To improve formulary design processes and support payers in providing more effective health care, policy makers should consider involving commercial payers in the development of comparative effectiveness research and creation of research and treatment guidelines.
Abstract
Purpose:
The perspective of commercial payers on comparative effectiveness research (CER) has not been well researched. This study aims to describe how US commercial payers use and value CER for formulary decision making in different disease states.
Methods:
We recruited 20 medical and pharmaceutical directors from national and regional plans who are involved in pharmaceutical and therapeutics committees to participate in the study. We conducted in-depth qualitative interviews with the payers and asked them to rate the usefulness of CER study types across various disease states and market conditions. The results were analyzed for thematic content.
Results:
Our findings indicate that payers are interested in a broad range of CER study types, are unsatisfied with the current state of CER, and would like to partner with research groups to develop research and treatment guidelines to better leverage CER. Payers value CER less so in oncology than in other disease states because of limitations in their ability to manage oncology therapies.
Conclusion:
To improve formulary design processes and support payers in providing more effective health care, policy makers should consider involving commercial payers in the development of CER as well as in the creation of research and treatment guidelines.
Introduction
Spending on pharmaceutical products from 2015 through 2020 is projected to increase an average of 7.2% per year, exceeding $500 billion by 2020.1 It has been suggested that comparative effectiveness research (CER) may improve the management and use of treatments, leading to more effective health care and decreased spending over time.2 Consequently, CER has received much attention recently. However, although two thirds of those insured in the United States are covered by private health insurance,3 most research into CER has neglected the viewpoint of private payers.4–6 Without the input and participation of commercial payers, comparative effectiveness data may not be optimal for making complex decisions on formulary design, access restrictions, and use controls for the bulk of US patients.
This study describes how US commercial payers use different types of CER to make reimbursement decisions in different disease states. Through qualitative interviews with commercial payers, we aim to provide useful insights into how these perspectives may improve CER.
Methods
We recruited 20 payers involved in pharmaceutical and therapeutic decision making. The sample was stratified by health plan scope (national v regional) and respondent role (medical v pharmacy director). Participants belonged to a diverse range of 15 different managed care organizations that collectively represented all regions within the United States. The payers participating in this study ranged from operating in one state to offering coverage spanning 50 states. The median number of lives covered was 2.5 million (range, < 500,000 to > 10 million), and the total number of lives covered totaled more than 95 million members. Lines of business ranged from being 100% commercial to more than 50% Medicare or Medicaid.
We probed payers on their views of CER studies to compare the value of pharmaceutical treatments within five common scenarios (Table 1; Data Supplement). Payers were then asked to independently rate the usefulness of six different CER study types (Table 1) on a scale of 1 to 7 and provide the rationale for their ratings. Ratings were averaged and stratified by respondent role and plan type; comments were transcribed and analyzed for thematic content.
Table 1.
Scenarios Tested and Comparative Effectiveness Methods6a
| Disease State | Prevalence | Current Situation | Cost of Therapy | New Situation | Comparative Factors |
|---|---|---|---|---|---|
| Chronic, degenerative | Moderate | Multiple therapies available | High | New biologic | Effectiveness |
| Safety | |||||
| Overall cost | |||||
| Chronic, prevalent | High | Multiple therapies available | Low | Branded drugs now generic | Overall cost |
| Adherence | |||||
| Genetic, orphan | Rare | Supportive therapies only | High | New targeted therapy | Effectiveness |
| Safety | |||||
| Advanced cancer | Rare | Multiple therapies available; poor efficacy | High | New targeted therapy | Effectiveness |
| Cost/benefit | |||||
| Vaccine preventable | Moderate | Prevention superior to treatment | Low (vaccine) | New vaccine with superior efficacy | Cost/benefit |
| Method | Strengths | Weaknesses | Examples |
|---|---|---|---|
| Experimental studies | Randomized | May not reflect real-world practice | Randomized controlled studies |
| High internal validity | Lengthy, costly | ||
| Pragmatic clinical trials | Real-world clinical setting | Larger sample size | Controlled studies within clinics |
| High internal validity | Longer follow-up | ||
| Prospective nonexperimental studies | Real-world setting | Subject to multiple biases | Registry study |
| Inexpensive | Data quality may be problematic | Cohort study | |
| Retrospective analyses | Use of secondary data | Subject to multiple biases | Claims analysis |
| Inexpensive and quick to conduct | Data quality may be problematic | Medical record analysis | |
| Decision modeling | Incorporates multiple data types | Involves complex analyses | Decision tree |
| Simulates complex situations | May be difficult to generalize | Markov model | |
| Budget impact model | |||
| Systematic reviews | Evidence from multiple sources | Limited to existing studies | Meta-analysis |
| Conducted quickly | Heterogeneous methods lead to difficulty combining studies |
Results
We identified four themes in payer perceptions of CER value: 1) different CER study types are valued; 2) dissatisfaction with the current state of CER; 3) importance of payer involvement in CER policy efforts; and 4) diminished impact of CER in oncology.
Different CER Study Types Are Valued
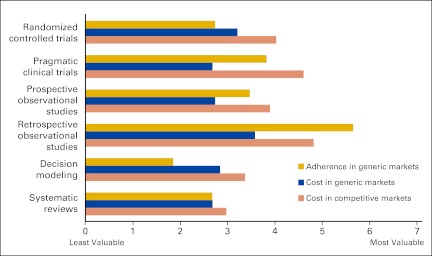
We confirmed the commonly accepted belief that payers prefer randomized controlled clinical trials (RCTs) when comparing drug effectiveness for diseases in which coverage decisions can be implemented. However, payers were interested in CER study designs beyond RCTs. Payers found prospective nonexperimental studies, such as registries, to be valuable in assessing effectiveness and safety. Most payers valued retrospective analyses, such as claims analyses, more highly than RCTs when comparing real-world costs and adherence to drugs (Fig 1). Payers reflected the view that observational studies are valuable for real-world data with larger study populations to detect rare adverse events, more representative of their member populations, and able to assess resource use and overall treatment cost.
Figure 1.
US commercial payers' perceived value of study designs for comparing the adherence to and cost of drugs, by market condition.
“Retrospective analyses are very useful; we're comfortable with it and do it with our own data. Volume of data is important.” —National Pharmacy Director
Generally, national payers valued retrospective analyses and modeling more highly than regional payers. Payers sometimes reviewed retrospective analyses from other research groups but preferred to analyze their own member population or review an analysis of a similar population. However, retrospective analyses require a significant amount of resources to extract and analyze large volumes of data from claims records. Regional payers often did not have sufficient resources and thus sometimes placed a lower value on retrospective analyses and were at a disadvantage in terms of application of CER.
“We're looking at real world utilization. We would like to use a large claims database. We don't have the resources to execute an analysis like this.” —Regional Pharmacy Director
National payers often had the resources to create, analyze, and review decision and economic models. Consequently, they were more likely to use models than were regional payers, who were sometimes less familiar with these tools.
“I'm biased against decision modeling. It is so complex with so many variables that it's hard to translate into your situation.” —Regional Pharmacy Director
Dissatisfaction With the Current State of CER
Although payers valued and used CER, much of the available research was inadequate to inform decision making because of a lack of relevant head-to-head comparisons, perceived lack of credibility of manufacturer-funded studies, and paucity of economic data. The lack of useful CER often translated into difficulties in coverage decisions and sometimes led to inefficiencies in treatment.
“We've been really hungry for head-to-head data. What is tremendously important is to give clinicians and patients information that they can use to select the right treatment. It will be difficult for drugs to get any kind of first-line consideration unless they have that kind of data available.” —National Medical Director
Throughout the interviews, payers expressed a deep suspicion of studies and models conducted by manufacturers. A few payers reported that they may discount manufacturer-funded studies entirely, because they expected intentionally biased studies to show products in a better light compared with competing drugs.
“Approximately 99% of the time [pharmaceutical-sponsored models] are skewed to make their product look better than competitors. We usually don't even look at them.” —National Pharmacy Director
“We don't want registries funded by pharma. Biases are inherent as they select certain people that may respond better to treatment groups; government-sponsored registries are more believable.” —Regional Pharmacy Director
Given the importance of industry funding to CER, it is essential that this evidence is seen as having the highest integrity in development, analysis, and reporting so that it is used by payers. The internal and external validity of CER should be underpinned by detailing assumptions, adjusting for biases, and reporting methodology in a transparent manner to dispel suspicion of biases. CER may be more broadly used when current observational research standards are followed and research is collaborative and published in top-tier journals.
“If data was authenticated by third parties in well-established peer-reviewed journals we could look at that. We will incorporate concepts as we look at our own data, as long as methodology makes sense.” —National Pharmacy Director
One of the most common concerns was the lack of cost data in CER. Payers preferred to have economic end points, such as resource use, when comparing drugs in competitive, mature markets. Although payers preferred head-to-head RCTs with economic end points, there are clear difficulties in designing and funding these studies both pre- and postlaunch. The reality is that much of the necessary comparative economic evidence will be generated in observational studies.
“[CER] has to include cost; if you don't have cost in there I don't know how you're going to use that information. We need to take into consideration the clinical and financial aspects when we make a formulary decision.” —Regional Pharmacy Director
Importance of Payer Involvement in CER Policy Efforts
The Patient-Centered Outcomes Research Institute (PCORI) aims to assist patients, providers, policy makers, and purchasers in making informed health decisions by prioritizing, funding, reviewing, and disseminating CER.7 The launch of PCORI, coupled with CER conducted by independent and manufacturer-based research groups, provides excellent opportunities for collaboration with payers. Partnerships with payers will facilitate communication about what CER data payers require to make more informed decisions.
“If [researchers] are going to do something, ask us what our needs are, what conclusions we want to draw. The most important thing is ask us what our needs are; don't assume.” —National Medical Director
One of the difficulties payers faced was comparing the results of different nonexperimental studies with varying methodologies and data quality. Payers preferred to see more comparative effectiveness studies with head-to-head comparisons with the current standard of care, economic end points, more diverse study populations, and transparent descriptions of unbiased methodologies. If research guidelines were developed by regulatory bodies or professional groups with the input of commercial payers to include these measures, CER would be more likely to generate data useful to payers. As CER becomes more standardized, payers would also be able to more easily synthesize data, further improving the utility of studies.
“There does need to be some standardization to determine the validity of the studies. Randomized controlled studies tend to be standardized, but when you look at other kinds of trials, I would hope there would be standardization proposed.” —Regional Medical Director
Diminished Impact of CER in Oncology
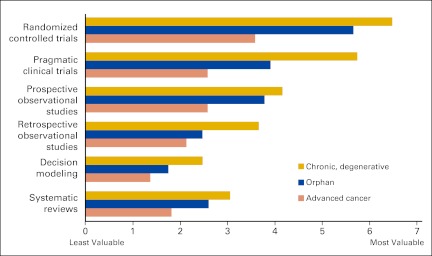
The value of CER was reduced when payers were not able to manage certain disease states because of limited treatment alternatives (ie, orphan diseases) or the politically sensitive nature of the disease (ie, cancer; Fig 2). Payers did not feel that they had the political standing to manage expensive treatments for sensitive disease states, such as cancer, as they did for other diseases. Payers were certainly wary of reimbursing oncology drugs that have marginal effectiveness but also wanted to avoid being perceived as interested only in profit at the expense of patients' health. Thus, payers were not able to use CER and were forced to cover all oncology drugs and attempt to manage oncology products through tiering, prior authorization, specialty pharmacies, and patient coinsurance. As a result, the value of CER was significantly diminished.
Figure 2.
US commercial payers' perceived value of study designs for comparing the adherence to and cost of drugs, by disease state.
“We don't manage [oncology] as well as we like. We don't have a choice of not covering [oncology drugs]. These study types may not be helpful.” —National Medical Director
“We've moved all chemotherapy to prior authorization. We've become much more driven by the huge treatment variations with no literature justification.” —National Medical Director
However, payers mentioned that they would find value in CER if they were able to use study results to inform their formulary decision making. Payers agreed that RCTs were the most useful in assessing the efficacy of oncology products. However, to assess factors like safety that contribute to the real-world effectiveness of oncology products payers preferred pragmatic clinical trials and prospective observational studies, namely registry studies.
“[Prospective observational studies] certainly have some use in oncology. I usually look at registries more from a safety perspective.” —Regional Pharmacy Director
In oncology, payers were looking toward professional and governmental organizations to develop more specific treatment guidelines to define which drugs are most effective and of reasonable cost. These guidelines would be fundamental in determining which drugs should be covered for diseases without a clear standard of care.
“We'd be able to manage [oncology products] if NCCN [National Comprehensive Cancer Network] decided to be more specific and take comparative cost effectiveness into play and created guidelines that were very drug specific. Then we would have something evidence based that we could hang our hat on.” —Regional Medical Director
Discussion
The input of US commercial payers regarding CER is of crucial importance, because they manage the health care of the majority of the US population. This study describes the perceptions that US commercial payers have of the value of CER in formulary placement decisions. We have shown that there are several areas in which US commercial payers would like CER to be improved. This study suggests that payers would like the inclusion of economic end points and other relevant data comparing new drugs with the standard of care. Payers would like to be involved in the development of rigorous standards for nonexperimental studies to ensure that they are trustworthy and comparable. Treatment guidelines would also help payers determine which drugs are most effective.
Previous studies focusing on US commercial payers have emphasized the need for payers to better manage oncology products but have not discussed how payers would like to apply or improve CER. The National Comprehensive Cancer Network conducted a study in which medical executives from the three largest payers in the United States were interviewed regarding the strategies they used to manage the increasing cost of cancer care.8 A follow-up study interviewed medical executives from 10 managed care organizations and provided a more in-depth description of payer cancer management strategies.9 Given the importance of CER in improving treatment effectiveness and controlling costs, it is surprising that CER is not relevant in oncology. Payers' inability to use CER to manage oncology products may help shed light on some of the difficulties in controlling cost in cancer. Developing oncology treatment guidelines using CER may be a way to increase the effectiveness of cancer treatment while controlling costs.
Our results are consistent with two recent commentaries outlining the challenges that PCORI will likely face. Specifically, Ommaya et al10 emphasized that PCORI should ensure that study methods be thoroughly reviewed and participate in the development of clinical practice guidelines. Krumholz11 stressed that researchers should build partnerships with those who will use study results and that relevant, timely, and methodologically sound studies should be produced by advancing study design and methodology. Our findings strongly suggest that commercial payers be included in these partnerships. It is unreasonable to assume that PCORI will be able to fund enough studies to fill the need for real-world CER. Thus, it will be essential for PCORI to develop CER guidelines for observational research and fund studies that demonstrate best practices in different types of settings and diseases for other research groups to follow. Furthermore, robust data must be available to conduct studies that produce relevant and unbiased results. Private payers are in a unique position to provide a significant amount clinical and health care data to supplement those collected by the federal government.12 It has been proposed that public-private partnerships be created to develop a national data infrastructure to provide health information for public health uses, effectiveness research, quality measurement, and health services research.12,13 By adopting more standardized observational research methods and analyzing robust data, manufacturer-funded studies may gain more credibility and value in the eyes of payers.
A strength of our study lies in the open-ended scenario-based approach used in the interviews. This provides the contexts of specific market conditions and disease states to allow payers to freely express their perceptions and opinions of CER within realistic situations. Thus, we believe our data provide a window into how US commercial payers value and use CER in real-world situations. Another strength is the diversity of payers in our study. Regional managed care organizations cover a significant portion of the US population, yet their views on CER are seldom heard.14 By including regional payers, we were able to gain an understanding of how they viewed and used CER.
A limitation of our study is that the study population was not a random sample. Thus, we cannot be certain that our findings can be generalized among all US commercial payers. Nevertheless, the broad range of payers we recruited represents plans that cover a significant portion of the US population, which we believe provides a reasonably accurate overview of how US commercial payers use CER to make formulary decisions.
To our knowledge, this study presents the most in-depth view of the opinions of US commercial payers regarding CER to date. This study reveals several important policy implications to help facilitate more efficient formulary design and more effective health care. Commercial payers are looking for the federal government and independent research groups, such as PCORI, to play a larger role in funding and conducting comparative effectiveness studies as well as developing treatment and research guidelines. This study provides evidence that although RCTs have long been considered the gold standard of CER, payers also consider other study designs to be of value when assessing the adherence to and effectiveness, safety, and overall cost of drugs.
Acknowledgment
Supported by PriceSpective. The authors have received no external funding for this research. Presented in part at the International Society for Pharmacoeconomics and Outcomes Research 14th Annual European Congress, Madrid, Spain, November 5-8, 2011.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Anthony Wang, Biotech Industry (C); Ronald J. Halbert, Biotech Industry (C); Tiffany Baerwaldt, Biotech Industry (C); Robert J. Nordyke, Biotech Industry (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: Anthony Wang, Ronald J. Halbert, Robert J. Nordyke
Financial support: Robert J. Nordyke
Administrative support: All authors
Provision of study materials or patients: Anthony Wang, Robert J. Nordyke
Collection and assembly of data: All authors
Data analysis and interpretation: Anthony Wang, Ronald J. Halbert, Robert J. Nordyke
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.National health expenditure projections 2010-2020. http://www.cms.gov/NationalHealthExpendData/downloads/proj2010.pdf.
- 2.Schoen C, Guterman S, Shih A, et al. Bending the curve: Options for achieving savings and improving value in U.S. health spending. http://www.commonwealthfund.org/usr_doc/Schoen_bendingthecurve_1080.pdf.
- 3.US Census Bureau. Health insurance highlights 2009. http://www.census.gov/hhes/www/hlthins/data/incpovhlth/2009/highlights.html.
- 4.Eichler HG, Bloechl-Daum B, Abadie E, et al. Relative efficacy of drugs: An emerging issue between regulatory agencies and third-party payers. Nat Rev Drug Discov. 2010;9:277–291. doi: 10.1038/nrd3079. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman A, Montgomery R, Aubry W, et al. How best to engage patients, doctors, and other stakeholders in designing comparative effectiveness studies. Health Aff (Millwood) 2010;29:1834–1841. doi: 10.1377/hlthaff.2010.0675. [DOI] [PubMed] [Google Scholar]
- 6.Bridges JF, Buttorff C. What outcomes should US policy makers compare in comparative effectiveness research? Expert Rev Pharmacoecon Outcomes Res. 2010;10:217–220. doi: 10.1586/erp.10.31. [DOI] [PubMed] [Google Scholar]
- 6a.Brookings Institution. Implementing comparative effectiveness research: Priorities, methods, and impact. http://www.brookings.edu/∼/media/Files/events/2009/0609_health_care_cer/0609_health_care_cer.pdf.
- 7. Patient Protection and Affordable Care Act. Pub L No. 111-148 §4205, 124 stat 573, 2010.
- 8.Hinkel J. “Cancer is now on the table”: Conversations with managed care executives. http://www.nccn.org/about/news/ebulletin/2009-06-09/table.asp.
- 9.Danielson E, Demartino J, Mullen JA. Managed care and medical oncology: The focus is on value. J Natl Compr Canc Netw. 2010;8(suppl 7):S28–S37. doi: 10.6004/jnccn.2010.0132. [DOI] [PubMed] [Google Scholar]
- 10.Ommaya AK, Kupersmith J. Challenges facing the US patient-centered outcomes research institute. JAMA. 2011;306:756–757. doi: 10.1001/jama.2011.1171. [DOI] [PubMed] [Google Scholar]
- 11.Krumholz HM. Real-world imperative of outcomes research. JAMA. 2011;306:754–755. doi: 10.1001/jama.2011.1170. [DOI] [PubMed] [Google Scholar]
- 12.Navathe AS, Conway PH. Optimizing health information technology's role in enabling comparative effectiveness research. Am J Manag Care. 2010;16(suppl 12):SP44–SP47. [PubMed] [Google Scholar]
- 13.Behrman RE, Benner JS, Brown JS, et al. Developing the Sentinel System: A national resource for evidence development. N Engl J Med. 2011;364:498–499. doi: 10.1056/NEJMp1014427. [DOI] [PubMed] [Google Scholar]
- 14.Health Strategies Group. Strategic accounts: Portraits of leading health plans. http://www.healthstrategies.net/download.cfm?d=5876.