A statewide collaboration between payers and providers to create a cancer clinical care pathways program is successful.
Abstract
Despite rising medical costs within the US health care system, quality and outcomes are not improving. Without significant policy reform, the cost-quality imbalance will reach unsustainable proportions in the foreseeable future. The rising cost of health care in part results from an expanding aging population with an increasing number of life-threatening diseases. This is further compounded by a growing arsenal of high-cost therapies. In no medical specialty is this more apparent than in the area of oncology. Numerous attempts to reduce costs have been attempted, often with limited benefit and brief duration. Because physicians directly or indirectly control or influence the majority of medical care costs, physician behavioral changes must occur to bend the health care cost curve in a sustainable fashion. Experts within academia, health policy, and business agree that a significant paradigm change in stakeholder collaboration will be necessary to accomplish behavioral change. Such a collaboration has been pioneered by Blue Cross Blue Shield of Michigan and Physician Resource Management, a highly specialized oncology health care consulting firm with developmental and ongoing technical, analytic, and consultative support from Cardinal Health Specialty Solutions, a division of Cardinal Health. We describe a successful statewide collaboration between payers and providers to create a cancer clinical care pathways program. We show that aligned stakeholder incentives can drive high levels of provider participation and compliance in the pathways that lead to physician behavioral changes. In addition, claims-based data can be collected, analyzed, and used to create and maintain such a program.
Introduction
The overall incidence of cancer in the United States is projected to increase by 45% in the next two decades from 1.6 million in 2010 to 2.3 million in 2030. Direct medical costs associated with cancer are also projected to increase exponentially from $104 billion in 2006 to more than $173 billion in 2020 as a result of increases in both the cost and quantity of cancer therapies.1 Newer cancer treatments are not only likely to be more expensive than the existing standard of care, but they will expand the pool of available treatment options.2,3 Despite these rising medical costs and treatment options, quality and outcomes are not improving.4 Without significant policy reform, the cost-quality imbalance will reach unsustainable proportions in the foreseeable future.1,4–6
Many factors play into the rising costs of health care, including an aging population, an expanding arsenal of therapeutics for chronic disease states, increasing regulatory demands on stakeholders, inefficiencies in delivery, and archaic information technologies. All of these factors result in significant variations in practice patterns among medical oncologists.7 Costs can vary dramatically because physicians often treat patients with the same condition differently in choice of drugs, referrals for surgery and radiation, referrals for palliative and end-of-life care, and types of supportive care. Strategies such as prior authorization and decreasing fee schedules have been implemented in an effort to lower health care costs.8–10 However, because neither of these strategies addresses practice variances or the rapidly rising cost of cancer therapeutics, their impact on health care costs has been of limited benefit and brief duration.11
Clinical pathways are a method to reduce unnecessary and costly treatment variation; however, physician participation is crucial for their success.1,12 The perceived challenge of cookbook-style medicine to physician autonomy from an external authority, such as insurance companies or academic advisory boards, can be one barrier to pathway adoption among others such as time constraints and comfort with previous practice patterns.13,14 Without accountability or incentives, physicians might comply partially with the terms of a pathway program or not participate at all. Therefore, a collaborative effort between pathway-developing parties and oncology groups is needed.15 Physicians directly or indirectly control or influence the majority of cancer care costs.1 Therefore, physician behavior change can be used as a surrogate marker of cost savings, which can be difficult to demonstrate as a result of evolving patterns of care, new drug technologies, patent expirations, and data capture problems from revenue code and charge bundling to name a few.
This article details a collaborative statewide cancer clinical pathway program in which provider network medical oncology physicians played an integral role in developing oncology clinical pathways. The success of the program was determined by evaluation of physician compliance to the pathways and their behavioral changes in the first year of the program resulting from the inherent difficulties in determining cost savings in this setting.
Collaborative Clinical Pathway Program
In mid-2009, three groups—Blue Cross Blue Shield (BCBS) of Michigan (BCBSM; Detroit, MI), a large single-state not-for-profit BCBS plan; Physician Resource Management (PRM; Novi, MI), a state physician organization; Cardinal Health Specialty Solutions (CHSS; Dublin, OH), an oncology benefit management company (previously P4 Healthcare)—partnered to develop a clinical pathway program whereby physicians would jointly develop the content, structure, and implementation of the program pathways. Involving physicians in the clinical pathways development process would provide an incentive for physicians to participate in the pathways themselves. Care pathways for breast, colon, and lung cancer were developed in the first year of the program and expanded by five additional malignancies in the second year. The program would benefit all parties involved in patient care, including patient, provider, and payer, by aiming to improve the consistency and quality of patient care while also reducing costs. The clinical cancer care pathways program was operated and funded through the Oncology Physicians Resources (OPR; West Bloomfield, MI) group, an existing statewide physician-owned general purchasing and management organization and subsidiary of the Michigan Society of Hematology and Oncology (MSHO; Rockingham Royal Oak, MI). PRM is an administrative arm of OPR and would manage the BCBSM pathway program.
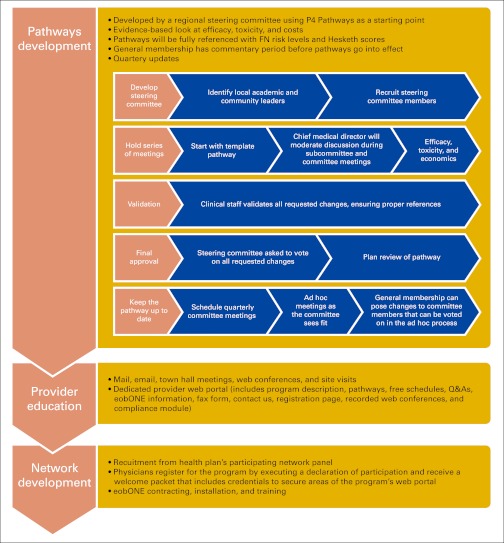
Before the development of this partnership, BCBSM instituted a pay-for-performance program in 2005 for Michigan physicians, the Physician Group Incentive Program (PGIP). This program added a physician organization fee component to each professional service payment. The physician organization component was held in an incentive pool and fully distributed twice each year to physicians who participated in PGIP. It was determined that the BCBSM cancer clinical care pathways program would be placed under the aegis of PGIP and that funding would be paid directly to OPR/PRM according to PGIP bylaws. Figure 1 presents a schematic of steps involved in the pathways development and the implementation of BCBSM oncology treatment pathways.
Figure 1.
Steps involved in pathways development and implementation of BCBSM oncology treatment pathways. FN, febrile neutropenia; Q&As, questions and answers.
Alignment of Stakeholders
One of the first critical steps in the development of the BCBSM pathway program was to align stakeholder incentives so that all interests would be taken into account. BCBSM wanted to develop a program that would improve clinical outcomes with more predictable costs, thereby bending the cost curve downward for future oncology care. Oncologists wanted reimbursement stability. CHSS wanted to prove its value-added benefits to the process by not only receiving a fee for service for their technical support but also sharing in both the upside and possible downside of the program results attributable to pathway compliance. All three stakeholders agreed that, given the appropriate incentives, provider behavior could be modified in a self-governing process in which claims data would be used to monitor compliance.
Methods
Provider Incentive Program
PRM and BCBSM agreed on ways to reward physicians for using pathways. BCBSM provided each physician participant a $5,000 payment for the first year of the pathways program to cover any extra costs involved and to provide a financial incentive to participate and meet approved compliance thresholds. In addition, the reimbursement rate for several generic therapies associated with the specific clinical pathways was increased to remove the perverse incentives created by average sales price–based reimbursement (13 drugs were modified in 2010). Evaluation and management codes would also be increased (10%) for pathway program participants as a result of compliance in year 1. Payments were also made to PRM to serve as the coordinating center for this statewide effort from the BCBSM PGIP incentive pool. With those monies, PRM elected to make payments to physician groups for their participation, support the development of clinical pathways, provide software developed by CHSS, and subcontract with CHSS for additional services. BCBSM incentives and fee schedules were also reviewed to assess how payer incentives aligned with best practices.
Pathway Development
PRM selected regional leaders to serve on a steering committee. The steering committee comprised 12 regional network oncologists from academic-based practices and large and small community-based practices. It was cochaired by the CHSS chief medical officer and an OPR officer as nonvoting members. On the basis of published scientific and clinical evidence and national guidelines, the steering committee developed the most up-to-date and effective oncology care pathways for breast, lung, and colon cancer and supportive care using granulocyte colony-stimulating factors, erythropoietin stimulating agents, and antiemetics. Treatment, efficacy, toxicity, and cost (in that order of priority) were considered in all pathway regimen selections. National Cancer Institute–designated clinical trials and/or decisions for palliative care were mutually agreed upon to be included in all pathways. The steering committee also embraced molecular diagnostics in breast, colon, and lung cancer as part of the pathways process.
The oncology pathways were introduced to the remaining oncologists within the broader BCBSM network for their review and input. All recommendations were evaluated by the steering committee. These steps were critical and ensured that the valuable experience and expertise of each network physician was considered before pathway adoption. The pathways are organized by line of therapy, and they include molecular profiling, histologic profiling, treatment indication, and stage. The steering committee continues to meet quarterly and on an ad hoc basis as needed.
Before the pathway finalization, the criteria for selection and protocols underwent review by BCBSM; however, BCBSM did not play a role in protocol selection. It was determined from the beginning of this partnership that this statewide initiative was best positioned for success if BCBSM kept its role to that of an objective third-party funder with a strong interest in the outcomes of the program but with no direct influence in the determination of what ultimately constituted the clinical pathways.
Compliance Monitoring
The BCBSM oncology care pathway program acknowledged that pathways are not a substitute for physician judgment and that some variance can and should be expected to allow for individual treatment on the basis of unique patient needs Therefore, it was agreed that compliance thresholds for participating physicians should not be set at 100%. For cancer treatment in the adjuvant and metastatic setting for breast, colon, and lung cancer, a 70% compliance rate was set as the threshold for the first year and 80% for subsequent years with reporting on both as a means of communication and education to the provider network. It was also agreed that an 80% rate of compliance would be appropriate for supportive care in the first and subsequent years. Most pathway deviations would be rendered noncompliant and part of the allowable 30% noncompliance rate. However, an appeals mechanism was put in place via portal. In rare instances, nonpathway approaches could be challenged proactively when standards of care are dynamic (eg, in triple-negative breast cancer). This could be addressed via an ad hoc steering committee meeting or a proactive appeal and would include oversight committee evaluation within 48 hours.
Compliance was measured through claims submitted to a proprietary claims cycle management software tool, eobONE (Cardinal Health; Dublin, OH). It was augmented and validated with data directly from the insurer for patients with breast, lung, and colon cancer who started new lines of antineoplastic therapy on or after January 2010. Additionally, physicians could submit information through a secure web portal or fax (eg, clinical trial schema and hospice care notifications). Collecting data in this manner created no additional work for the practices. Physicians were notified of their compliance scores through quarterly reports from CHSS and were allowed to reconcile noncompliant determinations by providing additional data through the appeal section of the CHSS web portal. Aggregated program compliance was presented to BCBSM at quarterly meetings and to participating physicians at semiannual conferences. OPR had the responsibility of notifying and counseling practices with compliance issues.
Results
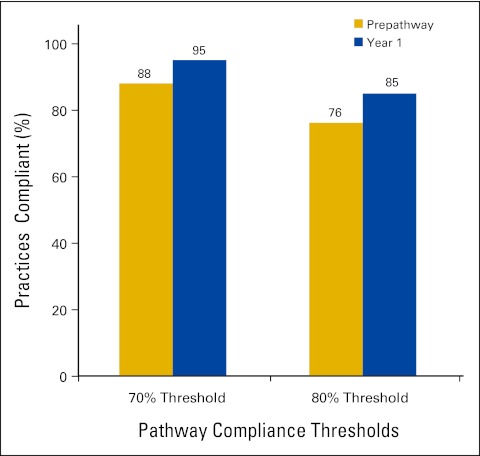
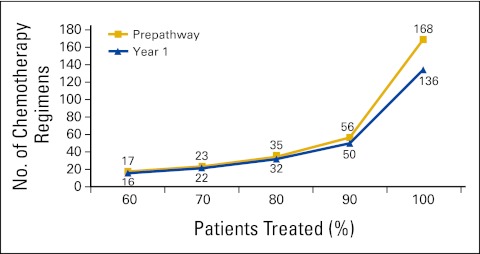
More than 80% of Michigan private practice medical oncologists participated in BCBSM's PGIP in the first year. Prepathway physician practices closely followed pathway guidelines, and baseline pathway compliance was therefore high at 88% when using the 70% threshold. This rate increased steadily in the first year of the program to 95% (Fig 2). Clearest among physician behavioral changes was a reduction in treatment variation. Although pathway guidelines allowed approximately 120 different chemotherapy combinations for breast, colon, and lung cancers, the vast majority of patients were treated with one of 30 regimens. Before pathways implementation, the participating physicians accounted for 168 distinct chemotherapy regimens for these cancers. By end of year 1, participating practices had reduced the total number to 136, with nearly all of the reduction affecting 10% of the treated population (Fig 3). The following are other behavioral changes adopted by participants: converting brand regimen to generic when equally effective and equitoxic; converting from more expensive to less expensive brand regimens when the same parameters apply; using molecular diagnostics to appropriately guide therapy; appropriate use of supportive care on the basis of evidence; decreasing lines of therapy when evidence is lacking; limiting late lines of therapy to single-agent cytotoxics; using biologics on the basis of labeling rather than irrational exuberance; and as the result of all the above, lower rates of emergency room and hospital use.
Figure 2.
Compliance levels for participating practices.
Figure 3.
Reduction in treatment variation by participating practices
Discussion
Behavior Change As a Surrogate for Cost Savings
A recent report by the congressional budget office as well as other peer-reviewed publications have challenged the savings opportunities in payer-mediated provider network guidelines.16,17 The absence of observed savings is a significant hurdle to overcome as the stakeholders of health care delivery explore solutions to bend the cost curve in cancer care. Although we are convinced by Gesme et al18 in our previously reported analyses that our cancer pathways programs can produce meaningful savings, we also recognize the complexity inherent in such analyses. An in-network concurrent provider control group is the ideal comparator for savings. Unfortunately, this control group is flawed by the small numbers of nonparticipants among community providers, which thereby creates wide CIs, the selection bias inherent in a voluntary participation program (eg, low-cost providers participate, high-cost providers choose not to), and the patient demographic differences among community and academic providers. Concurrent control groups, national or regional, are also compromised as has been made clear in the wide variations of resource use identified in the Dartmouth Atlas project.19 Historical controls are equally contentious, given that oncology in particular is such a dynamic specialty with evolving patterns of care, changing label indications, new drugs and technologies, molecular diagnostics, patent expirations, and so on; all of which contributes to an extent of variables that is nearly impossible to account for in comparative analytics. Finally, data capture problems around hospital-based care as a result of revenue codes and charge bundling and around oral therapeutics distributed among multiple plan vendors leave significant data vacuums. In summation, validated savings with the currently available data from payers and providers is virtually impossible. The authors believe that a sustainable reduction in variance inherently improves quality and cost and can only be achieved by changes in physician behavior.
Compliance Monitoring Challenges and Results
The physician-knows-best approach provided challenges in compliance monitoring. To accurately assess treatment practices, the pathways program relied on data provided by BCBSM, captured through eobONE, or submitted directly by physicians in paper-based forms. Even with this tripartite approach, data capture was incomplete. Revenue codes submitted from hospital settings lacked sufficient detail on the type of treatments received. The full treatment picture for patients with multiple insurance plans was incomplete in the BCBSM data. Data from eobONE included all treatments submitted for reimbursement but lacked information on therapies that were self-administered and obtained by the patient through a pharmacy (eg, oral chemotherapies). Fax data received from physicians provided clarity on oral treatments but was limited by accuracy and completeness of input.
Summary
Although we continue seek solutions to the conundrum of savings measurements in our programs, we believe that behavior change represents an appropriate surrogate that allows payers and providers to collaborate to bend the cost curve while improving quality in cancer care. The costs of cancer care are rapidly increasing and will soon be unsustainable.1 Many health care systems are considering clinical pathways as a method to reduce unnecessary and costly treatment variation; however, the extent to which they can effectively do so relies heavily on design and implementation. Some oncologists have embraced pathways whereas others have resisted, usually as a result of perceived challenges to their autonomy and decision-making ability.13–15
For effective cancer care pathway programs, developing parties and oncology groups must collaborate. Oncologists should participate in the development of pathways they believe reflect appropriate care in their community. Moreover, pathway compliance must be monitored and physician behavior validated to achieve success and best practice medicine. Feedback with continual discussions should be provided to participating physicians to keep pathways maintained and current. In addition, the mechanism to collect and disseminate this information must be a seamless, effortless process that health care providers perceive as unobtrusive to their office workflow. This article outlined a collaborative model between providers and payers to implement a clinical care pathway program that drives physician behavior. Compliance levels increased from pre– to post–pathways launch and was driven in part by reduced variation in treatment. The program provided standardized treatment options but allowed participating physicians the flexibility to use their own judgment for difficult treatment decisions. Putting this level of control into the physicians' hands played a significant role in the high level of physician participation and compliance.
Creating a standardized approach to patient care through clinical care pathways enables measurement of participation and compliance as well as treatment practices. Additional end points from this database—such as numbers of lines of treatment, brand to generic conversions, use of biologics in multiple lines of therapy, molecular diagnostics to govern care, and acute care interventions in emergency room and hospital—will be measured in future studies. The results of these analyses will help drive future program design.
Acknowledgment
We thank Phillips Gilmore Oncology Communications for their technical support.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Bruce A. Feinberg, Cardinal Health (C); James Lang, Blue Cross Blue Shield of Michigan (C); Thomas Leyden, Blue Cross Blue Shield of Michigan (C); Joseph Cooper, Cardinal Health (C); Thomas Ruane, Blue Cross Blue Shield of Michigan (C); Scott Milligan, Cardinal Health (C); Jeffrey A. Scott, Cardinal Health, P4 Pathways (C) Consultant or Advisory Role: None Stock Ownership: Bruce A. Feinberg, Cardinal Health Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: James Lang, Thomas Leyden
Financial support: James Lang
Administrative support: James Lang, James Grzegorczyk, Thomas Rybarczyk, Thomas Leyden, Thomas Ruane
Provision of study materials or patients: James Lang
Collection and assembly of data: Bruce A. Feinberg, Donna Stark, Thomas Rybarczyk, Joseph Cooper, Scott Milligan, Philip Stella, Jeffrey A. Scott
Data analysis and interpretation: Bruce A. Feinberg, James Grzegorczyk, Donna Stark, Joseph Cooper, Thomas Ruane, Scott Milligan, Philip Stella, Jeffrey A. Scott
Manuscript writing: Bruce A. Feinberg, Joseph Cooper, Scott Milligan, Philip Stella, Jeffrey A. Scott
Final approval of manuscript: All authors
References
- 1.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Elkin EB, Bach PB. Cancer's next frontier: Addressing high and increasing costs. JAMA. 2010;303:1086–1087. doi: 10.1001/jama.2010.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med. 2009;360:626–633. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 4.Advisory Board Company. Defining accountable cancer care: Unclear return on today's cancer care investment. http://www.advisory.com/Research/Oncology-Roundtable/Resources/2011/Posters/∼/media/Advisory-com/Screenshots/Posters/OR_HVCC_Infographic_Poster_big.jpg.
- 5.Smith TJ, Hillner BE. Bending the cost curve in cancer care. N Engl J Med. 2011;364:2060–2065. doi: 10.1056/NEJMsb1013826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan R, Peppercorn J, Sikora K, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011;12:933–980. doi: 10.1016/S1470-2045(11)70141-3. [DOI] [PubMed] [Google Scholar]
- 7.Fisher ES, Bynum JP, Skinner JS. Slowing the growth of health care costs: Lessons from regional variation. N Engl J Med. 2009;360:849–852. doi: 10.1056/NEJMp0809794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamel MB, Epstein AM. Prior-authorization programs for controlling drug spending. N Engl J Med. 2004;351:2156–2158. doi: 10.1056/NEJMp048294. [DOI] [PubMed] [Google Scholar]
- 9.MacKinnon N, Kumar R. Prior authorization programs: A critical review of the literature. J Manag Care Pharm. 2001;7:297–302. [Google Scholar]
- 10.Fischer MA, Schneeweiss S, Avorn J, et al. Medicaid prior-authorization programs and the use of cyclooxygenase-2 inhibitors. N Engl J Med. 2004;351:2187–2194. doi: 10.1056/NEJMsa042770. [DOI] [PubMed] [Google Scholar]
- 11.Holcombe D. Oncology management programs for payers and physicians: Evaluating current models and diagnosing successful strategies for payers and physicians. J Oncol Pract. 2011;7(suppl):e46s–e49s. doi: 10.1200/JOP.2011.000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoverman JR, Cartwright TH, Patt DA, et al. Pathways, outcomes, and costs in colon cancer: Retrospective evaluations in 2 distinct databases. Am J Manag Care. 2011;17(suppl 5):SP45–SP52. [PubMed] [Google Scholar]
- 13.Majumdar S, Simpson S, Marrie T. Physician-perceived barriers to adopting a critical pathway for community-acquired pneumonia. Jt Comm J Qual Saf. 2011;30:387–395. doi: 10.1016/s1549-3741(04)30044-4. [DOI] [PubMed] [Google Scholar]
- 14.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 15.Evans-Lacko S, Jarrett M, McCrone P, et al. Facilitators and barriers to implementing clinical care pathways. BMC Health Serv Res. 2010;10:182. doi: 10.1186/1472-6963-10-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson L. Washington, DC: Congressional Budget Office, Health and Human Resources Division; 2012. Lessons from Medicare's demonstration projects on disease management, care coordination, and value-based payment. http://cbo.gov/publication/42860. [Google Scholar]
- 17.Rauh SS, Wadsworth EB, Weeks WB, et al. The savings illusion: Why clinical quality improvement fails to deliver bottom-line results. N Engl J Med. 2011;365:e48. doi: 10.1056/NEJMp1111662. [DOI] [PubMed] [Google Scholar]
- 18.Gesme DH, Wiseman M. Strategic use of clinical pathways. J Oncol Pract. 2011;7:54–56. doi: 10.1200/JOP.2010.000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher ES, Goodman DC, Chandra A. Princeton, NJ: Robert Wood Johnson Foundation; 2008. Disparities in health and health care among Medicare beneficiaries: A brief report of the Dartmouth Atlas Project. http://www.dartmouthatlas.org/downloads/reports/AF4Q_Disparities_Report.pdf. [PubMed] [Google Scholar]