Abstract
Objectives
Assess the impact of patient population characteristics on accuracy by CT angiography (CTA) to detect obstructive coronary artery disease (CAD).
Background
The ability of CTA to exclude obstructive CAD in patients of different pretest probabilities and in presence of coronary calcification remains uncertain.
Methods
For the CorE-64 study 371 patients underwent CTA and cardiac catheterization for the detection of obstructive CAD defined as 50% or greater luminal stenosis by quantitative coronary angiography (QCA). This analysis includes 80 initially excluded patients with a calcium score ≥ 600. Area under the receiver-operating-characteristics curve (AUC) was used to evaluate CTA diagnostic accuracy compared to QCA in patients according to calcium score and pretest probability of CAD.
Results
Analysis of patient-based quantitative CTA accuracy revealed an AUC of 0.93 (95% confidence interval [CI] 0.90-0.95). AUC remained 0.93 (0.90-0.96) after excluding patients with known CAD but decreased to 0.81 (0.71-0.89) in patients with calcium score ≥ 600 (p=0.077). While AUC were similar (0.93, 0.92, and 0.93, respectively) for patients with intermediate, high pretest probability for CAD, and known CAD, negative predictive values were different: 0.90, 0.83, and 0.50, respectively. Negative predictive values decreased from 0.93 to 0.75 for patients with calcium score < or ≥ 100, respectively (p= 0.053).
Conclusions
Both pretest probability for CAD and coronary calcium scoring should be considered before using CTA for excluding obstructive CAD. CTA is less effective for this purpose in patients with calcium score ≥ 600 and in patients with a high pretest probability for obstructive CAD.
Keywords: Coronary Disease, Imaging, Angiography
INTRODUCTION
CT coronary angiography (CTA) is an emerging tool for the non-invasive assessment of coronary artery disease. Several expert consensus documents endorse the use of CTA for excluding coronary artery disease (CAD) in symptomatic patients with reference to numerous studies which have reported high negative predictive values (1, 2). However, predictive values heavily depend on disease prevalence within the study population, thus they cannot be applied outside the context of a defined patient group (3-5). Accordingly, an assessment of pretest probability of coronary artery disease may help predicting the value of CT angiography for excluding or confirming the presence of CAD. In this regard, coronary arterial calcification detected by non-contrast CT correlates well with CAD prevalence and therefore may help to identify patients in whom ruling out or confirming CAD by CT angiography is of low yield. Furthermore, coronary arterial calcification may also alter the diagnostic performance of CT angiography (6-9). Coronary calcium substantially attenuates X-ray penetration leading to “blooming” artifacts with current CT image reconstruction that may obscure the coronary lumen. Because of the perceived limitation of CTA in patients with severe coronary calcification, many investigators have favored obtaining a coronary calcium score to inform the decision of proceeding or not with CTA (10). However, the utilization of a coronary calcium score threshold for deciding to perform or not coronary CTA remains controversial (11).
We have previously reported the diagnostic performance of CTA among an international cohort of patients with a calcium score of less than 600 (12). Including all enrolled patients regardless of the presence and extent of coronary calcification, this investigation tested the following hypotheses: 1) Accuracy for CTA to detect obstructive CAD in patients with severe coronary arterial calcification is reduced compared to patients with low or moderate coronary calcification; 2) Predictive values for detecting obstructive CAD by CTA are highly variable according to calcium scores and disease prevalence in the study population; 3) CTA is ineffective for ruling out obstructive CAD in patients with severe coronary arterial calcification and in patients with high pretest probability of CAD.
METHODS
The study design has been described in detail previously (13). In brief, the Coronary Artery Evaluation Using 64-Row Multi-Detector Computed Tomography Angiography (CorE-64) study is a prospective, multicenter diagnostic study performed at nine hospitals in seven countries designed to evaluate the diagnostic accuracy of CTA for identifying coronary artery stenosis in patients with suspected or known significant CAD (12). All centers received study approval from their local institutional review boards, and all patients gave written informed consent.
Patient population
Eligible patients were at least 40 years of age, had suspected symptomatic CAD, and were referred for conventional coronary angiography. Patients were not eligible if they had a history of cardiac surgery, allergy to iodinated contrast material or contrast induced nephropathy, multiple myeloma, organ transplantation, elevated serum creatinine level (>1.5 mg per deciliter [133 μmol per liter]) or creatinine clearance less than 60 ml per minute, atrial fibrillation, New York Heart Association class III or IV heart failure, aortic stenosis, percutaneous coronary intervention within the past 6 months, intolerance to beta-blockers, or a body-mass index of greater than 40. Patients with Agatston calcium scores of 600 or greater were prespecified to be excluded from the primary analysis of the study but included for secondary analyses performed identically to the main cohort. The rationale by the steering committee for excluding patients with calcium score ≥ 600 was based in 1) the precedence of excluding such patients in a prior multicenter study (14) and 2) the concern of futility for CTA imaging in such patients based on the data available at the study design planning. This current investigation includes the results for the primary CorE-64 patient cohort and those patients with calcium score greater than 600 who were not included in the primary analysis of CorE-64 (12).
Acquisition and Analysis of Data from CT
Patients underwent two CT scans (coronary calcium scoring and angiography), before conventional coronary angiography was performed, using 64-row scanners with a slice thickness of 0.5 mm (Aquilion 64®, Toshiba Medical Systems). Calcium scoring was performed with the use of prospective electrocardiographic (ECG) gating with 400-msec gantry rotation, 120-kV tube voltage, and 300-mA tube current. For CT angiography, retrospective ECG gating was used, with heart rate adjusted gantry rotations of 350 to 500 msec to enable adaptive multisegmented reconstruction. Pitch and tube currents of 240 to 400 mA were determined by patients’ weight to ensure a sex-specific radiation dose of 12 to 15 mSv, with a maximum effective dose of 20 mSv, for the combination of multidetector CT calcium scoring and angiographic procedures. This was achieved by instituting a cap of 270 mA for women and 400 mA for men. Sublingual nitrates were given before CTA. Iopamidol (Isovue 370®, Bracco Diagnostics) was the intravenous contrast medium used for CTA. Beta-blockers were given if the resting heart rate was above 70 beats per minute. If the heart rate during acquisition was more than 80 beats per minute, the patient’s data were excluded from analysis.
Raw image data sets from all acquisitions were analyzed by an independent core laboratory. Multisegmental reconstruction was performed with 0.5-mm slice thickness, 0.3-mm overlap, multiple phases, and ECG editing. Images were reconstructed using both standard (FC43) and sharper (FC05) kernels particularly used to reduce artifacts from high density structures, such as coronary calcification. Two independent, blinded observers, using a modified coronary model, visually graded each of 19 nonstented segments that were 1.5 mm or more in diameter, according to an ordinal scale (no stenosis, 1 to 29% stenosis, 30 to 49% stenosis, 50 to 69% stenosis, 70 to 99% stenosis, or total occlusion). Then, segments with at least one visible stenosis of 30% or more were manually quantified with the use of commercially available software (Vitrea2 version 3.9.0.1, Vital Images), and results for the two readers were averaged. Interreader visual and quantitative differences for stenoses exceeding 50% were resolved by a third observer.
Data Acquisition and Analysis of Data from Conventional Coronary Angiography
Conventional coronary angiography was performed within 30 days after CTA using standard techniques made uniform across all centers for quantitative coronary angiography. Intracoronary nitroglycerin was administered (150 to 200 μg), and angiograms in DICOM (Digital Imaging in Communications in Medicine) format were transferred to the angiographic core laboratory. All coronary segments 1.5 mm or greater in diameter were analyzed quantitatively and visually by blinded readers using the 29-segment standard model condensed to 19 segments for comparison with data from CTA. Quantitative coronary angiography of the most severe stenosis was performed (CAASII® QCA Research version 2.0.1 software, Pie Medical Imaging) in all nonstented segments. After all measurements from CT angiography and conventional coronary angiography were finalized and stored in the database, a detailed adjudication process was performed to ensure the correct cross-modality correspondence of segments (i.e., that the same coronary arterial segments imaged by means of each method were compared).
Statistical Analysis
Data management and statistical analyses were performed in the statistical core laboratory (Bloomberg School of Public Health) with the use of SAS® software version 9.1, Stata software version 9, and S-PLUS software version 8.0. Computation of confidence limits for AUC values for vessel-level data took account of within-patient clustering through bootstrap resampling, with 2000 replicate samples. Confidence intervals were calculated according to the percentile method. P values of less than 0.05 were considered to indicate statistical significance. All P values are two-sided, and 95% confidence intervals are also presented. In addition to the results for the entire cohort, analysis was also performed in subgroups stratified according to pretest probability of CAD. For this purpose, patients were allocated a probability score according to Morise et al. (15). Since no information was available on chest pain characteristics other than typical angina, all symptomatic patients who did not have typical angina were assumed to have atypical angina. In addition, a secondary analysis was performed for which non-cardiac chest pain was assumed in this scenario to assess its effect on re-classification.
RESULTS
Of 405 patients consented, 34 patients were excluded for technical reasons or because of severe protocol deviations, resulting in a final study population of 371 patients. Demographics and clinical characteristics of study subjects are shown in Table 1. Average age was 61±10 years and 75 % were male. The majority of patients had risk factors for CAD such as arterial hypertension, diabetes mellitus, and hyperlipidemia. Of 371 study patients, only 3 (0.8%) had a low pretest probability for obstructive CAD (all of whom had a calcium score of < 600), whereas 172 (46%) were of intermediate pretest probability, and 98 (26%) had a high pretest probability for obstructive CAD. Twenty-six percent of patients (98) had known CAD. Pretest probability for CAD in the subgroup of patients with calcium score of ≥ 600 was intermediate in 26%, high in 35%, and known CAD was present in 39% of patients. Pretest probability was intermediate in 52%, high in 24%, and known CAD was present in 23% of patients with calcium score < 600. On quantitative coronary angiography, 163/291 (56%) patients with calcium score less than 600 had at least one obstructive stenosis of 50% or greater compared to 71/80 (89%) of patients with calcium score ≥ 600 and 234/371 patients (63%) of the entire cohort. Disease prevalence (50% or greater diameter stenosis by QCA) was 48% in patients with intermediate, 70% in patients with high pretest probability for CAD, and 84% in patients with known CAD.
Table 1.
Subject Characteristics
| All Participants N=371 |
CACS < 600 N=291 |
CACS ≥ 600 N=80 |
Intermediate Pretest Probability N=172 |
High Pretest Probability N=98 |
Known CAD N=98 |
|
|---|---|---|---|---|---|---|
| Age, median | 61 (53, 67) | 60 (52, 66) | 63 (58, 70) | 58 (52, 65) | 63 (58, 71) | 63 (55, 68) |
| Men (%) | 279 (75.2) | 214 (73.5) | 65 (81.3) | 121 (70.4) | 78 (79.6) | 80 (81.6) |
| Race | ||||||
| White | 260 (70.1) | 196 (67.4) | 64 (80.0) | 119 (69.2) | 71 (72.5) | 68 (69.4) |
| Black | 20 (5.4) | 18 (6.2) | 2 (2.5) | 9 (5.2) | 3 (3.1) | 7 (7.1) |
| Asian | 78 (21.0) | 66 (22.7) | 12 (15.0) | 37 (21.5) | 21 (21.4) | 20 (20.4) |
| Other | 13 (3.5) | 11 (3.8) | 2 (2.5) | 7 (4.1) | 3 (3.1) | 3 (3.1) |
| BMI†, median | 27 (25, 30) | 27 (25, 30) | 28 (25, 32) | 27 (25, 30) | 28 (25, 32) | 27 (24, 31) |
| <19 | 6 (1.6) | 6 (2.1) | 0 (0) | 3 (1.7) | 1 (1.0) | 2 (2.0) |
| 19-30 | 276 (74.4) | 223 (76.6) | 53 (66.3) | 139 (80.8) | 63 (64.3) | 71 (72.5) |
| >30 | 89 (24.0) | 62 (21.3) | 27 (33.8) | 30 (17.4) | 34 (34.7) | 25 (25.5) |
| Hypertension | 260 (70.1) | 192 (66.0) | 68 (85.0) | 100 (58.1) | 91 (92.9) | 67 (68.4) |
| Diabetes | 97 (26.2) | 68 (23.4) | 29 (36.3) | 17 (9.9) | 45 (45.9) | 35 (35.7) |
| Dyslipidemia | 236 (63.6) | 175 (60.1) | 61 (76.3) | 82 (47.7) | 82 (83.7) | 71 (72.5) |
| Smoking | ||||||
| Current | 67 (18.1) | 56 (19.2) | 11 (13.8) | 28 (16.3) | 17 (17.4) | 21 (21.4) |
| Past | 154 (41.5) | 119 (40.9) | 35 (43.8) | 63 (36.6) | 44 (44.9) | 46 (46.9) |
| Never | 150 (40.4) | 116 (39.9) | 34 (42.5) | 81 (47.1) | 37 (37.8) | 31 (31.6) |
| FH CAD | 111 (29.9) | 74 (25.4) | 37 (46.3) | 39 (22.7) | 35 (35.7) | 36 (36.7) |
| Previous MI | 83 (22.4) | 58 (19.9) | 25 (31.3) | n/a | n/a | 83 (84.7) |
| Prior PCI | 51 (13.8) | 28 (9.6) | 23 (28.8) | n/a | n/a | 51 (52.0) |
| Angina | 212 (57.1) | 169 (58.1) | 43 (53.8) | 76 (44.2) | 86 (87.8) | 50 (51.0) |
| Canadian Class N (%) |
||||||
| 0 | 7 (1.9) | 6 (2.1) | 1 (1.3) | 4 (2.3) | 2 (2.0) | 1 (1.0) |
| 1 | 40 (10.8) | 29 (10.0) | 11 (13.8) | 11 (6.4) | 18 (18.4) | 11 (11.2) |
| 2 | 128 (34.5) | 103 (35.4) | 25 (31.3) | 48 (27.9) | 52 (53.1) | 28 (28.6) |
| 3 | 24 (6.5) | 19 (6.5) | 5 (6.3) | 9 (5.2) | 11 (11.2) | 4 (4.1) |
| 4 | 13 (3.5) | 12 (4.1) | 1 (1.3) | 4 (2.3) | 3 (3.1) | 6 (6.1) |
| Median Calcium | ||||||
| Score (IQR): | 148 (8, 478) | 80 (1, 244) | 1066 (786,1539) | 49 (0, 337) | 251(65, 653) | 303 (106,764) |
| Mean (SD): | 396 (623) | 60 (8.6) | 1325 (781) | 263 (473) | 515 (756) | 522 (669) |
| CTA Heart Rate, median, bpm |
61 (55, 69) | 62 (55, 70) | 60 (55, 67) | 62 (55, 68.5) | 62 (55, 70) | 60 (55, 67) |
Data are presented as median or mean with the interquartile range in parentheses or as total numbers with percentages in parentheses unless indicated otherwise.
Calculated as weight in kilograms divided by height in meters squared.
Abbreviations: CACS, coronary artery calcium score; CAD, coronary artery disease; IQR, interquartile range; BMI, body mass index; FH, family history; MI, myocardial infarction; PCI, percutaneous coronary intervention. CTA, computed tomography angiography; bpm, beats per minute.
Patient Based Analysis CT Angiography
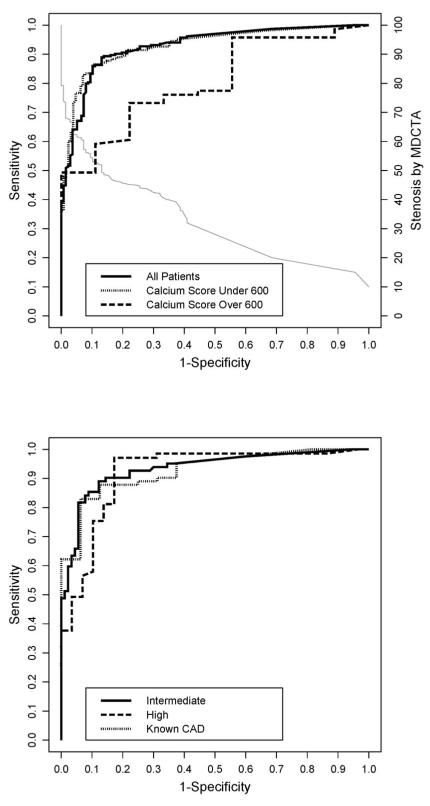
CT Diagnostic Accuracy Compared to QCA The area under the ROC curve (AUC) for detecting a 50% stenosis by quantitative CTA for the entire cohort was 0.93 (confidence interval [CI] 0.90-0-95) which is unchanged from the AUC reported in patients with calcium score <600 (0.93, CI 0.90-0.96) (13). In contrast, the AUC was only 0.81 for patients with a calcium score ≥ 600 (CI 0.71-0.89, p=0.077 vs. <600). The AUC curves are shown in Figure 1 including the calibration curve for CTA. The calibration curve allows assessing CTA performance at different thresholds for determining obstructive CAD. For example, when using a 45% stenosis threshold for determining CAD by CTA, sensitivity for the entire cohort rises to 92% while specificity decreases to 75% (Figure 1A). AUC values did not differ significantly in those patients with intermediate and high pretest probability (0.93 [0.89-0.97] vs. 0.92 [0.85-0.96], p =0.70). The AUC for patients with known CAD was also similar (0.93 [CI 0.86-0.97], Figure 1B). Furthermore, the AUC did not change (0.93 [0.90-0.96]) for the remaining 273 patients (disease prevalence 55.7%) after excluding patients with known CAD (sensitivity, specificity, positive and negative predictive values their CI were 91% [85-95], 87% [79-92], 90% [84-94], 88% [81-93]). The point statistics for CT diagnostic accuracy according to subgroups are listed in Table 2.
Figure 1. Panels A and B. Receiver-Operator-Characteristics Curves for CTA Diagnostic Accuracy According to Calcium Scores and Pretest Probability.
Panel A presents the receiver-operator-characteristic (ROC) curves for all patients, patients with calcium score less than 600, and patients with calcium score ≥ 600, describing the diagnostic performance of quantitative CT angiography (CTA) to identify a ≥ 50% coronary arterial stenosis in a patient when compared to quantitative coronary angiography (QCA). The dotted line is a calibration curve; to identify the corresponding CTA threshold point extend a vertical line from a point on the ROC curve to the calibration curve and then a horizontal line to the right ordinate, which gives the CTA threshold. For example, a sensitivity of 88% and a false positive rate (1 – specificity) of 13% correspond to a threshold point of 50% stenosis detected by CTA. The area under the curve (AUC) was 0.93 for all patients, 0.93 for patients with calcium score less than 600, and 0.80 for patients with calcium score ≥ 600 (p=0.063 vs. <600).
Panel B shows the ROC curves for patients with intermediate pretest probability of coronary artery disease, high pretest probability, and known coronary artery disease. The area under the curve (AUC) was 0.93 for patients with intermediate pretest probability of coronary artery disease, 0.92 for patients with high pretest probability, and 0.93 for patients with known coronary artery disease.
Table 2.
Diagnostic Accuracy of CTA For Detecting ≥ 50% Coronary Arterial Stenosis in Patients.
| N | CAD | Sensitivity | Specificity | PPV | NPV | AUC | |
|---|---|---|---|---|---|---|---|
|
Quantitative CTA: All |
371 | 63.1 % |
0.88 (0.83,0.92) |
0.87 (0.80,0.92) |
0.92 (0.88,0.95 |
0.81 (0.74, |
0.93 (0.90, |
|
Quantitative CTA: <600 |
291 | 56.0 % |
0.85 (0.79,0.90) |
0.90 (0.83,0.94) |
0.91 (0.86,0.95 |
0.83 (0.75,0.89 |
0.93 (0.90,0.96 |
|
Quantitative CTA: ≥600 |
80 | 88.8 % |
0.94 (0.86,0.98) |
0.44 (0.14,0.79) |
0.93 (0.85,0.98 |
0.50 (0.16,0.84 |
0.81 (0.71,0.89 |
|
Quant. CTA: w/o known CAD |
273 | 55.7 % |
0.91 (0.85,0.95) |
0.87 (0.79,0.92) |
0.90 (0.84,0.94 |
0.88 (0.81, |
0.93 (0.90, |
| Visual CTA: All | 371 | 63.1 % |
0.85 (0.80,0.89) |
0.90 (0.83,0.94) |
0.93 (0.89,0.96 |
0.78 (0.73,0.87 |
0.92 (0.89,0.95 |
|
Visual CTA: Calcium<600 |
291 | 56.0 % |
0.83 (0.76,0.88) |
0.91 (0.85,0.96) |
0.92 (0.87,0.96 |
0.81 (0.71,0.85 |
0.92 (0.89,0.95 |
|
Visual CTA: Calcium≥600 |
80 | 88.8 % |
0.96 (0.88,0.99) |
0.56 (0.21,0.86) |
0.94 (0.86,0.98 |
0.63 (0.24,0.91 |
0.86 (0.77,0.93 |
|
Quant. CTA: Intermed. PTP |
172 | 47.7 % |
0.89 (0.80,0.95) |
0.88 (0.79,0.94) |
0.87 (0.78,0.93 |
0.90 (0.81,0.95 |
0.93 (0.89,0.97 |
|
Quant. CTA: High PTP |
98 | 70.4 % |
0.93 (0.84,0.98) |
0.83 (0.64,0.94) |
0.93 (0.84,0.98 |
0.83 (0.64,0.94 |
0.92 (0.85,0.96 |
|
Quant. CTA: Known CAD |
98 | 83.7 % |
0.83 (0.73,0.90) |
0.88 (0.62,0.98) |
0.97 (0.90,1) |
0.50 (0.31,0.69 |
0.93 (0.86,0.97 |
Data are presented in all patients, patients with calcium score < 600, patients with calcium score ≥ 600, patients with intermediate pretest probability, high pretest probability, and known coronary artery disease including the 95% confidence intervals.
Abbreviations: AUC: area under the curve; CAD: coronary artery disease, Intermed.: intermediate; NPV: negative predictive value; CTA: computed tomography angiography; PTP: pretest probability; PPV: positive predictive value.
Visual CT Assessment
Visual and quantitative assessments by CTA of stenosis severity were similar (Table 2). AUC for the detection of obstructive CAD by visual assessment for the entire cohort, the initial group with calcium score < 600, and patient with calcium score ≥ 600 were 0.92, 0.93, and 0.86 respectively (p=0.36 < 600 vs. ≥ 600 calcium score group), which were not statistically significantly different from quantitative assessment (p=0.135 for ≥ 600 vs. quantitative CTA).
Analysis According to Calcium Scores and Pretest Probability of CAD
Table 3 presents the disease prevalence (50% or greater stenosis by QCA) according to calcium score brackets. While only 53 of 151 patients (35%) with calcium score < 100 had obstructive disease by QCA, 181 of 220 patients (82%) with calcium score ≥ 100 had significant disease. For patients with calcium score < 100 and intermediate pretest probability, disease prevalence was only 25% compared to 55% in patients with high pretest probability or known CAD (data were combined since they are similar in characteristics). For patients with calcium score ≥ 100, disease prevalence was similarly high for patients with intermediate and high pretest probability/known CAD (77% vs. 85%).
Table 3.
Distribution of Calcium Scores and Associated Disease Prevalences.
| Calcium Score | All | Intermediate Risk | High Risk or Known CAD |
|---|---|---|---|
| 0 | 72 (14+,58−) | 50 (7+,43−) | 20 (7+,13−) |
| 1-99 | 79 (39+,40−) | 47 (17+,30−) | 31 (21+,10−) |
| 100-199 | 52 (36+,16−) | 19 (15+,4−) | 33 (21+,12−) |
| 200-299 | 29 (25+,4−) | 11 (9+,2−) | 18 (16+,2−) |
| 300-399 | 32 (27+,5−) | 9 (5+,4−) | 23 (22+,1−) |
| 400-499 | 18 (14+,4−) | 11 (10+,1−) | 7 (4+,3−) |
| 500-599 | 9 (8+,1−) | 4 (3+,1−) | 5 (5+,0−) |
| 600+ | 80 (71+,9−) | 21 (16+,5−) | 59 (55+,4−) |
Given are the number of patients in each calcium score bracket (+/− indicates number of positive/negative findings by quantitative coronary angiography defined as 50% or greater coronary arterial stenosis)
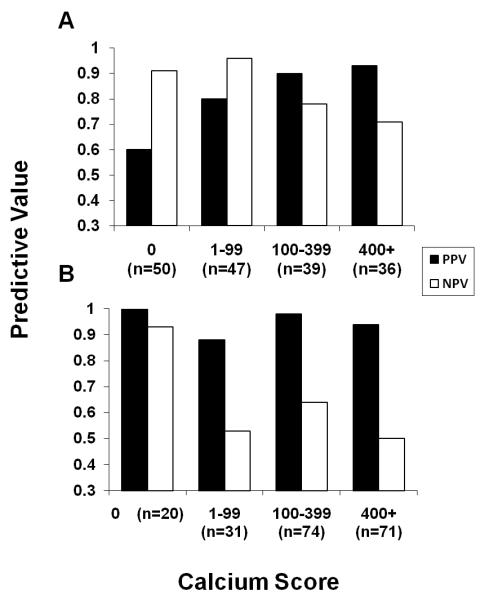
To assess the effect of coronary calcification on positive and negative predictive values, we separately analyzed data according to absent, mild, moderate, and severe calcification and pretest probability (Figure 2). Positive predictive values were very good (88-98%) and negative predictive values were poor (50-64%) for patients with high pretest probability/known CAD and any coronary calcification (calcium score > 0). Only 20/196 patients (10%) with high pretest probability/known CAD had no coronary calcification and both positive and negative predictive values were excellent for these patients (100% and 93% respectively). Among patients with intermediate pretest probability positive predictive values were modest (60-80%) and negative predictive values (91-96%) were excellent only with low calcium scores (0-99) whereas positive predictive values were very good (90-93%) and negative predictive values were poor (71-78%) with moderate and severe coronary calcification (calcium scores 100-399 and 400 and greater, Figure 2). When combining data to two groups, negative predictive value in patients with intermediate pretest probability was 93% for a calcium score of 0-99 and 75% for a calcium score 100 and greater (p=0.053). Because we assumed patients with non-anginal chest pain to have atypical rather than non-cardiac chest pain, we may have classified some patients into a higher pretest probability category. If we assumed non-cardiac chest pain instead, 10 patients would have been re-classified from high to intermediate, and 17 from intermediate to low pretest probability. The reclassifications did not result in different diagnostic accuracy for the groups: For 165 patients with intermediate pretest probability and 50.3 % disease prevalence sensitivity, specificity, positive and negative predictive values and AUC including their CI were 89% (80-95), 85% (76-92), 86% (77-93), 89% (79-95) and 0.93 (0.88-0.96). For 88 patients with high pretest probability and 70.5% disease prevalence sensitivity, specificity, positive and negative predictive values and AUC including their CI were 92% (82-97), 85% (65-96), 93% (84-98), 81% (62-94) and 0.92 (0.84-0.97). For 20 patients with low pretest probability after reclassification (disease prevalence of 35%) sensitivity, specificity, positive and negative predictive values and AUC including their lower confidence limits were 100% (59), 100% (75), 100% (59), 100% (75) and 1.00 (0.83). Figure 3 illustrates the dependency of predictive values on disease prevalence in the study population.
Figure 2. Panels A and B. Predictive Values According to Pretest Probability and Presence/Extent of Coronary Calcification.
Shown is a plot of positive and negative predictive values for patients with intermediate pretest probability of coronary artery disease (Panel A, n=172), and patients with either high pretest probability or known coronary artery disease (Panel B, n=196 combined), grouped into patients without (calcium score 0), mild (1-99), moderate (100-399), and severe coronary calcification (>400).
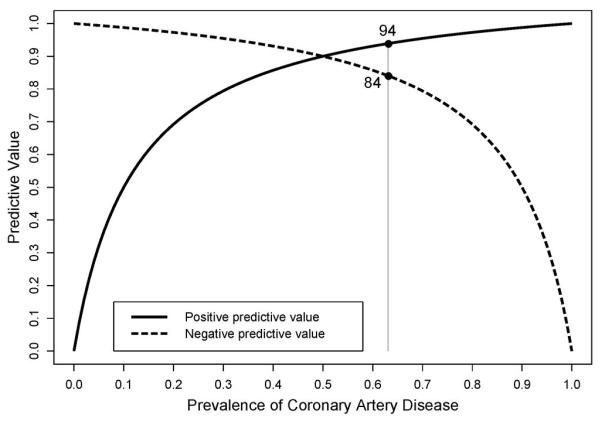
Figure 3. Predictive Values of a Diagnostic Test According to Disease Prevalence.
Shown are positive and negative predictive values as a function of disease prevalences ranging from 0-100% for a diagnostic test with a sensitivity and specificity of 90%, chosen as arbitrary but typical values. The reference line indicates the disease prevalence observed in this study (63%). One can appreciate the large shifts in predictive values according to low vs. high disease prevalence.
DISCUSSION
The main results of this investigation can be summarized as follows: 1. Despite poorer performance in patients with severe coronary calcification, inclusion of such patients did not alter the overall test performance of CT coronary angiography to detect a ≥ 50% stenosis in the CorE-64 multicenter study. 2. The diagnostic accuracy for CTA to detect obstructive CAD was reduced in patients with severe coronary calcification (calcium score of 600 or greater) compared to patients with a calcium score less than 600. 3. Pretest probability of CAD and coronary calcium score markedly affect negative predictive values for detecting obstructive CAD by CTA. 4. Noninvasive coronary angiography using CT best rules out obstructive CAD in patients with low-intermediate pretest probability of CAD and mild coronary calcification or in patients with a calcium score of zero.
CTA Diagnostic Accuracy
Compared to two other multicenter studies using 64-slice technology, our results revealed slightly lower sensitivity but higher specificity for detecting obstructive CAD by CTA, suggesting a higher reader threshold to call a given stenosis obstructive (7, 16). When combined with a relatively higher prevalence of obstructive CAD, positive predictive values were higher but negative predictive values were lower compared to other multicenter studies (7, 16). A unique feature of CorE-64 is the use of continuous quantitative CTA data in addition to semi-quantitative assessment, which allows assessing CTA performance with greater independence from reader bias. For example, if a more conservative approach was chosen, i.e., a lower threshold to call a stenosis significant such as typically employed in clinical practice, sensitivity for the entire cohort would increase from 88 to 94% for a 40% stenosis threshold, at expense of specificity which would decrease from 87 to 64% (Figure 1). These results are almost identical to reports from another multicenter study in a comparable population (16), demonstrating consistency of CTA accuracy results if methodology and patient characteristics are considered. On the other hand, comparison of our results to the ACCURACY multicenter study (7) is difficult since its disease prevalence and patient population were markedly different from ours. Nevertheless, the reported AUC, which depends only on sensitivity and specificity, of 0.96 [CI 0.94-0.98] was similar to what we found in our study.
In our study we demonstrate that predictive values critically depend on the disease prevalence in the study population. With rising disease prevalence positive predictive values increase while negative predictive values decrease. Thus, general statements regarding CTA’s or any other test’s positive or negative predictive value should be avoided as even a test with high sensitivity and specificity displays a remarkable variability for predictive values within a clinically applicable range of disease prevalences (Figure 3). Rather, a diagnostic test’s predictive value has to be seen in the context of the study population and should be applied accordingly. Both pretest probability and calcium score identify patients of different disease prevalence and may help recognizing the most adequate clinical scenario for the use of CTA.
Impact of Severe Coronary Arterial Calcification on CT Angiography Performance
Previous investigations have come to different conclusions in regards to whether severe coronary arterial calcification hampers CTA diagnostic performance. Several groups reporting same or better performance in patients with high calcium scores compared to patients with less calcification while others found poorer performance in the former (6-9). Our results revealed reduced CTA accuracy to detect obstructive CAD in patients with severe coronary calcification (≥ 600 calcium score) which was predominantly the result of markedly poorer specificity while sensitivity actually improved. The poor specificity was driven by the high disease prevalence of 89% in this subgroup, i.e., only 9 of 80 patients did not have obstructive CAD by QCA. Accordingly, the negative predictive value was low while the positive predictive value was excellent. CTA therefore is ineffective for ruling out coronary arterial stenoses in patients with severe coronary calcification who were referred for invasive angiography because of clinical suspicion of significant coronary artery disease.
Impact of Pretest Probability on CT Angiography Diagnostic Accuracy
Since predictive values are substantially influenced by the disease prevalence in the studied population, we separated our population by pretest probability for obstructive CAD. While overall test accuracy did not vary significantly among patients with intermediate and high pretest probability, and patients with known CAD, which was largely because of balanced shifts in sensitivity and specificity, negative predictive value was higher in patients with intermediate pretest probability than in other subgroups since disease prevalence was lower. If the purpose of CTA is to exclude obstructive CAD, a high negative predictive value is desired while a less than optimal positive predictive value may be acceptable. In patients with intermediate pretest probability, remarkably, both positive and negative predictive values were very good in our study with 87 and 90%, respectively. Our results reveal little on the use of CTA in patients with low pretest probability as they were poorly represented in our population. As predictive values are a function of a test’s sensitivity and specificity for a given disease prevalence, we can confidently state that the negative predictive value would exceed 95% for patients with low pretest probability (<20%) using CorE-64 data, confirming other studies documenting a high negative predictive value in a population which appears most applicable to the use of CTA at present. Our secondary analysis in 20 patients of low-intermediate pretest probability and 35% disease prevalence yielding a negative predictive value of 100% supported this notion. Conversely, our results confirm current recommendations to omit CTA for excluding obstructive CAD in patients with high pretest probability for CAD, with the possible exception of patients with zero calcium score, who, however, were a minority (10%) within this group.
Is There a Calcium Score Threshold Beyond Which CT Angiography is Not Effective?
Currently, the primary clinical value of CTA is perceived to be its ability to conclusively rule out obstructive CAD in patients of low-intermediate pretest probability with equivocal test results and atypical symptoms (1, 2). Since the probability of obstructive CAD increases with the coronary calcium score some clinicians withhold CTA in the setting of severe coronary arterial calcification (11). The coronary calcium score threshold beyond which CTA is deemed unnecessary, however, is controversial. Our analysis reveals that negative predictive values were 90% and greater in patients with intermediate pretest probability for CAD but not more than mild coronary calcification as well as in patients with any pretest probability and a calcium score of zero. Conversely, negative predictive value for CTA was highly variable and on average poor in patients with high pretest probability and known CAD with any coronary calcification, as well as in patients with intermediate pretest probability for CAD and more than mild coronary calcification (Figure 2). A coronary calcium score of ≥ 100 in patients referred for cardiac catheterization with clinical suspicion of CAD therefore identified patients who are very likely to have obstructive CAD and in whom CTA is less effective in ruling out disease. Conversely, the absence of coronary calcification is insufficient to dismiss the possibility of obstructive CAD (17) while CTA is highly effective in excluding significant coronary arterial stenoses in such patients.
Limitations
While we attempted to provide a comprehensive characterization of our patient population, our data are incomplete. We attempted to characterize the patient population using an established coronary artery disease pre-test probability score, but some factors influencing post-test probability may not have been accounted for. Our results therefore, may represent a conservative assessment of CTA’s utility in the presence of coronary calcification. Furthermore, we cannot provide data on how many patients were screened but not included in this cohort. Lastly, our patient population is international and not necessarily representative of a North American population per se.
Consistent with current guidelines (1, 2), statements in regards to the clinical usefulness of CTA are being made with respect to its ability to detect and particularly to rule out obstructive CAD. CTA on the other hand is capable of providing information about lesion location, plaque characteristics, remodeling status, and other features, which may prove to be useful for clinical management in selected situations. Therefore, while CTA’s accuracy to merely detect obstructive disease may be reduced compared to conventional angiography in patients with severe coronary calcification or high pretest probability, additional CAD assessment may outweigh this “deficiency”. This hypothesis is being tested in current investigations.
While it would be desirable from a clinical stand point to identify a calcium score “threshold” beyond which CTA is considered not useful, it has to be emphasized that such a threshold does not exist. Rather, there is a continuous shift from what one may consider an effective test to a less effective one with uncertainty of test performance in the transition zone. Therefore, as with all diagnostic test modalities, clinicians should interpret the calcium score in the greater context for guidance of how to interpret the CTA rather than making binary decisions based on particular score thresholds.
Lastly, some of the secondary analyses contained in this investigation contained relatively small numbers and results must be interpreted with caution.
Conclusions
A diagnostic test’s ability to predict or to exclude disease is critically dependent not only on its sensitivity and specificity but also on the disease prevalence within the study population. Accordingly, our results demonstrate that pretest probability for coronary artery disease and coronary calcium score, which are both predictive of disease prevalence, are important for the effectiveness of CT coronary angiography to exclude or confirm the presence of obstructive coronary artery disease in patients. CT angiography accurately rules out obstructive coronary artery disease in patients with low or intermediate pretest probability who have low coronary calcium scores as well as in patients with any pretest probability and a calcium score of zero. Conversely, CT angiography is less effective for this purpose in patients with high pretest probability, known coronary artery disease, and patients with extensive coronary calcification. In patients with clinical suspicion of coronary artery disease sufficient to consider cardiac catheterization, moderate or severe coronary calcification alone is highly predictive of obstructive coronary artery disease.
Acknowledgments
FUNDING SOURCES This investigation was supported by grants from Toshiba Medical Systems; the Doris Duke Charitable Foundation; the National Heart, Lung, and Blood Institute (RO1-HL66075-01 and HO1-HC95162-01); the National Institute on Aging (RO1-AG021570-01); and the Donald W. Reynolds Foundation.
Abbreviations
- CTA
Computed Tomography angiography
- CAD
Coronary Artery Disease
- AUC
Area Under The (ROC) Curve
- ROC
Receiver Operating Characteristics
- QCA
Quantitative Coronary Angiography
- ECG
Electrocardiogram
- CorE-64
Coronary Artery Evaluation Using 64-Row Multi-Detector Computed Tomography Angiography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES Dr. Arbab-Zadeh serves on the steering committee of the CORE-320 study which is sponsored by Toshiba Medical Systems. Drs. Miller, Dewey, Paul, Shapiro, Lardo, and Lima report receiving grant support from Toshiba Medical Systems; Drs. Dewey, Paul, Hoe, Lardo, Bush, and Lima, speakers’ fees from Toshiba Medical Systems; Dr. Dewey, speaker’s fees from Bayer and Schering and grant support from GE Healthcare and Bracco; and Dr. Paul, advisory fees from Vital Images. Dr. Hoe reports serving as director of the Cardiac CT Training Course sponsored by Toshiba Medical Systems, Asia, and receiving speaker’s fees from GE Biosciences. Dr. Lardo reports receiving grant support from CT Core Laboratory; Dr. Bush, speaker’s fees from Bristol-Myers Squibb and Sanofi-Aventis; and Dr. Lima, grant support from GE Medical Systems. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography: A report of the american college of cardiology foundation appropriate use criteria task force, the society of cardiovascular computed tomography, the american college of radiology, the american heart association, the american society of echocardiography, the american society of nuclear cardiology, the north american society for cardiovascular imaging, the society for cardiovascular angiography and interventions, and the society for cardiovascular magnetic resonance. J Am Coll Cardiol. 2010;56:1864–94. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: A scientific statement from the american heart association committee on cardiovascular imaging and intervention, council on cardiovascular radiology and intervention, and committee on cardiac imaging, council on clinical cardiology. Circulation. 2006;114:1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 3.Meijboom WB, van Mieghem CA, Mollet NR, et al. 64-slice computed tomography coronary angiography in patients with high, intermediate, or low pretest probability of significant coronary artery disease. J Am Coll Cardiol. 50:1469–75. doi: 10.1016/j.jacc.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Husmann L, Schepis T, Scheffel H, et al. Comparison of diagnostic accuracy of 64-slice computed tomography coronary angiography in patients with low, intermediate, and high cardiovascular risk. Acad Radiol. 2008;452:61. doi: 10.1016/j.acra.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Schenker MP, Dorbala S, Hong EC, et al. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: A combined positron emission tomography/computed tomography study. Circulation. 2008;117:1693–700. doi: 10.1161/CIRCULATIONAHA.107.717512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong TK, Chin SP, Liew CK, et al. Accuracy of 64-row multidetector computed tomography in detecting coronary artery disease in 134 symptomatic patients: Influence of calcification. Am Heart J. 2006;151:1323.e1–1323.e6. doi: 10.1016/j.ahj.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter ACCURACY (assessment by coronary computed tomographic angiography of individuals undergoing invasive coronary angiography) trial. J Am Coll Cardiol. 2008;52:1724–32. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Cordeiro MA, Miller JM, Schmidt A, et al. Non-invasive half millimetre 32 detector row computed tomography angiography accurately excludes significant stenoses in patients with advanced coronary artery disease and high calcium scores. Heart. 2006;92:589–97. doi: 10.1136/hrt.2005.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pundziute G, Schuijf JD, Jukema JW, et al. Impact of coronary calcium score on diagnostic accuracy of multislice computed tomography coronary angiography for detection of coronary artery disease. J Nucl Cardiol. 2007;14:36–43. doi: 10.1016/j.nuclcard.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Abbara S, Arbab-Zadeh A, Callister TQ, et al. SCCT guidelines for performance of coronary computed tomographic angiography: A report of the society of cardiovascular computed tomography guidelines committee. J Cardiovasc Comput Tomogr. 2009;3:190–204. doi: 10.1016/j.jcct.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Hecht HS, Bhatti T. How much calcium is too much calcium for coronary computerized tomographic angiography? J Cardiovasc Comput Tomogr. 2008:183–7. doi: 10.1016/j.jcct.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–36. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 13.Miller JM, Dewey M, Vavere AL, et al. Coronary CT angiography using 64 detector rows: Methods and design of the multi-centre trial CORE-64. Eur Radiol. 2009;816:28. doi: 10.1007/s00330-008-1203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia MJ, Lessick J, Hoffmann MH, CATSCAN Study Investigators Accuracy of 16-row multidetector computed tomography for the assessment of coronary artery stenosis. JAMA. 2006;296:403–11. doi: 10.1001/jama.296.4.403. [DOI] [PubMed] [Google Scholar]
- 15.Morise AP, Haddad WJ, Beckner D. Development and validation of a clinical score to estimate the probability of coronary artery disease in men and women presenting with suspected coronary disease. Am J Med. 1997;102:350–6. doi: 10.1016/s0002-9343(97)00086-7. [DOI] [PubMed] [Google Scholar]
- 16.Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: A prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–44. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 17.Gottlieb I, Miller JM, Arbab-Zadeh A, et al. The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol. 2010;55:627–34. doi: 10.1016/j.jacc.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]