Payment reform may be used to better align appropriate financial incentives with better quality of care.
Abstract
Purpose:
To assess the impact of 2005 and 2006 reductions in chemotherapy reimbursement, mandated in the Medicare Modernization Act, on patterns of chemotherapy receipt in the last 14 days of life.
Patients and Methods:
Included in the study were Medicare beneficiaries dying with poor-prognosis cancer from 2003 to 2007. We compared pre- and postreform probability and frequency of chemotherapy receipt in the last 14 days of life, a validated quality measure, using linear models. We assessed changes in chemotherapy use in physician offices (where prescribing is often directly linked to physician income) and hospital outpatient departments (where the link is indirect and likely weaker).
Results:
Among patients receiving chemotherapy in the 6 months before death in physicians' offices before the policy implementation (2003 to 2004), 18% received chemotherapy in the last 14 days of life. Those dying after implementation (2006 to 2007) were 3.5 percentage points (95% CI, −5.4 to −1.6; P < .001), or 20%, less likely to receive chemotherapy in the 14 days before death than those dying before implementation. By contrast, there was no significant change in the percentage of patients receiving chemotherapy in the last 14 days of life in hospital outpatient departments between 2003 and 2004 and 2006 to 2007.
Conclusion:
In physician offices, where drugs generate the majority of revenue and prescribing patterns can determine physician income, use of chemotherapy at the end of life fell significantly after reimbursement reductions; no concurrent change occurred in hospital outpatient departments. These results suggest that payment reform may be used to better align appropriate financial incentives with better quality of care.
Introduction
In the early 2000s, Medicare drug reimbursements provided high margins under an average wholesale price system, which permitted physicians to obtain drugs well below reimbursed rates.1 In response to concerns about rapid growth in the use of chemotherapy drugs with high margins, the Medicare Modernization Act (MMA) changed reimbursements to more closely reflect acquisition costs.2 The MMA reduced payments to 106% of manufacturer-reported average sales prices while increasing drug administration fees; the change took effect in physician offices in 2005 and in hospital outpatient departments in 2006. The Government Accountability Office estimated that the MMA reform would reduce the average profit margin on infused chemotherapy drugs from 22% to 6%.1 Payments for chemotherapy and anemia medications dropped by $1.2 billion between 2004 and 2005, after implementation of the reform in physicians' offices.3 This payment change had a large impact on oncologist practice revenue, because drug reimbursement accounted for 77% and drug administration accounted for approximately 10% of practice revenue during this time period.4
Policy makers have noted the potential conflicts of interest inherent in the pre-MMA system of payment for chemotherapy treatments.3,5–10 Studies assessing the effect of MMA reimbursement changes on access to and use of chemotherapy in patients with newly diagnosed cancer have generated mixed results.2,11–14 Despite fears that physicians would cease to offer chemotherapy services in their offices, restricting access to care and increasing volume in hospital outpatient departments,7 two studies found no meaningful effects on access to chemotherapy as measured by wait time, travel time, or infusion locale.13,14 Using Medicare data 6 and 10 months into the payment change, respectively, the Medicare Payment Advisory Commission2 and Jacobson et al12 found increased use of chemotherapy in patients with newly diagnosed cancer and evidence of switching to more costly agents. However, these studies focused on newly diagnosed patients cared for in a short window after MMA implementation and provide little sense of how changes affected quality of care.
The effect of financial incentives on service delivery may be most pronounced in cases of marginal or low value,11 such as chemotherapy at the end of life, where aggressive anticancer therapies may prove to be more toxic than beneficial, use is not related to probability of benefit, and appropriately timed cessation of chemotherapy is integral to a patient's terminal quality of life.15–17
We augment evidence on the impact of payment reform for Part B drugs and provider response to financial incentives through a study of end-of-life chemotherapy treatment.11–14,18,19 We expand current understanding in three ways. We examined a measure of the quality of cancer care—chemotherapy receipt in the last 14 days of life, a validated quality measure now included as a benchmark for improving clinical practice in the Quality Oncology Practice Initiative (QOPI).16,20 Second, we examined the effects of payment changes in physician office settings separately from hospital outpatient department settings to leverage differences in financial incentives. In office settings, chemotherapy use is often directly related to physician income and makes up a large portion of practice revenue; in the hospital outpatient setting, drug reimbursement makes up a much smaller proportion of the revenue mix, and the link between physician income and prescribing patterns is indirect and likely weaker. We hypothesized changes in chemotherapy payment were more likely to alter prescribing decisions in physicians' offices; modeling the groups separately allowed us to disentangle changes in behavior as a result of reimbursement from broader trends in end-of-life care. Finally, by examining data through 2007, we were able to observe effects of the policy change that occurred over a longer time horizon compared with previous studies and thus did not limit our assessment to immediate policy response.
Patients and Methods
We used Medicare claims data for beneficiaries dying with poor-prognosis cancer from 2003 to 2007 and assessed pre- (2003 to 2004) and post- (2006 to 2007) reform probability and frequency of chemotherapy receipt in the last 14 days and 3 months of life. We analyzed trends in treatment before and after reform by treatment location (physician offices and hospital outpatient departments).
Cohort Definition
From the 20% Medicare denominator files spanning 2003 to 2007, we identified fee-for-service Medicare beneficiaries who died between age 66 to 99 years and had continuous Parts A and B coverage in the last 6 months of life. Decedents were included in the study if they had at least one hospital claim or at least two clinician visits in the last 6 months of life with poor-prognosis cancer.21,22 Poor-prognosis cancer was defined by Iezzoni et al21 using claim diagnosis codes associated with high rates of death during hospital admissions, thus permitting us to create a cohort for whom providers likely understood prognosis to be poor in the last 6 months of life. We categorized decedents into one of 26 cancer types based on their predominant cancer diagnosis.20a
Outcomes
For each patient, we used billing codes to assess receipt of outpatient chemotherapy (administered by a clinician or facility) in the last 6 months of life, last 3 months of life, and last 14 days of life (codes defined in Appendix Table A1, online only). Our main outcome of interest was receipt of chemotherapy in the last 14 days of life, conditional on receipt in the last 6 months of life. We chose this measure because it is similar to the quality metric defined by Earle et al.16,23,24 We determined setting of chemotherapy infusion based on claim file type and place of service code during the last 6 months of life (hospital outpatient department, physician office, or both). We also assessed the number of chemotherapy treatments in each time window among those who received chemotherapy, defined as the number of days of treatment.
Patient Characteristics
From Medicare files, we obtained patient age at death, race (dichotomized as black or nonblack), state of residence, and sex. We used the methods of Iezzoni et al21 to assign patients up to eight noncancer chronic conditions based on one inpatient or two outpatient International Classification of Diseases, Ninth Revision, diagnosis claims occurring between 6 months and 1 month before death (Table 1).25 We categorized patients' metastatic cancer status using the definitions of Iezzoni et al.21 Each patient's residential zip code was used to assign an estimated household income based on US Census tract data and the proportion of the population in that area in poverty.26,27 Models included these patient characteristics as well as cancer type and age squared.
Table 1.
Descriptive Characteristics of End-of-Life Cancer Cohort of Medicare Beneficiaries Dying With Poor-Prognosis Cancer: 2003 to 2007
| Characteristic | Decedent Cancer Cohort |
Lung Cancer Cohort |
||||||
|---|---|---|---|---|---|---|---|---|
| Total | Received Chemotherapy in Last 6 Months of Life |
Total | Received Chemotherapy in Last 6 Months of Life |
|||||
| Physician Office Only | Outpatient Department Only | Both Settings | Physician Office Only | Outpatient Department Only | Both Settings | |||
| No. of patients | 235,821 | 55,450 | 15,503 | 4,073 | 74,581 | 18,857 | 4,610 | 1,155 |
| Age at death, years | 78.1 | 76.0 | 74.7 | 74.5 | 76.9 | 74.7 | 73.9 | 73.9 |
| Black race, % | 9.2 | 8.0 | 10.2 | 8.2 | 8.4 | 7.0 | 10.2 | 8.0 |
| Female sex, % | 48.6 | 41.7 | 46.9 | 44.1 | 44.4 | 39.2 | 41.2 | 38.5 |
| Zip code | ||||||||
| Income level, $ | 44,482 | 45,459 | 45,240 | 45,760 | 43,675 | 44,740 | 43,258 | 44,101 |
| Poverty rate, % | 10.5 | 10.0 | 10.5 | 9.9 | 10.6 | 10.0 | 11.1 | 10.4 |
| Type of cancer, % | ||||||||
| Metastatic | 54.7 | 66.2 | 67.1 | 67.1 | 48.0 | 61.2 | 62.1 | 64.7 |
| Lung | 31.6 | 34.0 | 29.7 | 28.4 | — | — | — | — |
| Breast | 5.3 | 7.5 | 8.0 | 7.4 | — | — | — | — |
| Prostate | 5.9 | 12.7 | 6.3 | 10.2 | — | — | — | — |
| Pancreatic | 6.4 | 6.4 | 6.5 | 6.7 | — | — | — | — |
| Hematologic | 8.9 | 7.1 | 8.3 | 10.7 | — | — | — | — |
| Colorectal | 8.1 | 9.5 | 9.4 | 10.2 | — | — | — | — |
| Liver | 3.1 | 1.3 | 1.8 | 1.5 | — | — | — | — |
| Unknown primary | 9.6 | 3.1 | 5.8 | 2.7 | — | — | — | — |
| Comorbidities, % | ||||||||
| Chronic pulmonary disease | 42.0 | 40.3 | 36.2 | 35.1 | 68.1 | 67.1 | 63.1 | 64.5 |
| Coronary artery disease | 31.2 | 29.6 | 25.7 | 26.2 | 34.2 | 32.9 | 30.3 | 30.4 |
| Congestive heart failure | 30.3 | 25.9 | 21.9 | 24.5 | 32.1 | 27.2 | 24.5 | 28.1 |
| Peripheral vascular disease | 9.8 | 8.3 | 7.0 | 6.7 | 12.3 | 10.8 | 9.9 | 9.4 |
| Severe chronic liver disease | 2.3 | 1.2 | 1.7 | 1.0 | 0.9 | 0.6 | 0.8 | 0.5 |
| Diabetes with end organ damage | 4.2 | 3.5 | 3.2 | 3.3 | 3.6 | 3.1 | 3.0 | 2.8 |
| Chronic renal failure | 12.2 | 10.5 | 9.0 | 8.5 | 9.7 | 7.2 | 7.2 | 5.2 |
| Dementia | 10.6 | 4.6 | 3.8 | 2.9 | 9.5 | 3.6 | 3.6 | 3.0 |
| Year of death, % | ||||||||
| 2003 | 20.4 | 20.8 | 17.2 | 23.4 | 20.4 | 21.0 | 17.4 | 22.9 |
| 2004 | 20.4 | 21.2 | 18.6 | 24.3 | 20.3 | 20.8 | 19.3 | 26.0 |
| 2005 | 20.4 | 20.8 | 20.2 | 24.1 | 20.5 | 21.0 | 20.0 | 22.6 |
| 2006 | 19.6 | 19.0 | 21.3 | 14.1 | 19.7 | 19.2 | 20.5 | 13.8 |
| 2007 | 19.2 | 18.2 | 22.7 | 14.1 | 19.2 | 18.0 | 22.8 | 14.7 |
| Outcomes, % | ||||||||
| Received chemotherapy in last 6 months of life | 32 | 100 | 100 | 100 | 33 | 100 | 100 | 100 |
| Received chemotherapy in last 3 months of life | 77 | 77 | 73 | 89 | 79 | 79 | 74 | 87 |
| Received chemotherapy in last 14 days of life | 15 | 16 | 12 | 19 | 17 | 18 | 13 | 21 |
| No. of chemotherapy treatments (among receivers) | ||||||||
| Last 6 months of life | 6.7 | 6.7 | 5.5 | 10.1 | 7.0 | 7.1 | 5.6 | 10.0 |
| Last 3 months of life | 4.1 | 4.1 | 3.4 | 5.5 | 4.3 | 4.3 | 3.5 | 5.7 |
| Last 14 days of life | 1.5 | 1.5 | 1.4 | 1.6 | 1.5 | 1.5 | 1.4 | 1.8 |
Analyses
We conducted two sets of analyses. We modeled the distinct individual monthly impacts of the 2005 and 2006 policy implementations and then considered their impact collectively as a single payment change, implementation of which spanned 12 months.
We plotted regression-adjusted mean predicted probabilities that a patient received chemotherapy in the last 2 weeks of life and last 3 months of life by setting of treatment (Fig 1). We estimated ordinary least squares models of the probability of chemotherapy receipt or frequency of chemotherapy receipt as a function of payment change, where the patient was the unit of analysis. We captured payment change with a linear time trend with coefficients to capture changes in the time trend in January 2005 and January 2006. We chose the linear time trend because it fit the raw data accurately and is readily interpretable. In sensitivity tests of the specification, we obtained similar results estimating probability of chemotherapy receipt using logistic regression. All regression models controlled for a linear time trend to capture trends in end-of-life treatment during the full time period. We estimated models for the entire cohort and separate models by site of chemotherapy receipt in the last 6 months (physician office or outpatient department). We also predicted each outcome without payment reform, assuming the time trend from the period before the payment change (2003 to 2004) persisted (ie, as if changes in trend in January 2005 and January 2006 were zero).
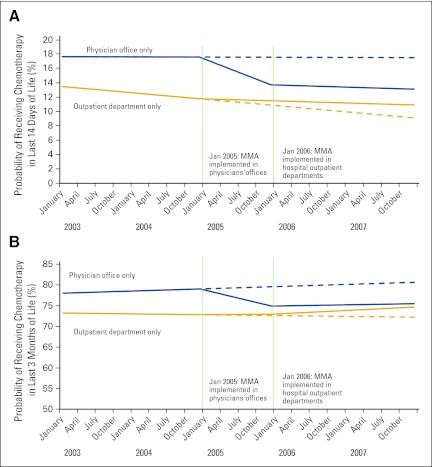
Figure 1.
Probability of receiving chemotherapy in (A) last 14 days of life and (B) last 3 months of life by location of chemotherapy receipt. Sample includes those who received chemotherapy in the last 6 months of life (physician office, n = 55,450; hospital outpatient department, n = 15,503). Mean predicted probabilities control for patient demographics, comorbidities, cancer type, metastatic disease, and linear time trend. The 2005 payment change is significant in physician offices but not significant in hospital outpatient departments; the 2006 payment change is not significant in either setting. Solid lines incorporate the linear effect of the payment change; dashed lines indicate the counterfactual, if prepayment reform trends had continued. Probabilities for those receiving care in both locations are not shown because the sample is small. MMA, Medicare Modernization Act.
We repeated these models after dropping patients who died in 2005 and estimated the cumulative effect of the payment change in January 2005 (physician offices) and January 2006 (hospital outpatient departments) as a single event, controlling for a linear time trend and the same covariates. This model has two advantages. It estimates the magnitude of the overall effect of the MMA on probability and frequency of chemotherapy receipt, allowing for a transition period as the payment change in physicians' offices had been in place for 1 year. Second, it excludes the cohort whose last 6 months before death spanned the pre-2005 and post-2005 periods, yielding a cleaner definition of the pre- and postperiod observations. We adjusted variance estimates for the correlation of observations within state and time period (before January 2005, after December 2005) using Huber-White sandwich estimators.28,29 Because previous publications studying effects of the MMA have focused specifically on incident lung cancer,12 we repeated our analysis for the subset of patients with lung cancer.
Results
Descriptive Characteristics
Overall, 235,821 patients met inclusion criteria; 32% received chemotherapy in the last 6 months of life (n = 75,026; Table 1). Mean age at death was 78.1 years; 49% were women; 9% were black. The most common cancer types were: lung cancer (32%), cancer of unknown primary (10%), and hematologic cancer (9%). Among patients who received chemotherapy within 6 months of death, 74% received all chemotherapy treatments in a physician's office, 21% received all treatments in a hospital outpatient department, and 5% received treatment in both places. Receipt of chemotherapy in hospital outpatient departments increased over the time period (from 18% in 2003 to 25% in 2007). Demographics varied by setting of administration, with those receiving chemotherapy in a physician's office significantly more likely to be older, nonblack, male, and from a wealthier zip code. Those with more comorbidities were treated in physicians' offices. Setting-specific population characteristics were similar among the subset of patients with lung, although these patients were more likely to be younger, nonblack, and poorer and have more comorbidities relative to the full sample.
Unadjusted Outcomes
Among those receiving chemotherapy in the last 6 months of life, 77% received chemotherapy in the last 3 months of life, and 15% received chemotherapy in the last 14 days of life over the full 2003 to 2007 time period (Table 1). Overall, chemotherapy receipt near the end of life was significantly more likely for those treated in physician office settings versus hospital outpatient departments. Of those treated in physician offices, 77% received chemotherapy in the last 3 months of life, and those who received chemotherapy in this window averaged 4.1 days of treatment. In physicians' offices, 16% received chemotherapy in the last 14 days of life and averaged 1.5 days of treatment. Of those using outpatient departments for treatment, 73% received chemotherapy in the last 3 months of life (averaging 3.4 days of treatment), and 12% received chemotherapy in the last 14 days (averaging 1.4 days of treatment). All of these differences across settings are statistically significant, with P values below .05. Patterns were similar among the subset of patients with lung cancer.
Regression-Adjusted Results
Figure 1 graphically presents results from models considering the two policy implementation events separately. It indicates that within both time windows (14 days and 3 months) before death, the probability of chemotherapy receipt dropped significantly after the payment change in physician offices, whereas it increased slightly in hospital outpatient departments. Patterns were similar among those with lung cancer.
Table 2 lists the coefficients on the payment change when transitional data from 2005 are dropped, and the payment change is measured as a one-time shift, controlling for patient demographics, comorbidities, cancer type, metastatic disease, and a linear time trend. Comparing 2006 and 2007 with 2003 and 2004, the rate of chemotherapy receipt in the last 14 days of life fell 2.6 percentage points (or 2.6 per 100 patients), a drop of approximately 20% (95% CI, −4.2 to −1.0; P = .002). The drop was driven entirely by reductions in terminal chemotherapy in physician offices, where the probability of chemotherapy in the last 14 days dropped 3.5 per 100 patients (95% CI, −5.4 to −1.6; P < .001) relative to the mean of 18% in the preperiod. For those who received treatment in a hospital outpatient department, there was no significant change over the same time period (95% CI, −2.4 to 4.4; P = .541). We also observed significant reductions in chemotherapy for the last 6 months of life (2.5 per 100 patients; 95%CI, −3.7 to −1.3; P < .001) and the last 3 months of life (4.2 per 100 patients; 95% CI, −6.1 to −2.2; P < .001). In the lung cancer cohort, there were no significant changes in chemotherapy in the last 14 days, but estimates of changes in probability in the last 3 and 6 months were similar in significance and magnitude to the full cohort. Because the composition of cancer type varied across the two settings, and differences in treatment for lung cancer (such as epidermal growth factor receptor inhibitors as a later line of therapy) could be a possible confounder, we estimated a model excluding patients with lung cancer. The results were unchanged.
Table 2.
Effect of MMA Reimbursement Change on Chemotherapy Receipt by Location of Administration in Medicare Beneficiaries Dying With Poor-Prognosis Cancer (2006 to 2007 v 2003 to 2004)
| Setting | Change in Probability of Receipt in Last: |
Change in No. of Treatments in Last:* |
||||
|---|---|---|---|---|---|---|
| 14 Days | 3 Months | 6 Months | 14 Days | 3 Months | 6 Months | |
| All patients | ||||||
| No. of patients | 57,656 | 57,656 | 182,426 | 8,602 | 44,203 | 57,656 |
| All settings | ||||||
| Mean (2003 to 2004) | 17% | 78% | 32% | 1.5 | 4.5 | 7.3 |
| Coefficient | −0.026† | −0.042† | −0.025† | −0.087 | −0.607† | −0.953† |
| SE | 0.008 | 0.012 | 0.005 | 0.050 | 0.098 | 0.164 |
| Physician office only | ||||||
| Mean (2003 to 2004) | 18% | 78% | 100% | 7.3 | 4.5 | 1.5 |
| Coefficient | −0.035† | −0.055† | −0.084 | −0.684† | −0.998† | |
| SE | 0.009 | 0.013 | 0.054 | 0.110 | 0.178 | |
| Outpatient department only | ||||||
| Mean (2003 to 2004) | 12% | 73% | 100% | 1.5 | 3.7 | 5.9 |
| Coefficient | 0.010 | 0.008 | −0.030 | 0.094 | −0.286 | |
| SE | 0.017 | 0.025 | 0.101 | 0.146 | 0.236 | |
| Physician office and outpatient department | ||||||
| Mean (2003 to 2004) | 20% | 89% | 100% | 1.6 | 6.0 | 10.9 |
| Coefficient | −0.024 | −0.004 | −0.113 | −0.979† | −1.131 | |
| SE | 0.035 | 0.030 | 0.260 | 0.372 | 0.671 | |
| Lung cancer cohort | ||||||
| No. of patients | 18,937 | 18,937 | 57,663 | 3,201 | 14,898 | 18,937 |
| All settings | ||||||
| Mean (2003 to 2004) | 19% | 80% | 33% | 1.5 | 4.8 | 7.8 |
| Coefficient | −0.014 | −0.046† | −0.023† | −0.038 | −0.877† | −1.373† |
| SE | 0.014 | 0.017 | 0.010 | 0.092 | 0.139 | 0.247 |
| Physician office only | ||||||
| Mean (2003 to 2004) | 20% | 81% | 100% | 1.5 | 4.8 | 7.9 |
| Coefficient | −0.007 | −0.051† | −0.004 | −0.948† | −1.389† | |
| SE | 0.017 | 0.019 | 0.108 | 0.164 | 0.272 | |
| Outpatient department only | ||||||
| Mean (2003 to 2004) | 15% | 74% | 100% | 1.5 | 4.1 | 6.2 |
| Coefficient | −0.047 | −0.022 | −0.201 | −0.173 | −0.806‡ | |
| SE | 0.034 | 0.036 | 0.227 | 0.273 | 0.399 | |
| Physician office and outpatient department | ||||||
| Mean (2003 to 2004) | 21% | 88% | 100% | 1.7 | 6.1 | 10.8 |
| Coefficient | −0.022 | −0.044 | 0.320 | −1.227 | −1.699 | |
| SE | 0.081 | 0.069 | 0.514 | 0.810 | 1.124 | |
NOTE. The all-settings group includes patients from each of the three mutually exclusive groups (ie, physician office only, outpatient department only, both). No. of patients applies to the all-settings group. Models control for patient demographics, comorbidities, cancer type, metastatic disease, and linear time trend. Heteroskedastic-robust SEs are clustered at the state level and pre-post time period.
Abbreviation: MMA, Medicare Modernization Act.
Beneficiaries were only included in No. of treatments model if they received chemotherapy in the specified time window.
Indicates significance at the 95% level.
Indicates significance at the 90% level.
In the full cohort who received chemotherapy during the window, the number of treatments per patient declined by 0.95 treatments in the last 6 months (95% CI, −1.3 to −0.6; P < .001) and 0.61 treatments in the last 3 months (95% CI, −0.8 to −0.4; P < .001). Taking into account the reductions in probability of receipt and frequency of receipt after the payment change in this cohort of patients with poor-prognosis cancers, we estimate that during a 2-year period, there were 546,000 fewer chemotherapy treatments in the last 6 months of life, and the number of patients treated in the last 14 days fell by 7,900. Using the postreform 2006 average payment per chemotherapy treatment ($720) leads to an estimated additional cost savings (through the reduction in quantity) of roughly $400 million.
Discussion
The MMA entailed sharp cutbacks in chemotherapy payments from 2005 to 2006. In this study, we found that chemotherapy receipt in the last 14 days of life fell 20% for patients treated in physicians' offices after the MMA legislation was implemented, with no corresponding decline among those treated in hospital outpatient departments. Previous articles have examined responses to the MMA in use of chemotherapy in newly diagnosed patients but have provided little evidence to determine whether treatment changes improved or degraded quality of care.2,12 Near the end of life, aggressive anticancer therapies may prove to be more toxic than beneficial, their use may not be related to probability of providing benefit, and appropriately timed cessation of chemotherapy is integral to a patient's terminal quality of life.15,16,24,30
Can chemotherapy in the last 14 days of life be used as an outcome measure? Clearly, the right rate of treatment in the last 14 days is not zero, because some deaths, even for those with metastatic cancer where chemotherapy is appropriate, occur unexpectedly. Yet chemotherapy in the last 14 days is a well-established and validated quality measure.15,16,24 Furthermore, there is little reason why the unanticipated mortality rate for patients being treated with chemotherapy should have declined between 2003 and 2004 and 2006 to 2007 only in physicians' offices but not in hospital outpatient departments.
Why are the effects of payment reform so much greater in physicians' offices compared with hospital outpatient departments? One reason may be that in hospital outpatient departments, physicians have no direct incentive to order chemotherapy of marginal benefit, and the fraction of total revenue composed of drug reimbursement is small. If the decrease in the use of end-of-life chemotherapy at this time were driven by broader trends such as diffusion of quality standards by oncology groups, the increasing acceptance of palliative care,31 or the introduction of new drugs, we would expect to see equivalent changes in both settings. Unfortunately, we cannot observe the differences between practices in treatment of drug revenue; some practices decouple personal income from chemotherapy use. This should also be considered as a policy option.
An important potential limitation to our study is the simultaneous cultural shift seen in the oncology community driven by end-of-life cost/benefit concerns and emerging models addressing the demands for better physician and patient communication.32 Hospice referral and discussions about end-of-life care have become recognized as critical needs for patients with cancer. We controlled for trends in chemotherapy that might be driven by this practice shift. A differential trend in hospice awareness for hospital outpatient and office settings could lead to biased estimates, but we saw no evidence of any such differential trends before the policy change. Chemotherapy at the end of life may be a sensitive metric with which to assess these cultural shifts. The American Society of Clinical Oncology (ASCO) QOPI addresses these metrics, but during our study period, it had limited penetration into oncology practices. (QOPI became available to all ASCO physicians in March 2006 as a voluntary quality measurement project, and 87 practices participated in 2006 [< 9% of practices currently participating and a smaller proportion of all oncology practices].) We detected a change in prescribing behavior in the last 6 months and last 3 months of life as well, where the 14-day quality metric would have had less impact. These reductions in use of chemotherapy 3 and 6 months before death indicate that payment reform in the MMA may have been too blunt of an instrument. Although quality of care may have improved in the 14 days before death, it may have been at the expense of quality in earlier time periods. As in all pre- and postpolicy analyses, a limitation of our study is possible omitted trends. Our results could potentially have been influenced by patient compositional changes in hospital outpatient departments versus physician offices. If the composition of those treated in physician offices shifted toward populations that typically receive less intense end-of-life therapy, then our findings could have resulted from composition changes. However, there were no appreciable changes in the composition of measured demographic characteristics such as race and sex in either setting, making it less likely that differential trends in unmeasured characteristics could explain our results.
Our study provides evidence that for a range of services with marginal value, a reduction in fee-for-service reimbursement can better align payment with quality-of-care goals. Our findings are in accordance with a study that found MMA reimbursement changes were associated with reduced use of discretionary or inappropriate androgen deprivation therapy but were not associated with any changes to appropriate use of androgen deprivation therapy.11 These studies provide support for removing incentives promoting drug use from cancer care through reform including value-based insurance design,33 bundled payment for chemotherapy,8,34 and capitated or prospective payment models.35,36
Acknowledgment
Supported by National Institutes on Aging Grant No. P01AG19783 and American Cancer Society Research Grant No. IRG-82-003-26. We thank Harold Sox for helpful comments on the manuscript.
Appendix
Table A1.
Codes Used to Identify Receipt of Chemotherapy
| Code | Description |
|---|---|
| ICD-9 codes V58.1x, V67.2 | Encounter for chemotherapy or postchemotherapy care |
| ICD-9 code 99.25 | Injection/infusion of chemotherapy |
| HCPCS beginning with J9xxx, 90,586, G0355, G0356, G0359-61, G9021-G9032, J8510, J8520, J8521, J8560, J8565, J8600, J8700 | Agent-specific chemotherapy administration codes |
| HCPCS J8999 | Prescription of oral chemotherapy |
| CPT 964xx, 96,542, 96,545, 96,549 | Outpatient chemotherapy administration |
| CPT 99,555 | Home infusion of chemotherapy |
| HCPCS Q-codes Q0083 Q0084 Q0085 | Home administration of chemotherapy |
| Revenue center codes 0331, 0332, 0335 | Oral, injected, or intravenous chemotherapy |
Abbreviations: CPT, Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System; ICD-9, International Classification of Diseases, Ninth Revision.
Author's Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: J. Russell Hoverman, Texas Oncology (C), US Oncology (C) Consultant or Advisory Role: J. Russell Hoverman, United Healthcare (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: All authors
Financial support: Carrie H. Colla, Jonathan S. Skinner
Administrative support: Carrie H. Colla
Provision of study materials or patients: Jonathan S. Skinner
Collection and assembly of data: Carrie H. Colla, Nancy E. Morden, Ellen Meara
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.US Government Accountability Office. Medicare chemotherapy payments: New drug and administration fees are closer to providers' costs. http://www.gao.gov/new.items/d05142r.pdf.
- 2.Medicare Payment Advisory Commission. Report to the Congress: Effects of Medicare payment changes on oncology services. http://www.medpac.gov/documents/Jan06_Oncology_mandated_report.pdf.
- 3.Berenson A. Incentives limit any savings in treating cancer. New York Times. 2007. Jun 12, http://www.nytimes.com/2007/06/12/business/12cancerpay.html.
- 4.Akscin J, Barr TR, Towle EL. Key practice indicators in office-based oncology practices: 2007 report on 2006 data. J Oncol Pract. 2007;3:200–203. doi: 10.1200/JOP.0743001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenson A. Cancer drug representatives spelled out the way to profit. New York Times. 2007. Jun 12, http://www.nytimes.com/2007/06/12/business/12cancerside.html?_r=1&ref=business.
- 6.Johnson A. In treating cancer, insurer tries new way to pay docs. Wall Street Journal. 2010. Oct 20, http://online.wsj.com/article/SB10001424052702303550904575562440652409512.html?KEYWORDS=medicare.
- 7.Harris G. Among cancer doctors, a Medicare revolt: New payment system spurs talk of return to hospital care and old drugs. New York Times. 2004. Mar 11, http://www.nytimes.com/2004/03/11/business/among-cancer-doctors-medicare-revolt-new-payment-system-spurs-talk-return.html?pagewanted=all.
- 8.Abelson R. Insurers test new cancer pay systems. New York Times. 2010. Oct 19, p. B1. http://www.nytimes.com/2010/10/20/health/policy/20cancer.html.
- 9.Berenson A. Cancer drugs offer hope but at a huge expense. New York Times. 2005. Jul 12, http://www.nytimes.com/2005/07/12/business/12cancer.html?pagewanted=all. [PubMed]
- 10.Berenson A. A stubborn case of spending on cancer care. New York Times. 2007 Jun 12;:C1. [Google Scholar]
- 11.Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010;363:1822–1832. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson M, Earle CC, Price M, et al. How Medicare's payment cuts for cancer chemotherapy drugs changed patterns of treatment. Health Aff (Millwood) 2010;29:1391–1399. doi: 10.1377/hlthaff.2009.0563. [DOI] [PubMed] [Google Scholar]
- 13.Shea AM, Curtis LH, Hammill BG, et al. Association between the Medicare Modernization Act of 2003 and patient wait times and travel distance for chemotherapy. JAMA. 2008;300:189–196. doi: 10.1001/jama.300.2.189. [DOI] [PubMed] [Google Scholar]
- 14.Friedman JY, Curtis LH, Hammill BG, et al. The Medicare Modernization Act and reimbursement for outpatient chemotherapy: Do patients perceive changes in access to care? Cancer. 2007;110:2304–2312. doi: 10.1002/cncr.23042. [DOI] [PubMed] [Google Scholar]
- 15.Earle CC, Neville BA, Landrum MB, et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care. 2005;17:505–509. doi: 10.1093/intqhc/mzi061. [DOI] [PubMed] [Google Scholar]
- 16.Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol. 2008;26:3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emanuel EJ, Young-Xu Y, Levinsky NG, et al. Chemotherapy use among Medicare beneficiaries at the end of life. Ann Intern Med. 2003;138:639–643. doi: 10.7326/0003-4819-138-8-200304150-00011. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson M, O'Malley AJ, Earle CC, et al. Does reimbursement influence chemotherapy treatment for cancer patients? Health Aff (Millwood) 2006;25:437–443. doi: 10.1377/hlthaff.25.2.437. [DOI] [PubMed] [Google Scholar]
- 19.Hillman AL, Pauly MV, Kerstein JJ. How do financial incentives affect physicians' clinical decisions and the financial performance of health maintenance organizations? N Engl J Med. 1989;321:86–92. doi: 10.1056/NEJM198907133210205. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson JO, Neuss MN, McNiff KK, et al. Improvement in oncology practice performance through voluntary participation in the Quality Oncology Practice Initiative. J Clin Oncol. 2008;26:1893–1898. doi: 10.1200/JCO.2007.14.2992. [DOI] [PubMed] [Google Scholar]
- 20a.Miesfeldt S, Murray K, Chang CH, et al. Association of age, gender, and race with intensity of end-of-life care for Medicine beneficiaries with cancer. J Palliat Med. doi: 10.1089/jpm.2011.0310. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iezzoni LI, Heeren T, Foley SM, et al. Chronic conditions and risk of in-hospital death. Health Serv Res. 1994;29:435–460. [PMC free article] [PubMed] [Google Scholar]
- 22.Berke EM, Smith T, Song Y, et al. Cancer care in the United States: Identifying end-of-life cohorts. J Palliat Med. 2009;12:128–132. doi: 10.1089/jpm.2008.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Earle CC, Ayanian JZ. Looking back from death: The value of retrospective studies of end-of-life care. J Clin Oncol. 2006;24:838–840. doi: 10.1200/JCO.2005.03.9388. [DOI] [PubMed] [Google Scholar]
- 24.Earle CC, Park ER, Lai B, et al. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol. 2003;21:1133–1138. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 25.Wennberg JE, Skinner J, Goodman DC, et al. Lebanon, NH: Dartmouth Institute for Health Policy and Clinical Practice; 2008. Tracking the Care of Patients With Chronic Illness: The Dartmouth Atlas of Health Care 2008. [PubMed] [Google Scholar]
- 26.US Census Bureau. Census 2000, American fact finder. http://factfinder.census.gov/servlet/DTGeoSearchByListServlet?ds_name=DEC_2000_SF3_U&_lang=en&_ts=283785721496.
- 27.Dartmouth Institute of Health Policy and Clinical Practice. Primary care service area project 2009. http://pcsa.dartmouth.edu/index.html.
- 28.White H. A heteroskedasticity-consistent covariance-matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- 29.Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 30.Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22:315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 31.MarketWatch. New poll: Doctors overwhelmingly support palliative care at end-of-life. Wall Street Journal. 2011 Nov 15; [Google Scholar]
- 32.Bruera E, Sweeney C, Calder K, et al. Patient preferences versus physician perceptions of treatment decisions in cancer care. J Clin Oncol. 2001;19:2883–2885. doi: 10.1200/JCO.2001.19.11.2883. [DOI] [PubMed] [Google Scholar]
- 33.Robinson JC. Applying value-based insurance design to high-cost health services. Health Aff (Millwood) 2010;29:2009–2016. doi: 10.1377/hlthaff.2010.0469. [DOI] [PubMed] [Google Scholar]
- 34.Bach PB, Mirkin JN, Luke JJ. Episode-based payment for cancer care: A proposed pilot for Medicare. Health Aff (Millwood) 2011;30:500–509. doi: 10.1377/hlthaff.2010.0752. [DOI] [PubMed] [Google Scholar]
- 35.Fisher ES, McClellan MB, Bertko J, et al. Fostering accountable health care: Moving forward in medicare. Health Aff (Millwood) 2009;28:w219–w231. doi: 10.1377/hlthaff.28.2.w219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClellan M, McKethan AN, Lewis JL, et al. A national strategy to put accountable care into practice. Health Aff (Millwood) 2010;29:982–990. doi: 10.1377/hlthaff.2010.0194. [DOI] [PubMed] [Google Scholar]