Summary
Complex social life requires individuals to recognize and remember group members [1] and, within those, to distinguish affiliates from nonaffiliates. Whereas long-term individual recognition has been demonstrated in some nonhuman animals [2–5], memory for the relationship valence to former group members has received little attention. Here we show that adult, pair-housed ravens not only respond differently to the playback of calls from previous group members and unfamiliar conspecifics but also discriminate between familiar birds according to the relationship valence they had to those subjects up to three years ago as subadult nonbreeders. The birds' distinction between familiar and unfamiliar individuals is reflected mainly in the number of calls, whereas their differentiation according to relationship valence is reflected in call modulation only. As compared to their response to affiliates, ravens responded to nonaffiliates by increasing chaotic parts of the vocalization and lowering formant spacing, potentially exaggerating the perceived impression of body size. Our findings indicate that ravens remember relationship qualities to former group members even after long periods of separation, confirming that their sophisticated social knowledge as nonbreeders is maintained into the territorial breeding stage.
Highlights
► Ravens remember former group members for extended time periods ► Ravens memorize relationship valence (affiliation) in addition to group membership ► Familiarity is coded in the number of calls given in response to playback ► Relationship valence is coded in the modulation of response calls
Results
Humans are able to visually recognize, remember, and think about hundreds of different faces over decades or longer, but the neural and evolutionary basis for this ability remains poorly understood. Nonhuman animals living in social groups and/or facing regular encounters with territorial neighbors may also recognize conspecifics on an individual basis [1, 6] and, sometimes, remember them for years [2–5, 7]. Sheep (Ovis aries), for example, are able to differentiate between conspecific faces for two years [4], and fur seal pups (Callorhinus ursinus) still remember their mother's calls after four years [2]. Long-term memory has been proposed to be particularly important in societies with a high degree of fission-fusion dynamics [8], as specific individuals may meet regularly but after unpredictably long periods of separation. Likewise, memory load may increase when, in addition to individual identity, the social relationships to (temporarily absent) group members need to be remembered [8]. Although dealing with social relationships is often considered a critical driving force in the evolution of advanced cognition [8–10], our knowledge about long-term memory for social relations is limited. So far, studies have tested only for long-term class-level recognition [1] by using in-group and out-group categories such as kin versus nonkin [11] and territorial neighbor versus unknown conspecific [3, 12].
Ravens Corvus corax spend their first years in nonbreeder flocks, engaging in sophisticated social maneuvers like recruitment to food [13, 14] and tactical deception for access to food caches [15]. Moreover, they regularly participate in complex interactions during and after conflicts [16, 17], providing support predominantly to those individuals with whom they share a valuable relationship [16]. The presence of valuable partners (kin and nonkin affiliates) also affects the birds' thoroughness in exploring novel objects [18] and the likelihood of acquiring skills through social learning [19]. Hence, the valence of relationship among individuals may explain much of the social behavior of nonbreeding ravens. Interestingly, nonbreeder flocks are not stable units but show high degrees of fission-fusion dynamics over the day and across months [20]. This complex system of coming and going coupled with the pattern of forming valuable relations would make an extensive memory for individuals and their relationships advantageous for nonbreeding ravens. In contrast to the vagrant life of nonbreeders, reproductively active ravens are highly local, defending a territory year-round. Yet they may encounter, and even join, nonbreeder groups from time to time [21], suggesting that memory for conspecifics may also be useful in the breeding stage.
Here we tested whether adult captive ravens remember their relationship valence to former group members. We made use of the fact that the birds were kept together in one social group as nonbreeders and subsequently were housed pairwise at different locations in Austria and Germany (see the Supplemental Experimental Procedures available online for details). At each current location, we played back calls from previously familiar ravens with whom the focal birds shared an affiliate or nonaffiliate relationship, and from unfamiliar ravens whom the birds had not encountered before (Figure 1). Affiliate relationships consisted of seven kin and eleven nonkin pairings. We predicted that birds would not only respond differently to the calls from familiar and unfamiliar conspecifics but also discriminate between familiar birds according to the relationship valence they had to those subjects up to three years prior as nonbreeders.
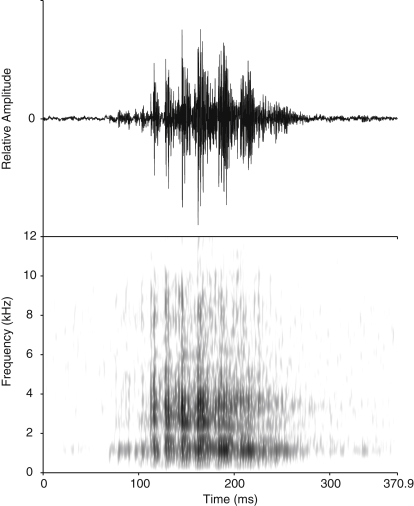
Figure 1.
Example Oscillogram and Spectrogram of a Stimulus Call
Amplitude modulation is best seen in the oscillogram; low frequency and noisiness are best seen in the spectrogram. (Spectrogram settings: fast Fourier transform with Gaussian window shape; window length 0.003 s; dynamic range 40 dB.) For details on stimulus presentation and experimental setup, see Table S3 and Figure S1.
Throughout the experiment, ravens emitted a total of 6,934 calls (mean = 630 per playback), of which 5,548 (mean = 504 per playback) were long-distance calls. The number of calls emitted by individuals when listening to the stimuli categories affiliate, nonaffiliate, or unknown differed significantly (generalized linear mixed model [GLMM]: F2,177 = 4.576, p = 0.012). Ravens called less often to unfamiliar individuals than to the two familiar ones (pairwise comparison: unfamiliar − nonaffiliate β = −3.765, SE = 1.615, df = 177, t = −2.332, p = 0.049; unfamiliar − affiliate β = −4.123, SE = 1.701, df = 177, t = −2.424, p = 0.049; affiliate − nonaffiliate β = 0.358, SE = 2.051, df = 177, t = 0.174, p = 0.862; estimated mean calls per minute: affiliate = 8.7, SE = 1.6; nonaffiliate = 8.3, SE = 1.5; unfamiliar = 4.6, SE = 0.8). A similar but even stronger effect was observed when we restricted our analysis to long-distance calls (GLMM: F2,177 = 5.805, p = 0.004; pairwise comparison: unfamiliar – nonaffiliate β = −4.509, SE = 1.640, df = 177, t = −2.750, p = 0.020; unfamiliar − affiliate β = −4.608, SE = 1.708, df = 177, t = −2.699, p = 0.020; affiliate − nonaffiliate β = 0.099, SE = 2.011, df = 177, t = 0.049, p = 0.961; estimated means for long-distance calls per minute: affiliate = 7.9, SE = 1.6; nonaffiliate = 7.8, SE = 1.5; unfamiliar = 3.3, SE = 0.9).
From the originally recorded response calls, we analyzed 1,294 calls without interfering background noises. From these remaining calls, we extracted six components from measured call parameters (Table 1). All six variables explained 66.5% of the overall variance of the data (for loadings of the parameters on each component, see Table 2). The final models including sex relation, kinship, and affiliation (Table S1) of components 1, 3, 4, 5, and 6 differed significantly between treatments. In the main text, we only present results for component 5 (for further details, see Table S2).
Table 1.
Explained Variance
| Component | Initial Eigenvalues |
Extraction Sums of Squared Loadings |
||||
|---|---|---|---|---|---|---|
| Total | % of Variance | Cumulative % | Total | % of Variance | Cumulative % | |
| 1 | 3.528 | 17.640 | 17.640 | 3.528 | 17.640 | 17.640 |
| 2 | 2.477 | 12.386 | 30.026 | 2.477 | 12.386 | 30.026 |
| 3 | 2.356 | 11.782 | 41.808 | 2.356 | 11.782 | 41.808 |
| 4 | 1.980 | 9.902 | 51.709 | 1.980 | 9.902 | 51.709 |
| 5 | 1.701 | 8.505 | 60.214 | 1.701 | 8.505 | 60.214 |
| 6 | 1.263 | 6.316 | 66.530 | 1.263 | 6.316 | 66.530 |
Six components with eigenvalues above 1.0 were extracted.
Table 2.
Component Matrix
| Component |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Call length | 0.275 | 0.220 | 0.161 | −0.124 | −0.580∗ | |
| Mean HNR | 0.369 | −0.376 | 0.560∗ | −0.440∗ | ||
| Minimum HNR | −0.212 | 0.345 | 0.642∗ | |||
| Maximum HNR | −0.247 | 0.115 | 0.286 | −0.270 | −0.418∗ | |
| Standard deviation of HNR | 0.222 | −0.483∗ | 0.173 | 0.435∗ | −0.223 | 0.293 |
| Formant 1 | −0.758∗ | 0.166 | ||||
| Formant 2 | −0.557∗ | −0.374 | 0.388 | 0.282 | −0.240 | |
| Formant 3 | −0.361 | −0.573∗ | 0.397 | 0.223 | 0.291 | |
| Formant 4 | −0.272 | −0.559∗ | 0.363 | 0.293 | 0.324 | |
| Formant 5 | 0.474 | 0.107 | −0.336 | 0.441∗ | 0.650∗ | −0.121 |
| Formant dispersal | 0.474 | 0.107 | −0.336 | 0.441∗ | 0.650∗ | −0.121 |
| Dominant frequency | −0.568∗ | 0.290 | −0.142 | 0.448∗ | −0.141 | |
| Dominant frequency of first third | −0.398 | 0.416∗ | −0.203 | 0.180 | ||
| Dominant frequency of second third | −0.490∗ | 0.298 | −0.126 | 0.348 | ||
| Dominant frequency of third third | −0.344 | 0.259 | −0.270 | 0.157 | ||
| Alpha 1000 | 0.527∗ | −0.220 | 0.200 | −0.509∗ | 0.176 | −0.140 |
| Alpha 2000 | 0.640∗ | −0.140 | −0.186 | 0.272 | −0.267 | 0.266 |
| First peak frequency of amplitude modulation | 0.279 | 0.474∗ | 0.669∗ | 0.122 | ||
| Second peak frequency of amplitude modulation | 0.261 | 0.510∗ | 0.725∗ | 0.176 | ||
| Third peak frequency of amplitude modulation | 0.223 | 0.464∗ | 0.661∗ | 0.188 | ||
Loadings of the original variables on the different components are shown. Loadings below ±0.1 are omitted, and loadings exceeding ±0.4 are marked with asterisks. HNR, harmonicity:noise ratio.
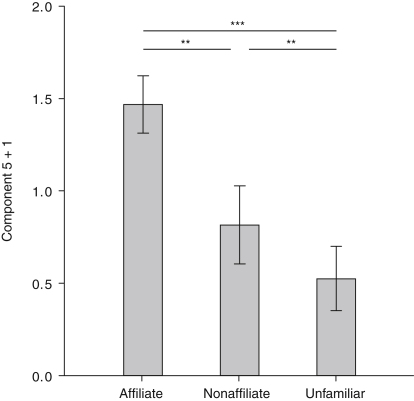
Component 5 revealed a highly significant difference between the three affiliation categories (GLMM: F2,1291 = 12.368, p ≤ 0.001) when corrected for the other two fixed factors, kinship and sex relation. Notably, pairwise comparison indicated a significant effect of relationship valence (affiliate – nonaffiliate: β = 0.651, SE = 0.235, df = 1291, t = 2.772, p = 0.011), along with the difference between familiar and unfamiliar birds (affiliate − unfamiliar: β = 0.942, SE = 0.212, df = 1291, t = 4.466, p ≤ 0.001; nonaffiliate − unfamiliar: β = 0.290, SE = 0.290, df = 1291, t = 0.116, p = 0.012). Interestingly, this component includes formant spacing and harmonicity (Table 2); it has the highest value in calls emitted in response to affiliates and the lowest value in calls emitted in response to unfamiliar individuals (Figure 2). Kinship (GLMM: F1,1288 = 7.992, p = 0.005) and sex combination (GLMM: F1,1288 = 5.509, p = 0.019) significantly influenced component 5. Pairwise comparisons revealed that ravens emit calls with lower component 5 values when calling back to kin affiliates (nonkin – kin: β = −0.194, SE = 0.241, df = 1288, t = −2.347, p = 0.019) and calls with lower component 5 values when calling back to same-sex combinations (same sex – different sex: β = −0.681, SE = −0.194, df = 1288, t = −2.827, p = 0.005).
Figure 2.
Differences between Playback Categories in Component 5 of Call Parameters
Analysis of call parameters shows that ravens react different to affiliate, nonaffiliate, and unfamiliar individuals. Error bars indicate the standard error of the estimates. ∗∗p ≤ 0.05; ∗∗∗p ≤ 0.001. See also Tables S1 and S2.
Discussion
Ravens separated for up to three years responded differently to playbacks of former group members according to the categorization into affiliated versus nonaffiliated and familiar versus unfamiliar individuals. This differentiation is expressed in call numbers and call modulation respectively and indicates that ravens possess long-term memory not only for categories based on familiarity (“former group members”) but also for the valence of their relationships to them (former affiliates or nonaffiliates). Distinctions between familiar and unfamiliar individuals are encoded mainly in the number of calls. The response to relationship valence is reflected in call modulation only (component 5).
Long-Term Memory
The ravens' ability to differentiate between familiar and unfamiliar individuals in our experiment exemplifies long-term memory at class-level recognition. These findings are in accordance with studies showing that songbirds remember pair partners [22] and territorial neighbors [3] as well as nesting and feeding sites for an extended time period [23, 24]. However, to our knowledge, this is the first study showing that nonhuman animals remember the relationship valence of former group members, that is, whether others were affiliates or nonaffiliates. Although the results were suggestive, our setup is not conclusive concerning memory for specific individuals [1]. It could be enough that the birds were memorizing former group members according to the categories “nice/affiliates” and “not nice/nonaffiliates.”
The time span tested for memory in our study is comparable to the time ranges known from social mammals (two years in sheep, four years in seals and cotton-top tamarins) [2, 4, 7]. So far, long-term memory in birds has been considered as remembering neighbors [3] or places from one breeding season to the next—roughly nine months [23, 24]—whereas long-term memory of social relationships during territorial defense, i.e., by wrens (Troglodytes troglodytes) [25] and nightingales (Luscinia megarhynchos) [26], has been considered as remembering individuals over a period of one night. In comparison to those results, our maximum separation of three years appears to be a rather long period; however, it represents only a fraction of a raven's maximum life span of 25–30 years in the wild [27]. Hence, the birds' ability to remember familiar individuals might well exceed the tested time span, particularly because the memory persists after the birds have made the transition from nonbreeder to breeder status, as was clearly the case in the current study. Tested individuals were aviary birds and thus lived under relatively stable conditions, which might have led to an enhanced memory for relationship valence. Nevertheless, recent field studies suggest that temporarily stable subgroups [20, 28] exist within nonbreeder flocks of ravens, providing opportunities for regular encounters. Although our findings are consistent with the idea that the high degree of fission-fusion dynamics found in raven nonbreeder flocks may have selected for enhanced memory capacities, further studies should test birds for longer time periods, for the ability to differentiate between a number of different individuals, and for a greater variety of relationship qualities.
An alternative explanation for our results might be that ravens were responding solely to subtle differences in call features of the playback stimuli. This is unlikely because our control birds, which were unfamiliar to all birds, were tested with the same acoustic stimuli as the nine birds that were housed together as nonbreeders; however, the control birds did not discriminate between stimulus categories. Calls used as stimuli possibly represent different dialects, and thus group members of the previous nonbreeder flock would differentiate only between same dialect versus different dialect, whereas control birds would not differentiate because all calls are from an unknown dialect. However, this alternative hypothesis for the differentiation between familiar and unfamiliar birds cannot explain why test subjects could discriminate within these supposed dialects, i.e., between affiliates and nonaffiliates.
A potential shortcoming of our setup could be seen in the fact that the affiliate category consisted of kin and nonkin, whereas the nonaffiliate category consisted of nonkin only. However, this distribution reflects the nature of many complex social systems, whereby kin share valuable relations of different kinds and degrees (e.g., [5, 29, 30]). From an evolutionary point of view, this makes sense because of the benefits of indirect inclusive fitness [31]. Finally, our birds could only be tested in pairs, raising the possibility that the individuals' responses during the test might have been influenced by the behavior of the pair partners. Note that we applied stimuli with similar relationship valence to both individuals of the pair (Table S3), so that the birds' responses could be enhanced but should not be inhibited or even annihilated by the pair partner.
Call Modulation
Interestingly, the ravens' ability to differentiate the relationship valence between familiar birds became evident only with a detailed acoustic analysis. Their response to a previously familiar and nonaffiliated individual, as compared to an affiliated one, was characterized by lowering the formant spacing and increasing the chaotic parts of the vocalization. This suggests that ravens exaggerated the impression of body size with former nonaffiliates but not with affiliates; with unfamiliar birds, the formant dispersion was the lowest, i.e., they increased their perceived size as compared to affiliates and exaggerated more than to nonaffiliates. It has been previously hypothesized that call structure correlates with social valence in birds and mammals [32], but to our knowledge, this is the first study showing how songbirds use formant dispersion to encode social valence.
In addition to responding to social valence, ravens adjusted call characteristics according to inter- and intrasex competition, as calls emitted in response to same-sex stimuli were lower in component 5. Similarly, when calling back to kin affiliates, ravens revealed lower values in component 5 than when calling back to nonkin affiliates. Ravens may profit from discriminating kin from nonkin during pair formation and territory defense; as nonbreeders, they seem to use this ability for sharing information [19] and providing social support during and after conflicts [17]. Note that about half of the affiliated stimuli played back were from nonkin, indicating that the ravens' differentiation according to relationship valence is not based on kinship relations only.
Numerous studies have demonstrated that animals can differentiate between various categories of vocalizations [6, 32, 33] and encode specific information in temporal and/or spectral call parameters [34, 35]. In our study, an important variable represented in component 5 is the relation of harmonic parts of the call to chaotic parts of the call. During enhanced excitement levels, ravens increase the chaotic parts of the call, as observed in mammalian vocalizations [36]. Notably, call parameters in component 5 may affect the acoustically perceived size of the calling individual [37]. The primary parameter determining formants is the length of the vocal tract [38]. Vocal tract length correlates in various mammal species with body size [37, 39]. The link between these components should enable calling individuals to manipulate the acoustically perceived size. Given that formant dispersion and vocal tract length seem to be negatively correlated in ravens (unpublished data), low loadings in component 5 represent lower formant spacing and thus appear to simulate longer vocal tract sizes. Our results reflect the acoustically perceived size exaggeration exhibited by red and fallow deer [37] and add a biologically meaningful interpretation to the findings of vocal tract resonances in avian vocal production [40, 41]. Even though ravens emitted calls differing in formant spacing as responses to playback stimuli, we have no evidence yet that the acoustic changes can be perceived. However, formant perception has already been demonstrated in some birds (e.g., Grus americana [42] and speech-producing parrots like Psittacus erithacus [43]) as well as in mammalian species (e.g., Cervus elaphus [44] and Macaca mulatta [45]).
Taken together, our findings demonstrate that ravens have an extensive memory of former group members and, via call modulation analysis, reveal that they differentiate between affiliates and nonaffiliates. Our study provides support for using natural communication and acoustic signals as a tool for addressing cognitive questions (e.g., [30, 46–48]). Applying this approach to bird species differing in degrees of social complexity seems promising and may expand our understanding of avian cognition and convergent evolution of sociocognitive skills.
Experimental Procedures
Subjects and Housing
We used a total of 16 adult ravens housed in male-female pairs in public zoos or by private keepers (see Supplemental Experimental Procedures). Nine of these birds were previously part of a nonbreeder group established in 2004 at the Konrad Lorenz Research Station for Behaviour and Cognition (KLF) in Grünau, Austria. After reaching sexual maturity, birds were allowed to form breeding pairs; pairs were then transported to their new enclosures and did not hear or see their former group members since (mean distance between enclosures 170 km, minimum 18 km, maximum 389 km). At the time of the experiment (October 2009–March 2010), they had been separated for an average of 24 months (minimum 8 months, maximum 36 months). We here considered them as previously “familiar” individuals. Importantly, records of the birds' social behavior were available for the entire period as nonbreeders (2004–2007). On the basis of these protocols on agonistic and affiliate interactions, the relationship valence among birds was calculated and the resulting components were labeled as value, compatibility, and security [16]. For the current study, we considered birds with high loadings in these components as affiliates and birds with low loadings as nonaffiliates, taking possible effects of kin, sex, and age into account (see Supplemental Experimental Procedures and Table S3 for details). Highest loadings were mostly found among siblings [16]. In addition to the nine familiar birds, we used seven individuals that had not been part of the nonbreeder group at KLF. These control birds had no experience with any of the other ravens and thus were considered as “unfamiliar” individuals.
The study complied with the current laws of the respective countries, received oversight by the KLF board, and was authorized by the central administration of Upper Austria due to its noninvasive character.
Stimulus Recording and Playback Presentation
For playbacks, we used a specific long-distance call “rab,” a short vocalization with low pitch, few harmonics, and amplitude modulation typically used in territorial defense (Figure 1). Stimuli were recorded shortly before the experiment, between March 2008 and October 2009, from ten individuals housed at seven different locations (see Supplemental Experimental Procedures and Table S3). Recordings of seven individuals were used more than once, depending on the relationship valence among test subjects.
Each raven pair was subjected to two playback sessions, featuring either male or female callers. We focused on behavioral responses of both male and female individuals to every playback. Sessions were conducted in the morning (between 9 and 11 a.m.) and in the afternoon (between 2 and 4 p.m.), respectively, with the order of males and females being counterbalanced across subjects. For those birds (n = 9) that had been part of the nonbreeder group at KLF, stimulus categories consisted of calls from a familiar raven with whom both birds of the pair previously shared an affiliate relationship (1) or nonaffiliate relationship (2) or from an unfamiliar raven whom they did not know at all (3). Control birds (n = 7) that had not been part of the nonbreeder group were presented with calls from the same individuals; however, to them, all three callers were unfamiliar. All playback stimuli used per session were roughly matched for the callers' age and broadcasted at naturally occurring sound pressure levels. The order of categories was counterbalanced across subjects (for details, see Supplemental Experimental Procedures and Figure S1). Stimuli were presented via speakers (Ion Block Rocker; frequency response 70 Hz–50 kHz ± 3 dB) connected to a MacBook Pro using QuickTime Player Pro (v7.6.9). All experiments were audio and video recorded (Marantz PMD660, Sennheiser K6/ME66, Sony Handycam DCR-HC23) by two human experimenters, each of whom focused on one member of the tested pair. This enabled us to identify all vocalizations that each bird made in response to the stimuli sets presented (when being the focal subject and when being the pair partner). Note that the responses to both sets of stimuli (own and partner) entered our statistical model.
Playback Analysis
We measured the acoustic features of these calls using custom-built programs in the PRAAT 5.2.10 DSP package [49] that automatically logged these variables in an output file. Call parameters measured were call length; dominant frequency; dominant frequency of the first, second, and third part of the call; mean, minimum, maximum, and standard deviation of the harmonicity:noise ratio; formant candidates 1–5; formant dispersal; alpha ratio 1000 and 2000; and first to third peak frequency of amplitude modulation. (For further details, see Supplemental Experimental Procedures.)
Statistical analysis was conducted using IBM SPSS for Mac 19. To reduce data dimensionality of call parameters, we calculated a principal component analysis using an unrotated correlation method and setting a minimum eigenvalue of 1.0 for components to be extracted. Differences between reactions to the different stimuli categories were calculated with type III GLMMs. To correct for differences between playback individuals, focal individuals, and presentation order, we included the nested term (focal(session(call))) and playback individual as a random factor. We used standard model selection procedures [50] for acoustic parameters. For post hoc tests, we used Student's t statistic with sequential Bonferroni correction for alpha because of repeated pairwise comparisons.
Acknowledgments
We thank Tecumseh Fitch, Kurt Kotrschal, and all coworkers at the Konrad Lorenz Research Station and the Department of Cognitive Biology for their support. Specifically, we thank Vera Brust, Orlaith Fraser, Aileen Hohnstein, Essi Kaartinen, Björn Schoas, Claudia Stephan, and Georgine Szipl for help with data collection; Orlaith Fraser for help with statistics; Christian Herbst for repeated support in improving our acoustic analysis; Björn Schoas for producing the sketched view of the setup; and Doris Preininger for her comments on the manuscript. Finally, we would like to thank the anonymous reviewers for their helpful comments, which contributed to the clarity of the paper. We are grateful to Alpenzoo Innsbruck, Cumberland Wildpark Grünau, Wildpark Altenfelden, Wildpark Goldau, Wildpark Haag, Wildpark Wels, Wildlife Enclosure in the National Park Center Lusen, Zoo Hellabrunn, Vogelpark Turnersee, Gerti Drack, Erich Hinterlehner, Martina and Michael Riess, and Mona and Kurt Trella for allowing our repeated visits, recording calls, and conducting playback experiments. This work was funded by the European Science Foundation (COCOR, EUROCORES framework TECT: I-105-G11) and the Austrian Science Fund (FWF, START: Y-366-B11).
Published online: April 19, 2012
Footnotes
Supplemental Information includes three tables, one figure, and Supplemental Experimental Procedures and can be found with this article online at doi:10.1016/j.cub.2012.03.023.
Supplemental Information
References
- 1.Tibbetts E.A., Dale J. Individual recognition: it is good to be different. Trends Ecol. Evol. (Amst.) 2007;22:529–537. doi: 10.1016/j.tree.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Insley S.J. Long-term vocal recognition in the northern fur seal. Nature. 2000;406:404–405. doi: 10.1038/35019064. [DOI] [PubMed] [Google Scholar]
- 3.Godard R. Long-term-memory of individual neighbors in a migratory songbird. Nature. 1991;350:228–229. [Google Scholar]
- 4.Kendrick K.M., da Costa A.P., Leigh A.E., Hinton M.R., Peirce J.W. Sheep don't forget a face. Nature. 2001;414:165–166. doi: 10.1038/35102669. [DOI] [PubMed] [Google Scholar]
- 5.McComb K., Moss C., Sayialel S., Baker L. Unusually extensive networks of vocal recognition in African elephants. Anim. Behav. 2000;59:1103–1109. doi: 10.1006/anbe.2000.1406. [DOI] [PubMed] [Google Scholar]
- 6.Wilson D.R., Mennill D.J. Black-capped chickadees, Poecile atricapillus, can use individually distinctive songs to discriminate among conspecifics. Anim. Behav. 2010;79:1267–1275. [Google Scholar]
- 7.Matthews S., Snowdon C.T. Long-term memory for calls of relatives in cotton-top tamarins (Saguinus oedipus) J. Comp. Psychol. 2011;125:366–369. doi: 10.1037/a0023149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunbar R.I.M. Neocortex size as a constraint on group size in primates. J. Hum. Evol. 1992;22:469–493. [Google Scholar]
- 9.Humphrey N.K. The social function of intellect. In: Bateson P.P.G., Hinde R.A., editors. Growing Points in Ethology. Cambridge University Press; Cambridge: 1976. pp. 303–317. [Google Scholar]
- 10.Jolly A. Lemur social behavior and primate intelligence. Science. 1966;153:501–506. doi: 10.1126/science.153.3735.501. [DOI] [PubMed] [Google Scholar]
- 11.Pitcher B.J., Harcourt R.G., Charrier I. The memory remains: long-term vocal recognition in Australian sea lions. Anim. Cogn. 2010;13:771–776. doi: 10.1007/s10071-010-0322-0. [DOI] [PubMed] [Google Scholar]
- 12.Mackin W.A. Neighbor–stranger discrimination in Audubon's shearwater (Puffinus l. lherminieri) explained by a “real enemy” effect. Behav. Ecol. Sociobiol. 2005;59:326–332. [Google Scholar]
- 13.Heinrich B. Winter foraging at carcasses by the three sympatric corvids, with emphasis on recruitment by the raven, Corvus corax. Behav. Ecol. Sociobiol. 1988;23:141–156. [Google Scholar]
- 14.Heinrich B., Marzluff J. Do common ravens yell because they want to attract others? Behav. Ecol. Sociobiol. 1991;28:13–21. [Google Scholar]
- 15.Bugnyar T., Kotrschal K. Observational learning and the raiding of food caches in ravens, Corvus corax: is it ‘tactical’ deception? Anim. Behav. 2002;64:185–195. [Google Scholar]
- 16.Fraser O.N., Bugnyar T. The quality of social relationships in ravens. Anim. Behav. 2010;79:927–933. doi: 10.1016/j.anbehav.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser O.N., Bugnyar T. Do ravens show consolation? Responses to distressed others. PLoS ONE. 2010;5:e10605. doi: 10.1371/journal.pone.0010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stöwe M., Bugnyar T., Loretto M.-C., Schloegl C., Range F., Kotrschal K. Novel object exploration in ravens (Corvus corax): effects of social relationships. Behav. Processes. 2006;73:68–75. doi: 10.1016/j.beproc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Schwab C., Bugnyar T., Schloegl C., Kotrschal K. Enhanced social learning between siblings in common ravens, Corvus corax. Anim. Behav. 2008;75:501–508. doi: 10.1016/j.anbehav.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber, B. (1988). Zur Sozialstruktur einer freilebenden Kolkrabenpopulation in der Schweiz. PhD thesis, Universität Bern, Bern, Switzerland.
- 21.Marzluff J., Heinrich B. Foraging by common ravens in the presence and absence of territory holders: an experimental analysis of social foraging. Anim. Behav. 1991;42:755–770. [Google Scholar]
- 22.Catchpole C.K., Slater P.J.B. Bird Song: Biological Themes and Variations. Second Edition. Cambridge University Press; Cambridge: 2008. Mate recognition. [Google Scholar]
- 23.Mettke-Hofmann C., Gwinner E. Long-term memory for a life on the move. Proc. Natl. Acad. Sci. USA. 2003;100:5863–5866. doi: 10.1073/pnas.1037505100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin T.E. Avian life history evolution in relation to nest sites, nest predation, and food. Ecol. Monogr. 1995;65:101–127. [Google Scholar]
- 25.Erne N., Amrhein V. Long-term influence of simulated territorial intrusions on dawn and dusk singing in the Winter Wren: spring versus autumn. J. Ornithol. 2008;149:479–486. [Google Scholar]
- 26.Schmidt R., Amrhein V., Kunc H.P., Naguib M. The day after: effects of vocal interactions on territory defence in nightingales. J. Anim. Ecol. 2007;76:168–173. doi: 10.1111/j.1365-2656.2006.01182.x. [DOI] [PubMed] [Google Scholar]
- 27.Ratcliffe D. Cambridge University Press; Cambridge: 1997. The Raven: A Natural History in Britain and Ireland. [Google Scholar]
- 28.Dall S.R.X., Wright J. Rich pickings near large communal roosts favor ‘gang’ foraging by juvenile common ravens, Corvus corax. PLoS ONE. 2009;4:e4530. doi: 10.1371/journal.pone.0004530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silk J.B., Beehner J.C., Bergman T.J., Crockford C., Engh A.L., Moscovice L.R., Wittig R.M., Seyfarth R.M., Cheney D.L. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proc. Biol. Sci. 2009;276:3099–3104. doi: 10.1098/rspb.2009.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheney D.L., Moscovice L.R., Heesen M., Mundry R., Seyfarth R.M. Contingent cooperation between wild female baboons. Proc. Natl. Acad. Sci. USA. 2010;107:9562–9566. doi: 10.1073/pnas.1001862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamilton W.D. The genetical evolution of social behaviour. I. J. Theor. Biol. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 32.Morton E.S. On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am. Nat. 1977;111:855–869. [Google Scholar]
- 33.Ryan M.J. Female mate choice in a neotropical frog. Science. 1980;209:523–525. doi: 10.1126/science.209.4455.523. [DOI] [PubMed] [Google Scholar]
- 34.Slocombe K.E., Zuberbühler K. Chimpanzees modify recruitment screams as a function of audience composition. Proc. Natl. Acad. Sci. USA. 2007;104:17228–17233. doi: 10.1073/pnas.0706741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer J., Hammerschmidt K., Cheney D.L., Seyfarth R.M. Acoustic features of male baboon loud calls: influences of context, age, and individuality. J. Acoust. Soc. Am. 2002;111:1465–1474. doi: 10.1121/1.1433807. [DOI] [PubMed] [Google Scholar]
- 36.Fitch W.T., Neubauer J., Herzel H. Calls out of chaos: the adaptive significance of nonlinear phenomena in mammalian vocal production. Anim. Behav. 2002;63:407–418. [Google Scholar]
- 37.Fitch W.T., Reby D. The descended larynx is not uniquely human. Proc. Biol. Sci. 2001;268:1669–1675. doi: 10.1098/rspb.2001.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fant G. Revised Edition (1970) Mouton & Co. N.V.; The Hague, the Netherlands: 1960. Acoustic Theory of Speech Production. [Google Scholar]
- 39.Liebermann P. Harvard University Press; Cambridge, MA: 1984. The Biology and Evolution of Language. [Google Scholar]
- 40.Nowicki S. Vocal tract resonances in oscine bird sound production: evidence from birdsongs in a helium atmosphere. Nature. 1987;325:53–55. doi: 10.1038/325053a0. [DOI] [PubMed] [Google Scholar]
- 41.Beckers G.J., Nelson B.S., Suthers R.A. Vocal-tract filtering by lingual articulation in a parrot. Curr. Biol. 2004;14:1592–1597. doi: 10.1016/j.cub.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 42.Fitch W.T., Kelley J. Perception of vocal tract resonances by whooping cranes Grus americana. Ethology. 2000;106:559–574. [Google Scholar]
- 43.Patterson D.K., Pepperberg I.M. A comparative study of human and parrot phonation: acoustic and articulatory correlates of vowels. J. Acoust. Soc. Am. 1994;96:634–648. doi: 10.1121/1.410303. [DOI] [PubMed] [Google Scholar]
- 44.Charlton B.D., Reby D., McComb K. Female red deer prefer the roars of larger males. Biol. Lett. 2007;3:382–385. doi: 10.1098/rsbl.2007.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitch W.T., Fritz J.B. Rhesus macaques spontaneously perceive formants in conspecific vocalizations. J. Acoust. Soc. Am. 2006;120:2132–2141. doi: 10.1121/1.2258499. [DOI] [PubMed] [Google Scholar]
- 46.Townsend S.W., Zöttl M., Manser M.B. All clear? Meerkats attend to contextual information in close calls to coordinate vigilance. Behav. Ecol. Sociobiol. 2011;65:1927–1934. [Google Scholar]
- 47.Pepperberg I.M. Harvard University Press; Cambridge, MA: 2002. The Alex Studies: Cognitive and Communicative Abilities of Grey Parrots. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balsby T.J., Bradbury J.W. Vocal matching by orange-fronted conures (Aratinga canicularis) Behav. Processes. 2009;82:133–139. doi: 10.1016/j.beproc.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Boersma, P., and Weenik, D. (2011). PRAAT: doing phonetics by computer, version 5.2.10 (computer program). http://www.praat.org.
- 50.Burnham K.P., Anderson D.R. Springer-Verlag; New York: 2010. Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.