Summary
The interplay between mitochondrial energetics, lipid balance and muscle insulin sensitivity has remained a topic of intense interest and debate for decades. One popular view suggests that increased oxidative capacity benefits metabolic wellness; based on the premise that it is healthier to burn fat than glucose. Attempts to test this hypothesis using genetically-modified mouse models have produced contradictory results; and instead link muscle insulin resistance to excessive fat oxidation, acylcarnitine production and increased mitochondrial H2O2 emitting potential. Here, we consider emerging evidence that insulin action in muscle is driven principally by mitochondrial load and redox signaling rather than oxidative capacity.
Introduction
Since the early discovery that type 2 diabetes is a disease intimately connected to whole body lipid imbalance, scientists in this field have been both fascinated and frustrated by studies aimed at understanding the interplay between glucose and fatty acid metabolism. A fundamentally important question still heavily debated is whether or not a shift in substrate preference towards fat oxidation lowers disease risk. There is no disputing that lipid oxidation confers a metabolic advantage during starvation and exercise, but the role of fuel selection per se in defending against metabolic disease remains elusive. This paper highlights emerging controversies surrounding the roles of mitochondrial function and fat oxidation in regulating insulin action, and questions the popular paradigm that it is healthier to burn fat than glucose. Instead, the etiology of metabolic disease is considered from the perspective of metabolic balance and mitochondrial bioenergetics.
Metabolic feedback: Substrate competition versus lipotoxicity
The idea that substrates compete with one another to support respiration likely dates back to the first calorimetry studies conducted in the early twentieth century (Benedict and Joslin, 1910). It was not until the early 1960s however before inhibition of glucose oxidation by fatty acids (and ketone bodies) was experimentally demonstrated in perfused hearts and diaphragm (Garland et al., 1962; Newsholme et al., 1962). In a subsequent set of now classic studies, Randle and colleagues proposed the concept of the glucose-fatty acid cycle (Randle et al., 1963). The model was developed based on the finding that increasing the supply and oxidation of fatty acids leads to cellular accumulation of acetyl-CoA, NADH and ATP, which allosterically inhibit pyruvate dehydrogenase (PDH), the mitochondrial enzyme complex that couples glycolysis to glucose oxidation. This same set of allosteric effectors activates a family of pyruvate dehydrogenase kinases that phosphorylate PDH, further inhibiting the catalytic activity of the enzyme (Sugden and Holness, 2006). Randle further proposed that a rise in cellular citrate would inhibit phosphofructokinase-1 (Denton and Randle, 1966), and that lowering of glycolysis and pyruvate oxidation would result in accumulation of glucose-6-phosphate, leading to allosteric inhibition of hexokinase and thus diminished glucose uptake.
In recent years the glucose-fatty acid substrate competition model of diabetes has been overshadowed by emphasis on alternative mechanisms that involve ectopic accumulation of triacylglycerol and other lipid molecules in liver and muscle. The spotlight on this area of investigation intensified when Shulman and co-workers challenged the idea that insulin resistance stems from fatty acid-induced inhibition of hexokinase II. Using stable isotopes and NMR methodology these studies pointed to glucose uptake rather than glucose phosphorylation as the rate-limiting barrier for muscle glucose disposal in type 2 diabetic subjects (Shulman, 2000). Subsequent studies identified intramyocellular lipid content as a strong predictor of insulin resistance. Together, these observations led to the conclusion that lipid-induced insulin resistance is not due to inhibition of glycolysis and glucose oxidation, but instead stems from a more direct and proximal mechanism wherein tissue accumulation of lipid signaling molecules such as diacylglycerol and ceramide disrupt insulin-stimulated translocation of the GLUT4 glucose transporter. Support for this model grew from numerous reports showing that intramuscular and/or hepatic content of triacylglycerol, diacylglycerol and/or ceramide correlate negatively with insulin sensitivity (Erion and Shulman, 2010; Goodpaster and Kelley, 2002; Holland and Summers, 2008). This relationship is evident in obese and diabetic humans as well as in several rodent and cell culture models of metabolic disease and/or chronic lipid exposure (An et al., 2004; Hulver et al., 2003; Nagle et al., 2009). Taken together, these observations have fostered widespread support for the idea that ectopic lipid accumulation (i.e. “lipotoxicity”) in muscle and liver is the principal contributor to obesity-associated insulin resistance.
Mitochondrial dysfunction: Cause or consequence of insulin resistance?
With evidence mounting in support of a role for ectopic fat accumulation as a primary cause of insulin resistance, attention turned to identifying potential mechanisms that underlie lipid imbalance within the muscle. Human studies that measured whole body substrate selection by indirect calorimetry, fatty acid flux across the leg, fatty acid oxidation in isolated skeletal muscle strips and/or maximal activities of various oxidative enzymes in biopsy specimens, strongly suggested that skeletal muscle capacity to oxidize fat is lower in obese compared to lean subjects; with the apparent mitochondrial insufficiencies being most pronounced in the severely obese and/or diabetic conditions (Hulver et al., 2003; Kelley et al., 2002; Kim et al., 2000; Noland et al., 2003; Simoneau et al., 1995; Simoneau and Kelley, 1997; Simoneau et al., 1999). A notable caveat of these and several similar animal studies is that the methods used did not account for potential label dilution effects due to hydrolysis and catabolism of endogenously-derived lipids, which are more abundant in the obese state (Boyle et al., 2011; Thyfault et al., 2010). Nonetheless, investigators found that insulin sensitivity correlated positively with skeletal muscle oxidative potential, assessed by enzyme activities as well as genome-wide expression arrays (Patti et al., 2003). Additional investigations employing non-invasive magnetic resonance spectroscopy (MRS) found that both mitochondrial substrate flux and/or ATP synthesis rates were depressed in muscle of elderly individuals with insulin resistance (Petersen et al., 2003) and in lean, insulin-resistant young subjects with a family history of type 2 diabetes (Petersen et al., 2004). These and other studies led to the proposal that insulin resistant humans have faulty muscle mitochondria and intrinsic deficiencies in oxidative metabolism that impinge on insulin signaling by diverting fatty acids away from oxidation and toward production of diacylglycerols and other toxic lipid species (Lowell and Shulman, 2005; Roden, 2005).
Several lines of evidence however do not support the idea that mitochondrial dysfunction is a root cause of lipotoxicity and insulin resistance. First, conceptually this hypothesis is incompatible with the principles of bioenergetics. Mitochondrial respiration in all cells is governed by energy demand; i.e., the energy utilized to support the basal or “idling” activity of the respiratory system plus that required for ATP regeneration. Lower rates of substrate flux and/or ATP synthesis measured at rest should not be interpreted as evidence of mitochondrial dysfunction nor blamed for local accumulation of lipid metabolites. In general, even obese and/or diabetic individuals have enough mitochondrial reserve to substantially increase substrate flux and ATP synthesis to meet the energy demands of light to moderate contractile activity. Similarly, individual differences in muscle mitochondrial content and the specific activities of β-oxidation enzymes should have little bearing on intramuscular lipid accumulation because oxidative phosphorylation is rarely operating at maximal capacity. Thus, regardless of mitochondrial oxidative capacity, intracellular lipids amass whenever the supply of fatty acids exceeds the energy needs of the cell. In other words, supply must adjust to demand, not vice versa. Secondly, high fat feeding in rodents actually increases rather than decreases muscle capacity to catabolize lipids due to upregulation of several key mitochondrial and peroxisomal enzymes (Hancock et al., 2008; Koves et al., 2005; Muoio; Noland et al., 2007; Turner et al., 2007). Despite adaptations that favor fat catabolism, ectopic lipids still accumulate because supply outpaces demand. It is only after several months on a high fat diet that mitochondrial oxidative capacity declines (Bonnard et al., 2008). Lastly, direct and rather compelling experimental evidence arguing against the mitochondrial dysfunction theory comes from several distinct transgenic mouse models with targeted disruptions in specific mitochondrial proteins or master transcriptional regulators of mitochondrial biogenesis. As anticipated these mice have severely compromised oxidative performance, but contrary to the predicted development of insulin resistance, many of these transgenic models display increased insulin sensitivity relative to wild type mice, even when challenged with a fat-enriched diet (reviewed in (Muoio, 2010)).
The potential impact of changes in skeletal muscle mitochondrial content however should not be lost in the cause or consequence debate. Electron microscopy images of skeletal muscle from mice on a prolonged high fat diet reveal profound changes in the appearance of mitochondria, including visible swelling and disarrayed cristae (Bonnard et al., 2008). Skeletal muscle from obese humans with or without diabetes is characterized by reduced overall mitochondrial content with evidence of morphological changes similar to those in the animal models (Chomentowski et al., 2011; Kelley et al., 2002; Ritov et al., 2005). These findings suggest that at some point in the etiology of diet-induced obesity/diabetes, mitochondrial architecture begins to deteriorate, ultimately leading to mitophagy and the complete dissolution of the organelle (Mitsuhashi et al., 2011). This loss of mitochondrial integrity is likely to compromise exercise tolerance. Moreover, because an estimated 10–25% of resting metabolic rate is attributed to respiration that supports mitochondrial proton leak (i.e., “idling”) (Rolfe and Brown, 1997), and because skeletal muscle represents a high percentage of body mass, a decline in mitochondrial density in skeletal muscle due to disease or aging could decrease overall basal metabolic rate and thus energy requirements (Brand, 1990). Without a corresponding reduction in caloric intake, the probability that fuel supply will exceed demand increases, not because of diminished oxidative capacity but because the costs of maintaining energy balance decrease.
Metabolic imbalance: Flooding the mitochondria
What does happen to muscle mitochondria during the initial phases of overnutrition and insulin resistance? Emerging evidence suggests that the carbon load on the muscle’s oxidative machinery begins to mount, such that the rate of fatty acid catabolism persistently surpasses the drive for ATP resynthesis. This situation first came to light with the discovery that both diet-induced and genetic forms of insulin resistance were associated with high rates of incomplete fat oxidation, evidenced by intramuscular accumulation of several mitochondrial-derived fatty acylcarnitine intermediates and reduced levels of TCA cycle intermediates (Koves et al., 2005; Koves et al., 2008). The acylcarnitines are produced by a family of acyltransferase enzymes that reside principally in the mitochondria and convert acyl-CoA molecules to their cognate carnitine esters. These metabolites are generally viewed as byproducts of mitochondrial metabolism that report on cellular fluctuations in substrate supply and flux limitations at specific catabolic enzymes. Interpretation of the foregoing metabolite profiles was informed by experiments in which fuel metabolism, mitochondrial function and respiratory capacity were assessed by several complementary methods, including whole body indirect calorimetry, in vitro flux analysis using radiolabeled tracers, and polarographic measurement of respiration. In aggregate, the results showed that the early stages of diet-induced insulin resistance are characterized by increasing β-oxidation and no change in respiratory capacity (Hancock et al., 2008; Kraegen et al., 2008; Noland et al., 2009). Similar abnormalities in acylcarnitine accumulation and incomplete β-oxidation have been identified in humans with obesity or type 2 diabetes (Adams et al., 2009; Huffman et al., 2009; Mihalik et al., 2010) and in primary human skeletal myocytes derived from obese compared to lean subjects (Bell et al., 2010; Kovalik et al., 2011). In the ensuing discussion we consider the biological relevance of these observations viewed against a backdrop of recent reports that have reshaped our current understanding of mitochondrial bioenergetics and insulin action.
Fueling the furnace: Accelerating versus overloading β-oxidation
Widespread speculation that a ‘defect’ in mitochondrial fatty acid oxidation is a major factor in the etiology of diet-induced insulin resistance raised the prospect that any intervention that promotes fatty acid oxidation should relieve the toxicity caused by accumulated lipid metabolites. This logic formed the basis of numerous studies that used genetic or pharmaceutical approaches to investigate the consequences of enhanced β-oxidation; the outcomes of which are seemingly conflicted. However, in many instances, the importance of accounting for all energy balance parameters was overlooked, which in turn compromised the original interpretation of the work and contributed to growing confusion in the literature. A careful reconsideration of some of these studies in the context of bioenergetics offers some surprising clarity.
An enlightening example that speaks to this discussion is the recent controversy over the utility of acetyl-CoA carboxylase 2 (ACC2) as a potential anti-obesity drug target. ACC2 appears to be localized on the outer mitochondrial membrane (Abu-Elheiga et al., 2001) where it catalyzes the synthesis of malonyl-CoA, a potent inhibitor of carnitine palmitoyltransferase-1 (CPT-1); the enzyme that functions as the gatekeeper for fatty acid entry into the mitochondria. Absence of ACC2 activity would thus be predicted to limit formation of malonyl-CoA and favor fatty acid oxidation. Indeed, ACC2−/− mice proved to have higher whole-body and muscle fatty acid oxidation rates (Abu-Elheiga et al., 2001; Abu-Elheiga et al., 2003). These animals were also hyperphagic but maintained normal body weight, glycemic control and muscle insulin sensitivity, even when fed a high fat diet (Abu-Elheiga et al., 2003; Choi et al., 2007). Both fat and carbohydrate oxidation were simultaneously increased in ACC2−/− mice, which was curiously credited as the impetus for increased total body energy expenditure without consideration of potential changes in energy demand (Choi et al., 2007). Nevertheless, based on the reduced fat mass and improved glucose metabolism evident in these mice, ACC2 became a prime target for pharmaceutical development (Keil et al., 2010). Intriguingly however, two other groups have recently generated whole body- and muscle-specific ACC2 knockout models and found no effect of the gene deletion on body weight, food intake, body composition and susceptibility to diet-induced obesity and glucose intolerance (Hoehn et al., 2010; Olson et al., 2010). Instead, the genetically engineered switch to fat catabolism was offset by diminished glucose oxidation and a reciprocal rise in de novo lipogenesis. In these second generation models total energy expenditure was either unaffected (Hoehn et al., 2010) or only slightly increased (Olson et al., 2010). Taken together, these findings suggest that simply shifting substrate selection in favor of fat oxidation does not protect against diet-induced insulin resistance when whole body energy balance is unchanged.
CPT-1 itself has been a favorite target of studies aimed at elucidating the relationship between fat oxidation and insulin action; however, this approach has produced mixed interpretations. In type 2 diabetic humans and in obese rodents, oral administration of CPT-1 inhibitors such as etomoxir and oxfenicine consistently elevates glucose oxidation and ameliorates hyperglycemia and hyperinsulinemia (Barnett et al., 1992; Deems et al., 1998; Dobbins et al., 2001; Fragasso et al., 2009; Hubinger et al., 1997). However, when insulin sensitivity was assessed by the euglycemic clamp technique, some studies reported improvements after etomoxir treatment whereas others found the opposite effect (Dobbins et al., 2001; Hubinger et al., 1997). Interpretation of these studies is further complicated by the observation that chronic administration of etoxomir causes severe hepatic steatosis, while also provoking a state of heightened oxidative stress (Vickers et al., 2006). Thus, potent inhibition of liver CPT-1 clearly leads to undesirable outcomes, whereas the potential utility of specifically inhibiting skeletal muscle CPT-1 remains unclear. Conversely, overexpression of CPT-1 in cultured myocytes or rat skeletal muscles using recombinant adenovirus or electroporation techniques has been shown to enhance insulin-stimulated glucose uptake (Bruce et al., 2009; Henique et al., 2010; Perdomo et al., 2004; Sebastian et al., 2007). In these studies investigators attributed improvements in insulin action to relatively modest increases in fat oxidation, assessed by CO2 production assays. Notably however, the genetic approaches used in these studies yield notoriously poor transfection efficiencies, thereby raising the question of whether enhanced insulin action measured in the entire population of myocytes/myofibers was actually due to events occurring within the transfected cells. It is possible that a minority population of myocytes harboring massive overexpression of CPT-1 protected neighboring cells by acting as a sink for surplus fatty acids, due not only to small increases in complete fat oxidation but also by virtue of their robust capacity to produce and export long chain acylcarnitines (Noland et al., 2009). Moreover, massive overexpression of any mitochondrial membrane protein tends to disrupt the native stoichiometry and integrity of the mitochondrial membrane, generating an artifactual acceleration in proton conductance and a concomitant increase in energy expenditure (Cadenas et al., 2002; Harper et al., 2002; Henique et al., 2010; Perdomo et al., 2004; Sebastian et al., 2007)
Another approach that is commonly deployed to evaluate the role of mitochondrial fatty acid oxidation in the etiology of insulin resistance centers on exploitation of the peroxisome proliferator-activated receptors (PPAR α, β/δ and ), a family of lipid-activated nuclear receptors that stimulate expression of genes involved in transport and oxidation of fatty acids (Desvergne et al., 1998). Administration of synthetic agonists of PPARα and PPARβ/δ have been shown to ameliorate lipid-induced insulin resistance in C2C12 cells and obese rodents (Coll et al., 2010; Guerre-Millo et al., 2000; Kramer et al., 2005; Tanaka et al., 2003). Based on these findings, muscle-specific transgenic overexpression of PPARα or PPARγ coactivator-1α (PGC-1α), a promiscuous nuclear receptor co-activator that promotes mitochondrial biogenesis, was expected to defend against metabolic disease. However, despite impressive upregulation of several oxidative enzymes and markedly enhanced capacity for fat oxidation in muscle, both of these transgenic mouse models were more susceptible to diet-induced insulin resistance (Choi et al., 2008; Finck et al., 2005).
Why do pharmacological and genetic activation of the same gene induce opposite responses? The answer probably relates to cellular bioenergetics. In cultured L6 myotubes, the repressive effects of PPARα overexpression on glucose uptake are reversed by addition of dinitrophenol (Finck et al., 2005), a protonophore that elevates energy demand by increasing proton conductance of the mitochondrial inner membrane. This implies that PPARα overexpression creates a situation wherein forced flux through β-oxidation outpaces the demand of the respiratory system, and that the added reducing pressure imposed by high rates of incomplete oxidation interferes with insulin action. This possibility is underscored by the finding that relief was gained simply by increasing energy demand. But why then do synthetic agonists of the PPARs appear to improve rather than impair insulin sensitivity? Here again, changes in the rates of β-oxidation must be considered in the context of energy demand. Treating mice with PPARα or PPARβ/δ agonists also induces, either directly or indirectly, an increase in whole body oxygen consumption without affecting caloric intake, thus accounting for lower rates of weight gain in response to high fat feeding (Guerre-Millo et al., 2000; Tanaka et al., 2003). Whereas treatments with PPAR agonists do indeed encourage a shift in metabolic currency towards fat oxidation, the drug-induced rise in total body energy expenditure is likely to underlie the absolute increase in flux through β-oxidation, thereby mitigating the “mitochondrial stress” normally associated with overnutrition.
Fuel selection and metabolic flexibility: Timing is everything
The concept of metabolic flexibility emphasizes the importance of choosing the right fuel at the right time. In recent years it has become increasingly apparent that nutrient-induced substrate switching is often compromised in the settings of obesity and type 2 diabetes (Kelley and Mandarino, 2000). This phenomenon, commonly known as “metabolic inflexibility”, was first introduced in a report showing that healthy individuals shift from robust fat oxidation in the fasted state to a high rate of glucose oxidation in response to a hyperinsulinemic-euglycemic clamp (measured by whole body or leg RQ), whereas obese and type 2 diabetic subjects showed little response (Kelley et al., 1999). Similar findings emerged from several subsequent studies in both humans and rodents (Koves et al., 2008; van Herpen et al., 2011). The molecular basis and pathophysiological relevance of metabolic inflexibility remain uncertain. One viewpoint suggests that the inability to appropriately activate glucose oxidation in response to a meal simply reflects a secondary consequence of impaired insulin action (Galgani et al., 2008). Evidence for a more complex scenario comes from the finding that obesity-induced perturbations in substrate switching are evident in isolated mitochondria (Koves et al., 2008; Noland et al., 2009), suggesting that defects in mitochondrial fuel selection are at least partly independent of insulin signaling and could contribute to impaired glucose disposal at the systemic level.
The metabolic inflexibility paradigm leads back to the question of whether chronic oversupply of lipid fuel results in persistent suppression of PDH activity, as originally proposed by Randle. Muscle accumulation of acylcarnitines and pyruvate observed in insulin resistant states (Boyle et al., 2011; Thyfault et al., 2010) is consistent with an increase in mitochondrial acyl CoA levels that impose negative feedback on PDH. The notion that pyruvate trafficking can indeed impact systemic glucose homeostasis is strengthened by studies in mice lacking PDH kinase 4, which have elevated PDH activity and enhanced glucose tolerance (Jeoung and Harris, 2008). Moreover, recent evidence suggests that lowering of mitochondrial acyl CoAs, resulting in increased PDH activity and improved metabolic flexibility, can be achieved via dietary supplementation with L-carnitine (Noland et al., 2009; Power et al., 2007). In these studies, improvements in metabolic function at the whole body and mitochondrial level corresponded with robust increases in plasma and urinary concentrations of acetylcarnitine (Noland et al., 2009), a metabolite that is produced from acetyl CoA and carnitine by the mitochondrial matrix enzyme carnitine acetyltransferase (CrAT). Unlike their acyl-CoA precursors, acylcarnitines can traverse cellular membranes. Accordingly, interconversion of these molecules is thought to play a key role in regulating mitochondrial acyl-CoA balance. Ensuing studies in mice with conditional deletion of CrAT confirmed an essential role for this enzyme in defending whole body glucose homeostasis (Muoio et al., (in press)). Thus, muscle-specific ablation of CrAT resulted in systemic glucose intolerance, complete loss of carnitine-stimulated PDH activity and blunted switching from fatty acid to glucose substrate at the whole body level and in isolated mitochondria. These results suggest that CrAT opposes the Randle cycle by permitting mitochondrial efflux of excess carbons, which thereby facilitates an efficient transition from fat to glucose during the fasted to fed transition.
Mitochondrial stress: A contemporary view of the Randle cycle
The acylcarnitine signature of insulin resistant states raises speculation that muscle insulin sensitivity is governed by mitochondrial load, rather than oxidative potential. Thus, disturbances in substrate selection, anaplerotic and cataplerotic carbon flux, production of reactive oxygen species (ROS), and intracellular redox balance might report on local energetic tone as a means to coordinate insulin sensitivity with cellular demand for glucose substrate. If true, how might lipid-overloaded mitochondria antagonize insulin action? Although decades of research have firmly established a reciprocal relationship between glucose and fat oxidation, the role of β-oxidation per se as an underlying cause of obesity-associated glucose intolerance remains a topic of heavy debate. Still uncertain is whether or not persistent suppression of PDH and PFK-1 leads to accumulation of glucose-6-phosphate and inhibition of hexokinase II. Whereas the aforementioned NMR-based studies in diabetic humans disputed this mechanism (Shulman, 2000), experiments employing a counter-transport technique concluded that limitations to muscle glucose uptake in rodents fed a high fat diet reside at the levels of glucose delivery and phosphorylation, as well as GLUT4 transport (Wasserman, 2009). These results fit a model of distributed flux control wherein the barrier to muscle glucose uptake shifts from the transport step to phosphorylation and metabolism once the GLUT4 transporters are mobilized by insulin or exercise (Wasserman, 2009). Accordingly, feedback inhibition of PDH and hexokinase might serve as the major mode of glucose disuse during the early phases of weight gain and hyperinsulinemia, prior to the onset of overt defects in insulin signaling.
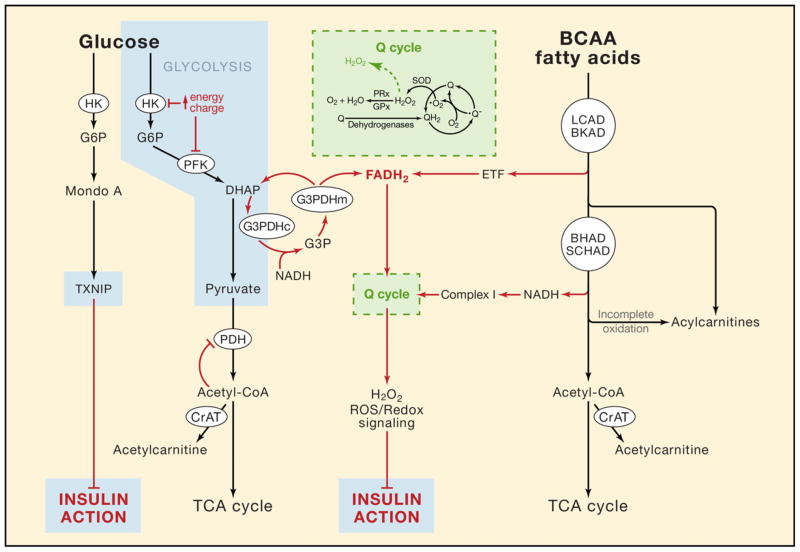
Compartmentalization of glycolytic intermediates might also factor into the foregoing discrepancies. For example, hexokinases I and II can translocate to the mitochondrial outer membrane where their catalytic activities are less sensitive to product inhibition (Azoulay-Zohar et al., 2004; Hashimoto and Wilson, 2002; John et al., 2011). Although the functional relevance of mitochondrial-associated hexokinase activity is unknown, a potential role in regulating glucose uptake is suggested by its intriguing connection to thioredoxin interacting protein (TXNIP), a redox-regulatory protein belonging to the α-arrestin family (Chutkow et al., 2010; Hui et al., 2008). TXNIP not only binds to and inhibits thioredoxin, but also antagonizes glucose uptake through a yet undefined mechanism (Parikh et al., 2007). In skeletal muscle, TXNIP gene expression is controlled by MondoA, a member of the bHLHZip family of transcription factors that dimerizes with another member of this family, MLX, to gain nuclear entry and DNA binding capacity. Interestingly, MondoA:MLX complexes are latently held at the outer mitochondrial membrane and translocate to the nucleus when glycolytic intermediates accumulate (Sloan and Ayer, 2010), an event that requires hexokinase activity (Stoltzman et al., 2008). TXNIP abundance is elevated in muscles of diabetic rodents and humans, suggesting that glycolytic intermediates are likewise increased under insulin resistant conditions. Taken together, these provocative new findings imply that co-localization of MondoA and hexokinase at the mitochondrial membrane reflects a mechanism that couples mitochondrial sensing of glycolytic flux to cellular glucose transport. Thus, competition between oxidative substrates might increase a mitochondrial-localized pool of glucose-6-phosphate that in turn triggers MondoA translocation and transcriptional induction of TXNIP to reduce uptake of unwanted glucose fuel (Figure 1).
Figure 1. Working model of nutrient-induced mitochondrial stress.
Fatty acids, branched chain amino acids (BCAA) and glucose are degraded to acetyl CoA, which serves as the universal substrate for the tricarboxylic acid cycle. Oversupply of these nutrients results in incomplete substrate catabolism and mitochondrial accumulation of acyl-CoA intermediates that are readily converted to their membrane permeant acylcarnitine counterparts. When substrate catabolism exceeds ATP demand, the high energy charge of the cell discourages glucose uptake by inhibiting hexokinase (HK), phosphofructokinase (PFK) and pyruvate dehydrogenase (PDH); and by activating thioredoxin interacting protein (TXNIP). Heightened reducing pressure (FADH2 and NADH) on the Q cycle of the electron transport chain promotes ROS generation (•O2 and H2O2) in excess of antioxidant capacity (e.g. superoxide dismutase (SOD), peroxiredoxin (PRx) and glutathione peroxidase (GPx)), thereby modulating metabolic enzymes and redox-sensitive signaling proteins that control fuel selection, glucose trafficking and insulin action. (BKAD, branched chain ketoacid dehydrogenase; BHAD, beta-hydroxyacyl-CoA dehydrogenase; CrAT, carnitine acetyltransferase; DHAP; dihydroxyacetone phosphate; ETF, electron transfer favoprotein; G3PDH, cytosolic(c)/mitochondrial(m) glycerol-3-phosphate dehydrogenase; LCAD, long chain acyl-CoA dehydrogenase; SCHAD, short chain hydroxyacyl-CoA dehydrogenase. See text for further detail.
Are there metabolic signals beyond glucose-6-phosphate that permit cross-talk between mitochondria and glucose transport? The acylcarnitines themselves are seemingly attractive candidates; however, the weight of evidence at this stage suggests that these metabolites function in a homeostatic capacity to relieve mitochondrial stress (Figure 1) (Muoio et al., (in press), Mingorance et al., 2011; Noland et al., 2009; Power et al., 2007). Another hypothesis that has gained momentum in recent years centers on the premise that mitochondrial-derived ROS modulate insulin signaling (Bloch-Damti and Bashan, 2005; Evans et al., 2005). This mechanism is attractive in that it could explain insulin resistance caused by overconsumption of sugar and protein, as well as fat. Thus, chronic elevations in incomplete oxidation of fatty acids and branched chain amino acids (Newgard, 2012) might foster a mitochondrial microenvironment that is conducive to ROS production. The initial steps in oxidation of fatty acids and amino acids (mediated by long chain acyl-CoA dehydrogenase (LCAD) and branched chain ketoacid dehydrogenase (BKAD), respectively) generate FADH2, which donates electrons directly to the ubiquinone (Q) cycle via the electron transfer protein (ETF). Two other FAD-linked complexes that feed into the Q cycle include complex II (succinate dehydrogenase), the entry point for amino acid-derived succinyl CoA, and the mitochondrial isoform of glycerol-3-phophate dehydrogenase, which shuttles electrons from cytosolically-localized glycolytic intermediates to the mitochondrial respiratory chain. Because these electrons bypass complex I, heightened flux through the foregoing enzyme complexes, in the absence of high ATP demand (i.e. exercise), is predicted to elevate reducing pressure within the system, particularly the backpressure on complex I, thereby increasing ROS production (reviewed in (Fisher-Wellman and Neufer, (in press)) (Figure 1). This sets the stage for a highly plausible scenario wherein chronic overabundance of these nutrients leads to persistent pressure on the ETC and resultant disruption of redox balance and ROS-signaling.
ROS are now viewed as bona fide signaling molecules and oxidant stress is known to activate several of the serine kinases and transcription factors that have been linked to insulin resistance, including c-jun amino-terminal kinases (JNK), IkB kinase catalytic subunit β (IKK-β), NF-kappa B transcription factor (NF-kB) and protein kinase C (PKC) (Bloch-Damti and Bashan, 2005; Chakraborti and Chakraborti, 1998)). Treatment of genetically obese/diabetic rodents with antioxidant nutrients has been shown to improve insulin action and glucose homeostasis (Henriksen, 2006; Hoehn et al., 2009; Houstis et al., 2006). A subsequent report provided compelling evidence linking insulin resistance to mitochondrial H2O2 emission (Anderson et al., 2009). In these studies, insulin resistant states were associated with increased H2O2 emission from muscle mitochondria and decreased tissue levels of reduced relative to oxidized glutathione (GSH:GSSG), a well-established biomarker of oxidative/reductive stress. Moreover, genetic or pharmacological manipulations that lowered mitochondrial ROS production preserved insulin action in lipid-treated myotubes (Hoehn et al., 2009) and in mice fed a high fat diet (Anderson et al., 2009; Hoehn et al., 2009; Lee et al., 2010). ROS signaling and redox sensing rely heavily on the interdependent glutathione and thioredoxin reducing systems. Both use the reducing power of NADPH to mitigate oxidative stress and to modulate reversible oxidation/reduction of protein thiols/disulfides. These so-called “sulfur switches” are gaining increasing recognition for their regulatory roles in cell signaling and metabolic control (Hui et al., 2008; Jones, 2008), and have recently been suggested to form an intracellular redox circuit linking mitochondrial H2O2 emission to the control of redox-sensitive phosphatases that target the insulin signaling pathway (reviewed in (Fisher-Wellman and Neufer, (in press))). This intriguing collection of findings calls for further investigation of ROS signaling and redox imbalance as an important connection between mitochondrial overload and insulin action.
Also relevant to the topic of mitochondrial stress are recent studies linking fuel metabolism and overnutrition to changes in the acylation state of mitochondrial proteins (Hirschey et al., 2009; Huang et al., 2010). Remarkably, application of mass spectrometry-based proteomics has led to the estimation that at least 20% of the mitochondrial proteome is acetylated. Moreover, every major pathway of intermediary metabolism was represented among the list of acetylated proteins. Subsequent studies identified three members of the sirtuin family of deacetylases; sirt3, sirt4 and sirt5, which localize to the mitochondria. Sirt3 is an established deacetylase whose targets include long chain acyl CoA dehydrogenase (LCAD), succinate dehydrogenase and Mn superoxide dismutase (SOD2) (Hirschey et al., 2010; Qiu et al., 2010). Interestingly, muscles of Sirt3 knockout mice have increased acetylation of LCAD, SOD2 and several ETC complexes, which is accompanied by elevated acylcarnitines, TBARS and pJNK (Jing et al., 2011; Qiu et al., 2010). These animals also develop multiple elements of the metabolic syndrome with aging (Hirschey et al., 2011). Moreover, recent studies have revealed novel roles for sirt4 and sirt5 in regulating protein malonylation and succinylation (Du et al., 2011; Peng et al., 2011; Zhang et al., 2011), implying that mitochondrial accumulation of various short chain acyl-CoAs could trigger broad-ranging metabolic consequences via their impact on protein modifications.
Stress relief: A matter of thermodynamics
In addition to those already discussed, a remarkable number of transgenic mouse models are resistant to high fat diet-induced obesity and insulin resistance (Chen and Farese, 2001; Reitman, 2002). The specific molecular targets are quite varied and too numerous to mention explicitly, but include genes involved in glucose metabolism (Hakimi et al., 2007), growth factor signaling (Molero et al., 2006), inflammation (Chiang et al., 2009), mitochondrial function (Li et al., 2000), lipid biosynthesis (Smith et al., 2000), lipid signaling (Mancuso et al., 2010) and transcriptional metabolic regulators (Fischer et al., 2009). Despite the diversity of these genetic targets, nearly all of these models have elevated whole-body VO2 with similar or even greater food intake relative their wildtype littermates. These findings suggest that increased energy expenditure is the common underlying mechanism accounting for the lean, insulin-sensitive phenotype. Elevated energy expenditure can arise only from an increase in thermogenesis (i.e., uncoupling), heavier reliance on inefficient ATP regenerating systems, enhanced carbon flux through futile energetic cycles, or increased physical/locomotor activity (Butler and Kozak, 2010). Also noteworthy, many of the same genetic manipulations resulted in the activation of 5’AMP-activated kinase (AMPK), which phosphorylates and inactivates ACC, thereby lowering cellular malonyl CoA levels and promoting fat oxidation. It is often presumed that antidiabetic protection associated with or afforded by heightened AMPK activity is due mainly to a rise in β-oxidation (Zhang et al., 2010). Seemingly overlooked, however, is that AMPK functions as a critical cellular sensor of energy deficit that in turn triggers a multifaceted cascade of catabolic responses at the levels of cell signaling, metabolism and transcriptional control (Kahn et al., 2005). Accordingly, its activation offers a strong hint that the maneuvers under consideration first elicited a negative energy balance in the affected tissues.
There are three points to draw from the preceding sections. First, because cellular and mitochondrial bioenergetics is governed by the rate of energy expenditure, any change in the rate of substrate oxidation must be considered in the context of energy demand. Second, because even a small increase in energy demand will completely relieve the reducing pressure and ROS production in mitochondria created by a positive energy balance, the increased energy expenditure evident in many of the mouse models that resist diet-induced metabolic abnormalities could represent the common underlying protective mechanism. The third point is that the flux rate through β-oxidation relative to energy demand appears to be a particularly important determinant of insulin sensitivity in skeletal muscle. Several lines of evidence support this contention. In the lean but severely insulin resistant muscle-specific PPARα transgenic mice, administration of the CPT-1 inhibitor oxfenicine blocked fatty acid oxidation and rescued insulin sensitivity, despite inducing profound lipid accumulation in muscle (Finck et al., 2005). Similarly, mice that were genetically-engineered to have restricted flux through β-oxidation in muscle maintain normal insulin sensitivity on a high fat diet even though lipid content of muscle, liver and/or adipose tissue remain elevated (Finck et al., 2005; Guerre-Millo et al., 2001; Koves et al., 2008; Tordjman et al., 2001). Moreover, whereas wildtype animals consuming a high fat diet manifest a persistent mismatch in mitochondrial lipid load relative to energy demand, resulting in muscle accumulation of partially oxidized fatty acylcarnitine intermediates (Koves et al., 2005; Koves et al., 2008), correction of this imbalance by one of three disparate strategies (lowering carbon import, increasing the rate of energy expenditure or enhancing rates of carbon efflux) restored both mitochondrial energetic balance and systemic glucose tolerance (Koves et al., 2005; Koves et al., 2008; Noland et al., 2009). Collectively, these data support the principle that increasing flux through β-oxidation without a corresponding boost in energy demand imposes unwarranted reducing pressure on the respiratory system, which in turn impinges upon redox balance and insulin action. Conversely, if flux through β-oxidation increases as a consequence of energy demand, the system then operates in metabolic balance and insulin sensitivity is maintained.
Closing remarks: Therapeutic implications
The past two decades of diabetes research have uncovered an intricate network of metabolic and molecular events that participate in obesity-related organ dysfunction and the subsequent demise of systemic glucose control. Prominent among these are perturbations in lipid signaling, inflammation, endoplasmic reticulum stress and mitochondrial stress. As revealed throughout this series of perspective articles, the signaling pathways involved in these responses intersect at multiple nodes and are likely to act in a collaborative fashion to dampen whole body insulin action. Interestingly, one common denominator that factors into each of the foregoing nutrient stress mechanisms is mitochondrial ROS production, which can be positioned both upstream and downstream of the foregoing cellular insults. Although a unifying mechanism of nutrient-induced insulin resistance seems improbable given the pathophysiological complexities of the disorder, ROS signaling might serve as a critical site of convergence and a potential avenue for nutraceutical and/or pharmaceutical intervention.
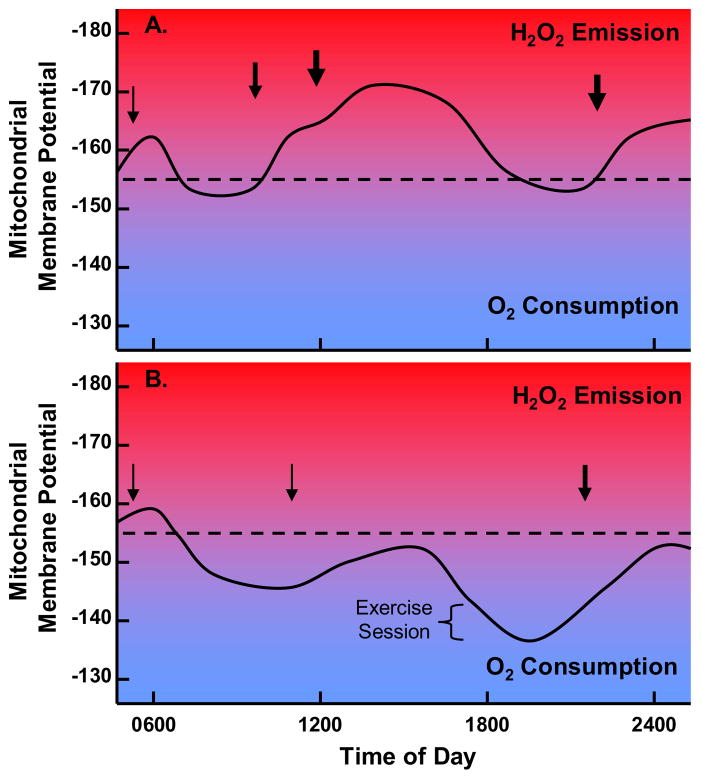
The relative contributions of various stress sensing mechanisms to the origin and progression of insulin resistance is likely to depend on tissue specificity, diet composition and genetic predisposition. The current article focused on the relationships between lipid stress, glucose disuse and mitochondrial metabolism in skeletal muscle. We highlighted a new wave of evidence in dispute of the simple prediction that lifestyle-related insulin resistance in muscle is caused by sluggish mitochondria with inherent deficits in fat oxidation. Instead, we suggest that the etiology of muscle insulin resistance is grounded in the fundamental principles that govern cellular and mitochondrial bioenergetics, and the redox pressures that are placed on the respiratory system when energy supply persistently outpaces energy demand (Figure 2). Careful consideration of these basic principles raises doubt about the popularized concept of “exercise in a pill”. Thus, mitochondrial adaptations that benefit exercise performance are those that favor energy efficiency and glucose sparing; whereas therapeutic agents targeted against obesity and diabetes should promote energetic inefficiencies and glucose utilization. This contrast implies that pharmaceuticals designed to evoke an exercise trained phenotype (e.g. heightened fat oxidation and/or mitochondrial biogenesis) without a corresponding increase in energy expenditure, might do more harm than good when administered to inactive, glucose intolerant patients.
Figure 2. Schematic illustration showing 24 hour predicted fluctuations in mitochondrial membrane potential.
for A. an individual out of metabolic balance due to excess caloric intake and sedentary lifestyle and B. an individual in metabolic balance due to appropriate caloric intake and active lifestyle. Dotted line indicates approximate threshold membrane potential at which electrons begin to leak to from superoxide. Arrows signify calorie intake. Red indicates progressively increasing H2O2 generation and blue indicates progressively increasing O2 consumption.
At least one general consensus in this field is that the recent surge in the prevalence of insulin resistance stems largely from inactivity, caloric excess and the poor nutrient quality of heavily processed foods. Chronic exposure to these adverse metabolic conditions imposes a persistent nutrient burden on most, if not all, subcellular compartments, leading inevitably to cellular distress and dysfunction at multiple levels. Where do the most attractive therapeutic opportunities lie? The obvious answer is to attack the root cause of the problem, rather than the constellation of molecular symptoms. The two therapies that are undeniably effective in combatting nutrient overload include dietary modification and habitual exercise. In practice however, long term maintenance of lifestyle modifications has proven challenging, evidenced by disappointingly high recidivism rates (Turk et al., 2009). These grim statistics call for the development of centrally acting drugs that facilitate behavior modification strategies, and/or peripherally-targeted compounds that promote energy wasting.
Whereas drug development to thwart obesity and diabetes is clearly necessary, this approach is neither practical nor sustainable as a first line of defense. Over two-thirds of U.S. adults are overweight or obese. Thus, the long term solution to the current epidemic of metabolic disease must encompass substantive efforts aimed at prevention in children and young adults. Progress toward this end will require that metabolic scientists engage in national discussions centering on the grave need for bold public health initiatives and environmental restructuring rather than the promise of a magic pill.
Acknowledgments
The authors are supported by grants from the United States Public Health Service: R01 AG028930 (DMM), R01 DK089312 (DMM), R01 HL101189R01 (DMM), DK073488 (PDN) and R01 DK074825 (PDN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- Abu-Elheiga L, Oh W, Kordari P, Wakil SJ. Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc Natl Acad Sci U S A. 2003;100:10207–10212. doi: 10.1073/pnas.1733877100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139:1073–1081. doi: 10.3945/jn.108.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, Koves TR, Stevens R, Millington D, Newgard CB. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. 2004;10:268–274. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. In self-defence: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem J. 2004;377:347–355. doi: 10.1042/BJ20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett M, Collier GR, O'Dea K. The longitudinal effect of inhibiting fatty acid oxidation in diabetic rats fed a high fat diet. Horm Metab Res. 1992;24:360–362. doi: 10.1055/s-2007-1003335. [DOI] [PubMed] [Google Scholar]
- Bell JA, Reed MA, Consitt LA, Martin OJ, Haynie KR, Hulver MW, Muoio DM, Dohm GL. Lipid partitioning, incomplete fatty acid oxidation, and insulin signal transduction in primary human muscle cells: effects of severe obesity, fatty acid incubation, and fatty acid translocase/CD36 overexpression. J Clin Endocrinol Metab. 2010;95:3400–3410. doi: 10.1210/jc.2009-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict F, Joslin E. Metabolism in Diabetes Mellitus. Vol. 136. Carnegie Institute of Washington Publication; 1910. pp. 3–324. [Google Scholar]
- Bloch-Damti A, Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid Redox Signal. 2005;7:1553–1567. doi: 10.1089/ars.2005.7.1553. [DOI] [PubMed] [Google Scholar]
- Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118:789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle KE, Canham JP, Consitt LA, Zheng D, Koves TR, Gavin TP, Holbert D, Neufer PD, Ilkayeva O, Muoio DM, Houmard JA. A high-fat diet elicits differential responses in genes coordinating oxidative metabolism in skeletal muscle of lean and obese individuals. J Clin Endocrinol Metab. 2011;96:775–781. doi: 10.1210/jc.2010-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD. The contribution of the leak of protons across the mitochondrial inner membrane to standard metabolic rate. J Theor Biol. 1990;145:267–286. doi: 10.1016/s0022-5193(05)80131-6. [DOI] [PubMed] [Google Scholar]
- Bruce CR, Hoy AJ, Turner N, Watt MJ, Allen TL, Carpenter K, Cooney GJ, Febbraio MA, Kraegen EW. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes. 2009;58:550–558. doi: 10.2337/db08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA, Kozak LP. A Recurring Problem With the Analysis of Energy Expenditure in Genetic Models Expressing Lean and Obese Phenotypes. Diabetes. 2010;59:323–329. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas S, Echtay KS, Harper JA, Jekabsons MB, Buckingham JA, Grau E, Abuin A, Chapman H, Clapham JC, Brand MD. The basal proton conductance of skeletal muscle mitochondria from transgenic mice overexpressing or lacking uncoupling protein-3. J Biol Chem. 2002;277:2773–2778. doi: 10.1074/jbc.M109736200. [DOI] [PubMed] [Google Scholar]
- Chakraborti S, Chakraborti T. Oxidant-mediated activation of mitogen-activated protein kinases and nuclear transcription factors in the cardiovascular system: a brief overview. Cell Signal. 1998;10:675–683. doi: 10.1016/s0898-6568(98)00014-x. [DOI] [PubMed] [Google Scholar]
- Chen HC, Farese RV. Turning WAT into BAT gets rid of fat. Nat Med. 2001;7:1102–1103. doi: 10.1038/nm1001-1102. [DOI] [PubMed] [Google Scholar]
- Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, White NM, Ma JT, Zhou J, Qi N, Westcott D, Delproposto JB, Blackwell TS, Yull FE, Saltiel AR. The Protein Kinase IKK[var epsilon] Regulates Energy Balance in Obese Mice. Cell. 2009;138:961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CS, Befroy DE, Codella R, Kim S, Reznick RM, Hwang YJ, Liu ZX, Lee HY, Distefano A, Samuel VT, Zhang D, Cline GW, Handschin C, Lin J, Petersen KF, Spiegelman BM, Shulman GI. Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci U S A. 2008;105:19926–19931. doi: 10.1073/pnas.0810339105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, Zhang D, Cline GW, Wakil SJ, Shulman GI. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A. 2007;104:16480–16485. doi: 10.1073/pnas.0706794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomentowski P, Coen PM, Radikova Z, Goodpaster BH, Toledo FG. Skeletal muscle mitochondria in insulin resistance: differences in intermyofibrillar versus subsarcolemmal subpopulations and relationship to metabolic flexibility. J Clin Endocrinol Metab. 2011;96:494–503. doi: 10.1210/jc.2010-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutkow WA, Birkenfeld AL, Brown JD, Lee HY, Frederick DW, Yoshioka J, Patwari P, Kursawe R, Cushman SW, Plutzky J, Shulman GI, Samuel VT, Lee RT. Deletion of the alpha-arrestin protein Txnip in mice promotes adiposity and adipogenesis while preserving insulin sensitivity. Diabetes. 2010;59:1424–1434. doi: 10.2337/db09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll T, Alvarez-Guardia D, Barroso E, Gomez-Foix AM, Palomer X, Laguna JC, Vazquez-Carrera M. Activation of peroxisome proliferator-activated receptor-{delta} by GW501516 prevents fatty acid-induced nuclear factor-{kappa}B activation and insulin resistance in skeletal muscle cells. Endocrinology. 2010;151:1560–1569. doi: 10.1210/en.2009-1211. [DOI] [PubMed] [Google Scholar]
- Deems RO, Anderson RC, Foley JE. Hypoglycemic effects of a novel fatty acid oxidation inhibitor in rats and monkeys. Am J Physiol. 1998;274:R524–528. doi: 10.1152/ajpregu.1998.274.2.R524. [DOI] [PubMed] [Google Scholar]
- Denton RM, Randle PJ. Citrate and the regulation of adipose-tissue phosphofructokinase. The Biochemical journal. 1966;100:420–423. doi: 10.1042/bj1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvergne B, AIJ, Devchand PR, Wahli W. The peroxisome proliferator-activated receptors at the cross-road of diet and hormonal signalling. J Steroid Biochem Mol Biol. 1998;65:65–74. doi: 10.1016/s0960-0760(97)00182-9. [DOI] [PubMed] [Google Scholar]
- Dobbins RL, Szczepaniak LS, Bentley B, Esser V, Myhill J, McGarry JD. Prolonged Inhibition of Muscle Carnitine Palmitoyltransferase-1 Promotes Intramyocellular Lipid Accumulation and Insulin Resistance in Rats. Diabetes. 2001;50:123–130. doi: 10.2337/diabetes.50.1.123. [DOI] [PubMed] [Google Scholar]
- Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, Lin H. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med. 2010;16:400–402. doi: 10.1038/nm0410-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistances. Antioxid Redox Signal. 2005;7:1040–1052. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- Finck BN, Bernal-Mizrachi C, Han DH, Coleman T, Sambandam N, LaRiviere LL, Holloszy JO, Semenkovich CF, Kelly DP. A potential link between muscle peroxisome proliferator- activated receptor-alpha signaling and obesity-related diabetes. Cell Metab. 2005;1:133–144. doi: 10.1016/j.cmet.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Bruning JC, Ruther U. Inactivation of the Fto gene protects from obesity. Nature. 2009;458:894–898. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- Fisher-Wellman KH, Neufer PD. Linking mitochondrial bioenergetics to the etiology of insulin resistance via redox biology. Trends in Endocrinology & Metabolism. doi: 10.1016/j.tem.2011.12.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragasso G, Salerno A, Spoladore R, Cera M, Montanaro C, Margonato A. Effects of metabolic approach in diabetic patients with coronary artery disease. Curr Pharm Des. 2009;15:857–862. doi: 10.2174/138161209787582093. [DOI] [PubMed] [Google Scholar]
- Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab. 2008;295:E1009–1017. doi: 10.1152/ajpendo.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland PB, Newsholme EA, Randle PJ. Effect of fatty acids, ketone bodies, diabetes and starvation on pyruvate metabolism in rat heart and diaphragm muscle. Nature. 1962;195:381–383. doi: 10.1038/195381a0. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Kelley DE. Skeletal muscle triglyceride: marker or mediator of obesity-induced insulin resistance in type 2 diabetes mellitus? Curr Diab Rep. 2002;2:216–222. doi: 10.1007/s11892-002-0086-2. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M, Gervois P, Raspe E, Madsen L, Poulain P, Derudas B, Herbert JM, Winegar DA, Willson TM, Fruchart JC, Berge RK, Staels B. Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000;275:16638–16642. doi: 10.1074/jbc.275.22.16638. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M, Rouault C, Poulain P, Andre J, Poitout V, Peters JM, Gonzalez FJ, Fruchart JC, Reach G, Staels B. PPAR-alpha-null mice are protected from high-fat diet-induced insulin resistance. Diabetes. 2001;50:2809–2814. doi: 10.2337/diabetes.50.12.2809. [DOI] [PubMed] [Google Scholar]
- Hakimi P, Yang J, Casadesus G, Massillon D, Tolentino-Silva F, Nye CK, Cabrera ME, Hagen DR, Utter CB, Baghdy Y, Johnson DH, Wilson DL, Kirwan JP, Kalhan SC, Hanson RW. Overexpression of the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) in skeletal muscle repatterns energy metabolism in the mouse. J Biol Chem. 2007;282:32844–32855. doi: 10.1074/jbc.M706127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci U S A. 2008;105:7815–7820. doi: 10.1073/pnas.0802057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JA, Stuart JA, Jekabsons MB, Roussel D, Brindle KM, Dickinson K, Jones RB, Brand MD. Artifactual uncoupling by uncoupling protein 3 in yeast mitochondria at the concentrations found in mouse and rat skeletal-muscle mitochondria. Biochem J. 2002;361:49–56. doi: 10.1042/0264-6021:3610049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Wilson JE. Kinetic and regulatory properties of HK I(+), a modified form of the type I isozyme of mammalian hexokinase in which interactions between the N- and C-terminal halves have been disrupted. Arch Biochem Biophys. 2002;399:109–115. doi: 10.1006/abbi.2001.2744. [DOI] [PubMed] [Google Scholar]
- Henique C, Mansouri A, Fumey G, Lenoir V, Girard J, Bouillaud F, Prip-Buus C, Cohen I. Increased Mitochondrial Fatty Acid Oxidation Is Sufficient to Protect Skeletal Muscle Cells from Palmitate-induced Apoptosis. Journal of Biological Chemistry. 2010;285:36818–36827. doi: 10.1074/jbc.M110.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen EJ. Exercise training and the antioxidant alpha-lipoic acid in the treatment of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2006;40:3–12. doi: 10.1016/j.freeradbiomed.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Huang JY, Verdin E. Acetylation of mitochondrial proteins. Methods Enzymol. 2009;457:137–147. doi: 10.1016/S0076-6879(09)05008-3. [DOI] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, Laakso M, Alt FW, Newgard CB, Farese RV, Jr, Kahn CR, Verdin E. SIRT3 Deficiency and Mitochondrial Protein Hyperacetylation Accelerate the Development of the Metabolic Syndrome. Mol Cell. 2011 doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn KL, Salmon AB, Hohnen-Behrens C, Turner N, Hoy AJ, Maghzal GJ, Stocker R, Van Remmen H, Kraegen EW, Cooney GJ, Richardson AR, James DE. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci U S A. 2009;106:17787–17792. doi: 10.1073/pnas.0902380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn KL, Turner N, Swarbrick MM, Wilks D, Preston E, Phua Y, Joshi H, Furler SM, Larance M, Hegarty BD, Leslie SJ, Pickford R, Hoy AJ, Kraegen EW, James DE, Cooney GJ. Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. Cell Metab. 2010;11:70–76. doi: 10.1016/j.cmet.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Huang JY, Hirschey MD, Shimazu T, Ho L, Verdin E. Mitochondrial sirtuins. Biochim Biophys Acta. 2010;1804:1645–1651. doi: 10.1016/j.bbapap.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Hubinger A, Knode O, Susanto F, Reinauer H, Gries FA. Effects of the carnitine-acyltransferase inhibitor etomoxir on insulin sensitivity, energy expenditure and substrate oxidation in NIDDM. Horm Metab Res. 1997;29:436–439. doi: 10.1055/s-2007-979072. [DOI] [PubMed] [Google Scholar]
- Huffman KM, Shah SH, Stevens RD, Bain JR, Muehlbauer M, Slentz CA, Tanner CJ, Kuchibhatla M, Houmard JA, Newgard CB, Kraus WE. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32:1678–1683. doi: 10.2337/dc08-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui ST, Andres AM, Miller AK, Spann NJ, Potter DW, Post NM, Chen AZ, Sachithanantham S, Jung DY, Kim JK, Davis RA. Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc Natl Acad Sci U S A. 2008;105:3921–3926. doi: 10.1073/pnas.0800293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulver MW, Berggren JR, Cortright RN, Dudek RW, Thompson RP, Pories WJ, MacDonald KG, Cline GW, Shulman GI, Dohm GL, Houmard JA. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab. 2003;284:E741–747. doi: 10.1152/ajpendo.00514.2002. [DOI] [PubMed] [Google Scholar]
- Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295:E46–54. doi: 10.1152/ajpendo.00536.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Verdin EM, Kahn CR. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A. 2011;108:14608–14613. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S, Weiss JN, Ribalet B. Subcellular localization of hexokinases I and II directs the metabolic fate of glucose. PLoS ONE. 2011;6:e17674. doi: 10.1371/journal.pone.0017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Keil S, Muller M, Zoller G, Haschke G, Schroeter K, Glien M, Ruf S, Focken I, Herling AW, Schmoll D. Identification and synthesis of novel inhibitors of acetyl-CoA carboxylase with in vitro and in vivo efficacy on fat oxidation. J Med Chem. 2010;53:8679–8687. doi: 10.1021/jm101179e. [DOI] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130–1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039–1044. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- Kovalik JP, Slentz D, Stevens RD, Kraus WE, Houmard JA, Nicoll JB, Lea-Currie YR, Everingham K, Kien CL, Buehrer BM, Muoio DM. Metabolic remodeling of human skeletal myocytes by cocultured adipocytes depends on the lipolytic state of the system. Diabetes. 2011;60:1882–1893. doi: 10.2337/db10-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Kraegen EW, Cooney GJ, Turner N. Muscle insulin resistance: a case of fat overconsumption, not mitochondrial dysfunction. Proc Natl Acad Sci U S A. 2008;105:7627–7628. doi: 10.1073/pnas.0803901105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer DK, Al-Khalili L, Perrini S, Skogsberg J, Wretenberg P, Kannisto K, Wallberg-Henriksson H, Ehrenborg E, Zierath JR, Krook A. Direct Activation of Glucose Transport in Primary Human Myotubes After Activation of Peroxisome Proliferator Activated Receptor. Diabetes. 2005;54:1157–1163. doi: 10.2337/diabetes.54.4.1157. [DOI] [PubMed] [Google Scholar]
- Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, Jurczak MJ, Zhang D, Woo DK, Shadel GS, Ladiges W, Rabinovitch PS, Santos JH, Petersen KF, Samuel VT, Shulman GI. Targeted Expression of Catalase to Mitochondria Prevents Age-Associated Reductions in Mitochondrial Function and Insulin Resistance. Cell Metabolism. 2010;12:668–674. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Nolte LA, Ju JS, Han DH, Coleman T, Holloszy JO, Semenkovich CF. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat Med. 2000;6:1115–1120. doi: 10.1038/80450. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- Mancuso DJ, Sims HF, Yang K, Kiebish MA, Su X, Jenkins CM, Guan S, Moon SH, Pietka T, Nassir F, Schappe T, Moore K, Han X, Abumrad NA, Gross RW. Genetic Ablation of Calcium-independent Phospholipase A2γ Prevents Obesity and Insulin Resistance during High Fat Feeding by Mitochondrial Uncoupling and Increased Adipocyte Fatty Acid Oxidation. Journal of Biological Chemistry. 2010;285:36495–36510. doi: 10.1074/jbc.M110.115766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, DeLany JP. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 2010;18:1695–1700. doi: 10.1038/oby.2009.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingorance C, Rodriguez-Rodriguez R, Justo ML, Alvarez de Sotomayor M, Herrera MD. Critical update for the clinical use of L-carnitine analogs in cardiometabolic disorders. Vasc Health Risk Manag. 2011;7:169–176. doi: 10.2147/VHRM.S14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi S, Hatakeyama H, Karahashi M, Koumura T, Nonaka I, Hayashi YK, Noguchi S, Sher RB, Nakagawa Y, Manfredi G, Goto Y-i, Cox GA, Nishino I. Muscle choline kinase beta defect causes mitochondrial dysfunction and increased mitophagy. Human Molecular Genetics. 2011;20:3841–3851. doi: 10.1093/hmg/ddr305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molero JC, Waring SG, Cooper A, Turner N, Laybutt R, Cooney GJ, James DE. Casitas b-Lineage Lymphoma-Deficient Mice Are Protected Against High-Fat Diet-Induced Obesity and Insulin Resistance. Diabetes. 2006;55:708–715. doi: 10.2337/diabetes.55.03.06.db05-0312. [DOI] [PubMed] [Google Scholar]
- Muoio DM. Intramuscular triacylglycerol and insulin resistance: guilty as charged or wrongly accused? Biochim Biophys Acta. 2010;1801:281–288. doi: 10.1016/j.bbalip.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM, Noland RC, Seiler SE, Kovalik JP, Davies M, Kraus WE, Debalsi KL, Ilkayeva OR, Stevens RD, Kheterpal I, Zhang J, Koves TR, Mynatt RL. Muscle-specific Deletion of Carnitine Acetyltransferase Compromises Glucose Tolerance and Metabolic Flexibility. Cell Metabolism. doi: 10.1016/j.cmet.2012.04.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. J Lipid Res. 2009;50(Suppl):S74–79. doi: 10.1194/jlr.R800053-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metabolism. 2012 doi: 10.1016/j.cmet.2012.01.024. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme EA, Randle PJ, Manchester KL. Inhibition of the phosphofructokinase reaction in perfused rat heart by respiration of ketone bodies, fatty acids and pyruvate. Nature. 1962;193:270–271. doi: 10.1038/193270a0. [DOI] [PubMed] [Google Scholar]
- Noland RC, Hickner RC, Jimenez-Linan M, Vidal-Puig A, Zheng D, Dohm GL, Cortright RN. Acute endurance exercise increases skeletal muscle uncoupling protein-3 gene expression in untrained but not trained humans. Metabolism. 2003;52:152–158. doi: 10.1053/meta.2003.50021. [DOI] [PubMed] [Google Scholar]
- Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O, Stevens RD, Hegardt FG, Muoio DM. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem. 2009;284:22840–22852. doi: 10.1074/jbc.M109.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland RC, Woodlief TL, Whitfield BR, Manning SM, Evans JR, Dudek RW, Lust RM, Cortright RN. Peroxisomal-mitochondrial oxidation in a rodent model of obesity-associated insulin resistance. Am J Physiol Endocrinol Metab. 2007;293:E986–E1001. doi: 10.1152/ajpendo.00399.2006. [DOI] [PubMed] [Google Scholar]
- Olson DP, Pulinilkunnil T, Cline GW, Shulman GI, Lowell BB. Gene knockout of Acc2 has little effect on body weight, fat mass, or food intake. Proc Natl Acad Sci U S A. 2010;107:7598–7603. doi: 10.1073/pnas.0913492107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh H, Carlsson E, Chutkow WA, Johansson LE, Storgaard H, Poulsen P, Saxena R, Ladd C, Schulze PC, Mazzini MJ, Jensen CB, Krook A, Bjornholm M, Tornqvist H, Zierath JR, Ridderstrale M, Altshuler D, Lee RT, Vaag A, Groop LC, Mootha VK. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007;4:e158. doi: 10.1371/journal.pmed.0040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, Luo H, Zhang Y, He W, Yang K, Zwaans BM, Tishkoff D, Ho L, Lombard D, He TC, Dai J, Verdin E, Ye Y, Zhao Y. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011;10:M111 012658. doi: 10.1074/mcp.M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdomo G, Commerford SR, Richard A-MT, Adams SH, Corkey BE, O'Doherty RM, Brown NF. Increased {beta}-Oxidation in Muscle Cells Enhances Insulin-stimulated Glucose Metabolism and Protects against Fatty Acid-induced Insulin Resistance Despite Intramyocellular Lipid Accumulation. J Biol Chem. 2004;279:27177–27186. doi: 10.1074/jbc.M403566200. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power RA, Hulver MW, Zhang JY, Dubois J, Marchand RM, Ilkayeva O, Muoio DM, Mynatt RL. Carnitine revisited: potential use as adjunctive treatment in diabetes. Diabetologia. 2007;50:824–832. doi: 10.1007/s00125-007-0605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, EAN The glucose fatty-acid cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet I. 1963:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Reitman ML. Metabolic lessons from genetically lean mice. Annu Rev Nutr. 2002;22:459–482. doi: 10.1146/annurev.nutr.22.010402.102849. [DOI] [PubMed] [Google Scholar]
- Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of Subsarcolemmal Mitochondria in Obesity and Type 2 Diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- Roden M. Muscle triglycerides and mitochondrial function: possible mechanisms for the development of type 2 diabetes. Int J Obes (Lond) 2005;29(Suppl 2):S111–115. doi: 10.1038/sj.ijo.0803102. [DOI] [PubMed] [Google Scholar]
- Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- Sebastian D, Herrero L, Serra D, Asins G, Hegardt FG. CPT I overexpression protects L6E9 muscle cells from fatty acid-induced insulin resistance. American Journal of Physiology - Endocrinology And Metabolism. 2007;292:E677–E686. doi: 10.1152/ajpendo.00360.2006. [DOI] [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneau JA, Colberg SR, Thaete FL, Kelley DE. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. Faseb J. 1995;9:273–278. [PubMed] [Google Scholar]
- Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol. 1997;83:166–171. doi: 10.1152/jappl.1997.83.1.166. [DOI] [PubMed] [Google Scholar]
- Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. Faseb J. 1999;13:2051–2060. doi: 10.1096/fasebj.13.14.2051. [DOI] [PubMed] [Google Scholar]
- Sloan EJ, Ayer DE. Myc, Mondo, and Metabolism. Genes Cancer. 2010;1:587–596. doi: 10.1177/1947601910377489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV., Jr Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet. 2000;25:87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- Stoltzman CA, Peterson CW, Breen KT, Muoio DM, Billin AN, Ayer DE. Glucose sensing by MondoA:Mlx complexes: a role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc Natl Acad Sci U S A. 2008;105:6912–6917. doi: 10.1073/pnas.0712199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden MC, Holness MJ. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch Physiol Biochem. 2006;112:139–149. doi: 10.1080/13813450600935263. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, Watanabe M, Magoori K, Ioka RX, Tachibana K, Watanabe Y, Uchiyama Y, Sumi K, Iguchi H, Ito S, Doi T, Hamakubo T, Naito M, Auwerx J, Yanagisawa M, Kodama T, Sakai J. Activation of peroxisome proliferator-activated receptor Î′ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyfault JP, Cree MG, Tapscott EB, Bell JA, Koves TR, Ilkayeva O, Wolfe RR, Dohm GL, Muoio DM. Metabolic profiling of muscle contraction in lean compared with obese rodents. Am J Physiol Regul Integr Comp Physiol. 2010;299:R926–934. doi: 10.1152/ajpregu.00093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman K, Bernal-Mizrachi C, Zemany L, Weng S, Feng C, Zhang F, Leone TC, Coleman T, Kelly DP, Semenkovich CF. PPARalpha deficiency reduces insulin resistance and atherosclerosis in apoE-null mice. J Clin Invest. 2001;107:1025–1034. doi: 10.1172/JCI11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE. Randomized clinical trials of weight loss maintenance: a review. J Cardiovasc Nurs. 2009;24:58–80. doi: 10.1097/01.JCN.0000317471.58048.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, Cooney GJ. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 2007;56:2085–2092. doi: 10.2337/db07-0093. [DOI] [PubMed] [Google Scholar]
- van Herpen NA, Schrauwen-Hinderling VB, Schaart G, Mensink RP, Schrauwen P. Three weeks on a high-fat diet increases intrahepatic lipid accumulation and decreases metabolic flexibility in healthy overweight men. J Clin Endocrinol Metab. 2011;96:E691–695. doi: 10.1210/jc.2010-2243. [DOI] [PubMed] [Google Scholar]
- Vickers AE, Bentley P, Fisher RL. Consequences of mitochondrial injury induced by pharmaceutical fatty acid oxidation inhibitors is characterized in human and rat liver slices. Toxicol In Vitro. 2006;20:1173–1182. doi: 10.1016/j.tiv.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab. 2009;296:E11–21. doi: 10.1152/ajpendo.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Christianson J, Liu ZX, Tian L, Choi CS, Neschen S, Dong J, Wood PA, Shulman GI. Resistance to high-fat diet-induced obesity and insulin resistance in mice with very long-chain acyl-CoA dehydrogenase deficiency. Cell Metab. 2010;11:402–411. doi: 10.1016/j.cmet.2010.03.012. Erratum in: Cell Metab. 2010 Jul 2014;2012(2011):2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011;7:58–63. doi: 10.1038/nchembio.495. [DOI] [PMC free article] [PubMed] [Google Scholar]