Abstract
Cyclic AMP response element binding protein (CREB) plays a critical role in fear memory formation. Here we determined the role of CREB selectively within the amygdala in reconsolidation and extinction of auditory fear. Viral overexpression of the inducible cAMP early repressor (ICER) or the dominant-negative mCREB, specifically within the lateral amygdala disrupted reconsolidation of auditory fear memories. In contrast, manipulations of CREB in the amygdala did not modify extinction of fear. These findings suggest that the role of CREB in modulation of memory after retrieval is dynamic and that CREB activity in the basolateral amygdala is involved in fear memory reconsolidation.
Reconsolidation, the process by which memories are restabilized and maintained after retrieval (Przybyslawski and Sara 1997; Nader et al. 2000), and extinction, the learning process by which conditioned responses are repressed (Bouton 1993; Myers and Davis 2002), are competing processes in the regulation of established memories. It has previously been shown that brief re-exposure to a conditioned stimulus (CS) triggers reconsolidation, whereas long re-exposures preferentially induce extinction (Pedreira and Maldonado 2003). Understanding the differential mechanisms of reconsolidation and extinction, and the molecular events that are involved in “switching” between these opposing processes, is crucial for understanding the dynamic regulation of established memory.
The cyclic AMP response element binding protein (CREB) has long been known for its critical role in memory consolidation (Dash et al. 1990; Bourtchuladze et al. 1994; Yin et al. 1994). Less well understood is the role of endogenous repressors of CREB including the inducible CREB early repressor (ICER). Activation of CREB leads to transcription of ICER, completing a negative feedback loop. Overexpression of ICER leads to impaired fear memory, and ICER knockout mice show enhanced fear memory (Mouravlev et al. 2006; Kojima et al. 2008). Thus, CREB repressor proteins are also critically important in regulating memory.
Previous studies using forebrain-deletion of CREB have demonstrated its critical involvement in the reconsolidation of hippocampal-dependent (Kida et al. 2002; Mamiya et al. 2009) and cued fear memory (Kida et al. 2002). These findings demonstrated a converging role of CREB in both consolidation and reconsolidation of fear. However, in addition to the hippocampus, CREB was also deleted after retrieval in other forebrain regions such as regions of the prefrontal cortex, including anterior cingulate cortex. The anterior cingulate is also activated by presentation of a fear-associated cue, and thus a potential contributor to reconsolidation processes (Thomas et al. 2002). These limitations, together with the lack of dissociation from extinction, makes it necessary to directly confirm the hypothesis that CREB in the amygdala is required for memory reconsolidation. It is therefore important to examine the specific role for CREB-regulated signaling in amygdala-dependent in fear memory processes.
Here we directly assessed the specific role of amygdalar CREB in memory reconsolidation and extinction. CREB is activated in the amygdala after either short or long re-exposure to a conditioned tone (Hall et al. 2001) or context (Mamiya et al. 2009). In addition, the upstream kinases, extracellular signal regulated kinase (ERK) (Duvarci et al. 2006) and protein kinase A (PKA) (Tronson et al. 2006; Sanchez et al. 2010), in the amygdala are required for reconsolidation of either fear or drug-paired cues. No direct evidence exists, however, for the role of CREB specifically in the amygdala in memory reconsolidation, and no studies have compared its role in reconsolidation with extinction in parallel.
The mechanisms of extinction are known to differ at a molecular (Myers and Davis 2002) and systems (Santini et al. 2008) level from those of consolidation. In extinction, evidence for the requirement of amygdalar CREB during memory extinction remains contradictory. Two studies demonstrate increased phospho-CREB after long, extinction-inducing trials (Hall et al. 2001; Mamiya et al. 2009) whereas other studies demonstrate a decrease in CREB activity after extinction (Lin et al. 2003; Izumi et al. 2008). Furthermore, the extracellular signal-regulated kinase (ERK), but not protein kinase A (PKA), is required for extinction in the amygdala (Herry et al. 2006; Matsuda et al. 2010). Thus, the role of amygdalar CREB in extinction of auditory fear is unknown. Here, we used specific manipulations of the amygdala to directly test the hypothesis that CREB within the basolateral amygdala (BLA) is necessary for reconsolidation, but not extinction, of auditory fear memories.
In all experiments Sprague-Dawley rats (Charles River Laboratories, USA) were conditioned on tone-dependent fear conditioning, consisting of a 5-min habituation in context A, followed by single 30-sec tone coterminating with a 1-sec, 2-mA footshock (as previously described in Tronson et al. 2006). HSV-mCREB, HSV-ICER, HSV-CREB, or HSV-LacZ were infused (1 µL/side at a rate of 0.25 µL/min) via cannulae (PlasticsOne) previously implanted into the BLA (Co-ordinates: −3.0 mm posterior to bregma, ±5.3 mm from the midline, and −8.0 mm ventral to skull surface; (Paxinos and Watson 1986). Injection cannulae were left in place for 2 min following the infusion to allow for diffusion. Viral titer was determined in prior studies from this laboratory, which demonstrated significant expression of the encoded proteins between 3 and 7 d after injection (Carlezon et al. 1998; Pliakas et al. 2001; Green et al. 2006). To examine the role of amygdala CREB in auditory fear memories, rats were given reactivation session consisting of a single 30-sec tone presentation in a novel context (context B) 72 h after viral vector injection, at the peak of expression. Fear was measured by scoring freezing as previously described (Tronson et al. 2006).
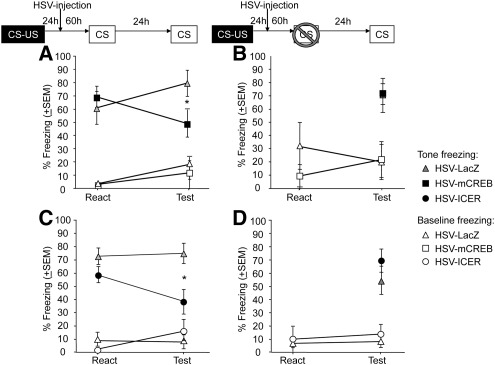
In the first experiment, HSV-mCREB injected into the BLA significantly reduced freezing during a test session 24 h after a reactivation trial (Fig. 1A, F(1,16) = 7.13, P < 0.05). Similarly, expression of HSV-ICER during reactivation disrupted freezing at a subsequent test (Fig. 1C, Day × Vector interaction F(1,15) = 6.61, P < 0.05). “No Reactivation” control groups consisted of rats expressing HSV-mCREB, HSV-ICER, or HSV-LacZ in the BLA, and exposed to the novel context without a reactivation trial (that is, no re-exposure to the tone CS). Neither HSV-mCREB nor HSV-ICER altered freezing in “No Reactivation” groups compared with HSV-LacZ (mCREB: Fig. 1B; F(1,11) < 1; ICER “No Reactivation”; Fig. 1D, F(1,15) = 2.06, P > 0.05), demonstrating that the deficits produced by inhibition of CREB activity or ICER overexpression were specific to a memory made labile by retrieval. All groups displayed high levels of freezing specific to the tone during the reactivation day, with low levels of nonspecific freezing to the context (Fig. 1A,B; F(1,16) = 106.38, P < 0.01; ICER Fig. 1C,D; all F(1,15) < 1), hence, CREB activity in the BLA is not required for retrieval of an auditory fear memory.
Figure 1.
Amygdalar CREB is required for fear memory reconsolidation. (A) mCREB overexpression in the BLA disrupts auditory fear memory after retrieval (*) P < 0.05; (B) fear memory retrieval is not disrupted without prior reactivation. (C) ICER overexpression in the BLA disrupts memory after retrieval (*) P < 0.05; (D) fear memory retrieval is not disrupted without prior reactivation; (A–D) open symbols represent baseline freezing in Context B. Neither HSV-mCREB (□) nor HSV-ICER (○) altered baseline freezing.
The disruption of fear memory reconsolidation by preventing amygdalar CREB activation is consistent with previous studies demonstrating that reconsolidation of auditory fear memories is dependent on ERK (Duvarci et al. 2005) and PKA (Tronson et al. 2006) in the amygdala, and that CREB throughout forebrain regions (including the amygdala, hippocampus, cortex, and striatum) is required for reconsolidation of contextual fear memories (Kida et al. 2002; Mamiya et al. 2009). The present study extends these findings and demonstrates, using two complementary experimental manipulations, that disruption of CREB specifically within the BLA is sufficient to impair reconsolidation of auditory fear memories.
It is important to note that inhibiting CREB activity did not disrupt retrieval of memory. Neither HSV-mCREB, nor HSV-ICER, prevented retrieval or freezing to the CS during the reactivation trial, nor did they affect freezing at test in animals in the “No Reactivation” control groups. The requirement for amygdalar CREB in reconsolidation of fear memories, together with the independence of retrieval processes from CREB function, provide strong support for the assertion that disruption of reconsolidation are due to post-retrieval storage failures and not impaired retrieval (Nader et al. 2000; Riccio et al. 2002; Alberini 2008; Hardt et al. 2009).
In addition, the use of viral vectors that continue to express throughout reactivation and test sessions means that there is no internal or perceptual context shift that would lead to impaired retrieval at a subsequent test (Hinderliter et al. 1975; Riccio et al. 2002). This procedural advantage means that disruption of memory after retrieval by HSV-mCREB or HSV-ICER cannot be explained by context-dependent memory retrieval. Together, these data strongly suggest that reconsolidation is a post-retrieval storage and maintenance process, the disruption of which can result in the permanent loss of a previously established memory. These data are consistent with the finding, extensively discussed in the literature, that although differences do occur, the molecular mechanisms underlying reconsolidation significantly overlap with those of consolidation (Tronson and Taylor 2007). The present findings provide further evidence that, like consolidation (e.g., Bourtchuladze et al. 1994; Han et al. 2007) amygdalar CREB activity is required for reconsolidation of fear memories.
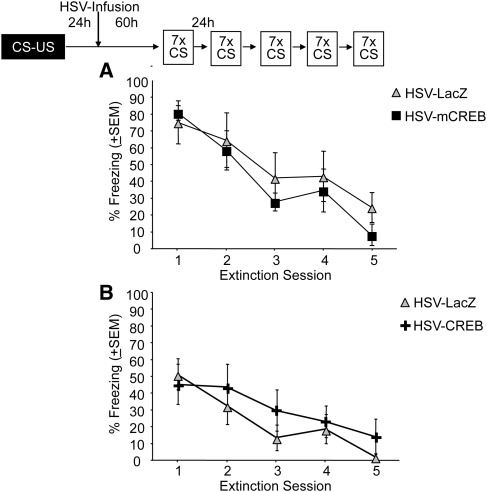
We next examined the role of CREB in the amygdala in the extinction of auditory fear. Here, we used an extinction protocol previously demonstrated to induce extinction without reconsolidation (Tronson et al. 2006). Briefly, long extinction sessions (seven presentations of 30-sec tone, 2-min ITI) were presented on each of five consecutive days. The first extinction session began 3 d after injection of HSV-mCREB, HSV-CREB, or HSV-LacZ, as described above. The long-reactivation sessions resulted in significant extinction in both HSV-LacZ and HSV-mCREB groups (Fig. 2A; Main effect Day F(4,56) = 21.77, P < 0.01), but no differences between the experimental groups (Interaction Day × Vector F(4,56) = 1.32, P > 0.05). Similarly, overexpression of CREB, using HSV-CREB, showed significant extinction with no difference compared with HSV-LacZ controls in the rate of extinction across days (Fig. 2B; Main effect Day F(4,64) = 10.05; P < 0.05; Day × Vector F(4,64) < 1. Viral vector expression shown in Fig. 3). Therefore, in contrast to the requirement for CREB in memory reconsolidation, extinction of fear was not altered by either disruption or overexpression of CREB.
Figure 2.
Amygdalar CREB is not required for extinction of auditory fear. (A) Expression of mCREB or (B) overexpression of CREB during retrieval does not alter extinction of conditioned fear.
Figure 3.
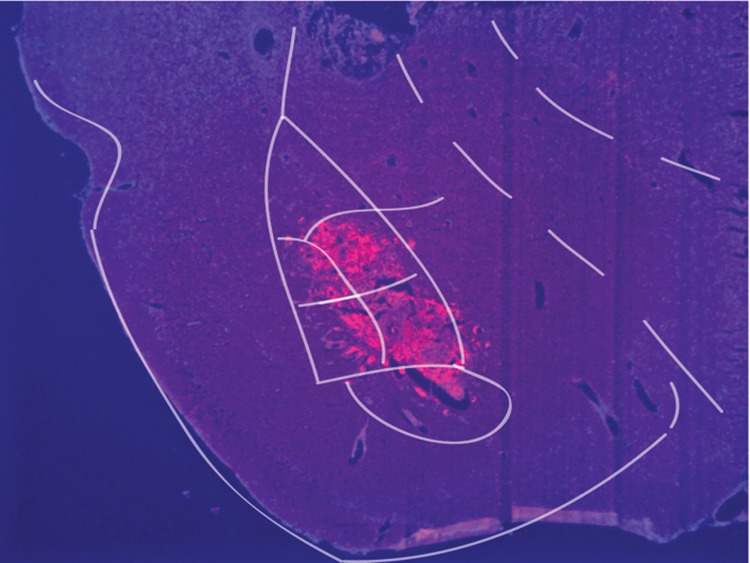
Expression of HSV-LacZ in the amygdala. For confirmation of viral expression, rats were transcardially perfused with 10% formalin 72 h after injection. Immunohistochemistry of 15-μm slide-mounted sections was conducted using a previously published protocol (Chao et al. 2002). Antibodies: goat anti-β-galactosidase (β-gal) (1:5000 Biogenesis England, UK); Cy3-conjugated secondary antibodies (1:200 Jackson ImmunoResearch, USA). DAPI (1:10000).
This finding is consistent with previous data suggesting that inhibition of neither PKA activity or protein synthesis within the BLA disrupts extinction of auditory fear (Anglada-Figueroa and Quirk 2005; Tronson et al. 2006). Together, these findings contrast with studies showing activation of CREB after long extinction sessions (Hall et al. 2001; Mamiya et al. 2009). These data support the hypothesis that extinction of auditory fear is mediated at a circuit level, where the medial prefrontal cortex plays a prominent role (Quirk et al. 2006). Within this circuit, the amygdala may primarily recruit a subset of neurons (Herry et al. 2006), and inhibitory processes, including those mediated by GABA (Chhatwal et al. 2009; Lin et al. 2009), endocannabinoids, and Akt signaling pathways (Chhatwal et al. 2009; Lin et al. 2009), but not PKA/CREB signaling.
The lack of effect on extinction here also clarifies the role of decreased phospho-CREB after extinction of fear (Lin et al. 2003; Izumi et al. 2008). If CREB dephosphorylation in the amygdala is required for extinction, then expression of the dominant-negative mCREB should enhance extinction. Thus, decreased CREB activity might correlate with, but not causally relate to, extinction of fear at the level of the amygdala. Finally, the lack of effect on extinction implies that the disruption of reconsolidation by ICER or mCREB was not due to enhanced extinction. Thus, the differential role of CREB in consolidation and reconsolidation vs. extinction suggest that, although extinction is a form of learning, it relies on fundamentally different processes in the mammalian brain.
Defining experimental protocols for selectively manipulating either reconsolidation or extinction has been a critical development in the study of reconsolidation and extinction (Eisenberg et al. 2003; Pedreira and Maldonado 2003; but, see also Duvarci et al. 2006). Using these parameters, mechanistic studies have demonstrated a different pattern of systems (Bahar et al. 2004) and molecular mechanisms (Suzuki et al. 2004; Lee et al. 2006; Tronson et al. 2006; Mamiya et al. 2009; Yamada et al. 2009) underlying reconsolidation and extinction (for further review, c.f. Tronson and Taylor 2007). The ability to experimentally determine whether reconsolidation or extinction after memory retrieval is the dominant process simply by changing the length of re-exposure to the conditioned stimulus suggests that there is one pathway, or multiple parallel processes (de la Fuente et al. 2011) that act as a “switch,” allowing for one process to take over from the other. These data presented here, together with previous evidence for the role of ICER in decreasing CREB after sustained activity (Sassone-Corsi 1995), suggest that regulation of CREB may contribute to the switch between reconsolidation and extinction. Induction of ICER may, under normal circumstances, act to suppress CREB, thereby reducing reconsolidation, and allowing extinction to proceed as the dominant process. This may be especially true after long reactivation sessions (Pedreira and Maldonado 2003) where CREB is first activated in a sustained manner (Hall et al. 2001; Mamiya et al. 2009), triggering transcription of CREB regulatory proteins including ICER (De Cesare and Sassone-Corsi 2000) and resulting in inhibition of CREB activity (Lin et al. 2003; Izumi et al. 2008).
Our results provide the first direct evidence using two complementary strategies that CREB activity in the BLA alone is required for reconsolidation of auditory memories, but is not required for retrieval or extinction of fear. Notably, the direct comparison of extinction and reconsolidation in rats with inhibited amygdalar CREB effectively rules out enhanced extinction as an explanation for disrupted memory after retrieval. Furthermore, these findings suggest that dysregulation of ICER/CREB in the amygdala may prevent the switch from reconsolidation to extinction, resulting in the maintenance of excessive fear states. Given that the amygdala and related circuitry have been implicated in pathological states associated with both fear and addiction, these mechanisms may similarly be involved in cue-induced maintenance of addiction.
Defining the differential molecular mechanisms of extinction and reconsolidation allows for the possibility of separately targeting both of these processes to reduce persistent memories and maladaptive behavioral responses. Together, enhancing extinction and disrupting reconsolidation might lead to effective treatments in anxiety disorders, including phobias and PTSD (Davis et al. 2006; Schiller et al. 2010) and drug addiction (Tronson and Taylor 2007; Taylor et al. 2009; Milton and Everitt 2010) thereby rapidly and persistently reducing invasive memories and subsequent relapse to anxiety, flashbacks, and drug-seeking.
Acknowledgments
This work was supported by PHS MH66172 (E.J.N.) and DA015222 (J.R.T.) and the Connecticut Department of Mental Health and Addiction Services (J.R.T.) We also acknowledge support from the Interdisciplinary Research Consortium on Stress, Self-control and Addiction (UL1-DE19586) and the NIH Roadmap for Medial Research/Common Fund (AA017537).
References
- Alberini CM 2008. The role of protein synthesis during the labile phases of memory: Revisiting the skepticism. Neurobiol Learn Mem 89: 234–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglada-Figueroa D, Quirk GJ 2005. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci 25: 9680–9685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar A, Dorfman N, Dudai Y 2004. Amygdalar circuits required for either consolidation or extinction of taste aversion memory are not required for reconsolidation. Eur J Neurosci 19: 1115–1118 [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ 1994. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79: 59–68 [DOI] [PubMed] [Google Scholar]
- Bouton ME 1993. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull 114: 80–99 [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ 1998. Regulation of cocaine reward by CREB. Science 282: 2272–2275 [DOI] [PubMed] [Google Scholar]
- Chao JR, Ni YG, Bolanos CA, Rahman Z, DiLeone RJ, Nestler EJ 2002. Characterization of the mouse adenylyl cyclase type VIII gene promoter: regulation by cAMP and CREB. Eur J Neurosci 16: 1284–1294 [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Gutman AR, Maguschak KA, Bowser ME, Yang Y, Davis M, Ressler KJ 2009. Functional interactions between endocannabinoid and CCK neurotransmitter systems may be critical for extinction learning. Neuropsychopharmacology 34: 509–521 [DOI] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER 1990. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature 345: 718–721 [DOI] [PubMed] [Google Scholar]
- Davis M, Barad M, Otto M, Southwick S 2006. Combining pharmacotherapy with cognitive behavioral therapy: Traditional and new approaches. J Trauma Stress 19: 571–581 [DOI] [PubMed] [Google Scholar]
- De Cesare D, Sassone-Corsi P 2000. Transcriptional regulation by cyclic AMP-responsive factors. Prog Nucleic Acid Res Mol Biol 64: 343–369 [DOI] [PubMed] [Google Scholar]
- de la Fuente V, Freudenthal R, Romano A 2011. Reconsolidation or extinction: Transcription factor switch in the determination of memory course after retrieval. J Neurosci 31: 5562–5573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE 2005. Activation of extracellular signal-regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci 21: 283–289 [DOI] [PubMed] [Google Scholar]
- Duvarci S, Mamou CB, Nader K 2006. Extinction is not a sufficient condition to prevent fear memories from undergoing reconsolidation in the basolateral amygdala. Eur J Neurosci 24: 249–260 [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y 2003. Stability of retrieved memory: Inverse correlation with trace dominance. Science 301: 1102–1104 [DOI] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Hommel JD, DiLeone RJ, Kumar A, Theobald DE, Neve RL, Nestler EJ 2006. Induction of inducible cAMP early repressor expression in nucleus accumbens by stress or amphetamine increases behavioral responses to emotional stimuli. J Neurosci 26: 8235–8242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ 2001. Fear memory retrieval induces CREB phosphorylation and Fos expression within the amygdala. Eur J Neurosci 13: 1453–1458 [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA 2007. Neuronal competition and selection during memory formation. Science 316: 457–460 [DOI] [PubMed] [Google Scholar]
- Hardt O, Wang SH, Nader K 2009. Storage or retrieval deficit: The yin and yang of amnesia. Learn Mem 16: 224–230 [DOI] [PubMed] [Google Scholar]
- Herry C, Trifilieff P, Micheau J, Luthi A, Mons N 2006. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci 24: 261–269 [DOI] [PubMed] [Google Scholar]
- Hinderliter CF, Webster T, Riccio DC 1975. Amnesia induced by hypothermia as a function of treatment-test interval and recooling in rats. Anim Learn Behav 3: 257–263 [Google Scholar]
- Izumi T, Inoue T, Kato A, Kitaichi Y, Nakagawa S, Koyama T 2008. Changes in amygdala neural activity that occur with the extinction of context-dependent conditioned fear stress. Pharmacol Biochem Behav 90: 297–304 [DOI] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, Pena de Ortiz S, Kogan JH, Chevere I, Masushige S, Silva AJ 2002. CREB required for the stability of new and reactivated fear memories. Nat Neurosci 5: 348–355 [DOI] [PubMed] [Google Scholar]
- Kojima N, Borlikova G, Sakamoto T, Yamada K, Ikeda T, Itohara S, Niki H, Endo S 2008. Inducible cAMP early repressor acts as a negative regulator for kindling epileptogenesis and long-term fear memory. J Neurosci 28: 6459–6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ 2006. Reconsolidation and extinction of conditioned fear: Inhibition and potentiation. J Neurosci 26: 10051–10056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu HY, Gean PW 2003. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J Neurosci 23: 8310–8317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Gean PW 2009. Block of γ-aminobutyric acid-A receptor insertion in the amygdala impairs extinction of conditioned fear. Biol Psychiatry 66: 665–673 [DOI] [PubMed] [Google Scholar]
- Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, Kida S 2009. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci 29: 402–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Matsuzawa D, Nakazawa K, Sutoh C, Ohtsuka H, Ishii D, Tomizawa H, Iyo M, Shimizu E 2010. d-serine enhances extinction of auditory cued fear conditioning via ERK1/2 phosphorylation in mice. Prog Neuropsychopharmacol Biol Psych 34: 895–902 [DOI] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ 2010. The psychological and neurochemical mechanisms of drug memory reconsolidation: Implications for the treatment of addiction. Eur J Neurosci 31: 2308–2319 [DOI] [PubMed] [Google Scholar]
- Mouravlev A, Dunning J, Young D, During MJ 2006. Somatic gene transfer of cAMP response element-binding protein attenuates memory impairment in aging rats. Proc Natl Acad Sci 103: 4705–4710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M 2002. Behavioral and neural analysis of extinction. Neuron 36: 567–584 [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE 2000. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406: 722–726 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C 1986. The rat brain in stereotaxic coordinates. Academic Press, Sydney, Australia; Orlando, FL [Google Scholar]
- Pedreira ME, Maldonado H 2003. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron 38: 863–869 [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA Jr 2001. Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci 21: 7397–7403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyslawski J, Sara SJ 1997. Reconsolidation of memory after its reactivation. Behav Brain Res 84: 241–246 [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F 2006. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 60: 337–343 [DOI] [PubMed] [Google Scholar]
- Riccio DC, Moody EW, Millin PM 2002. Reconsolidation reconsidered. Integr Physiol Behav Sci 37: 245–253 [DOI] [PubMed] [Google Scholar]
- Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR 2010. Reconsolidation of a cocaine-associated stimulus requires amygdalar protein kinase A. J Neurosci 30: 4401–4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT 2008. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci 28: 4028–4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P 1995. Transcription factors responsive to cAMP. Annu Rev Cell Dev Biol 11: 355–377 [DOI] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA 2010. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463: 49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S 2004. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci 24: 4787–4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM 2009. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology 56 (Suppl 1): 186–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KL, Hall J, Everitt BJ 2002. Cellular imaging with zif268 expression in the rat nucleus accumbens and frontal cortex further dissociates the neural pathways activated following the retrieval of contextual and cued fear memory. Eur J Neurosci 16: 1789–1796 [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR 2007. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci 8: 262–275 [DOI] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR 2006. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci 9: 167–169 [DOI] [PubMed] [Google Scholar]
- Yamada D, Zushida K, Wada K, Sekiguchi M 2009. Pharmacological discrimination of extinction and reconsolidation of contextual fear memory by a potentiator of AMPA receptors. Neuropsychopharmacology 34: 2574–2584 [DOI] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T 1994. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 79: 49–58 [DOI] [PubMed] [Google Scholar]