Abstract
Obesity, hypertension and type 2 diabetes are major contributing factors to the increase in the number of patients that have chronic kidney disease. The clustering of visceral obesity and cardiovascular risk factors has been designated metabolic syndrome or cardiometabolic syndrome. Cardiometabolic syndrome is associated with a complex systemic inflammatory state that has been implicated in medically important complications including endothelial dysfunction. Inflammation, endothelial dysfunction, and insulin resistance are interrelated and have reciprocal relationships that link cardiovascular and metabolic diseases. Ultimately, cardiometabolic syndrome increases the risk for cardiovascular events and end organ damage. Although the number of patients with cardiometabolic syndrome is escalating, therapeutic approaches have not been developed that provide protection to the kidney. Eicosanoids are altered in cardiometabolic syndrome and contribute the progression of renal injury. The anti-hypertensive and anti-inflammatory actions of epoxides and soluble epoxide hydrolase inhibitors make these attractive eicosanoid therapeutic targets for chronic kidney disease in patients with cardiometabolic syndrome.
Keywords: kidney, epoxyeicosatrienoic acids, epoxide hydrolase, obesity, cytokines, inflammation
Cardiometabolic Syndrome
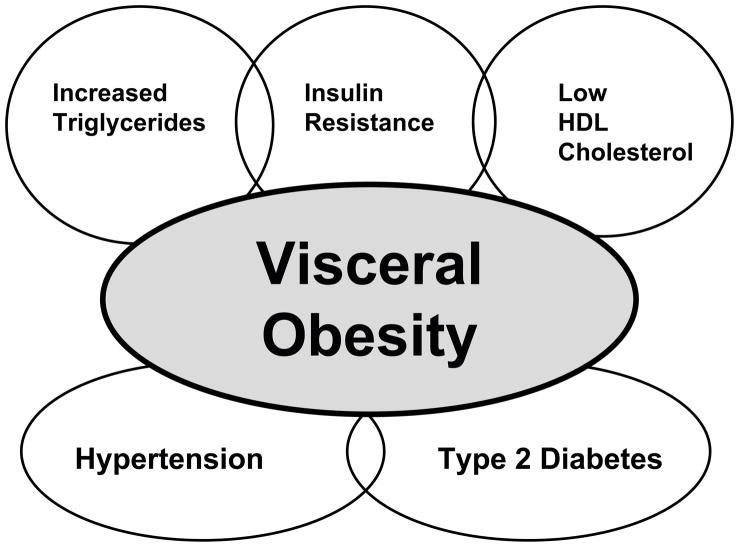
In recent years, the number of obese people in the world is growing rapidly and has reached epidemic status. Obesity is the central phenotype in metabolic syndrome, also known as cardiometabolic syndrome that clusters with other cardiovascular risk factors. These other cardiovascular risk factors include hypertension, type 2 diabetes, insulin resistance, low high-density lipoproteins (HDL) cholesterol, elevated triglycerides, microalbuminuria, and atherosclerosis [1,2,3] (Figure 1). The Adult treatment Panel II of the National Cholesterol Education Program has defined metabolic syndrome as any three of the following five traits: visceral obesity, hypertension, hypertriglyceridemia, low HDL cholesterol, and impaired fasting glucose [4]. Although obesity is the phenotypic hallmark of cardiometabolic syndrome, the other risk factors that manifest in individuals varies greatly. Blood pressure elevation in obese individuals is closely related to weight gain; however, not all obese individuals become hypertensive [5–8]. The relationship between obesity and hypertension is well established but the exact consequences to end organ damage remains unknown. Interestingly, hypertension and diabetes account for seventy percent of patients with end stage renal disease (ESRD) and the number of patients with ESRD will double in this decade [9,10]. One of the main reasons for the doubling in ESRD patients is the increase in obesity related type 2 diabetes and its co-existence with hypertension.
Figure 1.
Visceral obesity is the central component in cardiometabolic syndrome that clusters with other cardiovascular risk factors.
The clustering of cardiovascular risk factors such as inflammation, endothelial dysfunction and insulin resistance are intertwined in obesity and hypertension. These risk factors are currently under managed in the clinical practice and point for a need to understand the physiological systems and possible mechanistic interactions. Visceral obesity is the central component to cardiometabolic syndrome as it results in insulin resistance and glucose intolerance, which can progress to type 2 diabetes. Adipose tissue is in an active endocrine organ and functions as more than an energy storage depot. Adipocytes secrete a number of substances called adipokines. There is enhanced production of free fatty acids, leptin, resistin, and inflammatory cytokines in cardiometabolic syndrome [11]. On the other hand, the release of the anti-inflammatory protein adiponectin is decreased in obesity [12]. Therefore, inflammation and endothelial dysfunction are common complications associated with the increase in visceral fat.
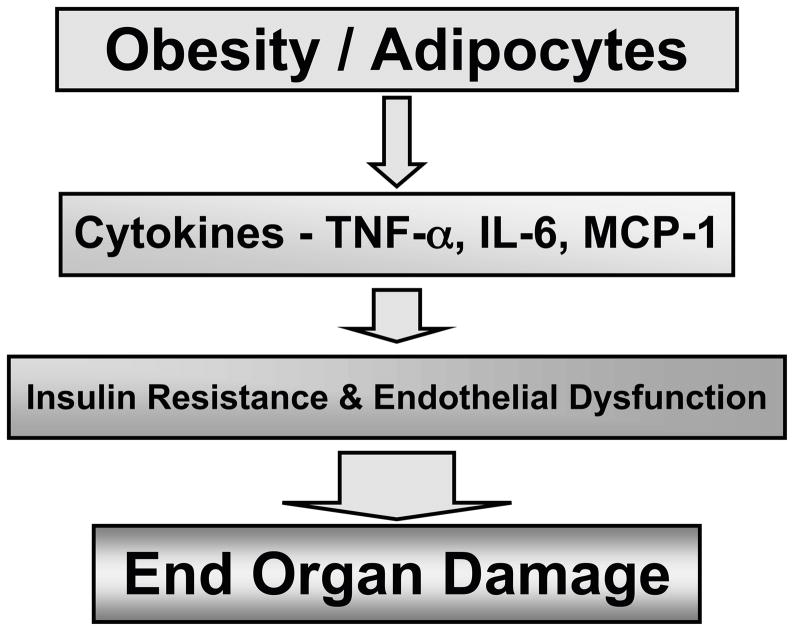
Inflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF) α are released by adipose tissue in response to increased adiposisity [13]. Adipocytes, monocytes and endothelial cells produce IL-6 and plasma levels correlate with adiposity and insulin resistance [14]. IL-6 has vascular actions including stimulating adhesion molecules and angiotensin type 1 receptors that can result in inflammation and oxidative stress [15,16]. TNF-α is an important cytokine secreted by adipose tissue that can induce insulin resistance and endothelial dysfunction in obesity [17–20]. Likewise, TNF-α activation of NFκB and the subsequent expression of cell adhesion molecules such as MCP-1 contribute to diminished endothelial responses in cardiovascular diseases [21,22]. Plasma TNF-α concentrations have reported to be increased in patients with type 2 diabetes and hypertension or cardiometabolic syndrome [17,23]. Clinically, inhibition of TNF-α inhibition with etanercept reduces C-reactive protein levels in patients with metabolic syndrome [17,18]. Monocyte chemoattractant protein 1 (MCP-1) is another chemokine that increases in proportion to adiposity in obese rodents and humans [17,24]. MCP-1 is a member of the CC chemokine family and a major ligand of chemokine receptor-2 (Ccr2). Interestingly, in mice with established obesity, short-term treatment with an antagonist of Ccr2 lowered macrophage content of adipose tissue and improved insulin sensitivity without altering body mass [24]. These inflammatory cytokines contribute to insulin resistance and to the progression of endothelial dysfunction that eventually leads to end organ damage like ESRD (Figure 2).
Figure 2.
Adipocytes generate inflammatory cytokines that promote insulin resistance and endothelial dysfunction resulting in end organ damage.
Renal Damage and Cardiometabolic Syndrome
A major cause of kidney failure is obesity-related metabolic syndrome that often contributes to the development of type 2 diabetes. Elevated blood glucose levels, hypertension and an increase in glomerular filtration (GFR) are major factors contributing to renal injury [2,25,26]. Obese individuals and obese mice have an elevated renal plasma flow and GFR that can lead to an increased glomerular capillary pressure and glomerular injury [27,28]. Obese animal models have significant glomerular injury, including increased mesangial matrix, basement membrane thickening and mesangial cell expansion [29]. The hyperinsulinemia associated with obesity and cardiometabolic syndrome could be contributing to glomerular hypertrophy. Cell proliferation and renal injury in response to increased growth factors such as transforming growth factor (TGF) β and decreased levels of matrix metalloproteinases (MMP) -2 occurs in states of hyperinsulinemia [30,31]. Elevated insulin levels have been demonstrated to cause glomerular hypertrophy in rats and increase extracellular matrix synthesis in cultured mesangial cells [10]. Other factors also contribute to renal and glomerular injury in patients with cardiometabolic syndrome.
Inflammatory cytokines that are associated with obesity are also contributors to the progression of renal disease in cardiometabolic syndrome. IL-6 is a cytokine that has been demonstrated to be associated with renal injury [32,33]. Renal IL-6 results in macrophage accumulation in the kidney, stimulates the expression of C-reactive protein, can increase TNF-α and inhibits nitric oxide [34,35]. TNF-α is another cytokine that has been suggested to mediate endothelial dysfunction and contribute to chronic kidney disease [36]. TNF-α can induce endothelial insulin resistance through inhibition of insulin-stimulated phosphorylation of insulin receptor substrate 1 [17,18]. TNF-α has also been demonstrated to contribute to the pathogenesis of chronic kidney disease. Others and we have also demonstrated that TNF-α blockade decreases kidney damage in hypertension [37,38]. TNF-α appears to be an important cytokine in the renal damage that occurs in cardiometabolic syndrome because it activates the transcription factor nuclear facto-κB (NFκB). There is increasing evidence that NFκB contributes to diabetic nephropathy. Obesity-mediated renal injury has been associated with activation of NFκB [39]. NFκB acts to increase inflammatory cytokines and adhesion molecules in the kidney, resulting in the progression of glomerular damage and inhibition of NFκB can decrease renal injury [40,41]. Interestingly, MCP-1 is a chemokine that is activated by NFκB activation and MCP-1 can increase infiltration of macrophages to the glomerulus that results in glomerular hypertrophy [42–44]. Taken together, there is convincing evidence that inflammation is an important contributing factor to the progression of renal injury that occurs in cardiometabolic syndrome. Oxidative stress and dyslipidemia are contributing factors to the progression of chronic kidney disease in the patients with cardiometabolic syndrome.
Oxidative stress is due to an imbalance between oxidants and antioxidants. Generation of the oxidant, reactive oxygen species (ROS) is elevated whereas the availability of the antioxidant, nitric oxide is reduced in diet-induced obesity [45]. There may also be a connection between ROS generation and the inflammation associated with cardiometabolic syndrome. ROS can also contribute to the inflammation and inflammation can increase ROS generation. Data suggests that patients with chronic kidney disease have increased oxidative stress that contributes to the renal injury [46]. Increased ROS levels have been found to contribute to endothelial dysfunction and renal injury in rat and animal models of obesity and insulin resistance [47–50]. Likewise, inhibition of NADPH oxidase to reduce ROS levels ameliorates glomerular damage in obese db/db mice [50]. Free fatty acids (FFA) and dyslipidemia may also be part of the cause for renal injury in cardiometabolic syndrome. In obesity there is an increase in circulating FFA levels that have been linked with the development of endothelial dysfunction [51]. Elevated levels of intracellular lipids will result in lipid products like diacylglyceride and ceramide that have been implicated as mediators of cell death or what is known as apoptosis [1,52]. Increased FFA levels have the potential to cause tubulointerstitital nephritis in metabolic syndrome [1,53]. Interestingly. FFA can generate ROS that also contribute to the renal injury [1,53]. As a whole, elevated ROS and FFA levels are factors that contribute to the endothelial dysfunction and progression of chronic kidney disease in cardiometabolic syndrome.
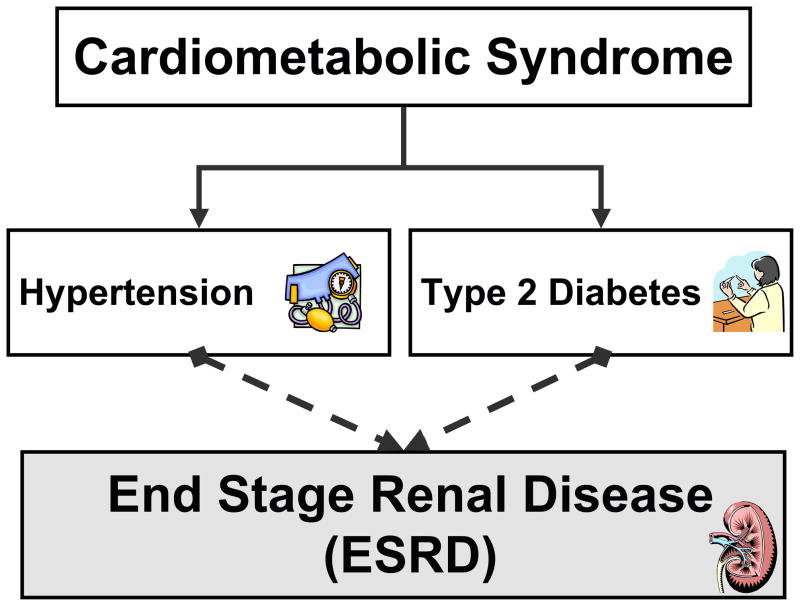
Hypertension is a cardiovascular risk factor that is linked to cardiometabolic syndrome and contributes to the progression of renal injury in this syndrome. It is estimated that the diabetes and hypertension co-exist in more than three million people in the United States [54]. This is most likely an underestimate because this does not include obese patients that are insulin resistant. The combination of high blood pressure and elevated blood glucose levels are thought to be contributing factors that predispose cardiometabolic syndrome patients to ESRD [54,55] (Figure 3). Likewise, renal complications associated with diabetes such as vascular hypertrophy and matrix deposition have been attributed to hypertension [56]. There is also evidence that adiposity independent of hypertension and diabetes is associated with increased incidence of chronic kidney disease [25,53,57]. Inflammation, oxidative stress, and lipid abnormalities that are associated with obesity are most likely contributing to the progression of renal damage. Because of this multitude of factors in cardiometabolic syndrome that can contribute to chronic kidney disease it is very difficult to predict if treatment of one or more of these factors will ameliorate renal damage.
Figure 3.
Cardiometabolic syndrome is associated with hypertension and type 2 diabetes that are key contributors to chronic kidney disease and progression to end stage renal disease.
Eicosanoids as a Therapeutic Target for Renal Damage in Cardiometabolic Syndrome
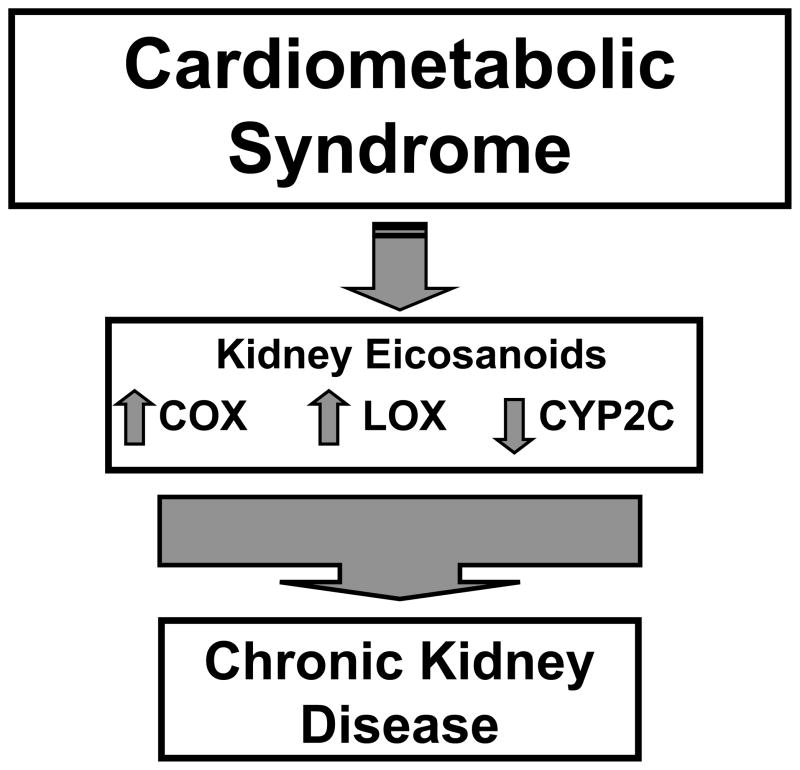
Arachidonic acid metabolites, also known as eicosanoids, represent a large biological class of lipids that have important activities for maintaining homeostasis. Eicosanoid metabolite levels are also altered in disease states and can contribute to the progression of disease processes. There are three distinct enzymatic pathways, cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP), that metabolize arachidonic acid. These three pathways are very important for renal function and renal damage in cardiometabolic syndrome has been attributed at least in part to eicosanoid metabolites [25,53,57] (Figure 4).
Figure 4.
Eicosanoids of the cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 (CYP) pathways are altered in cardiometabolic syndrome and contribute to chronic kidney disease.
COX metabolites have been implicated in the renal damage that occurs in animal models and patients with metabolic syndrome [58–62]. There are at least two COX enzymes, COX-1 and COX-2, expressed in the kidney. COX metabolites play a critical role in renal blood flow regulation, renin generation and vascular inflammation [25,58,63]. Alterations in COX metabolites contribute to endothelial dysfunction and renal damage in diabetes [56,64,65]. Increased thromboxane A2 (TXA2) can contribute to glomerular matrix deposition and stimulate glomerular mesangial expansion [61,66]. Prostaglandin I2 (PGI2) can oppose the deleterious TXA2 effects associated with chronic kidney disease [61]. A number of studies have linked obesity and type 2 diabetes to increased generation of COX-2 metabolites in the kidney [29,58,60]. A consistent finding in these studies has been a decreased PGI2/TXA2 ratio in diabetic patients and animal models of diabetes [62,67]. These previous findings have suggested that increased COX-2 and TXA2 and decreased PGI2 could contribute to chronic kidney disease that occurs in cardiometabolic syndrome.
The ability of TXA inhibitors and COX inhibitors to decrease renal damage in obesity has been examined. Diabetic patients treated with TX synthase inhibitors have decreased urinary protein and albumin levels [67–69]. Likewise, inhibition of TX synthase preserved renal blood flow and slowed the progression renal damage in diabetic rats [62,67,69]. Rofecoxib, a COX-2 inhibitor, decreases vascular and glomerular damage when administered to obese Zucker rats [29]. This renal protection occurred even though body weights, blood glucose, and cholesterol levels were not affected by COX-2 inhibition [29]. There could also be a connection between COX metabolites and oxidative stress in cardiometabolic syndrome. COX-2 inhibition has been demonstrated to decrease levels of the oxidative stress metabolite 8-isoprostane and this has been linked to decreased renal injury in obesity and hypertension [29,70]. Therefore, there is the potential for COX-2 inhibitors to be utilized as a treatment for renal injury associated with cardiometabolic syndrome.
Experimental evidence has implicated LOX enzymes in vascular and renal injury in diabetes [71–75]. The 12/15-LOX product 12-hydroxyeicosatetraenoic acid (12-HETE) is increased in the urine of diabetic patients with kidney disease [71,76]. Diabetic obese mice have increased endothelial cell expression of 12/15-LOX [77]. 12/15-LOX expression is also increased in the obese Zucker rats and contributes to vascular inflammation [78,79]. Increased 12/15-LOX expression is associated with increases in fibronectin and other mediators of diabetic nephropathy [80]. Administration of the LOX inhibitor masoprocol to insulin-resistant type 2 diabetic rats or high fat fed obese rats reduced triglycerides, free fatty acids and improved insulin sensitivity [81,82]. Extensive evaluation of LOX inhibitors for the treatment of renal damage associated with cardiometabolic syndrome awaits investigation.
CYP metabolites of arachidonic acid have biological actions on renal and cardiovascular tissue and regulation of specific tissue CYP enzymes contribute to organ function in renal and cardiovascular disease. Renal metabolites of the CYP4A hydoxylase enzyme have been implicated in vascular and tubular damage that occurs in diabetes, obesity and hypertension [29,53,83]. Obese Zucker rats have increased renal vascular CYP4A expression and increased generation of 20-HETE [29,83]. In contrast, renal tubular CYP4A expression is decreased in rats fed a high fat diet [84]. Intriguingly, obese hypertensive patients have decreased excretion of 20-HETE that is associated with increased circulating insulin [85]. These decreased 20-HETE levels at the renal tubular epithelium could impair handling of electrolytes and contribute to elevations in blood pressure that occurs in patients with cardiometabolic syndrome.
Renal vascular and tubular regulation of CYP epoxygenase enzymes during cardiometabolic syndrome can contribute to endothelial dysfunction. Renal CYP epoxygenase enzymes generate epoxyeicosatrienoic acids (EETs) that are metabolized by soluble epoxide hydrolase (sEH) to less active dihydroxyeicosatrienoic acids (DHETEs). Decreased renal expression of CYP2C epoxygenase enzymes has been observed in diabetes, obese Zucker rats, and high fat diet fed insulin resistance rats [29,83,84,86]. CYP2C enzyme expression is decreased in the kidney and mesenteric blood vessels of obese Zucker rats [86]. Additionally, sEH expression is increased in blood vessels of obese rats and could further contribute to the decrease in EET bioavailability [84]. Impaired endothelial dilator responses in obesity and diabetes have been attributed to decreased vascular EET levels [53,71,86]. A decrease in epoxygenase generation of EETs could be responsible for endothelial dysfunction associated with cardiometabolic syndrome. The endothelial dilator actions as well as the anti-inflammatory actions attributed to EETs have made this an attractive therapeutic target for the treatment of nephropathy associated with cardiometabolic syndrome.
Emerging Therapeutic Approaches for Epoxyeicosanoids and Renal Injury
Although the various eicosanoid metabolizing pathways, COX, LOX, and CYP all have therapeutic potential for the treatment of renal injury in cardiometabolic syndrome, this section will focus on aspects related to epoxygenase metabolites, epoxyeicosanoids. In recent years, the work in our laboratory as well as other laboratories has focused on the renal and cardiovascular protective actions of epoxygenase metabolites. Others and we have also established that EETs produced by CYP2C enzymes have anti-hypertensive, anti-inflammatory and profibrinolytic actions [87–89]. These cardiovascular actions have made EETs an attractive therapeutic target for the chronic kidney disease.
Inflammation is now recognized as a key factor contributing to end organ damage in obesity and cardiometabolic syndrome. Interestingly, there is mounting evidence that renal inflammation results in a decrease in epoxyeicosanoids. Cytokines have been demonstrated to decrease expression of CYP epoxygenase enzymes [90–92]. IL-6 has been demonstrated to downregulate CYP epoxygenase enzymes [91,92]. There are increased IL-6 levels and an inability to increase renal CYP epoxygenase expression in angiotensin salt-sensitive hypertension [33,93]. A recent study utilizing IL-6 gene deficient mice has demonstrated protection from renal damage in hypertension and that the decrease in injury was associated with an increased CYP expoxygenase expression [33]. Our laboratory has also demonstrated that interactions between TNF-α and regulation epoxyeicosanoid levels exist and can contribute to renal injury in hypertension [37]. A signaling pathway that could possible link TNF-α and epoxides to endothelial dysfunction and renal damage is NFκB activation of cell adhesion molecules such as MCP-1. Elevated MCP-1 levels have been reported to correspond to renal macrophage infiltration and urinary albumin excretion in type 2 diabetes [17,18,94,95]. The MCP-1/Ccr2 pathway also contributes to the inflammation associated with hypertension [18,37,96]. Recent evidence has demonstrated that Ccr2b inhibition decreases renal injury and improves renal afferent arteriolar vascular responses in salt-sensitive hypertension [97]. Furthermore, Ccr2b inhibition improves renal function via the decrease in NFκB inflammatory cascade and an increase in renal CYP2C23 expression [97]. EETs also have the ability to interfere with TNF-α activation of vascular cell adhesion molecules by inhibiting the activity of IKK and effectively blocking phosphorylation of IκB and preventing NFκB activation [98,99]. Thus, increasing epoxyeicosanoid levels could interfere with TNF-α, MCP-1, and IL-6 mediated inflammation, insulin resistance, endothelial function and progressive renal injury in cardiometabolic syndrome.
There are currently two pharmacological approaches being employed to target epoxyeicosanoids. The first approach is to inhibit the sEH enzyme that is responsible for the conversion of the biologically active EETs to the less active diols. Inhibitors of sEH have been demonstrated to elevate epoxyeicosanoid levels, decrease blood pressure, and provide renal protection in animal models of hypertension [100–102]. The second approach has been to develop EET analogs that resist β-oxidation and sEH conversion of the EET to a diol. EET analogs have been used successfully in vitro to oppose the inflammatory actions of TNF-α on endothelial cells and dilate blood vessels from various organ beds [87,89,99,103]. The use of EET analogs for the treatment and testing in cardiovascular diseases such as cardiometabolic syndrome awaits further development.
Conclusion
Cardiometabolic syndrome is a complex disease that is escalating in the world because of the increase in obesity. An obvious solution for combating this cardiovascular disease would be to decrease caloric intake, burn more calories through exercise and regulate body weight. Unfortunately, this approach has not been effective and the number of obese patients with cardiometabolic syndrome is increasing dramatically. Visceral obesity results in insulin resistance, hyperlipidemia, type 2 diabetes and hypertension. It is now recognized that the release of adipokines and inflammation is a key component to the progression of cardiometabolic syndrome. As a consequence, end organ damage like chronic kidney disease has become a major health issue in patients with cardiometabolic syndrome. The epoxyeicosanids are an interesting therapeutic target for cardiometabolic syndrome because these metabolites have anti-hypertensive, anti-inflammatory and other cardiovascular protective actions.
Expert Opinion
Cardiometabolic syndrome is a disease that involves the complex clustering of cardiovascular risk factors with visceral obesity being the central component. This type of disease presents a treatment dilemma for the physician. How do you treat hyperlipidemia, insulin resistance and type 2 diabetes and at the same time treat hypertension? In addition, lifestyle interventional treatment to combat the visceral obesity needs to be started. A multi-drug regimen is not the ideal solution; however, if not properly treated end organ damage will progress in patients with cardiometabolic syndrome. An area that is now being seen as a possible key factor in cardiometabolic syndrome is the release of adipokines from the visceral fat deposits. Therefore, therapeutic approaches that include interventions that are anti-inflammatory could hold significant promise as treatments for cardiometabolic syndrome patients.
The development of therapies that can treat a complex disease such as cardiometabolic syndrome is needed in the next five to ten years. An approach that holds the most promise is the development of therapies that can effectively treat more than a single component of cardiometabolic syndrome. One approach would be to develop combinational drugs such that it would treat multiple components. This approach is being tested and may provide promise for the treatment of cardiometabolic syndrome. Telimasartan is an anti-hypertensive angiotensin receptor blocker that was found to have PPARγ agonistic activities [104]. Thus, telimasartan can combat hypertension as well as increase insulin sensitivity. Other approaches are being tried with lipid lowering drugs and anti-hypertensive or lipid lowering drugs and insulin-sensitizing drugs. Another approach is to identify therapeutic targets that could affect multiple components of cardiometabolic syndrome. Key inflammatory cytokines and adipokines that have been identified as key mediators of dysfunction in cardiometabolic syndrome represent therapeutic targets. One such target has been MCP-1 and the CCR2 receptor because of its important role in obesity and end organ damage. The rigorous testing of cytokines and adipokine therapeutic targets has yet to be completed. Another target that can affect multiple components of cardiometabolic syndrome is epoxyeicosanoids utilizing EET analogs and sEH inhibitors. The epoxyeicosanids have anti-hypertensive, anti-inflammatory and other renal and cardiovascular protective actions. The possible cardiovascular benefits of EET analogs and sEH inhibitors in cardiometabolic syndrome require testing. Overall, the challenge for finding effective treatments for renal damage in cardiometabolic syndrome is the development of therapies with the capacity to combat the multiple components of this disease.
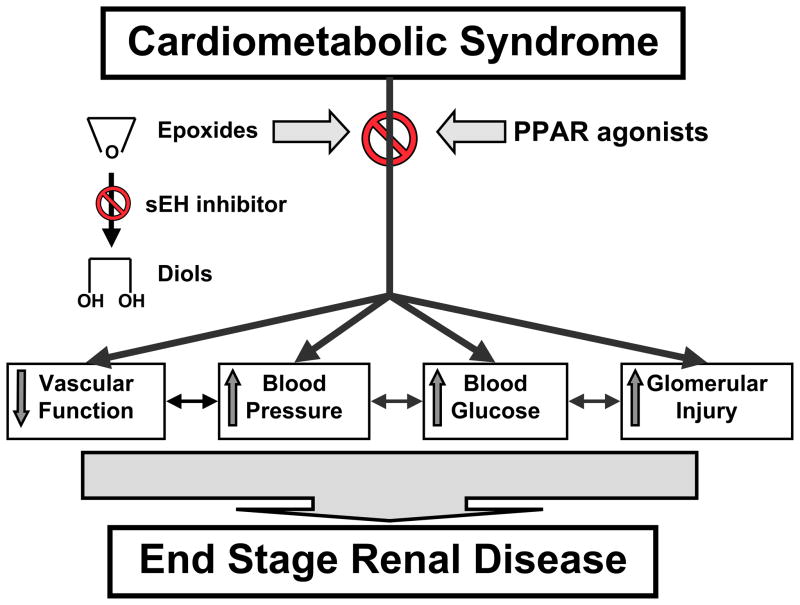
Figure 5.
Epoxyeicosanoids are therapeutic targets for end stage renal disease associated with cardiometabolic syndrome. Epoxides, soluble epoxide hydrolase (sEH) inhibitors alone or in combination with peroxisome proliferators-activated receptor (PPAR) agonists may provide protection to the kidney.
References
- 1.BAGBY SP. Obesity-initiated metabolic syndrome and the kidney: a recipe for chronic kidney disease? J Am Soc Nephrol. 2004;15:2775–2791. doi: 10.1097/01.ASN.0000141965.28037.EE. [DOI] [PubMed] [Google Scholar]
- 2.SOWERS JR, EPSTEIN M, FROHLICH ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053–1059. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 3.REAVEN GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 4.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 5.MASUO K, MIKAMI H, OGIHARA T, TUCK ML. Weight gain-induced blood pressure elevation. Hypertension. 2000;35:1135–1140. doi: 10.1161/01.hyp.35.5.1135. [DOI] [PubMed] [Google Scholar]
- 6.HUANG Z, WILLETT WC, MANSON JE, et al. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128:81–88. doi: 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- 7.JUHAERI J, STEVENS LE, CHAMBLESS HA, et al. Associations between weight gain and incident hypertension in a bi-ethnic cohort: the Atherosclerosis Risk in Communities Study. Int J Obes Relat Metab Disord. 2002;26:58–64. doi: 10.1038/sj.ijo.0801846. [DOI] [PubMed] [Google Scholar]
- 8.DYER AR, LIU K, WALSH M, KIEFE C, JACOBS DR, BILD DE. Ten-year incidence of elevated blood pressure and its predictors: the CARDIA study. Coronary Artery Risk Development in (Young) Adults. J Hum Hypertens. 1999;13:13–21. doi: 10.1038/sj.jhh.1000740. [DOI] [PubMed] [Google Scholar]
- 9.GARRISON RJ, KANNEL WB, STOKES J, CASTELLI WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1997;16:235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 10.ABRASS CK. Overview. obesity: what does it have to do with kidney disease? J Am Soc Nephrol. 2004;15:2768–2772. doi: 10.1097/01.ASN.0000141963.04540.3E. [DOI] [PubMed] [Google Scholar]
- 11.GUERRE-MILLO M. Adipose tissue hormones. J Endocrinol Invest. 2002;25:855–61. doi: 10.1007/BF03344048. [DOI] [PubMed] [Google Scholar]
- 12.DIEZ JJ, IGLESIAS P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 13.CINDIK N, BASKIN E, AGRAS PI, KINIK ST, TURAN M, SAATCI U. Effect of obesity on inflammatory markers and renal functions. Acta Paediatr. 2005;94:1732–1737. doi: 10.1111/j.1651-2227.2005.tb01845.x. [DOI] [PubMed] [Google Scholar]
- 14.BASTARD JP, JARDEL C, BRUCKERT E, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. The Journal of Clinical Endocrinology and Metabolism. 2000;85:3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 15.KIELAR ML, JOHN R, BENNETT M, et al. Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol. 2005;16:3315–3325. doi: 10.1681/ASN.2003090757. [DOI] [PubMed] [Google Scholar]
- 16.WASSMANN S, STUMPF M, STREHLOW K, et al. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res. 2004;94:534–541. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- 17.GRIMBLE RF. Inflammatory status and insulin resistance. Curr Opin Clin Nutr Metab Care. 2002;5:551–559. doi: 10.1097/00075197-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 18.KOFLER S, NICKEL T, WEIS M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clinical Science. 2005;108:205–213. doi: 10.1042/CS20040174. [DOI] [PubMed] [Google Scholar]
- 19.SCHALKWIJK CG, STEHOUWER CDA. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clinical Science. 2005;109:143–159. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- 20.PICCHI A, GAO X, BELMADANI S, et al. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 21.HIGUCHI Y, OTSU K, NISHIDA K, et al. Involvement of reactive oxygen species-mediated NF-kappa B activation in TNF-alpha-induced cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2002;34:233–240. doi: 10.1006/jmcc.2001.1505. [DOI] [PubMed] [Google Scholar]
- 22.GREENBERG AS, MCDANIEL ML. Identifying the links between obesity, insulin resistance and beta-cell function: potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur J Clin Invest. 2002;32:24–34. doi: 10.1046/j.1365-2362.32.s3.4.x. [DOI] [PubMed] [Google Scholar]
- 23.DOMINGUEZ H, STORGAARD H, RASK-MADSEN C, et al. Metabolic and vascular effects of TNF-α blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res. 2005;42:517–525. doi: 10.1159/000088261. [DOI] [PubMed] [Google Scholar]
- 24.WEISBERG SP, HUNTER D, HUBER R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.IMIG JD. Eicosanoids and renal vascular function in diseases. Clinical Science. 2006;111:21–34. doi: 10.1042/CS20050251. [DOI] [PubMed] [Google Scholar]
- 26.VIVIAN EM, RUBINSTEIN GB. Pharmacologic management of diabetic nephropathy. Clin Ther. 2002;24:1741–1756. doi: 10.1016/s0149-2918(02)80076-5. [DOI] [PubMed] [Google Scholar]
- 27.CHAGNAC A, WEINSTEIN T, KORZETS A, RAMADAN E, HIRSCH J, GAFTER U. Glomerular hemodynamics in severe obesity. American Journal of Physiology. 2000;278:817–822. doi: 10.1152/ajprenal.2000.278.5.F817. [DOI] [PubMed] [Google Scholar]
- 28.LEVINE DZ, IACOVITTI M, ROBERTSON SJ, MOKHTAR GA. Modulation of single-nephron GFR in the db/db mouse model of type 2 diabetes mellitus. American Journal of Physiology. 2006;290:R975–981. doi: 10.1152/ajpregu.00693.2005. [DOI] [PubMed] [Google Scholar]
- 29.DEY A, MARIC C, KAESEMEYER WH, et al. Rofecoxib decreases renal injury in obese Zucker rats. Clinical Science. 2004;107:561–570. doi: 10.1042/CS20040125. [DOI] [PubMed] [Google Scholar]
- 30.LUPIA E, ELLIOT SJ, LENZ O, et al. IGF-1 decreases collagen degradation in diabetic NOD mesangial cells: implications for diabetic nephropathy. Diabetes. 1999;48:1638–1644. doi: 10.2337/diabetes.48.8.1638. [DOI] [PubMed] [Google Scholar]
- 31.MORRISEY K, EVANS RA, WAKEFIELD L, PHILLIPS AO. Translational regulation of renal proximal tubular epithelial cell transforming growth factor-beta1 generation by insulin. Am J Pathol. 2001;159:1905–1915. doi: 10.1016/s0002-9440(10)63037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LEE D, STURGIS L, LABAZI H, et al. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol. 2006;290:H935–H940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 33.MANHIANI MM, QUIGLEY J, SOCHA MJ, MOTAMED K, IMIG JD. IL-6 suppression provides renal protection independent of blood pressure in a murine model of salt-sensitive hypertension. Kidney Blood Press Res. 2007;30:195–202. doi: 10.1159/000104094. [DOI] [PubMed] [Google Scholar]
- 34.HUBER SA, SAKKINEN P, CONZE D, HARDIN N, TRACY R. Interleukin-6 exacerbates early atherosclerosis in mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 35.PATEL NS, CHATTERJEE PK, DI PAOLA R, et al. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J Pharmacol Exp Ther. 2005;312:1170–1178. doi: 10.1124/jpet.104.078659. [DOI] [PubMed] [Google Scholar]
- 36.LYON CJ, LAW RE, HSUEH WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144:2195–200. doi: 10.1210/en.2003-0285. [DOI] [PubMed] [Google Scholar]
- 37.ELMARAKBY AA, QUIGLEY JE, POLLOCK DM, IMIG JD. TNF-α blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension. 2006;47:557–562. doi: 10.1161/01.HYP.0000198545.01860.90. [DOI] [PubMed] [Google Scholar]
- 38.MULLER DN, THEUER J, SHAGDARSUREN E, et al. A peroxisome proliferators-activated receptor-α activator induces renal CYP2C23 activity and protects from angiotensin II-induced renal injury. Am J Pathol. 2004;164:521–532. doi: 10.1016/s0002-9440(10)63142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.KLAHR S, MORRISSEY J. Progression of chronic renal disease. Am J Kidney Dis. 2003;41:S3–S7. doi: 10.1053/ajkd.2003.50074. [DOI] [PubMed] [Google Scholar]
- 40.LEE FT, CAO Z, LONG DM, et al. Interactions between angiotensin II and NF-kappaB-dependent pathways in modulating macrophage infiltration in experimental diabetic nephropathy. J Am Soc Nephrol. 2004;15:2139–2151. doi: 10.1097/01.ASN.0000135055.61833.A8. [DOI] [PubMed] [Google Scholar]
- 41.WILSON HM, CHETTIBI S, JOBIN C, WALBAUM D, REES AJ, KLUTH DC. Inhibition of macrophage nuclear factor-kappaB leads to a dominant anti-inflammatory phenotype that attenuates glomerular inflammation in vivo. Am J Pathol. 2005;167:27–37. doi: 10.1016/s0002-9440(10)62950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.BANBA N, NAKAMURA T, MATSUMURA M, KURODA H, HATTORI Y, KASAI K. Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int. 2000;58:684–690. doi: 10.1046/j.1523-1755.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 43.ROLLINS BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 44.KNIGHT SF, IMIG JD. Obesity, insulin resistance, and renal function. Microcirculation. 2007;14:349–362. doi: 10.1080/10739680701283018. [DOI] [PubMed] [Google Scholar]
- 45.MUNDY AL, HAAS E, BHATTACHARYA I, et al. Fat intake modifies vascular responsiveness and receptor expression of vasoconstrictors: Implications for diet-induced obesity. Cardiovas Res. 2007;73:368–375. doi: 10.1016/j.cardiores.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 46.VAZIRI ND, NI Z, OVEISI F, LIANG K, PANDIAN R. Enhanced nitric oxide inactivation and protein nitration by reactive oxygen species in renal insufficiency. Hypertension. 2002;39:135–141. doi: 10.1161/hy0102.100540. [DOI] [PubMed] [Google Scholar]
- 47.CHINEN I, SHIMABUKURO M, YAMAKAWA K, et al. Vascular lipotoxicity: endothelial dysfunction via fatty-acid-induced reactive oxygen species overproduction in obese Zucker diabetic fatty rats. Endocrinology. 2007;148:160–165. doi: 10.1210/en.2006-1132. [DOI] [PubMed] [Google Scholar]
- 48.MCCARTY MF, FALAHATI-NINI A. Neuroprotective potential of the Bahadori leanness program: A “mini-fast with exercise” strategy. Med Hypotheses. 2007;68:935–940. doi: 10.1016/j.mehy.2006.04.080. [DOI] [PubMed] [Google Scholar]
- 49.ROBERTS CK, BARNARD RJ, SINDHU RK, JURCZAK M, EHDAIE A, VAZIRI ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism: Clinical and Experimental. 2006;55:928–934. doi: 10.1016/j.metabol.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 50.SUSZTAK K, RAFF AC, SCHIFFER M, BOTTINGER EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 51.STEINBERG HO, CHAKER H, LEAMING R, JOHNSON A, BRECHTEL G, BARON AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.UNGER RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144:5159–5165. doi: 10.1210/en.2003-0870. [DOI] [PubMed] [Google Scholar]
- 53.IMIG JD, ZHAO X, DEY A, SHAW M. CYP450, COX-2 and obesity related renal damage. Toxicology Mechanisms and Methods. 2005;15:125–136. doi: 10.1080/15376520590918856. [DOI] [PubMed] [Google Scholar]
- 54.USRDS. the United States Renal Data System. Am J Kidney Dis. 2003;42:1–230. [PubMed] [Google Scholar]
- 55.SCHELLING JR, SEDOR JR. The metabolic syndrome as a risk factor for chronic kidney disease: more than a fat chance? J Am Soc Nephrol. 2004;15:2773–2774. doi: 10.1097/01.ASN.0000141964.68839.BB. [DOI] [PubMed] [Google Scholar]
- 56.TAYLOR AA. Pathophysiology of hypertension and endothelial dysfunction in patients with diabetes mellitus. Endocrinol Metab Clin North Am. 2001;30:983–997. doi: 10.1016/s0889-8529(05)70223-1. [DOI] [PubMed] [Google Scholar]
- 57.IMIG JD, ZHAO X. Eicosanoid inhibitors as therapeutic targets for metabolic syndrome related kidney disease. Current Enzyme Inhibition. 2006;2:73–77. [Google Scholar]
- 58.CHENG HF, WANG CJ, MOECEL GW, ZHANG MZ, MC KANNA JA, HARRIS RC. Cyclooxygenase-2 inhibitor blocks expression of mediators of renal injury in a model of diabetes and hypertension. Kidney Int. 2002;62:929–939. doi: 10.1046/j.1523-1755.2002.00520.x. [DOI] [PubMed] [Google Scholar]
- 59.FUJIHARA CK, ANTUNES GR, MATTAR AL, et al. Cyclooxygenase-2 (COX-2) inhibition limits abnormal COX-2 expression and progressive injury in the remnant kidney. Kidney Int. 2003;64:2172–2181. doi: 10.1046/j.1523-1755.2003.00319.x. [DOI] [PubMed] [Google Scholar]
- 60.KOMERS R, LINDLEY JN, OYAMA TT, et al. Immunohistochemical and functional correlations of renal cyclooxygenase-2 in experimental diabetes. J Clin Invest. 2001;107:889–898. doi: 10.1172/JCI10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.OKUMURA M, IMANISHI M, YAMASHITA T, et al. Renal production of thromboxane and prostaglandins in a rat model of type 2 diabetes. Life Sciences. 2000;66:371–377. doi: 10.1016/s0024-3205(99)00603-7. [DOI] [PubMed] [Google Scholar]
- 62.UMEDA F, KUROKI T, NAWATA H. Prostaglandins and diabetic nephropathy. J Diabetes Complications. 1995;9:334–336. doi: 10.1016/1056-8727(95)80035-d. [DOI] [PubMed] [Google Scholar]
- 63.IMIG JD. Eicosanoid regulation of the renal vasculature. Am J Physiol Renal Physiol. 2000;279:F965–F981. doi: 10.1152/ajprenal.2000.279.6.F965. [DOI] [PubMed] [Google Scholar]
- 64.QUILLEY J. Insulin resistance, oxidative stress and aspirin: therapeutic implications? J Hypertens. 2002;20:1279–1281. doi: 10.1097/00004872-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 65.SARUBBI D, MCGIFF JC, QUILLEY J. Renal vascular responses and eicosanoid release in diabetic rats. Am J Physiol. 1989;257:F762–F768. doi: 10.1152/ajprenal.1989.257.5.F762. [DOI] [PubMed] [Google Scholar]
- 66.STUDER RK, NEGRETE H, CRAVEN PA, DERUBERTIS FR. Protein kinase C signals thromboxane induced increases in fibronectin synthesis and TGF-beta bioactivity in mesangial cells. Kidney Int. 1995;48:422–430. doi: 10.1038/ki.1995.310. [DOI] [PubMed] [Google Scholar]
- 67.TAJIRI Y, UMEDA F, INOGUCHI T, NAWATA H. Effects of thromboxane synthetase inhibitor (OKY-046) on urinary prostaglandin excretion and renal function in streptozotocin-induced diabetic rat. J Diabetes Complications. 1994;8:126–132. doi: 10.1016/1056-8727(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 68.URIU K, KAIZU K, HASHIMOTO O, KOMINE N, ETOH S. Acute and chronic effects of thromboxane A2 inhibition on the renal hemodynamics in streptozotocin-induced diabetic rats. Kidney Int. 1994;45:794–802. doi: 10.1038/ki.1994.105. [DOI] [PubMed] [Google Scholar]
- 69.MASUMURA H, KUNITADA S, IRIE K, ASHIDA S, ABE Y. A thromboxane A2 synthetase inhibitor retards hypertensive rat diabetic nephropathy. Eur J Pharmacol. 1992;210:163–172. doi: 10.1016/0014-2999(92)90667-s. [DOI] [PubMed] [Google Scholar]
- 70.TOMIDA T, NUMAGUCHI Y, NISHIMOTO Y, et al. Inhibition of COX-2 prevents hypertension and proteinuria associated with a decrease of 8-iso-PGF2alpha formation in L-NAME-treated rats. J Hypertens. 2003;21:601–609. doi: 10.1097/00004872-200303000-00027. [DOI] [PubMed] [Google Scholar]
- 71.NATARAJAN R, NADLER JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1542–1548. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- 72.ANTONIPILLAI I, NADLER J, VU EJ, BUGHI S, NATARAJAN R, HORTON R. A 12-lipoxygenase product, 12-hydroxyeicosatetraenoic acid, is increased in diabetics with incipient and early renal disease. J Clin Endocrinol Metab. 1996;81:1940–1945. doi: 10.1210/jcem.81.5.8626861. [DOI] [PubMed] [Google Scholar]
- 73.HISHINUMA T, KOSEKI Y, MURAI Y, YAMAZAKI T, SUZUKI K, MIZUGAKI M. Urinary thromboxane A2/prostacyclin balance reflects the pathological state of a diabetic. Prostaglandins Other Lipid Mediat. 1999;58:263–271. doi: 10.1016/s0090-6980(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 74.HARDY G, STANKE-LABESQUE F, PEOC'H M, et al. Cysteinyl leukotrienes modulate angiotensin II constrictor effects on aortas from streptozotocin-induced diabetic rats. Arterioscler Thromb Vasc Biol. 2001;21:1751–1758. doi: 10.1161/hq1201.098769. [DOI] [PubMed] [Google Scholar]
- 75.KANG SW, ADLER SG, NAST CC, et al. 12-lipoxygenase is increased in glucose-stimulated mesangial cells and in experimental diabetic nephropathy. Kidney Int. 2001;59:1354–1362. doi: 10.1046/j.1523-1755.2001.0590041354.x. [DOI] [PubMed] [Google Scholar]
- 76.PARTHASARATHY S, WIELAND E, STEINBERG D. A role for endothelial cell lipoxygenase in the oxidative modification of low density lipoprotein. Proc Natl Acad Sci USA. 1989;86:1046–1050. doi: 10.1073/pnas.86.3.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.HATLEY ME, SRINIVASAN S, REILLY KB, BOLICK DT, HEDRICK CC. Increased production of 12/15 lipoxygenase eicosanoids accelerates monocyte/endothelial interactions in diabetic db/db mice. J Biol Chem. 2003;278:25369–25375. doi: 10.1074/jbc.M301175200. [DOI] [PubMed] [Google Scholar]
- 78.ALPERT E, GRUZMAN A, TOTARY H, KAISER N, REICH R, SASSON S. A natural protective mechanism against hyperglycaemia in vascular endothelial and smooth-muscle cells: role of glucose and 12-hydroxyeicosatetraenoic acid. Biochem J. 2002;362:413–422. doi: 10.1042/0264-6021:3620413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.TOKUYAMA Y, STURIS J, DEPAOLI AM, et al. Evolution of beta-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes. 1995;44:1447–1457. doi: 10.2337/diab.44.12.1447. [DOI] [PubMed] [Google Scholar]
- 80.GEORGE J, AFEK A, SHAISH A, et al. 12/15-Lipoxygenase gene disruption attenuates atherogenesis in LDL receptor-deficient mice. Circulation. 2001;104:1646–1650. doi: 10.1161/hc3901.095772. [DOI] [PubMed] [Google Scholar]
- 81.REED MJ, MESZAROS K, ENTES LJ, et al. Effect of masoprocol on carbohydrate and lipid metabolism in a rat model of Type II diabetes. Diabetologia. 1999;42:102–106. doi: 10.1007/s001250051121. [DOI] [PubMed] [Google Scholar]
- 82.SCRIBNER KA, GADBOIS TM, GOWRI M, AZHAR S, REAVEN GM. Masoprocol decreases serum triglyceride concentrations in rats with fructose-induced hypertriglyceridemia. Metabolism. 2000;49:1106–1110. doi: 10.1053/meta.2000.8604. [DOI] [PubMed] [Google Scholar]
- 83.DEY A, WILLIAMS RS, POLLOCK DM, et al. Altered kidney CYP2C and cyclooxygenase-2 levels are associated with obesity-related albuminuria. Obes Res. 2004;12:1278–1289. doi: 10.1038/oby.2004.162. [DOI] [PubMed] [Google Scholar]
- 84.WANG MH, SMITH A, ZHOU Y, et al. Downregulation of renal CYP-derived eicosanoid synthesis in rats with diet-induced hypertension. Hypertension. 2003;42:594–599. doi: 10.1161/01.HYP.0000090123.55365.BA. [DOI] [PubMed] [Google Scholar]
- 85.LAFFER CL, LANIADO-SCHWARTZMAN M, NASJLETTI A, ELIJOVICH F. 20-HETE and circulating insulin in essential hypertension with obesity. Hypertension. 2004;43:388–392. doi: 10.1161/01.HYP.0000112224.87290.3a. [DOI] [PubMed] [Google Scholar]
- 86.ZHAO X, DEY A, ROMANKO OP, et al. Decreased epoxygenase and increased epoxide hydrolase expression in the mesenteric artery of obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R188–R196. doi: 10.1152/ajpregu.00018.2004. [DOI] [PubMed] [Google Scholar]
- 87.IMIG JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005;289:F496–F503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 88.ZELDIN DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 89.CAMPBELL WB. New role for epoxyeicosatrienoic acids as anti-inflammatroy mediators. TiPS. 2000;21:125–127. doi: 10.1016/s0165-6147(00)01472-3. [DOI] [PubMed] [Google Scholar]
- 90.FITZPATRICK FA, ENNIS MD, BAZE ME, WYNALDA MA, MCGEE JE, LIGGETT WF. Inhibition of cyclooxygenase activity and platelet aggregation by epoxyeicosatrienoic acids. Influence of stereochemistry. J Biol Chem. 1986;261:15334–15338. [PubMed] [Google Scholar]
- 91.IBER H, CHEN Q, CHENG PY, MORGAN ET. Suppression of CYP2C11 gene transcription by interleukin-1 mediated by NF-kappaB binding at the transcription start site. Arch Biochem Biophys. 2000;377:187–194. doi: 10.1006/abbi.2000.1772. [DOI] [PubMed] [Google Scholar]
- 92.MORGAN ET, ULLRICH V, DAIBER A, et al. Cytochromes P450 and flavin monooxygenases--targets and sources of nitric oxide. Drug Metab Dispos. 2001;29:1366–1376. [PubMed] [Google Scholar]
- 93.ZHAO X, POLLOCK DM, INSCHO EW, ZELDIN DC, IMIG JD. Decreased renal CYP2C enzymes and impaired vasodilation are associated with salt-sensitive hypertension. Hypertension. 2003;41:709–714. doi: 10.1161/01.HYP.0000047877.36743.FA. [DOI] [PubMed] [Google Scholar]
- 94.GRANDALIANO G, GESUALDO L, RANIERI E, et al. Monocyte chemotactic peptide-1 expression in acute and chronic human nephritides: a pathogenic role in interstitial monocytes recruitment. J Am Soc Nephrol. 1996;7:906–913. doi: 10.1681/ASN.V76906. [DOI] [PubMed] [Google Scholar]
- 95.PRODJOSUDJADI W, GERRITSMA JS, ES LA, DAHA MR, BRUIJN JA. Monocyte chemoattractant protein-1 in normal and diseased human kidneys: an immunohistochemical analysis. Clin Nephrol. 1995;44:148–155. [PubMed] [Google Scholar]
- 96.CAPERS QT, ALEXANDER RW, LOU P, et al. Monocyte chemoattractant protein-1 expression in aortic tissues of hypertensive rats. Hypertension. 1997;30:1397–1402. doi: 10.1161/01.hyp.30.6.1397. [DOI] [PubMed] [Google Scholar]
- 97.ELMARAKBY AA, QUIGLEY JE, OLEARCZYK JJ, et al. Chemokine Receptor CCR2b Inhibition Provides Renal Protection in Angiotensin II-Salt Hypertension. Hypertension. 2007;00:000–000. doi: 10.1161/HYPERTENSIONAHA.107.098806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.NODE K, RUAN XL, DAI J, et al. Activation of Gαs mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem. 2001;276:15983–15989. doi: 10.1074/jbc.M100439200. [DOI] [PubMed] [Google Scholar]
- 99.NODE K, HUO Y, RUAN X, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.IMIG JD. Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovascular Drug Reviews. 2006;24:169–188. doi: 10.1111/j.1527-3466.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 101.IMIG JD, ZHAO X, CAPDEVILA JH, MORISSEAU C, HAMMOCK BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 102.ZHAO X, YAMAMOTO T, NEWMAN JW, et al. Soluble epoxide hydrolase inhibition protects the kidney from hypertension induced damage. J Am Soc Nephrol. 2004;15:1244–1253. [PubMed] [Google Scholar]
- 103.DIMITROPOULOU C, WEST L, FIELD MB, et al. Protein phosphatase 2A and Ca2+-activated K+ channels contribute to 11,12-epoxyeicosatrienoic acid analog mediated mesenteric arterial relaxation. Prostaglandins Other Lipid Mediat. 2007;83:50–61. doi: 10.1016/j.prostaglandins.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 104.BENSON SC, PERSHADSINGH HA, HO CI, et al. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]