Summary
Background
Vitamin D has a role in regulating immune function, and its deficiency is a suggested risk factor for childhood pneumonia. Our aim was to assess whether oral supplementation of vitamin D3 (cholecalciferol) will reduce the incidence and severity of pneumonia in a high-risk infant population.
Methods
We did a randomised placebo-controlled trial to compare oral 100 000 IU (2·5 mg) vitamin D3 with placebo given to children aged 1–11 months in Kabul, Afghanistan. Randomisation was by use of a computer-generated list. Vitamin D or placebo was given by fieldworkers once every 3 months for 18 months. Children presenting at the study hospital with signs of pneumonia had their diagnosis confirmed radiographically. Our primary outcome was the first or only episode of radiologically confirmed pneumonia. Our analysis was by intention to treat. This study is registered with ClinicalTrials.gov, number NCT00548379.
Findings
1524 children were assigned to receive vitamin D3 and 1522 placebo. There was no significant difference between the incidence of first or only pneumonia between the vitamin D (0·145 per child per year, 95% CI 0·129–0·164) and the placebo group (0.137, 0·121–0·155); the incidence rate ratio was 1·06 (95% CI 0·89–1·27). From 652 children during five separate periods of testing serum calcifediol, only one child in each of two testing periods had results greater than 375 nmol/L in the intervention group—a toxic level.
Interpretations
Quarterly bolus doses of oral vitamin D3 supplementation to infants are not an effective intervention to reduce the incidence of pneumonia in infants in this setting.
Funding
Wellcome Trust and British Council.
Introduction
Afghanistan has the third highest mortality of children younger than 5 years in the world (257 per 1000 livebirths); a leading cause of this is pneumonia. Two hospital-based case-control studies in Ethiopia1 and India2 suggest that vitamin D deficiency might substantially increase the risk of severe pneumonia in children. Our systematic review identified one randomised placebo controlled trial3 relating pneumonia or lower respiratory infections to vitamin D deficiency supplementation in young children. This trial of 100 000 IU (2·5 mg) vitamin D3 (cholecalciferol) supplementation along with antibiotic treatment of children younger than 3 years with clinically diagnosed pneumonia in Kabul showed no difference in the time to recovery between the groups. However, the risk of repeated pneumonia in the subsequent 3 months was 13% (relative risk [RR] 0·78, 95% CI 0·64–0·94; p=0·01) lower in the vitamin D3 compared with the placebo group.3 Other more recent studies assessing different outcomes in children or adults have conflicting findings on the effectiveness of vitamin D.
There is growing evidence of the vitamin D parancrine system enhancing innate immunity:4 the activation of Toll-like receptors on monocytes and macrophages by microbial pathogens results in increased expression of CYP27B1 and vitamin D receptor (VDR) genes within these cells. In the presence of adequate concentrations of calcifediol (25-hydroxyvitamin D), CYP27B1 leads to an increase synthesis of intracellular calcitriol (1,25-dihydroxyvitamin D). The binding of this to vitamin D receptors in turn results in increased intracellular formation of antimicrobial compounds, such as cathelicidins.5 The generation of intracellular antimicrobial compounds is impaired in patients who are vitamin D deficient (ie, low serum concentrations of calcifediol). It has been suggested that vitamin D might be used in the treatment of opportunistic and antibiotic-resistant infections.
There is debate between investigators regarding concentrations that relate to optimum stores of vitamin D in the body. In children, serum calcifediol concentrations should be maintained at 50 nmol/L (20 ng/mL) or greater for skeletal benefits.6 Vitamin D deficiency rickets in children younger than 5 years is an endemic problem in developing countries, ranging from 5–45%, even where sunlight is abundant,1,2,7,8 and has also re-emerged in some developed countries.7,9
In people, the main source of vitamin D is through the action of ultraviolet B radiation on 7-dehydrocholesterol in the skin, with small amounts derived from dietary sources. In Afghanistan, dietary intake of vitamin D is low10 and exposure of women to sunlight is limited by the widespread use of the burqa. Therefore, breastmilk, a normally poor source of vitamin D,11 becomes even more deficient. Furthermore, the common practice of swaddling infants might reduce exposure to sunlight.2 Thus young children are at a high risk of vitamin D deficiency. A cross-sectional survey during the high-risk winter months in 2005 showed that greater than 95% of children younger than 5 years in a socioeconomically deprived part of Kabul had serum calcifediol levels of less than 37·5 nmol/L.12
On the basis of findings that vitamin D is an immunomodulator, that deficiency is a risk factor for pneumonia, and that quarterly supplementation had better compliance than daily or weekly supplementation,13,14 we aimed to test the hypothesis that quarterly 100 000 IU vitamin D supplementation would reduce the incidence of radiologically confirmed pneumonia by 35% in our study population. Our secondary hypotheses were that supplementation would reduce all pneumonias (radiologically confirmed or clinically defined), repeat episodes of pneumonia, hospital admissions, and all cause mortality. We selected infants from inner-city Kabul because we knew the prevalence of vitamin D deficiency12 and incidence of pneumonia3,15 were high.
Methods
Participants
Between Nov 4 and Dec 4, 2008, we did a community based randomised placebo-controlled superiority trial in Kabul, Afghanistan, within the catchment area of the Maiwand Teaching Hospital, which serves an inner-city population. Follow up ended in May, 2009. The children we enrolled into our study largely came from five of the 18 socioeconomically deprived inner-city districts. We identified households with young children with detailed maps and advice from staff of the Agha Khan Trust for Culture, a non-governmental organisation working in the region. Furthermore, the study field-supervisors mapped the region independently to verify the accuracy of the maps of the Agha Khan Trust for Culture, and during recruitment they visited every house in the mapped region to identify families with young children.
20 pairs of female fieldworkers visited every home starting from streets closest to the hospital and radiating out until we reached our required sample size. Those homes where the carer or infant was absent were revisited again within 4 weeks of the recruitment period. Our inclusion criteria were infants aged 1–11 months and living in our study region. Our exclusion criteria were families expecting to move to another town within 18 months, diagnosis of rickets or treatment with vitamin D in the previous 3 months, and clinical diagnosis of Kwashiorkor or Marasmus. The fieldworkers obtained written informed consent from the mother, father, or another senior family member before recruitment; allocated the unique identification number (sequentially); and gave the first dose of vitamin D3 or placebo. Children with vomiting were excluded temporarily and enrolled 2 weeks later after recovery.
Thumbprint or signature consent was obtained from the child's parents (mother and father if in Kabul or another family member responsible for the child) at home if the child met our study criteria and after either the parent read the Dari consent form or it was explained to him or her by the fieldworker. Our study was approved by the ethics and review board of the Ministry of Public Health of Afghanistan (reference 422328; issued May 12, 2007) and the ethics committee of the London School of Hygiene and Tropical Medicine (application number 5117; issued May 29, 2008).
Randomisation and masking
An independent statistician (Shabbar Jaffar, London School of Hygiene and Tropical Medicine, London, UK) randomised unique identification numbers individually in fixed blocks of 20 to the vitamin D3 or placebo group by use of a random number generator with the SAS routine. By use of the randomisation list, a pharmacist in the Department of Pharmacy, Aga Khan University Hospital, Karachi prepared 100 000 IU (2·5 mg) of vitamin D3 (cholecalciferol) in olive oil (Sinochem Ningbo Laboratory, China) or placebo (olive oil) in sealed 2 mL plastic syringes labelled with the unique identification numbers. The vitamin D3 and the placebo were the same colour (pale yellow), taste, and quantity (0·5 mL) and therefore the study staff and the families did not know to which group the children were assigned. Fieldworkers allocated children to randomisation groups during recruitment and gave vitamin D or placebo.
Procedures
We chose a quarterly supplementation regimen because in routine programme conditions a quarterly regimen is likely to have better adherence than a daily regimen,13,14 and has been shown to be effective at maintaining the serum vitamin D concentration within normal ranges for 2–3 months in a French high-risk infant population.16 The syringes containing vitamin D or placebo were stored in conditions recommended by the manufacturer—a dry cool environment—between 2 and 24 weeks before administration.
We provided each child with an identification card, which included a photograph of the child, to enable easy access to outpatient and out-of-hours paediatric services at the study hospital. We encouraged families to bring their children to the study hospital for any illness for which quality treatment was offered free of charge. Four experienced paediatricians and 30 female fieldworkers (medical students, nurses, midwives, or community health workers) were rigorously trained in our study protocols, the Integrated Management of Childhood Illnesses strategy, and the assessment of signs and symptoms required by our study. The quality of clinical assessment in the clinics and during home visits was monitored weekly by study supervisors.
The fieldworkers followed up the children every 2 weeks until June, 2009, to obtain background information, assess illness (symptom history and examination of chest in-drawing, body temperature [Thermoval Classic Hartmann digital thermometer], signs of dehydration by skin pinching, respiratory rate count over 1 min with a stopwatch), and to refer to the study hospital if needed. Respiratory rate and anthropomorphic data were collected twice and we used the mean values in our analysis.
All children clinically diagnosed with pneumonia were offered free chest radiographs taken by radiographers trained by a WHO trainer. The masked radiographs were read by two independent paediatric radiologists (based at the Acute Respiratory Infections Unit of the Pakistan Institute of Medical Sciences), experienced in reading paediatric chest radiographs from WHO vaccine trials, with WHO proformas for standardised interpretation of paediatric chest radiographs for the diagnosis of pneumonia.17 In case of disagreement, radiographs were read by a third independent radiologist and most decisions were accepted. We ascertained causes of death through scrutiny of hospital notes, and verbal autopsy interviews with the WHO standard questionnaire and review of the interview data by two physicians independently.
We defined clinical pneumonia as a history of cough plus increased respiratory rate for age, chest in-drawing, or any danger sign (ie, not drinking or breastfeeding, convulsion, vomiting, lethargic or unconscious, stridor in a calm child). We defined severe pneumonia as cough plus chest in-drawing and very severe diseases as cough plus any danger signs. Our primary endpoint was the first episode of pneumonia from the time of enrolment confirmed by chest radiograph (consolidation or infiltrates). We defined as a new episode of pneumonia an episode happening 15 days or longer after the first. We judged an episode happening within 14 days to be continuation of the previous episode.
We collected venous blood samples from randomly selected blocks of children on the basis of the assumption that each block had an equal number of children from the vitamin D3 and placebo groups. This was to ascertain calcifediol serum concentrations 1 week after giving the first dose in the placebo group (a baseline value for the whole population) as well as early concentrations after the first vitamin D supplementation in the intervention group (70 in placebo and 69 in intervention groups). We also collected samples at other times (appendix) from a different set of randomly selected children to check fluctuations in serum concentrations. We stored the serum samples at −20°C and analysed them at the end of our study with IDS-iSYS Multi-Discipline Automated Chemiluminescent assay (Immunodiagnostic Systems Ltd, Tyne and Wear, UK) at the Manchester Royal Infirmary, Manchester, UK (Supra-Regional Vitamin D Reference Laboratories accredited to ISO9001:2000 and ISO13485:2003 and participating in the Vitamin D Quality Assurance Scheme).
Statistical analysis
We assumed that the incidence of the first or the only episode of pneumonia (first episode) in our placebo group would be 0·0585 per child per year based on the report that the incidence of acute lower respiratory infections in developing countries was greater than 0·65 per child per year,18 that 12% of these episodes were pneumonia, and 75% were first episodes. Given that 73% of children had vitamin D deficiency in the study region in 2005,12 and that the incidence of pneumonia was ten-times higher in vitamin D deficient children than in children without a deficiency in case-control studies1,2 we postulated that there would be at least a 35% reduction in pneumonia incidence in the vitamin D compared with the placebo group. Thus a study with 80% power and 95% significance needed 22 079 child-months per group (total of 2454 children for 18 months follow-up). Assuming a 20% loss to follow-up and allowing for protocol violations we intended to recruit 3050 children for 18 months follow-up.
Data were entered into a Microsoft Access database (version 2007) and data processing and analysis was done in STATA (version 11.0). We compared baseline characteristics and the distribution of our prestated confounders for intervention and placebo groups. To assess the potential problem of multicollinearity we used Pearsons correlation coefficient or Cramers V (for paired categorical variables). If a child was not seen for more than 45 days, at the two weekly visits, or at the hospital, they were censored for that period of our study. These children could re-enter the study when next seen. We calculated person-time at risk for each child up to the date a child reached our primary endpoint, was last seen at the end of our study, or when censored because they were lost to follow-up. For our intention-to-treat analysis we included all children randomly assigned to our study groups. For our per-protocol analysis we included children who in both groups received all doses with an interval between the doses of 60 and 120 days and had not violated the randomisation codes. We analysed the repeat episodes accounting for clustering within individuals. We made initial comparisons of time-to-an-episode between the two groups with log-rank tests and Kaplan–Meier plots. We estimated the incidence rate ratio (RR) for the episodes of pneumonia with Cox proportional hazard models. We assessed violation of the proportionality assumption with Schoenfeld residuals. This study is registered with ClinicalTrials.gov, number NCT00548379.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Figure 1 shows the trial profile. By the end of our trial 2616 of the 3046 recruited children were present in our study and 17 had died. The mortality rate in our study cohort was 4·2 per 1000 child years (95% CI 2·4–6·7): 4·2 per 1000 child years (2·4–6·7; ten cases) in our vitamin D group and 3·4 per 1000 child years (1·3–7·1; seven cases) in our placebo group. The difference was not statistically significant, although our study was not powered to detect differences in death rate. Six children in the vitamin D and four in the placebo group died of septicaemia or pneumonia. The remaining seven deaths were due to accidental or congenital causes. We did not record any immediate or other adverse events associated with vitamin D3 supplementation.
Figure 1.
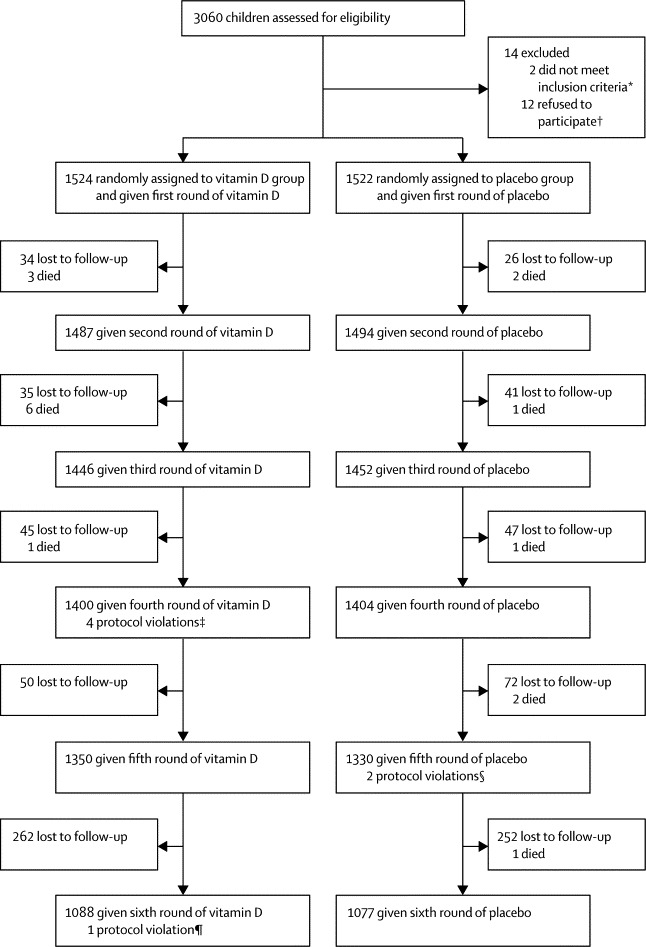
Trial profile
*One case was a severely malnourished child and another had received high-dose vitamin D within the past 3 months. †Did not want to be enrolled in the project for reasons such as did not want to go to Maiwand hospital, did not want the fieldworkers to come to their house, and other with no clear reasons. ‡The syringe with code number of 2706 was given to the child with study number of 2707, with 1070 to 1084, and with 2393 to 2939. §The syringe with code number of 1418 was given to the child with study number of 1814 and with 2335 to 2235. ¶The syringe with code number of 2215 was given to the child with study number of 2224.
The number of children lost to follow-up was low and many children lost at one time because of travel rejoined the study later with rates being similar between the two groups (figure 1). There was no statistically significant difference in any of the baseline characteristics between the groups (table 1), including reported sun exposure. Children that remained in our study had equally balanced characteristics. We did not identify any correlations between baseline variables.
Table 1.
Baseline characteristics of trial children and their families
| Vitamin D group (N=1524) | Placebo group (N=1522) | |
|---|---|---|
| Sex of child | ||
| Male | 811 (53%) | 780 (51%) |
| Female | 713 (47%) | 742 (49%) |
| Child age at recruitment (months) | ||
| <2 | 132 (9%) | 111 (7%) |
| 2–5 | 510 (34%) | 537 (35%) |
| 6–12 | 882 (58%) | 874 (57%) |
| Malnutrition (weight for age Z score) | ||
| −1 or greater | 812 (53%) | 832 (55%) |
| −2 or greater to less than −1 | 415 (27%) | 399 (26%) |
| −3 or greater to less than −2 | 183 (12%) | 195 (13%) |
| Less than −3 | 87 (6%) | 77 (5%) |
| Missing | 27 (2%) | 19 (1%) |
| Breastfeeding at recruitment | ||
| Yes | 894 (60%) | 902 (61%) |
| No | 589 (40%) | 580 (39%) |
| Ever breastfed | ||
| Yes | 1463 (99%) | 1457 (98%) |
| No | 22 (2%) | 28 (2%) |
| Reported child sun exposure up to 2 h | ||
| Everyday | 473 (35%) | 459 (34%) |
| Greater than 2 days per week | 315 (23%) | 344 (26%) |
| Rarely | 558 (42%) | 547 (41%) |
| Maternal age at recruitment (years) | ||
| Less than 20 | 98 (6%) | 111 (7%) |
| 20–39 | 1381 (91%) | 1352 (89%) |
| 40 or greater | 44 (3%) | 58 (4%) |
| Maternal years of formal education | ||
| None | 951 (62%) | 993 (65%) |
| 1–6 | 272 (18%) | 256 (17%) |
| 7–9 | 301 (20%) | 272 (18%) |
| 10–12 and higher education | 951 (62%) | 993 (65%) |
| Marital status | ||
| Married | 1521 (100%) | 1519 (100%) |
| Widowed, separated, or divorced | 3 (0%) | 3 (0%) |
| Paternal years of formal education | ||
| None | 439 (29%) | 449 (30%) |
| 1–6 | 279 (18%) | 263 (17%) |
| 7–9 | 250 (16%) | 256 (17%) |
| 10–12 and higher education | 514 (34%) | 541 (36%) |
| Number of indoor smokers in household | ||
| 0 | 900 (66%) | 889 (65%) |
| 1 or more | 459 (30%) | 476 (31%) |
| Indoor air pollution* | ||
| High pollution | 649 (48%) | 668 (49%) |
| Medium pollution | 350 (26%) | 332 (24%) |
| Low or no pollution | 360 (27%) | 365 (27%) |
| Number of people sleeping per room | ||
| Two or fewer | 223 (15%) | 238 (16%) |
| Three to four | 508 (34%) | 519 (35%) |
| More than four | 627 (42%) | 601 (40%) |
| Father's ethnic origin (family ethnic origin) | ||
| Tajik | 1063 (70%) | 1074 (71%) |
| Pashton | 352 (23%) | 336 (22%) |
| Uzbek | 27 (2%) | 21 (1%) |
| Hazara | 62 (4%) | 74 (5%) |
| Other | 19 (1%) | 16 (1%) |
| Do not know | 1 (0%) | 1 (0%) |
| Socioeconomic status† | ||
| Poorest | 254 (17%) | 235 (16%) |
| Very poor | 226 (15%) | 227 (15%) |
| Less poor | 226 (15%) | 247 (16%) |
| Least poor | 262 (17%) | 266 (18%) |
Data are n (%).
On the basis of the type of heating device, the fuel used for heating in the winter, and hours the heating devices were used on average in 24 h in the past week.
Based on principle component analysis by use of the household assets.
On intention-to-treat analysis, the incidence of the first episode of pneumonia was greater in the vitamin D rather than the placebo group, although this difference was not statistically significant (table 2). The incidence rate of repeat episodes of all type pneumonia was significantly higher in the vitamin D group (table 3). Restricting the definition of pneumonia confirmed with chest radiography to consolidation only, gave a rate of 0·011 per child per year (95% CI 0·008–0·017) in the vitamin D group and 0·008 (0·005–0·013) in the placebo group; this difference was not significant RR 1·44 (0·76–2·72). Extending the time between two consecutive episodes of pneumonia to 30 days to define a new repeat episode of pneumonia (used by the WHO Vaccine Trials group),19 gave similar results (data not shown).
Table 2.
Incidence rate of first or the only episode of pneumonia (intention-to-treat analysis)
|
Vitamin D group |
Placebo group |
Incidence rate ratio (95% CI) | p value | |||||
|---|---|---|---|---|---|---|---|---|
| Episode | Person-years at risk | Incidence rate (95% CI) | Episode | Person-years at risk | Incidence rate (95% CI) | |||
| Confirmed by chest radiograph | ||||||||
| All pneumonia | 260 | 1782 | 0·145 (0·129–0·164) | 245 | 1782 | 0·137 (0·121–0·155) | 1·065 (0·895–1·268) | 0·476 |
| Simple pneumonia | 230 | 1812 | 0·126 (0·111–0·144) | 219 | 1814 | 0·120 (0·105–0·137) | 1·055 (0·877–1·270) | 0·566 |
| Severe pneumonia | 45 | 1989 | 0·022 (0·016–0·030) | 40 | 1990 | 0·020 (0·014–0·027) | 1·127 (0·736–1·726) | 0·579 |
| Very severe diseases | 13 | 2021 | 0·006 (0·003–0·011) | 9 | 2018 | 0·004 (0·002–0·008) | 1·449 (0·619–3·391) | 0·389 |
| Confirmed and unconfirmed by chest radiograph | ||||||||
| All pneumonia | 1023 | 740 | 1·382 (1·299–1·469) | 1030 | 705 | 1·460 (1·373–1·552) | 0·953 (0·874–1·039) | 0·274 |
| Simple pneumonia | 1012 | 817 | 1·238 (1·164–1·317) | 997 | 791 | 1·259 (1·183–1·340) | 0·985 (0·903–1·075) | 0·748 |
| Severe pneumonia | 160 | 1847 | 0·086 (0·074–0·101) | 149 | 1854 | 0·080 (0·068–0·094) | 1·080 (0·864–1·350) | 0·499 |
| Very severe diseases | 151 | 1899 | 0·079 (0·067–0·093) | 167 | 1895 | 0·088 (0·075–0·102) | 0·903 (0·724–1·125) | 0·364 |
The number of child-months for those receiving at least four consecutive 3-monthly doses was 22 502 for the vitamin D group and 22 018 for the placebo group.
Table 3.
Person-time at risk and incidence rate of repeat episodes of pneumonia from all sources (intention-to-treat analysis)
|
Vitamin D group |
Placebo group |
Incidence rate ratio (95% CI) | p value | |||||
|---|---|---|---|---|---|---|---|---|
| Episode | Person-years at risk | Incidence rate (95% CI) | Episode | Person-years at risk | Incidence rate (95% CI) | |||
| Confirmed by chest radiograph | ||||||||
| All pneumonia | 138 | 2031 | 0·065 (0·055–0·078) | 82 | 2027 | 0·040 (0·032–0·050) | 1·685 (1·282–2·212) | <0·0001 |
| Simple pneumonia | 99 | 2031 | 0·047 (0·038–0·057) | 51 | 2027 | 0·025 (0·019–0·033) | 1·883 (1·341–2·644) | <0·0001 |
| Severe pneumonia | 10 | 2031 | 0·004 (0·002–0·009) | 8 | 2027 | 0·003 (0·001–0·007) | 1·252 (0·494–3·173) | 0·63 |
| Very severe diseases | 0 | 2031 | 0 | 0 | 2027 | 0 | NA | NA |
| Confirmed and unconfirmed by chest radiograph | ||||||||
| All pneumonia | 2338 | 2029 | 1·152 (1·106–1·199) | 2200 | 2025 | 1·086 (1·042–1·132) | 1·061 (1·001–1·125) | 0·04 |
| Simple pneumonia | 1945 | 2029 | 0·958 (0·916–1·001) | 1825 | 2025 | 0·901 (0·861–0·943) | 1·064 (0·998–1·134) | 0·05 |
| Severe pneumonia | 50 | 2029 | 0·024 (0·018–0·032) | 54 | 2025 | 0·026 (0·020–0·035) | 0·922 (0·627–1·354) | 0·68 |
| Very severe diseases | 43 | 2029 | 0·021 (0·015–0·028) | 38 | 2025 | 0·018 (0·013–0·025) | 1·126 (0·728–1·742) | 0·59 |
NA=not applicable.
The proportion of children without an episode of pneumonia during our 18 month study did not differ between the groups (figure 2). The incidence of first episodes of simple, severe or very severe pneumonias confirmed with chest radiography also did not differ (table 1, appendix). There were very few protocol violations and the results of our per-protocol and post-hoc analyses for children getting four and five consecutive doses were not different from that of our intention-to-treat analysis (table 2, 3; appendix) A further post-hoc analysis of the pneumonia rates by season also did not reveal any difference between the groups in our intention-to-treat or per-protocol analyses (data not shown).
Figure 2.
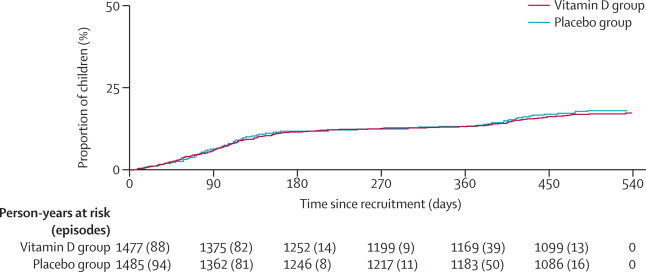
Proportion of children with a first episode of pneumonia over time
The mean serum calcifediol concentration was consistently and significantly higher in the vitamin D group than in the placebo group at 1 week, 4 weeks, and 3 months after administration of the first dose of vitamin D during the winter and at 2 weeks' post administration of dose 3 (spring; figure 3). One child in the first sample (1 week after the first dose in November, 2008) and one in the spring sample (a month after the second dose) of the intervention group had results greater than 375 nmol/L—a toxic level.6
Figure 3.
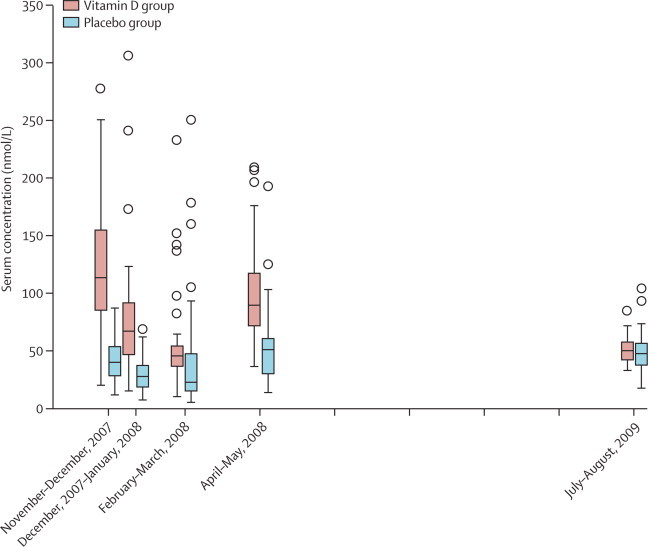
Boxplot of serum calcifediol concentration
Vertical lines represent the time of supplementation, and each placebo and vitamin D pair represent a sampling time. Boxes are medians with IQRs. Circles represent outliers. For November and December, 2008, p<0·001; for February, 2008, p=0·058; for May, 2008, p<0·001; and for July, 2009, p>1·0. One child in the vitamin D group in January, 2008, and one in May, 2008, had serum concentrations greater than 375 nmol/L (not shown).
Discussion
Our trial in a population known to be vitamin D deficient shows that 100 000 IU (2·5 mg) of vitamin D3 supplementation once every 3 months did not affect the incidence or severity of childhood pneumonia, in this population. Similarly, vitamin D3 supplementation did not affect hospital admissions and all-cause mortality.
Our trial had a large sample size with a low loss to follow-up that remained within our sample size estimates. The ascertainment of study outcomes was robust, and done clinically through masked experienced and retrained paediatricians with Integrated Management of Childhood Illnesses clinical definitions of pneumonia applied in other trials18 and radiologically through the interpretation of chest radiographs by masked, experienced, and external readers by use of WHO standards. Frequent home visits confirmed outcomes of interest. Supplementation raised the serum concentration of calcifediol in the intervention group at several points in our study. Thus the lack of a protective effect of vitamin D against pneumonia is probably not due to misclassification of outcomes or inadequate supplementation. Any misclassification of other causes of raised respiratory rate as pneumonia, under-reporting, or over-reporting would be distributed equally between the two groups since the families, doctors, radiographers, radiologists, and data entry staff were masked to the randomisation groupings and the randomisation was successful since the characteristics were balanced between the two groups. However, a limitation due to the high-risk nature of our population is that the generalisability of our findings to populations with low-to-moderate risk of vitamin deficiency is unknown. There were fewer deaths than we predicted in both groups, possibly due to the intense health surveillance possible during our study. Genotyping, which was not practical in our study setting, might have aided the interpretation of our findings, as shown in a vitamin D supplementation trial for tuberculosis.20
We accounted for nutritional factors through a diet questionnaire, weight at recruitment, and growth comparison at the end of our study—any nutritional deficiencies would have been equally balanced between the two groups. As with other studies, dietary calcium deficiency might have led to so-called wastage of body stores of vitamin D in both groups, affecting the maintenance of even higher vitamin D levels in the intervention group. Nevertheless, there was a higher (adequate level) mean serum concentration of calcifediol at the end of first supplementation period in the intervention group compared with the lower (not adequate) mean concentrations in the placebo group (data not shown). Furthermore, adult fracture-outcome supplementation studies21 show no benefit from adding calcium.
A further limitation of our study is that it was not feasible to undertake biochemical assessment of the supplements or assess serum concentrations in all participants after each and between supplementation. Our sample testing suggest that the vitamin D group maintained above mean adequate vitamin D concetrations verses consistently low concentrations in the placebo group. It can be argued that a steady-state adequate vitamin D concentration was not reached in some children because of fluctuations in serum concentrations due to our study regimen. Although this is probably true, a sufficient serum concentrations for the immunological effects of vitamin D is unknown and could be higher or lower than that of the skeletal system accepted concentrations.6 The need for a steady state is currently highly speculative and relates to vitamin D2 supplementation for negative cancer22 and bone outcomes.23
Our findings are at odds with smaller case-control hospital studies that show an enhanced rate of vitamin D deficiency or rickets in children with pneumonia and the increasing evidence suggesting that calcitriol, the biologically active metabolite of vitamin D, has an important role in the human immune system.4 A systematic review of the role of vitamin D supplementation in infectious diseases had mixed findings, concluding that more rigorously designed clinical trials are needed (panel).25 No studies report the effect of vitamin D on radiologically confirmed pneumonias. Other trials assessing infections of the upper respiratory tract also had mixed findings: one trial in post-menopausal women26 identified reduced self-reported episodes of infection of the upper respiratory tract, specifically in winter, whereas two others in elderly24 and general adults27 did not identify a difference between the groups with regards to self-reported episodes. One trial in schoolchildren28 (measuring influenza A antigen from swabs) found a statistically significant reduced incidence in the intervention group (RR 0·58, 95% CI 0·34–0·99).
Panel. Research in context.
Systematic Review
We searched PubMed/Medline with the terms “vitamin D supplement*”, “pneumonia”, “respiratory infections”, “immune*”, “cancer”, “heart disease”, and “diabetes”, supplemented with secondary citation (ie, assessing the references in the identified reports for more references). We did not limit our searches by language or date. No studies reported on radiologically confirmed pneumonias. One masked placebo trial assessed the effect of bolus supplementation of vitamin D3 in a high-risk population clinically diagnosed with pneumonia (100 000 IU [2·5 mg] vitamin D3 [cholecalciferol] supplementation along with antibiotic treatment to children younger than 3 years) in Kabul. Treatment had no effect on the time to recovery but reduced the risk of clinically defined pneumonia 13% up to 3 months after treatment.3 Trials assessing infections of the upper respiratory tract had mixed findings: one trial in post-menopausal women24 identified a reduction in self-reported episodes of infections during winter, whereas two other studies in adults did not identify effects on self-reported episodes. One trial in schoolchildren noted a 42% reduction in the risk of influenza A by use of antigen titre as a proxy measure. The reported in-vitro and case-control studies suggest a link between respiratory infections and vitamin D as an immunoregulator, but the evidence on the effect of vitamin D supplementation on acute respiratory infections is conflicting. Given that pneumonia is a leading cause of mortality in young children, the effects of vitamin D supplementation to infants on the incidence of pneumonia merits investigation.
Interpretation
Our findings show that bolus quarterly supplementation of 100 000 IU vitamin D3 did not reduce the rate or severity of radiologically confirmed or clinically defined pneumonia. This finding is surprising in view of evidence suggesting an association between vitamin D deficiency and respiratory infections. However, our findings are similar to some previous studies on the effect of supplementation on infections of the upper respiratory tract or other outcomes such as bone fractures, cancers, and heart disease, and they contribute to the debate on factors that determine the immunological effectiveness of vitamin D supplementation.
The benefits of supplementation for tuberculosis are similarly unclear. One paediatric trial showed 16% higher rates of tuberculosis symptom resolution,29 an adult trial showed 23% greater sputum conversion rate at 6 months,30 another trial showed 20% greater restriction of BCG-lux growth in blood,31 another trial found accelerated sputum conversion only in a certain genotype of the sample,20 whereas yet another found no significant difference in sputum conversion32 in the intervention compared with the placebo group. Vitamin D is linked with other non-bone health outcomes, but the few completed and adequately powered trials assessing the effect of supplementation have mixed findings in improving outcomes—ie, cancers, vascular disease, and diabetes. As in our studies, these inconsistent findings point to the existence of complicated pathways for the function of vitamin D in disease processes, or simply that the correct effective dose of vitamin D supplementation needs to be better defined. The optimum concentrations of vitamin D for adults and children6 are based on non-immunological and, mostly, bone outcomes. An associated issue is that, after the start of our trial, speculations arose that high fluctuations of natural or supplemented vitamin D intake might be linked to negative outcomes. These speculations relate two studies in which supplementation of a single large dose (300 00033 or 500 00023 IU) of vitamin D for reducing fractures in elderly women led to an increase in fractures, plus similar discrepancies in prostate cancer research with vitamin D.22 Our findings and particularly the slightly higher repeat episodes of pneumonia in the vitamin D group could be due to such fluctuations if the hypothesis is true. If so, then in terms of policy and guidelines, it is hard to know what regimen to recommend for future trials, given that clinical practice and evidence suggests that daily or weekly supplementations though ideal are not complied with13,14 and are impractical for families, especially in developing countries, and food fortification at the population level will not be an option during exclusive breastfeeding periods (aged <6 months), nor is it likely to be adequate in highly deficient populations.
Alternately, it might be that immunological effectiveness of supplementation is highly dependent upon other factors as yet unidentified, or other prevalent factors in this high-risk population (for example, other malnutrition factors and that 5·5% of the children in our study had a Z score of weight for age below −3). Further questions arise with respect to the previous treatment trial in the same region of Kabul, which showed that a single high-dose supplementation with 100 000 IU vitamin D3 given orally to young children, along with antibiotic treatment at the time of diagnosis, reduced repeat episodes of pneumonia in the subsequent 3 months.3 The inconsistent results could be related to the evidence that stimulation of Toll-like receptors on macrophages and monocytes by bacterial antigens is important in up-regulation of CYP27B1 and the vitamin D receptor,5 which are crucial in production of cathelicidins in vitamin-D-depleted individuals. In other words, this system might not be effective unless there is existing infection with pathogens when vitamin D supplementation is given. Finally, any possible reduction in the incidence of pneumonia in our trial might be restricted to only certain genotypes as recorded in a single tt genotype after the start of our trial in a study of tuberculosis.20 Both the pneumonia treatment and the population supplementation trials need to be repeated in other settings for confirmation, and frequent determination of serum calcifediol concentrations might guide the interpretation of the findings.
As with the trials in the older children, giving higher doses of vitamin D,25,34 we too did not record any adverse effects associated with vitamin D supplementation. However, the number of children and the length of follow-up involved in these studies could only detect common and early adverse effects. Although vitamin D overload is a theoretical possibility, 100 000 IU of vitamin D3 has been shown to provide the best protection against vitamin D deficiency without overload or side-effects in high-risk infants aged 0–9 months with normal baseline ranges of vitamin D.16 Furthermore, single intramuscular injection of three times this dose (300 000 IU) was safe in treating nutritional rickets in 6–30-month-old children residing in lower socioeconomic regions of sunny Istanbul.35 Higher than recommended doses of daily supplementation of vitamin D (500–1000 IU per day, adding up to 120 000 IU over 3 months) plus additional milk fortified with vitamin D, has been shown not to induce an overload,13 including in infants starting with normal ranges of vitamin D, even when supplementation continued during the summer (in France) and when mothers had antenatal vitamin D supplementation.36 It is important to note that in our study, where community supplementation was in a population with known high rates of vitamin D deficiency, 1% of children tested had a calcifediol concentration greater than 375 nmol/L—thought undesirably high.6 Although these did not produce any clinical signs and symptoms, these measurements raise a need for caution with doses for population supplementation. The speculative negative role of fluctuations is vitamin D needs to be considered with caution until further evidence is available.
We have shown that a quarterly 100 000 IU supplementation of vitamin D3 did not reduce the incidence of pneumonias in children living in regions with high vitamin D deficiency. In populations with similar characteristics, quarterly population level supplementation of vitamin D to infants and young children is not a policy option for reducing the burden of pneumonia in children.
Acknowledgments
Acknowledgments
We thank all the participating families in this study, the project field staff, especially the female Afghan fieldworkers (particularly supervisors Drs Ayob, Adel, and Khalid), the paediatricians (Drs Khesraw, Wali, Rashid, Mojahid, and Sadeq Faqiree), Mr Msjedi, and the radiology and outpatient departments. We thank Aga Khan Health Services Afghanistan staff, Dr Alawi and Dr Shams from the Ministry of Public Health, the Dean of the Medical School of Kabul, and Dr Kohdamani, the Director of the Maiwand Hospital. We acknowledge Latif Sheikh and Shamim Reza from the Pharmacy Department of the Aga Khan University Hospital, Karachi for providing the vitamin D and placebo, Arnold Rillera for training the radiographers and advising on radiographic techniques, Tabish Hazir and the ARC team at the Pakistan Institute of Medical Sciences, Islamabad, for reading the radiographs, Christian Diering from the German Medical Diagnostic Centre Ltd for processing and storing the blood samples, and J Jacqueline Berry from the Manchester Royal Infirmary for analysing the blood samples. We thank the Wellcome Trust and British Council Delphi programme for funding the study and USAID Afghanistan, and Washington States University (WSU) for providing a scholarship to ZM for the completion of his PhD based on this study.
Contributors
DC and SMH were co-principal investigators and were involved in the design, implementation, analysis, and reporting of the study. ZM was the trial manager, and managed the implementation of the study, analysed the results, and was involved in the writing of the report. JB was the trial statistician and involved in all aspects of statistics, analysis, and writing of the report. MIM was the trial Afghan collaborator and was involved in the design and implementation of the trial and the write up of the report. ZM, ZAB, and GW were all experts and involved in the design, interpretation, and reporting of the results, and advised throughout on methods and analysis.
Conflicts of interest
We declare that we have no conflicts of interest.
Supplementary Material
References
- 1.Muhe L, Lulseged S, Mason KE, Simoes EA. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349:1801–1804. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- 2.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 3.Manaseki-Holland S, Qader G, Isaq Masher M. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health. 2010;15:1148–1155. doi: 10.1111/j.1365-3156.2010.02578.x. [DOI] [PubMed] [Google Scholar]
- 4.White HJ. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76:3837–3843. doi: 10.1128/IAI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu PT, Stenger S, Li H. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 6.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 7.Pettifor JM. Vitamin D &/or calcium deficiency rickets in infants & children: a global perspective. Indian J Med Res. 2008;127:245–249. [PubMed] [Google Scholar]
- 8.Fraser DR. Vitamin D-deficiency in Asia. J Steroid Biochem Mol Biol. 2004;89–90:491–495. doi: 10.1016/j.jsbmb.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 9.Ward LM, Gaboury I, Ladhani M, Zlotkin S. Vitamin D-deficiency rickets among children in Canada. CMAJ. 2007;177:161–166. doi: 10.1503/cmaj.061377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tchibindat F, Ghafoori A, Maroof Z, Hedayatullah S, Qulam R. Care practices formative research: Ghozarah district community, Herat, Afghanistan. UNICEF and MOH Afghanistan; Herat: 2003. [Google Scholar]
- 11.Dawodu A, Agarwal M, Hossain M, Kochiyil J, Zayed R. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in summer: a justification for vitamin D supplementation of breast-feeding infants. J Pediatr. 2003;142:169–173. doi: 10.1067/mpd.2003.63. [DOI] [PubMed] [Google Scholar]
- 12.Manaseki-Holland S, Zulf Mughal M, Bhutta Z, Qasem Shams M. Vitamin D status of socio-economically deprived children in Kabul, Afghanistan. Int J Vitam Nutr Res. 2008;78:16–20. doi: 10.1024/0300-9831.78.1.16. [DOI] [PubMed] [Google Scholar]
- 13.Vervel C, Zeghoud F, Boutignon H, Tjani JC, Walrant-Debray O, Garabédian M. Fortified milk and supplements of oral vitamin D. Comparison of the effect of two doses of vitamin D (500 and 1000 UI/d) during the first trimester of life. Arch Pediatr. 1997;4:126–132. doi: 10.1016/s0929-693x(97)86154-4. (in French). [DOI] [PubMed] [Google Scholar]
- 14.Gallo S, Jean-Philippe S, Rodd C, Weiler HA. Vitamin D supplementation of Canadian infants: practices of Montreal mothers. Appl Physiol Nutr Metab. 2010;35:303–309. doi: 10.1139/H10-021. [DOI] [PubMed] [Google Scholar]
- 15.UNICEF . Multiple indicator cluster survey 2003, Afganistan. Central Statistics Office Afghanistan Transitional Authority and UNICEF; Kabul: 2004. [Google Scholar]
- 16.Zeghoud F, Ben-Mekhbi H, Djeghri N, Garabédian M. Vitamin D prophylaxis during infancy: comparison of the long-term effects of three intermittent doses (15, 5, or 2·5 mg) on 25-hydroxyvitamin D concentrations. Am J Clin Nutr. 1994;60:393–396. doi: 10.1093/ajcn/60.3.393. [DOI] [PubMed] [Google Scholar]
- 17.WHO Pneumonia Vaccine Trial Investigators' Group . Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children (WHO/V&B/01.35) WHO Department of Vaccine and Biologicals; Geneva: 2001. [Google Scholar]
- 18.Rudan I, Tomaskovic L, Boschi-Pinto C, Campbell H. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ. 2004;82:895–903. [PMC free article] [PubMed] [Google Scholar]
- 19.Enwere G, Cheung YB, Zaman SM. Epidemiology and clinical features of pneumonia according to radiographic findings in Gambian children. Trop Med Int Health. 2007;12:1377–1385. doi: 10.1111/j.1365-3156.2007.01922.x. [DOI] [PubMed] [Google Scholar]
- 20.Martineau AR, Timms PM, Bothamley GH. High-dose vitamin D3 during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2010;377:242–250. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bischoff-Ferrari HA, Willett WC, Wong JB. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169:551–561. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 22.Vieth R. How to optimize vitamin D supplementation to prevent cancer, based on cellular adaptation and hydroxylase enzymology. Anticancer Res. 2009;29:3675–3684. [PubMed] [Google Scholar]
- 23.Sanders KM, Stuart AL, Williamson EJ. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 24.Avenell A, Cook JA, Maclennan GS, Macpherson GC. Vitamin D supplementation to prevent infections: a sub-study of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438) Age Ageing. 2007;36:574–577. doi: 10.1093/ageing/afm091. [DOI] [PubMed] [Google Scholar]
- 25.Yamshchikov AV, Desai NS, Blumberg HM, Ziegler TR, Tangpricha V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract. 2009;15:438–449. doi: 10.4158/EP09101.ORR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aloia JF, Talwar SA, Pollack S, Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med. 2005;165:1618–1623. doi: 10.1001/archinte.165.14.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li-Ng M, Aloia JF, Pollack S. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect. 2009;137:1396–1404. doi: 10.1017/S0950268809002404. [DOI] [PubMed] [Google Scholar]
- 28.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 29.Morcos MM, Gabr AA, Samuel S. Vitamin D administration to tuberculous children and its value. Boll Chim Farm. 1998;137:157–164. [PubMed] [Google Scholar]
- 30.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38:3–5. [PubMed] [Google Scholar]
- 31.Martineau AR, Wilkinson RJ, Wilkinson KA. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 32.Wejse C, Gomes VF, Rabna P. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179:843–850. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 33.Smith H, Anderson FH, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women—a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford) 2007;46:1852–1857. doi: 10.1093/rheumatology/kem240. [DOI] [PubMed] [Google Scholar]
- 34.Rehman PK. Sub-clinical rickets and recurrent infection. J Trop Pediatr. 1994;40:58. doi: 10.1093/tropej/40.1.58. [DOI] [PubMed] [Google Scholar]
- 35.Kutluk G, Çetinkaya F, Banak M. Comparisons of oral calcium, high dose vitamin D and a combination of these in the treatment of nutritional rickets in children. J Trop Pediatr. 2002;48:351–353. doi: 10.1093/tropej/48.6.351. [DOI] [PubMed] [Google Scholar]
- 36.Zeghoud F, Vervel C, Guillozo H, Walrant-Debray O, Boutignon H, Garabédian M. Subclinical vitamin D deficiency in neonates: definition and response to vitamin D supplements. Am J Clin Nutr. 1997;65:771–778. doi: 10.1093/ajcn/65.3.771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


