Summary
The concept of “metabolic inflexibility” was first introduced to describe the failure of insulin resistant human subjects to appropriately adjust mitochondrial fuel selection in response to nutritional cues. This phenomenon has since gained increasing recognition as a core component of the metabolic syndrome, but the underlying mechanisms have remained elusive. Here, we identify an essential role for the mitochondrial matrix enzyme, carnitine acetyltransferase (CrAT), in regulating substrate switching and glucose tolerance. By converting acetyl-CoA to its membrane permeant acetylcarnitine ester, CrAT regulates mitochondrial and intracellular carbon trafficking. Studies in muscle-specific Crat knockout mice, primary human skeletal myocytes and human subjects undergoing L-carnitine supplementation support a model wherein CrAT combats nutrient stress, promotes metabolic flexibility and enhances insulin action by permitting mitochondrial efflux of excess acetyl moieties that otherwise inhibit key regulatory enzymes such as pyruvate dehydrogenase. These findings offer therapeutically relevant insights into the molecular basis of metabolic inflexibility.
Introduction
In light of the current obesity epidemic, research aimed at understanding and therapeutically targeting the interplay between intramuscular lipid balance, mitochondrial energetics and insulin action has taken on heightened urgency. This complex topic has been positioned at the forefront of diabetes research for the past fifty years. In 1963 Randle and colleagues proposed the glucose-fatty acid cycle to explain lipid-induced suppression of muscle glucose disposal (Randle et al., 1963). A central tenant of this model is that byproducts of fatty acid β-oxidation, including acetyl-CoA, NADH and ATP, act as potent allosteric inhibitors of glycolysis and pyruvate dehydrogenase (PDH), a unidirectional mitochondrial complex that couples glycolysis to glucose oxidation. Inhibition of PDH activity was presumed to slow glycolytic flux, resulting in negative feedback on glucose uptake. Although numerous studies in humans, animals and cultured cells have since confirmed that heavy supply of fatty acids suppresses glucose oxidation, the precise role of the glucose-fatty acid cycle in the etiology of glucose intolerance and insulin resistance remains a matter of controversy (Muoio, 2010; Samuel et al., 2010).
In recent years the substrate competition theory of insulin resistance has been challenged by alternative hypotheses centered on the idea that insufficient β-oxidation contributes to tissue accumulation of lipid signaling molecules that antagonize insulin signal transduction (Giancaterini et al., 2000; Samuel et al., 2010). Whereas the past decade of diabetes research has focused heavily on this so-called “lipotoxicity” paradigm, interest in the importance of mitochondrial fuel selection has been rekindled by studies showing that obese and/or diabetic humans fail to shift from fatty acid to glucose oxidation during the transition from fasting to feeding (Kelley and Mandarino, 2000). This phenomenon of “metabolic inflexibility” has gained increasing recognition as a hallmark of cardiometabolic disorders (Thyfault et al., 2006) but remains poorly understood at a molecular level. Additionally, the advent of metabolomics has provided new evidence linking muscle insulin resistance to overburdened mitochondria, as suggested by high rates of incomplete fat oxidation and tissue accumulation of mitochondrial-derived acylcarnitine metabolites detected in rodent models of obesity and diabetes (Koves et al., 2008). Notably, in some instances the obesity-associated accumulation of long and medium chain acylcarnitines in muscle was accompanied by a decline in free carnitine (Noland et al., 2009), whereas dietary L-carnitine supplementation enhanced whole body glucose tolerance and rescued metabolic flexibility (Noland et al., 2009; Power et al., 2007). Taken together, these findings lend merit to the glucose-fatty acid cycle as originally proposed by Randle and suggest a role for L-carnitine and acylcarnitine production in regulating glucose homeostasis.
L-carnitine is a conditionally essential nutrient that serves as a substrate for a family of acyltransferase enzymes that catalyze the reversible exchange of acyl groups between CoA and carnitine. Unlike their acyl-CoA precursors, acylcarnitines can traverse cellular membranes; thus, the interconversion of these molecules can impact cellular and inter-tissue carbon trafficking. The most prominent members of this family, carnitine palmitoyltransferase (CPT1) and CPT2, are localized on the outer and inner mitochondrial membranes, respectively, where together with carnitine acylcarnitine translocase they permit import of long chain fatty acyl-CoAs into the mitochondrial matrix for β-oxidation (Bieber, 1988; McGarry et al., 1991; Ramsay and Zammit, 2004). A third member of this family, carnitine acetyltransferase (CrAT), resides principally within the mitochondrial matrix where it acts selectively on short chain acyl-CoAs (Bieber, 1988; Cordente et al., 2004). In contrast to CPT1 and CPT2, the precise function of CrAT and its potential relevance to metabolic disease is poorly understood. Importantly however, a role for this enzyme in defending glucose homeostasis was strongly implicated in the aforementioned animal studies, wherein the therapeutic benefits of L-carnitine supplementation corresponded with robust tissue efflux and urinary excretion of acetylcarnitine (the major product of CrAT) (Noland et al., 2009; Power et al., 2007). Herein, we sought to test the hypothesis that CrAT plays a key role in regulating systemic glucose tolerance in both rodents and humans. In aggregate, our results provide definitive evidence that CrAT impacts whole body glucose homeostasis, and that muscle-specific loss of function compromises metabolic control in a manner that resembles the insulin resistant state.
Results
Tissue-specific targeting of CrAT activity
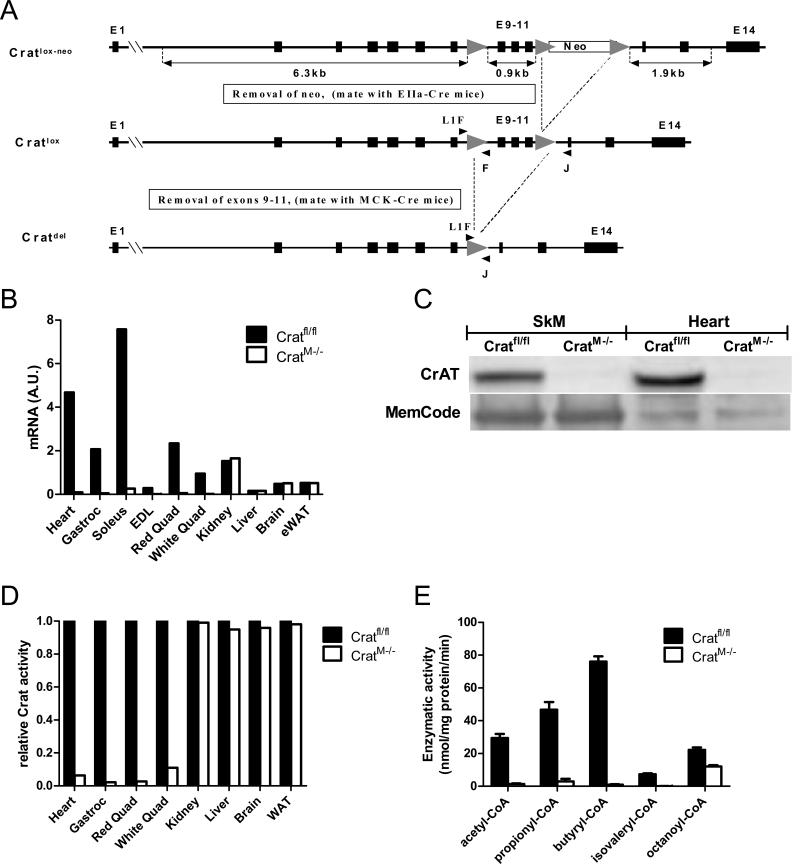
Considering that CrAT is most abundant in skeletal muscle and heart (Marquis and Fritz, 1965; Noland et al., 2009) and because these tissues are presumed to contribute substantially to the circulating pool of acetylcarnitine, we generated muscle-specific crat knockout mice (CratM-/-) by breeding animals harboring a floxed allele of the crat’ gene to those expressing Cre recombinase under the control of the muscle creatine kinase (MCK) promoter (Figure 1A and S. Methods). Analysis of crat abundance by qRT-PCR, Western blot and enzymatic activity confirmed loss of function in skeletal muscle and heart without effects on expression or activity in other tissues (Figure 1B-D). Figure 1E shows a near-complete loss (94-98%) of acyltransferase activity using various short chain acyl-CoA substrates in isolated mitochondria from skeletal muscle of CratM-/- compared to control littermates (Cratfl/fl). The high (54%) residual activity with octanoyl-CoA (C8) is likely attributable to carnitine octanoyltransferase and/or palmitoyltransferase 2 (Ramsay and Zammit, 2004).
Figure 1. Generation of mice with muscle-specific CrAT deficiency.
Panel A shows the genetic strategy for generating mice with muscle-specific ablation of CrAT (CratM-/-). Tissue-specific deletion was confirmed by analyzing CrAT mRNA (panel B), protein (panel C) and enzyme activity (panel D) in multiple tissues (n=3-6/group). Enzymatic activity in isolated mitochondrial lysates from mixed gastrocnemius skeletal muscle (SkM) was measured with several short and medium chain acyl-CoAs as substrates (panel E; n=6/group).
Muscle-specific deletion of CrAT alters whole body energy metabolism
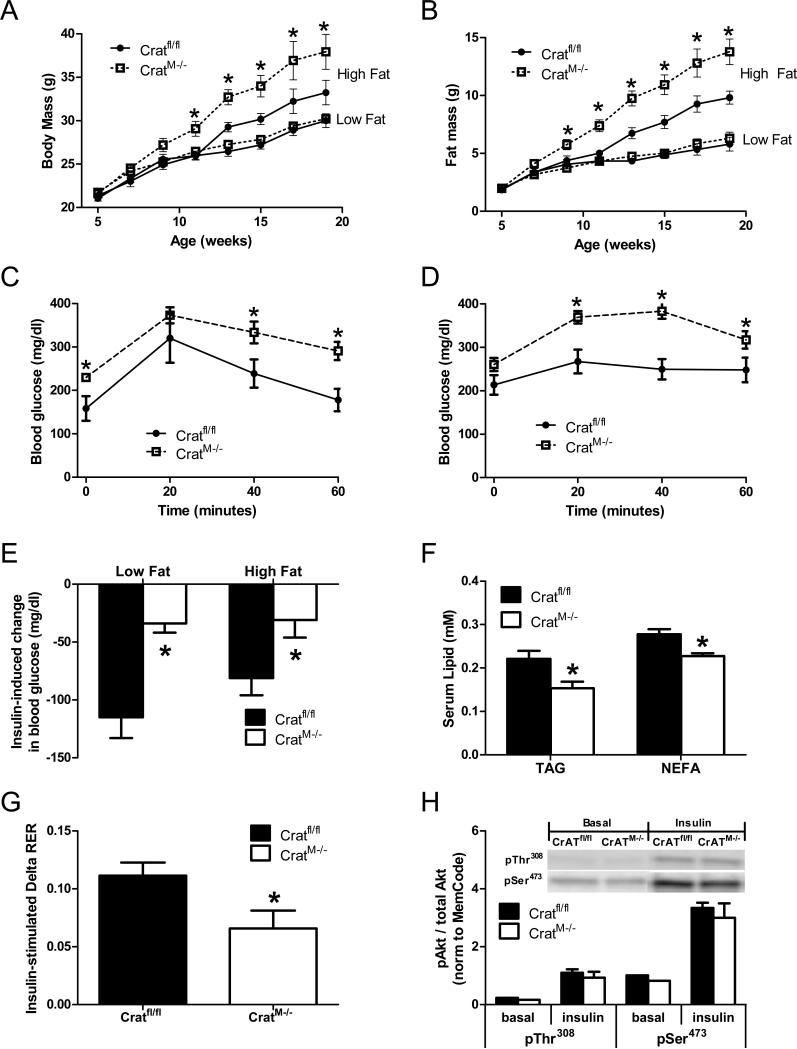
Between five and twenty weeks of age CratM-/- mice and control littermates (Cratfl/fl) fed a low fat diet gained total body weight and fat pad mass at similar rates (Figure 2A and 2B); however, when challenged with a high fat diet, CratM-/- mice gained more body weight and more fat mass than the control group. Interestingly, despite normal body weight when feeding on the low fat diet, blood glucose levels were higher in the CratM-/-, both at baseline and throughout an intra-peritoneal glucose tolerance test (Figure 2C and S. Figure 1). The glucose intolerant phenotype of CratM-/- mice was further exacerbated by high fat feeding (Figure 2D). Likewise, lowering of blood glucose in response to an acute insulin bolus was blunted in the CratM-/- mice, regardless of diet (Figure 2E and S. Figure 1).
Figure 2. Muscle-specific CrAT deficiency affects whole body metabolic homeostasis.
CratM-/- mice and control (Cratfl/fl) littermates were fed either a 10% fat (Low Fat) or a 45% fat (High Fat) diet beginning at 5 weeks of age. Body weight (panel A) and fat mass (panel B) were measured every two weeks (n=10-13/group). Glucose tolerance tests were performed using 40 mg glucose/mouse (panel C) or 20 mg glucose/mouse (panel D) (n=10/group). For insulin tolerance tests (panel E) blood glucose levels were measured 10 min after injection of 0.04U insulin/mouse (low fat diet) or 0.08U/mouse (high fat diet) in fed mice (n=10/group). The following measures were from mice fed a low fat diet. Plasma triglycerides (TAG) and non-esterified fatty acids (NEFA) were measured following a 4 h fast (panel F; n=12/group). Following a 7 day acclimatization period, respiratory exchange ratio (RER) was monitored by indirect calorimetry immediately after an insulin injection of 0.04U/mouse (panel G). Insulin signaling (Akt total, pThr308, and pSer473) was measured using lysates prepared from gastrocnemius muscles collected 10min after insulin injection (panel H). Quantified bands were normalized to a MemCode loading control and phosphorylated proteins were expressed relative to total Akt. * Genotype effect (p< 0.05) detected by Student's t-test.
To avoid potential confounding effects of increased body weight and adiposity, further analyses of the CratM-/- mice were performed in animals maintained on a low fat diet. Plasma triglycerides (TAG) and non-esterified fatty acids (NEFAs; Figure 2F) were lower in the knockout mice, suggesting potential alterations in lipid clearance and/or utilization. Whole body indirect calorimetry did not reveal differences in 24 h average RER or energy expenditure between knockout and control mice (data not shown). However, the increase in whole body carbohydrate oxidation that typically occurs in response to an insulin challenge (Figure 2G) or during the fasting-to-fed transition (S. Figure 1E and 1F) was diminished in the CratM-/- mice, implying that these mice are less metabolically flexible. Despite clear evidence of glucose intolerance in the CratM-/- mice, insulin-mediated phosphorylation of Akt (Figure 2H) and GSK-3β (not shown) were similar between genotypes, implicating other sites of dysregulation.
Acylcarnitine profiling of CratM-/- mice
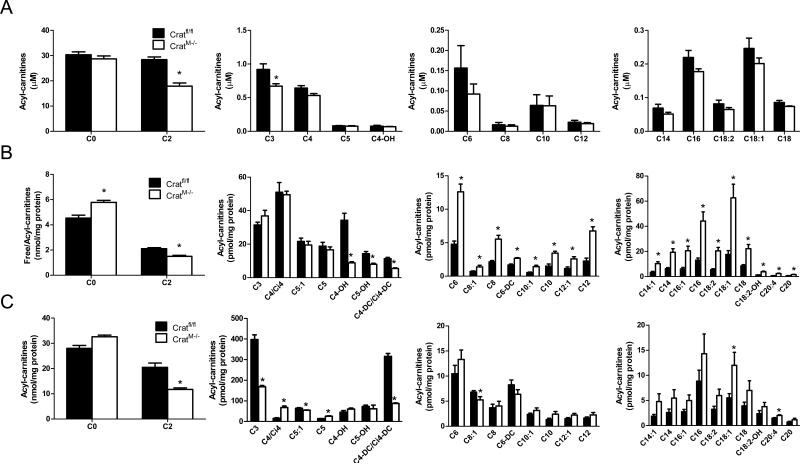
Muscle-specific loss of crat lowered acetylcarnitine levels 37-50% in plasma (Figure 3A), skeletal muscle (SkM; Figure 3B) and heart (Figure 3C). These reductions were less than predicted assuming that muscle is the major source of circulating acetylcarnitine. Likewise, tissue and plasma levels of propionylcarnitine (C3), another major product of CrAT, were only partially reduced, and butyrylcarnitine (C4) was actually elevated in the heart. Together, these findings indicate that tissues other than muscle contribute substantially to the circulating pool of short chain acylcarnitines. Notably, crat deficiency provoked marked accumulation of several medium and long chain acylcarnitines in skeletal muscle and to a lesser degree in heart, without affecting plasma levels of these metabolites. Additionally, several short, medium and long chain acyl-CoA species were elevated in CratM-/- hearts (S. Figure 2).
Figure 3. Free carnitine and acylcarnitine profiling in CratM-/- mice.
Tissue and blood specimens were obtained from anesthetized mice (n=6-12/group) following a 4 h fast. Free carnitine and acylcarnitine levels in plasma (panel A), gastrocnemius muscle (SkM; panel B) and heart (panel C) were measured by mass spectrometry. The acyl chain length (C) is denoted by the corresponding metabolite number (e.g. C0= free carnitine, C2= acetylcarnitine; C3= proprionylcarnitine). Data are expressed as mean ± SEM. * Genotype effect (p< 0.05) detected by Student's t-test.
CrAT deficiency disrupts mitochondrial pyruvate metabolism without inhibiting respiratory function
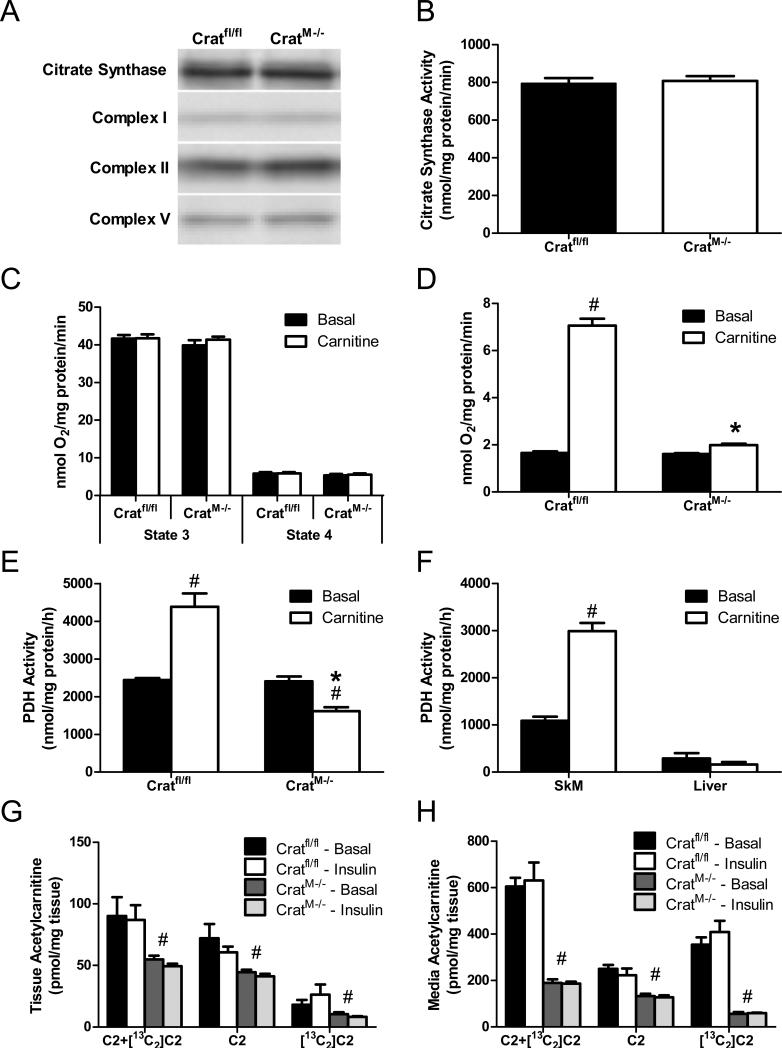
The foregoing accumulation of long and medium chain acylcarnitines in muscle of CratM-/- mice might reflect a distal bottleneck in β-oxidation, and/or heavier mitochondrial influx and catabolism of lipid substrate in muscle. To address the former possibility we evaluated the impact of crat deficiency on mitochondrial content and/or function. Tissue abundance of prominent mitochondrial marker proteins such as citrate synthase and electron transport complexes I, II and V was similar between genotypes (Figure 4A). Likewise, citrate synthase activity measured in skeletal muscle mitochondrial lysates was unchanged in the knockout mice (Figure 4B). Assessment of oxygen consumption in mitochondria respiring under state 3 and state 4 conditions in the presence of pyruvate/malate was also unremarkable, regardless of carnitine provision (Figure 4C). By contrast, when this assay was performed with pyruvate as the only carbon source (i.e. omitting malate as an anaplerotic substrate), addition of carnitine increased state 3 respiration 4.3-fold in mitochondria from Cratfl/fl mice, whereas this effect was completely absent in the crat null mitochondria (Figure 4D). We surmised that the carnitine-mediated increase in respiration might be due to CrAT-dependent stimulation of PDH, a reaction that produces NADH substrate for complex I of the electron transport chain. This possibility was confirmed via direct assessment of PDH activity, which was stimulated 1.8-fold by carnitine in the control group whereas a 33% inhibition occurred in the crat null mitochondria (Figure 4E). In contrast to muscle, hepatocytes use acetyl-CoA as a substrate for ketogenesis and de novo lipogenesis. Thus, in the liver, CrAT is absent from the mitochondrial compartment (S. Figure 3) and is instead localized mainly to the peroxisomes (Beenakkers and Klingenberg, 1964; Bloisi et al., 1990). Consistent with results in muscles from CratM-/- mice, carnitine failed to stimulate PDH activity in liver mitochondria (Figure 4F). Acetyl-CoA antagonizes PDH activity through direct allosteric inhibition of the enzyme complex and by stimulating a family of PDH kinases that inactive the E1a catalytic subunit via phosphorylation at multiple serine residues (Sugden and Holness, 2003). Accordingly, we examined E1a phosphorylation at Ser232, Ser293, and Ser300 in muscles from knockout and control mice, but differences between genotypes were not detected (not shown).
Figure 4. CrAT regulates PDH activity.
Gastrocnemius muscles were harvested after a 4 h fast and lysates were prepared for immunoblot analysis of the mitochondrial marker protein, citrate synthase (CS), and electron transport chain complexes I, II and V. (panel A). Mitochondria were isolated from gastrocnemius muscles and a portion of the suspension was used to assess citrate synthase activity (panel B; n=12). State 3 and state 4 mitochondrial respiration rates were measured using BD Oxygen Biosensor plates after addition of 10 mM pyruvate and 5 mM malate ± 1 mM carnitine (panel C; n=6). RCR values were 7.2-7.6 for all groups. State 3 respiration was also measured with 10 mM pyruvate ± 1 mM carnitine (panel D; n=6). PDH activity was determined by measuring 14CO2 produced from 1 mM [1-14C]pyruvate ± 5 mM carnitine in isolated muscle mitochondria from Cratfl/fl and CratM-/- mice (panel E; n=6). The acute effect of 5 mM carnitine on PDH activity was evaluated in mitochondria from rat gastrocnemius muscle compared to rat liver (n=3) (panel F). Soleus muscles (n=6) were incubated 2 h with 10 mM [13C6]glucose and 200 μM fatty acid ± 100 nM insulin, followed by LCMS-based mass analysis of [12C]acetylcarnitine (C2), [13C2]acetylcarnitine ([13C2]C2) and total acetylcarnitine (C2+([13C2]C2) in the muscle tissue (panel G) and incubation buffer (panel H). * Genotype and # carnitine effects (p< 0.05) detected by Student's t-test.
To assess trafficking of glucose-derived carbons in intact skeletal muscle we incubated isolated soleus muscles with [13C6]glucose followed by mass spectrometry-based analysis of acylcarnitines. In soleus muscles from Cratfl/fl mice the [13C2]acetylcarnitine species, which originated from PDH-generated acetyl-CoA, accounted for 20% and 30% of the total acetylcarnitine content under basal and insulin-stimulated conditions, respectively (Figure 4G). Interestingly, [13C2]acetylcarnitine was also detected in the crat null muscles, which could be attributable to residual CrAT activity in whole muscle tissue and/or another acylcarnitine transferase. Notably however, over 90% of the total [13C2]acetylcarnitine produced by the Cratfl/fl muscles was present in the incubation buffer (Figure 4H). In the control group, [13C2]acetylcarnitine accounted for 63% of total acetylcarnitine efflux, and absolute quantities were 692% of those measured in the incubation buffer from CratM-/- muscles. These results confirm an essential role for CrAT in permitting tissue efflux of PDH-derived acetylcarnitine.
CrAT deficiency disrupts substrate switching
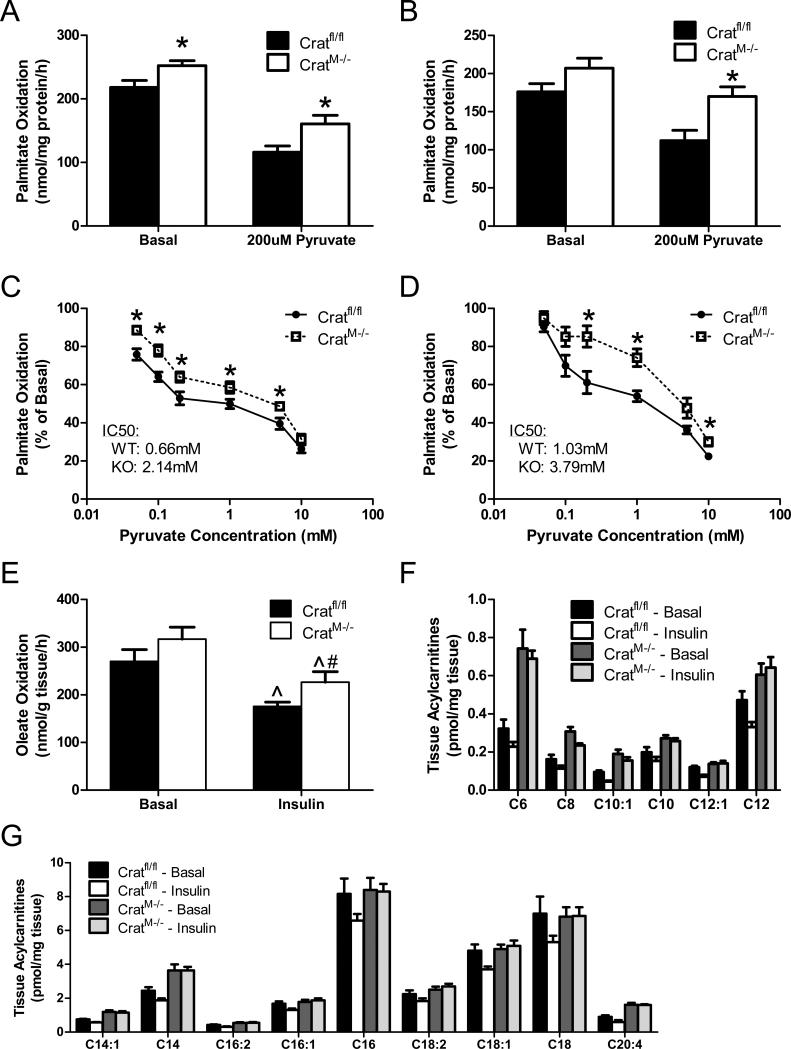
We next questioned whether the absence of CrAT affects fatty acid catabolism in isolated mitochondria exposed to moderate (100 μM) or high (300 μM) concentrations of [1-14C]palmitate. Rates of 14CO2 production were 16-18% greater in isolated muscle mitochondria from CratM-/- compared to Cratfl/fl mice (Figure 5A and 5B). The difference between genotypes was amplified by the addition of 200μM pyruvate, which inhibited fat oxidation more robustly in the control group (Figure 5A and 5B). To mimic the metabolic transition between fasting and feeding we evaluated dose-dependent pyruvate inhibition of fat oxidation. Absolute rates of palmitate oxidation were higher in the crat null mitochondria at nearly every dose of pyruvate. Moreover, crat deficiency resulted in a statistically significant rightward shift in the IC50 of pyruvate, an outcome that was exacerbated by increasing the palmitate concentration (Figures 5C & 5D, respectively). We then evaluated oxidation of [1-14C]oleate in intact mouse soleus muscles incubated ex-vivo under basal and insulin-stimulated conditions. Insulin suppressed soleus muscle fat oxidation in both groups. Two-way ANOVA revealed a main effect of the crat null genotype, which again corresponded with increased fat catabolism (Figure 5E). Likewise, ablation of CrAT increased soleus muscle content of medium and long chain acylcarnitines, and largely abolished the insulin-induced lowering of these species that was evident in the control group (Figures 5F and 5G). Thus, crat deficiency lowered mitochondrial and tissue efflux of glucose-derived carbons (Figure 4) while increasing rates of β-oxidation. These conditions are likely to promote intramitochondrial accumulation of fatty acyl-CoAs, giving rise to medium and long chain acylcarnitine species.
Figure 5. CrAT deficiency promotes fat oxidation.
Fatty acid oxidation to CO2 was measured in isolated muscle (gastrocnemius) mitochondria exposed to moderate (100 μM; panel A) or high (300 μM; panel B) doses of [1-14C]palmitate ± 200 μM pyruvate (n=12). Dose-dependent pyruvate inhibition of fatty acid oxidation was measured during exposure to 100 μM (panel C) or 300 μM (panel D) [1-14C]palmitate. Results were normalized to basal oxidation rates and IC50 values for the inhibitory effect of pyruvate on palmitate oxidation were calculated using GraphPad Prism software. Total oxidation of 500 μM [1-14C]oleate (panel E) was measured in intact soleus muscles (n=6) incubated 2 h in a modified KHB buffer containing 5 mM glucose ± 100 nM insulin (panel E). Soleus muscles (n=6) were incubated 2 h with 10 mM [13C6]glucose and 200 μM fatty acid ± 100 nM insulin, followed by LCMS-based mass analysis of medium (panel F) and long chain (panel G) acylcarnitines. Data are expressed as mean ± SEM. Main effects of insulin (^) and genotype (#) were detected by two-way ANOVA, p< 0.05; * post hoc test for genotype. Two-way ANOVA and MANOVA revealed main effects of insulin and genotype on the medium/long chain acylcarnitine profiles; symbols were omitted for simplicity.
CrAT counter-regulates glucose and fatty acid metabolism in cultured myocytes
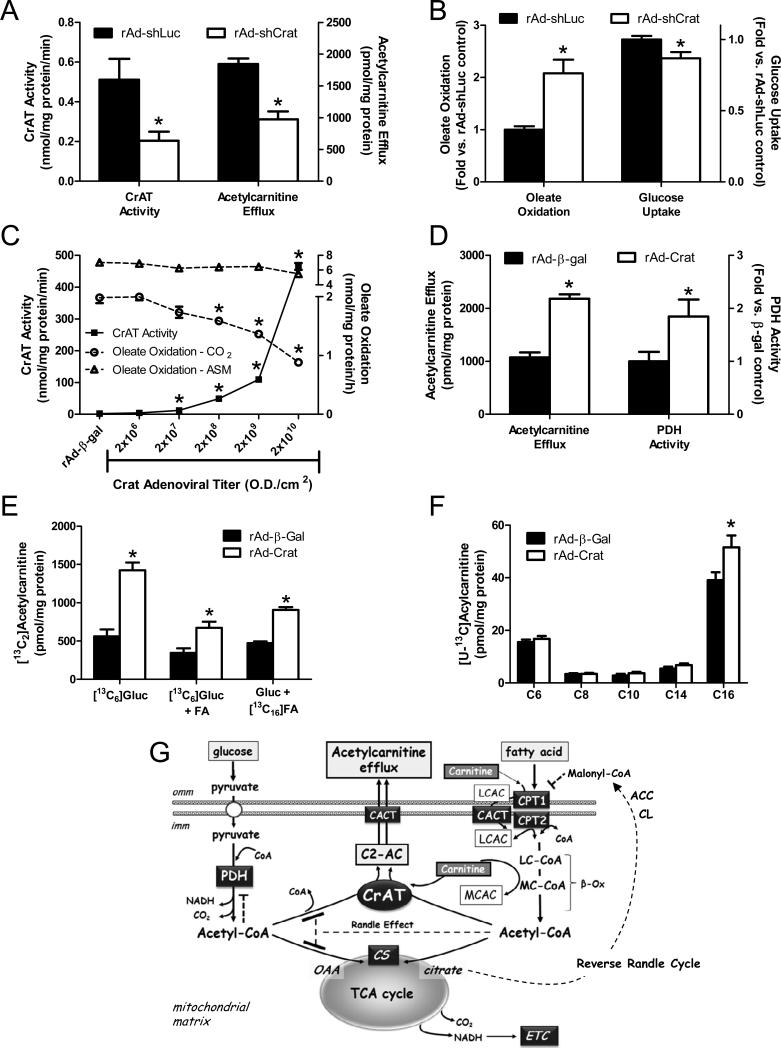
To gain further evidence that CrAT plays a direct role in counter-regulating glucose and fatty acid metabolism we performed loss- and gain-of-function experiments using recombinant adenoviruses engineered to silence (rAd-shCrat) or overexpress (rAd-Crat) the enzyme in primary human myocytes. Treatment of differentiated human myotubes with the rAd-shCrat virus decreased mRNA levels 60-80% (not shown), lowered CrAT specific activity 60% and reduced cellular export of acetylcarnitine by 47% (Figure 6A). This partial loss of Crat activity led to a 2-fold increase in oleate oxidation and a modest but significant 13% decrease in uptake of [3H]-2-deoxy-glucose(Figure 6B). Interestingly, this suppression of glucose uptake in the CrAT knockdown group was exacerbated by acute lipid exposure, both in the absence and presence of insulin (S. Figure 4). Conversely, treatment of human myotubes with rAd-Crat resulted in a dose-dependent induction of enzyme activity along with a progressive decline in complete oxidation of oleate, whereas production of acid soluble metabolites (an index of incomplete fat oxidation) was unchanged (Figure 6C). Treatment with the rAd-β-gal control virus had no effect. The decline in complete fat oxidation was accompanied by enhanced cellular acetylcarnitine efflux and increased PDH activity (Figure 6D).
Figure 6. CrAT counter-regulates glucose and fatty acid metabolism in primary human skeletal myocytes.
Treatment of primary human skeletal myotubes with Ad-shCrAT lowered CrAT activity and acetylcarnitine efflux into the culture media (panel A), increased oxidation of 100 μM [1-14C]oleate to CO2 and decreased uptake of [3H]-2-deoxy-glucose (panel B) as compared to the rAd-shLuc control. Treatment of myotubes treated with increasing doses of rat CrAT (rAd-CrAT) increased CrAT activity and lowered complete oxidation (14CO2) of 100 μM [1-14C]oleate without affecting incomplete oxidation (acid soluble metabolites; ASM). Overexpression of CrAT increased acetylcarnitine efflux into the culture medium and increased PDH activity measured in cell lysates (panel D). Cellular levels of glucose-derived acetylcarnitine was measured after 4 h exposure to [13C6]glucose/[13C3]pyruvate mixture (5 mM/0.1 mM) ± 100 μM palmitate (FA) (panel E). Fatty acid-derived acetylcarnitine (panel E) and medium/long chain acylcarnitines (panel F) were measured after 4 h exposure to [13C16]palmitate (100μM) in the presence of unlabeled glucose/pyruvate (5 mM/0.1 mM). Data are expressed as mean ± SEM and are representative of 2-4 independent experiments. * Effect of CrAT silencing or overexpression (p< 0.05) detected by Student's t-test. Proposed model of CrAT-mediated regulation of mitochondrial carbon trafficking and metabolic flexibility (panel G). During the early phases of re-feeding, muscle mitochondria are confronted with a heavy influx of both glucose and fatty acid fuel. By permitting mitochondrial efflux of excess acetyl moieties, CrAT allows PDH activity to increase despite high rates of β-oxidation. This eases the Randle effect and facilitates a rapid transition from fatty acid to glucose oxidation. CrAT deficiency and/or carnitine insufficiency imposes a more rigid version of the Randle cycle, wherein feeding-induced stimulation of PDH activity is heavily dependent on the production of malonyl-CoA and resultant inhibition of CPT1. Abbreviations: Acetyl-CoA carboxylase (ACC); acetylcarnitine (C2-AC); carnitine acylcarnitine translocase (CACT); carnitine palmitoyltransferase 1 (CPT1); carnitine palmitoyltransferase 2 (CPT2); citrate lyase (CL); carnitine acetyltransferase (CrAT); citrate synthase (CS); electron transport chain (ETC); inner mitochondrial membrane (imm); long chain acylcarnitine (LCAC); long chain acyl-CoA (LC-CoA); medium chain acyl-CoA (MC-CoA); outer mitochondrial membrance (omm); oxaloacetic acid (OAA); pyruvate dehydrogenase (PDH); tricarboxylic acid (TCA).
We next questioned whether CrAT acts exclusively on acetyl-CoA derived from the PDH complex. On the contrary, experiments in primary human skeletal myotubes exposed to [13C6]glucose or [13C16]palmitate showed that CrAT can use acetyl-CoA originating from either glucose or fatty acid (Figure 6E). Addition of fatty acid to the culture medium caused a pronounced shift in the carbon source of acetylcarnitine (in favor of fatty acid) (S. Figures 5 A-C), thus highlighting yet another way in which lipids antagonize muscle glucose metabolism. CrAT overexpression increased production of both glucose- and fatty acid-derived acetylcarnitine, but did not affect the relative enrichment of [13C], regardless of the carbon source (S. Figure 5C). Also noteworthy, incorporation of [13C16]palmitate into [U-13C]-labeled long and medium chain acylcarnitines was either unaffected or increased by CrAT overexpression (Figure 6F). Taken together, these data strongly suggest that CrAT-mediated lowering of fat oxidation to CO2 (Figure 6C) was not secondary to CPT1 inhibition, but instead due to rerouting of fatty acid-derived acetyl-CoA away from the TCA cycle and toward acetylcarnitine efflux (Figure 6G).
Carnitine supplementation improved glucose metabolism in insulin resistant human subjects
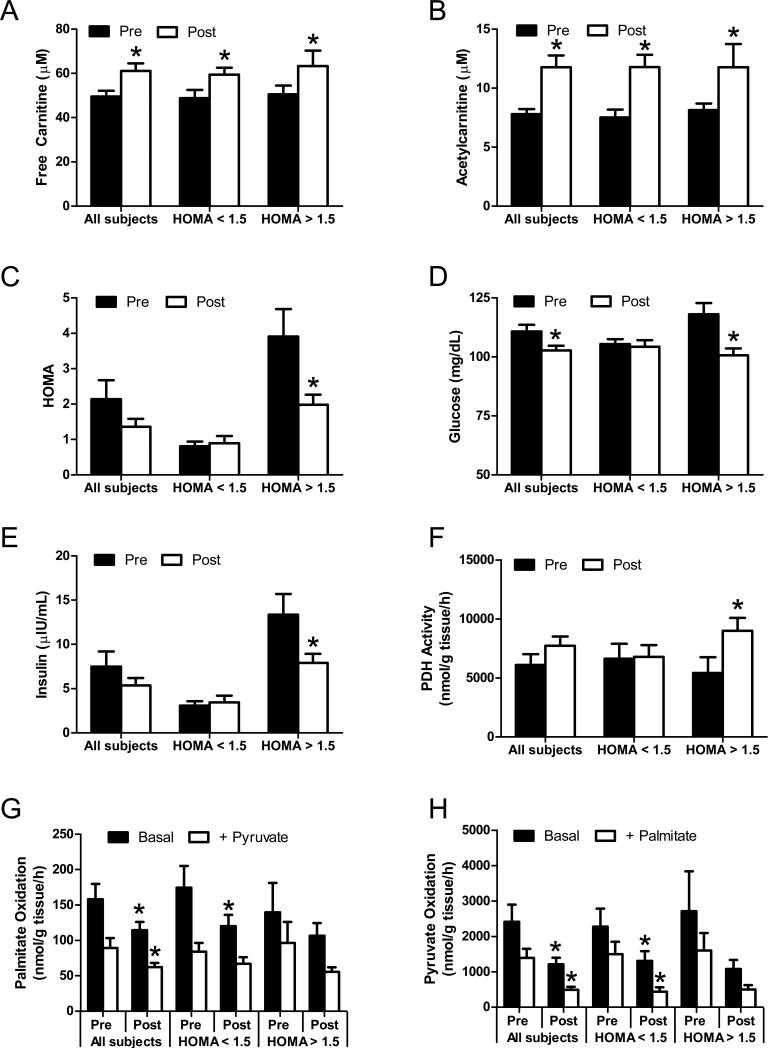
A key question is whether the foregoing findings in animal and cell culture models translate to humans. Real-time PCR analysis showed that CrAT gene expression is perturbed by metabolic disease, as mRNA abundance was 80% lower in skeletal muscle from human patients with type 2 diabetes as compared to normal weight or moderately obese subjects (S. Figure 6; S. Table 1). We then conducted a pilot study to assess the potential therapeutic actions of L-carnitine supplementation in older aged subjects with modestly elevated fasting blood glucose. Subject demographics are provided in the Supplemental Material. Major outcome measures included serum free carnitine and acylcarnitines, substrate oxidation measured in vitro using biopsied muscle specimens, and whole body glucose homeostasis assessed by the HOMA insulin resistance index. In Figure 7 results are presented for the entire cohort grouped together, and after stratifying subjects according to their HOMA-IR upon enrollment, using a conservative cut off of 1.5 (Dvorak et al., 1999). Six months of L-carnitine supplementation increased circulating levels of free carnitine and acetylcarnitine levels 26% and 54%, respectively (Figure 7A and 7B). Whereas the intervention did not appear to change whole body glucose control in the insulin sensitive subgroup, the HOMA-IR index decreased 50% in the insulin resistant group from an average of 3.9 to 1.8 (Figure 7C), due to lowering of both plasma glucose and insulin levels (Figures 7D and 7E).
Figure 7. Carnitine supplementation improves glucose homeostasis in insulin resistant humans.
Older aged human subjects (n=14) with modestly elevated fasting blood glucose levels received supplemental L-carnitine (2 g/day) for 6 months. Fasting serum samples were collected pre- and post-intervention for measurement of free carnitine (panel A), acetylcarnitine (panel B), HOMA (panel C), glucose (panel D) and insulin (panel E). Tissue homogenates prepared from vastus lateralis biopsies were used to measure PDH activity with 5 mM [1-14C]pyruvate (panel F). Substrate switching was determined by measuring oxidation of 50μM [1-14C]palmitate (panel G) or 5 mM [2-14C]pyruvate (panel H) to 14CO2 in the absence or presence of the unlabeled competing substrate (5 mM pyruvate or 100μM palmitate, respectively). Results were stratified according to HOMA at enrollment, using a cut off of 1.5; n=8 below and n=6 above. * Effect of carnitine supplementation analyzed by 1-tailed paired Student's t-test (p<0.05).
Similar to the animal and cell-based studies, enhanced acetylcarnitine efflux in the insulin resistant subjects was accompanied by increased PDH activity measured in muscle homogenates (Figure 7F). When all subjects were grouped together, in vitro oxidation rates of [1-14C]palmitate (Figure 7G) and [2-14C]pyruvate (Figure 7H) to 14CO2 were lower after six months of supplementation, whereas incomplete fat oxidation to ASM was unchanged (not shown). The trends were similar in the low and high HOMA groups, although statistical significance was achieved only in the former. These outcomes resembled the effects of CrAT overexpression in human myocytes and are consistent with enhanced mitochondrial efflux of acetylcarnitine. Metabolic flexibility assays were performed in vitro by measuring the capacity of pyruvate and palmitate to compete with and inhibit oxidation of [1-14C]palmitate and [2-14C]pyruvate, respectively. Fitting with our previous animal work (Noland et al., 2009), nutrient-induced substrate switching was blunted in the insulin resistant group and trended toward an improvement after L-carnitine therapy. Notably, these in vitro assays were performed with a surplus of L-carnitine, implying that the supplementation regimen replenished the mitochondrial pool of free carnitine and/or caused mitochondrial remodelling at some other level.
Discussion
The impetus for the current investigation stemmed from mounting evidence linking insulin resistance to aberrant carnitine metabolism, coupled with recent studies showing that carnitine supplementation promotes CrAT-mediated acetylcarnitine efflux and improves metabolic outcomes in obese rodents (Noland et al., 2009; Power et al., 2007). Whereas decades of intense research have been dedicated to understanding the roles of carnitine and CPT1 in regulating fat oxidation, the metabolic function of CrAT and the physiological relevance of acetylcarnitine efflux have remained comparatively understudied. Results described herein now provide compelling evidence that CrAT does indeed play an indispensable role in defending whole body glucose homeostasis. Thus, muscle-specific ablation of CrAT caused systemic glucose intolerance, interfered with insulin effectiveness and blunted the normal postprandial rise in glucose oxidation.
The primary substrate of CrAT, acetyl-CoA, holds a prominent position in intermediary metabolism as the universal end product of fatty acid, glucose and amino acid oxidation. As its major metabolic fate, acetyl-CoA typically enters the tricarboxylic acid (TCA) cycle where it drives production of reducing equivalents that in turn fuel the mitochondrial electron transport chain. Our findings suggest that CrAT functions as an outlet during conditions wherein the production of short chain acyl-CoAs exceeds TCA cycle flux. This could occur during the transition from fasting to feeding; from rest to vigorous exercise and vice versa; or in a chronically overfed state (Koves et al., 2008; Newgard et al., 2009; Thyfault et al., 2010). Although a role for CrAT in regulating mitochondrial acetyl-CoA/CoA balance and PDH activity had been suggested previously (Uziel et al., 1988); heretofore, this possibility had not undergone rigorous experimental testing, and the importance of the CrAT reaction at a systemic level was unknown. In the current study crat deficiency resulted in abnormal fuel selection, evident in whole animals and in isolated mitochondria, and a corresponding decay in systemic glucose homeostasis. These disruptions in energy metabolism were associated with aberrant control of PDH activity. Thus, loss of CrAT function completely obviated carnitine-mediated stimulation of PDH activity in muscle mitochondria while also hampering the capacity of pyruvate to compete with and inhibit fatty acid oxidation. Notably, the latter outcome bears strong resemblance to mitochondria from obese rats, which failed to reduce fat oxidation in response to a pyruvate challenge (Noland et al., 2009). These findings suggest that deficits in CrAT activity might contribute to diet-induced metabolic inflexibility by exacerbating the glucose-fatty acid cycle as originally described by (Randle et al., 1963).
The Randle hypothesis predicts that acetyl-CoA molecules derived from glucose and lipid substrates compete for entry into the TCA cycle. When β-oxidation increases, the mitochondrial pool of acetyl-CoA expands and inhibits PDH activity and glycolytic flux. This is an appropriate response during fasting conditions when glucose sparing becomes critical for survival. By contrast, the early phases of re-feeding are characterized by an ample supply of both glucose and fatty acid fuel, and the meal-induced rise in pancreatic insulin secretion serves as a fundamental “signal of plenty” that encourages peripheral tissues to re-engage in glucose catabolism. The prevailing view of substrate switching under this circumstance centers on nutrient repletion of the TCA cycle and a resultant surge in mitochondrial efflux of citrate, which is converted to cytosolic acetyl-CoA and then malonyl-CoA via the sequential actions of citrate lyase and acetyl-CoA carboxylase, respectively (Figure 6G). Malonyl-CoA in turn inhibits CPT1, thus restricting mitochondrial uptake and oxidation of fatty acid (McGarry et al., 1991). According to this so-called “reverse Randle cycle”, the pathways governing glucose and fatty acid oxidation are reciprocally regulated in a tightly coordinated fashion (Ruderman et al., 1999). The current study now adds a new dimension to this model by showing that CrAT-mediated acetylcarnitine efflux opposes the Randle cycle and allows PDH activity to increase without a corresponding rise in TCA cycle flux and/or a shutdown in CPT1 activity. We therefore surmise that CrAT affords a mechanism to ease inhibition of PDH during the fasted to fed transition, thereby facilitating a more rapid and efficient transition from fatty acid to glucose oxidation. Conversely, CrAT deficiency imposes a more rigid version of the Randle cycle, such that PDH is rendered more sensitive to counter-regulation by β-oxidation. In this scenario, the feeding-induced shift from lipid to glucose oxidation depends heavily on the gatekeeping function of the malonyl-CoA/CPT1 axis (Figure 6G).
Interestingly, impaired insulin action in the CratM-/- mice was not coupled to an overt defect in Akt phosphorylation. Thus, the block in insulin responsiveness in these mice appears to reside downstream of Akt, or alternatively, might be independent of insulin signaling all together. A number of studies examining the early phases of insulin resistance have likewise reported discrepancies between physiological changes in insulin-stimulated glucose disposal and activation of the canonical insulin signaling pathway (Hoeg et al., 2011; Hoy et al., 2009; Hoy et al., 2007). The notion that pyruvate trafficking per se could impact systemic glucose homeostasis is strengthened by studies in mice lacking PDH kinase 4, which have increased PDH activity and enhanced glucose tolerance (Jeoung and Harris, 2008). These findings fit with a model of distributed flux control wherein the barrier to muscle glucose uptake shifts from the transport step to phosphorylation and metabolism once the Glut4 transporters are mobilized by insulin or exercise (Wasserman, 2009). Whereas the conventional view of muscle glucose metabolism encompasses glucose oxidation, glycogen storage and glycerolipid synthesis, we found that PDH-derived acetylcarnitine is readily exported from both isolated soleus muscles and cultured human myocytes. Thus, acetylcarnitine efflux might represent a quantitatively important means of glucose disposal that in essence allows re-routing of carbons from muscle to other metabolically active tissues (Farrell et al., 1986; Lligona-Trulla et al., 1997). Further studies are necessary to fully delineate the ultimate energetic fate and function of circulating acetylcarnitine.
Another important and perhaps paradigm-shifting concept that emerges from this work is that carnitine positively regulates both lipid and carbohydrate oxidation in muscle. These dual actions of L-carnitine take effect through CPT1 and CrAT, respectively, which access two discrete pools of acyl-CoA substrates. In a previous study accumulation of long chain acylcarnitines in muscle of diabetic rats corresponded with lower free carnitine levels, raising suspicion that mild carnitine deficiency might permit the initial steps of β-oxidation while selectively compromising CrAT activity. Herein, we found that CrAT mRNA expression is severely decreased in humans with type 2 diabetes; and earlier studies reported that CrAT specific activity measured in vitro is inhibited by long chain acyl-CoAs (Chase, 1967). Taken together, these results suggest that metabolic disease and/or chronic lipid exposure might compromise mitochondrial acetylcarnitine efflux capacity. Also noteworthy, the accumulation of medium and long chain acylcarnitines in muscles from CratM-/- mice resembles the metabolite profile assayed in muscles from obese and/or diabetic rodents (Koves et al., 2005; Koves et al., 2008; Noland et al., 2009), thus building upon evidence linking glucose intolerance to overloaded mitochondria. Crat deficiency rendered the mitochondria more vulnerable to nutrient-induced stress and likewise increased susceptibility to diet-induced metabolic dysfunction (Figure 2); whereas carnitine therapy appears to mitigate this stress by shunting surplus acetyl-CoA toward the CrAT reaction. In the human pilot study carnitine therapy was most effective in subjects with the highest HOMA index, suggesting that nutritional and/or pharmacological strategies aimed at increasing acetylcarnitine efflux might benefit pre-diabetic patients. These findings are consistent with three previous reports showing that intravenous infusions of L-carnitine improved insulin sensitivity (measured by hyperinsulinemic euglycemic clamps) in diabetic subjects (Capaldo et al., 1991; Giancaterini et al., 2000; Mingrone et al., 1999). Surprisingly, the antidiabetic potential of chronic L-carnitine supplementation has yet to be rigorously tested in insulin resistant humans. Our results now provide strong justification for larger scale trials.
Lastly, whereas this investigation highlighted the interplay between acetylcarnitine efflux and PDH activity, the regulatory functions of CrAT are likely to extend beyond pyruvate metabolism. Acetyl-CoA and other short chain acyl-CoAs serve both as substrates and allosteric modulators of several metabolic enzymes. In addition, reversible protein acetylation is emerging as a central mechanism of nutrient sensing that in turn integrates metabolic control at multiple levels (Hirschey et al., 2009; Schlicker et al., 2008). This developing area of research tempts speculation that CrAT activity might have a broad-ranging impact on the acetylation state of mitochondrial proteins. Although the answers to this and a number of intriguing questions now await future investigation, our findings clearly establish a heretofore unappreciated role for CrAT in controlling whole body energy metabolism and raise promise that this enzyme might have utility as a therapeutic target.
Experimental Procedures
Animals studies
All procedures were approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee or the Duke University Institutional Animal Care and Use Committee. Mice had free access to food and water and were fed a standard chow (Purina Rodent Chow no. 5015, Purina Mills, St. Louis, MO, USA) prior to being fed a semi-purified 10% fat diet (Research Diets, D12450B) or a 45% fat diet (Research Diets, D12451). The genetic strategy used for generating Cratfl/fl and CratM-/- mice (Figure 1A) is further detailed in Supplemental Methods. Glucose and insulin tolerance tests were performed as stated in the figure legends and indirect calorimetry was measured as described (Albarado et al., 2004) after mice were acclimated for 7 days to a 16-chamber CLAMS system (Columbus Instruments, Columbus, OH, USA).
Metabolic profiling
Acylcarnitine measurements were made using flow injection tandem mass spectrometry and methods described in (An et al., 2004; Millington et al., 1990). [13C2]acetylcarnitine was quantified by standard isotope dilution methodology using D3 acetylcarnitine as the internal standardafter correcting for the natural abundance of the M+1peak of the [13C2]acetylcarnitine peak. This correction was determined empirically from a spectrum of [13C2]acetylcarnitine with no added internal standard. Acyl-CoAs were extracted from heart tissue and analyzed according to methods described in Supplemental Material. Plasma triacylglycerols, non-esterified fatty acids and insulin were measured using kits from Sigma (#337-B), Wako Chemicals and Linco Research, Inc., respectively.
Enzyme Activity Assays
Citrate synthase activity was assessed using a colorimetric assay (Srere, 1969). This assay was adapted for measurement of CrAT activity. Baseline measurements (2 min) were obtained using 10 L of sample incubated in 190 L reaction buffer (50mM Tris-HCl, 1M EDTA, 0.45mM acetyl-CoA, 0.1mM DTNB; pH=7.8). CrAT specific activity was determined by measuring the rate of reduction of DTNB (412 nm) by the free CoA liberated from acetyl-CoA after adding 5mM L-carnitine and monitoring for 10 min.
Substrate Oxidation and Mitochondrial Function
Mitochondria were isolated from mixed gastrocnemius muscles as described in Supplemental Methods and substrate oxidation experiments were performed as described (Kim et al., 2002). PDH activity was assessed ± 5mM L-carnitine by capturing 14CO2 from 1mM [1-14C]pyruvate, whereas palmitate oxidation was determined as 14CO2 released from 100 M or 300 M [1-14C]palmitate in the presence of 0-10mM pyruvate. IC50 values for the inhibitory effect of pyruvate on palmitate oxidation was determined using GraphPad Prism software (LaJolla, CA) after normalization to basal rates followed by log transformation [X=log(X)] of the pyruvate concentrations. Respiratory function was evaluated after mitochondrial resuspension in 0.5mg/ml in MiR05 respiration media (Boushel et al., 2007) using BD Oxygen Biosensor plates (BD Biosciences, MA) as detailed in Supplemental Methods.
Isolated Muscle Incubations
[1-14C]oleate oxidation in isolated soleus muscles was measured using methods adapted from (Muoio et al., 1999) as detailed in the figure legends and Supplemental Material. A similar protocol was followed for tracking metabolism of [13C6]glucose, except muscles were pre-incubated in KHB + 0.2mM oleate:palmitate (1:1) bound to 1% BSA, followed by incubation in the same buffer + 10 mM [13C6]glucose ± 100nM insulin.
Cell Culture Studies
Primary human skeletal myocytes were grown and differentiated (Muoio et al., 2002) in medium supplemented with 100μM L-carnitine. Cytomegalovirus (CMV) promoter-driven recombinant adenoviruses encoding either β-galactosidase (rAdCMV-β-gal) or Myc-tagged rat carnitine acetyltransferase (rAdCMV-Crat) were constructed, amplified and purified according to (Becker et al., 1994). Silencing of CrAT was accomplished using a siRNA target sequence of 5’-GCU CCG AUU UGC UGC CAA A-3’. H1 promoter-driven adenoviruses containing the siRNA sequences for luciferase (rAdH1-shLuc) and Crat (rAdH1-shCrat) were constructed as described by (Odegaard et al., 2010). On differentiation day 3, myotubes were exposed to 2 × 109 O.D./cm2 rAdCMV-Crat (unless otherwise noted) or 1.1 × 1010 O.D./cm2 rAdH1-shCrat for 24 h. Metabolic analyses were performed 3 days after virus treatment. PDH (Arumugam et al., 2010) and CrAT activities were measured in cell lysates. Oxidation of [1-14C]oleate (Koves et al., 2008) was measured during a 2 h incubation in α-MEM. Glucose uptake was measured after 2 h serum starvation in α-MEM, followed by a pre-incubation for 30 min at 37°C and then a 1 h incubation in uptake medium (α-MEM, [3H]-2-deoxyglucose (0.5μCi/ml; 11μM), 1mM L-carnitine) at 37°C. Cells were washed in ice cold buffer (PBS + 20mM glucose) and lysates underwent scintillation counting. Non-specific background counts were estimated from myotubes treated with 40μM cytochalasin B. Stable isotope tracer studies were performed using α-MEM containing [13C6]glucose/[13C3]pyruvate (5mM/0.1mM) ± palmitate (100μM/0.14% BSA), or similar concentrations of [13C16]palmitate with glucose/pyruvate present. Cell lysate and media samples were collected after 4 h and analyzed by mass spectrometry.
Protein Expression
Tissue lysates were prepared using protease and phosphatase inhibitors (Sigma-Aldrich, St. Louis, MO,). Protein concentrations were determined using the Pierce BCA protein assay (Thermo Fisher Scientific Inc., Rockford, IL). Proteins were separated on Criterion gels (Bio-Rad, Hercules, CA) and transferred to nitrocellulose membranes. Blots were blocked with 5% milk and exposed to primary antibodies to detect: CrAT (generously provided by Dr. Fausto Hegardt); total Akt, Akt pThr308 and Akt pSer473 (Cell Signaling, Danvers, MA); PDH-E1a and electron transport chain complexes I, II, and V (MitoSciences, Eugene, OR); PDH-E1a pSer232, PDH-E1a pSer293 and PDH-E1a pSer300 (Calbiochem, San Diego, CA); and citrate synthase (Alpha Diagnostics International, San Antonio, TX). Proteins were visualized using horseradish peroxidase-conjugated immunoglobulin G antibodies and ECL Plus chemiluminescence detection reagent with band intensity being quantified using ImageQuant software (Amersham Biosciences). MemCode (Pierce, Rockford, IL) protein staining served as a loading control for each blot.
Human carnitine supplementation
The experimental protocol was approved by the Duke University Institutional Review Board. Subjects (n=14) responded to ads placed in local papers and were screened for eligibility. Inclusion criteria were age ≥ 60, BMI 25-35.4, fasting blood glucose 95-125 mg/dl, HDL<40 for men and <45 for women and exercise < two times per week. Upon enrollment subjects were started on 2 g/day oral L-carnitine for six months. Compliance was monitored by phone call-ins. At enrollment and upon completion of the study overnight fasting blood samples were collected for measurement of glucose, insulin, free carnitine and acetylcarnitine levels. Muscle biopsy specimens were used for metabolic assays performed in tissue homogenates as detailed in the figure legend.
Statistical analysis
Data are presented as mean ± SEM. Results of animal studies were analysed by two-way ANOVA followed by pair-wise multiple comparison procedures with a Student Newman–Keuls test to evaluate effects of genotype, diet or their interaction using SigmaStat for Windows version 2.03 (SPSS, Chicago, IL, USA). Mitochondrial metabolism studies and isolated muscle incubation experiments were analyzed using a two-way repeated measures ANOVA with Bonferroni post hoc analysis (GraphPad Prism, La Jolla, CA). A Student's t-test was used for studies comparing two groups. Statistical significance was established a priori at p<0.05.
Supplementary Material
Highlights.
Comprehensive metabolic profiling links CrAT to whole body glucose homeostasis
Muscle-specific ablation of Crat disrupts systemic glucose tolerance in mice
CrAT deficiency disrupts nutrient control of PDH activity and substrate switching
L-carnitine supplements improve glucose control in insulin resistant humans
Acknowledgements
This work was supported by funding from the NIDDK; 1F32DK080609 (RN), 1R01DK089312 (DMM), 1R01AG028930 (DMM and WEK) and the American Diabetes Association (DMM and RLM), a Claude Pepper Older Americans Center grant AG028716 (DMM and TRK) and by a NORC Center Grant # 1P30 DK072476 (RLM and ER). We thank the dedicated staff of the Sarah W. Stedman Center Metabolomics and Biomarkers Core Facility; as well as Dr. Fausto Hegardt for providing the CrAT antibody. We also thank Dorothy Slentz, Steven Bond, Dieyun Ding and Estrellita Bermudez for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albarado DC, McClaine J, Stephens JM, Mynatt RL, Ye J, Bannon AW, Richards WG, Butler AA. Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice. Endocrinology. 2004;145:243–252. doi: 10.1210/en.2003-0452. [DOI] [PubMed] [Google Scholar]
- An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, Koves TR, Stevens R, Millington D, Newgard CB. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. NatMed. 2004;10:268–274. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- Arumugam R, Horowitz E, Noland RC, Lu D, Fleenor D, Freemark M. Regulation of islet beta-cell pyruvate metabolism: interactions of prolactin, glucose, and dexamethasone. Endocrinology. 2010;151:3074–3083. doi: 10.1210/en.2010-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TC, BeltrandelRio H, Noel RJ, Johnson JH, Newgard CB. Overexpression of hexokinase I in isolated islets of Langerhans via recombinant adenovirus. Enhancement of glucose metabolism and insulin secretion at basal but not stimulatory glucose levels. J Biol Chem. 1994;269:21234–21238. [PubMed] [Google Scholar]
- Beenakkers AM, Klingenberg M. Carnitine-Coenzyme a Transacetylase in Mitochondria from Various Organs. Biochim Biophys Acta. 1964;84:205–207. doi: 10.1016/0926-6542(64)90081-2. [DOI] [PubMed] [Google Scholar]
- Bieber LL. Carnitine. Annu Rev Biochem. 1988;57:261–283. doi: 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- Bloisi W, Colombo I, Garavaglia B, Giardini R, Finocchiaro G, Didonato S. Purification and properties of carnitine acetyltransferase from human liver. Eur J Biochem. 1990;189:539–546. doi: 10.1111/j.1432-1033.1990.tb15520.x. [DOI] [PubMed] [Google Scholar]
- Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50:790–796. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo B, Napoli R, Di Bonito P, Albano G, Sacca L. Carnitine improves peripheral glucose disposal in non-insulin-dependent diabetic patients. Diabetes Res Clin Pract. 1991;14:191–195. doi: 10.1016/0168-8227(91)90020-e. [DOI] [PubMed] [Google Scholar]
- Chase JF. The substrate specificity of carnitine acetyltransferase. Biochem J. 1967;104:510–518. doi: 10.1042/bj1040510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordente AG, Lopez-Vinas E, Vazquez MI, Swiegers JH, Pretorius IS, Gomez-Puertas P, Hegardt FG, Asins G, Serra D. Redesign of carnitine acetyltransferase specificity by protein engineering. JBiolChem. 2004;279:33899–33908. doi: 10.1074/jbc.M402685200. [DOI] [PubMed] [Google Scholar]
- Dvorak RV, DeNino WF, Ades PA, Poehlman ET. Phenotypic characteristics associated with insulin resistance in metabolically obese but normal-weight young women. Diabetes. 1999;48:2210–2214. doi: 10.2337/diabetes.48.11.2210. [DOI] [PubMed] [Google Scholar]
- Farrell S, Vogel J, Bieber LL. Entry of acetyl-L-carnitine into biosynthetic pathways. Biochim Biophys Acta. 1986;876:175–177. doi: 10.1016/0005-2760(86)90332-2. [DOI] [PubMed] [Google Scholar]
- Giancaterini A, De Gaetano A, Mingrone G, Gniuli D, Liverani E, Capristo E, Greco AV. Acetyl-L-carnitine infusion increases glucose disposal in type 2 diabetic patients. Metabolism. 2000;49:704–708. doi: 10.1053/meta.2000.6250. [DOI] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Huang JY, Verdin E. Acetylation of mitochondrial proteins. Methods Enzymol. 2009;457:137–147. doi: 10.1016/S0076-6879(09)05008-3. [DOI] [PubMed] [Google Scholar]
- Hoeg LD, Sjoberg KA, Jeppesen J, Jensen TE, Frosig C, Birk JB, Bisiani B, Hiscock N, Pilegaard H, Wojtaszewski JF, et al. Lipid-induced insulin resistance affects women less than men and is not accompanied by inflammation or impaired proximal insulin signaling. Diabetes. 2011;60:64–73. doi: 10.2337/db10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy AJ, Brandon AE, Turner N, Watt MJ, Bruce CR, Cooney GJ, Kraegen EW. Lipid and insulin infusion-induced skeletal muscle insulin resistance is likely due to metabolic feedback and not changes in IRS-1, Akt, or AS160 phosphorylation. AmJPhysiol EndocrinolMetab. 2009;297:E67–E75. doi: 10.1152/ajpendo.90945.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy AJ, Bruce CR, Cederberg A, Turner N, James DE, Cooney GJ, Kraegen EW. Glucose infusion causes insulin resistance in skeletal muscle of rats without changes in Akt and AS160 phosphorylation. AmJPhysiol EndocrinolMetab. 2007;293:E1358–E1364. doi: 10.1152/ajpendo.00133.2007. [DOI] [PubMed] [Google Scholar]
- Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. AmJPhysiol EndocrinolMetab. 2008;295:E46–E54. doi: 10.1152/ajpendo.00536.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- Kim JY, Koves TR, Yu GS, Gulick T, Cortright RN, Dohm GL, Muoio DM. Evidence of a malonyl-CoA-insensitive carnitine palmitoyltransferase I activity in red skeletal muscle. AmJPhysiol EndocrinolMetab. 2002;282:E1014–E1022. doi: 10.1152/ajpendo.00233.2001. [DOI] [PubMed] [Google Scholar]
- Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Lligona-Trulla L, Arduini A, Aldaghlas TA, Calvani M, Kelleher JK. Acetyl-L-carnitine flux to lipids in cells estimated using isotopomer spectral analysis. J Lipid Res. 1997;38:1454–1462. [PubMed] [Google Scholar]
- Marquis NR, Fritz IB. The Distribution of Carnitine, Acetylcarnitine, and Carnitine Acetyltransferase in Rat Tissues. The Journal of biological chemistry. 1965;240:2193–2196. [PubMed] [Google Scholar]
- McGarry JD, Sen A, Esser V, Woeltje KF, Weis B, Foster DW. New insights into the mitochondrial carnitine palmitoyltransferase enzyme system. Biochimie. 1991;73:77–84. doi: 10.1016/0300-9084(91)90078-f. [DOI] [PubMed] [Google Scholar]
- Millington DS, Kodo N, Norwood DL, Roe CR. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis. 1990;13:321–324. doi: 10.1007/BF01799385. [DOI] [PubMed] [Google Scholar]
- Mingrone G, Greco AV, Capristo E, Benedetti G, Giancaterini A, De Gaetano A, Gasbarrini G. L-carnitine improves glucose disposal in type 2 diabetic patients. J Am Coll Nutr. 1999;18:77–82. doi: 10.1080/07315724.1999.10718830. [DOI] [PubMed] [Google Scholar]
- Muoio DM. Intramuscular triacylglycerol and insulin resistance: guilty as charged or wrongly accused? Biochimica et biophysica acta. 2010;1801:281–288. doi: 10.1016/j.bbalip.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM, Dohm GL, Tapscott EB, Coleman RA. Leptin opposes insulin's effects on fatty acid partitioning in muscles isolated from obese ob/ob mice. Am J Physiol. 1999;276:E913–921. doi: 10.1152/ajpendo.1999.276.5.E913. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Way JM, Tanner CJ, Winegar DA, Kliewer SA, Houmard JA, Kraus WE, Dohm GL. Peroxisome proliferator-activated receptor-alpha regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes. 2002;51:901–909. doi: 10.2337/diabetes.51.4.901. [DOI] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O, Stevens RD, Hegardt FG, Muoio DM. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. JBiolChem. 2009;284:22840–22852. doi: 10.1074/jbc.M109.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard ML, Joseph JW, Jensen MV, Lu D, Ilkayeva O, Ronnebaum SM, Becker TC, Newgard CB. The mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway that mediates glucose- and glutamine-stimulated insulin secretion. J Biol Chem. 2010;285:16530–16537. doi: 10.1074/jbc.M109.092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power RA, Hulver MW, Zhang JY, Dubois J, Marchand RM, Ilkayeva O, Muoio DM, Mynatt RL. Carnitine revisited: potential use as adjunctive treatment in diabetes. Diabetologia. 2007;50:824–832. doi: 10.1007/s00125-007-0605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay RR, Zammit VA. Carnitine acyltransferases and their influence on CoA pools in health and disease. MolAspects Med. 2004;25:475–493. doi: 10.1016/j.mam.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Saha AK, Vavvas D, Witters LA. Malonyl-CoA, fuel sensing, and insulin resistance. The American journal of physiology. 1999;276:E1–E18. doi: 10.1152/ajpendo.1999.276.1.E1. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382:790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Srere PA. Citrate synthase. In: Lowenstein JM, editor. Methods in Enzymology. Academic Press; New York: 1969. pp. 3–5. [Google Scholar]
- Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. AmJPhysiol EndocrinolMetab. 2003;284:E855–E862. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- Thyfault JP, Cree MG, Tapscott EB, Bell JA, Koves TR, Ilkayeva O, Wolfe RR, Dohm GL, Muoio DM. Metabolic profiling of muscle contraction in lean compared with obese rodents. American journal of physiology Regulatory, integrative and comparative physiology. 2010;299:R926–934. doi: 10.1152/ajpregu.00093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyfault JP, Rector RS, Noland RC. Metabolic inflexibility in skeletal muscle: a prelude to the cardiometabolic syndrome? J Cardiometab Syndr. 2006;1:184–189. doi: 10.1111/j.1559-4564.2006.05629.x. [DOI] [PubMed] [Google Scholar]
- Uziel G, Garavaglia B, Di Donato S. Carnitine stimulation of pyruvate dehydrogenase complex (PDHC) in isolated human skeletal muscle mitochondria. Muscle Nerve. 1988;11:720–724. doi: 10.1002/mus.880110708. [DOI] [PubMed] [Google Scholar]
- Wasserman DH. Four grams of glucose. AmJPhysiol EndocrinolMetab. 2009;296:E11–E21. doi: 10.1152/ajpendo.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.