Abstract
Thousands of patients suffer from burn injuries each year, yet few therapies have been developed to accelerate the wound healing process. Most fibroblast growth factors (FGFs) have been extensively evaluated, but only a few have been found to participate in wound healing. In particular, FGF-10 is robustly increased in the wound microenvironment following injury and has demonstrated some ability to promote wound healing in vitro and in vivo. Glycosaminoglycans (GAGs) are linear carbohydrates that participate in wound repair by influencing cytokine/growth factor localization and interaction with cognate receptors. Dermatan sulfate (DS) is the most abundant GAG in human wound fluid and has been postulated to be directly involved in the healing process. Recently, the combination of FGF-10 and DS demonstrated the potential to accelerate wound healing via increased keratinocyte proliferation and migration. Based on these preliminary studies, DS may serve as a cofactor for FGF-10, and together, they are likely to expedite the healing process by stimulating keratinocyte activity. As a specific subtype of wounds, the overall healing process of burn injuries does not significantly differ from other types of wounds, where optimal repair results in matrix regeneration and complete re-epithelialization. At present, standard burn treatment primarily involves topical application of anti-microbial agents, while no routine therapies target acceleration of re-epithelialization, the key to wound closure. Thus, this novel therapeutic combination could be used in conjunction with some of the current therapies, but it would have the unique ability to initiate wound healing by stimulating keratinocyte epithelialization.
Keywords: Burns, wound healing, FGF-10, dermatan sulfate
Introduction
For the 45,000 burn patients admitted to the hospital each year (1), the average length of stay is more than eight days and costs more than $60,000 (2). Although the average burn patient is a young, previously healthy male (2), many older burn patients suffer from comorbid conditions, such as diabetes and/or vascular disease, prior to burn injury. These patients are consequently at a higher risk for developing chronic, non-healing wounds. While significant progress in topical antimicrobial agents and local wound care has substantially reduced the morbidity and mortality from burn wound sepsis (3), no routine therapies currently target keratinocytes and/or stimulating re-epithelialization, which could potentially accelerate wound closure.
Overview of FGF Structure and Function
One important area of interest in wound healing includes the normal biologic role and potential therapeutic application of fibroblast growth factors (FGFs). FGFs serve in numerous capacities, including regulation of cell proliferation, cell migration, cell differentiation, development, response to injury, wound healing, and tumorigenesis (4). FGFs are differentially expressed in many tissues with significant variability in the pattern and timing of expression, which has been shown to be important during development (4). In humans, there are 22 known FGFs, ranging in size from 17 to 34 kDa and sharing 13–71% amino acid identity (5). The prototypical FGF gene consists of three exons, and their protein structures are characteristically composed of multiple beta strands with a common core containing 28 highly conserved amino acid residues and six identical amino acid residues (5, 6).
Furthermore, ten of these highly conserved residues interact with one of the six known FGF receptors (FGFRs) (5), which allow for FGF signaling. In general, the receptor binding sites are believed to be distinct from the ligand binding sites (5). The typical structure of these tyrosine kinase receptors consists of an extracellular ligand-binding domain, a single transmembrane domain, and a cytoplasmic domain (7). The extracellular ligand-binding domain contains three immunoglobulin-like domains, designated D1-3. D2 is positively charged and contains a highly conserved region that serves as a binding site for ligands, such as glycosaminoglycans (7). Alternative splicing of FGFR mRNA is regulated in a tissue-specific manner, dramatically affecting ligand-receptor binding specificity. The variable region in the D3 segment of FGFR1-3 permits receptor-ligand binding and infers unique characteristics. For example, FGFR2-IIIb (also known as keratinocyte growth factor receptor, KGFR) is exclusively expressed in epithelial cells (8). It is activated during wound repair upon binding of FGF-7 or FGF-10, thus promoting receptor dimerization and inducing multiple transduction pathways, including the mitogen-activated protein kinase (MAPK) pathway (9). Expression of a dominant-negative form of FGFR2-IIIb results in significant wound healing delays from reduced keratinocyte proliferation and subsequently delayed re-epithelialization (10). A recent study showed that FGFR1-IIIb and FGFR2-IIIb are essential for the maintenance of skin appendages and epidermal barrier function, and they appear to cooperate in regulating epidermal homeostasis (11). Therefore, FGF signaling diversity is partially due to the different splice variants and gene products for FGFR.
FGFs are grouped into subfamilies based upon similarities and differences in their structural and functional characteristics. For example, FGF-7, -10, and -22 share similar genetics and functions, and all three FGFs strongly activate FGFR2-IIIb (8, 12). During homeostasis, FGF-7, -10, and -22 are significantly expressed in non-injured mouse skin. However, FGF-7 and FGF-10 protein levels robustly increase following wounding (13). In addition, the abundance of FGF-binding protein is amplified during wound healing and binds FGF-7, -10, and -22 to enhance ligand activity at low concentrations (14). FGF-22 shares ~40–45% amino acid homology with FGF-7 and FGF-10, and it has been shown to be preferentially expressed in keratinocytes (15), where it functions in an autocrine manner. FGF-7, also known as keratinocyte growth factor (KGF), is a paracrine growth factor for epithelial cells and is expressed mainly by fibroblasts (16). Both FGF-7 and FGF-10 appear to enhance cell migration via kinase activation to promote cortical cytoskeleton reorganization (17). Although FGF-7 has been shown to accelerate wound repair in several animal models, it showed only a modest improvement in re-epithelialization in a porcine deep partial-thickness burn model and showed no significant improvement after a full-thickness burn (18). Interestingly, aged mice have shown not only a decreased basal expression of FGF-7 and FGF-10, but they also demonstrated a blunted response after wounding (13), suggesting a possible contributory factor in the delayed healing and mortality observed in elderly burn patients. In parallel, elderly burn patients (≥ 65 years of age) tend to suffer from more severe burns at the time of admission, exhibiting a greater proportion of deep/superficial burns (41% vs. 23.3%) in a Chilean subpopulation (19). This resulted in a 12-fold higher mortality rate when adjusted for the %TBSA (total body surface area) burn and the TBSA/DTBSA (deep TBSA) proportion (19).
FGG-10 and its Role in Wound Healing
Under normal conditions, keratinocyte progenitor cells divide and proliferate to allow for the multi-layered epidermis. This intricate process of epidermal regeneration is dependent upon intracellular signaling cascades via keratinocyte-receptor interactions with the skin micro-environment through both autocrine and paracrine pathways. During the healing process, cross-talk between healthy keratinocytes on the periphery and other cells involved in wound repair is critical for optimal wound closure. Basal keratinocytes along the wound edges and dermal appendages, such as sweat glands, hair follicles, and sebaceous glands, are the primary cells responsible for the epithelialization phase of wound repair. Keratinocyte migration and proliferation is stimulated by cytokines and growth factors, such as FGF-10, produced during the inflammatory phase of wound healing. Injury also triggers an inflammatory keratinocyte response that results in secretion of cytokines, chemokines, and antimicrobial peptides required for immune defense against invading microbes. Thus, keratinocytes are considered active participants in both regeneration, where FGF-10 likely plays a key role, and in epidermal immune defense. [Reviewed in (20–22)]
FGF-10 is referred to as keratinocyte growth factor 2 (KGF-2), acts as a paracrine mediator of epithelial cell proliferation, and is expressed primarily by fibroblasts (22, 23). The human FGF-10 gene is located on 5p13-p12 and encodes a protein of 208 amino acids weighing ~19 kDa (23). Mouse FGF-10 mRNA was originally found to be expressed most abundantly in lung, skin, brain, and heart tissue (16). Notably, FGF-10 demonstrates significant thermal liability and is stabilized by a variety of polyanions, possibly affecting its functional capacity when not present (24, 25); thus, a small molecule, such as dermatan sulfate, may serve as an FGF-10 stabilizer and improve its functionality when readily available.
Comparable to FGFR2-IIIb-deficient mice, FGF-10-deficient mice die shortly after birth, demonstrate abnormal lung and limb development (26, 27), and exhibit defects in whisker formation and cutaneous epidermal differentiation resulting in hypoplasia (28, 29). More recently, FGF-10 was shown to have a stimulatory effect on hair growth in vitro, using isolated human hair follicles (30). Initial in vitro wound healing studies demonstrated that FGF-10 promotes both proliferation and differentiation of human keratinocytes, suggesting a likely role in epidermal growth and differentiation (31, 32). In addition, insertion of cells expressing wnt-2b/FGF-10 concurrently with wounding led to re-induction of the apical ectodermal ridge, suggesting new possibilities for limb regeneration in higher vertebrates (33). Using a rabbit model of corneal CO2 laser injury, topical FGF-10 was shown to accelerate corneal epithelial wound healing and to reduce inflammation, stromal edema, and fibrosis (34). A similar benefit was seen after applying topical FGF-10 to rabbit alkali burned cornea, noting a significantly enhanced rate of corneal epithelial wound healing (35).
With regard to cutaneous wound healing, the regulation of FGF-10 expression following injury has been controversial. Beer et al. concluded that FGF-10 mRNA expression was not induced during cutaneous wound repair using a murine excisional wound model, but noted that its protein form may be stored and released upon injury (16). However, Tagashira et al. demonstrated that FGF-10 mRNA was upregulated one day after injury, but normalized by day 3 in a mouse wounding model (36). Nevertheless, further studies have demonstrated that topical application of FGF-10 induces wound repair. A single topical application of FGF-10 to full-thickness incisional wounds in rats augmented and accelerated wound healing, characterized by an increase in the wounds’ mechanical strength and an increase in wound collagen content (37). Similarly, topical FGF-10 promoted re-epithelialization and enhanced granulation tissue formation in an ischemia-impaired rabbit ear model without a significant difference in scar formation (38). In order to further characterize the impact of FGF-10 on wound repair, one study employed three distinct animal models (39). These experimental models included human meshed skin grafts explanted to athymic “nude” rats, surgical incisions in rats, and contaminated excisional rat wounds. Using these models, FGF-10 accelerated epithelialization in the explanted meshed skin grafts and increased the gain in breaking strength of the surgical incisions (although no improvement in the contaminated excisional wounds was demonstrated). Based on these results, FGF-10 was suggested as a potential wound healing agent for stimulating epithelialization in venous stasis ulcers, partial-thickness burn wounds, skin graft donor sites, or other various wounds (39).
FGF-10 and its use in Clinical Trials
In 2001, preliminary clinical trials using a recombinant form of FGF-10 topically applied to venous ulcers in humans demonstrated an ability to expedite wound healing (40). However, this effect was no longer significant in follow-up studies, and the clinical trials were aborted (41). Although initial research also showed promise in the treatment of cancer therapy-induced mucositis, follow-up studies were again disappointing, and the trials were discontinued (42). Similarly, FGF-10 appeared to promote healing of small intestinal ulcerations in rats (43) and reduced mortality, weight loss, and stool score in a murine colitis model (44). Nevertheless, a randomized, prospective clinical trial evaluating its use in the treatment of active ulcerative colitis demonstrated no benefit, although it was well tolerated (45). Other preliminary studies have shown that FGF-10 accelerated healing of meshed skin grafts used to close deep burn wounds (46). Therefore, FGF-10 alone has demonstrated significant promise in its potential ability to promote re-epithelialization and wound healing in multiple models of tissue injury.
Overview of GAG Structure and Function
Glycosaminoglycans (GAGs) play a critical role in FGF signaling by facilitating the formation of the FGF-FGFR complex and enhancing FGF oligomerization (47). FGFs have a high affinity for GAGs, and as such, their interaction stabilizes FGFs against thermal denaturation and proteolysis (4). In addition, GAGs limit the ability of FGFs to diffuse and be released into interstitial spaces, thus generating a reservoir of growth factors for the surrounding environment (4, 5). Therefore, most FGFs require GAGs for binding and signaling through their FGF receptor to enhance stabilization and/or accumulation of growth factors (4, 48–51).
GAGs, the most abundant heteropolysaccharides in mammalian tissues, are composed of long, unbranched polysaccharides containing repeating disaccharide units and vary in molecular weight from 10–100 kDa on average (52). Their synthesis involves several enzymes, which ultimately determine the identity of the GAG being created (53). Subsequent epimerization, sulfation, or deacetylation modifications distinguish the specific GAG chains necessary for their particular activity. GAG classification includes heparin, heparan sulfate, chondroitin sulfate, dermatan sulfate, keratan sulfate, and hyaluronan. Most GAGs are linked to protein cores to form proteoglycans and include several sulfation sites, with the exception of hyaluronan. Typically, they are highly negatively charged at a physiologic pH and located on the cell surface or within the extracellular matrix (ECM) (52). Like FGFs, GAG functions vary widely and contribute to a variety of cellular processes, including development, homeostasis, wound repair, and disease. For example, some microorganisms such as herpes simplex virus and malaria recognize GAGs as cell surface receptors and exploit them to promote disease pathogenesis (54). They serve diverse roles and modulate biologic responses by acting as (1) stabilizers, cofactors, and/or coreceptors; (2) regulators of enzyme activity; (3) signaling molecules in response to cellular damage; and (4) targets for microbial virulence factors (55).
Hyaluronan is a large polymer (molecular weight ranging from 4–8,000 kDa) with shock absorbing and lubricating capacities, and it is typically found in synovial fluid, vitreous humor, and the ECM of loose connective tissue (52). Chondroitin sulfate is the most abundant GAG in mammalian tissues residing in cartilage, tendon, ligament, bone, and heart valves, where it binds large molecules to form proteoglycan aggregates. Considered the most heterogeneous GAG, keratan sulfate is expressed in cornea, bone, and cartilage, and frequently aggregates with chondroitin sulfates (52). Heparan sulfate is expressed primarily on cell surfaces and in the ECM as a part of proteoglycan complexes. Heparin, however, accumulates in granules of mast cells and is actually more sulfated than heparan sulfate (52). Both are well known for their ability to sustain FGF-2 signaling (56), while heparin is also commonly known for its ability to activate anti-thrombin II, which inhibits the serine proteases in the coagulation cascade (57). In addition, heparin participates in and stabilizes the interaction of specific FGF molecules and their FGF receptors (5, 52).
DS and its Role in Tissue Repair
Dermatan sulfate (DS), also known as chondroitin sulfate-B, is expressed in numerous mammalian tissues and is typically found on the cell surface or in the ECM. Structurally, DS is a linear polysaccharide composed of disaccharide units containing hexosamine, N-acetyl galactosamine, or glucuronic acid. The presence of iduronic acid, however, distinguishes DS from other forms of chondroitin sulfate and presumably plays a key role in the binding site specificity for GAG-binding proteins (55). DS also contains variable sulfation patterns on specific iduronic acid residues, which are critical for cell proliferation (9, 58). Altogether, the variability in the DS chain length, disaccharide composition, and iduronic acid residues likely influences its binding affinity and functional interactions (55, 57, 59). Moreover, some of its protein cores have demonstrated altered expression patterns during development, pathogenesis, and wounding (60–63). DS has been specifically shown to interact with numerous molecules important in several aspects of wound repair, including thrombin, activated protein C, collagen, fibronectin, α-defensin, interferon-γ, and transforming growth factor-β (54, 55). In addition to these distinct interactions, DS can participate in nonspecific binding with several plasma proteins, which likely influences its functional capacity (64).
Proteoglycans that contain DS as a major GAG include decorin, biglycan, versican, thrombomodulin, epiphycan, and endocan (55). DS and its related proteoglycans have been implicated in cardiovascular disease, tumorigenesis, infection, wound repair, and fibrosis (55, 65). For example, CS/DS can stimulate cancer progression and tissue regeneration in repair of the central nervous system and liver (54). In the skin, DS is the predominant glycan and is released at high concentrations during wound repair to serve as a cofactor for growth factors important in the proliferative phase of wound healing (59, 66). A shift in syndecan-1 GAG chains from predominantly HS to a mixture of DS/HS is observed during murine wound repair (67), which likely supports the ability of DS to potentiate keratinocyte proliferation via FGFs after injury to a greater extent than heparin-GAG moieties (9). These data suggest a pivotal role for DS in stimulating the wound repair process, particularly during the proliferative phase.
In mouse skin, decorin deficiency causes abnormal collagen morphology, increased skin fragility, and reduced tensile strength of the skin (68). In vitro, fibroblast cultures from post-burn hypertrophic scars were found to synthesize less decorin than normal dermal fibroblasts, which may have implications for the development of such scars (69). In more recent studies, DS blocked P-selectin activity, which may indicate a potential therapeutic target for DS to minimize thrombosis, inflammation, and metastasis (70). DS has also been shown to increase intercellular adhesion molecule-1 (ICAM-1) expression and nuclear factor-κb activity, suggesting a potential role in cell signaling during injury response (71). With regard to autoimmune disease, DS displays preferential affinity for apoptotic and dead cells. It has been suggested that molecules with an affinity for DS have a high propensity to become autoantigens, thus causing autoreactive B-1a cells to be positively selected and expanded by DS-autoantigen complexes (72, 73).
DS and its use in Wound Treatments and Clinical Trials
Using a murine induced-colitis model, a subcutaneous injection of DS limited colon inflammation by reducing inflammatory cytokine production, attenuating lymphocyte and macrophage recruitment, and reducing collagen-mediated fibrosis (74). Similar effects were noted using a murine unilateral ureteral obstruction model following 14 days of DS injections (75). Subsequent to arterial injury in mice, treatment with DS and bone marrow mononuclear cells inhibited the initial thrombotic and inflammatory processes, where DS is hypothesized to assist in the recovery of injured endothelium (76). Recent grafting of DS onto polyethylene terephtalate (i.e. Dacron ®), the main polymer component of vascular prostheses, demonstrated improved endothelial cell proliferation in vitro and enhanced biointegration following subcutaneous implantation in vivo (77).
DS has been shown to inactivate thrombin via heparin cofactor II (47), and it has been investigated for its antithrombotic properties (78, 79). Clinical studies have explored the utility of DS for patients undergoing dialysis for renal failure, with disseminated intravascular coagulation (DIC) syndrome, and/or with arterial atherothrombotic disease (80). These studies have revealed pharmacologic properties of DS with possible clinical relevance: a predictable dose-response based on its dose-proportional and linear, concentration-dependent pharmacokinetics (81), and overall clinical safety when used for venous thromboembolism prevention (78). To date, no studies have demonstrated bleeding complications associated with DS therapy, which makes DS a promising candidate for wound healing applications and in patients with blood clotting disorders (80).
Overview of Wound Healing and Current Therapeutic Targets
As a subtype of acute wounds, it is important to recognize that the overall healing process for most burn injuries does not significantly differ from other types of wounds. The inflammatory phase initiates wound healing, which is characterized by fibrin clot formation, platelet degranulation, infiltration of inflammatory cells, phagocytosis, and the robust release of growth factors and cytokines. Stimulated by factors produced during the inflammatory response, the proliferative phase involves wound closure, angiogenesis, and matrix deposition. The final remodeling phase encompasses the cross-linking of collagen, and the production of degradative enzymes and additional matrix proteins, which together contribute to further maturation of the injured tissue [reviewed in (20, 22, 82–87)]. With this fundamental basis, acute burns ultimately close by re-epithelialization, wound contraction, skin grafting, or a combination of these processes. A skin autograft is preferred for deeper burn wound closure, although it may also be achieved using biosynthetic dressings or skin/dermal substitutes (3, 88). In addition, donor autograft sites heal similar to a shallow burn, but can also result in a prolonged hospital stay if healing is delayed (86).
According to a rolling review by the American Burn Association, seven of the top ten clinically relevant complications among burn patients over the past decade were infectious (2). Consequently, most current therapies serve as anti-microbials targeting the most common infectious agents found in burn wounds: staphylococci, streptococci, and pseudomonas (89, 90). While these topical anti-microbial agents have significantly reduced the morbidity and mortality from burn wound sepsis (3), they may also prolong the healing time (3) and likely promote the development of multi-drug resistant bacteria. Furthermore, they do not address the process of burn wound repair itself. Several studies have been done using hyperbaric oxygen as a potential therapy to promote burn wound healing, but most reviews conclude that there is insufficient evidence to support or refute its effectiveness (91). Although a recent review suggested that cellular therapies using keratinocyte cultures are continuing to advance, several obstacles remain and prevent current practicality (92). Successful therapies, however, need to prevent infection and promote keratinocyte migration and proliferation early in the wound repair process. Few therapies today effectively target wound repair acceleration, but rather focus on preventing delays in wound healing from local infections or other systemic complications. Appropriate wound care currently requires multiple treatment modalities with variable timing to yield optimal repair (88). Therefore, the combination of FGF-10 and DS may serve as a novel adjunct by promoting keratinocyte proliferation and migration, and thus expediting re-epithelialization and wound healing.
The Potential of FGF-10 and DS in Wound Healing
Burn injuries constitute a subset of wounds that often heal by secondary intention, but may require more invasive treatment as well. Furthermore, they may occur in compromised individuals harboring local or systemic underlying conditions that hinder normal wound repair, such as diabetes and/or vascular disease. These comorbidities predispose them to the development of chronic, non-healing wounds which fail to respond to established medical and surgical therapies (93). In addition to burn wounds, ulcers from vascular disease, traumatic wounds, surgical wounds, pressure ulcers, and diabetic ulcers also frequently heal by secondary intention and represent a significant number of wounds treated on a daily basis. Specifically, more than 500,000 pressure ulcers were documented during hospitalizations in 2006, often resulting in longer hospital stays and higher mortality rates (94). Among the nearly 24 million diabetics in the United States (95), the estimated lifetime risk of developing a foot ulcer is 15% (96) with the average episode lasting 87 days and costing an average of $13,179 per episode (97). Unfortunately, secondary complications result in amputation for at least 15% of diabetics with foot ulcers (98). Although the incidence of such conditions would not be altered, accelerating the wound healing process of chronic wounds would likely decrease the subsequent development of their associated secondary complications, while also expediting the patient’s recovery and return to baseline functional status.
Similar to burn wounds, chronic wounds are often complicated and are steadily becoming more prevalent. In the industrialized world, an estimated 1% of the population is anticipated to battle a chronic wound during their lifetime (99). Chronic, non-healing wounds are believed to be a result of quiescent or damaged cells induced by wounding and inflammation. Keratinocytes secrete several growth factors, proteases, and basement membrane proteins necessary for the proliferative phase of wound healing to proceed (22). Thus, any defect in keratinocyte migration or proliferation may have detrimental effects on subsequent cellular responses during this phase (22). Furthermore, delayed epithelialization increases the risk for secondary infection and impairs the proliferative capacity of endothelial cells and fibroblasts responsible for angiogenesis and regeneration, respectively. Burn wounds specifically tend to have extensive areas of necrosis, increased inflammation, variable levels of inflammatory mediators, and increased levels of endogenous proteases, leading to a slower healing process (18). For current therapies to be successful, treatment needs to enhance re-epithelialization of the wound by further stimulating the regenerative capacity of keratinocytes.
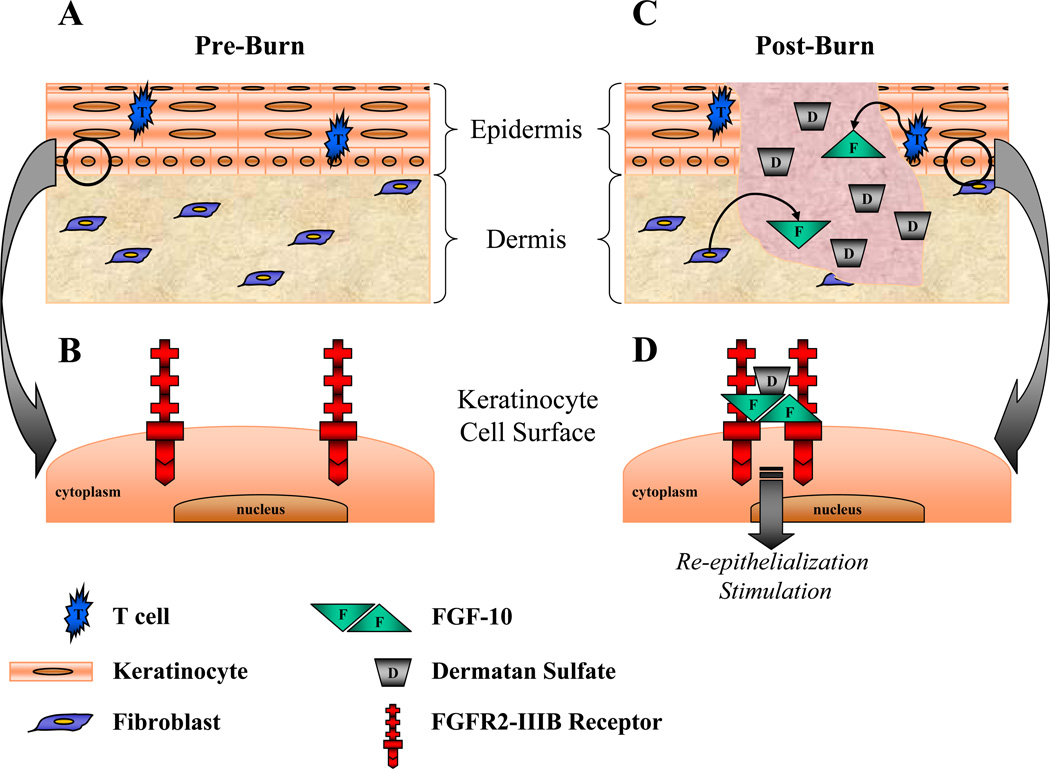
FGF-10 has been shown to promote keratinocyte migration and proliferation in preliminary studies (9), but no clinical trials to date have confirmed significant findings in vivo. Similar to the role heparin plays in stabilizing FGF-FGFR interactions, perhaps the addition of DS could enhance the ability of FGF-10 to further potentiate keratinocyte migration and proliferation, and consequently re-epithelialization. More specifically, it is known that resident T cells within the skin’s epidermis provide a robust supply of FGF-10, while mice lacking dendritic epidermal T cells display delayed keratinocyte proliferation and re-epithelialization, which correlated with decreased epidermal FGF-10 expression (100). Investigative studies have indicated that DS acts as a cofactor for FGF-10 to augment the ability of keratinocytes to migrate, which highlights the importance of DS as a cofactor for maximal cellular responsiveness to FGF-10 during wound repair (9). As proposed by Radek et al., DS fragments generated subsequent to wounding may act in a paracrine manner to facilitate, localize, and/or stabilize the FGF-10–FGFR2-IIIb interaction, resulting in receptor dimerization and subsequent signal transduction to promote burn wound re-epithelialization [as illustrated in Figure 1, modified from (9)]. Interestingly, desulfated DS preparations, even when combined with FGF-10, were no longer able to stimulate keratinocyte migration or proliferation in vitro, which suggests that a defect in DS sulfation in burn and/or chronic wounds may contribute to delayed or defective wound repair (9). Further studies are needed to asses the contribution of specific DS fragments as potential facilitators of the FGF-10–FGFR2-IIIb interaction in the wound, and to determine if this delicate balance is disturbed in abnormal wound repair. Collectively, these studies suggest that DS serves as a cofactor for FGF-10 in wound repair, and together, they have the potential to serve as a novel therapeutic agent in the treatment of burn injuries to stimulate re-epithelialization of the wound bed earlier in the process to minimize infection and further potentiate the proliferative phase of tissue repair.
FIGURE 1.
Theoretical model for the role of DS in FGF-10 signaling through FGFR2-IIIb in burn wound re-epithelialization [modified from Radek et al., (9)]. (A) In non-burn-injured skin, T cells and fibroblasts reside in the epidermis and dermis, respectively. (B) In keratinocytes, the FGF receptor, FGFR2-IIIb, exists as a monomer on the cell surface. (C) Following burn injury, T cells and fibroblasts secrete FGF-10 into the wound, while small fragments of DS are released into the wound from the extracellular matrix. (D) Enabled by DS, the increase in FGF-10 acts in a paracrine manner through FGFR2-IIIb on keratinocytes. DS promotes receptor dimerization by facilitating the interaction between FGF-10 and FGFR2-IIIb. This interaction ultimately results in downstream signaling that stimulates burn would re-epithelialization by keratinocytes. DS, dermatan sulfate; FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor.
Although many burn wounds heal without complications, most types of wounds could benefit from a therapy that would expedite the healing process. More specifically, the proposed combination of FGF-10 and DS could potentially eliminate the need for surgical intervention for some burn patients. Such a novel therapy may also have a significant advantage over other treatments, as most other therapies indirectly promote wound healing by targeting host microbial defense or inflammatory cascades to either enhance or suppress the body’s immune response, respectively. FGF-10 and DS could likely be used in combination with current therapies, but serve as a novel adjunct by promoting epithelialization and thus, wound healing directly. Furthermore, its therapeutic implications could be applied to numerous acute and chronic wounds currently debilitating thousands of Americans.
TABLE 1.
Selected articles highlighting significant findings related to FGF-10 and DS. GAG, glycosaminoglycan; DS, dermatan sulfate; FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; DSS, dextran sulphate sodium; ICAM, intercellular adhesion molecule; IdoA, iduronic acid.
| Author(Ref#) | Experimental Model |
Animal/Tissue/ Cells |
Treatment | Pertinent Conclusions (based on each Author's interpretation) |
|---|---|---|---|---|
| Beer (16) | Full-thickness excisional wounds |
Balb/c 3T3 mice | FGF-10 | No significant induction of FGF-10 mRNA expression was detected during wound healing and levels possibly declined after skin injury. |
| Han (43) | Intestinal ulcers | Rats | FGF-10 | Intravenous FGF-10 significantly decreased acute intestinal injury and chronic ulceration. |
| Jang (30) | Cell culture | Human hair- follicles |
FGF-10 | FGF-10 significantly stimulated human hair-follicle cell proliferation and may be a promising therapeutic agent for stimulating human hair growth. |
| Jimenez (37) | Full-thickness incisional wounding |
Sprague Dawley rats |
FGF-10 | A single topical application of FGF-10 at the time of wounding markedly accelerated healing, as suggested by an increased mechanical strength and increased wound collagen content. |
| Komi-Kuramochi (13) |
Wounding | Hairless mice | FGF-10 | FGF-10 mRNA is strongly expressed in healthy mice skin and expression is increased after wounding. Aged mice have a decreased basal expression and blunted response after wounding. |
| Liu (35) | Corneal wound (alkali burn) |
New Zealand white rabbits |
FGF-10 | Topical FGF-10 enhanced corneal epithelial healing in rabbit alkali burned cornea. |
| Marchese (31) | Primary cell culture | Human keratinocytes |
FGF-10 | FGF-10 is a potent mitogen for human keratinocytes and promotes the expression of both early and late differentiation markers |
| Miceli (44) | DSS-induced colitis | Mice | FGF-10 | FGF-10 enhanced weight recovery after discontinuation of DSS treatments. It also reduced mortality and stool scores, suggesting possible usefulness in treating inflammatory bowel disease. |
| Robson (40) | Chronic venous ulcers |
Humans (clinical trial) |
FGF-10 | Topical FGF-10 appeared to accelerate wound closure in chronic venous ulcers, and it was particularly effective in smaller wounds and wounds with shorter ages. |
| Sandborn (45) | Ulcerative colitis | Humans (clinical trial) |
FGF-10 | Intravenous FGF-10 was well tolerated, but demonstrated no improvement in clinical remission rates at the doses tested. |
| Smith (46) | Skin graft explantation |
Athymic "nude" rats |
FGF-10 | Wounds treated with topical FGF-10 showed a significantly increased rate of interstitial closure, suggesting acceleration of epithelialization. |
| Soler (39) | Skin graft explantation |
Athymic "nude" rats | FGF-10 | FGF-10 significantly accelerated the rate of epithelialization in the meshed skin graft model. |
| Surgical incisions | Sprague Dawley rats |
FGF-10 | FGF-10 resulted in a modestly more rapid gain in breaking strength of surgical incisions. | |
| Contaminated excisional wounds |
Sprague Dawley rats |
FGF-10 | Similar to FGF-7, FGF-10 did not accelerate wound closure by contraction of contaminated excisional wounds. | |
| Stump (41) | Chronic venous ulcers |
Humans (clinical trial) |
FGF-10 | Although it was well tolerated, FGF-10 did not significantly accelerate wound closure compared to placebo. |
| Stump (42) | Cancer therapy- induced mucositis |
Humans (clinical trial) |
FGF-10 | FGF-10 was not significantly effective at reducing the incidence of cancer therapy-induced mucositis, although it was well tolerated. |
| Tagashira (36) | Wounding | Mice | FGF-10 | FGF-10 mRNA was highly induced 1 day after injury and decreased rapidly by 3 days, suggesting a role in wound healing. |
| Wang (34) | Corneal wound (CO2 laser) |
Japanese white rabbits |
FGF-10 | Topical FGF-10 accelerated corneal epithelial wound healing, inhibited corneal neovascularization, and reduced inflammation, stromal edema, and fibrosis. |
| Xia (38) | Ischemic full- thickness dermal ulcer |
New Zealand white rabbits |
FGF-10 | Topical FGF-10 promoted re-epithelialization, increased dermal cell proliferation, and stimulated granulation tissue formation in full-thickness excisional wounds with no significant difference in scar formation. This effect was delayed in older rabbits. |
| Yang (32) | Primary cell culture | Human keratinocytes |
FGF-10 | FGF-10 had a significant proliferative effect on human keratinocytes, suggesting its ability to support the healing of chronic wounds. |
| Radek (9) | Primary cell culture | BaF3 cells | FGF-10, DS | DS promoted FGF-10-dependent cell proliferation, which was influenced by DS length, IdoA residues, and sulfation. |
| Wound scratch culture |
NHEK cells | FGF-10, DS | The combination of DS and FGF-10 resulted in maximal proliferation and migration of keratinocytes, suggesting a novel interplay between DS and FGF-10 in mediating wound repair. | |
| Belmiro (74) | Murine induced- colitis |
Wistar rats | DS | DS inhibited colon inflammation by reducing inflammatory cytokine production, attenuating lymphocyte and macrophage recruitment, and reducing collagen-mediated fibrosis. |
| Belmiro (75) | Murine unilateral ureteral obstruction |
Swiss Mice | DS | By reducing macrophage recruitment, myofibroblast population and fibrosis, DS attenuates kidney inflammation in mice subjected to unilateral ureteral obstruction. |
| Dhahri (77) | Subcutaneous implantation |
Rats | DS | Grafting of DS onto polyethylene terephtalate surfaces enhanced its biointegration following subcutaneous implantation in rats. |
| Godoy (76) | Murine arterial injury |
Mice | DS | Following arterial injury, DS inhibited the initial thrombotic and inflammatory processes, and promoted migration of bone marrow mononuclear cells to the lesion site to aid in recovery. |
| Kozlowski (70) | Murine metastasis | Mice | DS | DS attenuated metastasis of MC-38 colon carcinoma and B16-BL6 melanoma cells. |
| Murine peritonitis | Mice | DS | In a thioglycollate peritonitis model, DS attenuated inflammatory cell infiltration. | |
| Murine arterial injury |
Mice | DS | Following arterial injury, DS reduced platelet deposition and thrombus size, suggesting inhibition of P-selectin and thereby binding of activated platelets during thrombus formation. | |
| Lee (67) | Primary cell culture | NIH3T3 fibroblasts |
n/a | The transfer of fibroblasts from a monolayer to a 3D culture induced synthesis of decorin, and GAG extracts from the 3D culture had the ability to potentiate FGF-7 activity. |
| Incisional wounding | Balb/c mice | n/a | The incisional wounds demonstrated an increase in DS-GAG chain synthesis on the cell surface of the proteoglycan syndecan-1. | |
| Penc (66) | Primary cell culture | Human wound fluid; F32 Cells |
DS | Human wound fluid contains abundant amounts of DS, which supports the ability of FGF-2 to signal cell proliferation, suggesting DS as a mediator of FGF-2. |
| Penc (71) | Wounding | Human wound fluid |
DS | DS activated endothelial cells in the absence of supplemental costimulatory molecules. It increased mRNA and cell surface ICAM-1, and it was a significant activator of nuclear factor-κB. |
| Saivin (81) | Blood coagulation | Humans (clinical trial) |
DS | DS showed improved intramuscular absorption with plasma concentrations reaching steady state more quickly, suggesting continuous drug coverage with a once daily regimen. |
| Scott (69) | Post-burn hypertrophic scars |
Fibroblast cultures | n/a | Fibroblasts from post-burn hypertrophic scars have a reduced capacity to synthesize decorin. |
| Trowbridge (59) | Primary cell culture | Human wound fluid; BaF3 cells |
n/a | Both FGF-7 and FGF-1 showed a dose-dependent increase in cell proliferation in response to DS. In addition, the size, sulfation level, and concentration of DS affected both proliferation and receptor binding. |
Acknowledgments
Support Sources: Funding provided by NIH-1P30AA019373-010, NIH-T32-GM008750-11, and the Dr. Ralph and Marian C. Falk Medical Research Trust
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
REFERENCES
- 1.American Burn Association. Burn Incidence and Treatment in the United States: 2011 Fact Sheet. 2011. [cited 8/26/2011]. Available from: http://www.ameriburn.org/resources_factsheet.php. [Google Scholar]
- 2.American Burn Association. 2011 National Burn Repository: Report of Data from 2001–2010. Chicago, IL: 2011. [Google Scholar]
- 3.Atiyeh BS, Gunn SW, Hayek SN. State of the art in burn treatment. World Journal of Surgery. 2005;29(2):131–148. doi: 10.1007/s00268-004-1082-2. [DOI] [PubMed] [Google Scholar]
- 4.Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocrine-Related Cancer. 2000;7(3):165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 5.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biology. 2001;2(3):3005.1–3005.12. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ornitz DM. FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays. 2000;22(2):108–112. doi: 10.1002/(SICI)1521-1878(200002)22:2<108::AID-BIES2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 7.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine and Growth Factor Reviews. 2005;16(2):139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Igarashi M, Finch PW, Aaronson SA. Characterization of recombinant human fibroblast growth factor (FGF)-10 reveals functional similarities with keratinocyte growth factor (FGF-7) The Journal of Biological Chemistry. 1998;273(21):13230–13235. doi: 10.1074/jbc.273.21.13230. [DOI] [PubMed] [Google Scholar]
- 9.Radek KA, Taylor KR, Gallo RL. FGF-10 and specific structural elements of dermatan sulfate size and sulfation promote maximal keratinocyte migration and cellular proliferation. Wound Repair and Regeneration. 2009;17(1):118–126. doi: 10.1111/j.1524-475X.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werner S, Smola H, Krieg T, Liao X, Williams LT, Longaker MT, et al. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science. 1994;266(5186):819–822. doi: 10.1126/science.7973639. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Meyer M, Muller AK, Bohm F, Grose R, Dauwalder T, et al. Fibroblast growth factor receptors 1 and 2 in keratinocytes control the epidermal barrier and cutaneous homeostasis. The Journal of Cell Biology. 2010;188(6):935–952. doi: 10.1083/jcb.200910126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. The Journal of Biological Chemistry. 2006;281(23):15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komi-Kuramochi A, Kawano M, Oda Y, Asada M, Suzuki M, Oki J, et al. Expression of fibroblast growth factors and their receptors during full-thickness skin wound healing in young and aged mice. The Journal of Endocrinology. 2005;186(2):273–289. doi: 10.1677/joe.1.06055. [DOI] [PubMed] [Google Scholar]
- 14.Beer HD, Bittner M, Niklaus G, Munding C, Max N, Goppelt A, et al. The fibroblast growth factor binding protein is a novel interaction partner of FGF-7, FGF-10 and FGF-22 and regulates FGF activity: implications for epithelial repair. Oncogene. 2005;24(34):5269–5277. doi: 10.1038/sj.onc.1208560. [DOI] [PubMed] [Google Scholar]
- 15.Nakatake Y, Hoshikawa M, Asaki T, Kassai Y, Itoh N. Identification of a novel fibroblast growth factor, FGF-22, preferentially expressed in the inner root sheath of the hair follicle. Biochimica et biophysica acta. 2001;1517(3):460–463. doi: 10.1016/s0167-4781(00)00302-x. [DOI] [PubMed] [Google Scholar]
- 16.Beer HD, Florence C, Dammeier J, McGuire L, Werner S, Duan DR. Mouse fibroblast growth factor 10: cDNA cloning, protein characterization, and regulation of mRNA expression. Oncogene. 1997;15(18):2211–2218. doi: 10.1038/sj.onc.1201383. [DOI] [PubMed] [Google Scholar]
- 17.Ceccarelli S, Cardinali G, Aspite N, Picardo M, Marchese C, Torrisi MR, et al. Cortactin involvement in the keratinocyte growth factor and fibroblast growth factor 10 promotion of migration and cortical actin assembly in human keratinocytes. Experimental Cell Research. 2007;313(9):1758–1777. doi: 10.1016/j.yexcr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Danilenko DM, Ring BD, Tarpley JE, Morris B, Van GY, Morawiecki A, et al. Growth factors in porcine full and partial thickness burn repair. Differing targets and effects of keratinocyte growth factor, platelet-derived growth factor-BB, epidermal growth factor, and neu differentiation factor. The American Journal of Pathology. 1995;147(5):1261–1277. [PMC free article] [PubMed] [Google Scholar]
- 19.Albornoz C, Villegas J, Sylvester M, Pena V, Bravo I. Burns are more aggressive in the elderly: Proportion of deep burn area/total burn area might have a role in mortality. Burns. 2011;37(6):1058–1061. doi: 10.1016/j.burns.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 20.O'Toole EA. Extracellular matrix and keratinocyte migration. Clinical and Experimental Dermatology. 2001;26(6):525–530. doi: 10.1046/j.1365-2230.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 21.Pastar I, Stojadinovic O, Tomic-Canic M. Role of keratinocytes in healing of chronic wounds. Surgical Technology International. 2008;17:105–112. [PubMed] [Google Scholar]
- 22.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. The Journal of Investigative Dermatology. 2007;127(5):998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 23.Emoto H, Tagashira S, Mattei MG, Yamasaki M, Hashimoto G, Katsumata T, et al. Structure and expression of human fibroblast growth factor-10. The Journal of Biological Chemistry. 1997;272(37):191–194. doi: 10.1074/jbc.272.37.23191. [DOI] [PubMed] [Google Scholar]
- 24.Derrick T, Grillo AO, Vitharana SN, Jones L, Rexroad J, Shah A, et al. Effect of polyanions on the structure and stability of repifermin (keratinocyte growth factor-2) Journal of Pharmaceutical Sciences. 2007;96(4):761–776. doi: 10.1002/jps.20797. [DOI] [PubMed] [Google Scholar]
- 25.Kamerzell TJ, Unruh JR, Johnson CK, Middaugh CR. Conformational flexibility, hydration and state parameter fluctuations of fibroblast growth factor-10: effects of ligand binding. Biochemistry. 2006;45(51):15288–15300. doi: 10.1021/bi061712q. [DOI] [PubMed] [Google Scholar]
- 26.Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, et al. FGF10 acts as a major ligand for FGF receptor 2 IIIB in mouse multi-organ development. Biochem Biophys Res Commun. 2000;277:643–649. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- 27.Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, et al. FGF10 is essential for limb and lung formation. Nat Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 28.Ohuchi H, Tao H, Ohata K, Itoh N, Kato S, Noji S, et al. Fibroblast growth factor 10 is required for proper development of the mouse whiskers. Biochem Biophys Res Commun. 2003;302:562–567. doi: 10.1016/s0006-291x(03)00183-9. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki K, Yamanishi K, Mori O, Kamikawa M, Anderson B, Kato S, et al. Defective terminal differentiation and hypoplasia of the epidermis in mice lacking the FGF10 gene. FEBS Lett. 2000;481:53–56. doi: 10.1016/s0014-5793(00)01968-2. [DOI] [PubMed] [Google Scholar]
- 30.Jang JH. Stimulation of human hair growth by the recombinant human keratinocyte growth factor-2 (KGF-2) Biotechnology letters. 2005;27(11):749–752. doi: 10.1007/s10529-005-5624-y. [DOI] [PubMed] [Google Scholar]
- 31.Marchese C, Felici A, Visco V, Lucania G, Igarashi M, Picardo M, et al. Fibroblast growth factor 10 induces proliferation and differentiation of human primary cultured keratinocytes. The Journal of Investigative Dermatology. 2001;116(4):623–628. doi: 10.1046/j.0022-202x.2001.01280.x. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Fu X, Li J. Effect of keratinocyte growth factor-2 on proliferation of human adult keratinocytes. Chinese Journal of Traumatology. 2002;5(6):342–345. [PubMed] [Google Scholar]
- 33.Satoh A, Makanae A, Wada N. The apical ectodermal ridge (AER) can be re-induced by wounding, wnt-2b, and fgf-10 in the chicken limb bud. Dev Biol. 2010;342(2):157–168. doi: 10.1016/j.ydbio.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Zhou X, Ma J, Tian H, Jiao Y, Zhang R, et al. Effects of keratinocyte growth factor-2 on corneal epithelial wound healing in a rabbit model of carbon dioxide laser injury. Biological and pharmaceutical bulletin. 2010;33(6):971–976. doi: 10.1248/bpb.33.971. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Li Y, Huang S, Lin J, Zhang W. Keratinocyte growth factor-2 on the proliferation of corneal epithelial stem cells in rabbit alkali burned cornea. Eye Science. 2007;23(2):107–116. [PubMed] [Google Scholar]
- 36.Tagashira S, Harada H, Katsumata T, Itoh N, Nakatsuka M. Cloning of mouse FGF10 and up-regulation of its gene expression during wound healing. Gene. 1997;197(1–2):399–404. doi: 10.1016/s0378-1119(97)00187-x. [DOI] [PubMed] [Google Scholar]
- 37.Jimenez PA, Rampy MA. Keratinocyte Growth Factor-2 Accelerates Wound Healing in Incisional Wounds. Journal of Surgical Research. 1999;81(2):238–242. doi: 10.1006/jsre.1998.5501. [DOI] [PubMed] [Google Scholar]
- 38.Xia YP, Zhao Y, Marcus J, Jimenez PA, Ruben SM, Moore PA, et al. Effects of keratinocyte growth factor-2 (KGF-2) on wound healing in an ischaemia-impaired rabbit ear model and on scar formation. Journal of Pathology. 1999;188(4):431–438. doi: 10.1002/(SICI)1096-9896(199908)188:4<431::AID-PATH362>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 39.Soler PM, Wright TE, Smith PD, Maggi SP, Hill DP, Ko F, et al. In vivo characterization of keratinocyte growth factor-2 as a potential wound healing agent. Wound Repair and Regeneration. 1999;7(3):172–178. doi: 10.1046/j.1524-475x.1999.00172.x. [DOI] [PubMed] [Google Scholar]
- 40.Robson MC, Phillips TJ, Falanga V, Odenheimer DJ, Parish LC, Jensen JL, et al. Randomized trial of topically applied repifermin (recombinant human keratinocyte growth factor-2) to accelerate wound healing in venous ulcers. Wound Repair and Regeneration. 2001;9(5):347–352. doi: 10.1046/j.1524-475x.2001.00347.x. [DOI] [PubMed] [Google Scholar]
- 41.Stump DC, Parrott J. Human Genome Sciences reports results of clinical trial of repifermin in patients with chronic venous ulcers. Rockville, Maryland: Human Genome Sciences; 2003. Sep 25, 2003. [Google Scholar]
- 42.Stump DC, Parrott J. Human Genoma Sciences reports results of phase 2 clinical trial of repifermin in patients with cancer therapy-induced mucositis. Rockville, Maryland: Human Genome Sciences; 2004. Feb 2, 2004. [Google Scholar]
- 43.Han DS, Li F, Holt L, Connolly K, Hubert M, Miceli R, et al. Keratinocyte growth factor-2 (FGF-10) promotes healing of experimental small intestinal ulceration in rats. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2000;279(5):G1011–G1022. doi: 10.1152/ajpgi.2000.279.5.G1011. [DOI] [PubMed] [Google Scholar]
- 44.Miceli R, Hubert M, Santiago G, Yao DL, Coleman TA, Huddleston KA, et al. Efficacy of keratinocyte growth factor-2 in dextran sulfate sodium-induced murine colitis. The Journal of Pharmacology and Experimental Therapeutics. 1999;209(1):464–471. [PubMed] [Google Scholar]
- 45.Sandborn WJ, Sands BE, Wolf DC, Valentine JF, Safdi M, Katz S, et al. Repifermin (keratinocyte growth factor-2) for the treatment of active ulcerative colitis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Alimentary pharmacology and therapeutics. 2003;17(11):1355–1364. doi: 10.1046/j.1365-2036.2003.01589.x. [DOI] [PubMed] [Google Scholar]
- 46.Smith PD, Polo M, Soler PM, McClintock JS, Maggi SP, Kim YJ, et al. Efficacy of growth factors in the accelerated closure of interstices in explanted meshed human skin grafts. The Journal of Burn Care and Rehabilitation. 2000;21(1 Pt 1):5–9. doi: 10.1097/00004630-200021010-00003. [DOI] [PubMed] [Google Scholar]
- 47.Raman R, Sasisekharan V, Sasisekharan R. Structural Insights into Biological Roles of Protein-Glycosaminoglycan Interactions. Chemistry and Biology. 2005;12(3):267–277. doi: 10.1016/j.chembiol.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 48.Guimond S, Maccarana M, Olwin BB, Lindahl U, Rapraeger AC. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1, FGF-2, and FGF-4. The Journal of Biological Chemistry. 1993;268(32):23906–23914. [PubMed] [Google Scholar]
- 49.Kan M, Wang F, Xu J, Crabb JW, Hou J, McKeehan WL. An essential heparin-binding domain in the fibroblast growth factor receptor kinase. Science. 1993;259(5103):1918–1921. doi: 10.1126/science.8456318. [DOI] [PubMed] [Google Scholar]
- 50.Saksela O, Moscatelli D, Sommer A, Rifkin DB. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. The Journal of Cell Biology. 1988;107(2):743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vlodavsky I, Bar-Shavit R, Ishai-Michaeli R, Bashkin P, Fuks Z. Extracellular sequestration and release of fibroblast growth factor: a regulatory mechanism? Trends in Biochemical Sciences. 1991;16(7):268–271. doi: 10.1016/0968-0004(91)90102-2. [DOI] [PubMed] [Google Scholar]
- 52.Gandhi NS, Mancera RL. The Structure of Glycosaminoglycans and their Interactions with Proteins. Chemical Biology and Drug Design. 2008;72(6):455–482. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 53.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB. 2006;20(1):9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 54.Yamada S, Sugahara K. Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Current Drug Discovery Technologies. 2008;5(4):289–301. doi: 10.2174/157016308786733564. [DOI] [PubMed] [Google Scholar]
- 55.Trowbridge JM, Gallo RL. Dermatan sulfate: new functions from an old glycosaminoglycan. Glycobiology. 2002;12(9):117R–125R. doi: 10.1093/glycob/cwf066. [DOI] [PubMed] [Google Scholar]
- 56.Gospodarowicz D, Cheng J. Heparin protects basic and acidic FGF from inactivation. Journal of Cellular Physiology. 1986;128(3):475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- 57.Casu B, Guerrini M, Torri G. Structural and Conformational Aspects of the Anticoagulant and Anti-thrombotic Activity of Heparin and Dermatan Sulfate. Current Pharmaceutical Design. 2004;77(1):123–135. doi: 10.2174/1381612043452794. [DOI] [PubMed] [Google Scholar]
- 58.Taylor KR, Rudisill JA, Gallo RL. Structural and Sequence Motifs in Dermatan Sulfate for Promoting Fibroblast Growth Factor-2 (FGF-2) and FGF-7 Activity. The Journal of Biological Chemistry. 2005;280(7):5300–5306. doi: 10.1074/jbc.M410412200. [DOI] [PubMed] [Google Scholar]
- 59.Trowbridge JM, Rudisill JA, Ron D, Gallo RL. Dermatan Sulfate Binds and Potentiates Activity of Keratinocyte Growth Factor (FGF-7) The Journal of Biological Chemistry. 2002;277(45):42815–42820. doi: 10.1074/jbc.M204959200. [DOI] [PubMed] [Google Scholar]
- 60.Brown CT, Applebaum E, Banwatt R, Trinkaus-Randall V. Synthesis of stromal glycosaminoglycans in response to injury. Journal of Cellular Biochemistry. 1995;59(1):57–68. doi: 10.1002/jcb.240590108. [DOI] [PubMed] [Google Scholar]
- 61.Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Molecular Biology of the Cell. 1994;5(7):797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oksala O, Salo T, Tammi R, Hakkinen L, Jalkanen M, Inki P, et al. Expression of proteoglycans and hyaluronan during wound healing. Journal of Histochemistry and Cytochemistry. 1995;43(2):125–135. doi: 10.1177/43.2.7529785. [DOI] [PubMed] [Google Scholar]
- 63.Tumova S, Woods A, Couchman JR. Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. The International Journal of Biochemistry & Cell Biology. 2000;32(3):269–288. doi: 10.1016/s1357-2725(99)00116-8. [DOI] [PubMed] [Google Scholar]
- 64.Cosmi B, Fredenburgh JC, Rischke J, Hirsh J, Young E, Weitz JI. Effect of nonspecific binding to plasma proteins on the antithrombin activities of unfractionated heparin, low-molecular-weight heparin, and dermatan sulfate. Circulation. 1997;95(1):118–124. doi: 10.1161/01.cir.95.1.118. [DOI] [PubMed] [Google Scholar]
- 65.Malavaki C, Mizumoto S, Karamanos N, Sugahara K. Recent advances in the structural study of functional chondroitin sulfate and dermatan sulfate in health and disease. Connective Tissue Research. 2008;49(3):133–139. doi: 10.1080/03008200802148546. [DOI] [PubMed] [Google Scholar]
- 66.Penc SF, Pomahac B, Winkler T, Dorschner RA, Eriksson E, Herndon M, et al. Dermatan Sulfate Released after Injury Is a Potent Promoter of Fibroblast Growth Factor-2 Function. The Journal of Biological Chemistry. 1998;273(43):28116–28121. doi: 10.1074/jbc.273.43.28116. [DOI] [PubMed] [Google Scholar]
- 67.Lee PHA, Trowbridge JM, Taylor KR, Morhenn VB, Gallo RL. Dermatan Sulfate Proteoglycan and Glycosaminoglycan Synthesis Is Induced in Fibroblasts by Transfer to a Three-dimensional Extracellular Environment. The Journal of Biological Chemistry. 2004;279(47):48640–48646. doi: 10.1074/jbc.M407241200. [DOI] [PubMed] [Google Scholar]
- 68.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. Journal of Cell Biology. 1997;136(3):729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scott P, Dodd C, Ghahary A, Shen Y, Tredget E. Fibroblasts from post-burn hypertrophic scar tissue synthesize less decorin than normal dermal fibroblasts. Clin Sci (Lond) 1998;94(5):541–547. doi: 10.1042/cs0940541. [DOI] [PubMed] [Google Scholar]
- 70.Kozlowski E, Pavao M, Borsig L. Ascidian dermatan sulfates attenuate metastasis, inflammation and thrombosis by inhibition of P-selectin. J Thromb Haemost. 2011 doi: 10.1111/j.1538-7836.2011.04401.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 71.Penc SF, Pomahac B, Eriksson E, Detmar M, Gallo RL. Dermatan sulfate activates nuclear factor-kappab and induces endothelial and circulating intercellular adhesion molecule-1. The Journal of Clinical Investigators. 1999;103(9):1329–1335. doi: 10.1172/JCI4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J, Lee J, Yan M, Rho J, Roehrl M. Dermatan sulfate interacts with dead cells and regulates CD5(+) B-cell fate: implications for a key role in autoimmunity. Am J Pathol. 2011;178(5):2168–2176. doi: 10.1016/j.ajpath.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rho J, Zhang W, Murali M, Roehrl M, Wang J. Human proteins with affinity for dermatan sulfate have the propensity to become autoantigens. Am J Pathol. 2011;178(5):2177–2190. doi: 10.1016/j.ajpath.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belmiro C, Castelo-Branco M, Melim L, Schanaider A, Elia C, Madi K, et al. Unfractionated heparin and new heparin analogues from ascidians (chordate-tunicate) ameliorate colitis in rats. J Biol Chem. 2009;284(17):11267–11278. doi: 10.1074/jbc.M807211200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Belmiro C, Goncalves R, Kozlowski E, Werneck A, Takyia C, Leite M, Jr, et al. Dermatan sulfate reduces monocyte chemoattractant protein 1 and TGF-β production, as well as macrophage recruitment and myofibroblast accumulation in mice with unilateral ureteral obstruction. Braz J Med Biol Res. 2011;44(7):624–633. doi: 10.1590/s0100-879x2011007500077. [DOI] [PubMed] [Google Scholar]
- 76.Godoy J, Block D, Tollefsen D, Werneck C, Vicente C. Dermatan sulfate and bone marrow mononuclear cells used as a new therapeutic strategy after arterial injury in mice. Cytotherapy. 2011;13(6):695–704. doi: 10.3109/14653249.2010.548378. [DOI] [PubMed] [Google Scholar]
- 77.Dhahri M, Abed A, Lajimi R, Mansour M, Gueguen V, Abdesselem S, et al. Grafting of dermatan sulfate on polyethylene terephtalate to enhance biointegration. J Biomed Mater Res A. 2011;98(1):114–121. doi: 10.1002/jbm.a.33077. [DOI] [PubMed] [Google Scholar]
- 78.Nenci GG. Dermatan Sulphate as an Antithrombotic Drug. Pathophysiology of Haemostasis and Thrombosis. 2002;32(5–6):303–307. doi: 10.1159/000073586. [DOI] [PubMed] [Google Scholar]
- 79.Tovar A, de Mattos D, Stelling M, Sarcinelli-Luz B, Nazareth R, Mourao P. Dermatan sulfate is the predominant antithrombotic glycosaminoglycan in vessel walls: implications for a possible physiological function of heparin cofactor II. Biochimica et biophysica acta. 2005;1740(1):45–53. doi: 10.1016/j.bbadis.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 80.Volpi N. Therapeutic applications of glycosaminoglycans. Current Medicinal Chemistry. 2006;13(15):1799–1810. doi: 10.2174/092986706777452470. [DOI] [PubMed] [Google Scholar]
- 81.Saivin S, Cambus JP, Thalamas C, Lau G, Boneu B, Houin G, et al. Pharmacokinetics and pharmacodynamics of intramuscular dermatan sulfate revisited: a single- and repeated-dose study in healthy volunteers. Clinical Drug Investigation. 2003;23(8):533–543. doi: 10.2165/00044011-200323080-00006. [DOI] [PubMed] [Google Scholar]
- 82.DiPietro L. Wound healing: the role of the macrophage and other immune cells. Shock. 1995;4(4):233–240. [PubMed] [Google Scholar]
- 83.Guo S, DiPietro L. Factors affecting wound healing. Journal of Dental Research. 2010;89(3):219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hunt T, Hopf H, Hussain Z. Physiology of wound healing. Advances in Skin and Wound Care. 2000;13(2 Supplement):6–11. [PubMed] [Google Scholar]
- 85.Szpaderska AM, DiPietro L. Inflammation in surgical wound healing: Friend or foe? Surgery. 2005;137(5):571–573. doi: 10.1016/j.surg.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 86.Franz MG, Steed DL, Robson MC. Optimizing healing of the acute wound by minimizing complications. Current Problems in Surgery. 2007;44(11):691–763. doi: 10.1067/j.cpsurg.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 87.Spanholtz TA, Theodorou P, Amini P, Spilker G. Severe Burn Injuries. Deutsches Ärzteblatt international. 2009;106(38):607–613. doi: 10.3238/arztebl.2009.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kagan RJ, Peek MD, Ahrenholz DH, Hickerson WL, Holmes JH, Korentager RA, et al. Surgical Management of the Burn Wound and Use of Skin Substitutes: American Burn Association White Paper. 2009 doi: 10.1097/BCR.0b013e31827039a6. [DOI] [PubMed] [Google Scholar]
- 89.Polavarapu N, Ogilvie MP, Panthaki ZJ. Microbiology of burn wound infections. The Journal of Craniofacial Surgery. 2008;19(4):899–902. doi: 10.1097/SCS.0b013e318175b4f0. [DOI] [PubMed] [Google Scholar]
- 90.Ramakrishnan MK, Sankar J, Venkatraman J, Ramesh J. Infections in burn patients - experience in a tertiary care hospital. Burns. 2006;32(5):594–596. doi: 10.1016/j.burns.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 91.Villanueva E, Bennett MH, Wasiak J, Lehm JP. Cochrane database of systematic reviews (Online) The Cochrane Library; 2004. Hyperbaric oxygen therapy for thermal burns. reviewed 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Atiyeh BS, Costagliola M. Cultured epithelial autograft (CEA) in burn treatment: three decades later. Burns. 2007;33(4):405–413. doi: 10.1016/j.burns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 93.Lazarus G, Cooper D, Knighton D, Margolis D, Pecoraro R, Rodeheaver G, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Archives of Dermatology. 1994;130(4):489–493. [PubMed] [Google Scholar]
- 94.Russo CA, Steiner C, Spector W. HCUP Statistical Brief #64. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Dec, Hospitalizations Related to Pressure Ulcers, 2006. [PubMed] [Google Scholar]
- 95.Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2008, editors; 2007. [Google Scholar]
- 96.Reiber GE. The epidemiology of diabetic foot problems. Diabetic medicine. 1996;13(Supp 1):S6–S11. [PubMed] [Google Scholar]
- 97.Stockl K, Vanderplas A, Tafesse E, Chang E. Costs of Lower-Extremity Ulcers Among Patients With Diabetes. Diabetes Care. 2004;27(9):2129–2134. doi: 10.2337/diacare.27.9.2129. [DOI] [PubMed] [Google Scholar]
- 98.Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22(3):382–387. doi: 10.2337/diacare.22.3.382. %R 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- 99.Gottrup F. A specialized wound-healing center concept: importance of a multidisciplinary department structure and surgical treatment facilities in the treatment of chronic wounds. American Journal of Surgery. 2004;187(5A):38S–43S. doi: 10.1016/S0002-9610(03)00303-9. [DOI] [PubMed] [Google Scholar]
- 100.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, et al. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]