Abstract
Interleukin-32 (IL-32) is a pro-inflammatory cytokine conditionally produced by T cells, natural killer (NK) cells, monocytes, epithelial cells and keratinocytes, that plays an important role in host resistance against infectious disease. Interestingly, elevated levels of IL-32 transcripts in fine needle aspirates of tumor tissue have also been correlated with objective clinical responses in cancer patients receiving immunotherapy. To evaluate the anti-tumor impact of IL-32 gene therapy, we treated BALB/c mice bearing established s.c. CMS4 sarcomas with intratumoral (i.t.) injections of syngenic dendritic cells (DC) engineered to express human IL-32β cDNA (i.e. DC.IL32). While ectopic expression of IL-32β by DC resulted in only modest phenotypic changes in these antigen presenting cells (APC), DC.IL32 produced higher levels of IL-12p70 than control DC. DC.IL32 were more potent activators of Type-1 T cell responses in vitro and in vivo, with i.t. administration of DC.IL32 leading to the CD8+ T cell-dependent (but CD4+ T cell- and NK cell-independent) suppression of tumor growth. Effective DC.IL32-based therapy promoted infiltration of tumors by Type-1 (i.e. CXCR3+VLA-4+GrB+) CD8+ T cells and CD11b+CD11c+ host myeloid DC, but led to reductions in the prevalence of CD11b+Gr1+ myeloid-derived suppressor cells (MDSC) and CD31+ blood vessels.
Keywords: Interleukin-32, dendritic cells, gene therapy, CD8+ T cells, angiogenesis
INTRODUCTION
Dendritic cells (DC) are highly-efficient, specialized antigen-presenting cells (APC) that play a pivotal role in the cross-priming of protective anti-tumor immunity (1). We have previously shown that direct intratumoral (i.t.) delivery of DC may result in profound anti-tumor effects and durable CD8+ T cell-mediated immunity, particularly when these APC are conditioned or engineered to promote Type-1 immunity (2–4). In particular, we previously observed that i.t. injection of DC engineered to produce both IL-12p70 and IL-18 yielded acute, robust anti-tumor effects in the (H-2d) CMS4 sarcoma model that exceeded that observed for DC transduced with IL-12p70 cDNA or IL-18 cDNA alone (4).
Interestingly, the combination of IL-12 and IL-18 has been defined as a strong stimulus for production of the pro-inflammatory cytokine IL-32, also known as NK cell protein 4 (5–8). IL-32 exists in multiple isoforms (i.e. IL-32α, IL-32β, IL-32γ, IL-32δ and IL-32ζ) that appear structurally unique among the known cytokine families and are potent inducers of IL-1, IL-6, IL-8, TNF-α and MIP-2/CXCL2 (6, 7, 9). IL-32 activates the NF-κB, p38 mitogen-activated protein kinase (MAPK) and activating protein (AP)-1 signaling pathways (6, 10), important for DC maturation (11, 12) and the corollary cross-priming of inflammatory Type-1 T cell-mediated immunity (13, 14). Such immune responses appear to be causally or casually (positively) associated with inflammatory/autoimmune disorders such as rheumatoid arthritis, ulcerative colitis, Crohn’s disease, inflammatory bowel disease (IBD), atopic dermatitis and Wegener’s granulomatosis (8, 14–17). However, IL-32 also appears important for the development and maintenance of protective Type-1 immunity in the infectious disease setting; i.e. HIV-1 (10), HSV and VSV (18), HPV (19) and tuberculosis (20). In the cancer setting, IL-32 (aka NK4) has been identified as a marker associated with patient clinical responsiveness to IL-2-based immunotherapy as determined by gene array profiles obtained from fine needle aspirates of melanoma lesions pre-versus post-treatment (21).
Despite the lack of a homologous murine IL-32 molecule (6), human IL-32 (hIL-32) is bioactive in mice. Hence, the injection of hIL-32 into the knee joints of mice results in joint swelling, tissue infiltration by immune cells and tissue pathology (14) and provision of hIL-32β to mice leads to the expected increase in systemic levels of IL-1, TNF-α, and IL-6 in these animals (8). Given these preliminary data, we investigated the anti-tumor impact of delivering murine DC engineered to express hIL-32β (DC.IL32) into CMS4 sarcomas established in syngenic BALB/c mice.
MATERIALS AND METHODS
Mice
BALB/c (H-2d) mice (6–8 week-old, female) were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were maintained in micro-isolator cages within the pathogen-free, Central Animal Facility at the University of Pittsburgh. All studies were performed under an IACUC-approved protocol and in accordance with the recommendations for the proper care and use of laboratory animals.
Tumor cell lines and culture
The H-2d CMS4 and MethA sarcoma cell lines have been previously described (22). The murine Raw 264.7 macrophage cell line was purchased from the American Type Culture Collection (ATCC; Manassas, VA). All cell lines were cultured in RPMI 1640 supplemented with 10% heat-inactivated FCS, 100 U/ml penicillin, 100μg/ml streptomycin, 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate (designated as CM; all reagents from Life Technologies Inc., Grand Island, NY).
Recombinant adenovirus hIL-32β construction
cDNA encoding human hIL-32β protein was amplified by PCR from the plasmid pORF9-hIL-32β (Invivogen, San Diego, CA) using specific primers (Fwd: 5′-GCGGCATGCGGACACCATGGCGTGCTTCCCGAAG GTCCTCTCTG-3′ and Rev: 5′-GCGCCCGGGACCTCATTTTGAGGATTGGGGTTCAG-3′), and then sub-cloned into a SphI-XmaI restriction site in the pAdLox vector (Invitrogen, San Diego, CA). After sequence validation, recombinant adenovirus was generated by co-transfection of pAdLox.hIL-32β and helper virus DNA into the ecotropic adenoviral packaging cell line CRE8 (2–4). The harvested recombinant adenovirus hIL-32β (Ad.hIL-32β) was purified by cesium chloride density-gradient centrifugation and subsequent dialysis before storage in 3% threalose at −80°C. Titers of viral particles were determined by optical densitometry. The mock (empty) adenoviral vector Ad.ψ5 (2–4) was used as a negative control.
DC preparation and transduction with recombinant adenovirus
Bone marrow-derived DC were generated from BALB/c mice over 5 days in cultures containing rmIL-4 and rmGM-CSF (Peprotech, Rocky Hill, NJ) as previously described (2–4). On day 7, CD11c+ DC were purified using specific MACS beads (Miltenyi Biotec, Auburn, CA) and transduced with control adenovirus (Ad.ψ5) or Ad.IL32β at an MOI of between 50 and 500, as indicated in individual experiments. Infected DC were harvested after 48h and analyzed for their phenotype (using flow cytometry) and function in vitro and in vivo as indicated in the text.
Antibodies
The following mAbs were purchased from BD Biosciences (San Diego, CA): anti-H-2Kd mAb (SF1-1.1) FITC, anti-I-Ad mAb (39-10-8) FITC, anti-B220 (RA3-6B2) PE, anti-CD8α (53-6.7) PE, anti-mCD40 mAb (3/23) FITC, anti-mCD54 mAb (3E2) FITC, anti-mCD80 mAb (16-10A1) FITC, anti-mCD86 mAb (GL1) FITC, anti-mGr1 (RB6-8C5) FITC, and anti-mCD11b (M1/70) PE, anti-mCD11c (HL3) APC. Anti-mCD49d (VLA4; PS/2) APC was purchased from SouthernBiotech (Birmingham, AL). Anti-granzyme B (GrB; 16G6) Alexa Fluor® 647 was purchased from eBioscience (San Diego, CA). The following reagents were purchased from BioLegend (San Diego, CA): anti-mCCR7 (4B12) Alexa488, anti-mCXCR3 (CXCR3-173) APC, purified anti-hIL-32αβδ (KU32-56) and biotin-labeled anti-human IL-32αβγδ (KU32-52). These latter 2 reagents were used to establish a hIL-32β ELISA assay. Purified anti-mouse TNF-α (TN3-19.12) and biotinylated anti-mouse TNF-α (Poly5062) were used in ELISA assays. PE-labeled anti-mouse Foxp3 mAb (NRRF30) and its staining kit were obtained from eBioscience.
Cytokine ELISAs
DC culture supernatants and cell lysates were harvested for analysis of cytokine levels using specific ELISA. In some experiments, as indicated, DCs were stimulated by co-culture with CD40L+J558 cells (kindly provided by Dr. Pawel Kalinski, University of Pittsburgh) at a ratio of 1:1 for 24h, prior to harvest of supernatants for cytokine quantitation. Human IL-32β ELISA was constructed using probes from BioLegend as described in the preceding section, with a lower limit of detection of 7.5 pg/ml. As there was no current commercial source for rhIL-32β, we used rIL-32α (ProSpec, Rehovot, Israel) to establish the standard curve in this ELISA. DC and/or T cell culture supernatants were also evaluated using specific ELISAs for levels of secreted mIL-10 (BD Biosciences), mIL-12p70 (BD Biosciences), mIL-18 (R&D Systems, Minneapolis, MN), mTNF-α (BioLegend), mIFN-α (PBL InterferonSource, Piscataway, NJ) and mIFN-γ (BD Biosciences).
Assessment IL-32βbiologic activity
Human IL-32 bioactivity was evaluated by culturing cell-free supernatants harvested from DC.IL32 or control DC with murine Raw 264.7 cells (5 × 104 cells/well) for 18h at 37°C and 5% CO2. Supernatants were then harvested from these cultures and analyzed for mTNF-α content using a specific ELISA (BioLegend).
Mixed lymphocyte reaction (MLR)
To evaluate the allostimulatory function of control versus engineered DC, MLRs were performed as previously described, with minor modification (3). Control H-2b DC (DC.null) or Ad-infected DC (DC.ψ5 or DC.IL32) were seeded (2 × 104 cells/well) in round-bottom 96-well plates. CD8+ MACS (Miltenyi Biotec) splenic T cells from wild-type BALB/c (H-2d) mice were labeled with 0.5μM CFSE (Sigma-Aldrich) for 15 min at RT, after which, T cells were washed three times with CM and 2 × 105 cells added to control wells or wells containing DC in a total volume of 200 μl CM per well. In analyses of MDSC and Treg suppressor function, CD4+CD25neg BALB/c splenocytes were isolated by MACS (Miltenyi Biotec), labeled with CFSE as noted directly above and (105 cells) cultured with 2 × 104 H-2b control DC.null cells in the absence or presence of MDSC or Treg at a suppressor cell-to-CD4+ T cell ratio of between 0.5: 1 to 2:1, as indicated in text. After 72h of culture, cells were harvested and analyzed by flow cytometry for dilution of CFSE signal as an index of CD4+ T cell proliferation. Triplicate determinations were used in all instances, with data reported as the mean ± SD. Cell-free supernatants were also analyzed for production of IFN-γ and IL-10 using commercial ELISAs (all BD Biosciences).
CMS4 therapy model
For therapeutic experiments, BALB/c mice were injected s.c. with 5 × 105 CMS4 sarcoma cells in the right flank on day 0, with tumors allowed to establish for 7 days. On day 7 after tumor implantation, the mice were randomized into cohorts of 5 animals each (with each cohort exhibiting a mean tumor size of approximately 40 mm2) and left untreated or treated by i.t. injection of 1 – 2.5 × 106 control DC (DC.null or DC.ψ5) or DC.IL32 (106) in a total volume of 50 μl (in PBS) on days 7 and 14 post-tumor inoculation. Tumor size was assessed every 3 or 4 days and recorded in mm2 by determining the product of the largest perpendicular diameters measured by vernier calipers. In some experiments, as indicated, in vivo antibody depletions (on days 6, 13 and 20 post-tumor inoculation) of CD4+ T cells, CD8+ T cells and asialo-GM1+ NK cells were performed as previously described (3).
Evaluation of splenic CD8+ T-cell responses against CMS4 Tumors ex vivo
Spleens were harvested 1 week after the second treatment (i.e. day 21 after tumor inoculation), with 2 × 106 splenocytes stimulated with 2 × 105 irradiated (100 Gy) CMS4 cells for 5 days in the presence of low-dose (30 IU/ml) rhIL-2 (Peprotech). Responder CD8+ T cells were then isolated by specific MACS (Miltenyi Biotec), with CD8+ T cells (2 × 105) then co-cultured with 2 × 104 CMS4 or control (H-2d) MethA sarcoma cells for 48h at 37°C and 5% CO2. Cell-free supernatants were then harvested and analyzed for IFN-γ by ELISA (BD Biosciences). Data are reported as the mean ± SD of triplicate determinations.
Imaging of tumor tissues
Tumor samples were prepared and sectioned as previously reported (23). For analysis of T cell subsets, sections were first stained with purified rat anti-mouse CD8α or purified rat anti-mouse CD4 (both from BD-Pharmingen) mAbs for 1h. After washing, sections were stained with Alexa Fluor 488-conjugated goat anti-rat secondary antibody (Jackson ImmunoResearch, West Grove, PA). To detect DCs in tumor tissue, tissue sections were stained with FITC-conjugated anti-mouse CD11c antibody (BD Biosciences). In some assays, as indicated, DC were first labeled with 0.5 μM CFSE prior to injection, with tissue sections then stained using a PE-conjugated anti-mouse CD11c antibody (BD Bioscience) in order to discriminate injected from endogenous DC populations in the TME. For co-analysis of CD4 and Foxp3 expression, sections were washed and incubated with FITC-conjugated anti-mouse CD4 antibody and PE-conjugated anti-mouse Foxp3 antibody (eBioscience). For analysis of CD11b+Gr1+ MDSC, tissue sections were incubated with PE-conjugated rat anti-mouse CD11b and FITC-conjugated anti-mouse Gr1 (both from BD-Pharmingen). For analysis of CD31 and NG2 markers, the tissue sections were first incubated with rat anti-mouse CD31 and rabbit anti-mouse NG2 (both from Millipore, Bedford, MA) for 1h at RT, then washed with 0.5% BSA and stained with Alexa Fluor 488-conjugated goat anti-rat antibody and Cy3-conjugated goat anti-rabbit antibody (both from Invitrogen). To determine in situ cell death, the In Situ Cell Death Detection Kit (Roche Diagnostics Corp., Indianapolis, IN) was used. After staining with primary and secondary antibodies, the slides were washed and counterstained with 2 mg/ml Hoechst 33258 (Sigma-Aldrich) for 30 seconds. After washing, sections were then covered in Gelvatol (Monsanto, St. Louis, MO) and a coverslip applied. Slide images were acquired using an Olympus 500 scanning confocal microscope (Olympus America Inc., Center Valley, PA). The positively stained cells were quantified by analyzing the images at a final magnification of x20. Cell number and vascular area was analyzed using Metamorph Imaging software (Molecular Devices, Sunnyvale, CA).
RT-PCR
Reverse transcriptase-PCR (RT-PCR) was performed as previously described (23) using the following primer pairs; TNF-α (forward) 5′-ATGAGCACAGAAAGCATG-3′, (reverse) 5′-GAAGACTCCTCCCAGGTA-3′ [606 bp product]; CCL2 (forward) 5′-CTGTGCTGACCCCAAGAAGG-3′, (reverse) 5′-TGCTTGAGGTGGTTGTGGAA-3′ [191 bp product]; CCL7 (forward) 5′-AATGCATCCACATGCTGCTA-3′, (reverse) 5′-CTTTGGAGTTGGGGTTTTCA-3′ [204 bp product]; CCL20 (forward) 5′-CTTGCTTTGGCATGGGTACT-3′, (reverse) 5′-TCAGCGCACACAGATTTTCT-3′ [200 bp product]; β-actin (forward) 5′-CTGACGGCCAGGTCATCAC-3′, (reverse) 5′-GAGTACTTGCGCTCAGGAGG-3′ [286 bp product].
Flow cytometry analysis of tumor infiltrating cells
Surgically harvested tumors were enzymatically digested and viable single-cell suspensions analyzed by flow cytometry as previously described (24).
Statistical analysis
Statistical differences between groups were evaluated by applying a two-tailed Student’s t-test. All data are presented as the mean ± SD. P values < 0.05 were considered to be statistically significant.
RESULTS
Murine DC.IL32 produce bioactive hIL-32βprotein
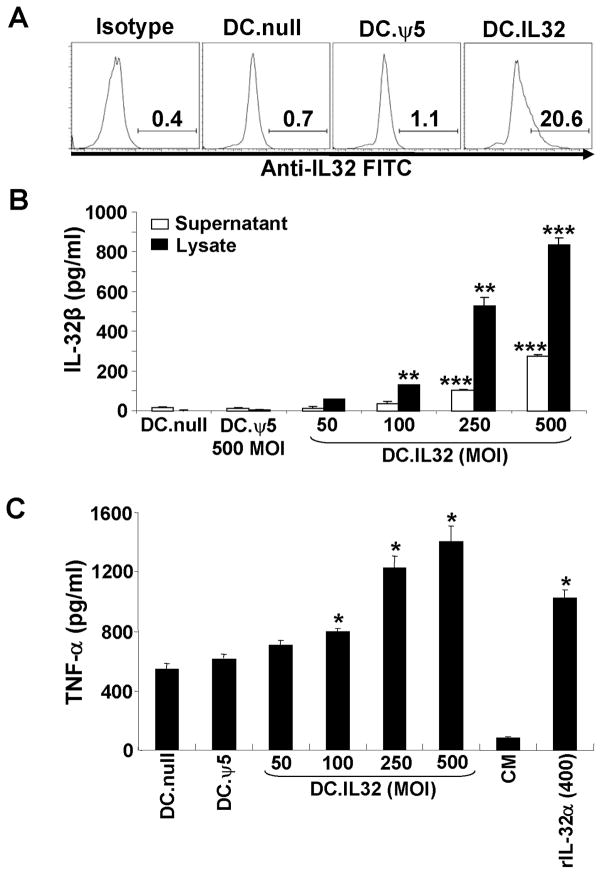
Murine bone marrow-derived DC were generated in 5 day cultures supported by rmIL-4 and rmGM-CSF, then either left untreated or they were infected at various MOIs with Ad.hIL-32β (produced as described in Materials and Methods) or control Ad.ψ5 for 48h. As depicted in Fig. 1, DC.IL32 express the specific transgenic protein based on intracellular staining monitored by flow cytometry (Fig. 1A), as well as, ELISA performed on DC.IL32 cell lysates and the cell-free supernatants harvested from these cultures (Fig. 1B). Notably, the levels of ectopic IL-32 protein expressed in DC.IL32 were directly related to the MOI used for viral infection, and control DC (DC.null and DC.ψ5) populations were deficient in IL-32β expression (Fig. 1A/B). To determine if the DC.IL32-secreted cytokine was bioactive, we applied the harvested supernatants to the murine Raw 264.7 macrophage cell line which has been reported to produce mTNF-α in response to hIL-32 (6). As shown in Fig. 1C, supernatants harvested from DC.IL32 cells promoted elevated levels (versus control DC) of TNF-α production from Raw 264.7 cells at MOIs > 100 (p < 0.05 versus DC.null and DC.ψ5). Based on the data obtained in Fig. 1B/C, we applied an “optimal” MOI of 250 (for Ad.IL-32β infections) in generating DC.IL32 in all prospective experiments.
Figure 1. Expression and bioactivity of hIL-32β in mouse CD11c+ DCs.
When compared to control DCs (DC.null and DC.ψ5), DC.IL-32 expressed higher intracellular expression levels of IL-32β protein as determined by flow cytometry (panel A; MOI = 250) and ELISA (panel B; MOI ranging from 50–500) as outlined in Materials and Methods. For the ELISA assays (B), both DC culture supernatants and DC lysates were analyzed for IL-32β concentration. In C, cell-free supernatants were harvested 48h after DC infection with no adenovirus, Ad.ψ5 (MOI = 500) or Ad.IL32β (at MOI ranging from 50–500), and then used to supplement cultures of the Raw 264.7 murine macrophage cell line. After 18h, supernatants were recovered and analyzed for TNF-α content by ELISA. Recombinant IL-32α (400 pg/ml) was used as positive control for induction of TNF-α from Raw 264.7 cells. * p < 0.05; ** p < 0.01; *** p < 0.001.
In vitro phenotype and functional analyses of DC.IL32
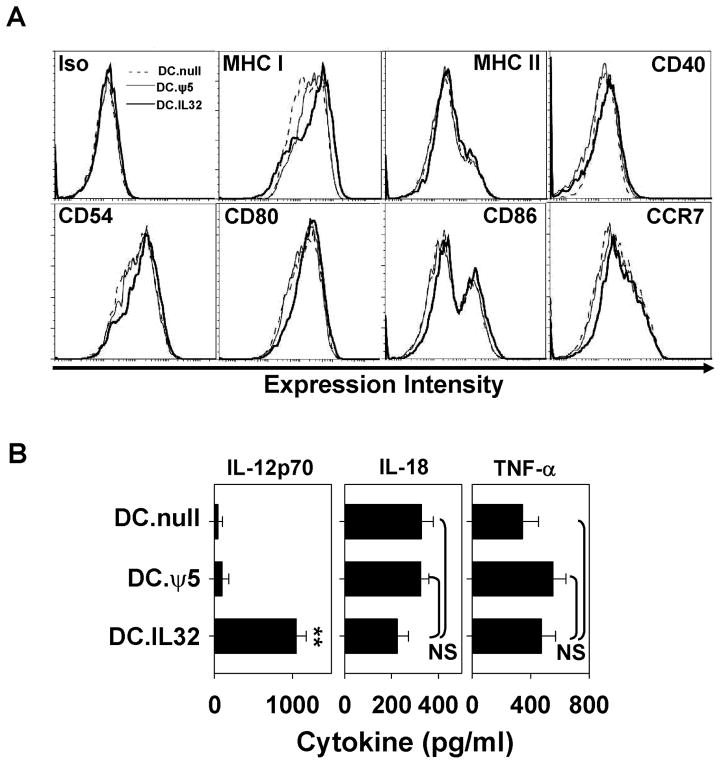
DC.IL32 and control DC were analyzed by flow cytometry for expression of cell surface molecules associated with antigen-presentation (MHC class I, MHC class II), T cell costimulation (CD40, CD54, CD80, CD86) and migration to secondary lymph nodes (CCR7). As shown in Fig. 2A, DC.IL32 expressed higher levels of many of these markers when compared to DC.null or DC.ψ5; i.e. MHC class I (MFI of 2575 versus 1570 and 1870, respectively), CD40 (MFI 185 versus 140 and 139, respectively), CD54 (MFI 1340 versus 974 and 1040, respectively), CD80 (MFI 1670 versus 1232 and 1240, respectively), CD86 (MFI 535 versus 303 and 309, respectively) and CCR7 (MFI 416 versus 319 and 323, respectively). MHC class II expression was not appreciably altered as a consequence of IL-32β cDNA gene insertion into DC (MFI 254 for DC.IL32 versus MFI 241 and 239 for DC.null and DC.ψ5, respectively). Consistent with our previous work (4), infection of DC with control Ad.ψ5 had minimal impact on the immunostimulatory phenotype of these APC.
Figure 2. Ectopic expression of IL-32β enhances the immunostimulatory phenotype of DC.
DC.IL32 (MOI = 250) or control DC (DC.null or DC.ψ5) were prepared as described in Materials and Methods. Forty-eight hours after adenoviral infection, DC were harvested and analyzed by flow cytometry for cell surface expression of MHC and costimulatory molecules, as well as, CCR7 required for DC migration to secondary lymphoid tissues (A). In B., cell-free supernatants were recovered from the individual DC cohorts 24h after stimulation with CD40L+J558 cells (as described in Materials and Methods) and analyzed for the indicated cytokines by specific ELISA. Results are reported as the mean ± SD of triplicate determinations. NS = not significant; **p < 0.01.
To address whether Ad.IL-32β infection of DC altered their capacity to secrete Type-1 polarizing cytokines, we stimulated DC.IL32 or control DC with CD40L+J558 cells and analyzed the cell-free supernatant for cytokine levels by ELISA. As shown in Fig. 2B, DC.IL32 produced significantly greater levels of IL-12p70, but not IL-18, when compared with DC.null or DC.ψ5 (p < 0.01). Interestingly, despite the ability of DC.IL32 elaborated cytokine to promote Raw 264.7 production of TNF-α, DC.IL32 cells themselves failed to produce higher levels of TNF-α versus control DC in response to CD40 ligation (Fig. 2B).
DC.IL32 promote superior development of allo-reactive Tc1 cells in vitro
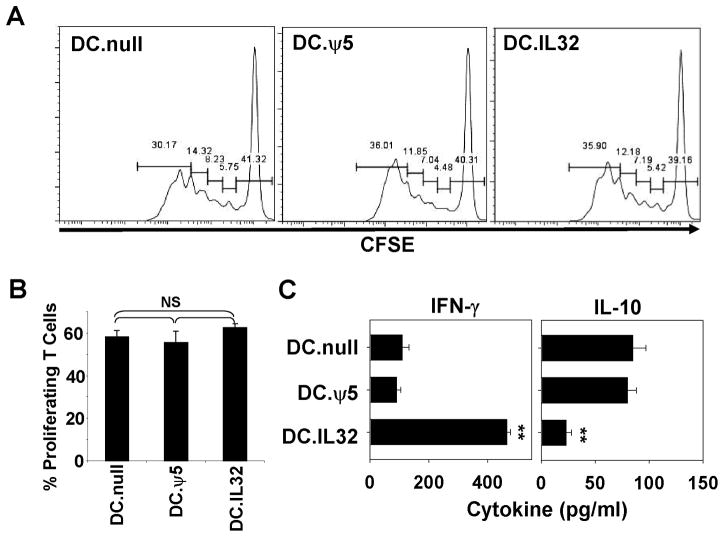
Given the observed up-regulation of MHC class I (but not class II) and costimulatory molecules on, as well as IL-12p70 production by, DC.IL32, we hypothesized that these APC could promote superior Type-1 CD8+ T cell (i.e. Tc1) responses in vitro. This was analyzed in mixed leukocyte reaction (MLR) experiments, using DC.IL32 (or control DC) prepared from C57BL/6 (H-2b) mice as stimulators cells and CFSE-labeled splenic CD8+ T cells isolated from BALB/c (H-2d) mice as responder cells. Although DC.IL32 failed to elicit differential proliferative responses from allogenic T cells when compared with control DC (Fig. 3A/B), these APC were stronger activators of alloreactive CD8+ T cells producing IFN-γ when compared with either DC.null or DC.ψ5 (Fig. 3C; p < 0.01). In marked contrast, IL-10 production was reduced when comparing allogenic T cells stimulated with DC.IL32 versus control DC (Fig. 3C, p < 0.05). IFN-γ and IL-10 were produced solely by T cells and could not be detected in any DC stimulator population (data not shown). These data suggest that ectopic IL-32β contributes predominantly (Type-1) differentiative, rather than proliferative, signals provided by DC.IL32 to responding CD8+ T cells.
Figure 3. DC.IL32 promote enhanced Type-1 polarization, but not proliferation, of alloreactive CD8+ T cells in vitro.
In A., CFSE-labeled (H-2d) CD8+ splenocytes were stimulated with H-2b control DC (DC.null or DC.ψ5) or DC.IL32for 3 days as outlined in Materials and Methods. Cells were then harvested and CD8+ gated cells analyzed for CSFE fluorescence dilution by flow cytometry. In B., the percentage of proliferating CD8+ T cells in each of the indicated cultures is reported as mean ± SD. Cell-free supernatants were also harvested from these DC-T cell cultures and analyzed for IFN-γ and IL-10 concentrations using commercial ELISA (panel C). Data are reported as mean ± SD from triplicate determinations. All experiments are representative of data obtained in 3 independent experiments. NS = Not significant; *p < 0.05; **p < 0.01.
Intratumoral (i.t.) delivery of DC.IL32 prevents CSM4 tumor growth by promoting tumor-specific Tc1 responses in vivo
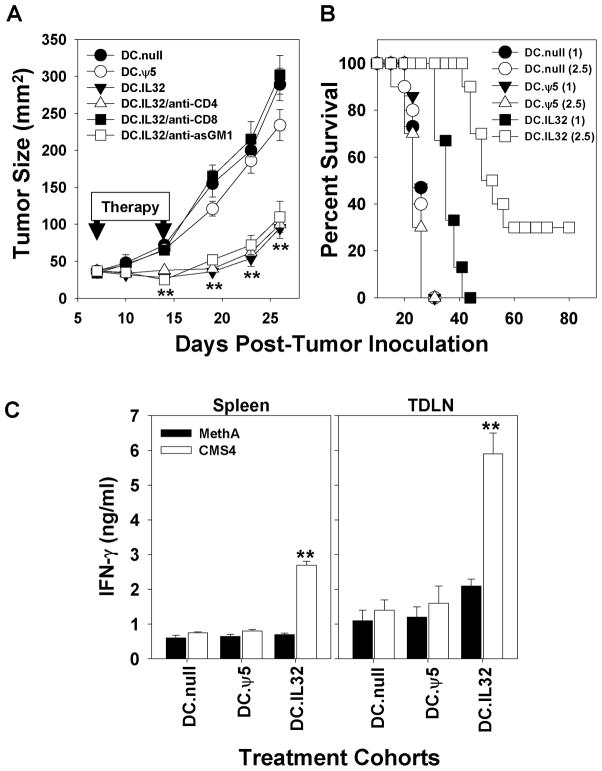
To evaluate the possible therapeutic benefits associated with IL-32β gene-modified DC, we treated BALB/c mice harboring day 7 established (s.c.) CMS4 sarcomas by i.t. injection with 1 × 106 DC.IL32 or control DC on days 7 and 14 post-tumor inoculation. As shown in Fig. 4A, all tumor-bearing mice treated with control DC exhibited progressively growing tumors, while animals treated using DC.IL32 displayed disease stabilization through day 19, after which, slow tumor growth was observed. While all tumor-bearing animals treated i.t. with 106 DC.IL32 ultimately succumbed to their disease, they exhibited extended overall survival (Fig. 4B). However, if the i.t. dose of DC.IL32 were increased to 2.5 × 106 cells per injection, 30% of treated animals exhibited complete tumor regression and long-term disease-free status (Fig. 4B). Antibody-based depletion of CD8+ T cells, but not CD4+ T cells or asialoGM1+ NK cells ablated protection associated with DC.IL32-based therapy in this model (Fig. 4A). The protective influence of therapy-induced Tc1 cells was further suggested by the ability of day 21 CD8+ T cells isolated from the spleens or tumor-draining lymph nodes (TDLN) of DC.IL32 (but not control DC)-treated mice to differentially recognize CMS4 tumor cells versus unrelated (H-2d) MethA sarcoma cells in vitro (Fig. 4C; p < 0.01 versus the DC.null or DC.ψ5 treatment groups).
Figure 4. Intratumoral delivery of DC.IL-32β is effective in suppressing CMS4 tumor growth in vivo via a CD8+ T cell-dependent mechanism.
In A., BALB/c mice bearing day 7 established CMS4 tumors (approximately 40 mm2 in size) received i.t. injections of 106 DC.IL32 or control DC, with a second identical injection of DC provided on day 14 post-tumor inoculation. To determine the relevance of CD4+ T cells, CD8+ T cells or asGM1+ NK cells in protection associated with i.t. delivery of DC.IL32, replicate cohorts of treated animals were also injected with specific depleting antibodies as described in Materials and Methods. Tumor growth was monitored every 4 days. Data are reported as the mean ± SD for 5 animals/group. **p < 0.01 versus DC.null or DC.ψ5. In B., Kaplan-Meier plots are provided for treatment with 1 × 106 (n = 15 mice) or 2.5 × 106 (n = 10 mice) DC.IL32 versus DC.null or DC.ψ5 treatment, respectively. p < 0.05 for DC.IL32 (106) versus DC.ψ5 (106) or DC.null (106); p < 0.001 for DC.IL32 (2.5 × 106) versus DC.ψ5 (2.5 × 106) or DC.null (2.5 × 106), and p < 0.01 for DC.IL32 (2.5 × 106) versus DC.IL32 (106). In C., to evaluate therapy-induced activation of anti-tumor Tc1 cells, on day 21 post-tumor inoculation, CD8+ T cells were isolated from the spleens and TDLN of CMS4-bearing animals treated with DC.null, DC.ψ5 or DC.IL32. These effector cells were then analyzed for their ability to recognize relevant CMS4 tumor cells versus unrelated H-2d MethA sarcoma cells based on IFN-γ secretion analyzed by ELISA as described in Materials and Methods. Data are reported as the mean ± SD of triplicate determinations. **p < 0.01 versus MethA.
Effective i.t. DC.IL32 therapy is associated with increased tumor infiltration by T cells, host CD11c+ DC and tumor/stromal cell apoptosis
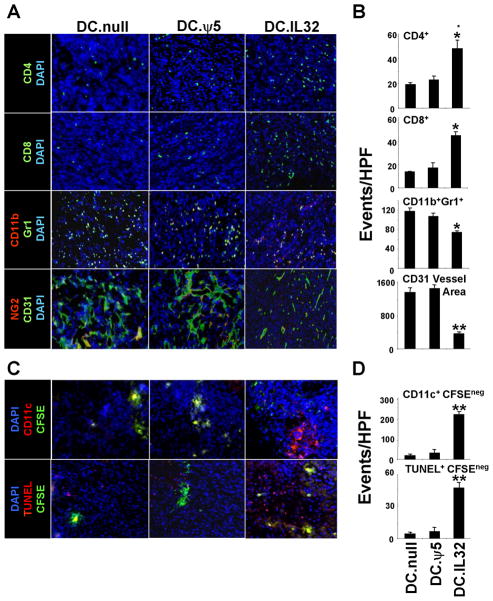
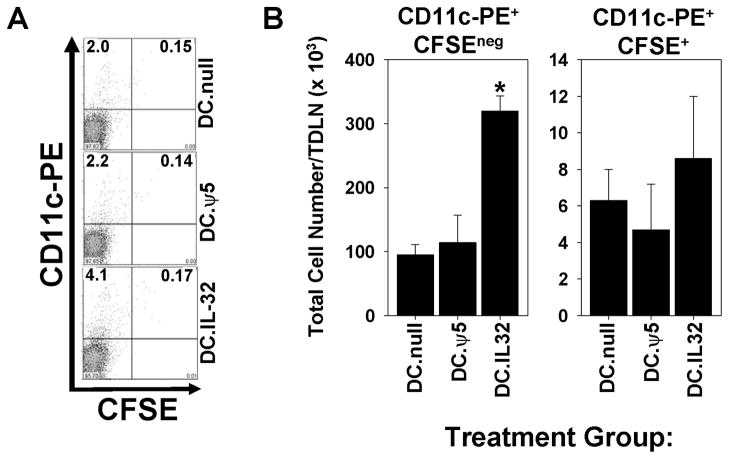
Since effective immunotherapy is believed to require increased tumor infiltration by (Type-1) T cells and improved cross-priming by host APC (25), we next evaluated whether DC.IL32-based therapy alters the prevalence of CD4+ T cells, CD8+ T cells and CD11c+ DC in the TME. CMS4 tumors were isolated from mice on day 21 post-tumor inoculation (i.e. one week after the second i.t. injection of DC) and tissue sections evaluated by immunohistochemistry. In some experiments, injected DC were pre-labeled with CFSE to allow for discrimination of these therapeutic DC from endogenous, host DC. Tumors in mice treated with DC.IL32 contained elevated levels of both T cell subsets (Fig. 5A/B; p < 0.05 versus control treated tumors), with the most profound increase (approximately 10 fold) noted for CD11c+CFSEneg/dim host DC (Fig. 5C/D; p < 0.01 versus control treated tumors). Additional phenotypic analysis of day 21 tumor infiltrating cells suggested that tumors treated with DC.IL32 were enriched in Type-1 (CXCR3+VLA4+Granzyme B (GrB)+) Tc1 cells (Fig. S1; p < 0.01 versus the DC.null or DC.ψ5 treatment groups) and the CD11b+CD11c+ myeloid DC subset (Fig. S2; p < 0.01 versus the DC.null or DC.ψ5 treatment groups). Based on a parallel analysis of CD11c+ populations in the tumor-draining lymph nodes of treated mice, i.t. delivery of DC.IL32 also appeared to increase the trafficking of host (CD11c+CFSEneg/dim) DC to secondary lymphoid sites (Fig. 6), consistent with the enhanced cross-priming of anti-tumor Tc1 in the periphery of these animals (Fig. 4C).
Figure 5. Intratumoral delivery of DC.IL32 promotes increased tumor infiltration by T cells and host DC, reductions in tumor vascularity and MDSC content, and the increased apoptotic death of tumor/stromal cells in the TME.
CMS4 tumor-bearing mice were treated as described in Fig. 4. On day 21 post-tumor inoculation, tumors were harvested and tissue sections analyzed for expression of CD4+ T cells, CD8+ T cells, CD11b+Gr1+ (MDSC) cells, and NG2+CD31+ vascular structures by immunofluorescence microscopy as outlined in the Materials and Methods. Representative images (A) and quantified numbers of events per high power field (HPF; reported as mean ± SD for 10 HPF per slide; panel B) are depicted. *p < 0.05; **p < 0.01 versus DC.null or DC.ψ5. CD31 vessel area is reported in microns2. For C, BALB/c mice bearing established day 7 CMS4 tumors were treated with i.t. delivery of DC.IL32 or control DC pre-labeled with CFSE (as described in Materials and Methods). On day 21 post-tumor inoculation, tumors were harvested and tissue sections analyzed for: the presence of injected DC (CD11c+CFSE+) versus host DC (CD11c+CFSEneg) and the presence of apoptotic DC (TUNEL+CFSE+) versus tumor/tumor stromal cells (TUNEL+CFSEneg) by immunofluorescence microscopy as outlined in Materials and Methods. **p < 0.01 versus DC.null or DC.ψ5. Quantified numbers of events per high power field (HPF; reported as mean ± SD for 10 HPF per slide) are reported in panel D. Similar data were obtained in 3 independent experiments performed.
Figure 6. Intratumoral delivery of DC.IL32 results in increased numbers of host-derived CD11c+ DC in the tumor-draining lymph nodes (TDLN).
BALB/c mice bearing established day 7 CMS4 tumors were treated with i.t. delivered DC.IL32 or control DC (pre-labeled with CFSE) on day 7. A, on day 8 post-tumor inoculation, TDLN were harvested and single-cell suspensions analyzed by flow cytometry for CD11c+ cells of host (CFSEneg/dim) versus therapy (CFSEbright+) origin. B, absolute numbers of each cell population were calculated as the product of total cell yield and the frequency of the indicated cell subset as deduced in panel A. Similar data were obtained in 3 independent experiments performed. *p < 0.05 versus DC.null or DC.ψ5.
Tumors effectively treated with DC.IL32 were also characterized by an increased number of TUNEL+CFSEneg (apoptotic) host cells (Fig. 5C/D; p < 0.01 versus control DC-treated tumors). Such apoptotic bodies are presumed to serve as the antigenic substrate for DC-mediated cross-priming of protective Tc1 responses fostered by i.t. IL-32 gene therapy. Despite reports that DC can directly mediate the apoptotic death of tumor cells (26, 27), we did not observe that IL-32β cDNA engineering of DC resulted in their improved capacity to mediate tumoricidal function in vitro (Fig. S3).
Cells bearing the phenotypes of myeloid-derived suppressor cells (MDSC), but not Treg, are reduced in DC.IL32-treated CMS4 tumors
CD11b+Gr1+ MDSC and CD4+Foxp3+ Treg are present in low levels in the circulation of healthy individuals, but have been reported to accumulate in the TME of cancer-bearing animals and patients (28–30), where they antagonize the activation, function and survival of T effector cells (28–30). To analyze whether DC.IL32-based therapy affects the levels of cells bearing the phenotype of MDSC and Treg in the TME, day 21 tumors were isolated from the various treatment cohorts and tissue sections analyzed by fluorescence microscopy. While the number of tumor-infiltrating cells bearing the CD11b+Gr1+ phenotype was reduced in tumors from the DC.IL32-treated group (Fig. 5A/B; p < 0.05 compared to tumors treated with either DC.null or DC.ψ5), levels of CD4+Foxp3+ TIL were not found to differ significantly between the groups (Fig. S4). Notably, the suppressive activity associated with Treg or MDSC isolated from CMS4 tumor lesions was not significantly affected by treatment with i.t. delivered DC.IL32 (Figs. S4 and S5).
DC.IL-32 mediates anti-angiogenic effects within the TME
Given the increased propensity of DC.IL-32 to produce higher levels of IL-12p70 (when compared with control DC), a potent anti-angiogenic cytokine (32, 33), we next determined whether i.t. delivery of DC.IL32 inhibits angiogenesis in the TME. Tumor sections were isolated from the various treatment groups one week after the second round of therapy (i.e. day 21 post-tumor inoculation) and analyzed by immunofluorescence microscopy for blood vessels based on the expression of pericyte (i.e. NG2) and VEC (i.e. CD31) markers. We observed that treatment with DC.IL32 versus control DC.null or DC.ψ5, resulted in tumor blood vessels that were fewer in number, as well as, less dynamic in their branching patterns and smaller in their overall total surface area (Fig. 5A/B).
DISCUSSION
We have shown that DC engineered to express the potent pro-inflammatory cytokine IL-32β defines a therapeutic modality when injected directly into murine tumor lesions. Therapeutic benefit is completely dependent on the development of treatment-induced CD8+ T effector cells characterized by their capacity to elaborate IFN-γ (i.e. Tc1) in response to tumor-specific stimulation. Despite increased infiltration of CD4+ T cells into the TME as a consequence of DC.IL32-based therapy, depletion of these T cells (or asialoGM1+ NK cells) had little impact on the anti-tumor benefits associated with this treatment regimen. Overall, the ability of DC.IL32 to foster enhanced cross-priming of anti-tumor Tc1 responses is consistent with the changes invoked in these APC upon ectopic expression of IL-32β; e.g. i.) increased production of IL-12p70, ii.) increased expression of MHC class I (but not class II) molecules, iii.) increased expression of costimulatory and integrin molecules (CD54, CD80, CD86) known to co-activate T cells and iv.) increased expression of CCR7, a secondary lymph node homing receptor required for the cross-priming of T cell responses (by DC emigrating from tumors) in tissue-draining lymph nodes. Although the preferential upregulation of MHC I over MHC II molecule expression by DC.IL32 was suggestive of the potential influence of Type-I IFNs in this model (34), DC.IL32 failed to produce/secrete detectable levels of these cytokines when monitored by commercial ELISA (data not shown). Other factors that may promote enhanced MHC class I (over class II) expression include TNF-α (35). While IL-32β biologic activity has been defined based on its ability to promote TNF-α (along with IL-1, IL-6, IL-8 and CXCL2 among others; ref. 6) from responder cell types, and IL-32 elaborated by DC.IL32 was able to activate TNF-α from the murine Raw264.7 macrophage cell line, it did not appear to promote autocrine TNF-α production from DC.IL32 themselves.
Our results suggesting the improved APC function of DC engineered to express transgene IL-32β contrast somewhat with a prior report by Netea et al. (36) who demonstrated that rIL-32γ inhibited the rIL-4 + rGM-CSF-driven differentiation of monocyte/macrophages into DC when added at high doses (i.e. 30 ng/ml) from the outset of cultures. In marked contrast, we have promoted IL-32β production from pre-differentiated DC. In this setting, one must appreciate that i.) the amount of IL-32β produced by DC.IL32 was only on the order of 1 ng/ml based on our ELISA results and ii.) IL-32β is the less biologically active splice product of IL-32γ (37). Under such lower dose conditions for IL-32β, any off-target (immuno)suppressive biology associated with IL-32β might well be avoided.
The most striking single change evoked by our IL-32 gene therapy was the (approximately 10–20 fold) enhanced presence of CD11b+CD11c+ myeloid DC detected within the tumor lesion post-treatment with DC.IL32. These DC were of host origin and total DC numbers in the TME were not significantly impacted by the therapeutically-injected DC population. Factors likely to be involved in the therapeutic recruitment of host DC into the effectively-treated, pro-inflammatory TME include the chemokines CCL2, CCL7 and/or CCL20 (38–41). In prospective experiments, we will analyze the impact of injecting neutralizing Abs against these chemokines on recruitment of host CD11c+ DC into the TME and whether these antagonists ablate Tc1 cross-priming in vivo and therapeutic benefit.
In preliminary MLR experiments, DC.IL32did not promote differential T cell proliferation when compared with control DC (DC.null or DC.ψ5), however, responder T cells harvested from DC.IL32 cultures were superior producers of IFN-γ and inferior producers of IL-10. Hence DC.IL32 likely provide differential developmental (i.e. functional polarizing), rather than proliferative, signals to cross-primed Type-1 CD8+ T cells in the TDLN. Interestingly, once Tc1 effector cells were activated systemically in treated mice, i.t.-delivered DC.IL32 also appeared to facilitate the recruitment of these T effector cells (approximately 2–3 fold) into tumor sites, likely via the enhanced production of CXCR3 ligand chemokines (such as Mig/CXCL9) in the TME (Fig. S6). We are currently investigating the impact of antagonizing CXCR3+ Tc1 infiltration into CMS4 tumors (using blocking anti-CXCR3 Abs) on the anti-tumor efficacy of DC.IL32-based therapy. Furthermore, IL-32β has been reported to up-regulate endothelial cell expression of VCAM-1 (42) which may facilitate tumor infiltration by VLA-4+ Tc1 cells in vivo (24). Such therapy-induced vascular changes are likely of clinical significance since we observed a profound enrichment in tumor infiltration by CD8+ T cells bearing a CXCR3+VLA-4+GrB+ phenotype after i.t. delivery of DC.IL32 (Fig. S1 and S2).
Somewhat surprisingly, DC.IL32-based therapy was effective despite promoting only a modest reduction (approximately 40%) in the number of cells bearing an MDSC phenotype, and no significant change in the number of cells bearing a CD4+Foxp3+ Treg phenotype within the treated TME. This suggests that the dominant influence of DC.IL32-based therapy on Type-1 CD8+ T cell-mediated immunity likely overrides the existing state of immune suppression and/or that the tumor-resident MDSC/Treg have been rendered less effective in their inhibitory activities towards protective Tc1 cells. In support of the former contention, our preliminary analyses of flow-sorted CD11b+Gr-1+ MDSC and CD4+CD25+ Treg cells from the TME of mice treated with DC.IL32 versus control DC indicate that these cell populations mediate a comparable level of immune suppressor activity (on a per-cell basis) regardless of the treatment cohort analyzed.
Although our studies suggest that transient expression of IL-32β by DC in the TME can be therapeutic, others have postulated that IL-32 expression (by tumor cells or infiltrating cells) in the context of chronic inflammation in the human TME may be involved in tumor progression and metastasis (43, 44). Indeed, IL-32 can be expressed by human breast, gastric, lung and pancreatic carcinomas, in addition to glioblastoma multiforme (GBM; refs. 43, 44). Even though murine CMS4 tumor cells are deficient in IL-32 transcripts(ref. 6 and RT-PCR data not shown), one is left to consider what determines the pro-/anti-tumor impact of IL-32β in the TME? Most simplistically, this could be related to whether IL-32 expression is “acute” versus “chronic”, with transient expression (as provided by DC.IL32) being associated with host protection (6, 43, 45). Conceptually, by avoiding prolonged production of IL-32 in the TME, compensatory immunoregulatory changes may not be enforced, allowing for improved treatment benefit. This could underlie the reported link between IL-32 (i.e. NK4) expression in the tumors of melanoma patients and superior therapy outcome after treatment with IL-2 (21).
In addition to promoting improved Type-1 anti-tumor immunity, i.t. delivery of DC.IL32 appears to alter the structure and activation status of blood vessels in the TME. We observed that tumors treated with DC.IL32 exhibited fewer and less dynamic blood vessel structures, which could relate to the potent anti-angiogenic activity of IL-12p70 (32, 33) that is produced at higher levels by DC.IL32 versus control DC. IL-32 has also been reported to inhibit COX-2 activity (46) thereby limiting VEGF-supported neoangiogenesis (47). While we did not observe elevated production of TNF-α from DC after infection with Ad.IL-32β, this does not preclude secretion of this anti-angiogenic cytokine (48) from activated tumor/stromal cells in the TME of DC.IL32-treated mice, a possibility that we are now investigating.
In conclusion, our results suggest pleiotropic protective effects resulting from i.t. delivery of DC.IL32 including the activation and recruitment of protective anti-tumor CD8+ T cells into the TME. Since IL-32-based therapy did not appear to profoundly deplete or inactivate MDSC or Treg in the TME, this suggests that combinational protocols employing agents that antagonize these suppressor cell populations (i.e. sunitinib, anti-CTLA4 mAb, etc.; refs. 31, 49) have potential to yield an even greater degree of anti-tumor benefit.
Supplementary Material
Acknowledgments
The authors wish to thank Drs. Devin Lowe, Per Basse and Pawel Kalinski for constructive comments provided during the generation of this manuscript. This work was supported by NIH grants P01 CA100327-05 and R01 CA140375-01 (to W.J.S.) and the University of Pittsburgh Cancer Center Support Grant (UP-CCSG; P30 CA047904).
Abbreviations used
- BM
bone marrow
- CM
culture media
- CFSE
carboxyfluorescein succinimidyl ester
- DC
dendritic cell(s)
- FCM
flow cytometry
- FITC
fluorescein isothiocyanate
- Foxp3
forkhead box protein 3
- MACS
magnetic activated cell selection
- MDSC
myeloid-derived suppressor cells
- NK
natural killer cell(s)
- PE
phycoerythrin
- Treg
regulatory T cell(s)
- TDLN
tumor-draining lymph node
- TIL
tumor infiltrating lymphocytes
- TME
tumor microenvironment
- VEC
vascular endothelial cell(s)
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES CITED
- 1.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Komita H, Zhao X, Katakam AK, Kumar P, Kawabe M, Okada H, et al. Conditional interleukin-12 gene therapy promotes safe and effective anti-tumor immunity. Cancer Gene Ther. 2009;16:883–891. doi: 10.1038/cgt.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qu Y, Chen L, Pardee AD, Taylor JL, Wesa AK, Storkus WJ. Intralesional delivery of dendritic cells engineered to express T-bet promotes protective type 1 immunity and the normalization of the tumor microenvironment. J Immunol. 2010;185:2895–2902. doi: 10.4049/jimmunol.1001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tatsumi T, Huang J, Gooding WE, Gambotto A, Robbins PD, Vujanovic NL, et al. Intratumoral delivery of dendritic cells engineered to secrete both interleukin (IL)-12 and IL-18 effectively treats local and distant disease in association with broadly reactive Tc1-type immunity. Cancer Res. 2003;63:6378–6386. [PubMed] [Google Scholar]
- 5.Dahl CA, Schall RP, He HL, Cairns JS. Identification of a novel gene expressed in activated natural killer cells and T cells. J Immunol. 1992;148:597–603. [PubMed] [Google Scholar]
- 6.Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNF-α. Immunity. 2005;22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Netea MG, Azam T, Ferwerda G, Girardin SE, Walsh M, Park JS, et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1β and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci USA. 2005;102:16309–16314. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoda H, Fujio K, Yamaguchi Y, Okamoto A, Sawada T, Kochi Y, et al. Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res Ther. 2006;8:R166. doi: 10.1186/ar2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nold MF, Nold-Petry CA, Pott GB, Zepp JA, Saavedra MT, Kim SH, et al. Endogenous IL-32 controls cytokine and HIV-1 production. J Immunol. 2008;181:557–565. doi: 10.4049/jimmunol.181.1.557. [DOI] [PubMed] [Google Scholar]
- 11.Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-α, and contact sensitizers. J Immunol. 2001;166:3837–3845. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- 12.Koski GK, Lyakh LA, Cohen PA, Rice NR. CD14+ monocytes as dendritic cell precursors: diverse maturation-inducing pathways lead to common activation of NF-κB/RelB. Crit Rev Immunol. 2001;21:179–189. [PubMed] [Google Scholar]
- 13.Melief CJ. Mini-review: Regulation of cytotoxic T lymphocyte responses by dendritic cells: peaceful coexistence of cross-priming and direct priming? Eur J Immunol. 2003;33:2645–2654. doi: 10.1002/eji.200324341. [DOI] [PubMed] [Google Scholar]
- 14.Joosten LA, Netea MG, Kim SH, Yoon DY, Oppers-Walgreen B, Radstake TR, et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci USA. 2006;103:3298–3303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shioya M, Nishida A, Yagi Y, Ogawa A, Tsujikawa T, Kim-Mitsuyama S, et al. Epithelial overexpression of interleukin-32α in inflammatory bowel disease. Clin Exp Immunol. 2007;149:480–486. doi: 10.1111/j.1365-2249.2007.03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer N, Zimmermann M, Bürgler S, Bassin C, Woehrl S, Moritz K, et al. IL-32 is expressed by human primary keratinocytes and modulates keratinocyte apoptosis in atopic dermatitis. J Allergy Clin Immunol. 2010;125:858–865. doi: 10.1016/j.jaci.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Csernok E, Holle JU, Gross WL. Proteinase 3, protease-activated receptor-2 and interleukin-32: linking innate and autoimmunity in Wegener’s granulomatosis. Clin Exp Rheumatol. 2008;26:S112–S117. [PubMed] [Google Scholar]
- 18.Zepp JA, Nold-Petry CA, Dinarello CA, Nold MF. Protection from RNA and DNA Viruses by IL-32. J Immunol. 2011;186:4110–4118. doi: 10.4049/jimmunol.1000081. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Kim JH, Kim H, Kang JW, Kim SH, Yang Y, et al. Activation of the interleukin-32 pro-inflammatory pathway in response to human papillomavirus infection and over-expression of interleukin-32 controls the expression of the human papillomavirus oncogene. Immunology. 2011;132:410–420. doi: 10.1111/j.1365-2567.2010.03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kundu M, Basu J. IL-32: an emerging player in the immune response network against tuberculosis? PLoS Med. 2006;3:e274. doi: 10.1371/journal.pmed.0030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panelli MC, Wang E, Phan G, Puhlmann M, Miller L, Ohnmacht GA, et al. Gene-expression profiling of the response of peripheral blood mononuclear cells and melanoma metastases to systemic IL-2 administration. Genome Biol. 2002;3:RESEARCH0035. doi: 10.1186/gb-2002-3-7-research0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayordomo JI, Loftus DJ, Sakamoto H, De Cesare CM, Appasamy PM, Lotze MT, et al. Therapy of murine tumors with p53 wild-type and mutant sequence peptide-based vaccines. J Exp Med. 1996;183:1357–1365. doi: 10.1084/jem.183.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komita H, Zhao X, Taylor JL, Sparvero LJ, Amoscato AA, Alber S, et al. CD8+ T-cell responses against hemoglobin-β prevent solid tumor growth. Cancer Res. 2008;68:8076–8084. doi: 10.1158/0008-5472.CAN-08-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki K, Zhao X, Pardee AD, Ueda R, Fujita M, Sehra S, et al. Stat6 signaling suppresses VLA-4 expression by CD8+ T cells and limits their ability to infiltrate tumor lesions in vivo. J Immunol. 2008;181:104–108. doi: 10.4049/jimmunol.181.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brossart P, Bevan MJ. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90:1594–1599. [PMC free article] [PubMed] [Google Scholar]
- 26.Huang J, Tatsumi T, Pizzoferrato E, Vujanovic N, Storkus WJ. Nitric oxide sensitizes tumor cells to dendritic cell-mediated apoptosis, uptake, and cross-presentation. Cancer Res. 2005;65:8461–8470. doi: 10.1158/0008-5472.CAN-05-0654. [DOI] [PubMed] [Google Scholar]
- 27.Wesa AK, Storkus WJ. Killer dendritic cells: mechanisms of action and therapeutic implications for cancer. Cell Death Differ. 2008;15:51–57. doi: 10.1038/sj.cdd.4402243. [DOI] [PubMed] [Google Scholar]
- 28.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–726s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 30.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and imunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 33.Wigginton JM, Wiltrout RH. IL-12/IL-2 combination cytokine therapy for solid tumours: translation from bench to bedside. Expert Opin Biol Ther. 2002;2:513–524. doi: 10.1517/14712598.2.5.513. [DOI] [PubMed] [Google Scholar]
- 34.Hermann P, Rubio M, Nakajima T, Delespesse G, Sarfati M. IFN-α priming of human monocytes differentially regulates gram-positive and gram-negative bacteria-induced IL-10 release and selectively enhances IL-12p70, CD80, and MHC class I expression. J Immunol. 1998;161:2011–2018. [PubMed] [Google Scholar]
- 35.Johnson DR, Pober JS. Tumor necrosis factor and immune interferon synergistically increase transcription of HLA class I heavy- and light-chain genes in vascular endothelium. Proc Natl Acad Sci USA. 1990;87:5183–5187. doi: 10.1073/pnas.87.13.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Netea MG, Lewis EC, Azam T, Joosten LA, Jaekal J, Bae SY, Dinarello CA, Kim SH. Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proc Natl Acad Sci USA. 2008;105:3515–3520. doi: 10.1073/pnas.0712381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinhuis B, Koenders MI, van de Loo FA, Netea MG, van den Berg WB, Joosten LA. Inflammation-dependent secretion and splicing of IL-32γ in rheumatoid arthritis. Proc Natl Acad Sci USA. 2011 Mar 7; doi: 10.1073/pnas.1016005108. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auray G, Lacroix-Lamandé S, Mancassola R, Dimier-Poisson I, Laurent F. Involvement of intestinal epithelial cells in dendritic cell recruitment during C. parvum infection. Microbes Infect. 2007;9:574–582. doi: 10.1016/j.micinf.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 39.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Aït-Yahia S, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell cross-priming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Cagnard N, Letourneur F, Essabbani A, Devauchelle V, Mistou S, Rapinat A, et al. Interleukin-32, CCL2, PF4F1 and GFD10 are the only cytokine/chemokine genes differentially expressed by in vitro cultured rheumatoid and osteoarthritis fibroblast-like synoviocytes. Eur Cytokine Netw. 2005;16:289–292. [PubMed] [Google Scholar]
- 42.Kobayashi H, Huang J, Ye F, Shyr Y, Blackwell TS, Lin PC. Interleukin-32β propagates vascular inflammation and exacerbates sepsis in a mouse model. PLoS One. 2010;5:e9458. doi: 10.1371/journal.pone.0009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi H, Lin PC. Molecular characterization of IL-32 in human endothelial cells. Cytokine. 2009;46:351–358. doi: 10.1016/j.cyto.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishida A, Andoh A, Inatomi O, Fujiyama Y. Interleukin-32 expression in the pancreas. J Biol Chem. 2009;284:17868–17876. doi: 10.1074/jbc.M900368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calabrese F, Baraldo S, Bazzan E, Lunardi F, Rea F, Maestrelli P, et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:894–901. doi: 10.1164/rccm.200804-646OC. [DOI] [PubMed] [Google Scholar]
- 46.Li W, Liu Y, Mukhtar MM, Gong R, Pan Y, Rasool ST, et al. Activation of interleukin-32 pro-inflammatory pathway in response to influenza A virus infection. PLoS One. 2008;3:e1985. doi: 10.1371/journal.pone.0001985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toomey DP, Murphy JF, Conlon KC. COX-2, VEGF and tumour angiogenesis. Surgeon. 2009;7:174–180. doi: 10.1016/s1479-666x(09)80042-5. [DOI] [PubMed] [Google Scholar]
- 48.Tian F, Grimaldo S, Fujita M, Cutts J, Vujanovic NL, Li LY. The endothelial cell-produced antiangiogenic cytokine vascular endothelial growth inhibitor induces dendritic cell maturation. J Immunol. 2007;179:3742–3751. doi: 10.4049/jimmunol.179.6.3742. [DOI] [PubMed] [Google Scholar]
- 49.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.