifest later during the life span. Recent landmark studies from Panici et al. (84) indicate that administration of GH from day 14 for 6 weeks to the long-lived Ames dwarf animal completely reversed the increased life span in these animals (Figure 2). Finally, studies from the Jackson Laboratory (84) comparing over 30 strains of mice indicated that IGF-1 levels at 6 months of age are inversely correlated with life span and that this correlation decreases as animals age. By 18 months of age, the correlation is essentially lost, suggesting that only levels of circulating IGF-1 earlier during the life span are predictive of life span (Figure 3). Given that circulating IGF-1 levels rise immediately prior to puberty (beginning around day 30 in rodent models), the levels at 6 months of age likely reflect these earlier time points. One of the potential conclusions from these studies is that the stage of life when GH and IGF-1 secretion are deficient is probably the most important variable in whether they affect life span. To date, the most robust effects on life span occur when GH and IGF-1 deficiency (or deficiency in their signaling pathways) is present when these hormones are regulating the development of specific organs and tissues. Based on the limited numbers of studies published to date, suppression of GH and IGF-1 or their signaling pathways after development has little effect on life span. Interesting in this regard is the observation that more rapid age-related declines in circulating IGF-1 levels in various inbred strains of mice tend to be associated with a shorter life span (Figure 4). The hypothesis that low IGF-1 levels in the postdevelopmental period delay aging would predict the opposite.
Figure 2.
In male Ames dwarf mice, short-term peripubertal treatment with bovine GH (6 μg/g bw/day injected subcutaneously) between 2 and 8 weeks of age prevents the expression of the longevity phenotype. The median life spans of Ames dwarf mice treated with GH, untreated wild-type controls, and untreated Ames dwarf mice are shown. Data are replotted from (83). *p = .0005.
Figure 3.
In genetically diverse inbred mouse strains, IGF-1 levels measured at 6 months of age are negatively correlated with median life span both in males (A) and females (B). Panels (C and D): No correlation exists between IGF-1 levels measured at 18 months of age and median life span of the mouse strains in either sex. These findings indicate that only levels of circulating IGF-1 early during the life span are predictive of life span. The data are derived from life-span studies conducted at the Jackson Aging Center. Data are replotted from previously published work of Yuan et al. (84,85).
Figure 4.
In genetically diverse inbred mouse strains, larger age-related declines in circulating IGF-1 levels (defined as the difference between IGF-1 levels measured at 18 months of age and 6 months of age) tend to be associated with shorter life span. The hypothesis that low IGF-1 levels in the postdevelopmental period delay aging would predict the opposite. The data are derived from life-span studies conducted in mice housed in a specific pathogen-free facility at the Jackson Aging Center. Data are replotted from previously published work of Yuan et al. (84,85).
In our opinion, it is plausible that the increase in life span observed in Ames and Snell dwarf animals as well as ghr knockout animals is the consequence of impairments in the developmental programming of key pathways that integrate energy metabolism and utilization and cellular stress resistance. This view is supported by the studies of Panici et al. (83) showing that injections of GH into Ames dwarf mice from day 14 for 6 weeks prevented the development of a cellular multi-stress resistance phenotype. Previous studies from the Miller laboratory (86–88) and our own recent results (89) showing that fibroblasts isolated from rodent models of GH/IGF-1 deficiency retain their unique stress resistance signatures in culture through many rounds of mitosis are consistent with the presence of epigenetic control mechanisms triggered in vivo by early GH and IGF-1 deficiency and maintained in extended culture. This concept is further supported by the finding that the stress resistance signatures fundamentally differ between cells isolated from newborn Snell dwarf mice and cells derived from the ∼3-month-old Snell dwarf mice in which postnatal changes in circulating GH and IGF-1 levels are manifest (83). Moreover, the ability of circulating IGF-1 deficiency in mice to exacerbate high-fat diet–induced inflammatory cytokine expression in the liver also depends on the onset (pre- or postpubertal) of the endocrine defect (90). It is tempting to speculate that low levels of GH/IGF-1 exposure during a short peripubertal period result in epigenetic modifications in the genome that ultimately affect health span and life span. Further studies are warranted to test this hypothesis. Importantly, the resulting long-term developmental changes in the cellular phenotype (eg, stress resistance, redox homeostasis, energy metabolism) may or may not be similar to the direct cellular effects of GH and IGF-1 in adults. The recent development of a novel mouse model of timed IGF-1 deficiency (adeno-associated viral knockdown of IGF-1 specifically in the liver of prepubertal vs postpubertal mice using Cre-lox technology; 64,91) will allow for the methodological dissection of the aforementioned developmental effects of IGF-1 in multiple target organs.
CONCLUSIONS
In the series of articles that follow in the present issue of Journal of Gerontology: Biological Sciences and Medical Sciences, research on the potential beneficial and deleterious actions of GH and IGF-1 and their potential contributions to health span and life span are reviewed. On the basis of this overview of the literature, numerous potential explanations can be offered for the apparent lack of consistency between the rodent models and humans with regard to the role of GH/IGF-1 signaling in regulation of life span. The picture that emerges is of a complex endocrine/paracrine regulatory network that has effects on tissue and organ development as well as tissue function and energy homeostasis throughout life.
The general consensus is that in the postdevelopmental stage of life, GH and IGF-1 have numerous beneficial/protective actions in skeletal muscle and the cardiovascular and nervous systems but nevertheless increase insulin insensitivity and cancer risk. Based on these studies, it would be expected that these hormones have differential effects on health-span and life-span based, in part, on the age-specific tissue dysfunction and pathologies evident for each species and strain. Future studies should test these hypotheses. Another important area for future investigations is the tissue-specific role of paracine IGF-1 and IGF-1 splice variants during aging. Based on the C elegans model, it will be essential to understand the role of paracrine IGF-1 signaling in the central nervous system in regulation of the aging process in mammals.
Although the actions of GH and IGF-1 through the aforementioned pathways may be responsible for a 15%–20% variation in life span in certain laboratory rodent strains, we find it unlikely that postdevelopmental decreases in circulating GH/IGF-1 per se account for the marked increase in life span found in the Ames dwarf, Snell dwarf, and ghr knockout mice. In contrast, exciting new data strongly suggest that circulating levels of GH and IGF-1 during development exert a key role in regulation of life span in rodent models. At the same time, one may argue that the rise in GH and IGF-1 during adolescence is critical for growth and maturation of numerous tissues resulting in an organism that is leaner, larger, and more competitive and reproductively competent in challenging environments. Recent data indicate that the increase in these hormones during adolescence has effects on brain and vascular function that are manifest throughout life and may even contribute to disease prevention at the end of life. Thus, tissue-specific effects of alterations in IGF-1 signaling during development should be addressed in future studies. These studies will have the potential to provide critical insight into the mechanisms by which alterations in IGF-1 signaling during development affect the pathophysiology of age-related diseases affecting mortality later in life.
Taken together, the perceived contradictory roles of GH and IGF-1 in the genesis of the aging phenotype should not be interpreted as a controversy on whether GH or IGF-1 increases or decreases life span but rather as an opportunity to explore the complex roles of these hormones during specific stages of the life span. Assessment of the mechanisms of these hormones during each stage of the life span and elucidation of the mechanisms by which GH and IGF-1 modulate pathways involved in life span regulation initiated during development will be essential in advancing research into the mechanisms of aging.
FUNDING
This work was supported by grants from the National Institutes of Health (NS056218, AG11370, AG27147, AG031085, AT006526), the American Federation for Aging Research, the Oklahoma Center for the Advancement of Science and Technology, the American Heart Association, the Ellison Medical Foundation, and Donald W. Reynolds Foundation.
Acknowledgments
The authors would like to express their gratitude for the support of the Donald W. Reynolds Foundation, which funds aging research at the University of Oklahoma Health Sciences Center under its Aging and Quality of Life Program. The editorial assistance of MaryAnn Sonntag in preparation of the manuscript is greatly appreciated.
References
- 1.Novosyadlyy R, LeRoith D. Insulin-like growth factors and insulin: at the crossroad between tumor development and longevity. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls065. In press. [DOI] [PubMed] [Google Scholar]
- 2.Higashi Y, Sukhanov S, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls102. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls072. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown-Borg HM, Bartke A. GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls086. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deak F, Sonntag WE. Aging, synaptic dysfunction and insulin-like growth factor-1. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls118. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang RAC. Elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 7.Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- 8.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. Daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 9.Ogg S, Paradis S, Gottlieb S, et al. The fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 10.Lin K, Dorman JB, Rodan A, Kenyon C. Daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 11.Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulin like signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- 12.Kimura KD, Riddle DL, Ruvkun G. The C. elegans DAF-2 insulin-like receptor is abundantly expressed in the nervous system and regulated by nutritional status. Cold Spring Harb Symp Quant Biol. 2011 doi: 10.1101/sqb.2011.76.010660. [DOI] [PubMed] [Google Scholar]
- 13.Broughton SJ, Piper MD, Ikeya T, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci U S A. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broughton S, Partridge L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem J. 2009;418:1–12. doi: 10.1042/BJ20082102. [DOI] [PubMed] [Google Scholar]
- 15.Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- 16.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 17.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 18.Sonntag WE, Ramsey MM. Of worms, flies, dwarfs, and things that go bump in the night. Sci Aging Knowledge Environ. 2002;2002:pe17. doi: 10.1126/sageke.2002.43.pe17. [DOI] [PubMed] [Google Scholar]
- 19.Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and IGF-1 in aging and lifespan. Trends Genet. 2002;18:295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]
- 20.Bartke A, Chandrashekar V, Bailey B, Zaczek D, Turyn D. Consequences of growth hormone (GH) overexpression and GH resistance. Neuropeptides. 2002;36:201–208. doi: 10.1054/npep.2002.0889. [DOI] [PubMed] [Google Scholar]
- 21.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 22.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 24.Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 25.Bokov AF, Garg N, Ikeno Y, et al. Does reduced IGF-1R signaling in Igf1r+/- mice alter aging? PLoS One. 2011;6:e26891. doi: 10.1371/journal.pone.0026891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conover CA, Bale LK. Loss of pregnancy-associated plasma protein A extends lifespan in mice. Aging Cell. 2007;6:727–729. doi: 10.1111/j.1474-9726.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- 27.Conover CA, Bale LK, Grell JA, Mader JR, Mason MA. Longevity is not influenced by prenatal programming of body size. Aging Cell. 2010;9:647–649. doi: 10.1111/j.1474-9726.2010.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selman C, Lingard S, Choudhury AI, et al. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- 29.Selman C, Partridge L, Withers DJ. Replication of extended lifespan phenotype in mice with deletion of insulin receptor substrate 1. PLoS One. 2011;6:e16144. doi: 10.1371/journal.pone.0016144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- 31.Shimokawa I, Higami Y, Utsuyama M, et al. Life span extension by reduction in growth hormone-insulin-like growth factor-1 axis in a transgenic rat model. Am J Pathol. 2002;160:2259–2265. doi: 10.1016/S0002-9440(10)61173-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonntag WE, Carter CS, Ikeno Y, et al. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- 33.Ladiges W, Van Remmen H, Strong R, et al. Lifespan extension in genetically modified mice. Aging Cell. 2009;8:346–352. doi: 10.1111/j.1474-9726.2009.00491.x. [DOI] [PubMed] [Google Scholar]
- 34.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laron Z. Do deficiencies in growth hormone and insulin-like growth factor-1 (IGF-1) shorten or prolong longevity? Mech Ageing Dev. 2005;126:305–307. doi: 10.1016/j.mad.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 36.Burgers AM, Biermasz NR, Schoones JW, et al. Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J Clin Endocrinol Metab. 2011;96:2912–2920. doi: 10.1210/jc.2011-1377. [DOI] [PubMed] [Google Scholar]
- 37.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 38.Bonafe M, Barbieri M, Marchegiani F, et al. Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. J Clin Endocrinol Metab. 2003;88:3299–3304. doi: 10.1210/jc.2002-021810. [DOI] [PubMed] [Google Scholar]
- 39.Suh Y, Atzmon G, Cho MO, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M, Zhang X, Zhao H, Wang Q, Pan Y. FoxO gene family evolution in vertebrates. BMC Evol Biol. 2009;9:222. doi: 10.1186/1471-2148-9-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burgering BM, Kops GJ. Cell cycle and death control: long live forkheads. Trends Biochem Sci. 2002;27:352–360. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 42.Stahl M, Dijkers PF, Kops GJ, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 43.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 44.Willcox BJ, Donlon TA, He Q, et al. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anselmi CV, Malovini A, Roncarati R, et al. Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res. 2009;12:95–104. doi: 10.1089/rej.2008.0827. [DOI] [PubMed] [Google Scholar]
- 46.Flachsbart F, Caliebe A, Kleindorp R, et al. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Wang WJ, Cao H, et al. Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum Mol Genet. 2009;18:4897–4904. doi: 10.1093/hmg/ddp459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soerensen M, Dato S, Christensen K, et al. Replication of an association of variation in the FOXO3A gene with human longevity using both case-control and longitudinal data. Aging Cell. 2010;9:1010–1017. doi: 10.1111/j.1474-9726.2010.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pawlikowska L, Hu D, Huntsman S, et al. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009;8:460–472. doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kleindorp R, Flachsbart F, Puca AA, Malovini A, Schreiber S, Nebel A. Candidate gene study of FOXO1, FOXO4, and FOXO6 reveals no association with human longevity in Germans. Aging Cell. 2011;10:622–628. doi: 10.1111/j.1474-9726.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- 51.Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 52.Back K, Brannmark C, Stralfors P, Arnqvist HJ. Differential effects of IGF-I, IGF-II and insulin in human preadipocytes and adipocytes—role of insulin and IGF-I receptors. Mol Cell Endocrinol. 2011;339:130–135. doi: 10.1016/j.mce.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Seely BL, Reichart DR, Takata Y, Yip C, Olefsky JM. A functional assessment of insulin/insulin-like growth factor-I hybrid receptors. Endocrinology. 1995;136:1635–1641. doi: 10.1210/endo.136.4.7895674. [DOI] [PubMed] [Google Scholar]
- 54.Tartare-Deckert S, Sawka-Verhelle D, Murdaca J, Van Obberghen E. Evidence for a differential interaction of SHC and the insulin receptor substrate-1 (IRS-1) with the insulin-like growth factor-I (IGF-I) receptor in the yeast two-hybrid system. J Biol Chem. 1995;270:23456–23460. doi: 10.1074/jbc.270.40.23456. [DOI] [PubMed] [Google Scholar]
- 55.Johansson GS, Arnqvist HJ. Insulin and IGF-I action on insulin receptors, IGF-I receptors, and hybrid insulin/IGF-I receptors in vascular smooth muscle cells. Am J Physiol Endocrinol Metab. 2006;291:E1124–E1130. doi: 10.1152/ajpendo.00565.2005. [DOI] [PubMed] [Google Scholar]
- 56.Ramsey MM, Ingram RL, Cashion AB, et al. Growth hormone-deficient dwarf animals are resistant to dimethylbenzanthracine (DMBA)-induced mammary carcinogenesis. Endocrinology. 2002;143:4139–4142. doi: 10.1210/en.2002-220717. [DOI] [PubMed] [Google Scholar]
- 57.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 58.Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- 59.Barton ER. The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab. 2006;31:791–797. doi: 10.1139/h06-054. [DOI] [PubMed] [Google Scholar]
- 60.Barton ER. Viral expression of insulin-like growth factor-I isoforms promotes different responses in skeletal muscle. J Appl Physiol. 2006;100:1778–1784. doi: 10.1152/japplphysiol.01405.2005. [DOI] [PubMed] [Google Scholar]
- 61.Barton ER, DeMeo J, Lei H. The insulin-like growth factor (IGF)-I E-peptides are required for isoform-specific gene expression and muscle hypertrophy after local IGF-I production. J Appl Physiol. 2010;108:1069–1076. doi: 10.1152/japplphysiol.01308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matheny RW, Jr, Nindl BC, Adamo ML. Minireview: mechano-growth factor: a putative product of IGF-I gene expression involved in tissue repair and regeneration. Endocrinology. 2010;151:865–875. doi: 10.1210/en.2009-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yakar S, Liu JL, Stannard B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitschelen M, Yan H, Farley JA, et al. Long-term deficiency of circulating and hippocampal insulin-like growth factor I induces depressive behavior in adult mice: a potential model of geriatric depression. Neuroscience. 2011;185:50–60. doi: 10.1016/j.neuroscience.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26:929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 66.Kawai M, Rosen CJ. The IGF-I regulatory system and its impact on skeletal and energy homeostasis. J Cell Biochem. 2010;111:14–19. doi: 10.1002/jcb.22678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baxter RC, Twigg SM. Actions of IGF binding proteins and related proteins in adipose tissue. Trends Endocrinol Metab. 2009;20:499–505. doi: 10.1016/j.tem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 68.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 69.Bunn RC, Fowlkes JL. Insulin-like growth factor binding protein proteolysis. Trends Endocrinol Metab. 2003;14:176–181. doi: 10.1016/s1043-2760(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 70.Conover CA, Bale LK, Mader JR, Mason MA, Keenan KP, Marler RJ. Longevity and age-related pathology of mice deficient in pregnancy-associated plasma protein-A. J Gerontol A Biol Sci Med Sci. 2010;65:590–599. doi: 10.1093/gerona/glq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kandalla PK, Goldspink G, Butler-Browne G, Mouly V. Mechano growth factor E peptide (MGF-E), derived from an isoform of IGF-1, activates human muscle progenitor cells and induces an increase in their fusion potential at different ages. Mech Ageing Dev. 2011;132:154–162. doi: 10.1016/j.mad.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 72.Delbono O. Regulation of excitation contraction coupling by insulin-like growth factor-1 in aging skeletal muscle. J Nutr Health Aging. 2000;4:162–164. [PubMed] [Google Scholar]
- 73.Gonzalez E, Messi ML, Zheng Z, Delbono O. Insulin-like growth factor-1 prevents age-related decrease in specific force and intracellular Ca2+ in single intact muscle fibres from transgenic mice. J Physiol. 2003;552:833–844. doi: 10.1113/jphysiol.2003.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bailey-Downs LC, Sosnowska D, Toth P, et al. Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci. 2009;64:516–521. doi: 10.1093/gerona/glp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sonntag WE, Steger RW, Forman LJ, Meites J. Decreased pulsatile release of growth hormone in old male rats. Endocrinology. 1980;107:1875–1879. doi: 10.1210/endo-107-6-1875. [DOI] [PubMed] [Google Scholar]
- 77.Carter CS, Ramsey MM, Ingram RL, et al. Models of growth hormone and IGF-1 deficiency: applications to studies of aging processes and life-span determination. J Gerontol A Biol Sci Med Sci. 2002;57:B177–B188. doi: 10.1093/gerona/57.5.b177. [DOI] [PubMed] [Google Scholar]
- 78.Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev. 1993;14:20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- 79.Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197(Pt 4):575–585. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramsey MM, Weiner JL, Moore TP, Carter CS, Sonntag WE. Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience. 2004;129:119–127. doi: 10.1016/j.neuroscience.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 81.Rudman D, Feller AG, Nagraj HS, et al. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323:1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- 82.Nieves-Martinez E, Sonntag WE, Wilson A, et al. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. J Endocrinol. 2010;204:31–36. doi: 10.1677/JOE-09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24:1–7. doi: 10.1096/fj.10-163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yuan R, Tsaih SW, Petkova SB, et al. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8:277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yuan R, Rosen CJ, Beamer WG. Aging study: IGF-1 and body weight for 33 inbred strains of mice. MPD: Yuan1. Mouse Phenome Database Web site. The Jackson Laboratory, Bar Harbor, ME. http://phenome.jax.org. Accessed: Feb 05, 2012. [Google Scholar]
- 86.Harper JM, Salmon AB, Leiser SF, Galecki AT, Miller RA. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell. 2007;6:1–13. doi: 10.1111/j.1474-9726.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murakami S, Salmon A, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17:1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- 88.Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289:E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- 89.Ungvari Z, Sosnowska D, Podlutsky A, Koncz P, Sonntag WE, Csiszar A. Free radical production, antioxidant capacity, and oxidative stress response signatures in fibroblasts from Lewis dwarf rats: effects of life span-extending peripubertal GH treatment. J Gerontol A Biol Sci Med Sci. 2011;66:501–510. doi: 10.1093/gerona/glr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu Y, Brodt P, Sun H, et al. Insulin-like growth factor-I regulates the liver microenvironment in obese mice and promotes liver metastasis. Cancer Res. 2010;70:57–67. doi: 10.1158/0008-5472.CAN-09-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bailey-Downs LC, Mitschelen M, Sosnowska D, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr164. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]