Abstract
The regenerating (Reg) protein family comprises C-type lectin-like proteins discovered independently during pancreatitis and pancreatic islet regeneration. However, an increasing number of studies provide evidence of participation of Reg proteins in the proliferation and differentiation of diverse cell types. Moreover, Reg family members are associated with various pathologies, including diabetes and forms of gastrointestinal cancer. These findings have led to the emergence of key roles for Reg proteins as anti-inflammatory, antiapoptotic and mitogenic agents in multiple physiologic and disease contexts. Yet, there are significant gaps in our knowledge regarding the regulation of expression of different Reg genes. In addition, the pathways relaying Reg-triggered signals, their targets and potential cross-talk with other cascades are still largely unknown. In this review, the expression patterns of different Reg members in the pancreas and extrapancreatic tissues are described. Moreover, factors known to modulate Reg levels in different cell types are discussed. Several signaling pathways, which have been implicated in conferring the effects of Reg ligands to date, are also delineated. Further efforts are necessary for elucidating the biological processes underlying the action of Reg proteins and their involvement in various maladies. Better understanding of the function of Reg genes and proteins will be beneficial in the design and development of therapies utilizing or targeting this protein group.
Keywords: Cancer, diabetes, regenerating (Reg) proteins, Reg expression regulation, signaling
Introduction
The first regenerating (Reg) protein was identified in pancreatic stones and was named pancreatic stone protein (PSP) (1). As later studies revealed, PSP could undergo cleavage by trypsin resulting in its insoluble form named pancreatic thread protein (PTP) and an 11-amino acid fragment (2, 3). PSP was also referred to as lithostathine due to its potential role in inhibiting pancreatic stone formation (4, 5). The protein was re-discovered independently in regenerating rat islets after pancreatectomy (6) and the term ‘regenerating protein’ was coined, although was reported subsequently that Reg (now known as Reg1), PSP and lithostathine refer to the same gene product.
In a similar manner, a related protein, the pancreatitis-associated protein (PAP) was independently found in acute pancreatitis samples (7), rat pituitary gland (peptide 23) (8, 9) and in hepatocellular carcinomas (hepatic intestinal pancreatic protein; HIP) (10). Other members of the Reg family include Reg2, detected in the mouse genome (11) and the islet neogenesis-associated protein (INGAP) normally expressed in the acinar cells of hamsters and mice (12). Despite their initial association with pancreas, most Reg proteins are expressed in multiple organs. The latest addition to the Reg group is Reg4 overexpressed in drug-resistant colon cancer cells (13). All genes encoding for Reg proteins are approximately 3 kb in size, contain six exons/five introns and are on the same chromosomal locus (2p12) except for the Reg4 gene (1p11-3).
The findings, thus far, categorically show that Reg genes/proteins comprise a versatile group with a multitude of significant activities in various cell types in normal and diseased states. Although the first member of the Reg family was discovered over three decades ago, many aspects of the functions of Reg proteins and the mechanisms they utilize are still unclear. In this review, the various –sometimes confusing– conventions followed in the literature for Reg gene/protein names are presented first. Then, the expression of different Reg members in the pancreatic endocrine and exocrine compartments along with their reported roles are discussed. This discussion is extended to extrapancreatic tissues where several Reg members have been identified. The actions of Reg proteins in various contexts are also described. Subsequently, several factors are summarized, which have been identified as modulators of different Reg isoforms in different cell types. An important and largely uncharted area is the signaling pathways utilized by Reg ligands in conferring their observed effects on cells including differentiation, proliferation and apoptosis. An account of the signaling events documented to date in conjunction with Reg actions is given. Finally, a brief outlook is provided regarding research on Reg members and pertinent challenges.
Nomenclature of Reg family members
The discovery of various Reg family members independently has contributed in large part to the nomenclatural redundancy of the corresponding genes/proteins. For instance, pancreatic stone protein (PSP), lithostathine, pancreatic thread protein (PTP), P19 and Reg1 refer to the same gene product (14). The terminology for Reg genes/proteins becomes more perplexing with the indiscriminate use of Arabic/Roman numerals and Greek letters (e.g. Reg3a, RegIIIa, RegIIIα or Reg3α) making more challenging the survey of pertinent findings in the literature. Efforts to standardize the names of different Reg proteins/genes across species led to their classification into four subfamilies based on DNA sequence and protein structure similarities (11, 15–17) (Table 1). According to the nomenclature suggested by Unno et al. (11) and Abe et al. (18): (i) Type 1 includes the mouse Reg1, rat Reg1, and human REG1α and REG1β (PSPα and PSPβ), (ii) Type 2 contains only the mouse and hamster Reg2, (iii) Type 3 encompasses the mouse Reg3α, Reg3β, Reg3γ and Reg3δ, the rat PAP/peptide 23 (p23), Reg3/PAP II and PAP III, and human HIP/PAP, islet neogenesis-associated protein-related protein (INGAP-rp). Another member discovered recently, human REG4 appears to be homologous (19) to both PAP/HIP/p23 and PSP/lithostathine/REG1 but was originally classified in a separate subfamily (type 4).
Table 1.
Reg family members in the mouse, rat and human.
| Name | Other names | Related tissue or organ |
|---|---|---|
| Mouse | ||
| Reg1 | Reg, PSP, PTP (cleaved form), Lithostathine |
Pancreas, gallbladder |
| Reg2 | PTP2, PSP2, Lithostathine 2 | Pancreas, Schwann cells, motor neuron cells |
| Reg3α | PAP II | Pancreas, intestinal tract |
| Reg3β | PAP I, PAP, HIP | Pancreas, small intestine, liver |
| Reg3γ | PAP III | Pancreas, intestinal tract |
| Reg3δ | INGAP-rp, INGAP | Pancreas, stomach, duodenum, skeletal muscle |
| Reg4 | RELP | Small intestine, gastrointestinal mucosa |
| Rat | ||
| Reg1 | Reg, PSP, PTP (cleaved form), Lithostathine |
Pancreas, stomach, duodenum |
| Reg3α | Reg3, PAP II | Pancreas |
| Reg3β | PAP, PAP I, HIP, Reg2, Peptide23 | Pancreas, small intestine, motor neurons |
| Reg3γ | PAP III | Pancreas, small intestine |
| Reg4 | RegIV | Small intestine |
| Human | ||
| REG1α | PSP, Lithostathine, PTP | Pancreas, gastric mucosa, colorectal cells, neurons |
| REG1β | REGH, REGL, Lithostathine, RS | Pancreas |
| REG3β | REG3A, PAP, HIP, PAP I, Reg2, PTP |
Pancreas, liver, hepatocellular carcinoma |
| REG3γ | Reg3, PAP IB, PAP II, PAP III | Pancreas, liver, hepatocellular carcinoma |
| REG4 | REGIV | Intestine, liver, pancreatic adenocarcinoma |
Abbreviations: RELP/REGL: Reg-like protein, REGH: Reg gene homologue, RS: Reg-related sequence. Other abbreviations are described in the text.
Based on amino acid sequence homology, Reg family members from the same species, e.g. human, mouse and rat, are highly conserved (16). The coding region of the human REG1α and REG1β cDNA, for example, share 78% homology with each other (14) but only 38% and 39%, respectively with REG4 (15). Interspecies (human, monkey, rodent, bovine, canine and porcine) homology is also evident for several Reg members (20). For instance, the rat Reg1 amino acid sequence is 68% homologous to the human REG1 (21) and 87% to the mouse Reg1 (11, 16). The Reg genes are considered to have evolved from a common ancestral species and also by duplication and divergence from common genes (17, 22, 23) given the high homology, similar intron-exon organization of the family members within and across species, and the presence of Reg members in the same chromosomal region in tandem order (except for Reg4). An evolutionary dendrogram created by Abe et al. (18) lists the evolutionary distance of the Reg family members from the gene of origin. Human, mouse and rat tissues or organs where Reg genes have been identified are listed in Table 1. Additional information on Reg proteins/genes such as their amino acid length and their chromosomal location can be found elsewhere (16).
Reg proteins in the pancreas
Type 1 Reg (Reg1, also known as PSP) is expressed in acinar cells under normal and pathological conditions including cancer and acute or chronic pancreatitis (11, 24–26). Immunoreactivity studies show the co-expression of Reg1 and pancreatic cell differentiation markers suggesting that Reg1 may play a role in the transdifferentiation of acinar cells to islets in patients with chronic pancreatitis (27). Reg1 was also proposed to inhibit pancreatic stone formation (4) but subsequent studies rejected this hypothesis (5, 28). When Reg1 is digested by trypsin, it forms insoluble fibrils (referred to as PTP) resistant to further cleavage but their physiological role remains unclear. It has been hypothesized that the PTP is generated in an attempt to protect the pancreas from further damage but the resulting protein plug deposits contribute to pancreatic stone formation and chronic calcifying pancreatitis (20). A receptor of rat Reg1 was identified as homologous to a human multiple exostoses-like (EXTL) gene with previously unknown functions (29) (see Signaling pathways and Reg proteins).
Probably the best-documented effect of Reg1 is on the proliferation of acinar and islet cells (30). Reg1 is absent from healthy islets, as evidenced by the negative Reg1 immunoreactivity of normal rat islets (31, 32) and the lack of Reg1 mRNA expression in normal mouse islets (11). Diabetes-prone rats also do not exhibit Reg1 immunoreactivity in their inflamed islets (33). However, the expression of Reg1 (6, 11, 31, 32) increases in regenerating or hyperplastic islets. The augmented Reg1gene expression (34, 35) during β-cell replication points to a role of Reg1as a marker for distinguishing replicating from differentiating β-cells. Besides β-cell proliferation, the expression of Reg1 only during islet regeneration or hyperplasia may suggest a possible involvement in islet cell ontogeny and maturation. In regenerating islet cells Reg1 is detected in the central core of secretory granules (31). The mitogenic effect of Reg1 has also been confirmed in transgenic rodent models where the enhanced Reg1 expression or exogenous Reg1 administration causes islet proliferation and the amelioration of diabetes (21). The islets of Reg−/− mice display the same morphology as normal islets but decreased [3H]thymidine incorporation. In contrast, transgenic mice with islet-specific overexpression of the Reg gene under the rat insulin promoter II (Ins-Reg) exhibit higher [3H]thymidine incorporation (36). Furthermore, non-obese diabetic (NOD) mice carrying the Ins-Reg transgene show a significantly delayed development of diabetes compared to non-transgenic NOD mice, most likely due to the increased regeneration of β-cells. Similar to these in vivo observations, growth factors (see Factors regulating Reg expression) inducing Reg1 gene expression in cultured islets increase cell proliferation (37). Exogenous Reg1 is also mitogenic (30, 38) for pancreatic cell lines and rat gastric epithelial cells (39). Despite promoting islet cell proliferation, Reg1 is not present in proliferating β-cells immediately after birth as immunostaining studies of rat islets show (33). Thus, it appears that Reg1 is expressed only during proliferation of β-cells after damage. Moreover, Reg1 co-localizes with islet differentiation markers (e.g. insulin, chromogranin A) in transdifferentiating cytokeratin 19 (CK19)+ acinoductular cells from human patients with chronic pancreatitis (27). Despite its islet proliferation- and regeneration-promoting effects, the tumor promoting activity of Reg1 may hinder its clinical use for diabetes treatment (40, 41). Overall, the roles of Reg1 members in islet regeneration and pancreatic cancer are still under intense investigation.
Reg2 has only been found in mice and hamsters (21). Nonetheless, mouse Reg2 exhibits 76% amino acid sequence homology with mouse Reg1 and 63% homology with both human REG1α and REG1β. Based on this comparison, Reg2 has been categorized as a member of the Reg1 subfamily in some reports (16, 42, 43). This may create confusion since a reportedly rat Reg2 protein has been considered a member of the type 3 subclass of the Reg gene family given its homology with mouse Reg3β 44, 45). Mouse Reg2 mRNA is strongly expressed in the normal pancreatic acini and weakly in liver. Reg2 expression is also evident in hyperplastic but not normal islets (11).
The third subclass of the Reg family consists of Reg3α, Reg3β, Reg3γ and Reg3δ. These are expressed in the pancreas but not in hyperplastic islets (18, 22). Similar to Reg1, Reg3β -also known as PAP-, can be cleaved by trypsin to form insoluble fibrils and both Reg1 and Reg3β are coordinately secreted as stress proteins during pancreatic inflammation (25, 26). The nuclear factor kappa B (NF-κB)/Rel, which is involved in inflammatory disease, upregulates Reg3β to protect acinar cells during infiltration based on in vivo evidence (46). In cultured acinar cells NF-κB-mediated upregulation of Reg3β during oxidative stress reduces apoptosis but not necrosis (47). Inhibition of acinar cell apoptosis by Reg3β is triggered by the addition of tumor necrosis factor alpha (TNFα) through an antiapoptotic mechanism involving NF-κB and mitogen-activated protein kinases (MAPK) (48). The transcription cofactor p8 is also implicated in upregulating Reg3β, which acts as an anti-inflammatory factor to improve pancreas resistance to acute pancreatitis inducers (49).
The mouse reg3δ mRNA has been detected only in normal pancreas but not in other tissues such as the pituitary gland (unlike other Reg3/PAP members) despite the presence of Pit-1 binding sites on its promoter (18). In the mouse pancreas, Reg3δ is detected in exocrine cells and endocrine non-β-cells predominantly located at the islet periphery (18, 50, 51). When human, rat and hamster genomes were subjected to Southern blot hybridization with the mouse reg3δ gene probe, bands appeared with sizes different than those of other Reg family members leading to the discovery of Reg3δ orthologs in these species. It should be noted that Reg3δ shows 72% amino acid sequence homology to the peptide-23/INGAP-rp (18, 52). Moreover, the reg3δ and INGAP cDNA sequences are 77% homologous suggesting that INGAP is the hamster ortholog of mouse reg3δ.
INGAP is detected in the healthy pancreas, duodenum, stomach and skeletal muscle (52). Hamster acinar cells express INGAP during islet neogenesis after cellophane wrapping of the pancreatic duct (12). Protein extract isolated from these hamsters contains INGAP and reverses diabetes in hamsters treated with streptozotocin. Sucrose administration to hamsters also increases the expression of INGAP and islet cell mass, concomitantly (53). In fact, the total number of INGAP+ cells rises mostly at the islet periphery (96%) as well as in the exocrine (3%) and ductal areas (<1%). Moreover, transgenic mice with a sustained INGAP expression in the acinar cells, are resistant to diabetogenic doses of streptozotocin and have improved islet function (51, 54).
Interestingly enough, INGAP has been hypothesized to stimulate the differentiation of putative pancreatic stem cells (55) (including ductal cells (27)) to islet cells in certain pancreatic pathologies, especially diabetes (12, 56, 57). Administration of INGAP or its pentadecapeptide fragment (INGAP104-118) leads to increased β-cell mass in diseased and healthy pancreases. For instance, islet neogenesis has been observed in healthy dogs after intramuscular injection of INGAP (58). Even islet cells induced to differentiate into duct-like epithelial cells expressing ductal/progenitor cell markers such as CK19, carbonic anhydrase, Ngn3 and nestin, can be coaxed back to functional islet cells in the presence of the INGAP104-118 (59). In addition, INGAP has been suggested to regulate islet ontogeny. Stem cell subpopulations positive for INGAP and pancreatic and duodenal homeobox-1 (PDX-1) at an early stage of development are highly activateable during neogenesis (60). Because of its induction of islet neogenesis, INGAP may be utilized in therapies for diabetes, where the β-cell mass is reduced (51).
Besides its role in islet cell proliferation and differentiation, INGAP may participate in other cellular processes as well. In cultured rat islets, the release of insulin stimulated by glucose or other amino acids is enhanced in the presence of the INGAP peptide (61). It is therefore possible that INGAP may act on islet genes involved in β-cell metabolism and insulin secretion. INGAP may also act in other organs outside the pancreas, although such roles are still largely unexplored.
The last subfamily, type 4, of Reg proteins contains only one member. Mouse Reg4 has 65% and 89% amino acid sequence similarities to the human and rat Reg4, respectively (16). Reg4 is expressed in rat acinar cells (62) and human insulin-producing β-cells (63). Overexpression of REG4 has been linked to the initiation and progression of pancreatic cancer leading to the notion of utilizing the protein as a marker for screening pancreatic adenocarcinoma (63, 64). Also, Reg4 has been suggested as a target for adjuvant therapy for pancreatic cancer (65).
Reg proteins in extrapancreatic tissues and cells
Reg family proteins are detected in several organs under normal and pathological conditions. At least one of the REG1α, REG1β and REG3β is detected in human fetal and adult pancreas, brain, stomach, intestine, and pituitary gland (66), but the expression patterns and relevance to diseases and/or tissue homeostasis remain unclear. Members of the Reg3 subclass are expressed in the intestinal tract (Reg3α, Reg3β, Reg3γ) (18, 22) and duodenum of mouse (Reg3δ) and hamster (INGAP) (52) as well as the columnar epithelial cells of rat ileum, jejunum and duodenum (Reg3β) (67). The expression of multiple Reg members in the same cell/tissue may indicate a compensatory mode of action although direct supporting evidence is still lacking.
Type 1 Reg members are detected in tissues from the duodenum, gallbladder (11), and human brain (68). The expression of rat Reg1 in the duodenum is augmented by the growth hormone-releasing hormone (69). Moreover, Reg1 regulates gastrin-induced cell growth (70) in the fundic mucosa where its expression coincides with that of the putative Reg1 receptor exostosin-like 3 (EXTL3) (29) (also known as exostosin-related 1 (EXTR1)) (71). In inflammatory bowel disease (IBD), the human REG1α is processed and exported, reducing cell apoptosis and contributing to colonic mucosal regeneration (72). Reg1-deficient mice have normal gastric development but Reg1 promotes gastric mucosal growth and restoration synergistically with gastrin (73). The loss of Reg1 is dispensable for the development of gastric tumors as shown in the gp130F/F mouse model of gastric cancer (74). The deficiency in Reg1 of gp130F/F/Reg1−/− mice is concomitant with the altered expression of other Reg family members, namely the decrease in Reg3β levels. However, Reg1 is also found in enterochromaffin-like cells of hypergastrinemic patients with mutations preventing its secretion (75) and may act as a paracrine/autocrine tumor suppressor.
Human REG1α, -1β and -3β are also upregulated in colorectal cancer tissues (76, 77). Similar to hyperplastic or regenerating cells, REG is expressed in human colon cancer cell lines during proliferation but is downregulated once enterocytic differentiation transpires (78). The expression of REG3β in human hepatocellular carcinoma samples but not in fetal or adult normal cells suggests its involvement in the proliferation or differentiation of liver cancer cells (10). This finding conflicts with observations of structural abnormalities in the liver and alimentary tract in Reg1 knockout mice (79) supporting a role for Reg1 in development. Reg3β (PAP) has also been implicated in lung inflammation during acute pancreatitis. Rats receiving PAP exhibit overexpression of hepatic TNFα leading to lung inflammation, which is curtailed upon injection of anti-TNFα antibodies (80).
Reg proteins are also expressed in cells of the central nervous system. PTP-like proteins (cleaved insoluble fibrils) referred to as neuronal thread proteins (NTPs) are found in the brain and have been associated with neuronal differentiation (20). They are also detected in primitive neuroectodermal tumor cell lines. The PTP/Reg1 is found in normal neurons while its expression is higher in those of Alzheimer patients (68). Moreover, Reg2 is found in developing and regenerating rat motor and sensory neurons. Livesey et al. (81) showed that Reg2/PAP may cause Schwann cell proliferation during regeneration of motor neurons. Reg2 also has anti-apoptotic effects because cytokines related to the motor neuron survival ciliary neurotrophic factor (CNTF) induce its expression (45). The INGAP104-118 peptide promotes neurite outgrowth in cultured mouse dorsal root ganglia (82) and ameliorates nerve damage due to streptozotocin-induced diabetes (83).
Another member of the Reg family, Reg4, is prominently expressed in the human gastrointestinal tract and its mRNA is significantly upregulated during mucosal injury due to active Crohn’s disease or ulcerative colitis (15). Reg4 expression is also linked to pancreatic cancer and malignancies of the stomach, intestine, colon, rectum, gallbladder and urogenital tract (13, 63, 84–86). In experiments with colorectal tumor cell lines, the expression of Reg4 was stronger than that of other Reg members (13). Whereas Reg4 expression is low in normal colon, its level in normal small intestine is on a par with that in colorectal tumor samples. In a comparative study (87), Reg4 expression was the highest in intestinal metaplasia tissues and gradually lower in adenoma, carcinoma and gastritis samples subjected to immunohistochemistry and RNA-DNA in situ hybridization. Like in pancreatic adenocarcinomas, Reg4 has also been proposed as a marker for gastric cancer (88) but its relevance of predicting the disease and survival probability of patients is still unresolved (88, 89).
The first account of Reg expression in embryonic stem cells (ESCs) was provided by Jing et al. (90). When probed for different Reg genes, mouse ESCs (mESCs) showed expression of Reg1 and Reg3γ under non-differentiating conditions. The putative Reg receptor Extl3 was also detected suggesting that Reg may act as an autocrine/paracrine factor in mESCs. When mESCs were treated with Wnt3a or LiCl (an activator of canonical Wnt), Reg1 gene expression was upregulated leading to an increase in secreted protein. Interestingly enough, the study findings also pointed to a possible role for Reg in the adoption of an endodermal fate by differentiating mESCs. Cells treated with exogenous Reg1 or overexpressing Reg1 after transduction with a recombinant adenoviral vector (AdReg1GFP) displayed enhanced expression of the endoderm genes sox17 and foxa2 (by qPCR), whereas the levels of genes characteristic of mesoderm and ectoderm lineages were not different from those in control cells. The adoption of endodermal fate was also corroborated by immunocytochemistry analysis. Whether Reg proteins can serve as factors for coaxing stem cells to particular lineages is still an open question. Moreover, there are only a few published studies (66, 69, 91) to date on the expression pattern of Reg family members during (primarily rodent) fetal development hampering efforts to address if these molecules stimulate in vivo differentiation. Nonetheless, Reg proteins are present in cells undergoing regeneration, differentiation and proliferation in the pancreas, intestine and other tissues under normal and/or stress conditions. Therefore, the prospect of Reg proteins acting as differentiation agents is intriguing and warrants further investigation.
Reg proteins and bacterial aggregation
Reg1 and Reg3β have been observed to promote bacterial aggregation, which is enhanced with Reg1 fibrils cleaved by trypsin. It has been postulated that these secretory stress proteins may control bacterial levels (92, 93). Due to the homology between the Reg1 fibril and carbohydrate-binding, Ca+2-dependent lectins (C-type lectins) (94–96), it was conjectured that bacterial aggregation is caused by putative carbohydrate-binding domains on Reg1 and Reg3β facilitating the attachment to carbohydrates on bacterial surfaces. However, this hypothesis was ruled out by the lack of: (i) a functional carbohydrate-binding site in a 3D model of the Reg1 protein (20) and, (ii) aggregation interference after carbohydrate addition (93).
Factors regulating Reg expression
The production of Reg proteins is observed during development and is induced after tissue damage or in various pathologies such as diabetes and cancer. The expression of Reg1 in the human embryonic pancreas is detected as early as 16 weeks during gestation (97, 98). Mouse islets become positive for Reg1 after induction of diabetes with streptozotocin (99). Reg2 is also overexpressed following administration of cyclophosphamide and in non-obese diabetic (NOD) mice (100), where it is believed to act as a β-cell-derived autoantigen (101). Another Reg, the HIP/PAP/Reg3, is expressed in the islets and ductal epithelium of pre-diabetic and diabetic NOD mice (102). Both Reg1 (REG1α) and Reg3 (REG3A/REG3α) are detected in human primary liver tumors with β-catenin mutations (103, 104).
In addition to identifying the Reg genes/proteins expressed during development, regeneration and/or in disease, efforts concentrated on the discovery of factors regulating their expression. Genes from the Reg family are activated in rat islets by cytokines, hormones and nutrients including glucose, amino acids, serum, insulin, growth hormone and the platelet-derived growth factor (PDGF) (37). Still, multiple factors may regulate Reg proteins in a combinatorial fashion. Dusetti et al. (105) reported two interleukin-6 (IL-6) response elements (T−266TCCCAG−260 and T−249TCCCAG−243 relative to the transcription initiation site) in the rat PAP I (Reg3β) promoter. Although incubation of acinar AR42J cells with IL-6 or IL-1 alone did not increase Reg expression, IL-6 synergistically with dexamethasone led to significant induction of PAP expression. Moreover, this activation was not completely abolished with mutations in both IL-6 response elements pointing to the presence of additional cis-regulatory elements. Similarly, lithostathin/Reg1 is upregulated in acinar cells by IL-6 and dexamethasone together but not by IL-6 or IL-1 alone or in combination (106). Besides acinar cells, activation of lithostathin/Reg1 by IL-6 and dexamethasone together was also observed in RINm5F β-cells (107) and was attributed to the presence of a promoter cis-element (T−81GCCCCTCCCAT−70). This is a binding site for the poly(ADP-ribose) synthetase/polymerase (PARP) and PARP inhibitors (e.g. nicotinamide) induce islet regeneration in depancreatized rats with concomitant increase in Reg expression and secretion. Another cytokine, IL-22 stimulates the PAP mRNA transcription in acinar cells (108). The action of IL-22 on PAP is via STAT3 activation and requires the IL-10 receptor β (IL-10Rβ), a common component of the IL-10 and IL-22 receptors. A hypothesis that IL-10 also activates PAP was proven in a subsequent study utilizing AR42J cells (109). In the same study, PAP stimulated its own mRNA expression indicative of the existence of a positive feedback mechanism.
Rat PAP/HIP/p23 and lithostathin/Reg1 are upregulated in pancreatic acinar AR42J cells treated with interferon gamma (IFNγ) and TNFα independently. Here however, the addition of dexamethasone to INFγ- or TNFα-treated cells curtails the expression of lithostathin (106). Inhibitors of MEK1 also reduce the TNFα-activated expression of PAP suggesting involvement of this pathway in PAP regulation (48). The proinflammatory and proapoptotic factor lipopolysaccharide (LPS) also boosts PAP/HIP expression both directly and indirectly via the upregulation of TNFα and interleukins (110). Similarly, PAP levels are enhanced by other apoptosis-associated factors such as troglitazone (111) (a peroxisome proliferator-activated receptor-gamma (PPAR-γ activator)), cycloheximide (112) (in conjunction with poly(ADP)-ribosylation), and arginine (113). Thus, the expression of PAP may be linked to the elicitation of apoptosis (and act as anti-apoptotic agent) but further studies are needed to elucidate the underlying mechanisms.
The transcription factors NeuroD, activator protein (AP)-1, STAT and transcription factor 3 (TCF3) bind to the hamster INGAP promoter and stimulate expression of the gene (114). PDX-1, a major transcriptional regulator of pancreatic β-cell development and homeostasis, binds directly to the INGAP promoter and stimulates its activity. When however, PDX-1 is combined with other inductive transcription factors, INGAP gene activation is suppressed. PDX-1 expression rises in duct cells when INGAP is administered and islet neogenesis occurs (56). Thus, PDX-1 may be operating in a feedback loop with INGAP to block uncontrolled islet cell expansion.
Extrapancreatic cells share the regulation of Reg genes observed in pancreatic cells. Similar to its regulation in acinar cells, PAP/HIP (Reg3β) is induced in intestinal cells treated with cytokines such as INF-γ and IL-6 linking directly the overexpression of Reg proteins to intestinal inflammatory maladies. Of note is the fact that Reg1 mRNA is expressed in the small intestine and Reg1−/− mice exhibit fewer proliferating (Ki67+ or proliferating cell nuclear antigen (PCNA+) intestinal cells (115). Lithostathin/REG1α mRNA is markedly upregulated in human gastric corpus cells (and AR42J acinar cells) after exposure to gastrin. This effect is weakened by the gastrin/cholecystokinin B receptor (CCKBR or CCK2R) antagonist L740093 (75). Targeting of Reg proteins by CCK2R activation was also demonstrated in an ElasCCK2 transgenic mouse expressing the human CCK2R under the control of the elastase I promoter in pancreatic acini (116). Reg stimulation by gastrin appears to be mediated by activation of protein kinase C (PKC) and RhoA. Exposure of gastric cancer AGS-GR cells to the PKC inhibitor Ro-32-0432 and the Rho family GTPase RhoA inhibitor C3-transferase (C. botulinum toxin), or transfection with a dominant negative form of RhoA suppresses Reg expression in the presence of gastrin (117). The Reg promoter contains a GATA site (−91 to −86) and C-rich sequence (−79 to −76). Mutation of the GATA site has little effect on the Reg response to gastrin but mutating the sequence G−80CCCCTCCCA−71 reduces basal expression and inhibits the response to gastrin by 50–80%.
Along with gastrin, the expression of Reg in gastric cells is also modulated by bacterial infection. Patients with Helicobacter pylori (H. pylori) infection show significantly increased expression of REG1α. Similarly, higher levels of Reg1 have been observed in a mouse model of gastric cancer (118) after infection with H. felis (119). Analysis of two promoter constructs (−2111 and −104 bp) revealed that distinct elements are required for Reg stimulation by H. pylori and H. felis. Furthermore, the gastrin interacting site on the Reg promoter is different from the one responsive to H. pylori infection. When a C-rich region (G−98GCTTT−93) of the promoter was mutated, the expression of Reg was rendered insensitive to H. pylori but responsiveness to gastrin was retained. Although the precise promoter elements regulating Reg gene expression require further elucidation, the link of H. pylori infection to Reg upregulation reveals an attractive target for therapeutic interventions.
As already mentioned, Reg proteins are also implicated in the proliferation of central nervous system cells. Leptin or IL-6 alone activates PAP (Reg3β) in PC12 cells (120) derived from a pheochromocytoma of the rat adrenal medulla. This is not surprising considering that the leptin receptor shares very high sequence similarity with the IL-6 receptor (121). The activation of PAP by leptin is mediated by STAT3 (similar to IL-22, see above) but not STAT1 (120). Yet, the adenylyl cyclase activator forskolin enhances the leptin-induced PAP expression but lessens IL-6 activation of PAP indicating potential differences in signaling mediating the actions of these stimuli. Rat Reg2/PAP I (Reg3β) (81) has been coined a Schwann cell or motor neuron mitogen facilitating regeneration after injury. Mouse embryos homozygous for a mutated leukemia inhibitory factor (LIF) receptor, do not express reg2 (i.e. reg3β) in their motor and sensory neurons. Therefore, Reg2 expression may depend on LIF family cytokines acting through the LIF receptor complex (gp130 and LIF receptor β). Indeed, the IL-6-related neurotrophic cytokines of the LIF/CNTF family including CNTF, cardiotrophin-1 (CT-1) and LIF binding to the same receptor complex induce the expression of Reg-2/PAP I (Fig. 1A) (45). In contrast, the glial cell line-derived neurotrophic factor (GDNF), neurotrophin-3, hepatocyte growth factor (HGF) and fibroblast growth factor-2 (FGF-2) do not alter Reg2/PAP I expression.
Figure 1.
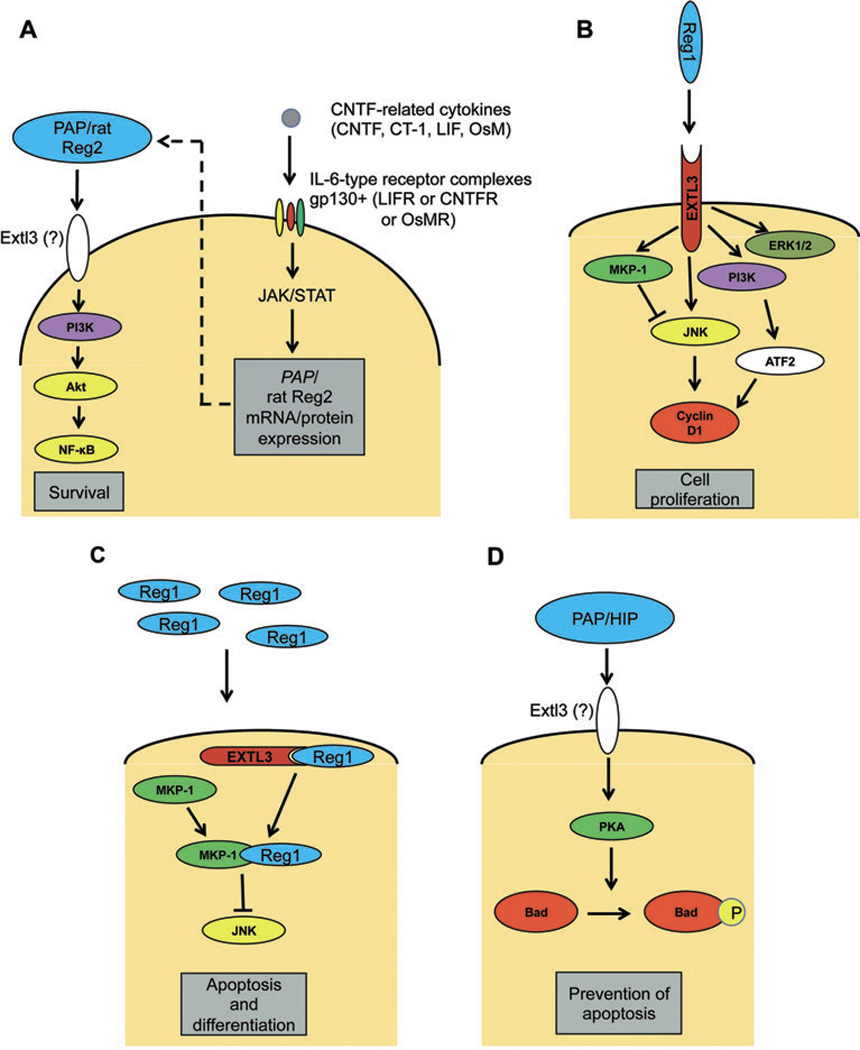
Potential mechanisms of Reg protein signaling. (A) PAP/rat Reg2 (Reg3β) mRNA and protein expression is stimulated by CTNF-related cytokines through the respective gp130 receptor complex and acts in a paracrine/autocrine manner, possibly via the Extl3 receptor activating (→) the PI3K/AKT/NF-κB cascade enhancing cell survival (45). (B) Reg1 stimulates PI3K which targets ATF-2 and the expression of cyclin D1 downstream (130). Alternatively, the Reg1 signal may be relayed via ERK1/2 (133). Signals triggered by extracellular Reg may cause direct activation of JNK and its indirect suppression (—|) via MKP1 (132). The net effect is promotion of cell proliferation. (C) At high concentrations of exogenous Reg, the ligand may form a complex with Extl3 or other internal receptors and interact with MKP-1 to inhibit JNK activity resulting in apoptosis and differentiation (132). (D) PAP/HIP (Reg3β) activates PKA, which promotes the phosphorylation of Bad thus preventing apoptosis (124).
The expression of Reg proteins is also detected in other endocrine tissues such as the pituitary gland and ovaries and is regulated by the aforementioned factors. Rat pituitary cells secrete PAP/HIP (Reg3β) when treated with growth hormone-releasing hormone, whereas exposure to somatostatin has the opposite effect (9). Additionally, Reg proteins (p23/PAP III, Reg3β and Reg3γ) in the ovaries and uterus are regulated by chorionic gonadotropin-induced progesterone synthesis (122) and estrogens (123), respectively.
Lastly, HIP/PAP (Reg3β) is not expressed in normal hepatocytes but its production is stimulated after partial hepatectomy or other liver injury (124). Transgenic mice with human HIP/PAP overexpression have higher fractions of BrdU+ hepatocytes than wild type littermates. The BrdU+ cell fraction is also higher in EGF-treated cultures of HIP/PAP overexpressing hepatocytes compared to normal liver cells. Hepatocyte apoptosis due to exposure to TNFα and actinomycin D is also ameliorated by HIP/PAP.
Signaling pathways and Reg proteins
Despite extensive evidence linking Reg proteins to cell differentiation, proliferation and protection from apoptosis under normal and pathologic conditions, little is known about the pathway(s) involved in relaying Reg signals. The putative receptor of Reg protein (29) exhibits over 97% homology to the human multiple exostosis-like gene 3 (EXTL3) (125). This gene encodes the α1,4-N-acetylglucosaminyltransferases I and II enzymes, which participate in heparan sulfate biosynthesis. Both the rat and human Reg proteins bind with high affinity to the rat Extl3 receptor upon overexpression of its cDNA in CHO cells. In the adult mouse, Extl3 transcripts are detected in the spleen, liver, testis, stomach and heart whereas the strongest expression is noted in the pancreas and brain (126). In E11.5–E18.5 mouse embryos, Extl3 mRNA is present in the neurons, gastrointestinal tract epithelia, liver, kidney, lung and pancreas. Particularly in the pancreas, Extl3 expression is substantial in the adult acini and exhibits a biphasic pattern of expression during development (E11.5–E18.5). Yet, developing and mature islets have a weaker expression of Extl3. In humans, the EXLT3 gene is expressed in skeletal muscle, liver, placenta, heart, brain and pancreas (125) similar to other EXT family genes (127).
The details of the interaction between Reg and its putative receptor are still unclear but overexpression of EXTL3 enhances the activity of nuclear factor-κB (NF-κB) induced by the TNFα (128). Activation of NF-κB, possibly through the PI3K/Akt kinase pathway, is suggested in regenerating motor neurons (81) after exposure to Reg2/PAP (Reg3β) (Fig. 1A) (45). When these cells are incubated with the PI3K inhibitor LY294002, the Reg2-induced increase in phosphorylated Akt is abolished. NF-κB is a target of Akt kinase (129) but its direct activation by Reg2 has not been shown yet. It should be noted that Reg3β upregulation, which limits the death of pancreatitis-inflamed mouse acinar cells and rat acinar AR42J cells (46, 48), has also been linked to the action of NF-κB/Rel factors.
The involvement of PI3K and other pathways in Reg activation in pancreatic and extrapancreatic cells has also been reported. Takasawa et al. (130) probed several pathways in pancreatic β-cells stimulated with mouse Reg (most likely Reg1). Cyclin D1 was upregulated by Reg in RINm5F rat insulinoma cells through the activating transcription factor-2 (ATF-2) cascade (Fig. 1B) but not via CREB, cJun, Elk1, CHOP and cFos. To that end, the levels of phospho-ATF-2 and cyclin D1 were suppressed in islets from Reg−/− mice. The addition of PI3K inhibitors wortmannin and LY294002 decreased the Reg-induced ATF-2 phosphorylation/activation in RINm5F β-cells, whereas inhibition of p38 MAPK, ERK1/2, CaM kinases II and IV, and PKA did not result in significant changes in the activity of the cyclin D1 promoter.
These findings, however, are not entirely aligned with results from other studies demonstrating the activation of MAPK and PKA by Reg proteins. The human Reg protein and a bioactive Reg fragment promoted the proliferation of rat ductal (ARIP) cells while activating p38 MAPK (131). Later, Mueller et al. (132) reported the activation of cyclin D1 by rat Reg1 in ARIP and rat insulinoma (RIN) cells. Microarray data analysis revealed increased expression of the MAPK phosphatase (MKP-1) after treatment with Reg1. At high concentrations of extracellular Reg, the protein was hypothesized to form intracellular complexes with its putative receptor and MKP-1 (Fig. 1C) (132). There were also increased levels of phosphorylated ERK1/2, SAPK/JNK, and p38 MAPK unlike the findings in the study by Takasawa et al. (130). This disagreement may be due to the different time frames utilized in the two studies (30 min-4 hrs (132) vs. 15 min (130)), although the exact reasons are undetermined. The mitogenic effect of Reg1 via the MAPK/ERK1/2 pathway was also demonstrated in human gastric adenocarcinoma cells (133), which produce Reg boosting their proliferation.
In primary hepatocytes in vivo and in culture, HIP/PAP (Reg3β) confers its mitogenic and anti-apoptotic effects via activation of the cyclic AMP (cAMP)/protein kinase A (PKA) pathway (124). The addition of forskolin to epidermal growth factor (EGF)-treated cultures increased the synthesis of DNA in hepatocytes from transgenic mice overexpressing HIP/PAP. In contrast, wild-type mouse hepatocytes (with no HIP/PAP expression) did not experience a change in DNA synthesis in response to forskolin pointing to a cooperative effect of HIP/PAP with cAMP. Furthermore, inhibition of PKA by KT5720 was counterbalanced by enhanced PKA activity due to its induction by HIP/PAP in transgenic mice overexpressing this protein. This led to reduced apoptosis of cells treated with TNFα. The results showed that HIP/PAP activates PKA and subsequently induces the PKA-dependent phosphorylation of Bad (Fig. 1D), a proapoptotic target in the PKA pathway.
The overexpression of REG4 was recently reported in pancreatic cancer biopsies and cell lines with upregulated glioma associated oncogene 1 (GLI1), a transactivator of Hedgehog signaling (134). There was a positive correlation in the change of expression of REG4 and GLI1. Analysis by chromatin immunoprecipitation (ChIP) revealed that GLI1 binds to the REG4 gene promoter at the G−528ATCATCCA−520 site, which is highly similar to the nucleotide GLI1-binding sequence (GATCATCCA) reported previously (135). These results were verified by electrophoretic mobility shift assay (EMSA) showing the GLI1 binding in vivo to the aforementioned site of the REG4 promoter. Despite these findings, further investigation is required to answer if REG4 is transcriptionally regulated by GLI1. Moreover, it is unclear whether signaling pathways, which are linked to both REG4 and Hedgehog, such as the EGF receptor/Akt/AP1 cascade (136), take part in this regulation.
Links between Reg and Wnt signaling have also been reported. Human primary hepatocellular carcinoma cells with β-catenin mutations subjected to subtractive hybridization with normal liver cells were found to overexpress REG1α and REG3α (103). Furthermore, hepatoma cells (Huh7) exposed to LiCl, which activates canonical Wnt signaling by inhibiting GSK-3β, exhibit increased REG3α mRNA. This increase was curtailed when the cells were transfected with siRNA against β-catenin prior to LiCl treatment. In the same experiments, the levels of REG1α mRNA were unaffected. Nonetheless, analysis of hepatocellular carcinomas from 42 patients by qRT-PCR showed overexpression of REG1α and REG3α correlating with β-catenin mutations. These findings were corroborated by immunostaining of biopsy sections. In a separate clinical study (104), 265 hepatocellular carcinoma samples with β-catenin mutations displayed high levels of REG1α and REG3α compared to normal biopsies. Synchronous upregulation of REG1α and REG3α has also been reported in colorectal carcinogenesis and colon adenomas with inactivated adenomatous polyposis coli (APC) gene (77, 137, 138). Collectively, these studies suggest that Reg genes serve as downstream targets of the canonical Wnt pathway.
As already mentioned, discovery of Reg expression in ESCs was recently reported (90). Considering the key roles of β-catenin/Wnt signaling in stem cell self-renewal and differentiation, we investigated whether a link between canonical Wnt and Reg exists in mESCs, similar to that in various cancer cells. Treatment with Wnt signaling activators such as LiCl or purified Wnt3a increased the expression of Reg1 but not Reg3γ. The increase in Reg1 was abrogated when mESCs were transfected with a plasmid encoding a dominant negative TCF4 prior to Wnt3a treatment. Other Reg genes were not detectable before or after Wnt activation. The observed upregulation in Reg 1 gene expression upon Wnt activation translated to increased amounts of secreted Reg1. These data support the association of Reg with Wnt signaling and merit further studies to unravel the underlying mechanisms and participating molecules.
Concluding remarks
Despite their initial discovery in pancreas inflammation and islet regeneration, Reg proteins have been found to be involved in the growth and differentiation of cells from various organs under normal and diseased states. In the pancreas, Reg proteins induce cell (trans)differentiation, especially to islet cells, and proliferation, while they reduce apoptosis due to damage or inflammation. The proliferative and anti-apoptotic effects of Reg members have also been documented in non-pancreatic tissues, mainly of the gastrointestinal tract. Reg expression is modulated by a diverse array of inducers including nutrients, hormones, growth factors and bacterial infection. Cytokines in particular are major regulators of the expression of Reg proteins making them integral in inflammatory responses. Cellular responses to Reg ligands are transduced via several pathways including the classical MAPK and canonical Wnt cascades. Although further elucidation is necessary of their biological roles and mechanisms of action, Reg proteins are emerging as a group with great potential for uses as screening markers and targets for therapies against diseases such as diabetes and various forms of cancer.
Outlook
The majority of Reg-related studies have been performed in the context of diabetes or cancer, leaving the contribution of Reg proteins to homeostasis or malignancies of other organs to be unveiled. Reg expression has been associated with islet regeneration, making these proteins attractive candidates for diabetes or pancreatic cancer therapies, even though the tumorigenic potential of Reg proteins should be considered as well. It should also be noted that Reg proteins are the smallest members of the superfamily of C-type lectins. Hence, there are opportunities for gaining a deeper insight of the biological roles and interactions of Reg proteins from function-structure studies and recent advances in glycobiology. Moreover, a mounting body of literature exists on factors controlling Reg expression but there are significant gaps in our knowledge of the signaling cascades involved in conferring the cellular responses triggered by Reg ligands. Detailed analysis of the pathways involved will lead to better understanding of the roles (and actions) of Reg proteins and will facilitate their therapeutic use.
Such studies will be expedited as antibodies with specificity against particular, highly homologous Reg members become widely available. Research in this field will also be aided by the generation of transgenic mice for the overexpression/knockdown of various Reg genes (besides Reg1 (40, 139)). To that end, coordinated efforts should be undertaken toward establishing and adopting a unified nomenclature system for Reg genes/proteins. This will lower the threshold for researchers interested in the field promoting progress at a faster pace.
Acknowledgements
This work was supported by grants from the New York State Stem Cell Science Trust (NYSTEM; contract # C024355) and the National Institutes of Health (R01HL103709 and R21HL092398) to E.S.T.
Footnotes
Disclosures
The authors declare no conflict of interest.
References
- 1.De Caro A, Lohse J, Sarles H. Characterization of a protein isolated from pancreatic calculi of men suffering from chronic calcifying pancreatitis. Biochem Biophys Res Commun. 1979;87(4):1176–1182. doi: 10.1016/s0006-291x(79)80031-5. [DOI] [PubMed] [Google Scholar]
- 2.De Caro AM, Bonicel JJ, Rouimi P, De Caro JD, Sarles H, Rovery M. Complete amino acid sequence of an immunoreactive form of human pancreatic stone protein isolated from pancreatic juice. Eur J Biochem. 1987;168(1):201–207. doi: 10.1111/j.1432-1033.1987.tb13405.x. [DOI] [PubMed] [Google Scholar]
- 3.Gross J, Carlson RI, Brauer AW, Margolies MN, Warshaw AL, Wands JR. Isolation, characterization, and distribution of an unusual pancreatic human secretory protein. The Journal of clinical investigation. 1985;76(6):2115–2126. doi: 10.1172/JCI112216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarles H, Dagorn JC, Giorgi D, Bernard JP. Renaming pancreatic stone protein as 'lithostathine'. Gastroenterology. 1990;99(3):900–901. doi: 10.1016/0016-5085(90)90999-h. [DOI] [PubMed] [Google Scholar]
- 5.Bimmler D, Graf R, Scheele GA, Frick TW. Pancreatic stone protein (lithostathine), a physiologically relevant pancreatic calcium carbonate crystal inhibitor? The Journal of biological chemistry. 1997;272(5):3073–3082. doi: 10.1074/jbc.272.5.3073. [DOI] [PubMed] [Google Scholar]
- 6.Terazono K, Yamamoto H, Takasawa S, Shiga K, Yonemura Y, Tochino Y, Okamoto H. A novel gene activated in regenerating islets. The Journal of biological chemistry. 1988;263(5):2111–2114. [PubMed] [Google Scholar]
- 7.Keim V, Rohr G, Stockert HG, Haberich FJ. An additional secretory protein in the rat pancreas. Digestion. 1984;29(4):242–249. doi: 10.1159/000199041. [DOI] [PubMed] [Google Scholar]
- 8.Tachibana K, Marquardt H, Yokoya S, Friesen HG. Growth hormone-releasing hormone stimulates and somatostatin inhibits the release of a novel protein by cultured rat pituitary cells. Mol Endocrinol. 1988;2(10):973–978. doi: 10.1210/mend-2-10-973. [DOI] [PubMed] [Google Scholar]
- 9.Katsumata N, Chakraborty C, Myal Y, Schroedter IC, Murphy LJ, Shiu RP, Friesen HG. Molecular cloning and expression of peptide 23, a growth hormone-releasing hormone-inducible pituitary protein. Endocrinology. 1995;136(4):1332–1339. doi: 10.1210/endo.136.4.7895644. [DOI] [PubMed] [Google Scholar]
- 10.Lasserre C, Christa L, Simon MT, Vernier P, Brechot C. A novel gene (HIP) activated in human primary liver cancer. Cancer research. 1992;52(18):5089–5095. [PubMed] [Google Scholar]
- 11.Unno M, Yonekura H, Nakagawara K, Watanabe T, Miyashita H, Moriizumi S, Okamoto H, Itoh T, Teraoka H. Structure, chromosomal localization, and expression of mouse reg genes, reg I and reg II. A novel type of reg gene, reg II exists in the mouse genome. The Journal of biological chemistry. 1993;268(21):15974–15982. [PubMed] [Google Scholar]
- 12.Rafaeloff R, Pittenger GL, Barlow SW, Qin XF, Yan B, Rosenberg L, Duguid WP, Vinik AI. Cloning and sequencing of the pancreatic islet neogenesis associated protein (INGAP) gene and its expression in islet neogenesis in hamsters. The Journal of clinical investigation. 1997;99(9):2100–2109. doi: 10.1172/JCI119383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Violette S, Festor E, Pandrea-Vasile I, Mitchell V, Adida C, Dussaulx E, Lacorte JM, Chambaz J, Lacasa M, Lesuffleur T. Reg IV, a new member of the regenerating gene family, is overexpressed in colorectal carcinomas. Int J Cancer. 2003;103(2):185–193. doi: 10.1002/ijc.10788. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe T, Yonekura H, Terazono K, Yamamoto H, Okamoto H. Complete nucleotide sequence of human reg gene and its expression in normal and tumoral tissues. The reg protein, pancreatic stone protein, and pancreatic thread protein are one and the same product of the gene. The Journal of biological chemistry. 1990;265(13):7432–7439. [PubMed] [Google Scholar]
- 15.Hartupee JC, Zhang H, Bonaldo MF, Soares MB, Dieckgraefe BK. Isolation and characterization of a cDNA encoding a novel member of the human regenerating protein family: Reg IV. Biochimica et biophysica acta. 2001;1518(3):287–293. doi: 10.1016/s0167-4781(00)00284-0. [DOI] [PubMed] [Google Scholar]
- 16.Liu JL, Cui W, Li B, Lu Y. Possible roles of reg family proteins in pancreatic islet cell growth. Endocr Metab Immune Disord Drug Targets. 2008;8(1):1–10. doi: 10.2174/187153008783928361. [DOI] [PubMed] [Google Scholar]
- 17.Laurine E, Manival X, Montgelard C, Bideau C, Berge-Lefranc JL, Erard M, Verdier JM. PAP IB, a new member of the Reg gene family: cloning, expression, structural properties, and evolution by gene duplication. Biochimica et biophysica acta. 2005;1727(3):177–187. doi: 10.1016/j.bbaexp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Abe M, Nata K, Akiyama T, Shervani NJ, Kobayashi S, Tomioka-Kumagai T, Ito S, Takasawa S, Okamoto H. Identification of a novel Reg family gene, Reg IIIdelta, and mapping of all three types of Reg family gene in a 75 kilobase mouse genomic region. Gene. 2000;246(1–2):111–122. doi: 10.1016/s0378-1119(00)00059-7. [DOI] [PubMed] [Google Scholar]
- 19.Iovanna JL, Dagorn JC. The multifunctional family of secreted proteins containing a C-type lectin-like domain linked to a short N-terminal peptide. Biochimica et biophysica acta. 2005;1723(1–3):8–18. doi: 10.1016/j.bbagen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 20.De Reggi M, Gharib B. Protein-X, Pancreatic Stone-, Pancreatic thread-, reg-protein, P19, lithostathine, and now what? Characterization, structural analysis and putative function(s) of the major non-enzymatic protein of pancreatic secretions. Curr Protein Pept Sci. 2001;2(1):19–42. doi: 10.2174/1389203013381233. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto H. The Reg gene family and Reg proteins: with special attention to the regeneration of pancreatic beta-cells. Journal of hepato-biliary-pancreatic surgery. 1999;6(3):254–262. doi: 10.1007/s005340050115. [DOI] [PubMed] [Google Scholar]
- 22.Narushima Y, Unno M, Nakagawara K, Mori M, Miyashita H, Suzuki Y, Noguchi N, Takasawa S, Kumagai T, Yonekura H, Okamoto H. Structure, chromosomal localization and expression of mouse genes encoding type III Reg, RegIII alpha, RegIII beta, RegIII gamma. Gene. 1997;185(2):159–168. doi: 10.1016/s0378-1119(96)00589-6. [DOI] [PubMed] [Google Scholar]
- 23.Lasserre C, Simon MT, Ishikawa H, Diriong S, Nguyen VC, Christa L, Vernier P, Brechot C. Structural organization and chromosomal localization of a human gene (HIP/PAP) encoding a C-type lectin overexpressed in primary liver cancer. Eur J Biochem. 1994;224(1):29–38. doi: 10.1111/j.1432-1033.1994.tb19991.x. [DOI] [PubMed] [Google Scholar]
- 24.Kimura N, Yonekura H, Okamoto H, Nagura H. Expression of human regenerating gene mRNA and its product in normal and neoplastic human pancreas. Cancer. 1992;70(7):1857–1863. doi: 10.1002/1097-0142(19921001)70:7<1857::aid-cncr2820700708>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Bimmler D, Schiesser M, Perren A, Scheele G, Angst E, Meili S, Ammann R, Graf R. Coordinate regulation of PSP/reg and PAP isoforms as a family of secretory stress proteins in an animal model of chronic pancreatitis. J Surg Res. 2004;118(2):122–135. doi: 10.1016/S0022-4804(03)00342-1. [DOI] [PubMed] [Google Scholar]
- 26.Graf R, Schiesser M, Lussi A, Went P, Scheele GA, Bimmler D. Coordinate regulation of secretory stress proteins (PSP/reg, PAP I, PAP II, PAP III) in the rat exocrine pancreas during experimental acute pancreatitis. J Surg Res. 2002;105(2):136–144. doi: 10.1006/jsre.2002.6387. [DOI] [PubMed] [Google Scholar]
- 27.Tezel E, Nagasaka T, Tezel G, Kaneko T, Takasawa S, Okamoto H, Nakao A. REG I as a marker for human pancreatic acinoductular cells. Hepatogastroenterology. 2004;51(55):91–96. [PubMed] [Google Scholar]
- 28.De Reggi M, Gharib B, Patard L, Stoven V. Lithostathine, the presumed pancreatic stone inhibitor, does not interact specifically with calcium carbonate crystals. The Journal of biological chemistry. 1998;273(9):4967–4971. doi: 10.1074/jbc.273.9.4967. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi S, Akiyama T, Nata K, Abe M, Tajima M, Shervani NJ, Unno M, Matsuno S, Sasaki H, Takasawa S, Okamoto H. Identification of a receptor for reg (regenerating gene) protein, a pancreatic beta-cell regeneration factor. The Journal of biological chemistry. 2000;275(15):10723–10726. doi: 10.1074/jbc.275.15.10723. [DOI] [PubMed] [Google Scholar]
- 30.Zenilman ME, Magnuson TH, Swinson K, Egan J, Perfetti R, Shuldiner AR. Pancreatic thread protein is mitogenic to pancreatic-derived cells in culture. Gastroenterology. 1996;110(4):1208–1214. doi: 10.1053/gast.1996.v110.pm8613011. [DOI] [PubMed] [Google Scholar]
- 31.Terazono K, Uchiyama Y, Ide M, Watanabe T, Yonekura H, Yamamoto H, Okamoto H. Expression of reg protein in rat regenerating islets and its co-localization with insulin in the beta cell secretory granules. Diabetologia. 1990;33(4):250–252. doi: 10.1007/BF00404804. [DOI] [PubMed] [Google Scholar]
- 32.Ishii C, Kawazu S, Tomono S, Ohno T, Shimizu M, Kato N, Fukuda M, Ito Y, Kurihara S, Murata K, Komeda K. Appearance of a regenerating (reg) gene protein in pancreatic islets of remission BB/Wor//Tky rats. Endocr J. 1993;40(2):269–273. doi: 10.1507/endocrj.40.269. [DOI] [PubMed] [Google Scholar]
- 33.Graf R, Schiesser M, Reding T, Appenzeller P, Sun LK, Fortunato F, Perren A, Bimmler D. Exocrine meets endocrine: pancreatic stone protein and regenerating protein--two sides of the same coin. J Surg Res. 2006;133(2):113–120. doi: 10.1016/j.jss.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 34.Otonkoski T, Mally MI, Hayek A. Opposite effects of beta-cell differentiation and growth on reg expression in human fetal pancreatic cells. Diabetes. 1994;43(9):1164–1166. doi: 10.2337/diab.43.9.1164. [DOI] [PubMed] [Google Scholar]
- 35.Zenilman ME, Magnuson TH, Perfetti R, Chen J, Shuldiner AR. Pancreatic reg gene expression is inhibited during cellular differentiation. Annals of surgery. 1997;225(3):327–332. doi: 10.1097/00000658-199703000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unno M, Nata K, Noguchi N, Narushima Y, Akiyama T, Ikeda T, Nakagawa K, Takasawa S, Okamoto H. Production and characterization of Reg knockout mice: reduced proliferation of pancreatic beta-cells in Reg knockout mice. Diabetes. 2002;51(Suppl 3):S478–S483. doi: 10.2337/diabetes.51.2007.s478. [DOI] [PubMed] [Google Scholar]
- 37.Francis PJ, Southgate JL, Wilkin TJ, Bone AJ. Expression of an islet regenerating (reg) gene in isolated rat islets: effects of nutrient and non-nutrient growth factors. Diabetologia. 1992;35(3):238–242. doi: 10.1007/BF00400923. [DOI] [PubMed] [Google Scholar]
- 38.Levine JL, Patel KJ, Zheng Q, Shuldiner AR, Zenilman ME. A recombinant rat regenerating protein is mitogenic to pancreatic derived cells. J Surg Res. 2000;89(1):60–65. doi: 10.1006/jsre.1999.5800. [DOI] [PubMed] [Google Scholar]
- 39.Fukui H, Kinoshita Y, Maekawa T, Okada A, Waki S, Hassan S, Okamoto H, Chiba T. Regenerating gene protein may mediate gastric mucosal proliferation induced by hypergastrinemia in rats. Gastroenterology. 1998;115(6):1483–1493. doi: 10.1016/s0016-5085(98)70027-7. [DOI] [PubMed] [Google Scholar]
- 40.Yamaoka T, Yoshino K, Yamada T, Idehara C, Hoque MO, Moritani M, Yoshimoto K, Hata J, Itakura M. Diabetes and tumor formation in transgenic mice expressing Reg I. Biochem Biophys Res Commun. 2000;278(2):368–376. doi: 10.1006/bbrc.2000.3813. [DOI] [PubMed] [Google Scholar]
- 41.Zhou L, Zhang R, Wang L, Shen S, Okamoto H, Sugawara A, Xia L, Wang X, Noguchi N, Yoshikawa T, Uruno A, Yao W, Yuan Y. Upregulation of REG Ialpha accelerates tumor progression in pancreatic cancer with diabetes. Int J Cancer. 2010;127(8):1795–1803. doi: 10.1002/ijc.25188. [DOI] [PubMed] [Google Scholar]
- 42.Castellarin ML, Petropavlovskaia M, Lipsett MA, Rosenberg L. The identification and sequence analysis of a new Reg3gamma and Reg2 in the Syrian golden hamster. Biochimica et biophysica acta. 2007;1769(9–10):579–585. doi: 10.1016/j.bbaexp.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Huszarik K, Wright B, Keller C, Nikoopour E, Krougly O, Lee-Chan E, Qin HY, Cameron MJ, Gurr WK, Hill DJ, Sherwin RS, Kelvin DJ, Singh B. Adjuvant immunotherapy increases beta cell regenerative factor Reg2 in the pancreas of diabetic mice. J Immunol. 2010;185(9):5120–5129. doi: 10.4049/jimmunol.1001596. [DOI] [PubMed] [Google Scholar]
- 44.Tebar LA, Geranton SM, Parsons-Perez C, Fisher AS, Bayne R, Smith AJ, Turmaine M, Perez-Luz S, Sheasby A, De Felipe C, Ruff C, Raivich G, Hunt SP. Deletion of the mouse RegIIIbeta (Reg2) gene disrupts ciliary neurotrophic factor signaling and delays myelination of mouse cranial motor neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(32):11400–11405. doi: 10.1073/pnas.0711978105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishimune H, Vasseur S, Wiese S, Birling MC, Holtmann B, Sendtner M, Iovanna JL, Henderson CE. Reg-2 is a motoneuron neurotrophic factor and a signalling intermediate in the CNTF survival pathway. Nat Cell Biol. 2000;2(12):906–914. doi: 10.1038/35046558. [DOI] [PubMed] [Google Scholar]
- 46.Algul H, Treiber M, Lesina M, Nakhai H, Saur D, Geisler F, Pfeifer A, Paxian S, Schmid RM. Pancreas-specific RelA/p65 truncation increases susceptibility of acini to inflammation-associated cell death following cerulein pancreatitis. The Journal of clinical investigation. 2007;117(6):1490–1501. doi: 10.1172/JCI29882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ortiz EM, Dusetti NJ, Vasseur S, Malka D, Bodeker H, Dagorn JC, Iovanna JL. The pancreatitis-associated protein is induced by free radicals in AR4-2J cells and confers cell resistance to apoptosis. Gastroenterology. 1998;114(4):808–816. doi: 10.1016/s0016-5085(98)70595-5. [DOI] [PubMed] [Google Scholar]
- 48.Malka D, Vasseur S, Bodeker H, Ortiz EM, Dusetti NJ, Verrando P, Dagorn JC, Iovanna JL. Tumor necrosis factor alpha triggers antiapoptotic mechanisms in rat pancreatic cells through pancreatitisassociated protein I activation. Gastroenterology. 2000;119(3):816–828. doi: 10.1053/gast.2000.16491. [DOI] [PubMed] [Google Scholar]
- 49.Vasseur S, Folch-Puy E, Hlouschek V, Garcia S, Fiedler F, Lerch MM, Dagorn JC, Closa D, Iovanna JL. p8 improves pancreatic response to acute pancreatitis by enhancing the expression of the antiinflammatory protein pancreatitis-associated protein I. The Journal of biological chemistry. 2004;279(8):7199–7207. doi: 10.1074/jbc.M309152200. [DOI] [PubMed] [Google Scholar]
- 50.Flores LE, Garcia ME, Borelli MI, Del Zotto H, Alzugaray ME, Maiztegui B, Gagliardino JJ. Expression of islet neogenesis-associated protein in islets of normal hamsters. J Endocrinol. 2003;177(2):243–248. doi: 10.1677/joe.0.1770243. [DOI] [PubMed] [Google Scholar]
- 51.Taylor-Fishwick DA, Bowman A, Korngiebel-Rosique M, Vinik AI. Pancreatic islet immunoreactivity to the Reg protein INGAP. J Histochem Cytochem. 2008;56(2):183–191. doi: 10.1369/jhc.7A7365.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sasahara K, Yamaoka T, Moritani M, Yoshimoto K, Kuroda Y, Itakura M. Molecular cloning and tissue-specific expression of a new member of the regenerating protein family, islet neogenesis-associated protein-related protein. Biochimica et biophysica acta. 2000;1500(1):142–146. doi: 10.1016/s0925-4439(99)00095-2. [DOI] [PubMed] [Google Scholar]
- 53.Del Zotto H, Massa L, Rafaeloff R, Pittenger GL, Vinik A, Gold G, Reifel-Miller A, Gagliardino JJ. Possible relationship between changes in islet neogenesis and islet neogenesis-associated protein-positive cell mass induced by sucrose administration to normal hamsters. J Endocrinol. 2000;165(3):725–733. doi: 10.1677/joe.0.1650725. [DOI] [PubMed] [Google Scholar]
- 54.Taylor-Fishwick DA, Bowman A, Hamblet N, Bernard P, Harlan DM, Vinik AI. Islet neogenesis associated protein transgenic mice are resistant to hyperglycemia induced by streptozotocin. J Endocrinol. 2006;190(3):729–737. doi: 10.1677/joe.1.06698. [DOI] [PubMed] [Google Scholar]
- 55.Bonner-Weir S, Sharma A. Pancreatic stem cells. J Pathol. 2002;197(4):519–526. doi: 10.1002/path.1158. [DOI] [PubMed] [Google Scholar]
- 56.Rosenberg L, Lipsett M, Yoon JW, Prentki M, Wang R, Jun HS, Pittenger GL, Taylor-Fishwick D, Vinik AI. A pentadecapeptide fragment of islet neogenesis-associated protein increases beta-cell mass and reverses diabetes in C57BL/6J mice. Annals of surgery. 2004;240(5):875–884. doi: 10.1097/01.sla.0000143270.99191.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenberg L, Vinik AI, Pittenger GL, Rafaeloff R, Duguid WP. Islet-cell regeneration in the diabetic hamster pancreas with restoration of normoglycaemia can be induced by a local growth factor(s) Diabetologia. 1996;39(3):256–262. doi: 10.1007/BF00418339. [DOI] [PubMed] [Google Scholar]
- 58.Pittenger GL, Taylor-Fishwick DA, Johns RH, Burcus N, Kosuri S, Vinik AI. Intramuscular injection of islet neogenesis-associated protein peptide stimulates pancreatic islet neogenesis in healthy dogs. Pancreas. 2007;34(1):103–111. doi: 10.1097/01.mpa.0000240609.56806.43. [DOI] [PubMed] [Google Scholar]
- 59.Jamal AM, Lipsett M, Sladek R, Laganiere S, Hanley S, Rosenberg L. Morphogenetic plasticity of adult human pancreatic islets of Langerhans. Cell Death Differ. 2005;12(7):702–712. doi: 10.1038/sj.cdd.4401617. [DOI] [PubMed] [Google Scholar]
- 60.Gagliardino JJ, Del Zotto H, Massa L, Flores LE, Borelli MI. Pancreatic duodenal homeobox-1 and islet neogenesis-associated protein: a possible combined marker of activateable pancreatic cell precursors. J Endocrinol. 2003;177(2):249–259. doi: 10.1677/joe.0.1770249. [DOI] [PubMed] [Google Scholar]
- 61.Borelli MI, Stoppiglia LF, Rezende LF, Flores LE, Del Zotto H, Boschero AC, Gagliardino JJ. INGAP-related pentadecapeptide: its modulatory effect upon insulin secretion. Regul Pept. 2005;131(1–3):97–102. doi: 10.1016/j.regpep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Azman J, Starcevic Klasan G, Ivanac D, Picard A, Jurisic-Erzen D, Nikolic M, Malnar D, Arbanas J, Jerkovic R. Reg IV protein and mRNA expression in different rat organs. Acta histochemica. 2010 doi: 10.1016/j.acthis.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 63.Oue N, Mitani Y, Aung PP, Sakakura C, Takeshima Y, Kaneko M, Noguchi T, Nakayama H, Yasui W. Expression and localization of Reg IV in human neoplastic and non-neoplastic tissues: Reg IV expression is associated with intestinal and neuroendocrine differentiation in gastric adenocarcinoma. J Pathol. 2005;207(2):185–198. doi: 10.1002/path.1827. [DOI] [PubMed] [Google Scholar]
- 64.Takayama R, Nakagawa H, Sawaki A, Mizuno N, Kawai H, Tajika M, Yatabe Y, Matsuo K, Uehara R, Ono K, Nakamura Y, Yamao K. Serum tumor antigen REG4 as a diagnostic biomarker in pancreatic ductal adenocarcinoma. J Gastroenterol. 2010;45(1):52–59. doi: 10.1007/s00535-009-0114-y. [DOI] [PubMed] [Google Scholar]
- 65.Legoffic A, Calvo E, Cano C, Folch-Puy E, Barthet M, Delpero JR, Ferres-Maso M, Dagorn JC, Closa D, Iovanna J. The reg4 gene, amplified in the early stages of pancreatic cancer development, is a promising therapeutic target. PloS one. 2009;4(10):e7495. doi: 10.1371/journal.pone.0007495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartoli C, Baeza N, Figarella C, Pellegrini I, Figarella-Branger D. Expression of peptide-23/pancreatitis-associated protein and Reg genes in human pituitary and adenomas: comparison with other fetal and adult human tissues. J Clin Endocrinol Metab. 1998;83(11):4041–4046. doi: 10.1210/jcem.83.11.5217. [DOI] [PubMed] [Google Scholar]
- 67.Chakraborty C, Sharma S, Katsumata N, Murphy LJ, Schroedter IC, Robertson MC, Shiu RP, Friesen HG. Plasma clearance, tissue uptake and expression of pituitary peptide 23/pancreatitis-associated protein in the rat. J Endocrinol. 1995;145(3):461–469. doi: 10.1677/joe.0.1450461. [DOI] [PubMed] [Google Scholar]
- 68.de la Monte SM, Ozturk M, Wands JR. Enhanced expression of an exocrine pancreatic protein in Alzheimer's disease and the developing human brain. The Journal of clinical investigation. 1990;86(3):1004–1113. doi: 10.1172/JCI114762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chakraborty C, Katsumata N, Myal Y, Schroedter IC, Brazeau P, Murphy LJ, Shiu RPC, Friesen HG. Age-Related-Changes in Peptide-23 Pancreatitis-Associated Protein and Pancreatic Stone Protein Reg Gene-Expression in the Rat and Regulation by Growth Hormone-Releasing Hormone. Endocrinology. 1995;136(5):1843–1849. doi: 10.1210/endo.136.5.7720628. [DOI] [PubMed] [Google Scholar]
- 70.Chiba T, Fukui H, Kinoshita Y. Reg protein: a possible mediator of gastrin-induced mucosal cell growth. J Gastroenterol. 2000;35(Suppl 12):52–56. [PubMed] [Google Scholar]
- 71.Kazumori H, Ishihara S, Fukuda R, Kinoshita Y. Localization of Reg receptor in rat fundic mucosa. The Journal of laboratory and clinical medicine. 2002;139(2):101–108. doi: 10.1067/mlc.2002.120796. [DOI] [PubMed] [Google Scholar]
- 72.Dieckgraefe BK, Crimmins DL, Landt V, Houchen C, Anant S, Porche-Sorbet R, Ladenson JH. Expression of the regenerating gene family in inflammatory bowel disease mucosa: Reg Ialpha upregulation, processing, and antiapoptotic activity. J Investig Med. 2002;50(6):421–434. doi: 10.1136/jim-50-06-02. [DOI] [PubMed] [Google Scholar]
- 73.Peterson AJ, Nguyen N, Okamoto H, Giraud AS, van Driel IR, Judd LM. Loss of RegI in conjunction with gastrin deficiency in mice facilitates efficient gastric ulcer healing but is dispensable for hyperplasia and tumourigenesis. Regul Pept. 2010;160(1–3):9–18. doi: 10.1016/j.regpep.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 74.Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F, Heath JK, Ernst M. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8(10):1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- 75.Higham AD, Bishop LA, Dimaline R, Blackmore CG, Dobbins AC, Varro A, Thompson DG, Dockray GJ. Mutations of RegIalpha are associated with enterochromaffin-like cell tumor development in patients with hypergastrinemia. Gastroenterology. 1999;116(6):1310–1318. doi: 10.1016/s0016-5085(99)70495-6. [DOI] [PubMed] [Google Scholar]
- 76.Zenilman ME, Kim S, Levine BA, Lee C, Steinberg JJ. Ectopic expression of reg protein: A marker of colorectal mucosa at risk for neoplasia. J Gastrointest Surg. 1997;1(2):194–201. doi: 10.1016/s1091-255x(97)80109-6. discussion -2. [DOI] [PubMed] [Google Scholar]
- 77.Rechreche H, Montalto G, Mallo GV, Vasseur S, Marasa L, Soubeyran P, Dagorn JC, Iovanna JL. pap, reg Ialpha and reg Ibeta mRNAs are concomitantly up-regulated during human colorectal carcinogenesis. Int J Cancer. 1999;81(5):688–694. doi: 10.1002/(sici)1097-0215(19990531)81:5<688::aid-ijc3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 78.Bernard-Perrone FR, Renaud WP, Guy-Crotte OM, Bernard P, Figarella CG, Okamoto H, Balas DC, Senegas-Balas FO. Expression of REG protein during cell growth and differentiation of two human colon carcinoma cell lines. J Histochem Cytochem. 1999;47(7):863–870. doi: 10.1177/002215549904700703. [DOI] [PubMed] [Google Scholar]
- 79.Okamoto H, Takasawa S. Recent advances in the Okamoto model: the CD38-cyclic ADP-ribose signal system and the regenerating gene protein (Reg)-Reg receptor system in beta-cells. Diabetes. 2002;51(Suppl 3):S462–S473. doi: 10.2337/diabetes.51.2007.s462. [DOI] [PubMed] [Google Scholar]
- 80.Folch-Puy E, Garcia-Movtero A, Iovanna JL, Dagorn JC, Prats N, Vaccaro MI, Closa D. The pancreatitis-associated protein induces lung inflammation in the rat through activation of TNFalpha expression in hepatocytes. J Pathol. 2003;199(3):398–408. doi: 10.1002/path.1307. [DOI] [PubMed] [Google Scholar]
- 81.Livesey FJ, O'Brien JA, Li M, Smith AG, Murphy LJ, Hunt SP. A Schwann cell mitogen accompanying regeneration of motor neurons. Nature. 1997;390(6660):614–618. doi: 10.1038/37615. [DOI] [PubMed] [Google Scholar]
- 82.Tam J, Rosenberg L, Maysinger D. Islet-neogenesis-associated protein enhances neurite outgrowth from DRG neurons. Biochem Biophys Res Commun. 2002;291(3):649–654. doi: 10.1006/bbrc.2002.6497. [DOI] [PubMed] [Google Scholar]
- 83.Tam J, Rosenberg L, Maysinger D. INGAP peptide improves nerve function and enhances regeneration in streptozotocin-induced diabetic C57BL/6 mice. FASEB J. 2004;18(14):1767–1769. doi: 10.1096/fj.04-1894fje. [DOI] [PubMed] [Google Scholar]
- 84.Nanakin A, Fukui H, Fujii S, Sekikawa A, Kanda N, Hisatsune H, Seno H, Konda Y, Fujimori T, Chiba T. Expression of the REG IV gene in ulcerative colitis. Laboratory investigation; a journal of technical methods and pathology. 2007;87(3):304–314. doi: 10.1038/labinvest.3700507. [DOI] [PubMed] [Google Scholar]
- 85.Tamura H, Ohtsuka M, Washiro M, Kimura F, Shimizu H, Yoshidome H, Kato A, Seki N, Miyazaki M. Reg IV expression and clinicopathologic features of gallbladder carcinoma. Human pathology. 2009;40(12):1686–1692. doi: 10.1016/j.humpath.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 86.Hayashi T, Matsubara A, Ohara S, Mita K, Hasegawa Y, Usui T, Arihiro K, Norimura S, Sentani K, Oue N, Yasui W. Immunohistochemical analysis of Reg IV in urogenital organs: Frequent expression of Reg IV in prostate cancer and potential utility as serum tumor marker. Oncology reports. 2009;21(1):95–100. [PubMed] [Google Scholar]
- 87.Zheng HC, Xu XY, Yu M, Takahashi H, Masuda S, Takano Y. The role of Reg IV gene and its encoding product in gastric carcinogenesis. Human pathology. 2010;41(1):59–69. doi: 10.1016/j.humpath.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 88.Mitani Y, Oue N, Matsumura S, Yoshida K, Noguchi T, Ito M, Tanaka S, Kuniyasu H, Kamata N, Yasui W. Reg IV is a serum biomarker for gastric cancer patients and predicts response to 5-fluorouracil-based chemotherapy. Oncogene. 2007;26(30):4383–4393. doi: 10.1038/sj.onc.1210215. [DOI] [PubMed] [Google Scholar]
- 89.Ohara S, Oue N, Matsubara A, Mita K, Hasegawa Y, Hayashi T, Usui T, Amatya VJ, Takeshima Y, Kuniyasu H, Yasui W. Reg IV is an independent prognostic factor for relapse in patients with clinically localized prostate cancer. Cancer science. 2008;99(8):1570–1577. doi: 10.1111/j.1349-7006.2008.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jing D, Kehoe DE, Tzanakakis ES. Expression of Reg family proteins in embryonic stem cells and its modulation by Wnt/beta-catenin signaling. Stem Cells Dev. 2010;19(9):1307–1319. doi: 10.1089/scd.2009.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsumoto S, Konishi H, Maeda R, Kiryu-Seo S, Kiyama H. Analysis on expression of the regenerating gene (Reg) family members Reg-IIIbeta and Reg-IIIgamma in the mouse during development. The Journal of comparative neurology. 2011 doi: 10.1002/cne.22705. [DOI] [PubMed] [Google Scholar]
- 92.Iovanna J, Frigerio JM, Dusetti N, Ramare F, Raibaud P, Dagorn JC. Lithostathine, an inhibitor of CaCO3 crystal growth in pancreatic juice, induces bacterial aggregation. Pancreas. 1993;8(5):597–601. doi: 10.1097/00006676-199309000-00011. [DOI] [PubMed] [Google Scholar]
- 93.Iovanna J, Orelle B, Keim V, Dagorn JC. Messenger RNA sequence and expression of rat pancreatitis-associated protein, a lectin-related protein overexpressed during acute experimental pancreatitis. The Journal of biological chemistry. 1991;266(36):24664–24669. [PubMed] [Google Scholar]
- 94.Patthy L. Homology of human pancreatic stone protein with animal lectins. Biochem J. 1988;253(1):309–311. doi: 10.1042/bj2530309b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Petersen TE. The amino-terminal domain of thrombomodulin and pancreatic stone protein are homologous with lectins. FEBS letters. 1988;231(1):51–53. doi: 10.1016/0014-5793(88)80700-2. [DOI] [PubMed] [Google Scholar]
- 96.Drickamer K. C-type lectin-like domains. Curr Opin Struct Biol. 1999;9(5):585–590. doi: 10.1016/s0959-440x(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 97.Mally MI, Otonkoski T, Lopez AD, Hayek A. Developmental gene expression in the human fetal pancreas. Pediatr Res. 1994;36(4):537–544. doi: 10.1203/00006450-199410000-00022. [DOI] [PubMed] [Google Scholar]
- 98.Sanchez D, Figarella C, Marchand-Pinatel S, Bruneau N, Guy-Crotte O. Preferential expression of reg I beta gene in human adult pancreas. Biochem Biophys Res Commun. 2001;284(3):729–737. doi: 10.1006/bbrc.2001.5033. [DOI] [PubMed] [Google Scholar]
- 99.Anastasi E, Ponte E, Gradini R, Bulotta A, Sale P, Tiberti C, Okamoto H, Dotta F, Di Mario U. Expression of Reg and cytokeratin 20 during ductal cell differentiation and proliferation in a mouse model of autoimmune diabetes. Eur J Endocrinol. 1999;141(6):644–652. doi: 10.1530/eje.0.1410644. [DOI] [PubMed] [Google Scholar]
- 100.Baeza N, Sanchez D, Vialettes B, Figarella C. Specific reg II gene overexpression in the non-obese diabetic mouse pancreas during active diabetogenesis. FEBS letters. 1997;416(3):364–368. doi: 10.1016/s0014-5793(97)01241-6. [DOI] [PubMed] [Google Scholar]
- 101.Gurr W, Shaw M, Li Y, Sherwin R. RegII is a beta-cell protein and autoantigen in diabetes of NOD mice. Diabetes. 2007;56(1):34–40. doi: 10.2337/db06-0669. [DOI] [PubMed] [Google Scholar]
- 102.Gurr W, Yavari R, Wen L, Shaw M, Mora C, Christa L, Sherwin RS. A Reg family protein is overexpressed in islets from a patient with new-onset type 1 diabetes and acts as T-cell autoantigen in NOD mice. Diabetes. 2002;51(2):339–346. doi: 10.2337/diabetes.51.2.339. [DOI] [PubMed] [Google Scholar]
- 103.Cavard C, Terris B, Grimber G, Christa L, Audard V, Radenen-Bussiere B, Simon MT, Renard CA, Buendia MA, Perret C. Overexpression of regenerating islet-derived 1 alpha and 3 alpha genes in human primary liver tumors with beta-catenin mutations. Oncogene. 2006;25(4):599–608. doi: 10.1038/sj.onc.1208860. [DOI] [PubMed] [Google Scholar]
- 104.Yuan RH, Jeng YM, Chen HL, Hsieh FJ, Yang CY, Lee PH, Hsu HC. Opposite roles of human pancreatitis-associated protein and REG1A expression in hepatocellular carcinoma: association of pancreatitis-associated protein expression with low-stage hepatocellular carcinoma, beta-catenin mutation, and favorable prognosis. Clin Cancer Res. 2005;11(7):2568–2575. doi: 10.1158/1078-0432.CCR-04-2039. [DOI] [PubMed] [Google Scholar]
- 105.Dusetti NJ, Ortiz EM, Mallo GV, Dagorn JC, Iovanna JL. Pancreatitis-associated protein I (PAP I), an acute phase protein induced by cytokines. Identification of two functional interleukin-6 response elements in the rat PAP I promoter region. The Journal of biological chemistry. 1995;270(38):22417–22421. doi: 10.1074/jbc.270.38.22417. [DOI] [PubMed] [Google Scholar]
- 106.Dusetti NJ, Mallo GV, Ortiz EM, Keim V, Dagorn JC, Iovanna JL. Induction of lithostathine/reg mRNA expression by serum from rats with acute pancreatitis and cytokines in pancreatic acinar AR-42J cells. Arch Biochem Biophys. 1996;330(1):129–132. doi: 10.1006/abbi.1996.0234. [DOI] [PubMed] [Google Scholar]
- 107.Akiyama T, Takasawa S, Nata K, Kobayashi S, Abe M, Shervani NJ, Ikeda T, Nakagawa K, Unno M, Matsuno S, Okamoto H. Activation of Reg gene, a gene for insulin-producing beta -cell regeneration: poly(ADP-ribose) polymerase binds Reg promoter and regulates the transcription by autopoly(ADP-ribosyl) ation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(1):48–53. doi: 10.1073/pnas.240458597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aggarwal S, Xie MH, Maruoka M, Foster J, Gurney AL. Acinar cells of the pancreas are a target of interleukin-22. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2001;21(12):1047–1053. doi: 10.1089/107999001317205178. [DOI] [PubMed] [Google Scholar]
- 109.Folch-Puy E, Granell S, Dagorn JC, Iovanna JL, Closa D. Pancreatitis-associated protein I suppresses NF-kappa B activation through a JAK/STAT-mediated mechanism in epithelial cells. J Immunol. 2006;176(6):3774–3779. doi: 10.4049/jimmunol.176.6.3774. [DOI] [PubMed] [Google Scholar]
- 110.Vaccaro MI, Calvo EL, Suburo AM, Sordelli DO, Lanosa G, Iovanna JL. Lipopolysaccharide directly affects pancreatic acinar cells: implications on acute pancreatitis pathophysiology. Digestive diseases and sciences. 2000;45(5):915–926. doi: 10.1023/a:1005521007609. [DOI] [PubMed] [Google Scholar]
- 111.Masamune A, Satoh K, Sakai Y, Yoshida M, Satoh A, Shimosegawa T. Ligands of peroxisome proliferator-activated receptor-gamma induce apoptosis in AR42J cells. Pancreas. 2002;24(2):130–138. doi: 10.1097/00006676-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 112.Bodeker H, Vasseur S, Dusetti NJ, Dagorn JC, Iovanna JL. PAP gene transcription induced by cycloheximide in AR4-2J cells involves ADP-ribosylation. Biochem Biophys Res Commun. 1998;251(3):710–713. doi: 10.1006/bbrc.1998.9533. [DOI] [PubMed] [Google Scholar]
- 113.Motoo Y, Taga K, Su SB, Xie MJ, Sawabu N. Arginine induces apoptosis and gene expression of pancreatitis-associated protein (PAP) in rat pancreatic acinar AR4-2J cells. Pancreas. 2000;20(1):61–66. doi: 10.1097/00006676-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 114.Taylor-Fishwick DA, Shi W, Pittenger GL, Vinik AI. PDX-1 can repress stimulus-induced activation of the INGAP promoter. J Endocrinol. 2006;188(3):611–621. doi: 10.1677/joe.1.06108. [DOI] [PubMed] [Google Scholar]
- 115.Ose T, Kadowaki Y, Fukuhara H, Kazumori H, Ishihara S, Udagawa J, Otani H, Takasawa S, Okamoto H, Kinoshita Y. Reg I-knockout mice reveal its role in regulation of cell growth that is required in generation and maintenance of the villous structure of small intestine. Oncogene. 2007;26(3):349–359. doi: 10.1038/sj.onc.1209799. [DOI] [PubMed] [Google Scholar]
- 116.Gigoux V, Clerc P, Sanchez D, Coll MG, Corominola H, Leung-Theung-Long S, Penicaud L, Gomis R, Seva C, Fourmy D, Dufresne M. Reg genes are CCK2 receptor targets in ElasCCK2 mice pancreas. Regul Pept. 2008;146(1–3):88–98. doi: 10.1016/j.regpep.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 117.Ashcroft FJ, Varro A, Dimaline R, Dockray GJ. Control of expression of the lectin-like protein Reg-1 by gastrin: role of the Rho family GTPase RhoA and a C-rich promoter element. Biochem J. 2004;381(Pt 2):397–403. doi: 10.1042/BJ20031793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ, Fox JG. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118(1):36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 119.Steele IA, Dimaline R, Pritchard DM, Peek RM, Jr, Wang TC, Dockray GJ, Varro A. Helicobacter and gastrin stimulate Reg1 expression in gastric epithelial cells through distinct promoter elements. American journal of physiology Gastrointestinal and liver physiology. 2007;293(1):G347–G354. doi: 10.1152/ajpgi.00076.2007. [DOI] [PubMed] [Google Scholar]
- 120.Broekaert D, Eyckerman S, Lavens D, Verhee A, Waelput W, Vandekerckhove J, Tavernier J. Comparison of leptin- and interleukin-6-regulated expression of the rPAP gene family: evidence for differential co-regulatory signals. European cytokine network. 2002;13(1):78–85. [PubMed] [Google Scholar]
- 121.Zabeau L, Lavens D, Peelman F, Eyckerman S, Vandekerckhove J, Tavernier J. The ins and outs of leptin receptor activation. FEBS letters. 2003;546(1):45–50. doi: 10.1016/s0014-5793(03)00440-x. [DOI] [PubMed] [Google Scholar]
- 122.Yoshioka S, Fujii S, Richards JS, Espey LL. Gonadotropin-induced expression of pancreatitis-associated protein-III mRNA in the rat ovary at the time of ovulation. J Endocrinol. 2002;174(3):485–492. doi: 10.1677/joe.0.1740485. [DOI] [PubMed] [Google Scholar]
- 123.Chakraborty C, Vrontakis M, Molnar P, Schroedter IC, Katsumata N, Murphy LJ, Shiu RP, Friesen HG. Expression of pituitary peptide 23 in the rat uterus: regulation by estradiol. Molecular and cellular endocrinology. 1995;108(1–2):149–154. doi: 10.1016/0303-7207(94)03470-e. [DOI] [PubMed] [Google Scholar]
- 124.Simon MT, Pauloin A, Normand G, Lieu HT, Mouly H, Pivert G, Carnot F, Tralhao JG, Brechot C, Christa L. HIP/PAP stimulates liver regeneration after partial hepatectomy and combines mitogenic and anti-apoptotic functions through the PKA signaling pathway. FASEB J. 2003;17(11):1441–1450. doi: 10.1096/fj.02-1013com. [DOI] [PubMed] [Google Scholar]
- 125.Van Hul W, Wuyts W, Hendrickx J, Speleman F, Wauters J, De Boulle K, Van Roy N, Bossuyt P, Willems PJ. Identification of a third EXT-like gene (EXTL3) belonging to the EXT gene family. Genomics. 1998;47(2):230–237. doi: 10.1006/geno.1997.5101. [DOI] [PubMed] [Google Scholar]
- 126.Osman NM, Kagohashi Y, Udagawa J, Otani H. Alpha1,4-N-acetylglucosaminyltransferase encoding gene EXTL3 expression pattern in mouse adult and developing tissues with special attention to the pancreas. Anat Embryol (Berl) 2003;207(4–5):333–341. doi: 10.1007/s00429-003-0348-z. [DOI] [PubMed] [Google Scholar]
- 127.Stickens D, Clines G, Burbee D, Ramos P, Thomas S, Hogue D, Hecht JT, Lovett M, Evans GA. The EXT2 multiple exostoses gene defines a family of putative tumour suppressor genes. Nat Genet. 1996;14(1):25–32. doi: 10.1038/ng0996-25. [DOI] [PubMed] [Google Scholar]
- 128.Mizuno K, Irie S, Sato TA. Overexpression of EXTL3/EXTR1 enhances NF-kappaB activity induced by TNF-alpha. Cell Signal. 2001;13(2):125–130. doi: 10.1016/s0898-6568(00)00144-3. [DOI] [PubMed] [Google Scholar]
- 129.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401(6748):86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 130.Takasawa S, Ikeda T, Akiyama T, Nata K, Nakagawa K, Shervani NJ, Noguchi N, Murakami-Kawaguchi S, Yamauchi A, Takahashi I, Tomioka-Kumagai T, Okamoto H. Cyclin D1 activation through ATF-2 in Reg-induced pancreatic beta-cell regeneration. FEBS letters. 2006;580(2):585–591. doi: 10.1016/j.febslet.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 131.Zenilman ME, Zheng QH, Wu H, Rengabashyam P. Pancreatic Reg And A Conserved Bioactive Fragment Are Mitogenic Through The MAPK P38 Pathway. Surgical forum. 2000;191(4 S1):S29. doi: 10.1016/S1072-7515(00)00456-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mueller CM, Zhang H, Zenilman ME. Pancreatic reg I binds MKP-1 and regulates cyclin D in pancreatic-derived cells. J Surg Res. 2008;150(1):137–143. doi: 10.1016/j.jss.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kadowaki Y, Ishihara S, Miyaoka Y, Rumi MA, Sato H, Kazumori H, Adachi K, Takasawa S, Okamoto H, Chiba T, Kinoshita Y. Reg protein is overexpressed in gastric cancer cells, where it activates a signal transduction pathway that converges on ERK1/2 to stimulate growth. FEBS letters. 2002;530(1–3):59–64. doi: 10.1016/s0014-5793(02)03398-7. [DOI] [PubMed] [Google Scholar]
- 134.Wang F, Xu L, Guo C, Ke A, Hu G, Xu X, Mo W, Yang L, Huang Y, He S, Wang X. Identification of RegIV as a novel GLI1 target gene in human pancreatic cancer. PloS one. 2011;6(4):e18434. doi: 10.1371/journal.pone.0018434. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Kinzler KW, Vogelstein B. The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. Molecular and cellular biology. 1990;10(2):634–642. doi: 10.1128/mcb.10.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bishnupuri KS, Luo Q, Murmu N, Houchen CW, Anant S, Dieckgraefe BK. Reg IV activates the epidermal growth factor receptor/Akt/AP-1 signaling pathway in colon adenocarcinomas. Gastroenterology. 2006;130(1):137–149. doi: 10.1053/j.gastro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 137.Macadam RC, Sarela AI, Farmery SM, Robinson PA, Markham AF, Guillou PJ. Death from early colorectal cancer is predicted by the presence of transcripts of the REG gene family. Br J Cancer. 2000;83(2):188–195. doi: 10.1054/bjoc.2000.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Buckhaults P, Rago C, St Croix B, Romans KE, Saha S, Zhang L, Vogelstein B, Kinzler KW. Secreted and cell surface genes expressed in benign and malignant colorectal tumors. Cancer research. 2001;61(19):6996–7001. [PubMed] [Google Scholar]
- 139.Miyaoka Y, Kadowaki Y, Ishihara S, Ose T, Fukuhara H, Kazumori H, Takasawa S, Okamoto H, Chiba T, Kinoshita Y. Transgenic overexpression of Reg protein caused gastric cell proliferation and differentiation along parietal cell and chief cell lineages. Oncogene. 2004;23(20):3572–3579. doi: 10.1038/sj.onc.1207333. [DOI] [PubMed] [Google Scholar]


