Abstract
Study Design
An in vitro study using ovine intervertebral discs to correlate the effects of advanced glycation end-products (AGEs) with disc hydration evaluated by magnetic resonance imaging (MRI).
Objective
To determine the relationship between the level of AGEs and tissue water content in intervertebral discs using T2 relaxation MRI.
Summary of Background Data
AGEs result from nonenzymatic glycation, and AGEs have been shown to accumulate in the IVD tissue with aging and degeneration. AGEs can alter biochemical properties, including the hydrophobicity of the extracellular matrix. Since one of the degenerative signs of the IVD is the reduced hydration, it was hypothesized that increased levels of tissue AGEs may contribute to disc hydration. T2 relaxation MRI has been shown to be sensitive to the hydration status of the disc, and may be valuable in detecting the changes in the IVD mediated by the increase of AGEs.
Methods
Thirty-eight IVDs were obtained from 4 ovine spines, and the annulus fibrosis (AF) and nucleus pulposus (NP) tissues were isolated from these discs. The tissues were incubated in either a ribosylation or control solution for up to 8 days to induce the formation of AGEs. These tissues were subsequently analyzed for tissue water content and concentration of AGEs. T2 relaxation times were obtained from these tissues after ribosylation.
Results
Ribosylation led to the increased accumulation of AGEs and reduced water content in both the AF and NP in a dose-dependent manner. When analyzed by MRI, ribosylation significantly altered the mean T2 relaxation times in the NP (p=0.001), but not in the AF (p=0.912). Furthermore, the mean T2 values in the NP significantly decreased with increasing periods of incubation time (p<0.001).
Conclusion
This study demonstrates that levels of AGEs in the IVD may affect the tissue water content. Moreover, these ribosylation-mediated changes in tissue hydration were detectable using T2 relaxation MRI. T2 relaxation MRI may provide a non-invasive tool to measure in vivo changes in disc hydration that are negatively correlated with the accumulation of AGEs.
Keywords: Non-enzymatic glycation, advanced glycation end products, magnetic resonance imaging, intervertebral disc, degenerative disc disease
Introduction
Degenerative disc disease is a major public health problem in the United States with significant economic and social costs [1,2]. Degeneration of the intervertebral disc (IVD) is characterized by a loss of cellularity, degradation of extracellular matrix, and dehydration of disc tissue leading to the loss of structural integrity in the functional spine segment [3]. Advanced glycation end-products (AGEs) are formed through the non-enzymatic glycation (NEG) of amino residues and oxidation of fatty acids [4,5]. Tissues that undergo relatively low levels of biological turnover containing long-lived structural proteins, such as collagen, are known to accumulate AGEs [5]. Thus, the modest vascularity combined with the limited tissue remodeling that occurs within the IVD make it particularly susceptible towards accumulating AGEs. This accumulation of AGEs has been associated with the narrowing of intradiscal space [6,7]. At the tissue level, increased AGEs affect the mechanical behavior of disc tissues including reduced strain energy dissipation and increased stiffness of the IVD matrix [8]. The loss disc hydration is a common finding in disc degeneration although the exact mechanisms are not known. The formation of AGEs is known to cause changes in the charge-density characteristics, membrane permeability, and tissue hydrophobicity in the extracellular matrix [4,9,10]. Since AGEs are known to accumulate in the degenerating disc, and increased AGEs can alter proteoglycan charge density and hydrophobicity of tissues, we thus hypothesized that the accumulation of AGEs in the IVD tissue may affect its tissue water content.
Magnetic resonance imaging (MRI) is a valuable noninvasive clinical tool for assessing the morphology of the IVD [11–13]. The Pfirmann and Modic grading systems use T1 and T2 weighted MRI sequences to classify the level of IVD degeneration in the lumbar spine [12–16]. T2 relaxation MRI has been shown to correlate with IVD tissue water content [11]. Furthermore the T2 signal intensity, a surrogate measure for hydration, has been shown to be a useful indicator of disc degeneration [12,16]. The ability to measure disc hydration non-invasively makes T2 relaxation MRI an ideal tool to monitor the AGEs-mediated changes in tissue water content, and may be useful in monitoring therapies targeted towards the rehydration of the IVD. We thus further hypothesize that the AGEs-mediated changes in IVD tissue water content is detectable by T2 relaxation MRI. Using in vitro ribosylation system on ovine discs, we investigate the effects of AGEs on the water retention characteristics of the IVD and the ability of MRI to detect these changes.
Materials and Methods
Sample Preparation
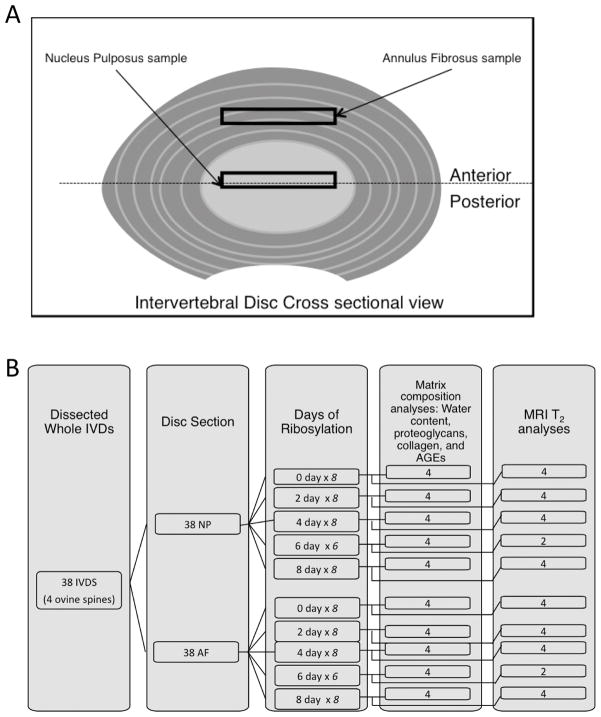
Four 6-month old ovine spines were obtained from Colorado State University in accordance to the Institutional Animal Care and Use Committee (IUCAC) protocols. Thirty-eight IVDs were obtained from the thoracic and lumbar regions of these spines. Care was taken to maintain the intact tissue integrity during the dissection process. The nucleus pulposus (NP=38) and the annulus fibrosus (AF=38) were separated from each sample, resulting in a total of 76 specimens (Figure 1). Specimens were dissected in the transverse direction from the nucleus pulposus and along the anterior region of the annulus fibrosis (Figure 1). Tissues were stored in a 0.15M phosphate buffered solution (PBS) with protease inhibitors at 4°C to minimize swelling.
Figure 1.
(A) The intervertebral disc tissues were dissected from the annulus fibrosus (AF) and nucleus pulposus (NP). (B) A schematic illustrating the allocation of sample sizes in each experiment.
In vitro ribosylation of IVD tissues
The specimens were paired by disc level and incubated in ribosylation solution for 0, 2, 4, 6, or 8 days in 37°C. Once the tissues have undergone the designated incubation time points, it is then removed from the ribosylation solution, placed into a control solution, and placed back at 37°C until all the specimens have undergone 8-days of incubation. The ribosylation solution contained 0.6M ribose, 25mM e-amino-n-caproic acid, 5mM benzamidine, 10mM N-ethylmaleimide, and 30mM Hepes in Hanks buffer [17]. The control solution had the same composition as the ribosylation solution but without ribose. In preliminary studies, these ribosylation parameters achieve a four to five-fold increase in AGEs of the IVD tissue that is comparable to the levels observed in aging and degeneration [6]. After all specimens have been subjected to an 8 day incubation period, the samples were then stored back in 0.15M PBS at 4°C.
Biochemical analyses of the IVD matrix
Forty samples of disc tissues (NP-20, AF-20) were massed before and after speedvac dessication. The tissues were first digested by papain (Sigma Aldrich, 18 mg/ml, 26 U/mg) for 16 hours, and then assayed using 1,9-dimethylmethylene blue dye-binding assay (DMMB) to determine the normalized glycosaminoglycan (GAG) concentration. The remaining papain-digested tissue lysates were hydrolyzed in 6N HCl at 60°C for 24 hours and the autofluorescence of the hydrolysates, a measure of AGEs [5, 17], was determined at an excitation wavelength of 370 nm and an emission wavelengths of 440 nm and normalized to a quinine standard. The collagen content, determined by a colorimetric assay for hydroxyproline, was determined from these same lysates [18]. The final AGEs measurement is then given by the fluorescence in nanograms quinine and divided by the collagen content of the tissue. The GAG content is calibrated by a standard curve (optical density read at a 525 nm) of chondroitin sulfate C from shark cartilage (chondroitin 6-sulfate; Sigma-Aldrich) and normalized by the detected hydroxyproline mass present in an equal volume of tissue. The collagen content is reported as the assayed collagen amount over the dry tissue weight.
Magnetic Resonance Imaging
The remaining 36 specimens were subjected to the same ribosylation protocol and then analyzed by MRI. The disc samples were placed in conical tubes and immersed in PBS. Imaging was performed on a clinical 3.0 T GE Excite MRI scanner (GE Medical Systems, Milwaukee, WI) using a 7-turn solenoidal small animal coil built in-house. Multi-echo spin echo T2-weighted images were acquired using a 4 cm x 2 cm field of view, 256 x 128 matrix, 1 mm slice thickness and 2000 ms repetition time (TR). Individual echo times (TE) were varied at intervals of 20 ms between 20 and 160 ms for samples. Histogram analysis of the AF and NP MRI data was performed using in-house software written with IDL 7.0 (ITT Visual; Boulder, CO). Nonlinear fits containing additive Gaussians were used separate background and tissue voxels. The means for T2 values were averaged over the tissue voxels.
Statistical Analyses
The analysis of variance (ANOVA) was used to determine the effects incubation time on the resulting AGEs concentration and tissue water content in the IVDs; and the effects of in vitro ribosylation on T2 relaxation times. The Fisher’s Least Squares Differences test was used to determine post-hoc comparisons between groups. Pearson’s correlation coefficient between AGEs and water content of IVD was determined for both the AF and NP. Statistical analyses were conducted using MiniTab (MiniTAB Inc. State College, PA).
Results
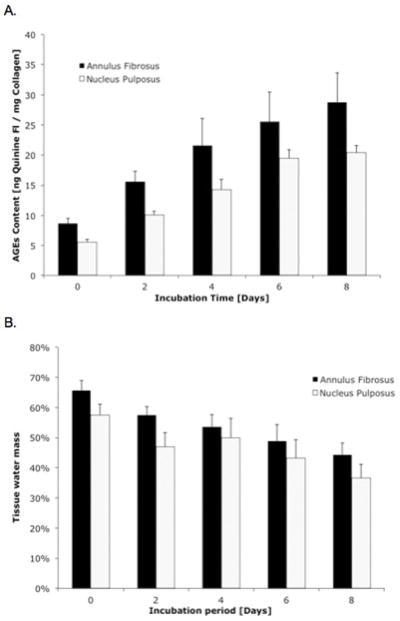
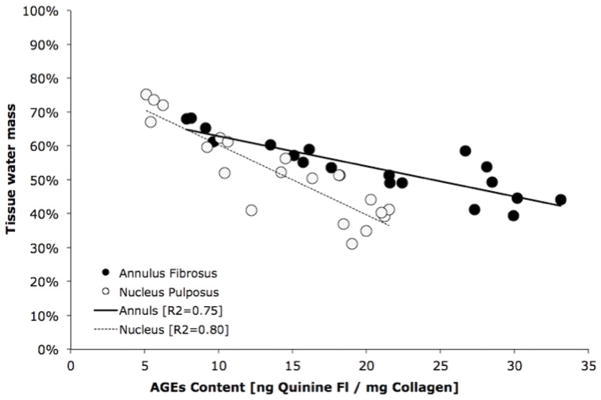
Increasing durations of ribosylation incubation times resulted in the increased concentration of AGEs in both the AF (p<0.001; ANOVA) and NP tissues (p<0.001; ANOVA; Figure 2A). The water content in these tissues also decreased significantly with incubation time in both the AF (p<0.001; ANOVA) and NP (p<0.001; ANOVA; Figure 2B). The reduction of tissue water content in the intervertebral disc correlated negatively with the concentration of AGEs (p<0.001; Pearson’s correlation; Figure 3). The GAG content and collagen content were not statistically different between the tissues that underwent ribosylation and the 0-day controls (Table I).
Figure 2.
(A) The accumulation of AGEs increased with ribosylation incubation time in both the annulus fibrosis (p<0.001; ANOVA) and the nucleus pulposus (p<0.001; ANOVA). (B) The increases in AGEs also corresponded with the decrease in the tissue water mass in these IVD tissues. Water content in the intervertebral disc tissues decreased significantly with incubation time in both the annulus fibrosis (p<0.001; ANOVA) and the nucleus pulposus (p<0.001; ANOVA).
Figure 3.
The loss of water content in the intervertebral disc tissues was significantly correlated with the accumulation of AGEs in both the annulus and the nucleus.
Table I.
Average glycosaminoglycan (GAG) and collagen content (standard deviations in parenthesis) of interverbral disc samples after in vitro ribosylation.
| Ribosylation incubation period | p-values | |||||
|---|---|---|---|---|---|---|
| Control | 2 days | 4 days | 6 days | 8 days | Ribosylated vs 0-day control | |
| AF GAG content [GAG/hydroxyproline] | 8.22 (2.13) | 7.14 (1.96) | 6.98 (1.74) | 8.42 (2.01) | 7.85 (1.65) | p = 0.472 |
| AF Collagen content [assayed/dry mass - %] | 65.3 (11.0) | 69.4 (9.11) | 63.2 (12.3) | 61.2 (13.6) | 67.5 (8.87) | p = 0.562 |
| NP GAG content [GAG/hydroxyproline] | 34.2 (7.87) | 33.8 (5.45) | 29.4 (6.51) | 30.1 (8.13) | 28.3 (9.35) | p = 0.627 |
| NP Collagen content [assayed/dry mass - %] | 25.1 (11.0) | 28.2 (9.11) | 22.8 (12.3) | 21.2 (13.6) | 24.5 (8.87) | p = 0.366 |
AF = Annulus fibrosus; NP = Nucleus pulposus.
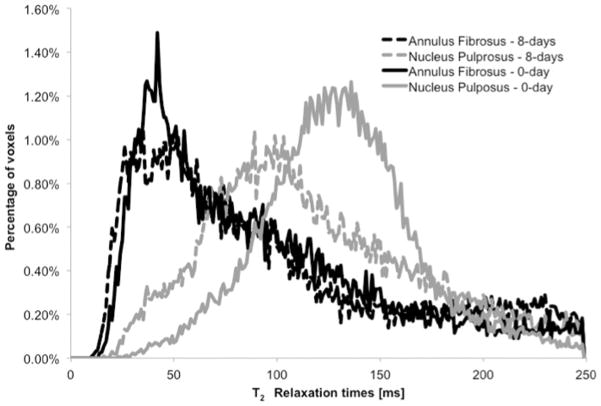
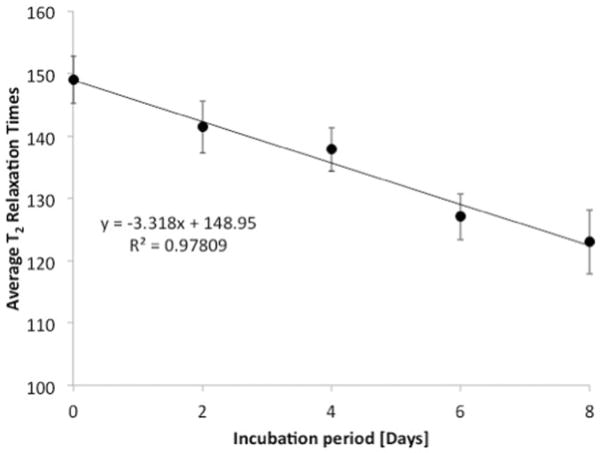
In vitro ribosylation significantly altered the mean peak T2 relaxation times (Table II; Figure 4) in the NP (p=0.001; ANOVA), but not in the AF (p=0.912; ANOVA) when compared with the respective 0-day controls. Furthermore, the mean T2 values of the NP samples significantly decreased (p<0.001; Pearson’s correlation) in a dose-dependent manner with increasing periods of incubation time (Figure 5).
Table II.
Average MR T2 relaxation times (standard deviations in parenthesis) of interverbral disc samples after in vitro ribosylation.2
| Ribosylation incubation period | p-values | |||||
|---|---|---|---|---|---|---|
| Control | 2 days | 4 days | 6 days | 8 days | Ribosylated vs 0-day control | |
| Annulus fibrosus [ms] | 97.6 (11.1) | 99.5 (3.47) | 99.7 (13.3) | 103 (11.5) | 83.3 (9.96) | p = 0.912 |
| Nucleus pulposus [ms] | 149 (5.35) | 141 (7.19) | 137* (6.01) | 127* (3.73) | 123* (8.87) | p < 0.001 |
Denotes significantly lower than the control as determined by the Fisher’s LSD test.
Figure 4.
Representative T2 relaxation distributions of AF and NP samples at 0 days of ribosylation (control) and at 8 days of ribosylation. Ribosylation has little effect on the MR T2 relaxation times of the annulus fibrosus samples, while reducing the MR T2 relaxation times of the nucleus pulposus samples.
Figure 5.
NP tissues exhibited a loss in T2 relaxation times in a dose-dependent manner with increasing ribosylation times (p<0.001; Pearson’s correlation).
Discussion
We demonstrate for the first time that the loss of water content in IVD can be mediated through the accumulation of AGEs by nonenzymatic glycation, and these changes in tissue water content were detectable by T2 magnetic resonance imaging. The results of this study suggest that the increases of AGEs observed in aging and degenerating intervertebral disc may be contributing to the loss of hydration in the tissue.
The Maillard reaction that occurs with NEG creates AGEs that structurally crosslink the amino residues of matrix components including collagen [5, 6] and proteoglycans [4]. At the molecular level, these crosslinks inhibit stretching of the collagen network resulting in reduced energy dissipation capabilities of the tissue [5,10]. The increased accumulation of AGEs also alters the molecular characteristics of proteoglycans by reducing the buoyant density of the aggrecan subfractions [4], causing a shift in the balance between hydrophobic and hydrophilic interactions that are necessary for optimal permeation of fluids [4,15]. Because the intervertebral disc is a biphasic material consisting of matrix proteins and ionic fluids whose deformation resistance is based on the intricate interactions between the two phases [19], it is likely that the changes in hydration due to AGEs observed in this study would have adverse consequences for IVD tissue mechanical behavior [20]. Because AGEs do not directly imbibe water, the loss of water content may also be a consequence of the changes in the extracellular matrix due to alterations in the inability of the collagen network to swell under crosslinked conditions [21], or the reduced ability of the proteoglycans to reversibly bind to water due to AGEs-mediated crosslinking with collagen [22]. Furthermore, it is likely that the permeability of the intervertebral disc, which is critical towards the nutrition and viability of the disc tissue [20], affects the tissues’ general health and ability to retain water [23].
The detrimental effects of AGEs on the IVD extend beyond tissue hydration and material properties of the disc. AGEs have pleotropic effects on cellular function through advanced glycation end-product receptors (r-AGEs) that are found on macrophages, mesangial, and endothelial cells. The activation of r-AGEs have been implicated in inflammatory pathways including those of cytokines TNF alpha, IL-1 alpha, growth factors, endocytosis, and proteolytic enzymes [24]. Tsuru et al. showed that AGEs cause macrophage apoptosis that leads to further disc extrusion during human disc herniation [25]. An in vitro study by Yokosuka et al. showed that culture of nucleus pulposus cells in an oxidative environment with AGEs resulted in the reduced cellular protein expression of proteoglycan and aggrecan [3].
Given these pathologic effects of AGEs on the degeneration of the intervertebral disc, treatment strategies for intervertebral disc rehydration may require, at least in part, the reduction or cleavage of AGEs concentration in the extra-cellular matrix. There are several compounds that can inhibit the formation or breakdown of these advanced glycation end-products. One such compound, aminoguanidine has been shown to both decrease AGE levels through inhibition of NEG and reverse the characteristic damage to tissue such as crosslinking of collagen fibers and increased permeability of cellular basement membrane [26, 27]. Other compounds such as aspirin and pyridoxamine have also been shown to reverse AGEs formation [21, 28].
Magnetic resonance imaging operates on the principle of proton realignment of water molecules within a shifting magnetic field, and the spin-echo relaxation times (such as T1ρ and T2) are commonly used as surrogates for tissue hydration in the intervertebral disc [11]. The observed decreases in the T2 relaxation times in the nucleus pulposus suggests that the AGEs-mediated changes in water content of the tissue may be detectable by non-invasive biomedical imaging [11–14]. The decreasing T2 relaxation times observed in the ribosylated nucleus pulposus tissues are consistent with the trends observed in the pathologic degeneration of the IVD [12, 14], and the increased AGE concentration reduced tissue water content in the NP in a dose-dependent manner. On the other hand, the changes in AF hydration were not detectable by MR imaging, confirming the findings by Chatani et al [29]. The lack of detectable difference by MR in AF hydration may be a limitation of the sensitivity range of MR imaging [11,29].
Individuals with elevated glycemic levels, such as those afflicted with diabetes mellitus, are known to have increased accumulations of AGEs in their tissues [5,10,26,27]. These individuals are three times as likely to have disc herniation even after adjusting for age and weight, suggesting that AGEs may increase the risk of disc degeneration [30]. Discs from diabetic animal models have reduced levels of disc hydration, fixed charged density, and osmotic resistance [31], all of which are consistent with AGEs-mediated modifications of the extra-cellular matrix. These changes in the disc are coupled with reduced buoyant density in the disc proteoglycans and increased under-sulfated glycosaminoglycans [32]. Our findings presented here demonstrate the causal relationship between increased AGEs and the reduced ability of the IVD matrix to retain water.
There are several notable limitations to this study. First, although the ovine spine is a commonly used model for the human spine biomechanics [33, 34], subtle differences exist in the matrix components of the intervertebral disc, including the species of glycosaminglycans with different molecular confirmations [35]. However, because nonenzymatic glycation does not target specific proteins, but rather exposed amino residues, it is likely that the formation kinetics and resulting species of AGEs within human and ovine tissues are quite similar. Secondly, the current study examines only the effects of AGEs within the IVD tissue, and does not account for other age-related and pathological changes such as matrix degradation and cellular function. With these additional changes occurring in the degenerating disc, it is likely that the changes in water content and T2 relaxation times will be more pronounced in tissues that exhibit all of the degenerative changes.
The current results suggest that AGEs may directly mediate the tissue water content, and these changes are detectable using magnetic resonance imaging. Because AGEs may have a multifactorial role in the pathogenesis of degenerative disc disease, it is critical to investigate and understand the role of AGEs on the intervertebral disc, and develop therapeutic strategies targeted towards the inhibition of AGEs. Clinical MR shows great promise in its ability to monitor these AGEs-mediated changes.
Key Points.
Increased incubation time in ribose solution causes reduced water retention in the ovine IVD.
AGEs accumulation is significantly correlated with tissue water content of IVD.
AGEs accumulation reduces T2 relaxation time in the nucleus pulposus.
Acknowledgments
The authors gratefully thank Kim Menges for providing the ovine tissues. Funding is in part supported by UCSF Program for Breakthrough Biomedical Research and NIH F32-AR059497 (ST).
Footnotes
Disclosures: None.
The manuscript submitted does not contain information about medical device(s)/drug(s). Institutional funds were received to support this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1.United States Bone and Joint Decade: The Burden of Musckoskeletal Diseases in the United States. Amer Acad Ortho Surg. 2008;Ch 9 [Google Scholar]
- 2.Praemer A, Furner S, Rice DP. Costs of musculoskeletal conditions in the US. Amer Acad Ortho Surg. 1992:145–170. [Google Scholar]
- 3.Yokosuka K, Park JS, Jimbo K, et al. Advanced glycation end-products downregulating intervertebral disc cell production of proteoglycans in vitro. J Neurosurg: Spine. 2006;5(4):324–9. doi: 10.3171/spi.2006.5.4.324. [DOI] [PubMed] [Google Scholar]
- 4.Sivan SS, Tsitron E, Wachtel E, et al. Age-related accumulation of pentosidine in aggrecan and collagen from normal and degenerate human intervertebral discs. Biochem J. 2006;399:29–35. doi: 10.1042/BJ20060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiser KM. Nonenzymatic glycation of collagen in aging and diabetes. Proc Soc Exp Biol Med. 1991;196(1):17–29. doi: 10.3181/00379727-196-43158c. [DOI] [PubMed] [Google Scholar]
- 6.Pokharna HK, Phillips FM. Collagen crosslinks in human lumbar intervertebral disc aging. Spine. 1998;23(15):1645–8. doi: 10.1097/00007632-199808010-00005. [DOI] [PubMed] [Google Scholar]
- 7.Sharan AD, Tang SY, Quirno M, et al. Decreased height and increased tissue browning of degenerated intervertebral discs are associated with the accumulation of advanced glycation end-products. Trans Amer Ortho Assoc. 2007:495. [Google Scholar]
- 8.Wagner DR, Reiser KM, Lotz JC. Glycation increases human annulus fibrosus stiffness in both experimental measurements and theoretical predictions. J Biomech. 2006;39:1021–9. doi: 10.1016/j.jbiomech.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Vlassara H, Striker LJ, Teichberg S, et al. Advanced glycosylation end-products induce sclerosis and albuminuria in normal rats. Proc Natl Acad Sci. 1994;91:11704–11708. doi: 10.1073/pnas.91.24.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998;22(3):535–42. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 11.Majumdar S. Magnetic resonance imaging and spectroscopy of the intervertebral disc. NMR Biomed. 2006;19(7):894–903. doi: 10.1002/nbm.1106. [DOI] [PubMed] [Google Scholar]
- 12.Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 13.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–8. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 14.Luoma K, Vehas T, Riihimaki H, et al. Disc height and signal intensity of the nucleus pulposus on magnetic resonance imaging as indicators of lumbar disc degeneration. Spine. 2001:680–686. doi: 10.1097/00007632-200103150-00026. [DOI] [PubMed] [Google Scholar]
- 15.Antoniou J, Demers CN, Beaudoin G, et al. Apparent diffusion coefficient of intervertebral discs related to matrix composition and integrity. Mag Reson Imag. 2004;22:963–72. doi: 10.1016/j.mri.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Jones A, Clarke A, Freeman BJ, et al. The Modic classification: inter- and intraobserver error in clinical practice. Spine. 2005;30(16):1867–9. doi: 10.1097/01.brs.0000173898.47585.7d. [DOI] [PubMed] [Google Scholar]
- 17.Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007:1144–1151. doi: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Ar Biochem Biophysics. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 19.Klisch SM, Lotz JC. A special theory of biphasic mixtures and experimental results for human annulus fibrosus tested in confined compression. J biomech. 2000;122(2):180–8. doi: 10.1115/1.429640. [DOI] [PubMed] [Google Scholar]
- 20.Iatridis JC, Setton LA, Weidenbaum M, et al. Alterations in the mechanical behavior of the human lumbar nucleus pulposus with degeneration and aging. J Orthop Res. 1997;15(2):318–22. doi: 10.1002/jor.1100150224. [DOI] [PubMed] [Google Scholar]
- 21.Hadley J, Malik N, Meek K. Collagen as a model system to investigate the use of aspirin as an inhibitor of protein glycation and crosslinking. Micron. 1993;32(3):307–315. doi: 10.1016/s0968-4328(00)00032-9. [DOI] [PubMed] [Google Scholar]
- 22.Phillips FM, Pottenger LA, Hay RV. In situ cross-linking of cartilage proteoglycans. J Orthop Res. 1990;8(2):189–198. doi: 10.1002/jor.1100080206. [DOI] [PubMed] [Google Scholar]
- 23.Katz MM, Hargens AR, Garfin SR. Intervertebral disc nutrition: diffusion versus convection. Clin Orthop. 1986;210:243–5. [PubMed] [Google Scholar]
- 24.Raj DS, Choudhury D, Welbourne TC, et al. Advanced glycation end products: a Nephrologist’s perspective. Am J Kidney Dis. 2000;35(3):365–80. doi: 10.1016/s0272-6386(00)70189-2. [DOI] [PubMed] [Google Scholar]
- 25.Tsuru M, Nagata K, Jimi A, et al. Effect of AGEs on human disc herniation: intervertebral disc hernia is also effected by AGEs. Kurume Med J. 2002;49(1–2):7–13. doi: 10.2739/kurumemedj.49.7. [DOI] [PubMed] [Google Scholar]
- 26.Brownlee M. Lilly Lecture 1993. Glycation and diabetic complications. Diabetes. 1994;43(6):836–41. doi: 10.2337/diab.43.6.836. [DOI] [PubMed] [Google Scholar]
- 27.Bailey AJ. Molecular mechanisms of ageing in connective tissue. Mech Ageing Dev. 2000;122(2001):735–55. doi: 10.1016/s0047-6374(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 28.Booth AA, Khalifah RG, Todd P, et al. In vitro kinetic studies of formation of antigen advanced glycation end-products (AGEs). Novel inhibition of post-Amadori glycation pathway. J Biol Chem. 1997;272:5430–37. doi: 10.1074/jbc.272.9.5430. [DOI] [PubMed] [Google Scholar]
- 29.Chatani K, Kusaka Y, Mifune T, et al. Topographic differences of 1H-NMR relaxation times (T1, T2) in the normal intervertebral disc and its relationship to water content. Spine. 1993;18:2271–75. doi: 10.1097/00007632-199311000-00022. [DOI] [PubMed] [Google Scholar]
- 30.Sakellaridis N. The influence of diabetes mellitus on lumbar intervertebral disk herniation. Surg Neuro. 2006;66(2):152–54. doi: 10.1016/j.surneu.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Ziv I, Moskowitz RW, Kraise I, et al. Physicochemical properties of the aging and diabetic sand rat intervertebral disc. J Orthop Res. 1992;10(2):205–10. doi: 10.1002/jor.1100100207. [DOI] [PubMed] [Google Scholar]
- 32.Robinson D, Mirovsky Y, Halperin N, et al. Changes in proteoglycans of intervertebral disc in diabetic patients. A possible cause of increased back pain. Spine. 1998;23(8):849–55. doi: 10.1097/00007632-199804150-00001. [DOI] [PubMed] [Google Scholar]
- 33.Easley NE, Wang M, McGracy LM, et al. Biomechanical and radiographic evaluation of an ovine model for the human lumbar spine. J Eng Med. 2008;222(6):915–22. doi: 10.1243/09544119JEIM345. [DOI] [PubMed] [Google Scholar]
- 34.Hoogendoorn Roel, Doulabi Behrouz Z, et al. Molecular changes in the degenerated goat intervertebral disc. Spine. 2008;33(16):1714–1721. doi: 10.1097/BRS.0b013e31817d2468. [DOI] [PubMed] [Google Scholar]
- 35.Melrose J, Ghost P, Taylor TKF. Proteoglycan heterogeneity in the normal adult ovine intervertebral disc. Mat Bio. 1994;14 (1):61–75. doi: 10.1016/0945-053x(94)90030-2. [DOI] [PubMed] [Google Scholar]