SUMMARY
A major obstacle in the application of cell-based therapies for the treatment of neuromuscular disorders is obtaining the appropriate number of stem/progenitor cells to produce effective engraftment. The use of embryonic stem (ES) or induced pluripotent stem (iPS) cells could overcome this hurdle. However to date, derivation of engraftable skeletal muscle precursors that can restore muscle function from human pluripotent cells has not been achieved. Here we applied conditional expression of Pax7 in human ES/iPS cells to successfully derive large quantities of myogenic precursors, which upon transplantation into dystrophic muscle, are able to engraft efficiently, producing abundant human-derived dystrophin-positive myofibers that exhibit superior strength. Importantly, transplanted cells also seed the muscle satellite cell compartment and engraftment is present over 11 months post-transplant. This study provides the proof-of-principle for the derivation of functional skeletal myogenic progenitors from human ES/iPS cells, and highlights their potential for future therapeutic application in muscular dystrophies.
Keywords: Human Embryonic stem cells, Human induced pluripotent stem cells, Pax7, Skeletal muscle progenitors, engraftment, regeneration, satellite cells, contractility, muscular dystrophy
INTRODUCTION
Muscle wasting affects millions of individuals worldwide, and is caused by a variety of conditions, including cachexia, sarcopenia, and muscular dystrophies (MD). The latter comprises more than 30 genetically distinct disorders that culminate in paralysis, and in many instances, cardiopulmonary complications (Emery, 2002). Current treatment options are only palliative, and thus far there is no cure for any type of MD. Therapeutic strategies that focus on the replacement of the diseased muscle tissue with stem cells that can give rise to healthy myofibers as well as self-renew are particularly attractive. This strategy has been used in the hematopoietic system for the past 40 years with great success. A major caveat with muscle tissue is the impossibility of obtaining enough skeletal muscle stem cells (satellite cells) without causing severe and permanent damage to the muscle of the donor, in contrast to hematopoietic stem cells (HSCs), which can be harvested from mobilized peripheral blood and bone marrow with minimal harm to the donor. Small muscle biopsies allow for the ex vivo expansion of satellite cell progeny, however as observed for HSCs (Guenechea et al., 1999), ex vivo expansion of myoblasts from satellite cells results in loss of engraftment ability (Montarras et al., 2005). Consistently, early clinical trials involving the transplantation of ex vivo expanded myoblasts failed to improve strength in patients with Duchenne’s MD (Mendell et al., 1995; Vilquin, 2005). Therefore alternate sources of early skeletal muscle progenitors are required for the feasibility of a stem cell therapy approach for MD.
One of the major advantages of pluripotent stem cells is the prospect of generating large quantities of specific cell populations for regenerative purposes. In particular with the recent breakthrough of reprogramming somatic cells (Park et al., 2008; Takahashi et al., 2007; Yu et al., 2007), ethical concerns associated with human ES cells are eliminated, and the possibility of generating patient-specific iPS cells for autologous therapies is enabled. Whereas safety issues still need to be carefully addressed before these cells can be used in the clinical setting, a critical prerequisite for a potential therapeutic application is the generation of abundant engraftable tissue-specific cell preparations. Although the use of mouse iPS-derived cells to correct a disease phenotype has been documented for several models of disease through derivation of hematopoietic (Hanna J, 2007), endothelial (Xu et al., 2009), neural (Wernig et al., 2008), pancreatic (Alipio et al., 2010), liver (Espejel et al., 2010), and myogenic precursor cells (Darabi et al., 2011a; Mizuno et al., 2010), the human iPS field lags far behind in this regard. To date there is only one study documenting functional improvement from human iPS cells, using a rat model of Parkinson disease (Hargus et al., 2010). Thus there is clearly a huge gap between transplantation studies involving mouse and human pluripotent stem cells. Proof-of-principle studies using human iPS cells are required in order to begin seriously considering potential therapeutic applications of these cells.
Here we describe for the first time the efficient derivation of a proliferating population of human skeletal myogenic progenitors from both ES and iPS cells, which upon transplantation into dystrophin-deficient mice, promote extensive and long-term regeneration that is accompanied by functional improvement.
RESULTS
Pax7 Induces the Myogenic Program in Differentiating Human ES and iPS Cells
To assess whether Pax7, a paired-box transcription factor well known for its role in the maintenance of the adult satellite cell compartment (Oustanina et al., 2004; Seale et al., 2000), can efficiently induce the myogenic program in human ES- and iPS-derived embryoid bodies, as observed in mouse cultures (Darabi et al., 2011a; Darabi et al., 2011b), we modified the human H9 ES cell line, and two well characterized human iPS cell lines, IPRN13.13 and IPRN14.57 (Figure S1A–F), generated from fibroblasts from normal donors, with a doxycycline-inducible lentiviral vector encoding Pax7 (iPax7). Expression of the transgene was detected by incorporating an ires-GFP reporter downstream of the Pax7 gene (Figure S1G). Further confirmation of Pax7 induction in these cells was provided by immunofluorescence analyses, which showed co-expression of Pax7 and GFP upon doxycycline (dox) induction (Figure S1H). Genetic modification did not alter the morphology of the pluripotent cells or their ability to differentiate into embryoid bodies (EBs) (Figure 1A).
Figure 1. Myogenic induction of human ES/iPS cells by Pax7.
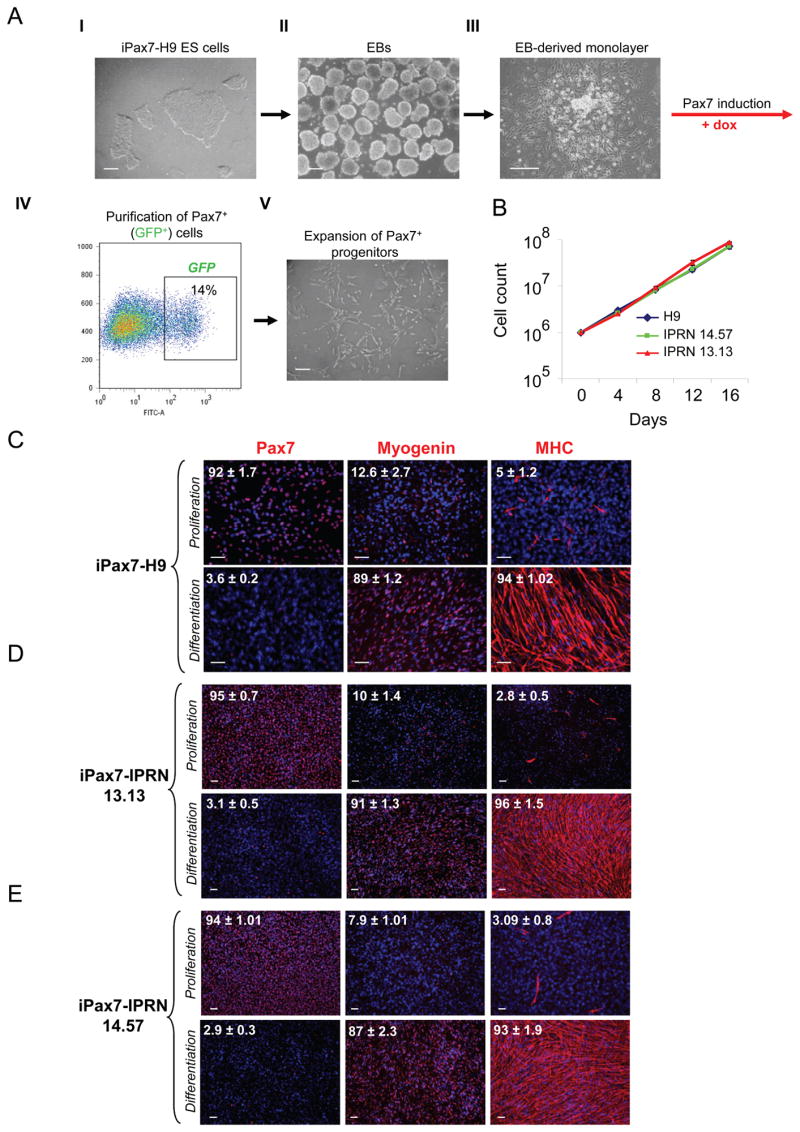
(A) Schematic of differentiation protocol with representative morphological aspects of iPax7 H9: in the undifferentiated state as ES cell colonies in mTeSR medium (I), and in the EB stage (II). At D7 of differentiation, EBs are collected and plated on a gelatinized flask to grow as a monolayer (III). Pax7 induction is initiated at D10 of differentiation by adding dox to the myogenic medium. GFP+ (Pax7+) cells emerge in these cultures and begin to proliferate. GFP+ cells are purified by FACS (IV). Representative FACS profile shows Pax7 (GFP) expression after 4 days of dox induction in H9 differentiating ES cells. The percentage indicated represents the fraction of GFP+ cells. (IV). Pax7+ myogenic progenitors are expanded in myogenic induction medium supplemented with dox and human bFGF (V). Scale bars, 100 μm.
(B) Growth curve of Pax7-induced ES- and iPS-derived myogenic progenitors during in vitro expansion. Data represent Mean ± S.E. of four independent experiments.
(C–E) Immunostaining of Pax7-induced human ES- (C) and iPS- (D–E) derived myogenic cells for Pax7, Myogenin and Myosin Heavy Chain (MHC) in proliferation (upper) and differentiation (lower) conditions. With Pax7 induction under proliferation conditions, most cells express Pax7 and only a few express markers of terminal differentiation (upper panels), while under differentiation conditions (and dox withdrawal), almost all the cells become positive for Myogenin and MHC, forming multinucleated myotubes (lower panels). Cells were co-stained with DAPI (blue). Numbers on each panel represent the percentage of cells expressing Pax7, Myogenin, or MHC. Data are mean ± SE. For each condition, 4 slides were used for quantification. Scale bars, 100 μm. See also Figure S1.
In embryogenesis, Pax7 and its homologue Pax3 act to confer myogenic fate within paraxial mesoderm. We therefore differentiated iPax7 human (h) ES and iPS cells for 7 days as EBs followed by 3 days in monolayer before inducing Pax7 with dox (Figure 1A). This time point is well into the peak of mesoderm generation as indicated by Brachyury expression (Figure S1I). Following four days of induction, Pax7+GFP+ cells were purified by FACS and expanded in secondary monolayer culture in proliferation medium containing dox and bFGF (Figure 1A and Figure S1J). Both ES- and iPS-derived myogenic progenitors demonstrated notable expansion potential, averaging 86-fold by week 2 (Figure 1B), with a total of 6–7 doublings during this period. Under these proliferation conditions, iPax7 hES and hiPS cells expressed Pax7 abundantly (Figure 1C–E and Figure S1K–L). Myogenin and myosin heavy chain (MHC), markers of terminal muscle differentiation, were barely detectable (Figure 1C–E). This profile changed when iPax7 hES and hiPS cells were subjected to differentiation (5% horse serum and withdrawal of dox and bFGF; differentiation medium). In these culture conditions for 2 weeks, human myogenic progenitors differentiated into multinucleated myotubes, with abundant expression of myogenin and MHC while rare cells expressed Pax7 (Figure 1C–E). These results were confirmed by gene expression analyses, which showed high levels of Pax7 expression solely under proliferation conditions (in the presence of dox) (Figure S1M), and up-regulation of MyoD and late skeletal muscle specific markers, Myogenin, Dystrophin, and MHC when these myogenic progenitors had undergone final maturation (Figure S1M).
Human ES- and iPS-Derived Myogenic Progenitors Display Similar Surface Marker Profile
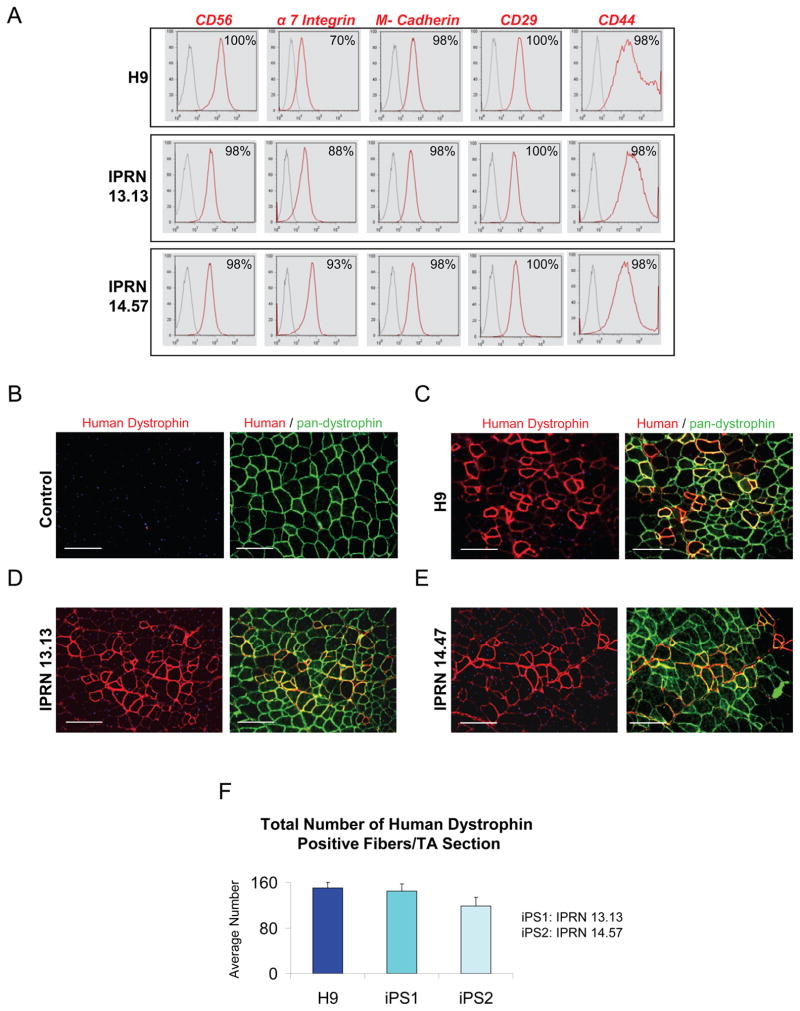
We characterized surface marker expression of these myogenic progenitors by FACS using a panel of antibodies. Our results show a remarkable similarity between hES- (Figure 2A and Figure S2A) and hiPS-derived myogenic progenitors (Figure 2A and Figure S2B–C). Cells in each preparation showed homogenous expression of CD56, CD29, CD44, M-cadherin, and α7-integrin. Although most of these markers are associated with murine satellite cells and early myogenic progenitors (Cornelison and Wold, 1997; Sacco et al., 2008; Sherwood et al., 2004), the human satellite cell has not yet been defined by flow cytometry. Only CD56 has been considered a reliable marker of human satellite cells (Péault et al., 2007). These cells were also found to express high levels of CD63, CD146, CD105, CD90, and CD13; the last three are antigens known to be present in mesenchymal stem cells (Pittenger and Martin, 2004). CD34 labeled a discrete sub-fraction of these cells. Other screened antigens, including CD45, CD33, KDR, and CD31 were undetectable in these myogenic progenitor populations (Figure S2), indicating the absence of hematopoietic and endothelial cells. The adhesion molecules CXCR4 and CD106 were also not detected. We examined major histocompatibility complex (MHC) expression because the lack on MHC class I expression on other embryonic and ES-derived cells has limited engraftability, even in immunodeficient mice, due to NK cell-mediated responses (Rideout et al., 2002; Tabayoyong et al., 2009). This analysis revealed that, regardless of ES or iPS origin, proliferating myogenic progenitors express MHC class I molecules (Figure S2). This pattern is beneficial from the perspective of avoiding an NK-mediated lack of self MHC response, but indicates the importance of HLA-matching.
Figure 2. Phenotypic profile and regenerative potential of human ES/iPS-derived myogenic cells.
(A) Representative FACS profile of Pax7-induced human ES- and iPS-derived proliferating myogenic progenitors. Histogram plots show isotype control staining profile (gray line) versus specific antibody staining profile (red line). Percentages represent the fraction of cells that express a given surface antigen. See also Figure S2.
(B–F) Transplantation of myogenic progenitors into cardiotoxin-injured NSG mice. (B) PBS-injected control muscles show no staining for human-specific dystrophin (right, in red), but as expected, uniform expression of mouse dystrophin (left, in green) as evidenced by the use of a pan-dystrophin antibody. (C–E) Engraftment of proliferating myogenic progenitors obtained from Pax7-induced human ES- (C) and iPS-derived (D–E) cells in TA muscles of NSG (n = 4 for each cell line) two months after intramuscular transplantation. Immunofluorescence staining with anti-human (in red) and anti-pan dystrophin (in green) antibodies reveals presence of donor-derived myofibers expressing human dystrophin in recipient muscles (in red). Scale bars, 100 μm. (F) Quantification of human dystrophin positive fibers in engrafted muscles shows similar engraftment of human ES- versus iPS-derived myogenic progenitors. For this, the total number of human dystrophin positive fibers in cross-sections of TA muscles (sections spanned entire muscles) was counted. Data are shown as Mean ± SE.
In vivo Regenerative Potential of Human ES/iPS-Derived Myogenic Progenitors
Next we examined the in vivo skeletal muscle regenerative potential of iPax7 hES- and hiPS-derived myogenic progenitors by transplanting these cells directly into the tibialis anterior (TA) muscles of NOD/SCID gamma-c (NSG) mice, an immune deficient strain commonly used as a recipient of human hematopoietic cells. The gamma-c mutation (IL2Rg) ablates NK cells, rendering NSG mice unable to reject human cells due to lack of self-MHC presentation, resulting in better hematopoietic engraftment than in mice bearing the NOD/SCID mutation alone (Shultz et al., 2005). NSG mice were injured with cardiotoxin (CTX) 24 hours prior to cell transplantation. The contralateral TA muscle, which served as a control, was also pre-injured with CTX, but injected only with PBS. Two months after transplantation, muscle sections were harvested and evaluated for engraftment by immunostaining with both pan-dystrophin and human-specific dystrophin antibodies. No expression of human dystrophin could be detected in PBS-injected control muscles (Figure 2B); staining was only observed with a pan-dystrophin antibody (Figure 2B). On the other hand, muscles that had been treated with iPax7 hES- (Figure 2C) and hiPS-derived (Figure 2D–E) myogenic progenitors demonstrated engraftment of human-derived myofibers, as evidenced by the clear expression of human-specific dystrophin in recipient muscles (Figure 2C–E). We did not observe major differences in terms of engraftment between ES- and iPS-derived myogenic progenitors (Figure 2F). No tumor formation was observed in transplanted mice, even in a long-term (46 weeks) cohort.
Functional improvement in dystrophic mice
To determine the regenerative potential of these myogenic progenitors in the context of muscular dystrophy, we transplanted them into mdx mice engineered to lack B, T, and NK cells. These mice were generated by crossing mice carrying the mdx4Cv mutation, an ENU-induced stop codon in exon 53 (Im et al., 1996) with very low reversion frequency (Danko et al., 1992), to NSG mice. Recombinant X-chromosomes bearing both mdx4Cv and IL2RgΔ were brought to homozygosity with the NOD/SCID mutation, and the stock was maintained by sib-mating. The genetic background is thus mixed-inbred, distinct from either C57BL/6Rox (of mdx4Cv) or NOD/ShiLtJ (of NSG). These NSG-mdx4Cv mice, similarly to conventional mdx mice (Coulton et al., 1992; Durbeej and Campbell, 2002), lack dystrophin (Figure S3) and are characterized by extensive regeneration, as evidenced by the presence of centrally nucleated myofibers (Figure S3).
Intramuscular transplantation of TA muscles with ES- or iPS-derived myogenic progenitors resulted in considerable engraftment, clearly shown by the large number of myofibers expressing human dystrophin (Figure 3A), while PBS-injected muscles lacked dystrophin (Figure 3A). This engraftment was confirmed by the use of a second human specific antibody, Lamin AC (Figure 3A). Human nuclei were exclusive to cell-transplanted muscle and mainly found within human dystrophin+ fibers (Figure 3A). We observed comparable engraftment between iPax7 hES- versus hiPS- derived myogenic cells (Figure 3B), suggesting similar regenerative potential between ES- and iPS-derived myogenic progenitors. As controls, we transplanted dermal fibroblasts as well as myoblasts using the same cell number into the TA muscles of NSG-mdx4Cv mice. While no engraftment was detected following injection of fibroblasts (Figure S4A), dystrophin+ myofibers could be observed in myoblast-transplanted mice (Figure S4A), although at a much lower level (Figure S4B) than ES- and iPS-derived myogenic progenitors using our protocol (p < 0.001).
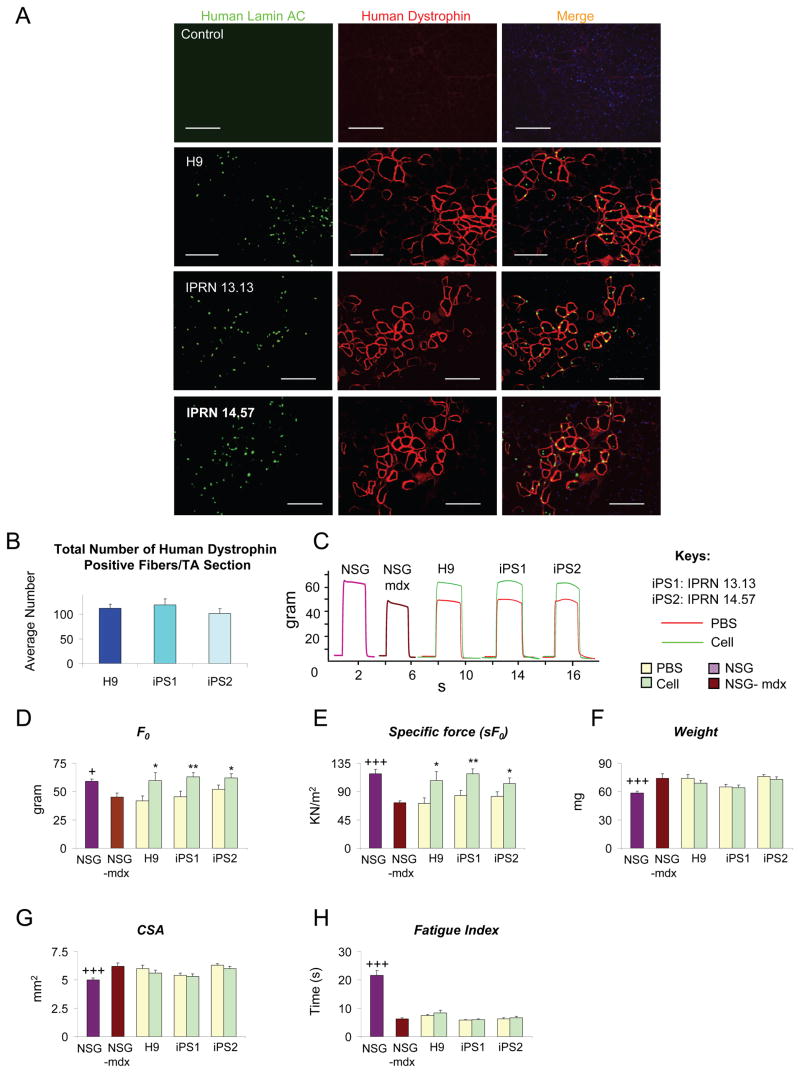
Figure 3. Efficient engraftment and functional recovery following transplantation of human ES/iPS-derived myogenic progenitorsinto dystrophic mice.
(A) No staining for human Lamin AC or dystrophin is detected in PBS-injected control TA muscles of NSG-mdx mice. Cell engraftment in NSG −mdx4Cv mice (n=5 for H9, n=6 for IPRN13.13 and n=7 for IPRN 14.47). Abundant expression for human Lamin AC (in green) and dystrophin (in red) is observed in dystrophic muscles treated with human ES/iPS-derived myogenic progenitors one month after the transplantation. Note that nuclear Lamin AC staining occurs predominantly within human dystrophin+ myofibers. Scale bars, 100 μm. See also Figure S3.
(B) Quantification of human dystrophin positive fibers in NSG-mdx4Cv engrafted mice shows comparable engraftment of human ES- versus iPS-derived myogenic progenitors. For this, the total number of human dystrophin positive fibers in cross-sections of TA muscles (sections spanned entire muscles) was counted. Data are shown as Mean ± SE.
(C) Representative example of force tracings in TA muscles of non-treated non-injured NSG (purple line) and NSG-mdx4Cv (brown line) mice as well as CTX-injured NSG-mdx4Cv mice that had been injected with PBS (control, red line) or human ES/iPS-derived myogenic progenitors (green line).
(D–E) Effect of iPax7 human ES/iPS-derived myogenic cell transplantation on absolute and specific (sF0: F0 normalized to CSA) force, respectively. Values for non-treated, non-injured NSG (purple) and NSG-mdx4Cv (brown) mice are shown for reference.
(F–G) Weight and CSA of control and transplanted muscles, respectively. Values for non-treated, non-injured NSG (purple) and NSG-mdx4Cv (brown) mice are shown for reference. See also Figure S4.
(H) Fatigue index: time for force to decline to 30% of its maximal value shows no significant recovery with cell treatment compared to PBS control.
* P<0.05, ** P<0.01 compared to its PBS control
+P<0.05, +++ P<0.001 compared to NSG-mdx4Cv
Next we investigated whether muscle contractile parameters were altered following transplantation. Similarly to conventional mdx mice (Darabi et al., 2008), TA muscles from immunodeficient dystrophic mice were weak and hypertrophic, as shown in untreated or PBS-injected controls (Figure 3C–G). On the other hand, dystrophic muscles that had been transplanted with human ES- and iPS-derived myogenic progenitors demonstrated significant functional improvement, as demonstrated by superior isometric tetanic force (Figure 3C), increased absolute (Figure 3D), and specific force (Figure 3E) when compared to their respective contralateral PBS-injected TA muscle. Weight and CSA parameters remained unchanged (Figure 3F–G). No changes were observed when transplanted muscle was subjected to fatigue test (Figure 3H), suggesting that levels of engraftment were not sufficient to restore this parameter. Meanwhile, transplantation of fibroblasts or myoblasts did not result in improvement of any of these functional parameters (Figure S4E–G).
Engraftment of the satellite cell compartment
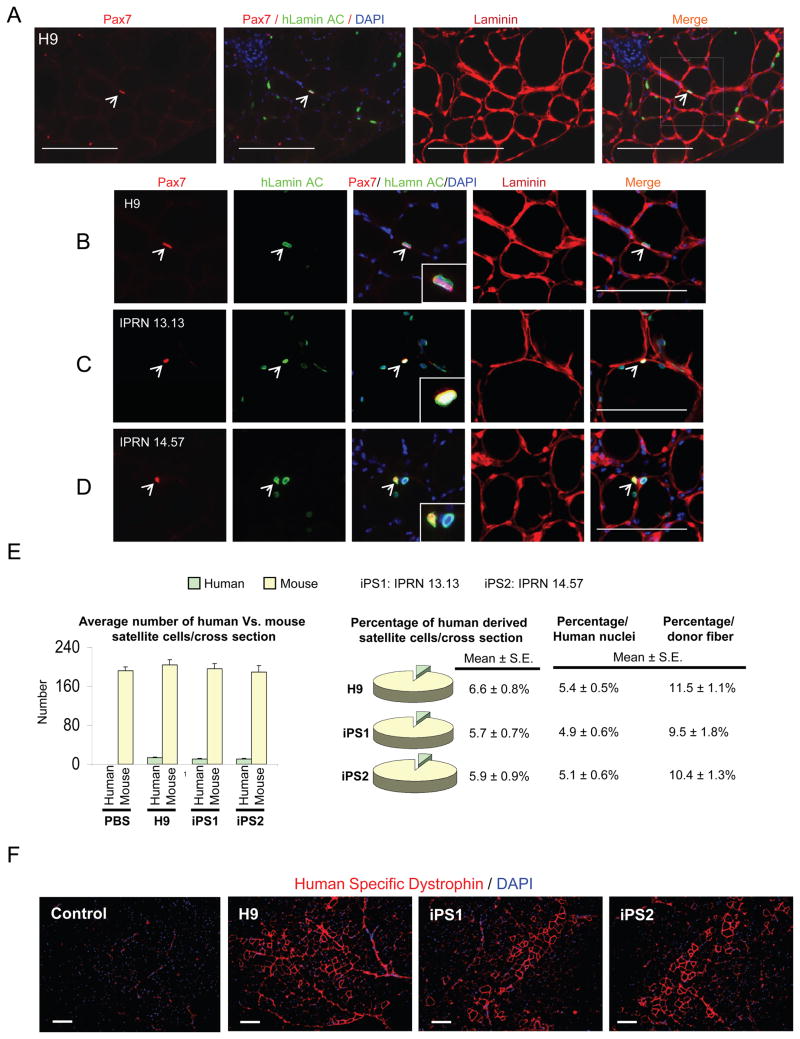
Finally, we investigated whether ES- and iPS-derived myogenic progenitors have the ability to seed the satellite cell compartment following their transplantation into NSG-mdx4Cv. We performed these analyses by staining muscle cryosections with Pax7 (satellite cell marker), human Lamin AC (specific antibody to track human cells), Laminin (to identify position within the sarcolemma), and DAPI. The majority of human nuclei were Pax7- negative and within human dystrophin+ myofibers. This is expected as the majority of transplanted human myogenic progenitors differentiate into myofibers. However we also detected a significant number of Pax7+ human Lamin AC+ cells, representing donor-derived satellite cells. These results were quantified and are shown in Figure 4E. The data clearly show that human ES- and iPS-derived myogenic progenitors are able to seed the satellite cell compartment (Figure 4A–D). As expected, since muscles were not previously irradiated, the majority of the satellite cell pool was of recipient origin, with only a small fraction being donor-derived (Figure 4E).
Figure 4. Satellite cell engraftment by human ES/iPS-derived myogenic cells.
(A–B) Representative images show staining for satellite cells in muscle sections from NSG-mdx4Cv mice that had been transplanted with H9 human ES-derived myogenic progenitors. Images are shown at lower (A) and higher (B) magnification. Immunostaining shows the presence of human Lamin AC+ (in green) cells in engrafted regions. Arrows shows the presence of human-derived satellite cells in engrafted muscles, as evidenced by the presence of Pax7+ (in red) Lamin AC+ (in green) cells under the basal lamina. Scale bars, 100 μm.
(C–D) Similar satellite cell engraftment was observed upon transplantation of human iPS-derived myogenic progenitors, IPRN 13.13 (C) and IPRN 14.57 (D). Scale bars, 100 μm.
(E) Quantification of Pax7+Lamin AC+ and Pax7+Lamin AC− cells in transplanted muscles, representative of donor human derived and host satellite cells, respectively. The total number of Pax7+Lamin AC+ and Pax7+Lamin AC− cells in cross-sections of TA muscles was counted. Sections spanned entire muscles. Data are shown as Mean ± SE. Left panels show absolute numbers and right panels indicate respective percentages. Data are also shown as percentage per human nuclei as well as percentage per donor fiber.
(F) Assessment of long-term engraftment at 46 weeks after transplantation in NSG mice. Immunofluorescence staining with anti-human dystrophin antibody reveals the presence of donor-derived myofibers expressing human dystrophin (in red) in NSG recipient muscles. Scale bars, 100 μm. See also Figure S4.
To determine whether the engraftment of ES- and iPS-derived myogenic progenitors was durable, we assessed the presence of human dystrophin+ myofibers at 46 weeks after transplantation into NSG mice. Immunostaining of this long-term experimental cohort revealed significant engraftment in muscles of NSG mice (n=9) that had been transplanted with human ES/iPS derived skeletal myogenic progenitors (11 months post-transplantation) (Figure 4F and Figure S4H). Quantification of human dystrophin+ myofibers demonstrated that the engraftment level at 46 weeks was about 60%–80% (Figure S4I) of the levels at 8 weeks (Figure 2F). This sustained long-term engraftment data is remarkable, as to our knowledge, no study has followed engraftment for such a long period with human myogenic cells.
DISCUSSION
There has been increasing enthusiasm about the possibility of applying iPS technology to generate autologous cells for therapeutic purposes. Some of the advantages associated with these pluripotent stem cells include: i) the absence of ethical concerns, as cells are derived from adult tissue; ii) the potential for an off-the-shelf supply of HLA-matched or patient-specific stem cell preparations; iii) the possibility of correcting genetic defects by homologous recombination, and iv) in the case of autologous cell transplantations, immunosuppression might be dispensable. Although significant progress has been made in terms of generating integration-free iPS cells through the use of safer transient vectors (Kaji et al., 2009; Okita et al., 2008; Stadtfeld et al., 2008; Woltjen et al., 2009; Yu et al., 2009), transduction of recombinant proteins (Kim et al., 2009; Zhou et al., 2009) or the use of synthetic modified mRNA (Warren et al., 2010), proof-of-principle studies demonstrating functional recovery following transplantation of human iPS-derived stem cell preparations into animal models of disease are still lacking. Although one study has previously documented evidence of skeletal muscle differentiation after intra-muscular transplantation of human ES cells (Barberi et al., 2007), this was very limited and only a few myogenic cells were observed in vitro and in vivo. Moreover, these were not performed in a dystrophic mouse model, making it difficult to assess therapeutic relevance.
In this study we demonstrate for the first time the feasibility of generating large quantities of human ES- and iPS-derived early skeletal myogenic progenitors that are endowed with the ability to promote regeneration in vivo, restoring not only dystrophin expression in an immunodeficient model of Duchenne muscular dystrophy, but also improving the force generation of engrafted muscles. We also show that Pax7-induced human ES- and iPS-derived myogenic progenitors contribute to the satellite cell pool, and that engraftment is durable, being sustained for around half the lifespan of the animal, and most likely longer. It will be interesting to determine whether engraftment levels can be increased with different delivery or conditioning strategies. As irradiation was not used in these studies, in addition to the human ES/iPS-derived regeneration, there was also ongoing regeneration by host satellite cells. Thus it might be possible to improve engraftment levels by pre-conditioning muscles with irradiation (Skuk et al., 2010). Moreover, these cell preparations demonstrate significant scalability in response to maintained Pax7 expression. In the experiments described here, >80-fold expansion was achieved over two weeks, and much greater expansion is likely possible, potentially facilitating delivery of much larger numbers of cells. It will then be interesting to determine whether in addition to improved contractility, ES-/iPS-myogenic transplantation can improve more complex functional parameters such as resistance to eccentric exercise-induced injury, general motility, or, in more severe models such as mdx/mTR mice (Sacco et al., 2010), lifespan.
In the system we describe, the in vitro expansion potential and the in vivo functional regeneration of Pax7-derived myogenic progenitors allows one to envision producing therapeutic quantities of myogenic progenitor cells for clinical evaluation in muscular dystrophies. However before this is attempted, it will be necessary to establish non-genetic methods of delivering Pax7 to generate equivalent myogenic progenitors. These could include the utilization of safer transient vectors, transduction of recombinant proteins or the use of synthetic modified mRNA, approaches that have been used with success to generate integration-free iPS cells.
EXPERIMENTAL PROCEDURES
Human iPS induction
Human iPS cells were generated from adult human fibroblasts, as previously described (Dimos et al., 2008). Detailed information on the generation and fully characterization of the two iPS clones studied here, IPRN13.13 and IPRN14.57, is provided in Supplemental Experimental Procedures.
Generation of human inducible Pax7 ES and iPS cell lines
Human H9 ES cells and the iPS clones referred above were grown in feeder free conditions using mTeSR medium on human ESC qualified Matrigel (BD Biosciences) coated plates. To generate iPax7 pluripotent cells, ES and iPS cell cells were transduced with a lentiviral vector expressing the reverse tet-transactivator (rtTA) (Bosnakovski et al., 2008). The full length human Pax7 cDNA (Clone ID. 40121582, Open Biosystems) was sub-cloned into pSAM2, a lentiviral construct containing the transactivator, a second-generation tet-responsive element (sgTRE), which allows the expression of the target gene upon doxycycline (dox) induction, and IRES-EGFP, which allows confirmation of integration and inducible expression (Bosnakovski et al., 2008). Vectors were co-transfected with packaging and coat protein constructs Δ8.91 and pVSVG into 293T cells using the FuGENE 6 transfection reagent (Roche). Virus-containing supernatant was collected 48 hours after transfection, filtered through a 0.45 μm filter and used for infection. Human ES cells and iPS cells were co- infected with rtTA and pSAM2-Pax7 simultaneously. ES/iPS cells containing the Pax7 insert were purified by FACS based on GFP expression following an overnight incubation with dox (Sigma) at 0.75μg/ml.
Differentiation of human ES/iPS cells into myogenic progenitors
Detailed information is provided in Supplemental Experimental Procedures.
Real Time PCR analysis
Real time PCR for muscle specific genes was performed using probe sets from Applied Biosystems.
FACS characterization
A detailed description is provided in Supplemental Experimental Procedures.
NSG-mdx4Cv mice
Mdx4Cv (B6Rox.Cg-Dmdmdx-4Cv/J) and NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice were purchased from Jackson Laboratories (stock numbers 002378 and 005557), respectively. The dystrophin and IL2Rg genes are both X-linked: recombinant X chromosomes were isolated by crossing females bearing these mutations in trans with wild-type males. One male and one female recombinant were identified. The line was then established by sib-mating, selecting for homozygosity of the Prkdcscid mutation, and kept as mixed-inbred.
Transplantation studies
Animal experiments were carried out according to protocols approved by the University of Minnesota Institutional Animal Care and Use Committee. 5–8 week old male NSG mice from Jackson labs (stock number 002378) and NSG-mdx4Cv mice (above) were used for these in vivo studies. Before intramuscular cell transplantation, mice were pre-injured with cardiotoxin as previously described (Darabi et al., 2008). 24 hrs after cardiotoxin damage into both tibialis anterior (TA) muscles, myogenic progenitors from iPax7 ES or iPS cells (3 x 105 cells/15μl PBS) were injected into left TA muscles, while right leg received same volume of PBS as negative control.
Muscle preparation for mechanical studies
For the measurement of contractile properties, mice were anaesthetized with avertin (250 mg/kg I.P.) and intact tibialis anterior (TA) muscles were dissected and placed in an experimental organ bath as previously described in detail (Darabi et al., 2008). Detailed information is provided in Supplemental Experimental Procedures.
Immunofluorescence staining of cultured cells and tissue sections
Two months after transplantation, muscles were harvested and frozen in isopentane cooled in liquid nitrogen. Serial 8 to 12μm cryosections were collected. For immunofluorescence staining, cells cultured on slides and tissue cryosections either were fixed using cold acetone or 4% PFA or unfixed (in case of human dystrophin staining), permeabilized with 0.3 % Triton X-100 (Sigma), and blocked with 3% BSA and 0.01% Triton X-100 in PBS, and then incubated with appropriate antibodies. For satellite cell quantification, slides were stained with DAPI, Pax7, human Lamin AC, and Laminin (Skuk et al., 2010), and the number of mouse- (Pax7+Lamin AC−) vs. human-derived (Pax7+Lamin AC+) satellite cells were quantified in pan-cross sections of TA muscles (2 pan sections/mice- 10 per group). Absolute numbers and respective percentages were calculated and plotted. All antibodies are listed in Supplemental Experimental Procedures.
Statistical analysis
Differences between samples were assessed by using the Student’s two-tailed t test for independent samples.
Supplementary Material
HIGHLIGHTS.
Pax7 drives generation and expansion of muscle progenitors from human ES/iPS cells
ES/iPS-derived muscle progenitors restore dystrophin in immunodeficient mdx mice
Engraftment is accompanied by increase in muscle strength
Long-term engraftment and satellite cell contribution indicate clinical relevance
Acknowledgments
This project was supported by NIH grants RC1 AR058118, R01 AR055299, RC2 AR058919, R01 AR055685, R21 AG034370, and P01 GM081627. We also thank the generous support from the Dr. Bob and Jean Smith Foundation. The monoclonal antibody to MHC was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa. We thank Cynthia Dekay for assistance in graphic design, and members of iPierian’s R&D team for technical support.
Footnotes
AUTHORSHIP STATEMENT CONTRIBUTION
R.D. designed and conducted the in vitro and in vivo experiments with iPax7 ES and iPS cells, performed final analysis of the data, and contributed to writing the paper. S.I, J.D. and M.G. designed and conducted experiments regarding the generation and characterization of iPS cells. R.W.A and M.K. developed the NSG-mdx4Cv mice and contributed to writing the paper. R.C.R.P. supervised the overall project, designed experiments, analyzed the data and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alipio Z, Liao W, Roemer EJ, Waner M, Fink LM, Ward DC, Ma Y. Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic beta-like cells. Proc Natl Acad Sci U S A. 2010;107:13426–13431. doi: 10.1073/pnas.1007884107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- Bosnakovski D, Xu Z, Gang EJ, Galindo CL, Liu M, Simsek T, Garner HR, Agha-Mohammadi S, Tassin A, Coppee F, et al. An isogenetic myoblast expression screen identifies DUX4-mediated FSHD-associated molecular pathologies. EMBO J. 2008;27:2766–2779. doi: 10.1038/emboj.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Coulton GR, Rogers B, Strutt P, Skynner MJ, Watt DJ. In situ localisation of single-stranded DNA breaks in nuclei of a subpopulation of cells within regenerating skeletal muscle of the dystrophic mdx mouse. J Cell Sci. 1992;102(Pt 3):653–662. doi: 10.1242/jcs.102.3.653. [DOI] [PubMed] [Google Scholar]
- Danko I, Chapman V, Wolff JA. The frequency of revertants in mdx mouse genetic models for Duchenne muscular dystrophy. Pediatr Res. 1992;32:128–131. doi: 10.1203/00006450-199207000-00025. [DOI] [PubMed] [Google Scholar]
- Darabi R, Gehlbach K, Bachoo RM, Kamath S, Osawa M, Kamm KE, Kyba M, Perlingeiro RC. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat Med. 2008;14:134–143. doi: 10.1038/nm1705. [DOI] [PubMed] [Google Scholar]
- Darabi R, Pan W, Bosnakovski D, Baik J, Kyba M, Perlingeiro RC. Functional Myogenic Engraftment from Mouse iPS Cells. Stem Cell Rev. 2011a;7:948–957. doi: 10.1007/s12015-011-9258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi R, Santos FNC, Filareto A, Pan W, Koene R, Rudnicki M, Kyba M, Perlingeiro RCR. Assessment of the Myogenic Stem Cell Compartment Following Transplantation of Pax3/Pax7-Induced Embryonic Stem Cell-Derived Progenitors. Stem Cells. 2011b;29:777–790. doi: 10.1002/stem.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev. 2002;12:349–361. doi: 10.1016/s0959-437x(02)00309-x. [DOI] [PubMed] [Google Scholar]
- Emery AE. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- Espejel S, Roll GR, McLaughlin KJ, Lee AY, Zhang JY, Laird DJ, Okita K, Yamanaka S, Willenbring H. Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice. J Clin Invest. 2010;120:3120–3126. doi: 10.1172/JCI43267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenechea G, Segovia JC, Albella B, Lamana M, Ramírez M, Regidor C, Fernández MN, Bueren JA. Delayed engraftment of nonobese diabetic/severe combined immunodeficient mice transplanted with ex vivo-expanded human CD34(+) cord blood cells. Blood. 1999;93:1097–1105. [PubMed] [Google Scholar]
- Hanna JWM, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- Hargus G, Cooper O, Deleidi M, Levy A, Lee K, Marlow E, Yow A, Soldner F, Hockemeyer D, Hallett PJ, et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci U S A. 2010;107:15921–15926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im WB, Phelps SF, Copen EH, Adams EG, Slightom JL, Chamberlain JS. Differential expression of dystrophin isoforms in strains of mdx mice with different mutations. Hum Mol Genet. 1996;5:1149–1153. doi: 10.1093/hmg/5.8.1149. [DOI] [PubMed] [Google Scholar]
- Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JR, Kissel JT, Amato AA, King W, Signore L, Prior TW, Sahenk Z, Benson S, McAndrew PE, Rice R, et al. Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. N Engl J Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Chang H, Umeda K, Niwa A, Iwasa T, Awaya T, Fukada S, Yamamoto H, Yamanaka S, Nakahata T, et al. Generation of skeletal muscle stem/progenitor cells from murine induced pluripotent stem cells. FASEB J. 2010;24:2245–2253. doi: 10.1096/fj.09-137174. [DOI] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Oustanina S, Hause G, Braun T. Pax7 directs postnal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 2004;23:3430–3439. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Péault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, Gussoni E, Kunkel LM, Huard J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- Rideout WM, 3rd, Hochedlinger K, Kyba M, Daley GQ, Jaenisch R. Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell. 2002;109:17–27. doi: 10.1016/s0092-8674(02)00681-5. [DOI] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143:1059–1071. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cellls. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Sherwood RI, Christensen JL, Weissman IL, Wagers AJ. Determinants of skeletal muscle contributions from circulating cells, bone marrow cells, and hematopoietic stem cells. Stem Cells. 2004;22:1292–1304. doi: 10.1634/stemcells.2004-0090. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- Skuk D, Paradis M, Goulet M, Chapdelaine P, Rothstein DM, Tremblay JP. Intramuscular transplantation of human postnatal myoblasts generates functional donor-derived satellite cells. Mol Ther. 2010;18:1689–1697. doi: 10.1038/mt.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabayoyong WB, Salas JG, Bonde S, Zavazava N. HOXB4-transduced embryonic stem cell-derived Lin-c-kit+ and Lin-Sca-1+ hematopoietic progenitors express H60 and are targeted by NK cells. J Immunol. 2009;183:5449–5457. doi: 10.4049/jimmunol.0901807. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Vilquin JT. Myoblast transplantation: clinical trials and perspectives. Acta Myol. 2005;24:119–127. [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci U S A. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hamalainen R, Cowling R, Wang W, Liu P, Gertsenstein M, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Alipio Z, Fink LM, Adcock DM, Yang J, Ward DC, Ma Y. Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proc Natl Acad Sci U S A. 2009;106:808–813. doi: 10.1073/pnas.0812090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.