Abstract
There is substantial evidence implicating N-methyl-d-aspartate receptors (NMDARs) in memory and cognition. It has also been suggested that NMDAR hypofunction might underlie the cognitive deficits observed in schizophrenia since morphological changes, including alterations in the dendritic architecture of pyramidal neurons in the prefrontal cortex (PFC), have been reported in the schizophrenic brain post mortem. Here, we used a genetic model of NMDAR hypofunction, a serine racemase knockout (SR−/−) mouse in which the first coding exon of the mouse serine racemase gene has been deleted, to explore the role of d-serine in regulating cognitive functions as well as dendritic architecture. SR −/− mice exhibited a significantly disrupted representation of the order of events in distinct experiences as revealed by object recognition and odor sequence tests; however, SR −/− animals were unimpaired in the detection of novel objects and in spatial displacement, and showed intact relational memory in a test of transitive inference. In addition, SR −/− mice exhibited normal sociability and preference for social novelty. Neurons in the medial PFC of SR−/− mice displayed reductions in the complexity, total length, and spine density of apical dendrites. These findings demonstrate that d-serine is important for specific aspects of cognition, as well as in regulating dendritic morphology of pyramidal neurons in the mPFC. Moreover, they suggest that NMDAR hypofunction might, in part, be responsible for the cognitive deficits and synaptic changes associated with schizophrenia, and highlight this signaling pathway as a potential target for therapeutic intervention.
Keywords: D-serine, NMDA receptors, dendritic morphology, temporal order memory, sequence memory, schizophrenia
Introduction
Substantial evidence has demonstrated that N-methyl-d-asparate receptors (NMDARs), a type of ionotropic glutamate receptor, are essential for multiple aspects of episodic memory and cognition (Tsien et al., 1996; Nakazawa et al., 2003; Rajji et al., 2006). NMDARs are considered molecular “coincidence detectors,” since activation requires postsynaptic depolarization, and the binding of two agonists, glutamate, and either glycine or d-serine, at the glycine modulatory site (GMS; Tsien, 2000). d-serine is enriched in corticolimbic regions of the brain, where its localization closely parallels that of NMDARs (Schell et al., 1995). Reduced levels of d-serine in serum and in cerebrospinal fluid (CSF), as well as alterations in the level of NMDAR expression, have been associated with cognitive impairment (Turpin et al., 2009; Yang et al., 2005; Potier et al., 2010), as well as schizophrenia (Akbarian et al., 1996; Hashimoto et al., 2003; Bendikov et al., 2007; Morita et al., 2007).
Patients suffering from schizophrenia exhibit significant impairments in memory, problem solving, and executive functions (Mesholam-Gately et al., 2009), which are associated with both structural and functional abnormalities within the hippocampus and prefrontal cortex (PFC). In particular, the complexity of dendritic branching, total dendritic length, and dendritic spine density of pyramidal neurons is reduced in the PFC of patients with schizophrenia (Garey et al., 1998; Rajkowska et al., 1998; Glantz and Lewis, 2000; Kalus et al., 2000). Disruptions in dendritic morphology alter the cortical and/or thalamic circuitry, which in turn might be the neurobiological substrate underlying the cognitive deficits observed in patients with schizophrenia (Lewis and Gonzalez-Burgos, 2008).
Animals lacking serine racemase (SR −/−), the enzyme that synthesizes d-serine from l-serine, provide an important tool for exploring the contribution of d-serine to memory and cognitive function (Morita et al., 2007; Verrall et al., 2007). A prior assessment of SR −/− mice, an animal with reduced NMDAR-dependent glutamatergic transmission, has demonstrated that these animals exhibit disruptions in anxiety as well as impairment in spatial memory (Basu et al., 2009). In order to expand upon these findings, here, we assessed several domains of higher-level cognitive function, including episodic memories, which are known to engage the hippocampus and PFC, and examined dendritic plasticity of pyramidal neurons in the PFC as a biological marker of neurotransmission. We report here that D-serine plays a critical and selective role in memory for temporal order and is important for proper dendritic patterning and maintenance of spine density on excitatory neurons.
Materials and Methods
Animals
Serine racemase (SR) heterozygous [Het; SR +/−] mice were originally generated at McLean Hospital in Belmont, MA, as previously described (Basu et al., 2009). A colony of SR+/− mice is maintained at McLean Hospital by continuous backcrossing of each generation to a C57BL/6J background. Subjects of this study were bred from SR+/− mice of F10 or higher backcross generation. C57BL/6J breeders were obtained from the Jackson Laboratory (Bar Harbor, ME) and bred in-house at McLean Hospital. New C57BL/6J breeders were imported every 5 generations. Mice on which post mortem analyses were performed were bred and housed at McLean Hospital as previously described (Basu et al., 2009). Briefly, non-sibling heterozygous (SR+/−) offspring of backcrosses were mated to each other to produce homozygous [KO; SR −/−] and wildtype [WT; SR +/+] experimental subjects. The SR+/− strain was re-derived by embryo transfer to pseudopregnant C57BL/6J surrogate mothers at the Jackson Laboratory (Bar Harbor, ME) before transfer to Boston University. Homozygous [KO; SR −/−] and wildtype [WT; SR +/+] mutants tested in behavioral experiments were generated from heterozygous breeding pairs at Boston University. At weaning, tissue samples were sent to the Charles River Laboratory for genotyping.
All animals at Boston University were maintained on a reverse 12-hour light/dark cycle [09:00 off; 21:00 on], while those housed at McLean Hospital were maintained on a regular 12-hour light/dark cycle. Animals were given ad libitum access to food and water, unless otherwise specified in behavioral methods. Only age-matched, littermate males were used in experiments reported here. Animals were used for more than one behavioral experiment in order to reduce the overall number of animals used in these studies. The group sizes were as follows for each behavioral experiment: object recognition: 21 WT and 17 SR −/− mice; object displacement: 12 WT and 12 SR −/− mice; object order: 13 WT and 11 SR −/− mice; odor sequence memory: 12 WT and 11 SR −/− mice; transitive inference: 8 WT and 10 SR −/− mice; sociability: 20 WT and 16 SR −/− mice; social novelty: 19 WT and 14 SR −/− mice. The group sizes for Golgi-staining are: 5 WT and 5 SR−/− mice. The IACUCs of Boston University and McLean Hospital approved the treatment and use of the animals in these experiments.
Memory for objects: Object recognition, object displacement, and object order memory
Episodic memory is commonly characterized as the ability to remember specific events in the place and time that they occurred (Clayton and Dickinson, 1998). We assessed these components of episodic memory by using a series of tests of memory for objects, their spatial locations, and the order in which they were experienced (Barker et al., 2007). Animals were first habituated to a rectangular Plexiglas apparatus with proximal visual cues on the walls for a 5-minute period (40cm length X 20cm width X 37cm height). Exploratory behavior was recorded via a WebCam (Gigaware®, UK) positioned above the testing arena and later scored for investigation time of each object in the sample sessions and the test session. Investigation was measured as the time over a 5-minute test in which the animal directly explored each object by sniffing or whisking, when the animal’s nose was facing and within 2 cm of the object. Investigation also included rearing or climbing onto the object. Two researchers blind to the subject’s genotype coded each video for investigation of each object using a stopwatch. Any animal showing a significant spatial bias, as defined by the greatest amount of exploration occurring at the same location relative to all other locations in successive trials, was removed from analysis. Only one WT mouse was removed from analysis of the object recognition test for exhibiting a significant spatial bias. Discrimination ratios based on exploration of the objects were used for measures of each type of memory.
Object recognition
In the object recognition assay, mice were presented with a set of two identical objects A (small glass beaker; Corning, Lowell, MA), to which they had not been exposed previously, in a rectangular Plexiglas apparatus (40cm length X 20cm width X 37cm height; Figure 1A, left panel). The objects were presented on the east and west walls of the testing apparatus, mid-way between the north and south corners. Mice were allowed 5 minutes to explore both objects. After this exploration period, they were briefly returned to their home cages for sufficient time to clean the apparatus and replace the objects (approximately one minute) and then placed back into the testing apparatus. In the test phase, one object was replaced by a copy of object A (left-right position was counter-balanced) and the other object was replaced in the same location by a different object B (plastic cylinder; Nalgene, Rochester, NY). The animals were then given another 5-minute exploration period and a discrimination ratio was calculated as the difference between the exploration time for object B and the exploration time for object A, divided by the sum of those times. Pilot studies ensured that there were no innate biases for the objects used in this study (t(14)=0.024, p=0.981).
Figure 1.
Performance of the WT mice and SR −/− mice on object recognition, object displacement, and object order. A. Arrangement of stimuli in the object recognition test. A discrimination ratio was used to measure preference for a novel vs. a familiar object. B. Arrangement of stimuli in the object location test. A discrimination ratio was used to measure preference for a displaced vs. a stationary object. C. Arrangement of stimuli in the object order test. A discrimination ratio was used to measure preference for an older vs. a more recent object. *indicates p<.05 discrimination ratio less than zero; # indicates p<.05 difference in discrimination ratio between groups.
Object displacement
In the object displacement assay, animals were presented with two identical objects C (small plastic container; Nalgene, Rochester, NY), different from those they experienced in the object recognition task (Figure 1B, left panel). The objects were presented on the northeast and northwest walls of the same testing apparatus used in the object recognition task, and animals were allowed 5 minutes to explore both objects. After this exploration period, they were briefly returned to their home cages for sufficient time to clean the apparatus and replace the objects (approximately one minute), and then placed back into the testing apparatus. In the test phase, one object (left-right position was counter-balanced) was replaced by a copy of object C in a different location, while the other object was replaced by another copy of object C in its original location. Then the animals were allowed to explore the objects for 5 minutes and the discrimination ratio was calculated as the difference between the exploration time for the relocated object C and the exploration time for the stationary object C, divided by the sum of those times.
Object order memory
The object order assay was conducted in two phases (Figure 1C, top panel). In Sample 1, animals were allowed 5 minutes to explore two identical objects D (glass container; VWR, West Chester, PA) in the east and west corners of the same testing apparatus used in the previously described tests. After a 50-minute delay, in Sample 2, animals were allowed 5 minutes to explore 2 new identical objects E (small plastic bottle; VWR, West Chester, PA) presented in the same locations. After another 50-minute delay, in the test phase, animals were presented with a copy of object D and a copy of object E in the same location (left-right position was counter-balanced) and allowed to explore the objects for 5 minutes. The discrimination ratio was calculated as the difference between the exploration time for the less recent object D and the more recent object E, divided by the sum of those times. Pilot studies ensured that there were no innate biases for the objects used in this study (t(14)= −0.987, p=0.340).
Memory for the order of events in distinct sequences
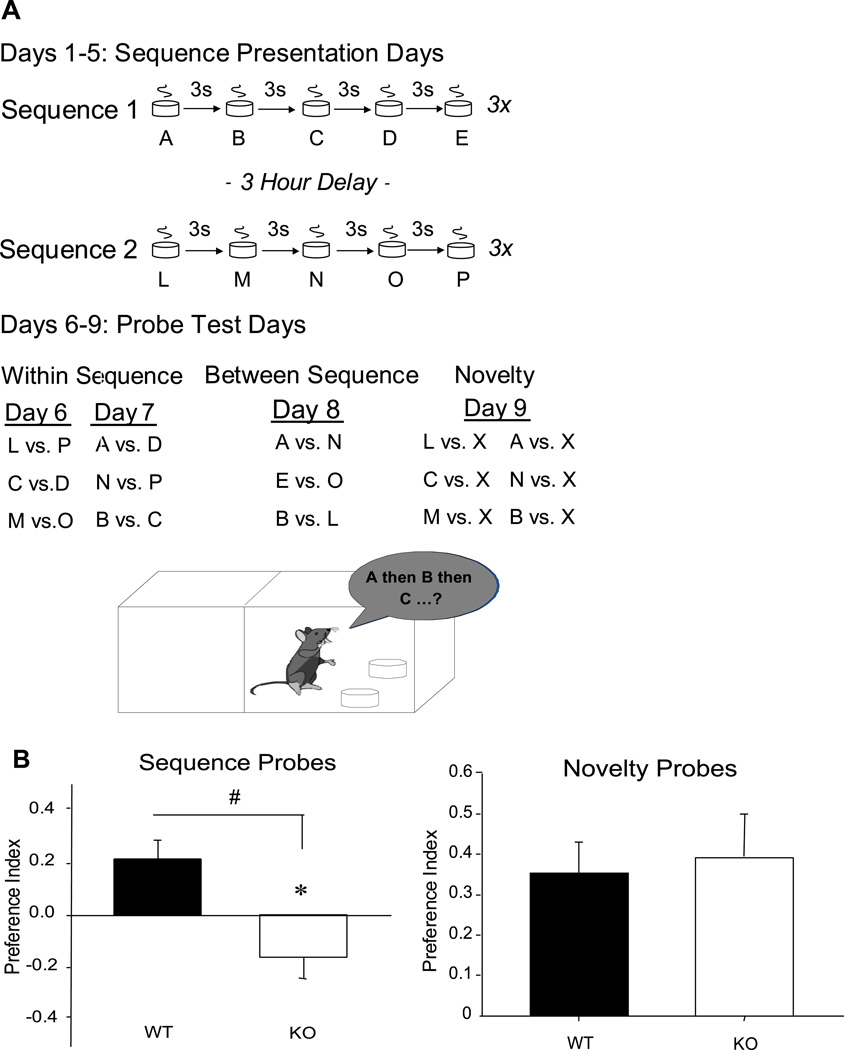
Mice were tested on a sequence memory task that assessed the ability to remember the order of specific odors presented within two different series (DeVito and Eichenbaum, under review). Animals were placed on food restriction and maintained at 85% of free feeding weight. Over a 3-day period, animals were introduced to Plexiglas cages (26cm length X 15cm width X 13cm height) in which they were allowed to dig for chocolate sprinkle rewards buried in small plastic cups filled with sand. Once all subjects exhibited reliable digging behavior, the animals were exposed to two different odor sequences each day over a 5-day period. All odors were prepared at 1% concentration by weight in sand.
Sequence 1, with the odors referred to as A, B, C, D, E, consisted of the following odors: A=cilantro (Spice Islands Trading Company, San Francisco, CA), B=marjoram (Frontier Natural Products Co-op, Norway, IA), C=dill (Frontier Natural Products, Norway, IA), D=parsley (Frontier Natural Products Co-op, Norway, IA), and E=allspice (Spice Islands Trading Company, San Francisco, CA). Sequence 2, referred to as L, M, N, O, P, consisted of the following odors: L=lemon, (McCormick, Hunt Valley, MD), M=cinnamon (McCormick, Hunt Valley, MD), N=tarragon [(Frontier Natural Products Co-op, Norway, IA), O=anise (McCormick, Hunt Valley, MD), and P=orange (McCormick, Hunt Valley, MD). These odors were selected as stimuli for which mice show no strong natural attraction or aversion.
In the first session each day, animals were presented with Sequence 1. At the beginning of each sequence, the animal was presented with the first odor, which contained a buried chocolate sprinkle that the mouse could obtain by digging in the sand (Figure 2A). This process was repeated for the remaining four odors in that sequence until the animal had been exposed to all five odors successively with a 3 second inter-stimulus interval. Each animal was presented with Sequence 1 three times with a 3-minute inter-trial interval. Three hours after the first session was completed, the animals were exposed to Sequence 2 in the same manner. Each odor was presented in one of several locations within the testing apparatus in order to prevent the development of spatial biases. This procedure for sequence presentation was repeated for 5 days. After these 5 days, the animals were not subsequently exposed to the odor sequences.
Figure 2. 56.
Performance of the WT mice and SR −/− mice on odor sequence memory. A. Schematic of the apparatus and odors used to test the animals on sequence memory. B. A preference index was used to assess the amount of time spent digging in the item occurring earlier in the sequence compared to an item occurring later in the sequence. *indicates p<.05 discrimination ratio less than zero; # indicates p<.05 difference in discrimination ratio between groups.
Beginning the next day, the animals were administered probe tests that assessed memory for the odors within and between each of the sequences. On each of these probe tests, two odors were selected, and both were presented without buried rewards. We measured the amount of time the animals spent digging in each cup and the two digging times were used to calculate a preference index (PI; Bunsey and Eichenbaum, 1996); PI = (Time spent digging in the odor that occurred earlier in the sequence – time spent digging in the odor that occurred later in the sequence) / Total time digging in both odors). In order to prevent digging behavior from extinguishing on these probe trials, the animal was presented with a cup of unscented sand baited with a chocolate sprinkle on the surface of the cup immediately after each probe trial.
On the first two days of probe testing (days 6 and 7), the animals were presented with odors selected from the same sequence with which they were presented earlier. These were considered “within sequence” probe tests as they evaluated the ability of the animals to remember the order of odors that were presented within one of the specific sequences. On the first day of probe testing (day 6), the animals were presented with L vs. P, C vs. D, and M vs. O. On the second day of probe testing (day 7), the animals were presented with A vs. D, N vs. P, and B vs. C.
On the third day of probe testing (day 8), the animals were presented with pairs consisting of one odor from Sequence 1 and one odor from Sequence 2. These were considered “between sequence” probe tests because they evaluated the ability of the animals to compare the times at which the odors were presented over the course of each day, i.e., earlier in Sequence 1 versus 3 hours later in Sequence 2. The animals were presented with A vs. N, E vs. O, and B vs. L.
Finally, on the fourth day (day 9), the animals were presented with an odor from either sequence against a novel odor they had never experienced. These were considered “novelty” probe tests because they evaluated the ability of the animals to recognize novel as compared to familiar odors. The animals were presented with L vs. caraway (McCormick, Hunt Valley, MD), C vs. mace (McCormick, Hunt Valley, MD), and M vs. sage (McCormick, Hunt Valley, MD). Following a 3-hour delay, the animals were presented with A vs. baby powder (Shaw’s Supermarkets, East Bridgewater, MA), N vs. onion (McCormick, Hunt Valley, MD), and B vs. strawberry (Nestle, Glendale, CA).
We did not counterbalance for the assignment of the order of odor presentations during training because there would be a very large number of permutations of the order of 5 items in each sequence. And we did not counterbalance the order of probe tests on within sequence and between sequence preferences. However, our previous study showed that animals that are not trained on these sequences do not exhibit within sequence or between sequence order preferences, and the pattern of performance on the two types of probe tests does not depend upon the order of their presentation (DeVito and Eichenbaum, under review).
Relational memory
The transitive inference task assesses the ability of animals to learn relations among stimuli formulated as a series of overlapping discrimination problems that share common elements (Dusek and Eichenbaum, 1997). In the classic test for relational memory, subjects are initially trained on a series of premise problems (e.g., choose A over B, then chose B over C, then chose C over D, then chose D over E), then tested for the capacity to infer the transitive relation between items that had never appeared together (B vs. D). The choice of B over D indicates the existence of a relational hierarchy (A over B over C over D over E).
Mice were trained on the premise problems and probe tests for transitive inference in a series of stages. Initially, animals were placed on food restriction and maintained at 85% of free feeding weight. Over a 3-day period they were trained to dig for chocolate sprinkle rewards buried in sand that filled small plastic cups placed in Plexiglas cages (26cm length X 15cm width X 13cm height). Once all subjects exhibited reliable digging and reward retrieval behavior, they were trained on a preliminary olfactory discrimination. All odors were prepared at a concentration of 1% by weight. The reward-baited odor cup was presented at one of several locations randomly selected on each trial, in order to prevent the development of spatial biases.
Following the preliminary discrimination, animals were trained on a series of premise problems formulated as overlapping discriminations (A+ vs. B−, B+ vs. C−, C+ vs. D−, and D+ vs. E−; where A = paprika [CVS, Woonsocket, RI], B = coffee [Folger’s, Cincinnati, OH], C = basil [McCormick, Hunt Valley, MD], D = cumin [McCormick, Hunt Valley, MD], and E = cocoa [Hershey’s, Hershey, PA]; + and − refer to rewarded and non-rewarded odors, respectively; Figure 3A). Animals were trained in two 8 trial sessions per day across four training stages that began with large blocks of trials of the same type and involved progressively including more of the trial types. Training continued until animals reached a criterion of 75% accuracy (6/8 trials) across two consecutive days at each stage of training.
Figure 3.
Performance of WT mice and SR −/− mice on transitive inference. A. Schematic of the apparatus and odors used to train and test the animals on transitive inference. B. The number of days to reach criterion across the four stages of training was used to assess acquisition of the odor pairs. Percentage correct was used to assess performance on the training pairs during the transitive inference test. C. A preference index was used to assess the amount of time spent digging in B versus D (“transitive pair”) and A versus E (“non-transitive pair”) on the probe tests.
The day after reaching criterion on the last training stage, animals were given probe tests for transitive (B vs. D) as well as non-transitive (A vs. E) judgments, composed as novel discriminations between items that had not previously been presented together. Four BD and four AE probe tests were intermixed within the training pairs over a two-day period, given at trials 3, 6, 11, and 14 within the regular 16 trials. A correct judgment (choosing B over D) on the BD pair required that animals had linked the odor premises into an integrated hierarchy so that they could make the inference across the missing overlapping element C. In contrast, a correct judgment of A over E could be made without reference to the structure of the odor premises because odor A was always rewarded and odor E was never rewarded; the AE pair served as a control for the ability to make a judgment about a novel pairing in which performance did not depend on existence of the hierarchical representation. Neither cup was baited during probe trials and we measured the amount of time the animals spent digging in each cup. These digging times were used to calculate a preference index (PI; Bunsey and Eichenbaum, 1996). For the B vs. D test, PI = (B−D)/(B+D); for A vs. E, PI = (A−E)/(A+E).
Social recognition: Sociability and preference for social novelty
A social recognition paradigm was adapted from Moy et al. (2004). The main difference between the Moy et al. (2004) protocol and the methods described here is the use of littermates as stimulus animals as compared to animals from different litters. Our version of this paradigm was used in a previous study and demonstrated that this protocol is robust at detecting differences in sociability and preferences for social novelty (DeVito et al., 2009). The apparatus was a rectangular Plexiglas apparatus (40cm length X 20cm width X 37cm height), divided into three areas of equal width by a thin plastic mesh (20 cm height X 20cm length) that allowed the animals to explore one another but prevented aggressive interactions. A stimulus animal, an age-matched, naive male SR +/− mouse from a different litter, was placed in one of the outer compartments (left and right placement was counter-balanced). The test animal was then placed in the center compartment and allowed to investigate both outer compartments. The apparatus was cleaned with 70% ethanol after each test.
Sociability
In this test animals were exposed to one compartment containing a familiar littermate while the other compartment was empty (Figure 4A, left panel). A stimulus animal, an age-matched, naive male SR +/− mouse from a different litter, was placed in one compartment and the other compartment was empty. Investigation was measured as the time over a 5-minute test in which the animal directly explored each compartment by sniffing, when the animal’s nose was facing and within 2 cm of the compartment, and climbing the mesh divider. Each animal’s behavior was recorded following a one-minute period of habituation to the testing apparatus. An animal expressing normal sociability will spend a greater amount of time investigating the compartment containing the familiar littermate as compared to the empty compartment. The score for sociability was calculated as the difference between the exploration time for the compartment containing the familiar littermate and that for the empty compartment, divided by the sum of those times.
Figure 4.
Performance of WT mice and SR −/− mice on sociability and social novelty. A. Apparatus used to assess sociability. A discrimination ratio was used to assess preference for a compartment containing a littermate as compared to an empty compartment. B. Apparatus used to assess social novelty. A discrimination ratio was used to compare the amount of time spent investigating a familiar versus novel animal.
Preference for social novelty
The day following sociability testing, the animals were assessed on preference for a novel conspecific animal (Figure 4B, left panel). A stimulus animal, an age-matched, naive male SR +/− mouse from a different litter, was placed in one compartment and a littermate (different from the animal used in the sociability test) was placed in the other compartment. As in the test for sociability, investigation of each compartment was defined as direct exploration by sniffing and climbing the mesh divider. Each animal’s behavior was recorded following a one-minute period of habituation to the testing apparatus. An animal expressing a normal preference for social novelty will spend a greater amount of time investigating the compartment containing the animal from a different litter (novel) as compared to the compartment containing the littermate (familiar). The score for social novelty was calculated as the difference between the exploration times for the compartment containing the novel animal and that for the familiar littermate divided by the sum of those times.
Golgi Staining
Golgi staining was performed on adult, male WT (n = 5) and SR−/− (n = 5) mice using the FD Rapid GolgiStain Kit (FD NeuroTechnologies, Ellicot City, MD) according to the manufacturer’s instructions. Brains were removed, immersed in fixative (5 ml; equal volume of Solutions A/B) for 40 hours, and then transferred into Solution C and stored at 4°C until slicing. The fixed brains were sliced on a freezing microtome (American Optical 860, Buffalo, NY) at a thickness of 120 µm. Sections were mounted with Solution C on 3% gelatin-coated slides (75×25 mm; FD NeuroTechnologies, Ellicot City, MD). Excess Solution C was immediately removed from the slide using a Pasteur pipette and a fine paintbrush. Sections were kept at room temperature overnight, protected from light. Slides were then stained, dehydrated, and cleared with xylenes according to the manufacturer’s protocol, without counterstaining.
Quantification of Dendritic Morphology
Pyramidal neurons in the mPFC chosen for reconstruction were located approximately 2.22 mm to 1.54 mm anterior to bregma and included the cingulate, prelimbic, and infralimbic cortices (Paxinos and Franklin, 2001). Neurons selected for reconstruction were located in the middle third of the section and were not obscured by neighboring neurons and glia. Within each region examined, a minimum of 3 neurons was reconstructed for each mouse. Neurons were drawn at 40× on a Zeiss Axioskop40, and the morphology of apical and basilar arbors was quantified in three dimensions using Neurolucida (MBF Bioscience, Williston, VT), with the experimenter blind to genotype. Sholl analysis was performed using the Neurolucida Explorer software to assess dendritic complexity (concentric circles increased in diameter by 10 µm increments).
Spine Density Analysis
Spines were counted on dendritic branches from a minimum of three neurons per region in each animal. For apical dendrites, spines were counted on the apical trunk (last 40 µm) and oblique segments separately. Neurons were visualized at 63× (oil-immersion) using a Leitz Laborlux12 microscope. One to two branches at each order were selected and spines were counted using BioQuant Nova Prime (BioQuant Image Analysis Corp., Nashville, TN). Spines were counted on branches from the same neurons that were reconstructed at 40× for dendritic analysis.
Statistical Analysis of Morphometric Data
The effect of genotype on total dendritic length and segment analyses was analyzed using two-tailed, unpaired Student’s t test. Dendritic complexity was analyzed using two-factor ANOVA with repeated measures, while spine density and maturity were compared using two-factor ANOVA. Significant F tests were followed up with Student’s t test comparisons. p < 0.05 was considered statistically significant.
Results
Memory for objects: Object recognition, object displacement, and object order memory
A 2-Way ANOVA comparing performance across the three tests revealed a significant effect of test type and group X test type interaction (group F(1,85)=2.096, p=0.152; test type F(2,85)=6.043, p=0.004; group X test type interaction F(2,85)=4.76, p=0.011). Post hoc tests revealed that there was a significant difference between groups on the object order task only (t(22)=3.70, p=0.001; Figure 1C, bottom panel). Further analyses revealed that WT mice exhibited a significant preference for an earlier experienced object over a more recently experienced object (t(24)=2.346, p=0.028). In striking contrast, SR −/− mice demonstrated the opposite preference, that is, greater exploration of the more recently experienced object than the less recently experienced object (t(20)= −3.016, p=0.007). Therefore, although the SR −/− mice can discriminate objects previously experienced at different times, which clearly reflects some form of memory for the order of object presentations, the expression of that order memory is abnormal.
Both WT and SR −/− mice exhibited a significant preference for the novel object as compared to the familiar object (WT mice: t(40)=2.32, p=0.025; SR −/− mice: t(32)=4.31, p<0.001) and there was no significant difference in performance between the two groups (t(36)=−1.23, p=0.225; Figure 1A, right panel). In addition, both WT and SR −/− mice also exhibited a significant preference for the displaced object over the stationary object (WT mice: t(22)=2.88, p=0.009; SR −/− mice: t(22)=2.25, p=0.034) and there was no significant difference in performance between the two groups (t(22)=0.441, p=0.663; Figure 1B, left panel). Therefore, the SR −/− mice demonstrated intact memory for object identity and object location.
Analysis of total exploration time during both the training sessions and test sessions revealed that there was a significant difference in exploration time between the WT and SR −/− mice on the training session of object recognition, such that the SR −/− mice explored the objects for a greater period of time than did the WT mice (t(74)=8.198; p=0.005); however, there were no differences between exploration in the test phase of the object recognition test (t(74)=0.378, p= −0.888). There were also no differences in exploration between the WT and SR −/− mice at any other phase of the object displacement (training session: t(46)=0.189, p=0.851; test session: t(46)= −1.07, p=0.290) and object order tasks (training session 1: t(48)=−1.354, p=0.182; training session 2: t(48)= −1.002, p=0.321; test session: t(48)= −1.097, p=0.278).
Memory for the order of events in distinct sequences
WT mice significantly preferred odors presented earlier within a sequence over odors presented later in the same sequence (t(22)=3.205, p=0.004). In contrast, SR −/− mice exhibited the reverse preference, preferring late-presented odors over earlier-presented odors in the same sequence (t(20)= −2.086, p=0.05). Correspondingly, the preference index differed between the groups (t(21)= −3.69, p=0.001; Figure 2B, left panel). These findings indicate that SR −/− mice exhibit some form of memory for the order of experienced odors, in that they discriminate between odors previously experienced in different ordinal positions, but they express this memory abnormally.
Neither the WT (t(22)= −0.617, p=0.544) nor the SR −/− mice (t(20)=1.882, p=0.074) exhibited a significant preference for items that had been experienced in different sequences. The preference index for the WT mice was −0.07 +/− 0.11 and the preference index for the SR −/− mice was 0.21 +/− 0.11; thus, the SR −/− mice demonstrated a marginal preference for items occurring in Sequence 1 compared to Sequence 2 (t(20)=1.882, p=0.074, as noted above). However, the difference in group mean preference scores failed to reach statistical significance (t(21)= −1.769, p=0.091).
We also tested whether animals could recognize the previously experienced odors using recognition tests in which the animals were given a choice between a novel odor and odors they had experienced in the trained sequences. Both groups performed at levels that were significantly different from chance (WT mice: t(22)=4.546, p<0.001; SR −/− mice: t(20)=3.807, p=0.001), and there was no difference in performance between the groups (t(21)=−0.288, p=0.776; Figure 2B, right panel). Finally, there was a significant difference across groups in the total amount of time spent digging during probe tests for items within each sequence (t(284)=2.851, p=0.005), such that the WT mice spent a greater time digging in the probe trials; however, there was no difference in the total amount of time spent digging during probe trials for items between each sequence (t(136)=1.311, p=0.192) or during novelty judgments (t(274)=−0.414, p=0.679).
Relational memory
SR −/− and WT mice learned the four odor pair discriminations (premise problems) successfully and there was no difference between the two groups in the number of days required to reach criterion across the four stages of training (2-Way ANOVA: group F(1,71)=0.19, p=0.659; stage F(3,71)=0.77, p=0.514; group X stage interaction F(3,71)=0.08, p=0.967). This indicates that SR −/− mice have an intact ability to learn a series of overlapping odor discriminations.
Both groups also performed similarly on the premises when tested along with the probe pairs BD and AE (2-Way ANOVA: group F(1,71)=0.54, p=0.923; pair type F(3,71)=6.50, p=0.001; group X pair type interaction F(3,71)=0.22; p=0.881; Figure 3B). Although there were significant differences in performance on different premise pairs, there was no difference in performance between the two groups on performance of any of the premise pairs (AB: t(16)= −0.630, p=0.537; BC: t(16)= −0.172, p=0.865; CD: t(16)=0.351, p=0.730, DE: t(16)=0.776, p=0.449). Therefore, in addition to performing similarly on each odor pair, WT and SR −/− mice also exhibited a similar pattern of performance across the pair types.
In the probe tests (Figure 3C), both WT and SR −/− mice exhibited a significant preference for B over D (WT: t(14)=3.822, p=0.002; SR −/− mice: t(18)=3.344, p=0.004) and A over E (WT: t(14)=13.858, p<0.001; SR −/− mice: t(18)=12.246, p<0.001) at levels that are significantly different from chance. Correspondingly, there was no difference in performance between the two groups on the BD (t(16)=0.321, p=0.752) or AE probe tests (t(16)=0.910, p=0.376). In addition, the total amount of time spent digging in all probe cups was comparable, indicating that both the WT mice and SR −/− mice were equally motivated and displayed no differences in activity levels (t(284)=−0.676, p=0.499).
Social recognition: Sociability and preference for social novelty
The sociability test assesses the animal’s level of social motivation and interest in social interaction. WT and SR −/− mice spent more time investigating the compartment containing the littermate versus the empty compartment, and there was no significant difference between the group scores (t(34)=−1.679, p=0.102; Figure 4A, right panel). The social preference of WT mice was significantly different from zero (t(38)=3.209, p=0.003), as was the social preference of the SR −/− mice (t(30)=4.204, p<0.001).
The social novelty test assesses the animal’s social memory, reflected by a preference for investigating a novel conspecific over a familiar one. WT and SR −/− mice spent considerably more time investigating the compartment containing the novel animal versus the compartment containing the familiar littermate, and there was no difference between groups (t(31)=0.486, p=0.630; Figure 4B, right panel). Both the WT mice (t(36)=3.449, p=0.001) and the SR −/− mice (t(26)=2.830, p=0.009) exhibited preference scores that were significantly greater than zero.
Dendritic morphology of pyramidal neurons in the medial prefrontal cortex
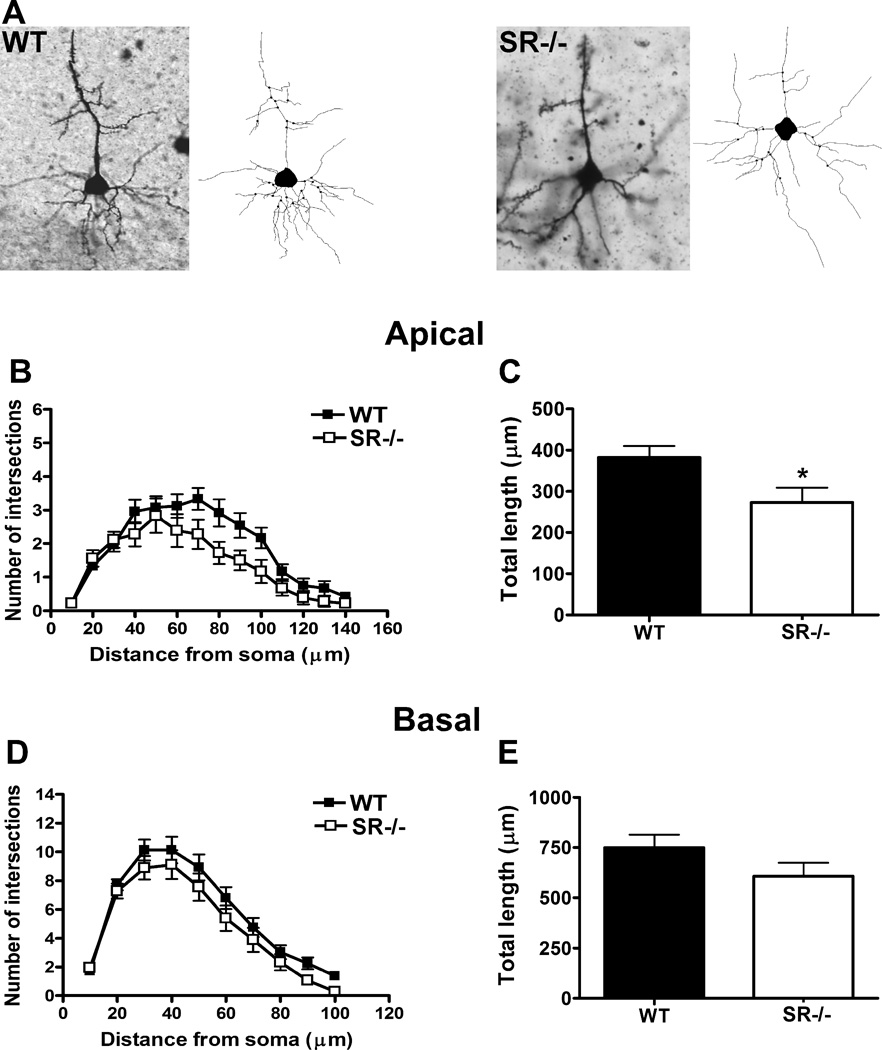
Multiple parameters were compared between WT and SR−/− mice across the apical and basilar dendritic systems of pyramidal neurons. Sholl analysis revealed that the neurons in SR−/− had less complex branching of their apical dendrites (distance from soma: F(13,40)= 28.80, p<0.0001; genotype: F(1,40)=5.01, p=0.03; distance X genotype: F(9,40)=1.57, p=0.09; Figure 5A,B). The neurons of SR−/− mice also had significantly less total apical dendritic length than WT mice (t(40)=2.45, p=0.02; Figure 5C); however, their basal dendrites did not show significant reductions in complexity (distance from soma: F(9,40)=107.7, p<0.0001; genotype: F(1,40)=1.641, p=0.21; distance × genotype: F(9,40)=0.59, p=0.81 Figure 5D;) or total dendritic length (t(40)=1.51, p=0.14; Figure 5E).
Figure 5.
Apical and basilar dendritic morphology of pyramidal neurons in the medial prefrontal cortex (mPFC). A. Golgi stained pyramidal neurons and computer-assisted reconstructions of representative neurons in WT (left) and SR−/− (right) mice. B., C. Sholl analysis was used to compare the apical dendrites of neurons from WT and SR−/− mice. D., E. Sholl analysis was also used to compare the basal dendrites of neurons from WT and SR−/− mice. *indicates p < 0.05 between groups.
Segment analysis was performed to determine whether there were certain areas of the apical dendritic tree where these perturbations were concentrated in the neurons of SR−/− mice (Table 1). The total length (t(40)=2.45, p=0.02) and number of 5th order branches (t(40)=2.50, p=0.02) were significantly lower in SR−/− mice, while the average length of 4th order branches (t(40)=2.07, p=0.04) was also reduced in the SR−/− animals. Although there were no overall differences in basilar dendritic morphology, segment analysis revealed that pyramidal neurons of SR−/− mice had significant reductions specifically in the total length (t(40)=2.18, p=0.03) and number (t(40)=2.39, p=0.02) of 4th order basal dendrites (Table 1).
Table 1.
Detailed segment analysis of apical and basal dendrites of neurons in the mPFC from wild type (WT) and SR−/− mice. The total length (µm), number of segments, and average length of segments (µm) were compared across each of the branch orders. N/A (not applicable) indicates that no data were collected at that branch order.
| Branch Order | |||||||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | |
| Total length (µm) | |||||||
| Apical | |||||||
| WT | 23 ± 4 | 48 ± 4 | 85 ± 11 | 120 ± 18 | 62 ± 12 | 32 ± 11 | 11 ± 6 |
| SR−/− | 17 ± 4 | 61 ± 11 | 72 ± 11 | 74 ± 21 | 24 ± 8 * | 15 ± 7 | 10 ± 8 |
| Basal | |||||||
| WT | 124 ± 12 | 180 ± 19 | 227 ± 38 | 151 ± 23 | 51 ± 16 | N/A | N/A |
| SR−/− | 105 ± 13 | 150 ± 25 | 170 ± 28 | 73 ± 20 * | 35 ± 11 | N/A | N/A |
| Number of segments | |||||||
| Apical | |||||||
| WT | 1.0 ± 0.0 | 2.0 ± .04 | 2.9 ± 0.3 | 2.9 ± 0.4 | 2.1 ± 0.4 | 1.1 ± 0.3 | 0.4 ± 0.2 |
| SR−/− | 1.0 ± 0.0 | 2.0 ± 0.2 | 2.6 ± 0.4 | 2.1 ± 0.6 | 0.9 ± 0.2* | 0.4 ± 0.2 | 0.2 ± 0.2 |
| Basal | |||||||
| WT | 5.1 ± 0.3 | 6.9 ± 0.5 | 7.3 ± 0.8 | 4.0 ± 0.5 | 1.3 ± 0.4 | N/A | N/A |
| SR−/− | 4.2 ± 0.3 | 5.5 ± 0.5 | 5.6 ± 0.8 | 2.3 ± 0.5* | 1.4 ± 0.3 | N/A | N/A |
| Average segment length (µm) | |||||||
| Apical | |||||||
| WT | 23 ± 4 | 24 ± 2 | 27 ± 3 | 40 ± 5 | 20 ± 4 | 11 ± 3 | 4 ± 2 |
| SR−/− | 17 ± 4 | 29 ± 5 | 25 ± 4 | 25 ± 5 * | 12 ± 4 | 7 ± 4 | 5 ± 4 |
| Basal | |||||||
| WT | 24 ± 2 | 27 ± 3 | 27 ± 2 | 32 ± 3 | 16 ± 4 | N/A | N/A |
| SR−/− | 23 ± 3 | 24 ± 3 | 25 ± 3 | 20 ± 5 | 15 ± 5 | N/A | N/A |
indicates p < 0.05 between groups.
Spine density on pyramidal neurons was compared across genotypes on the last 40µm of the primary apical dendrite and on oblique apical dendrites, as well as on basal dendrites. SR−/− mice had reduced spine density on both primary and oblique branches, with the distal primary dendrite more densely occupied with spines than oblique branches in both genotypes (branch type: F(1,32)=11.19, p=0.002; genotype: F(1,32)=7.708, p=0.009; branch × genotype: F(1,32)=0.14, p=0.72; Figure 6A,B). However, spine density on all branch orders of basal dendrites was unaffected on the neurons of SR−/− mice (branch order: F(1,32)=0.42, p=0.52; genotype: F(2,32)=0.05, p=0.95; branch × genotype: F(2,32)=0.19, p=0.82 Figure 6C).
Figure 6.
Spine density analysis of pyramidal neurons in the medial prefrontal cortex (mPFC). A. Apical dendritic spines of a Golgi-stained pyramidal neuron in a WT (left) and a SR−/− mouse (right). B. Spine density analysis of both oblique branches and the distal end of apical dendrites in WT and SR−/− mice. C. Spine density analysis of branch order of basilar dendrites of pyramidal neurons in WT and SR−/− mice. Spine density was normalized to the number of spines per 10 µm of dendrite. *indicates p < 0.05 between groups.
Discussion
NMDARs play an important role in memory and cognition, and hypofunction of these receptors contribute to the pathophysiology of schizophrenia (Goff and Coyle, 2001; Konradi and Heckers, 2003; Lisman et al., 2008). Here, we demonstrate that SR−/− mice, in which NMDAR signaling is hypofunctional (Basu et al., 2009), display significant alterations in their representation for the order of events that is consistent with compromised function of the PFC. Moreover, pyramidal neurons in the mPFC of SR −/− mice exhibit dendritic abnormalities reminiscent of the pathological changes observed in schizophrenia.
SR −/− mice demonstrate impaired representation of the order of events in distinct experiences
Tulving (1983) characterized episodic memory as having a temporal organization, involving the ability to remember the order of events in distinct experiences, and this ability depends on the hippocampus (Fortin et al., 2002; Gelbard-Sagiv et al., 2008; Lehn et al., 2009). Episodic memory has also been characterized as the ability to remember what happened in a particular place and time (Clayton and Dickinson, 1998). In the present study, we observed that SR −/− mice have intact memory for object identity, odors (in the odor sequence test), and object location. In contrast, SR −/− mice exhibited an abnormal pattern of preference for the order of events in distinct experiences in two distinct tests, where unlike wild type, SR −/− mice demonstrated preference for the objects and odors that were presented more recently during the task. Additionally, in the odor sequence task, despite exhibiting a preference for items occurring later within each sequence, the SR −/− mice displayed a marginal preference for items occurring in Sequence 1 compared to Sequence 2, again, the opposite pattern to that exhibited by WT mice. This finding of abnormal preference in order tests is unlike the loss of order memory following hippocampal damage (Fortin et al., 2002). Instead, these findings suggest that the SR −/− mice have some representation for the order of events, but it reflects an interference with the normal expression of temporal organization of events in distinct experiences. One possible explanation for the reversal of order preference is that, in the absence of a functional prefrontal cortex, other brain systems may bias the animals to abnormally prefer the stimulus with the greater relative level of familiarity in object order test, that is, to prefer the object they had less time to forget. In the odor sequence test, one possibility is that, having been repeatedly rewarded for digging in odors in a particular order may dispose the mice to spend considerable time searching for food in the odor for which digging had always previously been rewarded first; that is, they may have had a high expectancy of reward in the earlier odor, as was the case repeatedly during the exposure phase. Conversely, they might switch quickly away from digging in a non-rewarded probe odor that had only previously been rewarded after reward on the alternate probe in the exposure phase; that is, they may have had a low expectancy of reward in an odor that had always been preceded by the alternate odor and reward.
The pattern of abnormality in order memory observed here is similar to that observed in animals with damage to the prefrontal cortex (Mitchell and Laiacona, 1998; Hannesson et al., 2004). We have previously reported that while hippocampal damage eliminates memory for order, damage to the prefrontal cortex results in the pattern of preference for more recently experienced odors when the lag between items is short, similar to our current findings in the SR −/− mice (DeVito and Eichenbaum, under review). Thus, these cognitive impairments are most likely due to a disruption within the hippocampal-prefrontal circuit. Additionally, this disruption in information processing is similar to the clinical presentation of cognitive disorganization in schizophrenia.
Intact relational memory and social recognition
The hippocampus and surrounding cortical structures are importantly involved in relational memory, the ability to bind together overlapping items in order to recreate the full episode (Dusek and Eichenbaum, 1997; Heckers et al., 2004). The SR −/− mice learned the overlapping odor pairs as readily as their wild type littermates and performed at comparable levels on both the transitive and non-transitive probe tests, demonstrating intact relational memory. These results contrast with previous findings indicating that animals with hippocampal damage (Van der Jeugd et al., 2009; DeVito et al., 2010) and schizophrenic patients (Titone et al., 2004) have profound deficits in transitive inference. Although the ability to associate overlapping events is an important aspect of episodic memory in that it allows for the flexible expression of information in novel situations, it seems to be an aspect of memory with which these animals display little difficulty.
SR −/− mice demonstrated normal social preferences in both the sociability and social novelty tests, which is in contrast to previous reports indicating that NMDAR hypofunction disrupts social behaviors (Labrie et al., 2009; Mohn et al., 1999). Although all of these models, including ours, aim to induce NMDAR hypofunction, the various strategies ultimately affect NMDAR signaling differently and could therefore produce distinct behavioral phenotypes.
Dendritic abnormalities in the mPFC of SR −/− mice
SR −/− mice had less complex dendrites with reduced spine density in the mPFC. This findings bears similarity with the observation that, in schizophrenic subjects, neurons in deep layer 3 of the dorsolateral PFC (DLPFC) have specific reductions in both basilar dendritic material and spine density, whereas no perturbations were found in the primary visual cortex (Glantz and Lewis, 2000). Whether this same pattern holds true in our genetic model has yet to be determined; however, importantly, similar dendritic abnormalities were observed in the somatosensory cortex of the SR −/− mice (Balu et al., 2009). It is possible that somatosensory cortex could have contributed to performance on the object tests, in that whisking may have provided cues to identify the objects. However, if this was the case, one would expect similar deficits in SR−/− mice on all the object-cued tasks, and not a selective effect on object order memory, as was observed. Combining this finding with the similar results on the odor sequence task, wherein somatosensory cortex is unlikely to have contributed significantly, we conclude it is most likely that the cognitive deficits are due to the abnormalities in the prefrontal cortex.
Interestingly, the SR−/− mice had pyramidal neurons in the mPFC with abnormal apical, but not basal, dendrites. Others have reported divergence in the effects of genetic manipulation on the patterning of apical and basilar dendrites in the infralimbic region of the mPFC in serotonin transporter KO mice (Wellman et al., 2007). The soma and basal dendritic tree of layer II/III neurons are innervated by thalamic projections (Shibata, 1993), while the apical dendrites are preferentially contacted by afferents from limbic structures, such as the hippocampus (Swanson and Cowan, 1977). Therefore, as SR is highly expressed in limbic regions, including hippocampus, but virtually absent in the thalamus (Basu et al., 2009), it is plausible that the apical dendrites of mPFC pyramidal neurons would be the most severely affected since they receive projections from areas where d-serine plays a more prominent role in NMDAR-dependent transmission.
The reduced spine density of pyramidal neurons in the cortex of SR−/− mice would be expected to lead to abnormalities in glutamatergic transmission (Fiala et al., 2002). The complexity of dendritic branching is thought to represent the extent of neuronal connectivity. Dendritic arborization is enhanced by NMDAR-dependent transmission and downstream Ca2+ sensitive signaling pathways (Wayman et al., 2006; Wayman et al., 2008). We previously demonstrated NMDAR hypofunction in SR-KO mice by showing that the mutants had reduced occupancy of the GMS, reduced global NMDAR-mediated transmission by a biotin-switch assay, and reduced hippocampal LTP (Basu et al., 2009). Although we have not measured NMDAR expression in our mice, NMDAR protein levels were found to be unaltered in whole tissue homogenates in other mouse models of SR deficiency (Labrie et al., 2009; Inoue et al., 2008). As excitatory activity influences the density of dendritic spines (Zito et al., 2009), reduced NMDAR-dependent signaling in the SR−/− mice could be responsible for the reductions in spine density. Future studies should shed light on molecular and physiological abnormalities specifically in the mPFC of SR−/− mice.
Conclusion
SR−/− mice demonstrated an altered representation of the order of events in distinct experiences, a feature of episodic memory associated with PFC function. In addition, pyramidal neurons in the mPFC of SR−/− mice displayed dendritic perturbations and reduced spine density, suggesting that there is significantly altered glutamatergic signaling in this region. The results presented here demonstrate that d-serine modulation of NMDAR function is important for specific aspects of cognition, as well as the dendritic characteristics of cortical pyramidal neurons, and suggest that NMDAR hypofunction might, in part, be contributing to the cognitive and synaptic changes observed in schizophrenia (Coyle, 1996; Coyle, 2006; Javitt, 2006).
Acknowledgements
We would like to thank Dr. Sabina Berretta and Dr. Francine M. Benes for the generous use of their equipment and software. We also thank Linda Hassinger, Matthew Demers, Daniel C. Liu, and Harry Pantazopoulos for technical assistance, as well as Will Sauls and Jiamin Feng for animal colony maintenance and genotyping. This work was supported by grants from the Conte Center for Schizophrenia Research NIH MH60450, R01MH05190, and P50MH0G0450, as well as an unrestricted grant from Bristol-Myers Squibb to JTC.
References
- Akbarian S, Sucher N, Bradley D, Tafazzoli A, Trinh D, Hetrick W, Potkin S, Sandman C, Bunney W, Jones E. Selective alterations in gene expression for NMDA subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16(1):19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Basu AC, Coyle JT. Altered cortical dendritic morphology in serine racemase knockout mice, a genetic model of NMDA receptor hypofunction. Program No. 443.16 Chicago, Il. Society for Neuroscience. 2009 [Google Scholar]
- Barker G, Bird F, Alexander V, Warburton E. Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27(11):2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Tsai G, Ma C, Ehmsen J, Mustafa A, Han L, Jiang Z, Benneyworth M, Froimowitz M, Lange N, Snyder S, Bergeron R, Coyle J. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mole Psychiatry. 2009:1–9. doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendikov I, Nadri C, Amar S, Panizzutti R, De Miranda J, Wolosker H, Agam G. A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophrenia Res. 2007;90:41–51. doi: 10.1016/j.schres.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Conservation of the hippocampal memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- Clayton N, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3(5):241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: Beyond the dopamine hypothesis. Cell and Mole Neurobio. 2006;26(4–6):365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito LM, Kanter BR, Eichenbaum H. The contribution of the hippocampus to memory expression in transitive inference in mice. Hippocampus. 2010;20(1):208–217. doi: 10.1002/hipo.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito LM, Eichenbaum H. Memory for the order of events in specific sequences: Contributions of the hippocampus and medial prefrontal cortex. 2010 doi: 10.1523/JNEUROSCI.4202-10.2011. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito LM, Konigsberg R, Lykken C, Sauvage M, Young WS, III, Eichenbaum H. Vasopressin 1b receptor knockout impairs memory for temporal order. J Neurosci. 2009;29(9):2676–2683. doi: 10.1523/JNEUROSCI.5488-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek J, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. PNAS USA. 1997;94(13):7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Fortin N, Agster K, Eichenbaum H. Critical role of the hippocampus in memory for sequence of events. Nature Neurosci. 2002;5(5):458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Goff D, Coyle J. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psych. 2001;158(9):1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Hannesson D, Vacca G, Howland J, Phillips A. Medial prefrontal cortex is involved in spatial temporal order memory but not spatial recognition memory in tests relying on spontaneous exploration in rats. Behav Brain Res. 2004;153:273–285. doi: 10.1016/j.bbr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Hasegawa H, Imai K, Iyo M. Decreased serum levels of D-serine in patients with schizophrenia: Evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psych. 2003;60:572–576. doi: 10.1001/archpsyc.60.6.572. [DOI] [PubMed] [Google Scholar]
- Heckers S, Zalesak M, Weiss AP, Ditman T, Titone D. Hippocampal activation during transitive inference in humans. Hippocampus. 2004;14:153–162. doi: 10.1002/hipo.10189. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Is the glycine site half saturated or half unsaturated? Effects of glutamatergic drugs in schizophrenia patients. Curr Opin Psychiatry. 2006;19:151–157. doi: 10.1097/01.yco.0000214340.14131.bd. [DOI] [PubMed] [Google Scholar]
- Kalus P, Muller TJ, Zuschratter W, Senitz D. The dendritic architecture of prefrontal pyramidal neurons in schizophrenic patients. Neuroreport. 2000;11:3621–3625. doi: 10.1097/00001756-200011090-00044. [DOI] [PubMed] [Google Scholar]
- Konradi C, Heckers S. Molecular aspects of glutamatergic dysregulation: implications for schizophrenia and its treatment. Pharm and Therapeutics. 2003;97:153–179. doi: 10.1016/s0163-7258(02)00328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie V, Fukumura R, Rastogi A, Fick L, Wang W, Boutros P, Kennedy J, Semeralul M, Lee F, Baker G, Belsham D, Barger S, Gondo Y, Wong A, Roder J. Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Human Mole Genetics. 2009;18(17):3227–3243. doi: 10.1093/hmg/ddp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn H, Steffenach H, Strien N, Veltman D, Witter M, Haberg A. A specific role of the human hippocampus in recall of temporal sequences. J Neurosci. 2009;29(11):3475–3484. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Mitchell J, Laiacona J. The medial frontal cortex and temporal memory: Tests using spontaneous exploratory behaviour in the rat. Behav Brain Res. 1998;97:107–113. doi: 10.1016/s0166-4328(98)00032-1. [DOI] [PubMed] [Google Scholar]
- Mohn A, Gainetdinov R, Caron M, Koller B. Mice with reduced NMDA receptors display behavior related to schizophrenia. Cell. 1999:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Morita Y, Ujike H, Tanaka Y, Otani K, Kishimoto M, Morio A, Kotaka T, Okahisa Y, Matsushita M, Morikawa A, Hamase K, Zaitsu K, Kuroda S. A genetic variant of the serine racemase gene is associated with schizophrenia. Biol Psychiatry. 2007;61:1200–1203. doi: 10.1016/j.biopsych.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Moy S, Nadler J, Perez A, Barbaro R, Johns J, Magnuson T, Piven J, Crawley J. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3(5):287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Sun L, Quirk M, Rondi-Reig L, Wilson M, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of a one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Potier B, Turpin F, Sinet P, Rouaud E, Mothet J, Videau C, Epelbaum J, Dutar P, Billard M. Contribution of the D-Serine-dependent pathway to the cellular mechanisms underlying cognitive aging. Frontiers in Neurosci. 2010;2(1):1–11. doi: 10.3389/neuro.24.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajji T, Chapmann D, Eichenbaum H, Greene R. The role of CA3 hippocampal NMDA receptors in paired associate learning. J Neurosci. 2006;26(3):908–915. doi: 10.1523/JNEUROSCI.4194-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H. Efferent projections from the anterior thalamic nuclei to the cingulate cortex in the rat. J Comp Neurol. 1993;330:533–542. doi: 10.1002/cne.903300409. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Titone D, Ditman T, Holzman P, Eichenbaum H, Levy D. Transitive inference in schizophrenia: Impairments in relational memory organization. Schizophrenia Res. 2004;68(2–3):235–247. doi: 10.1016/S0920-9964(03)00152-X. [DOI] [PubMed] [Google Scholar]
- Tsien J, Huerta P, Tonegawa S. The essential role of hippocampal CA1 NMDAR-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Tsien J. Linking Hebb’s coincidence-detection to memory formation. Current Op in Neurobio. 2000;10:266–273. doi: 10.1016/s0959-4388(00)00070-2. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of episodic memory. London: Oxford UP; 1983. [Google Scholar]
- Turpin R, Potier B, Dulong J, Sinet P, Alliot J, Oliet S, Dutar P, Epelbaum J, Mothet J, Billard J. Reduced serine racemase expression contributes to age-related deficits in hippocampal cognitive function. Neurobio of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.09.001. in press. [DOI] [PubMed] [Google Scholar]
- Van der Jeugd A, Goddyn H, Laeremans A, Arckens L, D'Hooge R, Verguts T. hippocampal involvement in the acquisition of relational associations, but not in the expression of a transitive inference task in mice. Behavioral Neuroscience. 2009;123(1):109–114. doi: 10.1037/a0013990. [DOI] [PubMed] [Google Scholar]
- Verrall L, Walker M, Rawlings N, Benzel I, Kew J, Harrison P, Burnet P. D-amino acid oxidase and serine racemase in human brain: Normal distribution and altered expression in schizophrenia. Euro J Neurosci. 2007;26:1657–1669. doi: 10.1111/j.1460-9568.2007.05769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, Cheng HY, Marks D, Obrietan K, Soderling TR, Goodman RH, Impey S. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci U S A. 2008;105:9093–9098. doi: 10.1073/pnas.0803072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27(3):684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Qiao H, Wen L, Zhou W, Zhang Y. D-Serine enhances impaired long-term potentiation in CA1 subfield of hippocampal slices from aged senescence-accelerated mouse prone/8. Neurosci Lett. 2005;379:7–12. doi: 10.1016/j.neulet.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Zito K, Scheuss V, Knott G, Hill T, Svoboda K. Rapid functional maturation of nascent dendritic spines. Neuron. 2009;61:247–258. doi: 10.1016/j.neuron.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]