Abstract
Insulin-like growth factor (IGF)-1 is an important neurotrophic hormone. Deficiency of this hormone has been reported to influence the genesis of cognitive impairment and dementia in the elderly patients. Nevertheless, there are studies indicating that cognitive function can be maintained into old age even in the absence of circulating IGF-1 and studies that link IGF-1 to an acceleration of neurological diseases. Although IGF-1 has a complex role in brain function, synaptic effects appear to be central to the IGF-1–induced improvement in learning and memory. In this review, synaptic mechanisms of learning and memory and the effects of IGF-1 on synaptic communication are discussed. The emerging data indicate that synaptic function decreases with age and that IGF-1 contributes to information processing in the brain. Further studies that detail the specific actions of this important neurotrophic hormone will likely lead to therapies that result in improved cognitive function for the elderly patients.
Keywords: IGF-1, Brain aging, Synaptic function, Memory, Learning
HUMANS as a species have achieved astonishing success in prolonging life span. Recent estimates suggest that the number of people older than 90 years in the United States will double to around 2 million in this decade (1). However, the emerging problem of the 21st century is the increasing number of individuals with mild cognitive impairment or dementia within the aging population. It is estimated that one of every two individuals older than 85 years of age will suffer from some type of dementia. By 2050, the number of elderly patients with dementia may increase from 5.2 to 16 million in the United States (data from U.S. Census Bureau and 2011 Facts and Figures of Alzheimer’s Association). Worldwide predicted numbers are similarly staggering.
The purpose of this review is to assess the complex role of growth hormone and insulin-like growth factor (IGF)-1 in the genesis of cognitive impairment and dementia. The overarching conclusion from numerous studies is that IGF-1 deficiency is an important contributing factor in cognitive impairment both in adults and aged humans as well as rodent models of aging. Nevertheless, there are important studies in rodent models (eg, Ames, Snell, and ghr−/− mice) indicating that cognitive function (and neurogenesis) can be maintained into old age in the absence of circulating IGF-1. Based on the limited number of studies available in these models, it is not possible to easily reconcile differences between studies indicating that growth hormone and IGF-1 deficiency prevent central nervous system (CNS) dysfunction with age and those suggesting that growth hormone and IGF-1 deficiency are part of the etiology of the decline in learning and memory with age. In this review, we present data on the known actions of IGF-1 on brain structure and function and propose several areas where further research studies are warranted to resolve differences in the field. In several earlier reviews, we have presented data related to the effects of IGF-1 on age-related deficits in cerebrovascular density and function, neurogenesis and neuronal survival, as well as neuronal number and oxidative stress in brain regions important for learning and memory. For more information on these topics, please refer to other reviews in this series (2–5), an earlier review from our laboratory (6), as well as excellent reviews by Torres-Aleman and colleagues (7–9). In this chapter, we focus primarily on the role of IGF-1 and age-related IGF-1 deficiency on synaptic function because these changes appear to be essential for our understanding of the age-related decline in learning and memory induced by IGF-1 deficiency and have the greatest potential for therapeutic intervention.
SYNAPTIC DYSFUNCTION AND AGING
Overview
Aging has long been associated with impairments in the nervous system. Vision and hearing are impaired, there is a dampened sense of smell, changes in appetite, lapses of short-term memory, forgetfulness, and difficulty in finding adequate expressions and words. Symptoms may also extend to concretization of idioms and delays in critical thinking and/or executive functions (10–13). Although the morphological basis for these effects is not well understood, studies on brains from a variety of mammalian species including humans conclude that a reduced number of synaptic connections among neurons is the most consistent correlate with aging (14–19) and specifically cognitive decline (20–22). Most importantly, it is clear that even though many morphological and biochemical changes are associated with age, disorders that affect cognitive function of the elderly patients (including Alzheimer’s disease [AD]) are markedly different than the normal course of aging (1,23) and do not represent an “accelerated aging” phenotype. For example, normal aging does not appear to be accompanied by significant neuron death or the profound loss of synapses typically observed in end stage AD (24,25). Nonetheless, it is important to emphasize that in both humans and animal models, deficits of spatial learning and memory are manifest in a significant portion of the aged population. The emerging view is that standard age-related alterations in neuronal cytostructure and neurochemical composition are, by themselves, not sufficient to cause mild cognitive impairment or dementia. This finding was emphasized in recent studies from the Freeman laboratory when they reported that a set of neurotransmission-regulating proteins decrease in expression with age but are not specifically associated with cognitive impairment (18,22). Importantly, age-related cognitive impairment is associated with a specific set of synaptic proteins with roles in functional and structural plasticity (26). Thus, there appear to be independent cellular and molecular events that lead to cognitive impairment that are distinct from the changes that are part of healthy aging.
Inhibitory/Excitatory Synaptic Balance in Aging
An evolving area of research in brain aging is focused on the balance between excitatory and inhibitory synaptic systems (27,28). Although there is overwhelming evidence related to the reduction of synaptic markers with age (18,27), it is not clear whether both inhibitory and excitatory synapses contribute equally to these impairments (14). The importance of this debate is highlighted by the view that in many neuropsychiatric conditions (eg, autism, schizophrenia, epilepsy), an imbalance of excitation and inhibition is the major underlying pathological mechanism (29,30). If inhibition is better preserved than excitation during aging, long-term synaptic plasticity could be hindered (8). Indeed, in models of learning and memory, deficits in long-term potentiation (LTP), an electrophysiological correlate of memory trace formation, are corrected after pharmacologically reducing synaptic inhibition. Importantly, in liver-specific IGF-1–deficient mice, perforant path LTP deficits appear to be the result of a selective loss of excitatory inputs and the relative abundance of inhibitory neurons (31). The relatively stronger excitatory drive could be a factor in the rising incidence of epilepsy after the age of 65 years (32). One possible explanation for these seemingly contradictory scenarios is that individual brain regions may react differently with age. Therefore, at the local level, the excitatory to inhibitory balance could be different than in a neighboring region.
A PRIMER ON THE CELLULAR AND MOLECULAR BASIS OF MEMORY
Neurotransmission at Chemical Synapses
According to the current theory of learning and memory, new information is stored by modifications of the strength of synaptic connections between neurons of cortical networks. There are three major components of the chemical synapse: the presynaptic site, the synaptic cleft, and the postsynaptic site. Classically, the presynaptic site is activated by a depolarizing action potential, which opens voltage-gated calcium channels. The result is calcium ion influx into the terminal or synaptic bouton. Elevated calcium ion concentration triggers the exocytosis machinery. This machinery consists of calcium sensor(s) (synaptotagmins), SNARE proteins (synaptobrevin/VAMP, syntaxin1, and SNAP-25), and other regulatory binding partners (rab3, rabphilin, munc13, and munc18) that are essential for the proper spatial and temporal execution of synaptic vesicle fusion at the active zone (33–41). Lipid membranes of the vesicle and the plasma membrane are forced into close proximity, and in turn, this creates the fusion pore through which the neurotransmitter molecules are released into the cleft. The synaptic cleft, an extremely narrow almost “virtual” gap, has a highly organized structure made of various extracellular parts of membrane receptors as well as matrix proteins. Diffusion of small neurotransmitters (eg, glutamate and gamma-aminobutyric acid [GABA]) through this gap requires only microseconds. At the postsynaptic site, neurotransmitters are captured selectively by their receptors. Two major classes of these receptors exist as ionotropic (functioning as ion channels) and metabotropic (communicating with intracellular G protein signals). For glutamate, the ionotropic receptors are divided into three groups: AMPA (2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl)propanoic acid), NMDA (N-methyl D-aspartic acid), and kainate receptors. AMPA receptors are composed of four types of subunits, designated as GluR1, 2, 3, and 4. Various combinations of those subunits assemble into tetramers that form the functional glutamate receptor and conduct cations if activated and open. Importantly, if the GluR2 subunit is present, it renders the channel impermeable to calcium ions. NMDA receptors are also composed of four subunits forming a tetramer. The constant subunit of the NMDA receptor is NR1, which is accompanied by NR2 subunits (NR2A, NR2B, NR2C, or NR2D). For the inhibitory GABA transmitter, the ionotropic receptor is referred to as GABA-A-type, whereas the metabotropic is GABA-B-type.
LTP as the Cellular Process in Memory Trace Formation
A permanent increase in synaptic strength after a stimulus or learning task is termed LTP (42). LTP was discovered in hippocampal excitatory synaptic connections between perforant path synapses and dentate gyrus granule cells (43) but is a ubiquitous phenomenon in a wide variety of brain regions and at many different synaptic connections including inhibitory synapses (42,44). The opposite effect leading to a permanent decrease in synaptic strength is termed long-term depression (LTD). Established new memory traces of spatial location could partially saturate synaptic LTP when later tested in a region of hippocampus representing the map of the field to navigate (45). Moreover, spatial memory can be extinguished by blocking the maintenance of hippocampal LTP (46) or by inhibition of the atypical protein kinase (PK) C isoform PKMzeta in the CA1 field. These results provide direct and convincing support for the role of LTP of synaptic responses as the neuronal mechanism of memory.
The Classical Trisynaptic Hippocampal Pathway
A highly specialized and phylogenetically ancient part of the temporal lobe is Ammon’s horn, which contains three major structures that are interconnected: the entorhinal cortex with the subiculum, the dentate gyrus, and the hippocampus proper. The latter is subdivided into three fields, for example, CA1, CA2, and CA3. Neuronal connections from the entorhinal cortex reach both the CA1 region and dentate gyrus via the perforant pathway (Figure 1A). The principal cells of the dentate gyrus are excitatory granule cells that stimulate the pyramidal neurons of the CA3 field through mossy fibers (mf). CA3 pyramidal neurons are connected to each other, to the CA3 field of the opposite site and through the Schaffer collaterals of their axons to the pyramidal cells in the CA1 field. This synaptic connection between the CA3 and CA1 fields of the hippocampus is probably the most studied for synaptic plasticity (48,49).
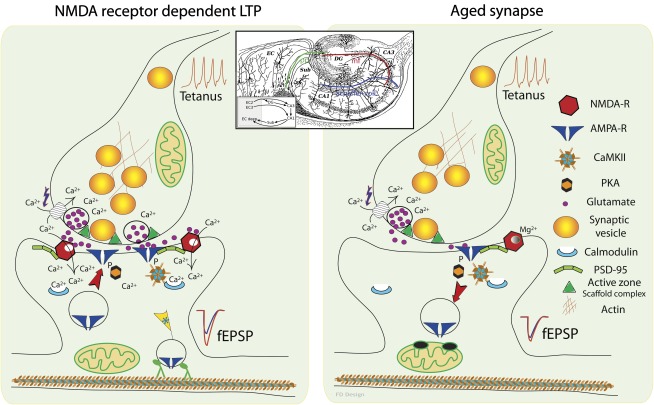
Figure 1.
Basic mechanism of NMDA receptor–dependent postsynaptic long-term potentiation (LTP). The left panel depicts the molecular events in the synapse during a high-frequency stimulation train of action potentials (tetanus) that induces LTP of excitatory postsynaptic potentials (EPSPs), as typically seen in the Schaffer collateral synapses onto pyramidal neurons CA1 field of hippocampus. Note the influx of calcium ions through NMDA receptors and the subsequent change of phosphorylation state of AMPA receptors. Postsynaptic potentials are further increased by insertion of new AMPA receptors into the postsynaptic density. Recent results suggest that there is a special “tagging system” of the activated synapses that act as a flag for cargo vesicles delivering new proteins, including AMPA receptors. Taken together, these changes induce a significant increase in synaptic responses for incoming action potentials (shown here as field EPSPs before in blue and after in red) even hours after tetanic stimulation. Insert illustrates the classical view of the trisynaptic pathway in the hippocampus based on Cajal's drawing of the hippocampal circuitry (47). Signals from specific layers of the entorhinal cortex travel to the dentate gyrus through the perforant paths (pp in green) and then to CA3 through the mossy fibers (mf in red) and to CA1 via the Schaffer collaterals (in blue) before returning to other layers of the entorhinal cortex. Right panel depicts the events in a synapse impaired by aging. Neurotransmitter release resulting from tetanus is hindered by decreased presynaptic vesicle number and slower mobilization. Lower number of NMDA and AMPA receptors and their modified subunit composition further decrease the probability of LTP induction. Note also that under such conditions, internalization of AMPA receptors is accelerated, which could be one of the mechanisms leading to long-term depression of synaptic communication with age. The drawings of Santiago Ramón y Cajal are reproduced with permission of Legado Cajal, Instituto Cajal (CSIC), Madrid, Spain.
LTP in the Hippocampal CA1 Field—Role of Postsynaptic AMPA and NMDA Glutamate Receptors
Possible modifications of the postsynaptic site that contribute to LTP of synaptic transmission can be summarized overall as increased total current through neurotransmitter receptors (Figure 1). Multiple factors could result in such an elevated excitatory postsynaptic potential (EPSP), more receptors, better affinity to the transmitter, increased opening probability and/or mean open (dwell) time, decreased inactivation, and higher unitary conductance. In synapses between Schaffer collaterals and CA1 pyramidal cells, both exchange of glutamate receptor components, modifications of receptors through phosphorylation, and addition of more receptors to the area of the postsynaptic density contribute to LTP (50). These changes are triggered by calcium influx through the postsynaptic NMDA receptors during tetanic stimulation (51). Similarly, effective is a single incoming stimulus if it reaches the postsynaptic cell within a critical time window of 10–20 ms when the dendrites are strongly depolarized, and thus, the Mg2+ blockade of NMDA receptors is removed (52,53). Therefore, NMDA receptors function as coincidence detectors sensing both the state of the postsynaptic neuron and the specific synaptic input through local glutamate concentration. This mechanism is referred to as the Hebbian model of synaptic plasticity since it was first proposed by Donad O. Hebb in 1949 (54,55). Although the most important kinase for LTP induction through NMDA receptors is the calcium-calmodulin–dependent protein kinase, CaMKII, other signaling pathways exert modulatory effects, for example, GluR1 phosphorylation by PKA, requires the protein, AKAP150, to anchor PKA at the postsynaptic density (56).
Presynaptic LTP—NMDA Receptor–Independent LTP of Mossy Fibers in the Hippocampal CA3 Field
mf-LTP depends on a rise in presynaptic calcium (48) induced by strong repetitive field stimulation (also known as tetanus). Once calcium has entered the presynaptic terminal, it binds to a calcium–calmodulin complex, which in turn activates adenylyl cyclase I, the type 8 form of which is highly enriched in mf synapses (57). Thus, adenylyl cylcase I transiently elevates 3’-5’-cyclic adenosine monophosphate (cAMP) levels (58,59), which then causes an activation of protein kinase A (57).
There are multiple specific targets of PKA during LTP induction. RIM1alpha was proposed as the most likely synaptic candidate because its phosphorylation at Ser 143 allows the binding of the synaptic isoform of 14-3-3 (Figure 2). Both RIM1 and 14-3-3 are large scaffold proteins with multiple protein-interacting domains, but recently, it was shown that they selectively bind to each other only at phosphoserine143 of RIM1alpha. Surprisingly, RIM1alpha KI mice with mutation of S143A (which is unable to be phosphorylated) had no defect in mf-LTP (60). The controversy on RIM1 could be resolved by the observation that recruitment of 14-3-3 to the active zone was observed with tetanus causing LTP in this KI model. Together, these findings raise the possibility that a decline of 14-3-3 expression levels in aged rats may contribute to cognitive decline—a finding supported by recent studies from the Freeman laboratory (22).
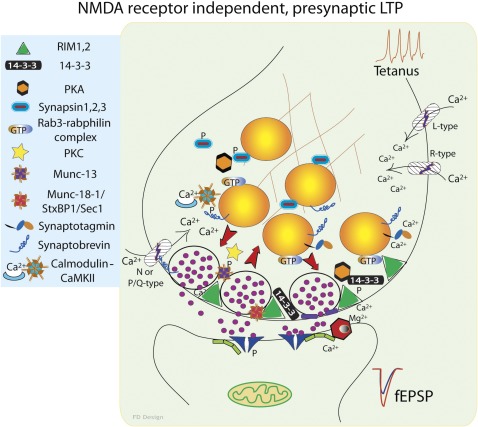
Figure 2.
Presynaptic form of long-term potentiation (LTP). The best-studied example of the presynaptic NMDA receptor–independent LTP is the mossy fiber synapse on CA3 hippocampal pyramidal neurons. In this case, NMDA receptor activation is not required for LTP induction. The most important changes in this form of synaptic plasticity happen in the presynaptic bouton leading to increased neurotransmitter release after tetanic stimulation. The molecular mechanism involves activation of multiple kinases including protein kinase (PK) A and PKC. Phosphorylation of synapsin releases synaptic vesicles from the actin cytoskeleton. Mobilized vesicles bind to the active zone and undergo priming to become ready for release. This process can be facilitated by RIM1, munc13, and munc18 protein phosphorylation. Activated RIM protein attracts the scaffold protein of 14-3-3 and thus could create new landing regions for vesicles. Synaptic vesicle delivery is accelerated by the activated GTP bound form of rab3 small G protein, probably by shedding its binding partner, rabphilin. Primed vesicles undergo fusion when elevated intracellular calcium levels trigger SNARE complex (synaptobrevin2/VAMP2, syntaxin1, and SNAP-25) activation via binding to the calcium sensor synaptotagmin on the primed vesicle. Increased neurotransmitter release is detected as larger excitatory postsynaptic potential amplitude. For clarity, the endocytosis part of synaptic vesicle cycle was omitted.
Importantly, another binding partner of RIM1 is rab3, a small GTPase protein associated with synaptic vesicles and a good candidate for regulation of presynaptic plasticity. Indeed, mice lacking rab3a have been shown to have a severe deficit of LTP in CA3 (61). Moreover, expression of rab3a declines with age, and the rate of rab3 decline is accelerated in brain samples from human AD patients (62), similar to syntaxin1a and synaptogyrin3 expression. The molecular mechanism of LTP is further refined after inclusion of other targets of PKA phosphorylation. Most notably, rabphilin is both a binding partner of rab3 and phosphorylated by PKA on Ser 234 (63–65). Rabphilin regulates recovery of synaptic transmission from short-term depression by interacting with SNAP-25 (66). Another noteworthy target of PKA phosphorylation is the hyperpolarization-activated cation channel Ih, which has a significant role in maintaining mf potentiation after tetanic or chemical (forskolin) stimulation (67) through the depolarization of granule cells.
Finally, synapsins are also presynaptic regulators of transmitter release. In mammalian neurons, three types of synapsins are expressed: synapsin I, II, and III plus their alternative spliced variants (68–70). Synapsins are phosphorylated by PKA (Figure 2) that is thought to disrupt their role as a bridge between the actin cytoskeleton and vesicles (71) and thus increase the mobility of synaptic vesicles. Studies on mice lacking one or more synapsin isoform(s) revealed specific effects not only on vesicle mobilization and recycling (72) but also on synaptic plasticity (73). For example, triple null mutant mice for all known synapsins had a remarkably different synaptic release phenotype in excitatory and inhibitory hippocampal neurons (74). Evoked responses from inhibitory neurons were decreased, whereas those from excitatory neurons were maintained (at least at the beginning of a 10 Hz stimulus train). After approximately 50 pulses, a significant acceleration of excitatory post-synaptic current depression was observed. Overall, the amount of released transmitter was less than half of wildtype control for both types of synapses. This is an interesting example of the difference in molecular regulation of excitatory and inhibitory transmission and highlights the need for detailed functional experiments on the synaptic changes that occur with age. Age-dependent decreases in levels of synapsin I are accompanied by increased expression of synapsin II in rats (18). Nevertheless, the role of synapsins in hippocampal long-term synaptic plasticity remains unclear because synapsin I and II double knockout mice have normal LTP in both the Schaffer collateral and mf synapses (75). It is also noteworthy that at least one alternative pathway circumvents the PKA regulation of presynaptic release; cAMP-GEFII, a cAMP sensing nucleotide exchange factor, is reported to directly bind to RIM2 (76) as well as activate the ras pathway through Rap1A in the presence of elevated cAMP (77). Moreover, the possible contribution of the postsynaptic site to mf-LTP has been proposed by multiple groups, but it is still debated in part because there is no well-identified retrograde messenger, although a direct transsynaptic protein bridge through ephrinB and its receptor was described (78).
Protein Kinases Regulate the Kinetics of Neurotransmitter Release
The calcium-calmodulin–dependent protein kinase, CaMKII, is essential for postsynaptic changes during hippocampal CA1 LTP. Also, CaMKII phosphorylates many presynaptic proteins, including members of the SNARE complex. Vesicular SNARE, synaptobrevin (also known as vesicle-associated membrane protein), VAMP-1 and -2 are phosphorylated at Ser61 by CaMKII (79). Synaptobrevin2 is a key protein in action potential–evoked exocytosis of synaptic vesicles (35,38), and it couples neurotransmitter release to rapid endocytosis of vesicles after fusion (37). Although age-dependent decreases in levels of SNARE proteins (synaptobrevin2 and SNAP-25) are not associated with cognitive dysfunction, lower expression of CaMKIIalpha is only detected in animals that have impaired performance in the water maze (22), suggesting that CaMKIIalpha has an important role in cognitive decline in rodents.
Calcium signal–dependent activation of PKC is coupled to phosphorylation of SNAP-25 (80) and munc18-1 (33), and both SNAP-25 and munc18-1 are essential for effective evoked synaptic release (34,36,39,41). In the case of SNAP-25, phosphorylation by PKC on Ser184 recruits catecholamine vesicles of adrenal gland chromaffin cells, whereas SNAP-25 phosphorylation by PKA is necessary to keep the vesicles in a primed release ready state (81). In hippocampal neurons, however, Ser184 phosphorylation of SNAP-25 is not essential for PKC to stimulate neurotransmission (82).
To become a stable and durable memory trace, long-lasting late-phase LTP requires activation of the transcription factor cAMP response element-binding protein (83) and is dependent on new protein synthesis, similar to long-term memory. Pharmacological inhibition of protein synthesis completely blocks late-phase LTP in both the CA1 (84,85) and CA3 fields (86–88). The specific protein synthesis products responsible for the induction and maintenance of LTP remain to be identified.
Summary
There is ample evidence that long-term synaptic plasticity (LTP and LTD) is the key biochemical correlate of learning and memory. Based on these studies and recent advances in the field, there is a sufficient justification to pursue the underlying synaptic mechanisms of cognitive aging. Many of these potential mechanisms are detailed earlier; however, other factors likely contribute to age-related changes in synaptic function but are beyond the focus of this review. It must be mentioned, however, that synaptic protein expression can be modified by posttranscriptional modifications (eg, RNA editing of GluR2 subunit of AMPA-R), posttranslational processes including palmitoylation, prenylation, glycosylation, and enzymatic cleavage of protein precursors (most relevantly for the IGF-1 receptor), and assembly of protein complexes into functional macromolecules (eg, receptors and channels). Specific localization of these processes requires regulated transport to and from the synapse. Finally, damaged components must be removed, cleared, or repaired (emphasizing an important role of chaperons). It is likely that minor defects in multiple processes mentioned earlier cumulate during aging and thus add to the complexity of synaptic dysfunction and cognitive decline in the individual patient. Using our current understanding of the molecular basis of learning and memory, it is now possible to dissect the specific cellular changes that occur with age and establish the mechanisms through which interventions that improve learning and memory are manifest.
IGF-1 DEFICIENCY, COGNITIVE FUNCTION, AND AGING
Growth Hormone, IGF-1, and Cognitive Function in Humans
In humans, childhood-onset or adult-onset growth hormone deficiency has been associated with attention and memory deficits along with mood disorders (89–92). In addition, impaired iconic, short-term, and long-term memory were reported in men with childhood-onset growth hormone deficiency compared with normal participants (93). In this study, replacement of growth hormone (at doses sufficient to raise serum IGF-1 levels above normal participants) resulted in improvements in short- and long-term memory within 6 months. Furthermore, restoration of IGF-1 to the levels of normal adults resulted in improvements after 1 year, and these effects persisted for 2 years after treatment (94). Individuals with childhood-onset growth hormone deficiency also exhibit delayed verbal memory recall and deficits in the Trail Making Test A (a test of planning, processing speed, and attention), and these deficits were associated with reductions in brain N-acetyl-aspartate/choline ratio, a marker of neuronal integrity (95). The optimal growth hormone supplementation strategy to improve deficits in cognition in growth hormone–deficient patients is still highly debated, but there is some evidence that discontinuation of supplementation during the transitional adolescent period may affect somatic development (96). Growth hormone substitution therapy has also been reported to improve long-term and working memory in 27-year-old childhood-onset growth hormone–deficient patients treated for 6 months (97).
Multiple studies have established that IGF-1 and its signaling pathway have an important role in cognitive decline in aged participants. Aleman and colleagues (98) reported an association between perceptual motor performance, information processing speed, and IGF-1 levels in participants 65–76 years of age. A similar association was noted between IGF-1 levels and measures of fluid intelligence (99). In addition, high IGF-1/IGF binding proteins-3 ratios were associated with better maintenance of cognition determined by scores on the 30-point Mini-Mental State Examination (100), and a similar correlation was noted among centenarians (101). In addition, in a 3-year longitudinal study, Dik and colleagues (102) noted an association between low levels of IGF-1 and reduced processing speed. Other studies find that IGF-1 messenger RNA levels are lower in the frontal cortex of middle age humans compared with young (103). Importantly, one of the conclusions of this paper was that it was not age per se that correlated highly with cognitive deficits but the diminished level of IGF-1 and decreased signaling through the insulin response substrate-PI3K and Akt pathways. These conclusions are supported by recent data indicating an important association between cognitive function and IGF-1 levels in healthy fit older adults (104).
Although there have been limited studies of growth hormone or IGF-1 replacement to older individuals, the studies conducted to date suggest a complex relationship between IGF-1 levels and cognitive function. For example, Papadakis and colleagues (105) demonstrated that treatment of older men with growth hormone improved performance on the Trails B test. However, Friedlander and colleagues (106) using memory of name face or word list recall found no effect of IGF-1 treatment for 1 year in women. Certainly, additional studies on the role of growth hormone and/or IGF-1 replacement on cognitive function are required.
Growth Hormone, IGF-1, and Cognitive Function in Rodents
Assessment of learning and memory in rodent models also suggests that there are complex interactions that occur in response to growth hormone/IGF-1 deficiency and/or replacement. As noted earlier in this review, both growth hormone and IGF-1 decrease with age (6), and studies indicate a close temporal association between the decrease in these circulating hormones and spatial and working memory performance. Furthermore, intracerebroventricular IGF-1 replacement to older F344 × Brown Norway rats, which increases concentrations of IGF-1 in the hippocampus to levels found in young animals, reverses these deficits (107). This increase in learning and memory also occurs in response to administration of growth hormone (6), and injection of growth hormone–releasing hormone from 9 to 30 months of age (sufficient to maintain elevated IGF-1 levels in older animals) prevents the age-related decline in cognitive performance (108). These studies clearly support the hypothesis that reductions in growth hormone and IGF-1 contribute to cognitive dysfunction with age.
The studies of growth hormone and IGF-1 replacement to aged animals are supported by experiments indicating that a deficiency of IGF-1 in adult animals results in deficits in learning and memory. For example, the liver IGF-1–deficient mouse demonstrates impairments in spatial learning that can be reversed by administration of IGF-1 (109). Interestingly, rats with a primary deficiency in growth hormone secretion that results in a 50% reduction in circulating IGF-1 appear to have normal spatial learning at 8 months of age but exhibit a reduction in learning and memory by 18 months of age. This “accelerated” decline in capacity for learning and memory was prevented by administration of growth hormone for 10 weeks during adolescence (110). These results not only support the role of IGF-1 as an important neurotrophic hormone but also suggest that the time during the life span when IGF-1 is reduced may be important for the genesis of the impairments in learning and memory at later stages of the life span.
Despite the data suggesting a positive relationship between IGF-1 and cognitive function in adults and aged animals, data from the Ames dwarf and growth hormone receptor knockout mouse suggest a more complex relationship. In these models, memory assessments using inhibitory avoidance tasks and the water maze suggest better performance in IGF-1–deficient animals (111,112). Although the specific molecular basis for these differences is unresolved, several possibilities exist. One of the most important differences is that although circulating IGF-1 levels are reduced in these models, brain levels of IGF-1 appear to be maintained through a paracrine mechanism (113). Although the specific mechanisms that contribute to this increase have not been investigated to our knowledge, the decline in these hormones during prenatal and neonatal life appears to be essential for this effect because circulating IGF-1 does not appear to influence expression of brain IGF-1 levels at later ages (114). Therefore, additional studies are required to assess the specific role of IGF-1 and IGF-1 deficiency in learning and memory.
CNS as Both Source and Target of IGF-1
Studies of paracrine secretion of IGF-1 in Ames dwarf animals emphasize some key aspects of IGF-1 regulation. Production and secretion of IGF-1 by the CNS have been observed for decades (115–118). The importance of IGF-1 in normal development of the brain is demonstrated by the striking CNS phenotype of IGF-1 knockout mice. igf-1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin–containing neurons (119). Moreover, transgenic mice overexpressing IGF-1 have a significantly larger brain as well as increased myelin content (120). IGF-1 has a major role in neuronal development as it supports neuronal stem cell differentiation (121), axonal path finding (122), and dendritic outgrowth (123,124). Studies on the role of the IGF-1 receptor elicit very similar phenotype. A homozygous null mutation of the IGF-1 receptor causes neonatal lethality in mice (125,126). Finally, the brain-specific IGF-1 receptor knockout mice are viable but exhibit severe developmental abnormalities including dwarfism and microcephalia (127).
IGF-1 production in the brain is not only a response to neuronal injury (ischemia or focal brain injury), but it is also dependent on neuronal activity (128). Recently, an important regulatory mechanism for IGF-1 secretion has been reported (124). Examining synaptic transmission in the olfactory bulb of Synaptotagmin10 (syt10) knockout mice, Cao and colleagues (124) demonstrated that syt10 is essential for IGF-1 release. Previous work of the Sudhof group had established that synaptotagmins are the major calcium sensor protein for action potential–evoked synchronized neurotransmitter release (129,130). Although syt1, 2, and 9 are involved in classical neurotransmission (131–136), recent results on syt10 indicated that it is required to couple-enhanced neuronal firing to IGF-1 release. Consequently, IGF-1 acts locally to augment synaptic connections and maturation of developing neurons in the olfactory bulb. In syt10 knockout mice, mitral and granule neurons are smaller with less extensive dendritic arborizations and fewer synaptic connections. Importantly, treatment with exogenous IGF-1 completely reversed the syt10 knockout phenotype. Similarly, viral transduction with functional syt10 was effective, but Ca binding mutations of syt10 abolished the effect. Taken together, these data indicate that IGF-1 is produced in mitral neurons of the olfactory bulb and stored in vesicles containing syt10, which triggers exocytosis of the vesicles and IGF-1 if dendritic calcium is sufficiently elevated during rapid neuronal firing (Figure 3). Additionally, increased neuronal activity resulting in nuclear calcium signaling likely activates the transcription of IGF binding proteins (137), thus further modifying the complex interactions of IGF-1 signaling. Despite the decrease in brain IGF-1 levels with age, to date, there are no studies to our knowledge of the regulation of brain IGF-1 levels through this mechanism.
Figure 3.
Overview of the potential synaptic effects of insulin-like growth factor (IGF)-1. This figure depicts the potential effects of IGF-1 on a glutamatergic synapse, which may be responsible for the positive effect of IGF-1 on synaptic transmission and prevention of cognitive decline. IGF-1 receptor signaling leads to the phosphorylation of voltage-gated calcium channels, thus increasing calcium influx and neurotransmitter release. In addition to this indirect effect, it is currently unknown whether IGF-1 also exerts a direct effect on synaptic vesicle mobilization and the core complex of SNARE proteins during exocytosis. Interestingly, at the postsynaptic site, IGF-1 may inhibit GSK3beta activity. GSK3beta is suspected of being a major factor in hyperphosphorylation of microtubule-associated protein tau. By reducing GSK3beta activity, IGF-1 could potentially prevent the formation of neurofibrillary tangles, a major pathological hallmark of Alzheimer's disease. Moreover, through PI3K activation, IGF-1 may enhance the delivery and incorporation of glutamate receptors into the dendritic spine. Further studies are required to explore the interactions between IGF-1 signaling and other kinase pathways including PKA, PKC, and CaMKII. Finally, the new insights into the molecular regulation of IGF-1 secretion must be investigated for hippocampal and cortical neurons.
Age-Related Changes in Synapses, Synaptic Transmission, and Glutamate Receptors
As noted previously, there is compelling evidence that the decrease in learning and memory with age results from changes that occur within the synapse. Marked alterations of both the pre- and postsynaptic structures with age were described both in human (20,138) and animal models (139). The expression level of the presynaptic marker synaptophysin is decreased in the elderly patients, which is likely the result of synapse loss. Because synaptophysin is a synaptic vesicular protein, reduced levels of this protein also can be interpreted as a decrease in the number of vesicles in synaptic boutons assuming the overall number of synapses is unchanged. In addition, synaptic morphology is altered consistent with a decrease in overall synaptic function (139).
Studies also indicate that synapses are more likely to become depressed as induction of LTD is shifted, and as a result, aged animals are more prone to reversal of LTP at synapses within the CA1 field (140,141). This phenomenon appears to be partly the result of intracellular calcium signal dysregulation (142) and also is associated with the redox state of aged neurons that exhibit a selective reduction in the NMDA response to field stimulation (143).
In addition to synaptic loss and LTD dysfunction, postsynaptic NMDA receptor subunits of NR1, NR2A, and NR2B are downregulated in aging rats without change in NR2C levels (139,144). Moreover, GluR2 and NR1 levels are dramatically reduced in the hippocampus of aged rats, whereas NR2A is only decreased in the parahippocampal subregion (145). Interestingly, learning deficits are associated with changes in NMDA receptor subunit expression in the hippocampal CA3 field (139,146). These age-dependent changes can be reversed by systematic IGF-1 treatment (6,139,144,147), suggesting that the effects of IGF-1 on learning and memory are mediated, at least in part, through modulation of synaptic function in general and, specifically, NMDA receptors.
An important unanswered question is whether the new neurons that form in aged animals can form functional synapses. Interestingly, a recent study (148,149) reported that the oligodendritic factor, Nogo-A, was highly enriched in CA3 axons. Also, Nogo-A was expressed in neuronal nuclei in the pyramidal and granule cell layers. Although it is possible that the nuclear Nogo-A localization reflects Nogo-B reactivity, which has been previously reported in hippocampal pyramidal cells (150), overexpression of Nogo-A has the potential to downregulate expression of synaptic scaffolding proteins and trigger synaptic disassembly (151). Previous studies indicate that inhibition of Nogo-A increases axon complexity and shifts synaptic morphology to more plastic phenotypes (152). In addition, pharmacological suppression of NgR1 signaling by antibody- and peptide-based blockade of MAI binding induces formation of functional synapses and reorganization of axon arbors (153–155). Therefore, increased Nogo-A expression in older animals likely is an important factor that inhibits the genesis of new synapses.
POTENTIAL MECHANISMS FOR IGF-1 ACTIONS ON BRAIN SYNAPSES
Hippocampal Synaptic Plasticity
Previous studies indicate that 22-month-old rodents with impaired IGF-1 signaling could not sustain perforant path LTP, whereas others with normal IGF-1 levels of the same age responded with LTP similar to young rats (156). In addition, the relevance of circulating IGF-1 to CNS function is best described in liver-specific IGF-1 knockout mice that exhibit 60% of normal IGF-1 plasma levels (comparable to the decreases observed in aged animals and humans). These animals have a perforant path LTP deficit due to the selective loss of excitatory inputs and the relative abundance of inhibition (31). Interestingly, the potentiation of perforant path synapses also could be blocked by γ-interferon injected intracerebroventricularly in rats—an effect that was abrogated by IGF-1 treatment (intracerebroventricular) in vivo (156) suggesting that inflammatory responses present in the brain with age may contribute to impaired LTP and be reversed by IGF-1 application.
LTD and IGF-1 Signaling
LTD in CA1 hippocampal pyramidal cells can be induced by brief (10 minutes) application of high dose of insulin, which induces the endocytosis of the GluR2 subunit containing AMPA receptors (157). Interestingly, synaptic plasticity of parallel fibers onto Purkinje cells in the cerebellum is regulated by IGF receptor Tyr kinase signaling. Acute addition of IGF-1 (or high concentrations of insulin) causes a robust reduction in postsynaptic AMPA receptor numbers through endocytosis (158). Although the details of the signaling pathway are still elusive, it is worth noting that the JNK1 kinase pathway regulates the phosphorylation state of the main scaffold protein, postsynaptic density protein-95. This process is blocked during hippocampal LTD (159) supporting a role of IGF-1 signaling in synaptic receptor trafficking.
Acute Synaptic Effects of IGF-1
Although the effects of IGF-1 are generally considered to result from long-term exposure, acute application of des-IGF-1 (lacking the first three amino residues from the N-terminal of the peptide, 40 ng/mL) increased field EPSP amplitudes by 40% in CA1 field of hippocampal slices from young rats (160). The enhancement was selective to current through AMPA receptors reversible and dependent on PI3K pathway activation. Also, IGF-1 administration has acute effects on calcium currents (Figure 3) through L- and N-type voltage-gated channels (161) of cerebellar granule cells. Augmentation of current through these calcium channels was voltage dependent and selective as P/Q- and R-type channels were unaffected. The effect on N-type channels was independent of Akt signaling, whereas Akt kinase activity was essential for the potentiation of L-type currents (162). In the case of L-type channels, phosphorylation of the alpha1 subunit at Y2122 by PI3K/Akt or src pathways was necessary for the potentiation (163).
The Role of Other Elements of the Insulin/IGF-1/Insulin Response Substrate Signaling
Impairment of signaling molecules in the insulin, IGF-1 pathway also has an important effect on synaptic plasticity. For example, Irs2 knockout mice have impaired CA1 LTP. Despite a normal basal synaptic activity, these animals cannot maintain CA1 potentiation that is most likely the result of inadequate activation of NMDA receptors during tetanic stimulation (164). Interestingly, multiple receptor kinase pathways are hindered in this model, and the NMDA receptor B subtype is absent just as NMDA receptor phosphorylation. Furthermore, NMDA responses are further reduced after tetanus, a possible sign of receptor removal from the postsynaptic plasma membrane. Surprisingly, the IRS2 knockout phenotype is more severe than IGF-1 deficiency as LTP is not induced even after the blockade of synaptic inhibition by the GABA-A receptor antagonist, bicuculline (164). It must be noted that a novel regulation of NMDA receptor surface density has been discovered, induced by low intensity (25 Hz instead of 100 Hz) stimulation (165–167). Such changes in the NMDA receptors may be ideal to modify and fine-tune the threshold for LTP (Figure 3), a phenomenon termed metaplasticity (168). Taken together, one could view the phenotype of reduced IGF-1/insulin response substrate signaling as a special case of shifted metaplasticity with a significantly elevated threshold for LTP.
IGF-1 and Neuronal Networks
To better understand neuronal regulation of behavior, learning and memory studies have been extended to neuronal circuits and complex networks both in vivo and in vitro. In these studies, IGF-1–activated cultured hippocampal networks indicated by selectively increasing somatic excitatory postsynaptic potential frequency (169). However, data should be interpreted with caution because of the experimental design. Although a concentration-dependent activation of mitogen-activated protein kinase/extracellular signal-regulated kinase pathway (originally ERK) has been reported even the lowest IGF-1 concentration tested (1 ng/mL) boosted network activity to maximal frequency after 24-hour treatment. Moreover, shape, size, and frequency of individual quantal release events (mEPSCs), all were unchanged by IGF-1 in this test. In contrast, in another study (170), IGF-1 treatment (300 ng/mL) of hippocampal neurons increased spontaneous mEPSC frequency almost fourfold. This effect was probably indirect, as it relied on the activation of hypoxia-inducable factor 1alpha and required the expression of vascular endothelial growth factor. Many questions remain unanswered by these latest studies, and further experiments will be required to gain important insight into the molecular mechanism of IGF-1 action on presynaptic neurotransmitter release.
THE CONTROVERSIAL ISSUE OF THERAPEUTIC BENEFITS OF IGF-1 IN AD
Synaptic loss and/or alterations in synaptic function are considered to be the main pathological features of cognitive decline in aging. In addition, recent results strongly support the concept that synaptic loss is a structural correlate of very early cognitive decline in AD (171). A similar correlation was found with tau fibrillary tangles but not with amyloid plaque load in AD brains (172). IGF-1 appears to have many synaptic effects and protects against toxic Aβ oligomer–induced synaptic disruption (173). Both insulin and IGF-1 reduce Aβ oligomer binding, protect synapses from synaptotoxic effects of Aβ oligomers, and prevent the Aβ oligomer–induced surface insulin receptor loss. Furthermore, insulin/IGF-1 receptor signaling may be a part of the neuroprotective effect of rosiglitazone (174) Rosiglitazone is known as a peroxisome proliferator–activated receptor-γ agonist, which can reduce Aβ toxicity and improves memory test results in AD models (175–177). These results suggest that brain insulin resistance and dysfunction of IGF-1 receptors contribute to toxic Aβ oligomer accumulation and subsequent synaptic loss.
Nevertheless, there are studies indicating that activation of IGF-1 signaling accelerates the decline in learning and memory in AD mice (178–180). Although the mechanism for this effect was proposed as a reduced aggregation of amyloid into inert compact senile plaques, some alternative explanations are possible. For instance, IGF-1 appears to increase neuronal network activity in cultured neurons, and amyloid production correlates with neuronal activity (181–183). Therefore, the possible beneficial effects of IGF-1 could be hindered in AD patients through elevated Aβ secretion and amyloid accumulation if IGF-1 further activates the default network in AD. Moreover, pharmacological inhibition of PI3K with wortmannin was reported to decrease amyloid accumulation and Aβ levels in the brain of Tg2576 mice (184). This is surprising, as the main amyloid-degrading enzyme also known as insulin-degrading enzyme is upregulated through the insulin/IGF-1 PI3K/Akt pathway (185). Extension of the original study to larger cohorts could provide better understanding of this complex phenotype. Increased inflammation with aging within the CNS is another crucial pathophysiological mechanism proposed for increased AD risk in the elderly patients. The long list of IGF-1 effects on inflammatory responses is beyond the scope of this review; however, it is noteworthy that recent findings support a role of IGF-1 treatment in protecting synaptic plasticity from inflammatory agents (156). Furthermore, some reactions including increased interleukin-6 clear amyloid from the rodent brain (186) supporting the conclusion that inflammation per se is not a major driving force for amyloid deposition.
Unfortunately, a definitive assessment on the benefits and risks of IGF-1 treatment in AD cannot be expected in the near future. Additional in vivo studies similar to the elegant work from the Torres-Aleman group (187) on the Tg2576 AD model expressing human mutant amyloid precursor protein where cognitive decline was prevented by IGF-1 treatment are needed. One possible explanation for the contradictory results from the various in vivo studies on IGF-1 signaling in AD amyloid toxicity is that a negative feedback relationship exists between systemic IGF-1 and local intracerebral IGF-1 production. However, we find this unlikely in light of recent detailed analysis on the correlation of serum and cerebrospinal fluid levels of IGF-1 (188). Another potential explanation may be related to the important differences between the AD model used in studies of AD (eg, presenilin mutations [179] or only amyloid precursor protein mutations [187]).
CONCLUSIONS
In summary, IGF-1 has multiple effects on the nervous system and specifically on synaptic structure and plasticity that are beneficial in maintaining cognitive function during aging. Although IGF-1 has multiple independent and interacting effects on many aspects of brain function, further molecular and electrophysiological studies are required to identify the specific molecular pathways that contribute to the effects of IGF-1 at the synapse and ultimately on the mechanisms that regulate learning and memory. To illustrate, an understanding of the possible interactions of IGF-1, glutamate receptors, and the Akt pathway will require integration of new data on synaptic activity with neuronal survival. In a recent article (189), a moderate increase in NMDA activity resulted in calcium signaling, activation of PI3K/Akt, and inhibition of FOXO transcription factor. These changes reduced apoptosis and thus prevented cell death. A plausible scenario is that IGF-1 has a similar effect through an increase in NMDA receptor expression and/or Akt activation. Interestingly, IGF-1 also protects retinal rod photoreceptor cells from degeneration induced by okadaic acid (190). However, despite the well-recognized positive effects of IGF-1 on synaptic function and mild cognitive impairment, the complex role of IGF-1 in neurodegenerative diseases is understudied and advances in our knowledge of brain aging and interactions with disease progression will require additional research. Because IGF-1 is one of only a few interventions that have consistently been reported to reverse cognitive decline with age, we expect that further research in this area will identify targets for selective therapeutic interventions that improve cognitive function in the elderly patients.
FUNDING
This work was supported by grants from the National Institutes of Health (NS056218, AG11370, AG27147, AG031085, and AT006526), the American Federation for Aging Research, the Oklahoma Center for the Advancement of Science and Technology, the American Heart Association, the GHR Foundation, and the Ellison Medical Foundation.
Acknowledgments
The authors would like to express their gratitude for the support of the Donald W. Reynolds Foundation, which funds aging research at the University of Oklahoma Health Sciences Center under its Aging and Quality of Life Program. The editorial assistance of MaryAnn Sonntag in preparation of this manuscript is greatly appreciated.
References
- 1.Nelson PT, Head E, Schmitt FA, et al. Alzheimer's disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011;121:571–587. doi: 10.1007/s00401-011-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ungvari ZCA. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls072. (this issue) Advance Access March 26, 2012 doi:10.1093/gerona/gls072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novosyadlyy RLD. Insulin-like growth factors and insulin: at the crossroad between tumor development and longevity. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls065. (this issue) Advance Access March 15, 2012 doi:10.1093/gerona/gls065. [DOI] [PubMed] [Google Scholar]
- 4.Higashi YSS, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls102. (this issue) Advance Access April 5, 2012 doi:10.1093/gerona/gls102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown-Borg HMBA. GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls086. (this issue) Advance Access March 30, 2012 doi:10.1093/gerona/gls086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Piriz J, Muller A, Trejo JL, Torres-Aleman I. IGF-I and the aging mammalian brain. Exp Gerontol. 2011;46:96–99. doi: 10.1016/j.exger.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Muller AP, Fernandez AM, Haas C, Zimmer E, Portela LV, Torres-Aleman I. Reduced brain insulin-like growth factor I function during aging. Mol Cell Neurosci. 2012;49:9–12. doi: 10.1016/j.mcn.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Torres-Aleman I. Toward a comprehensive neurobiology of IGF-I. Dev Neurobiol. 2010;70:384–396. doi: 10.1002/dneu.20778. [DOI] [PubMed] [Google Scholar]
- 10.Katsumata Y, Todoriki H, Higashiuesato Y, et al. Metabolic syndrome and cognitive decline among the oldest old in Okinawa: in search of a mechanism. The KOCOA Project. J Gerontol A Biol Sci Med Sci. 2012;67:126–134. doi: 10.1093/gerona/glr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66:582–590. doi: 10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang SS, Weiss CO, Xue QL, Fried LP. Patterns of comorbid inflammatory diseases in frail older women: the Women's Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2010;65:407–413. doi: 10.1093/gerona/glp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klusmann V, Evers A, Schwarzer R, et al. Complex mental and physical activity in older women and cognitive performance: a 6-month randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2010;65:680–688. doi: 10.1093/gerona/glq053. [DOI] [PubMed] [Google Scholar]
- 14.Brunso-Bechtold JK, Linville MC, Sonntag WE. Age-related synaptic changes in sensorimotor cortex of the Brown Norway X fischer 344 rat. Brain Res. 2000;872:125–133. doi: 10.1016/s0006-8993(00)02515-4. [DOI] [PubMed] [Google Scholar]
- 15.Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giorgio A, Santelli L, Tomassini V, et al. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51:943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soghomonian JJ, Sethares C, Peters A. Effects of age on axon terminals forming axosomatic and axodendritic inhibitory synapses in prefrontal cortex. Neuroscience. 2010;168:74–81. doi: 10.1016/j.neuroscience.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VanGuilder HD, Yan H, Farley JA, Sonntag WE, Freeman WM. Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J Neurochem. 2010;113:1577–1588. doi: 10.1111/j.1471-4159.2010.06719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters A, Kemper T. A review of the structural alterations in the cerebral hemispheres of the aging rhesus monkey. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickson DW, Crystal HA, Bevona C, Honer W, Vincent I, Davies P. Correlations of synaptic and pathological markers with cognition of the elderly. Neurobiol Aging. 1995;16:285–298. doi: 10.1016/0197-4580(95)00013-5. ; discussion 298–304. [DOI] [PubMed] [Google Scholar]
- 21.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 22.VanGuilder HD, Farley JA, Yan H, et al. Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline. Neurobiol Dis. 2011;43:201–212. doi: 10.1016/j.nbd.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannakopoulos P, Hof PR, Michel JP, Guimon J, Bouras C. Cerebral cortex pathology in aging and Alzheimer's disease: a quantitative survey of large hospital-based geriatric and psychiatric cohorts. Brain Res Brain Res Rev. 1997;25:217–245. doi: 10.1016/s0165-0173(97)00023-4. [DOI] [PubMed] [Google Scholar]
- 24.Morrison JH, Hof PR. Selective vulnerability of corticocortical and hippocampal circuits in aging and Alzheimer's disease. Prog Brain Res. 2002;136:467–486. doi: 10.1016/s0079-6123(02)36039-4. [DOI] [PubMed] [Google Scholar]
- 25.Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Vanguilder HD, Freeman WM. The hippocampal neuroproteome with aging and cognitive decline: past progress and future directions. Front Aging Neurosci. 2011;3:8. doi: 10.3389/fnagi.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu T, Pan Y, Kao SY, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 28.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa E, Davis JM, Dong E, et al. A GABAergic cortical deficit dominates schizophrenia pathophysiology. Crit Rev Neurobiol. 2004;16:1–23. doi: 10.1615/critrevneurobiol.v16.i12.10. [DOI] [PubMed] [Google Scholar]
- 30.Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol. 2003;23:601–640. doi: 10.1097/01.jcp.0000095349.32154.a5. [DOI] [PubMed] [Google Scholar]
- 31.Trejo JL, Piriz J, Llorens-Martin MV, et al. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol Psychiatry. 2007;12:1118–1128. doi: 10.1038/sj.mp.4002076. [DOI] [PubMed] [Google Scholar]
- 32.Leppik IE, Birnbaum AK. Epilepsy in the elderly. Ann N Y Acad Sci. 2010;1184:208–224. doi: 10.1111/j.1749-6632.2009.05113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujita Y, Sasaki T, Fukui K, et al. Phosphorylation of Munc-18/n-Sec1/rbSec1 by protein kinase C: its implication in regulating the interaction of Munc-18/n-Sec1/rbSec1 with syntaxin. J Biol Chem. 1996;271:7265–7268. doi: 10.1074/jbc.271.13.7265. [DOI] [PubMed] [Google Scholar]
- 34.Verhage M, Maia AS, Plomp JJ, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 35.Schoch S, Deák F, Konigstorfer A, et al. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- 36.Washbourne P, Thompson PM, Carta M, et al. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- 37.Deák F, Schoch S, Liu X, Südhof TC, Kavalali ET. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat Cell Biol. 2004;6:1102–1108. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- 38.Deák F, Shin OH, Kavalali ET, Südhof TC. Structural determinants of synaptobrevin 2 function in synaptic vesicle fusion. J Neurosci. 2006;26:6668–6676. doi: 10.1523/JNEUROSCI.5272-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bronk P, Deák F, Wilson MC, Liu X, Südhof TC, Kavalali ET. Differential effects of SNAP-25 deletion on Ca2+ -dependent and Ca2+ -independent neurotransmission. J Neurophysiol. 2007;98:794–806. doi: 10.1152/jn.00226.2007. [DOI] [PubMed] [Google Scholar]
- 40.Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deák F, Xu Y, Chang WP, et al. Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming. J Cell Biol. 2009;184:751–764. doi: 10.1083/jcb.200812026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 43.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castillo PE, Chiu CQ, Carroll RC. Long-term plasticity at inhibitory synapses. Curr Opin Neurobiol. 2011;21:328–338. doi: 10.1016/j.conb.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 46.Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- 47.Cajal Ry., editor. Histologie du Systeme Nerveux de l’Homme et des Vertebretes. Paris, France: A. Maloine; 1911. [Google Scholar]
- 48.Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 49.Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- 50.Ahmad M, Polepalli JS, Goswami D, et al. Postsynaptic complexin controls AMPA receptor exocytosis during LTP. Neuron. 2012;73:260–267. doi: 10.1016/j.neuron.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collingridge GL, Bliss TV. Memories of NMDA receptors and LTP. Trends Neurosci. 1995;18:54–56. [PubMed] [Google Scholar]
- 52.Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- 53.Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- 54.Brown RE, Milner PM. The legacy of Donald O. Hebb: more than the Hebb synapse. Nat Rev Neurosci. 2003;4:1013–1019. doi: 10.1038/nrn1257. [DOI] [PubMed] [Google Scholar]
- 55.Cooper SJ. Donald O. Hebb's synapse and learning rule: a history and commentary. Neurosci Biobehav Rev. 2005;28:851–874. doi: 10.1016/j.neubiorev.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Lu Y, Allen M, Halt AR, et al. Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. EMBO J. 2007;26:4879–4890. doi: 10.1038/sj.emboj.7601884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H, Pineda VV, Chan GC, Wong ST, Muglia LJ, Storm DR. Type 8 adenylyl cyclase is targeted to excitatory synapses and required for mossy fiber long-term potentiation. J Neurosci. 2003;23:9710–9718. doi: 10.1523/JNEUROSCI.23-30-09710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang YY, Li XC, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 59.Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- 60.Kaeser PS, Kwon HB, Blundell J, et al. RIM1alpha phosphorylation at serine-413 by protein kinase A is not required for presynaptic long-term plasticity or learning. Proc Natl Acad Sci U S A. 2008;105:14680–14685. doi: 10.1073/pnas.0806679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castillo PE, Janz R, Sudhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature. 1997;388:590–593. doi: 10.1038/41574. [DOI] [PubMed] [Google Scholar]
- 62.Saetre P, Jazin E, Emilsson L. Age-related changes in gene expression are accelerated in Alzheimer's disease. Synapse. 2011;65:971–974. doi: 10.1002/syn.20933. [DOI] [PubMed] [Google Scholar]
- 63.Numata S, Shirataki H, Hagi S, Yamamoto T, Takai Y. Phosphorylation of Rabphilin-3A, a putative target protein for Rab3A, by cyclic AMP-dependent protein kinase. Biochem Biophys Res Commun. 1994;203:1927–1934. doi: 10.1006/bbrc.1994.2413. [DOI] [PubMed] [Google Scholar]
- 64.Fykse EM, Li C, Südhof TC. Phosphorylation of rabphilin-3A by Ca2+/calmodulin- and cAMP-dependent protein kinases in vitro. J Neurosci. 1995;15:2385–2395. doi: 10.1523/JNEUROSCI.15-03-02385.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lonart G, Südhof TC. Characterization of rabphilin phosphorylation using phospho-specific antibodies. Neuropharmacology. 2001;41:643–649. doi: 10.1016/s0028-3908(01)00126-5. [DOI] [PubMed] [Google Scholar]
- 66.Deak F, Shin OH, Tang J, et al. Rabphilin regulates SNARE-dependent re-priming of synaptic vesicles for fusion. EMBO J. 2006;25:2856–2866. doi: 10.1038/sj.emboj.7601165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mellor J, Nicoll RA, Schmitz D. Mediation of hippocampal mossy fiber long-term potentiation by presynaptic Ih channels. Science. 2002;295:143–147. doi: 10.1126/science.1064285. [DOI] [PubMed] [Google Scholar]
- 68.Trimble WS, Scheller RH. Molecular biology of synaptic vesicle-associated proteins. Trends Neurosci. 1988;11:241–242. doi: 10.1016/0166-2236(88)90098-7. [DOI] [PubMed] [Google Scholar]
- 69.Südhof TC, Czernik AJ, Kao HT, et al. Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science. 1989;245:1474–1480. doi: 10.1126/science.2506642. [DOI] [PubMed] [Google Scholar]
- 70.Hosaka M, Südhof TC. Synapsin III, a novel synapsin with an unusual regulation by Ca2+ J Biol Chem. 1998;273:13371–13374. doi: 10.1074/jbc.273.22.13371. [DOI] [PubMed] [Google Scholar]
- 71.Hosaka M, Hammer RE, Südhof TC. A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron. 1999;24:377–387. doi: 10.1016/s0896-6273(00)80851-x. [DOI] [PubMed] [Google Scholar]
- 72.Gitler D, Cheng Q, Greengard P, Augustine GJ. Synapsin IIa controls the reserve pool of glutamatergic synaptic vesicles. J Neurosci. 2008;28:10835–10843. doi: 10.1523/JNEUROSCI.0924-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosahl TW, Geppert M, Spillane D, et al. Short-term synaptic plasticity is altered in mice lacking synapsin I. Cell. 1993;75:661–670. doi: 10.1016/0092-8674(93)90487-b. [DOI] [PubMed] [Google Scholar]
- 74.Gitler D, Takagishi Y, Feng J, et al. Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J Neurosci. 2004;24:11368–11380. doi: 10.1523/JNEUROSCI.3795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spillane DM, Rosahl TW, Südhof TC, Malenka RC. Long-term potentiation in mice lacking synapsins. Neuropharmacology. 1995;34:1573–1579. doi: 10.1016/0028-3908(95)00107-h. [DOI] [PubMed] [Google Scholar]
- 76.Ozaki N, Shibasaki T, Kashima Y, et al. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol. 2000;2:805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- 77.Kawasaki H, Springett GM, Mochizuki N, et al. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 78.Armstrong JN, Saganich MJ, Xu NJ, Henkemeyer M, Heinemann SF, Contractor A. B-ephrin reverse signaling is required for NMDA-independent long-term potentiation of mossy fibers in the hippocampus. J Neurosci. 2006;26:3474–3481. doi: 10.1523/JNEUROSCI.4338-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirling H, Scheller RH. Phosphorylation of synaptic vesicle proteins: modulation of the alpha SNAP interaction with the core complex. Proc Natl Acad Sci U S A. 1996;93:11945–11949. doi: 10.1073/pnas.93.21.11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nagy G, Matti U, Nehring RB, et al. Protein kinase C-dependent phosphorylation of synaptosome-associated protein of 25 kDa at Ser187 potentiates vesicle recruitment. J Neurosci. 2002;22:9278–9286. doi: 10.1523/JNEUROSCI.22-21-09278.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nagy G, Reim K, Matti U, et al. Regulation of releasable vesicle pool sizes by protein kinase A-dependent phosphorylation of SNAP-25. Neuron. 2004;41:417–429. doi: 10.1016/s0896-6273(04)00038-8. [DOI] [PubMed] [Google Scholar]
- 82.Finley MF, Scheller RH, Madison DV. SNAP-25 Ser187 does not mediate phorbol ester enhancement of hippocampal synaptic transmission. Neuropharmacology. 2003;45:857–862. doi: 10.1016/s0028-3908(03)00283-1. [DOI] [PubMed] [Google Scholar]
- 83.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 84.Stanton PK, Sarvey JM. Blockade of long-term potentiation in rat hippocampal CA1 region by inhibitors of protein synthesis. J Neurosci. 1984;4:3080–3088. doi: 10.1523/JNEUROSCI.04-12-03080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- 86.Barea-Rodriguez EJ, Rivera DT, Jaffe DB, Martinez JL., Jr. Protein synthesis inhibition blocks the induction of mossy fiber long-term potentiation in vivo. J Neurosci. 2000;20:8528–8532. doi: 10.1523/JNEUROSCI.20-22-08528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barnes SJ, Opitz T, Merkens M, et al. Stable mossy fiber long-term potentiation requires calcium influx at the granule cell soma, protein synthesis, and microtubule-dependent axonal transport. J Neurosci. 2010;30:12996–13004. doi: 10.1523/JNEUROSCI.1847-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Calixto E, Thiels E, Klann E, Barrionuevo G. Early maintenance of hippocampal mossy fiber—long-term potentiation depends on protein and RNA synthesis and presynaptic granule cell integrity. J Neurosci. 2003;23:4842–4849. doi: 10.1523/JNEUROSCI.23-12-04842.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frankel JJ, Laron Z. Psychological aspects of pituitary insufficiency in children and adolescents with special reference to growth hormone. Isr J Med Sci. 1968;4:953–961. [PubMed] [Google Scholar]
- 90.Laron Z, Galatzer A. Effect of hGH on head circumference and IQ in isolated growth hormone deficiency. Early Hum Dev. 1981;5:211–214. doi: 10.1016/0378-3782(81)90054-2. [DOI] [PubMed] [Google Scholar]
- 91.Falleti MG, Maruff P, Burman P, Harris A. The effects of growth hormone (GH) deficiency and GH replacement on cognitive performance in adults: a meta-analysis of the current literature. Psychoneuroendocrinology. 2006;31:681–691. doi: 10.1016/j.psyneuen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 92.van Nieuwpoort IC, Drent ML. Cognition in the adult with childhood-onset GH deficiency. Eur J Endocrinol. 2008;159(suppl 1):S53–S57. doi: 10.1530/EJE-08-0279. [DOI] [PubMed] [Google Scholar]
- 93.Deijen JB, de Boer H, Blok GJ, van der Veen EA. Cognitive impairments and mood disturbances in growth hormone deficient men. Psychoneuroendocrinology. 1996;21:313–322. doi: 10.1016/0306-4530(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 94.Deijen JB, de Boer H, van der Veen EA. Cognitive changes during growth hormone replacement in adult men. Psychoneuroendocrinology. 1998;23:45–55. doi: 10.1016/s0306-4530(97)00092-9. [DOI] [PubMed] [Google Scholar]
- 95.van Dam PS, de Winter CF, de Vries R, et al. Childhood-onset growth hormone deficiency, cognitive function and brain N-acetylaspartate. Psychoneuroendocrinology. 2005;30:357–363. doi: 10.1016/j.psyneuen.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 96.Clayton P, Gleeson H, Monson J, Popovic V, Shalet SM, Christiansen JS. Growth hormone replacement throughout life: insights into age-related responses to treatment. Growth Horm IGF Res. 2007;17:369–382. doi: 10.1016/j.ghir.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 97.Arwert LI, Veltman DJ, Deijen JB, van Dam PS, Drent ML. Effects of growth hormone substitution therapy on cognitive functioning in growth hormone deficient patients: a functional MRI study. Neuroendocrinology. 2006;83:12–19. doi: 10.1159/000093337. [DOI] [PubMed] [Google Scholar]
- 98.Aleman A, Verhaar HJ, De Haan EH, et al. Insulin-like growth factor-I and cognitive function in healthy older men. J Clin Endocrinol Metab. 1999;84:471–475. doi: 10.1210/jcem.84.2.5455. [DOI] [PubMed] [Google Scholar]
- 99.Aleman A, de Vries WR, Koppeschaar HP, et al. Relationship between circulating levels of sex hormones and insulin-like growth factor-1 and fluid intelligence in older men. Exp Aging Res. 2001;27:283–291. doi: 10.1080/036107301300208718. [DOI] [PubMed] [Google Scholar]
- 100.Kalmijn S, Janssen JA, Pols HA, Lamberts SW, Breteler MM. A prospective study on circulating insulin-like growth factor I (IGF-I), IGF-binding proteins, and cognitive function in the elderly. J Clin Endocrinol Metab. 2000;85:4551–4555. doi: 10.1210/jcem.85.12.7033. [DOI] [PubMed] [Google Scholar]
- 101.Paolisso G, Ammendola S, Del Buono A, et al. Serum levels of insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 in healthy centenarians: relationship with plasma leptin and lipid concentrations, insulin action, and cognitive function. J Clin Endocrinol Metab. 1997;82:2204–2209. doi: 10.1210/jcem.82.7.4087. [DOI] [PubMed] [Google Scholar]
- 102.Dik MG, Pluijm SM, Jonker C, Deeg DJ, Lomecky MZ, Lips P. Insulin-like growth factor I (IGF-I) and cognitive decline in older persons. Neurobiol Aging. 2003;24:573–581. doi: 10.1016/s0197-4580(02)00136-7. [DOI] [PubMed] [Google Scholar]
- 103.Erraji-Benchekroun L, Underwood MD, Arango V, et al. Molecular aging in human prefrontal cortex is selective and continuous throughout adult life. Biol Psychiatry. 2005;57:549–558. doi: 10.1016/j.biopsych.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 104.Bellar D, Glickman EL, Juvancic-Heltzel J, Gunstad J. Serum insulin like growth factor-1 is associated with working memory, executive function and selective attention in a sample of healthy, fit older adults. Neuroscience. 2011;178:133–137. doi: 10.1016/j.neuroscience.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 105.Papadakis MA, Grady D, Black D, et al. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996;124:708–716. doi: 10.7326/0003-4819-124-8-199604150-00002. [DOI] [PubMed] [Google Scholar]
- 106.Friedlander AL, Butterfield GE, Moynihan S, et al. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. J Clin Endocrinol Metab. 2001;86:1496–1503. doi: 10.1210/jcem.86.4.7377. [DOI] [PubMed] [Google Scholar]
- 107.Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559–569. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- 108.Thornton PL, Ingram RL, Sonntag WE. Chronic [D-Ala2]-growth hormone-releasing hormone administration attenuates age-related deficits in spatial memory. J Gerontol Biol Sci. 2000;55:B106–B112. doi: 10.1093/gerona/55.2.b106. [DOI] [PubMed] [Google Scholar]
- 109.Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37:402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 110.Nieves-Martinez E, Sonntag WE, Wilson A, et al. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. J Endocrinol. 2010;204:31–36. doi: 10.1677/JOE-09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav. 2001;72:653–660. doi: 10.1016/s0031-9384(01)00423-1. [DOI] [PubMed] [Google Scholar]
- 112.Kinney-Forshee BA, Kinney NE, Steger RW, Bartke A. Could a deficiency in growth hormone signaling be beneficial to the aging brain? Physiol Behav. 2004;80:589–594. doi: 10.1016/j.physbeh.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 113.Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26:929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 114.Mitschelen M, Yan H, Farley JA, et al. Long-term deficiency of circulating and hippocampal insulin-like growth factor I induces depressive behavior in adult mice: a potential model of geriatric depression. Neuroscience. 2011;185:50–60. doi: 10.1016/j.neuroscience.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lund PK, Moats-Staats BM, Hynes MA, et al. Somatomedin-C/insulin-like growth factor-I and insulin-like growth factor-II mRNAs in rat fetal and adult tissues. J Biol Chem. 1986;261:14539–14544. [PubMed] [Google Scholar]
- 116.Adamo M, Werner H, Farnsworth W, Roberts CT, Jr, Raizada M, LeRoith D. Dexamethasone reduces steady state insulin-like growth factor I messenger ribonucleic acid levels in rat neuronal and glial cells in primary culture. Endocrinology. 1988;123:2565–2570. doi: 10.1210/endo-123-5-2565. [DOI] [PubMed] [Google Scholar]
- 117.Rotwein P, Burgess SK, Milbrandt JD, Krause JE. Differential expression of insulin-like growth factor genes in rat central nervous system. Proc Natl Acad Sci U S A. 1988;85:265–269. doi: 10.1073/pnas.85.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ayer-le Lievre C, Stahlbom PA, Sara VR. Expression of IGF-I and -II mRNA in the brain and craniofacial region of the rat fetus. Development. 1991;111:105–115. doi: 10.1242/dev.111.1.105. [DOI] [PubMed] [Google Scholar]
- 119.Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron. 1995;14:717–730. doi: 10.1016/0896-6273(95)90216-3. [DOI] [PubMed] [Google Scholar]
- 120.Carson MJ, Behringer RR, Brinster RL, McMorris FA. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993;10:729–740. doi: 10.1016/0896-6273(93)90173-o. [DOI] [PubMed] [Google Scholar]
- 121.Vicario-Abejon C, Yusta-Boyo MJ, Fernandez-Moreno C, de Pablo F. Locally born olfactory bulb stem cells proliferate in response to insulin-related factors and require endogenous insulin-like growth factor-I for differentiation into neurons and glia. J Neurosci. 2003;23:895–906. doi: 10.1523/JNEUROSCI.23-03-00895.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scolnick JA, Cui K, Duggan CD, et al. Role of IGF signaling in olfactory sensory map formation and axon guidance. Neuron. 2008;57:847–857. doi: 10.1016/j.neuron.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cheng CM, Mervis RF, Niu SL, et al. Insulin-like growth factor 1 is essential for normal dendritic growth. J Neurosci Res. 2003;73:1–9. doi: 10.1002/jnr.10634. [DOI] [PubMed] [Google Scholar]
- 124.Cao P, Maximov A, Sudhof TC. Activity-dependent IGF-1 exocytosis is controlled by the Ca(2+)-sensor synaptotagmin-10. Cell. 2011;145:300–311. doi: 10.1016/j.cell.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 126.Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 127.Kappeler L, De Magalhaes Filho C, Dupont J, et al. Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol. 2008;6:e254. doi: 10.1371/journal.pbio.0060254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hughes PE, Alexi T, Walton M, et al. Activity and injury-dependent expression of inducible transcription factors, growth factors and apoptosis-related genes within the central nervous system. Prog Neurobiol. 1999;57:421–450. doi: 10.1016/s0301-0082(98)00057-4. [DOI] [PubMed] [Google Scholar]
- 129.Südhof TC. Synaptotagmins: why so many? J Biol Chem. 2002;277:7629–7632. doi: 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- 130.Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 131.Li C, Ullrich B, Zhang JZ, Anderson RG, Brose N, Südhof TC. Ca(2+)-dependent and -independent activities of neural and non-neural synaptotagmins. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 132.Li C, Davletov BA, Südhof TC. Distinct Ca2+ and Sr2+ binding properties of synaptotagmins. Definition of candidate Ca2+ sensors for the fast and slow components of neurotransmitter release. J Biol Chem. 1995;270:24898–24902. doi: 10.1074/jbc.270.42.24898. [DOI] [PubMed] [Google Scholar]
- 133.Fernandez-Chacon R, Konigstorfer A, Gerber SH, et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 134.Sugita S, Shin OH, Han W, Lao Y, Südhof TC. Synaptotagmins form a hierarchy of exocytotic Ca(2+) sensors with distinct Ca(2+) affinities. EMBO J. 2002;21:270–280. doi: 10.1093/emboj/21.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shin OH, Maximov A, Lim BK, Rizo J, Südhof TC. Unexpected Ca2+-binding properties of synaptotagmin 9. Proc Natl Acad Sci U S A. 2004;101:2554–2559. doi: 10.1073/pnas.0308477100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pang ZP, Melicoff E, Padgett D, et al. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J Neurosci. 2006;26:13493–13504. doi: 10.1523/JNEUROSCI.3519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang SJ, Zou M, Lu L, et al. Nuclear calcium signaling controls expression of a large gene pool: identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS Genet. 2009;5:e1000604. doi: 10.1371/journal.pgen.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Honer WG, Dickson DW, Gleeson J, Davies P. Regional synaptic pathology in Alzheimer's disease. Neurobiol Aging. 1992;13:375–382. doi: 10.1016/0197-4580(92)90111-a. [DOI] [PubMed] [Google Scholar]
- 139.Adams MM, Shi L, Linville MC, et al. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp Neurol. 2008;211:141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci. 1996;16:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kumar A, Thinschmidt JS, Foster TC, King MA. Aging effects on the limits and stability of long-term synaptic potentiation and depression in rat hippocampal area CA1. J Neurophysiol. 2007;98:594–601. doi: 10.1152/jn.00249.2007. [DOI] [PubMed] [Google Scholar]
- 142.Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A. Calcineurin links Ca2+ dysregulation with brain aging. J Neurosci. 2001;21:4066–4073. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bodhinathan K, Kumar A, Foster TC. Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2010;30:1914–1924. doi: 10.1523/JNEUROSCI.5485-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sonntag WE, Bennett SA, Khan AS, et al. Age and insulin-like growth factor-1 modulate N-methyl-D-aspartate receptor subtype expression in rats. Brain Res Bull. 2000;51:331–338. doi: 10.1016/s0361-9230(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 145.Liu P, Smith PF, Darlington CL. Glutamate receptor subunits expression in memory-associated brain structures: regional variations and effects of aging. Synapse. 2008;62:834–841. doi: 10.1002/syn.20563. [DOI] [PubMed] [Google Scholar]
- 146.Adams MM, Smith TD, Moga D, et al. Hippocampal dependent learning ability correlates with N-methyl-D-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J Comp Neurol. 2001;432:230–243. doi: 10.1002/cne.1099. [DOI] [PubMed] [Google Scholar]
- 147.Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197(Pt 4):575–585. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Van Kirk CA, VanGuilder HD, Young M, Farley JA, Sonntag WE, Freeman WM. Age-related alterations in retinal neurovascular and inflammatory transcripts. Mol Vis. 2011;17:1261–1274. [PMC free article] [PubMed] [Google Scholar]
- 149.Vanguilder HD, Bixler GV, Sonntag WE, Freeman WM. Hippocampal expression of myelin-associated inhibitors is induced with age-related cognitive decline and correlates with deficits of spatial learning and memory. J Neurochem. 2012 doi: 10.1111/j.1471-4159.2012.07671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huber AB, Weinmann O, Brosamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aloy EM, Weinmann O, Pot C, et al. Synaptic destabilization by neuronal Nogo-A. Brain Cell Biol. 2006;35:137–156. doi: 10.1007/s11068-007-9014-3. [DOI] [PubMed] [Google Scholar]
- 152.Zagrebelsky M, Schweigreiter R, Bandtlow CE, Schwab ME, Korte M. Nogo-A stabilizes the architecture of hippocampal neurons. J Neurosci. 2010;30:13220–13234. doi: 10.1523/JNEUROSCI.1044-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hanell A, Clausen F, Bjork M, et al. Genetic deletion and pharmacological inhibition of Nogo-66 receptor impairs cognitive outcome after traumatic brain injury in mice. J Neurotrauma. 2010;27:1297–1309. doi: 10.1089/neu.2009.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li S, Liu BP, Budel S, et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mehta NR, Lopez PH, Vyas AA, Schnaar RL. Gangliosides and Nogo receptors independently mediate myelin-associated glycoprotein inhibition of neurite outgrowth in different nerve cells. J Biol Chem. 2007;282:27875–27886. doi: 10.1074/jbc.M704055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Maher FO, Clarke RM, Kelly A, Nally RE, Lynch MA. Interaction between interferon gamma and insulin-like growth factor-1 in hippocampus impacts on the ability of rats to sustain long-term potentiation. J Neurochem. 2006;96:1560–1571. doi: 10.1111/j.1471-4159.2006.03664.x. [DOI] [PubMed] [Google Scholar]
- 157.Man HY, Lin JW, Ju WH, et al. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 158.Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- 159.Kim MJ, Futai K, Jo J, Hayashi Y, Cho K, Sheng M. Synaptic accumulation of PSD-95 and synaptic function regulated by phosphorylation of serine-295 of PSD-95. Neuron. 2007;56:488–502. doi: 10.1016/j.neuron.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 160.Ramsey MM, Adams MM, Ariwodola OJ, Sonntag WE, Weiner JL. Functional characterization of des-IGF-1 action at excitatory synapses in the CA1 region of rat hippocampus. J Neurophysiol. 2005;94:247–254. doi: 10.1152/jn.00768.2004. [DOI] [PubMed] [Google Scholar]
- 161.Blair LA, Marshall J. IGF-1 modulates N and L calcium channels in a PI 3-kinase-dependent manner. Neuron. 1997;19:421–429. doi: 10.1016/s0896-6273(00)80950-2. [DOI] [PubMed] [Google Scholar]
- 162.Blair LA, Bence-Hanulec KK, Mehta S, Franke T, Kaplan D, Marshall J. Akt-dependent potentiation of L channels by insulin-like growth factor-1 is required for neuronal survival. J Neurosci. 1999;19:1940–1951. doi: 10.1523/JNEUROSCI.19-06-01940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bence-Hanulec KK, Marshall J, Blair LA. Potentiation of neuronal L calcium channels by IGF-1 requires phosphorylation of the alpha1 subunit on a specific tyrosine residue. Neuron. 2000;27:121–131. doi: 10.1016/s0896-6273(00)00014-3. [DOI] [PubMed] [Google Scholar]
- 164.Martin ED, Sanchez-Perez A, Trejo JL, et al. IRS-2 deficiency impairs NMDA receptor-dependent long-term potentiation. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kerr AM, Jonas P. The two sides of hippocampal mossy fiber plasticity. Neuron. 2008;57:5–7. doi: 10.1016/j.neuron.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 166.Kwon HB, Castillo PE. Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron. 2008;57:108–120. doi: 10.1016/j.neuron.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 168.Thiagarajan TC, Lindskog M, Malgaroli A, Tsien RW. LTP and adaptation to inactivity: overlapping mechanisms and implications for metaplasticity. Neuropharmacology. 2007;52:156–175. doi: 10.1016/j.neuropharm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 169.Xing C, Yin Y, Chang R, Gong X, He X, Xie Z. Effects of insulin-like growth factor 1 on synaptic excitability in cultured rat hippocampal neurons. Exp Neurol. 2007;205:222–229. doi: 10.1016/j.expneurol.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 170.Huang YF, Yang CH, Huang CC, Tai MH, Hsu KS. Pharmacological and genetic accumulation of hypoxia-inducible factor-1alpha enhances excitatory synaptic transmission in hippocampal neurons through the production of vascular endothelial growth factor. J Neurosci. 2010;30:6080–6093. doi: 10.1523/JNEUROSCI.5493-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 172.Giannakopoulos P, Herrmann FR, Bussiere T, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- 173.Zhao WQ, Lacor PN, Chen H, et al. Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric a{beta} J Biol Chem. 2009;284:18742–18753. doi: 10.1074/jbc.M109.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.De Felice FG, Vieira MN, Bomfim TR, et al. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A. 2009;106:1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Watson GS, Cholerton BA, Reger MA, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 176.Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol. 2006;199:265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 177.Rodriguez-Rivera J, Denner L, Dineley KT. Rosiglitazone reversal of Tg2576 cognitive deficits is independent of peripheral gluco-regulatory status. Behav Brain Res. 2011;216:255–261. doi: 10.1016/j.bbr.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 179.Cohen E, Paulsson JF, Blinder P, et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139:1157–1169. doi: 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dillin A, Cohen E. Ageing and protein aggregation-mediated disorders: from invertebrates to mammals. Philos Trans R Soc Lond B Biol Sci. 2011;366:94–98. doi: 10.1098/rstb.2010.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kamenetz F, Tomita T, Hsieh H, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 182.Cirrito JR, Yamada KA, Finn MB, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 183.Cirrito JR, Kang JE, Lee J, et al. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Haugabook SJ, Le T, Yager D, et al. Reduction of Abeta accumulation in the Tg2576 animal model of Alzheimer's disease after oral administration of the phosphatidyl-inositol kinase inhibitor wortmannin. FASEB J. 2001;15:16–18. doi: 10.1096/fj.00-0528fje. [DOI] [PubMed] [Google Scholar]
- 185.Zhao L, Teter B, Morihara T, et al. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer's disease intervention. J Neurosci. 2004;24:11120–11126. doi: 10.1523/JNEUROSCI.2860-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chakrabarty P, Jansen-West K, Beccard A, et al. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010;24:548–559. doi: 10.1096/fj.09-141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med. 2002;8:1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- 188.Yan H, Mitschelen M, Bixler GV, et al. Circulating IGF1 regulates hippocampal IGF1 levels and brain gene expression during adolescence. J Endocrinol. 2011;211:27–37. doi: 10.1530/JOE-11-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Al-Mubarak B, Soriano FX, Hardingham GE. Synaptic NMDAR activity suppresses FOXO1 expression via a cis-acting FOXO binding site: FOXO1 is a FOXO target gene. Channels (Austin) 2009;3:233–238. doi: 10.4161/chan.3.4.9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Adao-Novaes J, de Cassia Belem Guterrres C, Linden R, Sholl-Franco A. Rod photoreceptor cell death is induced by okadaic acid through activation of PKC and L-type voltage-dependent Ca2+ channels and prevented by IGF-1. Neurochem Int. 2010;57:128–135. doi: 10.1016/j.neuint.2010.04.021. [DOI] [PubMed] [Google Scholar]