This cost-utility analysis reports on the effect of quality of life on the value of screening all new patients with colorectal cancer for Lynch syndrome.
Abstract
Purpose:
Patients and relatives have varying preferences for genetic testing and interventions related to hereditary cancer syndromes. We examined how the impact of these services on quality of life (QoL) affects the cost-effectiveness of screening for Lynch syndrome among probands newly diagnosed with colorectal cancer and their relatives.
Methods:
We constructed a state-transition model comparing screening strategies (clinical criteria, prediction algorithms, tumor testing, and upfront germline testing) with no screening to identify Lynch syndrome. The model incorporated individuals' health state utilities after screening, germline testing, and risk-reducing surgeries, with utilities persisting for 12 months in the base case. Outcomes consisted of quality-adjusted life-years (QALYs), costs, and cost per QALY gained. Sensitivity analyses assessed how the duration and magnitude of changes in QoL influenced results.
Results:
Multiple screening strategies yielded gains in QALYs at acceptable costs compared with no screening. The preferred strategy—immunohistochemistry of tumors followed by BRAF mutation testing (IHC/BRAF)—cost $59,700 per QALY gained in the base case. The duration and magnitude of decreases in QoL after decisions related to germline testing and surgeries were key determinants of the cost-effectiveness of screening. IHC/BRAF cost > $100,000 per QALY gained when decrements to QoL persisted for 21 months.
Conclusion:
Screening for Lynch syndrome in the population is likely to yield long-term gains in life expectancy that outweigh any short-term decreases in QoL, at acceptable costs. Counseling for individuals should aim to mitigate potential negative impact of genetic testing and risk-reducing interventions on QoL.
Introduction
Lynch syndrome increases the risk of developing colorectal, endometrial, ovarian, and other cancers. Inherited mutations in DNA mismatch repair genes MLH1, MSH2, MSH6, and PMS2 constitute the molecular basis of Lynch syndrome. Stakeholders have recommended screening for Lynch syndrome using clinical criteria, mutation risk–prediction algorithms, and tumor testing.1,2
Screening is likely to decrease cancer incidence and improve life expectancy in affected families at acceptable costs to payers.3–6 The favorable impact is attributed to cancer risk stratification based on germline testing, followed by preventive interventions including colonoscopy and risk-reducing total abdominal hysterectomy/oophorectomy.7–9
Economic evaluations have not considered the potential effects of these services on quality of life (QoL) among patients with colorectal cancer with Lynch syndrome (probands) and their relatives. The interplay of preferences for screening, germline testing, and preventive care for probands and relatives at risk for developing cancer likely determines the impact of these services on QoL. For example, some may not value germline testing because identifying increased cancer risk may cause distress.10 Others may value total abdominal hysterectomy/oophorectomy highly because it decreases cancer risk and possibly worry.11 Probands' desires and decisions about germline testing affect relatives, but preferences among relatives may differ from those of probands.
Our aim was to examine how changes in individuals' QoL resulting from medical services for Lynch syndrome affect the cost-effectiveness of screening for Lynch syndrome among persons newly diagnosed with colorectal cancer and their relatives. We performed a cost-utility analysis (CUA) with a decision analytic model using health state utilities from our recent study of patient preferences related to Lynch syndrome to estimate gains in quality-adjusted life-years (QALYs) and incremental cost per QALY gained with screening.4
Methods
CUA evaluates the effects of health care services in terms of both costs and QALYs gained (not just crude number of life-years gained). We refer readers to the Data Supplement for additional information about CUA and our study methods. The statistical code and data set are available by written agreement.
Model
We adapted a state-transition Markov model of screening for Lynch syndrome, described in detail previously, to incorporate health state utilities related to germline testing and risk-reducing interventions.4 The model compares 16 screening strategies to a referent strategy of no active effort to identify Lynch syndrome. Persons newly diagnosed with colorectal cancer enter the simulation at a mean age of 48 years in the base case, and relatives enter at a mean age of 25 years.
We compared different strategies for identifying Lynch syndrome among persons with new diagnoses of colorectal cancer. The strategies included clinical criteria, such as Amsterdam II and revised Bethesda guidelines,12,13 which require an extensive family history of Lynch-associated cancer (colorectal, endometrial, or other associated cancer) and consider age of cancer onset. We included risk-prediction algorithms, such as MMRpro, PREMM126, and MMRpredict,14–17 which estimate the probability of having a genetic mutation based on factors like family history of cancer, tumor characteristics, histology, and age at diagnosis among patients and relatives. We also examined several tumor testing strategies (eg, microsatellite instability testing, immunohistochemistry [IHC], microsatellite instability testing combined with IHC, and IHC followed by BRAF testing).18–22 Testing a tumor sample for microsatellite instability can identify impairment in the DNA replication and repair system, which may result from mutations in mismatch repair (MMR) genes. IHC determines MMR protein expression in tumor samples by applying antibodies against the MMR proteins. Loss of IHC expression of specific MMR proteins guides germline testing. For example, IHC showing loss of expression of MSH2 but not MLH1, MSH6, or PMS2 would suggest germline testing of only the MSH2 gene. Combining BRAF testing with IHC identifies sporadic loss of MLH1 protein expression that is not related to mutations in the MMR genes but may result from hypermethylation of the MLH1 promoter. Finally, germline testing of a blood sample can identify inherited mutations in MMR genes through sequencing, deletion or duplication analysis, or rearrangement analysis. When a specific mutation is identified in a proband, subsequent germline testing can focus on identifying the same mutation in relatives. We modeled different clinical management programs and acceptance rates among probands and relatives until age 75 years based on their germline testing results and cancer risk.23
Major events were first colorectal, endometrial, or ovarian cancer; metachronous colorectal cancer; cancer treatment complications; and death resulting from cancer or other causes. We accounted for the more favorable cancer prognosis associated with Lynch syndrome versus sporadic cancers.24 Persons were observed until death or age 100 years.
Health State Utilities
Health state utilities (herein referred to as utilities) represent the strength of an individual's preferences for specific health-related outcomes and can be used as preference weights to make QoL adjustments to the number of life-years gained after a health intervention. Our model includes utilities that reflect how views about germline testing and management of Lynch syndrome affect QoL among probands and relatives (Table 1).24a Utilities related to Lynch syndrome and living with cancer were measured in our recent study using preference-elicitation exercises and the time tradeoff metric (Data Supplement).
Table 1.
Separate Health States and Utilities Related to Lynch Syndrome Among Probands and Relatives, by Medical Service
| Treatment | Probands |
Relatives |
||
|---|---|---|---|---|
| Health States* | Utilities | Health States* | Utilities | |
| Screening | Men and women who received false-negative screening result | 0.760 | ||
| Germline testing | Men and women who declined germline testing | 0.660 | Men and women who declined germline testing | 0.719 |
| Men and women who accepted germline testing, without Lynch syndrome | 0.760 | |||
| Male relatives who accepted germline testing, with Lynch syndrome | 0.739 | |||
| Germline testing and risk-reducing surgery | Men and women who accepted germline testing, with Lynch syndrome, and: | Female relatives who accepted germline testing, with Lynch syndrome, and: | ||
| Declined TAH/BSO at age 40 years or colectomy at time of diagnosis | 0.622 | Declined TAH/BSO at age 40 years | 0.669 | |
| Accepted TAH/BSO at age 40 years or colectomy at time of diagnosis | 0.672 | Accepted TAH/BSO at age 40 years | 0.697 | |
| Cancer treatment | Colorectal cancer | 0.601 | Colorectal cancer | 0.601 |
| Endometrial cancer | 0.728 | Endometrial cancer | 0.728 | |
| Ovarian cancer | 0.593 | Ovarian cancer | 0.593 | |
Abbreviation: TAH/BSO, total abdominal hysterectomy/oophorectomy.
Separate health states represented in the model by their own utility weight.
We applied utilities related to Lynch syndrome for 12 months in the base case and assumed that decrements to QoL resulting from Lynch syndrome–related services were transient. We chose 12 months based on literature and to avoid bias in favor of screening.10,25–33 Furthermore, studies of hereditary cancer syndromes have similarly applied utilities in a time-limited fashion.34,35 To test how our assumption affected cost-effectiveness, we varied time in health states related to Lynch syndrome, testing from 0 to 36 months in sensitivity analyses.
After living for 12 months in health states related to Lynch syndrome, utilities for both probands and relatives reverted to those of the general population, as measured and adjusted for age and sex starting at age 45 years by Fryback et al.36 We assigned a utility of 1 (representing perfect health) to relatives entering the simulation at age 25 years, as suggested by Kwon et al,35 and interpolated values through age 44 years based on values reported for age 45 years by Fryback et al.
We accounted for changes in QoL for individuals living with colorectal, endometrial, and ovarian cancers based on findings from our utilities study (Kuppermann et al, manuscript submitted for publication). Utilities for persons living with cancer were applied for 5 years; they then reverted to general population utilities.
Clinical and Cost Inputs
We derived clinical inputs from an Evaluation of Genomic Applications in Practice and Prevention meta-analysis.37 Surveillance, Epidemiology, and End Results data informed cancer risk estimates.4,38
We adjusted costs from published sources and Medicare schedules to 2010 US dollars using the medical component of the Consumer Price Index.39 Costs reflected all direct expenses associated with screening, germline testing and genetic counseling, preventive interventions, complications, and cancer care (Data Supplement).
Outcomes and Cost-Utility Analyses
Applying a third-party payer perspective and an annual 3% discount rate, we calculated primary outcomes of mean QALYs per person, mean cost per person, incremental QALYs gained, and incremental cost per QALY gained (ie, incremental cost-effectiveness ratio [ICER]). Analyses focused on families with a representative number of at-risk relatives, and results reflect weighted averages for probands and relatives with and without Lynch syndrome.4
To examine effects of changes in QoL associated with germline testing and management decisions on outcomes, we focused on the tumor testing strategy of IHC followed by BRAF mutation testing. Both our previous cost-effectiveness analysis and this current cost-utility analysis found this to be the preferred strategy.4 This strategy also reflects the current movement toward reflexive screening of all colon cancer samples for Lynch syndrome.2,5,40
Sensitivity Analyses
We varied model inputs individually in one-way sensitivity analyses to assess their impact on the base case results (Data Supplement). Lower and upper bounds for health state utilities were based on interquartile ranges (IQRs) from our recent study. We conducted a sensitivity analysis varying all cost inputs simultaneously using values that were 50% and 100% higher than Medicare costs. Finally, we performed a probabilistic sensitivity analysis using a Monte Carlo simulation with 1,000 iterations and inputs with appropriate parameters for beta, gamma, and normal distributions.4,41
Results
Base Case QALYs, Costs, and ICERs
Screening yielded better outcomes (ie, more QALYs per person) than no screening but at higher cost (Table 2; Data Supplement). The referent strategy of no screening had the worst outcome (mean QALYs per person, 21.0649) and the lowest mean cost per person ($11,200). Upfront germline testing had the best outcome (mean QALYs per person, 21.2500) and highest cost per person ($33,500).
Table 2.
Base Case Results
| Strategy | QALYs Per Person | Cost Per Person | Discounted Incremental Cost Per QALY Gained | Discounted Incremental Cost Per QALY Gained* |
|---|---|---|---|---|
| Referent strategy | 21.0649 | $11,242 | — | — |
| Clinical criteria and algorithm strategies | ||||
| Amsterdam/IHC | 21.0949 | $12,933 | — | — |
| Amsterdam/germline | 21.1010 | $13,282 | — | — |
| MMRpredict/IHC | 21.1448 | $15,319 | — | — |
| MMRpredict/germline | 21.1612 | $16,375 | — | — |
| MMRpro/IHC | 21.1680 | $16,455 | $50,562 | |
| PREMM/IHC | 21.1692 | $16,920 | — | |
| Bethesda/IHC | 21.1767 | $17,021 | $65,347 | |
| MMRpro/germline | 21.1891 | $17,873 | $68,384 | |
| PREMM/germline | 21.1905 | $18,829 | — | |
| Bethesda/germline | 21.1995 | $18,737 | $82,864 | — |
| Tumor-testing strategies | ||||
| IHC | 21.2012 | $19,381 | — | — |
| IHC/BRAF | 21.2012 | $19,551 | — | $59,719 |
| MSI | 21.2045 | $21,155 | — | — |
| MSI plus IHC | 21.2249 | $23,833 | — | $179,576 |
| MSI plus IHC/BRAF | 21.2249 | $23,642 | $193,343 | — |
| Upfront germline testing | 21.2500 | $33,492 | $393,303 | $271,219 |
Abbreviations: IHC, immunohistochemistry; MSI, microsatellite instability testing; QALY, quality-adjusted life-year.
Excluding clinical criteria strategies.
Table 2 lists ICERs after excluding dominated strategies. The ICER for IHC followed by BRAF testing was $59,700 per QALY gained after excluding the clinical criteria and algorithm strategies. This represents settings in which reflex tumor testing can be ensured, but not application of clinical criteria or risk-prediction algorithms.
Duration of Time in Health States Related to Lynch Syndrome
Assuming changes in QoL related to germline testing and management decisions last only 12 months, all screening strategies produced better outcomes (more QALYs per person) than no screening (Table 2). That is, long-term gains in life expectancy attributable to screening outweighed near-term decreases in QoL in the base case.
The QALYs gained per person with screening compared with no screening decreased when disutility related to germline testing and management decisions lasted for more than 12 months. For example, IHC followed by BRAF testing yielded 0.14 and 0.06 QALYs gained per person compared with no screening if QoL decrements lasted 12 (base case) and 24 months, respectively. There was an absolute loss of QALYs per person (−0.004) when decreases in QoL lasted 36 months. Thus, screening could result in net harm if adverse effects on QoL lasted substantially longer than the average 12-month estimate reported in literature.
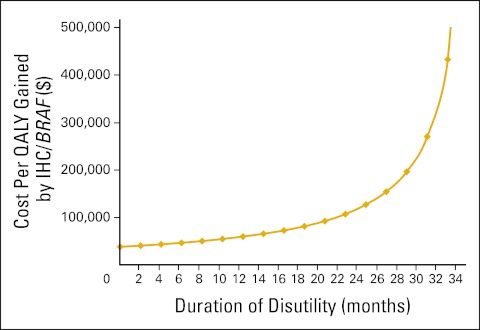
Accordingly, there was an exponential increase in the ICER for screening compared with no screening as the duration of disutility related to germline testing and management decisions increased (Fig 1). The cost per QALY gained by IHC followed by BRAF was $38,100 if QoL was affected for a negligible period of time. In contrast, screening cost > $100,000 per QALY gained compared with no screening when decrements to QoL related to germline testing and management decisions persisted for 21 months, and > $1 million per QALY gained when disutility lasted for 34 months.
Figure 1.
Sensitivity analysis on duration of health state disutility related to decisions about germline testing and management of Lynch syndrome: immunohistochemistry (IHC) followed by BRAF testing versus referent. QALY, quality-adjusted life-year.
Scenario Analyses
We varied clusters of related utilities to model clinical scenarios representing changes in QoL among probands or relatives (Data Supplement). When only probands experienced relatively low QoL after any decision about medical services, the ICER for screening compared with no screening was $82,000 per QALY gained. The ICER also increased to slightly > $100,000 per QALY gained when only relatives with Lynch syndrome experienced relatively low QoL after decisions about medical services.
Next, we simulated scenarios representing changes in QoL after specific decisions about germline testing to reflect uncertainty about cancer risk and Lynch syndrome status. When both probands and relatives experienced low QoL after declining germline testing, screening compared with no screening cost > $190,000 per QALY gained.
Another scenario represented probands and relatives who experienced low QoL after declining risk-reducing surgery. The result for screening compared with no screening was > $117,000 per QALY gained. In contrast, screening compared with no screening cost only $65,000 per QALY gained when probands and relatives experienced low QoL after deciding to undergo risk-reducing surgery.
To estimate the results from a commerical payer perspective, we conducted a sensitivity analysis multiplying all cost inputs simultaneously by factors of 1.5 and 2. When costs were 1.5× higher than those of Medicare, the incremental cost per incremental QALY gained was $90,800 for IHC followed by BRAF testing compared with no screening. When costs were 2× those of Medicare, the ICER increased to $122,000 per QALY gained.
Additional Sensitivity Analyses
Varying most inputs in one-way sensitivity analyses did not substantially affect incremental QALYs gained or ICERs (Data Supplement). Findings were consistent with those of our cost-effectiveness analysis.4
We conducted probabilistic sensitivity analyses to calculate mean, median, IQRs, and 95% CIs for incremental QALYs gained and ICERs for all strategies. IQRs were relatively narrow and within ranges generally accepted as cost-effective. ICERs in this simulation showed wide 95% CIs, in contrast to the results of our recent cost-effectiveness analysis.4 We attribute the current results to the relatively wide distributions for the health state utility estimates, which reflect the small sample size of our utility elicitation study.
Discussion
To our knowledge, our cost-utility analysis is the first to demonstrate how incorporating QoL related to medical services for Lynch syndrome affects the cost-effectiveness of screening for Lynch syndrome. Our results demonstrate that the duration of decreased QoL related to germline testing and management of Lynch syndrome is a key determinant of the cost-effectiveness of screening for Lynch syndrome.
By comparing current results with those from our recent cost-effectiveness analysis, one can appreciate the importance of incorporating patient preferences in estimating the cost-effectiveness of screening for a hereditary cancer syndrome. Using IHC followed by BRAF testing compared with no screening as an example, we previously estimated the cost per life-year gained to be $36,200, absent consideration of QoL.4 When we included only cancer state and general population utilities in one of our current analyses, we calculated a similar cost of screening of $38,100 per QALY gained. When we accounted for the potential short-term decreases in QoL related to germline testing and management of Lynch syndrome, we estimated a higher ICER of $59,700 per QALY gained. Multiplying base case Medicare costs by 1.5 and 2 to model commercial payer costs increased the ICER to $90,800 and $122,000 per QALY gained, respectively. These ICERs may be acceptable to third-party payers and policy makers, as evidenced by coverage of cancer services with comparable ICERs.42
Our cost-utility analysis identifies three factors that underscore the importance of including preferences to estimate the value of screening. First, the cost-effectiveness of screening depended on how long people experienced decrements in QoL related to germline testing and management of Lynch syndrome. Screening was relatively cost-effective if decrements to QoL lasted ≤ 12 months. The ICER worsened as the duration of disutility increased, exceeding $100,000 per QALY gained after 21 months. As duration approached 36 months, the decrements in QoL after germline testing and management decisions outweighed increases in overall survival when measured in QALYs.
Although a number of small studies have reported transience of distress associated with Lynch syndrome testing lasting from 2 weeks to 12 months,10,25–33 specific subgroups defined by demographic factors and cancer risk may experience longer intervals of disutility. One study has shown that young men may continue to feel anxiety about Lynch syndrome over time.33 In addition, persons who do not receive counseling may experience long-term disutility. Therefore, strategies to improve the effectiveness and cost-effectiveness of screening may include ensuring that genetic counseling is offered as part of genetic testing and target additional follow-up and counseling services to persons most likely to experience disutility longer than 12 months. Further research to better identify at-risk individuals is required.
Second, screening became less cost-effective if either probands or relatives with Lynch syndrome experienced extreme disutility after decisions about medical services. Studies have documented differences in distress between persons with a personal history of cancer compared with relatives at risk for cancer.27,28,30 To examine whether cost-effectiveness was more sensitive to changes among probands or relatives, we conducted scenario analyses that showed the ICER was sensitive to variation in utilities for both groups. Although our results represent mean estimates at the population level, we suggest that personalized clinical care and counseling addressing the individual needs of both probands and relatives may enhance the effectiveness and cost-effectiveness of screening.
Third, disutility after declining services had negative effects on the value of screening. Persons may forego germline testing because of concerns about family and abilities to manage the emotional aspects of receiving genetic test results.43 Although counseling is intended largely to help persons who undergo testing understand and manage their cancer risk, counseling may also benefit persons who decline testing by addressing their concerns, thus further improving the value of screening.
In summary, we suggest tailoring interventions to persons who experience long-term disutility, probands and relatives, and persons who decline services. We recommend additional research to validate whether tailoring interventions can preserve the cost-effectiveness of screening.
We acknowledge limitations in our study that can be addressed in future research. We have incorporated health state utilities representing potential QoL outcomes after decisions about medical services among probands and relatives. However, our model does not distinguish utilities for specific subgroups, such as younger versus older men.33 Studies that measure QoL among probands and relatives with different demographic characteristics may provide data for incorporation into future cost-effectiveness models.
We found that cost-effectiveness was sensitive to duration of disutility, but we assumed that everyone experienced the same duration. Probands, relatives with Lynch syndrome, and relatives without Lynch syndrome may each experience distress for different lengths of time. Modeling this would help to identify specific subpopulations that could affect the overall cost-effectiveness of screening as well as persons to target for additional monitoring and counseling.
Finally, our model assumes that everyone who considers germline testing accrues a specific cost for genetic counseling, but this does not account for variable clinical or economic consequences for people who decline counseling or for differences in QoL depending on the intensity of counseling. When relevant data become available, cost-effectiveness studies can include longer-term counseling as an intervention among those who experience unusually long-term decreases in QoL.
In conclusion, our study suggests that universal screening of all patients with newly diagnosed colorectal cancer for Lynch syndrome is cost-effective, even after accounting for the impact of decisions about germline testing and management of Lynch syndrome on short-term QoL. In addition to increasing tumor screening rates, germline testing rates, and adherence to intensive management,4 policy makers and clinicians can maximize the value of screening for Lynch syndrome by attending to the psychosocial needs of probands with colorectal cancer and their relatives who face decisions about germline testing and management of Lynch syndrome.
Acknowledgment
Supported by Grant No. P01CA130818 from the National Cancer Institute.
The study protocol is available from U.L. (e-mail: uri.ladabaum@stanford.edu).
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
Conception and design: Grace Wang, Miriam Kuppermann, Kathryn A. Phillips, Uri Ladabaum
Provision of study materials or patients: Miriam Kuppermann
Collection and assembly of data: Grace Wang, Miriam Kuppermann, Uri Ladabaum
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Vasen HF, Möslein G, Alonso A, et al. Recommendations to improve identification of hereditary and familial colorectal cancer in Europe. Fam Cancer. 2010;9:109–115. doi: 10.1007/s10689-009-9291-3. [DOI] [PubMed] [Google Scholar]
- 2.Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: Genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11:35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinh TA, Rosner BI, Atwood JC, et al. Health benefits and cost-effectiveness of primary genetic screening for Lynch syndrome in the general population. Cancer Prev Res (Phila) 2011;4:9–22. doi: 10.1158/1940-6207.CAPR-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the lynch syndrome among patients with colorectal cancer: A cost-effectiveness analysis. Ann Intern Med. 2011;155:69–79. doi: 10.7326/0003-4819-155-2-201107190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gudgeon JM, Williams JL, Burt RW, et al. Lynch syndrome screening implementation: Business analysis by a healthcare system. Am J Manag Care. 2011;17:e288–e300. [PubMed] [Google Scholar]
- 6.Mvundura M, Grosse SD, Hampel H, et al. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med. 2010;12:93–104. doi: 10.1097/GIM.0b013e3181cd666c. [DOI] [PubMed] [Google Scholar]
- 7.Stupart DA, Goldberg PA, Algar U, et al. Surveillance colonoscopy improves survival in a cohort of subjects with a single mismatch repair gene mutation. Colorectal Dis. 2009;11:126–130. doi: 10.1111/j.1463-1318.2008.01702.x. [DOI] [PubMed] [Google Scholar]
- 8.Jarvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 9.Koornstra JJ, Mourits MJ, Sijmons RH, et al. Management of extracolonic tumours in patients with Lynch syndrome. Lancet Oncol. 2009;10:400–408. doi: 10.1016/S1470-2045(09)70041-5. [DOI] [PubMed] [Google Scholar]
- 10.Claes E, Denayer L, Evers-Kiebooms G, et al. Predictive testing for hereditary nonpolyposis colorectal cancer: Subjective perception regarding colorectal and endometrial cancer, distress, and health-related behavior at one year post-test. Genet Test. 2005;9:54–65. doi: 10.1089/gte.2005.9.54. [DOI] [PubMed] [Google Scholar]
- 11.Hadley DW, Ashida S, Jenkins JF, et al. Colonoscopy use following mutation detection in Lynch syndrome: Exploring a role for cancer screening in adaptation. Clin Genet. 2011;79:321–328. doi: 10.1111/j.1399-0004.2010.01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 14.Barnetson RA, Tenesa A, Farrington SM, et al. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N Engl J Med. 2006;354:2751–2763. doi: 10.1056/NEJMoa053493. [DOI] [PubMed] [Google Scholar]
- 15.Balmana J, Balaguer F, Castellvi-Bel S, et al. Comparison of predictive models, clinical criteria and molecular tumour screening for the identification of patients with Lynch syndrome in a population-based cohort of colorectal cancer patients. J Med Genet. 2008;45:557–563. doi: 10.1136/jmg.2008.059311. [DOI] [PubMed] [Google Scholar]
- 16.Kastrinos F, Steyerberg EW, Mercado R, et al. The PREMM(1,2,6) model predicts risk of MLH1, MSH2, and MSH6 germline mutations based on cancer history. Gastroenterology. 2011;140:73–81. doi: 10.1053/j.gastro.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green RC, Parfrey PS, Woods MO, et al. Prediction of Lynch syndrome in consecutive patients with colorectal cancer. J Natl Cancer Inst. 2009;101:331–340. doi: 10.1093/jnci/djn499. [DOI] [PubMed] [Google Scholar]
- 18.Martin SA, Lord CJ, Ashworth A. Therapeutic targeting of the DNA mismatch repair pathway. Clin Cancer Res. 2010;16:5107–5113. doi: 10.1158/1078-0432.CCR-10-0821. [DOI] [PubMed] [Google Scholar]
- 19.Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: Part I—The utility of immunohistochemistry. J Mol Diagn. 2008;10:293–300. doi: 10.2353/jmoldx.2008.080031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 21.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 22.Zhang L. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: Part II—The utility of microsatellite instability testing. J Mol Diagn. 2008;10:301–307. doi: 10.2353/jmoldx.2008.080062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 24.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Kuppermann M, Wang G, Wong S, et al. Preferences for outcomes associated with decisions to undergo or forego genetic testing for Lynch syndrome. Cancer. doi: 10.1002/cncr.27634. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aktan-Collan K, Haukkala A, Mecklin JP, et al. Psychological consequences of predictive genetic testing for hereditary non-polyposis colorectal cancer (HNPCC): A prospective follow-up study. Int J Cancer. 2001;93:608–611. doi: 10.1002/ijc.1372. [DOI] [PubMed] [Google Scholar]
- 26.Collins VR, Meiser B, Ukoumunne OC, et al. The impact of predictive genetic testing for hereditary nonpolyposis colorectal cancer: Three years after testing. Genet Med. 2007;9:290–297. doi: 10.1097/gim.0b013e31804b45db. [DOI] [PubMed] [Google Scholar]
- 27.Gritz ER, Peterson SK, Vernon SW, et al. Psychological impact of genetic testing for hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2005;23:1902–1910. doi: 10.1200/JCO.2005.07.102. [DOI] [PubMed] [Google Scholar]
- 28.Shiloh S, Koehly L, Jenkins J, et al. Monitoring coping style moderates emotional reactions to genetic testing for hereditary nonpolyposis colorectal cancer: A longitudinal study. Psychooncology. 2008;17:746–755. doi: 10.1002/pon.1338. [DOI] [PubMed] [Google Scholar]
- 29.Claes E, Evers-Kiebooms G, Decruyenaere M, et al. Surveillance behavior and prophylactic surgery after predictive testing for hereditary breast/ovarian cancer. Behav Med. 2005;31:93–105. doi: 10.3200/BMED.31.3.93-106. [DOI] [PubMed] [Google Scholar]
- 30.Keller M, Jost R, Haunstetter CM, et al. Psychosocial outcome following genetic risk counselling for familial colorectal cancer: A comparison of affected patients and family members. Clin Genet. 2008;74:414–424. doi: 10.1111/j.1399-0004.2008.01089.x. [DOI] [PubMed] [Google Scholar]
- 31.Meiser B, Collins V, Warren R, et al. Psychological impact of genetic testing for hereditary non-polyposis colorectal cancer. Clin Genet. 2004;66:502–511. doi: 10.1111/j.1399-0004.2004.00339.x. [DOI] [PubMed] [Google Scholar]
- 32.Keller M, Jost R, Haunstetter CM, et al. Comprehensive genetic counseling for families at risk for HNPCC: Impact on distress and perceptions. Genet Test. 2002;6:291–302. doi: 10.1089/10906570260471822. [DOI] [PubMed] [Google Scholar]
- 33.Hasenbring MI, Kreddig N, Deges G, et al. Psychological impact of genetic counseling for hereditary nonpolyposis colorectal cancer: The role of cancer history, gender, age, and psychological distress. Genet Test Mol Biomarkers. 2011;15:219–225. doi: 10.1089/gtmb.2010.0165. [DOI] [PubMed] [Google Scholar]
- 34.Holland ML, Huston A, Noyes K. Cost-effectiveness of testing for breast cancer susceptibility genes. Value Health. 2009;12:207–216. doi: 10.1111/j.1524-4733.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 35.Kwon JS, Sun CC, Peterson SK, et al. Cost-effectiveness analysis of prevention strategies for gynecologic cancers in Lynch syndrome. Cancer. 2008;113:326–335. doi: 10.1002/cncr.23554. [DOI] [PubMed] [Google Scholar]
- 36.Fryback DG, Lawrence WF, Martin PA, et al. Predicting quality of well-being scores from the SF-36: Results from the Beaver Dam Health Outcomes Study. Med Decis Making. 1997;17:1–9. doi: 10.1177/0272989X9701700101. [DOI] [PubMed] [Google Scholar]
- 37.Palomaki GE, McClain MR, Melillo S, et al. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11:42–65. doi: 10.1097/GIM.0b013e31818fa2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horner MJ, Ries LAG, Krapcho M, et al., editors. SEER cancer statistics review, 1975-2006. http://seer.cancer.gov/csr/1975_2006.
- 39.US Department of Labor, Bureau of Labor Statistics. Consumer price index. http://www.bls.gov/cpi.
- 40.The Ohio State University. OSU James screening for hereditary colon cancer syndrome. http://www.internalmedicine.osu.edu/nephrology/article.cfm?ID=3068.
- 41.Briggs A, Claxton K, Sculpher M. Cambridge, MA: Oxford University Press; 2006. Making decision models probabilistic, in Decision Modelling for Health Economic Evaluation; pp. 77–120. [Google Scholar]
- 42.Hillner BE, Schrag D, Sargent DJ, et al. Cost-effectiveness projections of oxaliplatin and infusional fluorouracil versus irinotecan and bolus fluorouracil in first-line therapy for metastatic colorectal carcinoma. Cancer. 2005;104:1871–1884. doi: 10.1002/cncr.21411. [DOI] [PubMed] [Google Scholar]
- 43.Hadley DW, Jenkins J, Dimond E, et al. Genetic counseling and testing in families with hereditary nonpolyposis colorectal cancer. Arch Intern Med. 2003;163:573–582. doi: 10.1001/archinte.163.5.573. [DOI] [PubMed] [Google Scholar]