Abstract
Previously we demonstrated profound effects of dopamine transporter (DAT) SLC6A3 genotype on limbic responses to smoking cues (SCs). Probands carrying at least one copy of the 9-repeat allele (9-repeat carriers) had greater neural responses to SCs in the anatomically interconnected rostral ventral striatum/medial orbitofrontal cortex (VS/mOFC), compared with homozygotes for the 10-repeat allele (10/10-repeats). To test the reliability of the initial findings, we examined perfusion functional magnetic resonance images acquired during SC exposure in a new cohort of smokers (N = 26) who were genotyped for the SLC6A3 polymorphism. In smokers overall, activity was enhanced in the VS/mOFC (t = 3.77). Contrasts between allelic groups revealed that 9-repeat carriers had a greater response to SCs in the VS (t = 3.12) and mOFC (t = 3.19). In separate groups, 9-repeat carriers showed increased activity in the VS (t = 5.47) and mOFC (T = 4.96), while no increases were observed in 10-repeats. Subjective reports of craving correlated with increased activity in reward-related structures including the extended amygdala, insula and post-central gyrus, and decreased activity in the dorsolateral prefrontal cortex, and were DAT-genotype dependent (r = 0.63–0.96). In secondary analyses, we found that The Fagerström Test for Nicotine Dependence scores correlated with enhanced SC-induced perfusion in 10/10-repeats in the insula, mOFC, medial temporal and superior frontal gyri (r = 0.50–0.82), while correlations were absent in 9-repeat carriers. Despite heterogeneity introduced by a host of factors, including variance in other genes involved in smoking behavior, we confirm that DAT genotype predicts the direction and location of neural responses to SCs.
Keywords: Addiction, dopamine transporter, genetic variability, neuroimaging, relapse vulnerability, smoking cues
INTRODUCTION
Mortality rates related to cigarette smoking are higher than any preventable cause of death in our nation, and diseases such as glaucoma, diabetes, chronic obstructive pulmonary disease and emphysema are directly linked to smoking. During 2000–2004, cigarette smoking was estimated to be responsible for $193 billion in annual health-related economic losses in the United States ($96 billion in direct medical costs and approximately $97 billion in lost productivity) (Prevention CfDCa 2008). The dire health and societal costs associated with smoking stress the importance of identifying and understanding the underlying neurobiology to implement strategies to improve smoking cessation treatment outcomes.
There are a host of factors involved in the motivation to smoke and that promote relapse, including stress, peer pressure, availability, menstrual cycle phase and even weight management (Perkins 2001; Perkins et al. 2001; Sinha & Li 2007; Franklin et al. 2008; Dagher et al. 2009). However, smoking cue- (SC) and withdrawal-induced ‘cravings’ are posited to be the two major motivators to continued smoking and relapse (Baker, Morse & Sherman 1986; Caggiula et al. 2001; Le Foll & Goldberg 2005; Payne et al. 2006; Rose 2006). Inability to combat withdrawal-induced craving, which declines within a month (Hughes 2007), plays a major role in relapse in the first few weeks after quitting. However, smokers report that SCs, such as the smell of a burning match, seeing another person smoking and even internal mood states repeatedly associated with smoking, can trigger relapse months or even years after quitting. Some smokers, who are thought to possess high ‘cue reactivity’ are especially vulnerable and have an increased probability of relapse initiated by exposure to SCs (Drummond 2000). However, studies are just beginning to explore medications to treat cue-vulnerable smokers. Thus, a greater understanding of the neurobiological underpinnings of SC reactivity is crucial in the identification and development of effective therapeutics.
In previous work, we observed increased cerebral blood flow (perfusion) during SC exposure in reward-related mesocorticolimbic structures, including the ventral striatum (VS), orbitofrontal cortex (OFC), insula, thalamus, amygdala, anterior cingulate and parahippocampal gyrus, extending findings of other SC reactivity studies (Brody et al. 2002; Due et al. 2002; McBride et al. 2006), and supporting hypotheses implicating the mesocorticolimbic system in stimulus-evoked craving and relapse (Di Chiara 2000; Franklin & Druhan 2000). Despite robust results, we noted substantial inter-individual variability in brain and associated SC-induced craving responses. As there is a substantial genetic component to smoking behaviors and mesolimbic dopamine (DA) is critical for reward and its predictors, we hypothesized that genetically driven variation in the mesolimbic dopaminergic system may account for the variability. Specifically, we hypothesized that heterogeneity resulting in increased synaptic DA during SC exposure might reveal a SC-vulnerable subtype.
The dopamine transporter (DAT) SLC6A3 gene is a positional candidate gene in several linkage and association studies examining cigarette addiction (Ho & Tyndale 2007). It rapidly removes DA from the synapse after its phasic release in response to rewarding substances and cues that predict them. The DAT has two common alleles with either a 9 or 10 variable number tandem repeat (VNTR) of a 40 base pair sequence in its 3′ untranslated region (Vandenbergh et al. 1992). Evidence suggests that the 9-repeat allele is associated with lower expression of the DAT (Vandenbergh et al. 1992; Heinz et al. 2000; although see van Dyck et al. 2005), which may lead to slower DA clearance (Fuke et al. 2001; Mill et al. 2002). In support, Brody and colleagues showed that 11C raclo-pride competition in striatal regions was greater in 9-repeat carriers immediately after smoking, which might indicate either compromised DAT function or reduced availability in the 9-repeat probands (Brody et al. 2006). We initially hypothesized that genetic variation in the DAT would lead to greater or prolonged ventral striatal synaptic DA, enhancing the salience of drug-associated cues, and that this would be reflected in greater neural responses during SC exposure in the VS and related mesocorticolimbic circuitry. To examine this possibility, we evaluated the impact of genetic variation in the DAT gene on brain and behavioral responses to SCs in sated smokers. Smokers were grouped according to whether they carried a 9-repeat allele (9-repeat carriers) or were homozygous for the 10-repeat allele (10/10-repeats). We found that 9-repeat carriers had greater brain responses to SCs (versus Non-SCs) than 10/10-repeats, bilaterally in the VS, mOFC, parahippocampal gyrus, dorsolateral prefrontal cortex (DLPFC) and other brain regions (Franklin et al. 2009). These results provided evidence to support our hypothesis that 9-repeat carriers might represent a smoking cue-vulnerable endophenotype.
As the sample size was relatively small in our initial study, we examined the influence of DAT genotype on SC reactivity in a new cohort of smokers. Thus, as before, we linked a candidate gene approach with functional neuroimaging using perfusion fMR images acquired during exposure to highly appetitive SC video clips in smokers. Brain data was compared across DAT genotype groups with respect to responses to SCs (compared with non-SCs).
In secondary hypotheses, we predicted that severity of nicotine dependence would be related to the degree of insula activation during exposure to SCs. The insula has received considerable attention in the drug addiction field. Smokers with lesions to the insula spontaneously quit smoking and reported an absence of craving for cigarettes (Naqvi et al. 2007). Neuroimaging studies examining smokers and cocaine dependent individuals support a role for the insula as a key neurobiological substrate underlying cue-induced craving (Wang et al. 1999; Brody et al. 2002). In Franklin et al. 2009 we found that recruitment of the insula was DAT genotype dependent: 10/10-repeats had significantly greater activity in both left and right insula compared to 9-repeat carriers and showed strong correlations between reported craving and brain activity (Franklin et al. 2007; Franklin et al. 2009). Given that cigarette smoking is prevalent in both allelic groups, that both genotypes experience difficulty quitting, and that brain responses to SCs were reduced in 10/10-repeats, we hypothesized that 10/10-repeat smoking behavior may be influenced more by pharmacological withdrawal from nicotine (nicotine dependent) rather than by exposure to SCs (cigarette dependent). The Fagerström Test for Nicotine Dependence (FTND) is a measure of dependence that focuses primarily on the assessment of dependence related to nicotine withdrawal, with less emphasis on the motivation to smoke provoked by SC exposure (Fagerstrom & Schneider 1989; Kozlowski et al. 1994; Pomerleau et al. 1994; Weinberger et al. 2007). Thus, we used the FTND to test the hypothesis that greater insula activation during SC exposure would be associated with greater dependence on nicotine in 10/10-repeats, and that the FTND would not be predictive of nicotine dependence in 9-repeat carrier cue-vulnerable, and thus cigarette dependent smokers.
The perfusion fMRI technique is particularly well-suited to our paradigm, wherein cue sets are minutes-long videos: first, it has stable noise characteristics over the entire frequency spectrum conducive to capturing signal related to sustained brain states such as craving, which once triggered can persist for several minutes; second, it has the potential to provide enhanced visualization of limbic regions located in regions of high susceptibility and third, similar to positron emission tomography, it provides a quantitative measure of cerebral blood flow (CBF; ml of blood/100 g of tissue/minute) permitting separate acquisitions of responses to smoking cues and nonsmoking cues (Aguirre et al. 2005; Detre & Wang 2002). As neural activation generated by drug cues may persist via carry-over effects (Waters et al. 2005; Wilson et al. 2007; Sharma & Money 2009), this technique has the potential for providing a stronger, sharper signal. In contrast, SC and non-SCs are presented in a repeated counterbalanced mode over the course of a scan using the blood oxygenation level dependent (BOLD) technique. Thus, the differential responses to cue sets may be obscured as neural responses to the SCs escalate, the result of which may be weaker signal acquisition. The appropriateness of perfusion fMRI for our paradigm is evinced by our recent SC reactivity studies wherein all of the regions consistently and repeatedly implicated in the preclinical literature that are observable with the resolution of fMRI, such as the amygdala, VS, mOFC, insula, thalamus and hippocampus, were activated during SC exposure (Franklin et al. 2007; Franklin et al. 2009). Our findings differ from BOLD fMRI studies in which activation in regions known to be involved in conditioned drug reward did not corroborate with the substantial animal literature to the same degree as our perfusion fMRI studies (Brody et al. 2002; Due et al. 2002; David et al. 2005; McBride et al. 2006; Dagher et al. 2009),(Okuyemi et al. 2006; McClernon, Kozink, Rose 2008). Alternatively, disparities in neural responses to SCs between our paradigm and the work of others is more likely related to additional differences between the study designs, as well as heterogeneity in the populations that were examined (described more fully in the discussion).
METHODS
Subjects
Twenty-six physically healthy and mentally stable smokers between the ages of 18 and 60 (37.6 ± 2.3) participated in the study. Subjects smoked between 10 and 30 cigarettes per day (17.7 ± 1.6). The sample was 29% female, 29% African American, 67% Caucasian and 4% multiple ethnicity. The Edinbergh Handedness Inventory identified 82% right-handed, 11% left-handed and 7% ambidextrous. Additional demographics and smoking history characteristics are listed in Table 1.
Table 1.
General characteristics.
| All n = 26 | 10/10-repeats n = 17 | 9-repeat carriers n = 9 | P | |
|---|---|---|---|---|
| Sex | 19 M (73%) | 12 M (71%) | 7 M (78%) | 0.69 |
| Race | 8 AA 17 EA 1 Multi | 5 AA 11 EA 1 Multi | 3 AA 6 EA 0 Multi | 0.76 |
| Means ± (SEMs) | ||||
| Age | 37.8 ± 2.1 | 36.7 ± 2.7 | 39.8 ± 3.5 | 0.50 |
| Education | 14.5 ± 0.4 | 14.4 ± 0.5 | 14.8 ± 0.8 | 0.62 |
| CPD | 17.5 ± 1.5 | 17.8 ± 1.8 | 16.8 ± 2.8 | 0.74 |
| Pack yearsa | 15.7 ± 2.5 | 13.9 ± 2.4 | 18.9 ± 5.8 | 0.35 |
| FTND scores | 4.7 ± 0.3 | 4.9 ± 0.5 | 4.5 ± 0.5 | 0.63 |
| Desire to quitb | 84.5% | 85.8% | 82.1% | 0.65 |
Pack years calculation: CPD (÷) cigarettes in a pack (X) years smoking. FTND scores ranged from 1 to 9.
Desire to Quit was assessed by asking subjects to rate their agreement with the phrase ‘I want to quit smoking.’ Scores ranged from 0 to 100, where 0 indicates strongly disagree and 100 indicates strongly agree. 10/10-repeats = homozygotes for the 10-repeat variable number tandem repeat (VNTR) allele of the DAT; 9-repeat carriers = carriers of 1 or 2 copies of the 9-VNTR of the DAT; AA = African American; EA = European American; Multi = reported more than one race.
Subjects were recruited through radio advertisements and local listservs that specifically stated the study was intended for smokers who may be contemplating quitting but were not ready to quit in the near future. Subjects were screened, tested on study knowledge and consented prior to psychological and physical evaluations. The Mini International Neuropsychiatric Interview (Sheehan, Lecrubier & Sheehan 1998) was used to determine current DSM-IV diagnosis of substance dependence other than nicotine, and current severe psychiatric symptoms. Individuals with other current substance dependence, current Axis I DSM-IV psychiatric diagnoses, significant medical conditions, an intellectual ability estimate score of ≤80 on the Weschler Abbreviated Scale of Intelligence (Weschler 1999), an abnormal structural MRI, a history of head trauma or injury causing loss of consciousness lasting greater than 3 minutes or associated with skull fracture or inter-cranial bleeding, or who had irremovable magnetically active objects on or within their body were excluded. Smokers were compensated $100.00 for an initial consenting appointment and completion of the MRI scanning session. The study, approved by the University of Pennsylvania Institutional Review Board, adhered to the Declaration of Helsinki.
Severity of nicotine dependence was ascertained from the Fagerström Test for Nicotine Dependence (Fagerstrom & Schneider 1989) (FTND), a six-item, self-report measure of nicotine dependence derived from the Fagerström Tolerance Questionnaire. This reliable and validated measure of nicotine dependence has been widely used in the field for over 30 years (Fagerstrom & Schneider 1989; Kozlowski et al. 1994; Pomerleau et al. 1994; Weinberger et al. 2007) and is predictive of treatment outcome (Kozlowski et al. 1994; Dijkstra & Tromp 2002).
Imaging approach
Continuous arterial spin labeled (CASL) perfusion fMRI, a quantitative estimate of CBF and as such, an indirect measurement of neural activity (Floyd et al. 2003), was used to examine brain activation in response to SC exposure. Prior to scanning, subjects smoked ad lib to minimize any nicotine withdrawal-induced craving that might accrue over the scanning session. Subjects were scanned approximately 20–25 minutes after smoking, to ensure dissipation of the acute cardiovascular effects of smoking. Images were acquired during a 35-minute scanning session that included, in sequence, a 5-minute resting baseline scan (unprovoked by task, subjects were instructed to lie still in the scanner with their eyes open), a 10-minute non-SC CASL scan, a high resolution structural scan and a 10-minute SC CASL scan. We administered the Shiffman–Jarvik Scale, a measure designed to assess craving related to withdrawal from nicotine (Shiffman & Jarvik 1976), immediately before and after scanning sessions to determine whether withdrawal accrued over time in the scanner. Given that expectancy enhances SC reactivity (Droungas et al. 1995; McBride et al. 2006), subjects were informed of the opportunity to smoke a cigarette after the scanning session.
Stimuli
Stimuli were 10-minute audio-video clips. The SC video was comprised of individuals differing in race, age and sex who are smoking and using explicit language designed to induce appetitive desire for a cigarette (e.g. ‘I love a cigarette right after a hard day at work’). The non-SC video was similar in content, except individuals’ related short stories that did not portray cigarette smoking or smoking reminders. Two different but similarly-valenced cue sets were employed (counterbalanced across scanning sessions) to control for habituation.
Imaging parameters and data processing
Imaging data were acquired on a 3.0 Tesla Trio whole-body scanner (Siemens AG, Erlangen, Germany), using a Bruker volume coil (volume coils are designed to provide a homogenous receiving sensitivity and are 1 channel; Bruker Biospin, Billerica, MA). A T1-weighted 3D high resolution MPRAGE scan was acquired (FOV = 250 mm, TR/TE = 1620/3 ms, 192 × 256 matrix, slice thickness 1 mm) for anatomical co-registration and spatial normalization. CASL perfusion fMRI sequence was used for resting baseline, SC and non-SC data acquisition. Interleaved images with and without labeling were obtained using a gradient echo echo-planar imaging sequence with a delay of 1000 ms inserted between the end of the labeling pulse and image acquisition (FOV =11 a 2 mm inter-slice gap.
Imaging data processing and statistical analyses
Processing
A statistical parametric mapping (SPM)-based optimized ASL data processing toolbox was used for data analyses (Wellcome Department of Cognitive Neurology, London, UK) (Wang et al. 2008). CASL image pairs were realigned to the mean of all control images and spatially smoothed with a 3D isotropic Gaussian kernel (FWHM10 mm). CBF image series were generated using a simplified two-compartment model with the sinc interpolated method to acquire CBF values (Aguirre, Detre & Wang 2005). The mean control image of each subject’s data was co-registered to the structural image using the mutual information based co-registration algorithm provided by SPM5. The same co-registration parameters were also used to co-register the CBF maps to the structural image. The structural image was then spatially normalized to the Montreal Neurological Institute (MNI) standard space template. The same parameters were used to normalize the CBF images to the MNI standard space. Normalized, segmented mean images were used for calculating global CBF.
fMRI statistical analyses
Whole brain voxel-wise analyses of the CBF data were conducted on each subject using a general linear model (GLM). Contrasts between cue sets were defined in the GLM model to assess the voxel by voxel CBF difference. Using the corresponding parametric maps of the contrasts, random effects analysis was employed to test for a significant main effect of condition (SCs versus non-SCs) with a statistical parametric map of the t statistic at each voxel for population inference (second-level analysis). Areas of significance were identified at the cluster level for the P value less than 0.01 and the cluster extent size of ≥ 20 contiguous 2 mm3 voxels. Reported activations are those with P values ≤ 0.0001 at the voxel level, uncorrected.
Simple regression analyses were conducted on CBF with the change in craving scores [post-SC—pre-SC craving scores)], FTND scores, cigarettes smoked per day, years smoking and age as covariates of interest to test for brain/behavioral correlations.
Demographic and behavioral statistical analyses
Continuous demographic variables were summarized, by calculating means and standard error measurements (X ± SEMs). Nominal demographic variables were summarized by calculating proportions and compared across groups using χ2 analyses. Analysis of variance (ANOVA) was used to assess main effect (DAT genotype) by time (pre- versus post-SCs) differences on the Craving and Withdrawal Questionnaire and DAT genotype across time (pre- versus post-scanning session) differences and on the Shiffman–Jarvik Withdrawal Scale. Statistical analyses were conducted in Microsoft Excel 2008 and SPSS version 16.0.
Genotyping
Blood samples were acquired during the initial physical examination. Genomic DNA was extracted from antico-agulated venous blood samples using a standard salting out method (Lahiri & Nurnberger 1991). Genotyping of the SLC6A3 40 bp repeat polymorphism (rs28363170) was performed as previously described (Vandenbergh et al. 1992). Briefly, PCR was performed using a mix of 100 ng genomic DNA, 1x Amplitaq buffer containing MgCl2, 200 nM dNTP mix, 150 nmol for each primer (Fwd: 5′-TGT GGT GTA GGG AAC GGC CTG AG-3′ and Rev: 5′-CTT CCT GGA GGT CAC GGC TCAAGG-3′) and 2.5 units AmpliTaq per reaction. The PCR conditions included an initial melting step (94ºC; 4 minutes) followed by 40 cycles of melting (94ºC; 1 minute), annealing (65ºC; 1 minute) and extending (72ºC; 1 minute). A final extension step was used (72ºC; 5 minutes). Reaction products were separated by agarose gel electrophoresis, and product sizes were determined by comparison to molecular weight standards and known sequenced samples. In addition, four samples were confirmed by sequencing. All samples were run in duplicate with 100% concordance rate and were read independently by two blinded investigators.
RESULTS
Demographics
There were no significant differences between genotypes in ethnicity, sex, cigarettes smoked per day, pack years (a measure to quantify intensity of chronic cigarette exposure since smoking initiation), FTND scores or other general demographic items (Table 1)
Group assignment
Twenty-six smokers were genotyped for variance in the SLC6A3 gene. Seventeen subjects were homozygous for the 10-repeat allele. Seven were heterozygous and two were homozygous for the 9-repeat allele. Genotype frequencies did not deviate from Hardy–Weinberg Equilibrium (P = 0.325). Smokers who carried at least one 9-repeat allele were classified and grouped together for analyses, as homozygotes for the 9-repeat allele are rare, and 10/10-repeat probands were classified as 10/10-repeats. Allele frequencies were 0.212 for 9-repeat carriers and 0.788 for 10/10-repeats, which is similar to what has been observed in other studies (Vandenbergh et al. 2002).
Shiffman–Jarvik withdrawal scale
Prior to and immediately after scanning sessions, subjects were administered the Shiffman–Jarvik Withdrawal Scale. To assess whether withdrawal had accrued over time in the scanner, we calculated the difference between pre- and post-scanning composite scores from the five factors (psychological discomfort, physical symptoms, withdrawal-induced craving, stimulation and appetite). Paired t-tests demonstrated that withdrawal did not accrue over the course of the scanning session in all subjects or within each allelic group. Mean change scores ± SEMS were 4.71 ± 2.67 in all subjects (P = 0.09), 0.44 ± 2.40 (P = 0.86) in 9-repeat carriers, and 7.27 ± 3.93 in 10/10-repeats (P = 0.09).
Subjective craving and withdrawal scale
Subjects rated their craving and withdrawal before and after stimulus presentations using the Craving and Withdrawal Questionnaire (CWQ, Table 2), which was used in our previous SC neuroimaging studies. During brief (60 seconds) intermissions between scans, smokers were asked to rate their feelings when asked each item of the questionnaire inserted into the phrase ‘On a scale from 1 to 7 … with 1 corresponding to Not at all and 7 corresponding to Extremely’. CWQ items were analyzed using a GLM repeated-measures ANOVA with group by time effects (DAT genotype × pre-SC and post-SC scores). In smokers as a group and in separate allelic groups, there were no group by time differences in items that were designed to probe scanner-induced anxiety (items 1 and 2). However, there were significant group by time increases in the items designed to assess desire for a cigarette (item 3–6) in all groups. Note that although both allelic groups desired to smoke a cigarette (item 3), 9-repeat carriers desired to smoke ‘just to smoke’ (item 4) and for pleasure (item 6), while 10/10-repeats desired to smoke because they were edgy (item 5) and for relief (item 7). Non-SCs did not elicit changes in any of the CWQ items in all subjects or in separate allelic groups. There were no significant group by time interactions between 10/10-repeats and 9-repeat carriers in responses to any of the items for either SCs or non-SCs (not shown).
Table 2.
Craving and withdrawal questionnaire items.
| Craving and withdrawal questionnaire | All subjects | P | 9-repeats | P | 10/10-repeats | P |
|---|---|---|---|---|---|---|
| (1) How calm are you right now? | −0.08 ± 0.30 | 0.92 | 0.11 ± 0.31 | 0.73 | −0.18 ± 0.44 | 0.69 |
| (2) How content do you feel right now? | −0.27 ± 0.17 | 0.26 | 0.00 ± 0.29 | 1.00 | −0.41 ± 0.21 | 0.07 |
| (3) How much do you desire a cigarette right now? | 1.04 ± 0.30 | 0.003* | 1.11 ± 0.42 | 0.03* | 1.00 ± 0.41 | 0.03* |
| (4) Right now, if you could, would you smoke a cigarette? | 1.23 ± 0.42 | 0.003* | 2.00 ± 0.80 | 0.04* | 0.82 ± 0.46 | 0.09 |
| (5) Right now, do you feel edgy, as if you have not had a cigarette in a while? | 0.81 ± 0.28 | 0.02* | 0.67 ± 0.41 | 0.14 | 0.88 ± 0.38 | 0.03* |
| (6) How much of an urge or desire do you have to smoke right now for pleasure? | 1.23 ± 0.30 | 0.001* | 1.33 ± 0.53 | 0.04* | 1.18 ± 0.37 | 0.01* |
| (7) How much do you need to smoke right now for relief? | 1.12 ± 0.31 | 0.004* | 0.89 ± 0.48 | 0.10 | 1.24 ± 0.41 | 0.01* |
| (8) How interested were you in the video you just watched? | 3.15 ± 0.43 | 3.78 ± 0.80 | 2.82 ± 0.51 | 0.37 |
For items 1–7, scores were generated from the difference between pre-smoking cue exposure to post-smoking cue exposure. Each item was inserted into the phrase ‘On a scale from 1 to 7 … with 1 corresponding to Not at all and 7 corresponding to Extremely’. Starred items (*) are significant at P < 0.05. There were no group by time differences between allelic groups in any of the first seven items.
Imaging results
Random effects analysis
Representative saggital, axial and coronal sections co-registered to the MNI brain and centered on the suprathreshold voxel in all subjects, are shown in Fig. 1. Coordinates listed are those chosen from the suprathresh-old voxel of each region using the Duvernoy Brain Atlas and the Atlas of the Human Brain Mai, Paxinos and Voss as references (Duvernoy 1999; Mai, Voss & Paxinos 2008). Table 3 lists all SC-elicited neural activity. An interactive visual display of all brain data in all three planes can be found at http://franklinbrainimaging.com.
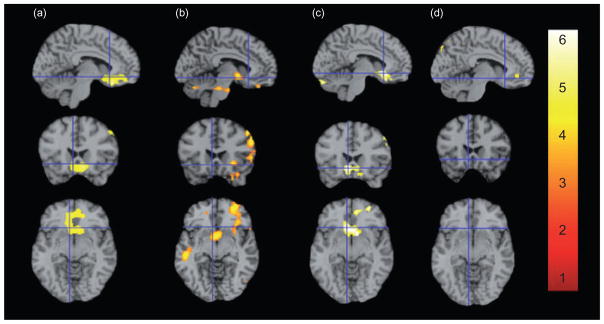
Figure 1.
Brain activity during SC versus Non-SC exposure in all subjects, in 9-repeat carriers greater than 10-repeats, and when grouped by DAT genotype. Representative fMR saggital, axial and coronal brain slices, analyzed in SPM5, and overlain on the MNI brain are displayed with crosshairs centered on −6 22 −8 for all comparisons at P = 0.01 and 20 contiguous voxels. [Note that the chosen coordinate is not the suprathreshold voxel for all comparisons (see Table 3 for a complete list of coordinates and t-values).] All subjects (a; t = 3.77), 9-repeat carriers greater than 10-repeats (b; t = 3.19) and 9-repeat carriers (c; t = 5.47) show heightened responses to SCs in VS and mOFC. Responses in 10/10-repeats (d) were non-significant. Images are displayed neurologically (left is left). An interactive visual display of all brain data in all three planes can be found at http://franklinbrainimaging.com
Table 3.
Brain perfusion during smoking cue exposure relative to non-smoking cue exposure.
| Region | All n = 26 | 9-repeat carriers > 10/10-repeats | 9-repeat carriers n = 9 | 10/10-repeats n = 17 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Increases | X | Y | Z | t Val | X | Y | Z | t Val | X | Y | Z | t Val | X | Y | Z | t Val |
| VS/mOFC bilateral | −6 | 28 | 18 | 3.77 | −6 | 22 | −8 | 5.47 | ||||||||
| VS | −4 | 4 | −6 | 3.12 | ||||||||||||
| mOFC | −10 | 42 | −22 | 3.19 | 16 | 50 | −6 | 4.96 | ||||||||
| 8 | 46 | −28 | 3.19 | |||||||||||||
| M frontal G | −44 | −4 | 52 | 3.21 | 46 | −6 | 60 | 4.41 | ||||||||
| M frontal G | −36 | 32 | 46 | 4.55 | ||||||||||||
| Inf temp G | 52 | −26 | −24 | 3.00 | ||||||||||||
| DM insula | −38 | −4 | 14 | 3.76 | ||||||||||||
| Anterior cingulate | −4 | 44 | −4 | 2.99 | ||||||||||||
| DLPFC | 30 | 66 | 12 | 3.56 | 32 | 64 | 8 | 4.87 | ||||||||
| Lat OFC | 42 | 42 | −16 | 3.83 | ||||||||||||
| Fusiform | 40 | −28 | −30 | 3.12 | ||||||||||||
| Decreases | X | Y | Z | t Val | X | Y | Z | t Val | X | Y | Z | t Val | ||||
| DP insula | 62 | −26 | 14 | −5.10 | ||||||||||||
| DP insula | −60 | −14 | 2 | −4.69 | ||||||||||||
| DP insula | 58 | −30 | 18 | −6.13 | ||||||||||||
| DM insula | −4.69 | −38 | −4 | 14 | −4.12 | −32 | 8 | 10 | −3.64 | −50 | −34 | 12 | −5.15 | |||
| DM insula | −50 | 0 | 4 | −4.06 | −6.13 | |||||||||||
| P cingulate M | 2 | −44 | 52 | −4.02 | ||||||||||||
| Lat OFC | 26 | 62 | −12 | −3.89 | ||||||||||||
| Parahippocampal G | −48 | −6 | 46 | −3.74 | ||||||||||||
| Inf frontal G | −38 | 18 | 28 | −3.70 | −44 | −34 | −24 | −3.71 | ||||||||
| Inf temp G | 40 | −24 | −30 | −4.29 | ||||||||||||
| Mid temp G | −48 | −52 | 2 | −4.23 | 56 | 24 | 6 | −3.72 | ||||||||
D = dorsal; DLPFC = dorsolateral prefrontal cortex; G = gyrus; Inf = inferior; Lat = lateral; M = medial; Mid = middle; P = posterior; Temp = temporal; VS/mOFC = ventral striatum/medial orbitofrontal cortex. Coordinates are in MNI. t-Values are from the suprathreshold voxel in the cluster.
In all subjects (N = 26), significantly greater perfusion to SCs compared with non-SCs was observed selectively and bilaterally, in the anatomically interconnected rostral ventral striatum and medial orbitofrontal cortex (VS/mOFC; t = 3.77, peak voxel, −6, 28, −18).
In comparison to 10/10-repeats, 9-repeats exhibited greater responses in the VS (t = 3.12, peak voxel −4, 4, −6) and mOFC (t = 3.19, −10 42–22). Greater responses were also observed in the DLPFC, the fusiform gyrus, and the inferior temporal and middle frontal gyri.
In 9-repeat carriers, greater perfusion was observed bilaterally in the VS/mOFC (t = 5.47; peak voxel, −6, 22, −8; P < 0.0001 at both the voxel and cluster levels). Increased perfusion was also observed in a slightly lateral and more dorsal region of the ventromedial prefrontal cortex, and in the dorsolateral prefrontal and lateral orbitofrontal cortices.
In contrast to the SC-elicited increased VS/mOFC activity in 9-repeat carriers, perfusion to this region was not significantly different across SC versus non-SC conditions in 10/10-repeats. Brain perfusion was enhanced in the middle frontal gyrus, the dorsal medial insula and the anterior cingulate (marginally significant) in 10/10-repeats.
All groups had reductions in perfusion to the insula, but not in the anterior ventral portion of the insula, which has been linked with craving and the ability to quit smoking (Naqvi et al. 2007) and which was activated during SC exposure in our earlier studies (Franklin et al. 2007; Franklin et al. 2009). Decreased perfusion was observed in more dorsal and posterior parts of the insula in smokers overall and in both allelic groups (t-values ranged from 3.64 to 6.13). In all smokers and in 10/10-repeats, the regional reductions in perfusion were much greater in both magnitude and extent.
Results were not different when age, cigarettes smoked per day or years smoking were entered into the analyses as nuisance variables or covariates of interest. However, correlations were observed between cigarette dependence as assessed by the FTND and SC-induced neural activity (see below).
Resting baseline perfusion (subjects were asked to lie in the scanner with their eyes open) was not different between allelic groups in any regions evincing that the divergent responses were specifically related to SC exposure and not differences related to dopaminergic tone prior to viewing the stimuli.
Brain/behavioral correlational analyses
Associations between cue-induced craving and neural activity. In 25 subjects (one subject’s scores were unavailable), subjective scores of SC-induced craving correlated with increased SC-induced activity in a region that has been characterized as the sublenticular extended amygdala (SLEA), which includes the posterior VS, ventral pallidum and the central and medial amygdaloid nuclei, and is bound by the bed nucleus of the stria terminalis (Heimer 2003). Positive correlations between SC reactivity and reported SC-induced craving were also observed in the dorsal medial insula and post central gyrus, while inverse correlations were observed in the DLPFC and the middle temporal gyrus. In 9-repeat carriers, the association between craving and increased brain activity was localized to the SLEA area, while decreased activity was observed in the DLPFC. In contrast to increased activity in the SLEA in the 9-repeat carriers, activity in 10/10-repeats included the VS, the dorsal medial insula and the pre- and post-central gyri, with decreased activity observed in the middle temporal gyrus. There were no significant positive or negative correlations between subjective craving and perfusion in any other regions (Table 4).
Table 4.
Correlations with craving during smoking cue exposure relative to non-smoking cue exposure.
| Region | All (n = 25) | 10/10-repeats (n = 16) | 9-repeats (n = 9) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Increases | X | Y | Z | t Val | r | X | Y | Z | t Val | r | X | Y | Z | t Val | r |
| SLEA | 12 | −2 | −10 | 3.56 | 0.60 | 14 | −10 | −14 | 5.89 | 0.91 | |||||
| SLEA | −16 | −6 | −14 | 9.05 | 0.96 | ||||||||||
| DM insula | 38 | −14 | 2 | 3.26 | 0.56 | 38 | −12 | 4 | 3.50 | 0.68 | |||||
| Post central G | 26 | −32 | 56 | 3.09 | 0.51 | −48 | −28 | 50 | 4.33 | 0.76 | |||||
| Pre central G | 64 | −28 | 36 | 3.98 | 0.73 | ||||||||||
| VS | 16 | 8 | 0 | 3.07 | 0.63 | ||||||||||
| Decreases | |||||||||||||||
| DLPFC | 42 | 48 | 0 | −3.60 | −0.60 | 42 | 38 | 14 | −3.98 | −0.83 | |||||
| DLPFC | −36 | 42 | 0 | −4.13 | −0.84 | ||||||||||
| Mid temp G | 64 | −8 | −4 | −4.44 | −0.67 | 64 | −8 | −4 | −5.51 | −0.83 | |||||
| Mid temp G | −56 | −52 | 4 | −4.99 | −0.80 | ||||||||||
DLPFC = dorsolateral prefrontal cortex; DM = dorsal medial; G = gyrus; Lft = left; Mid = middle; Rt = right; SLEA = sublenticular extended amygdala; Temp = temporal; VS = ventral striatum. Coordinates are in MNI. t-Values are from the suprathreshold voxel in the cluster. One subject’s craving reports were unavailable.
Associations between FTND scores and neural activity
In all subjects the mean of the FTND scores was 4.80 (±0.3) and ranged from 1 (minimal dependence) to 9 (high dependence). For the group as a whole, a regression analysis showed a significant positive relationship between the severity of nicotine dependence and perfusion in the left anterior insula, medial OFC, precuneus and medial and superior temporal and superior frontal gyri in response to SCs (See Table 5). Perfusion in the insula was significant at the suprathreshold voxel (P = 0.001) and at the cluster level (P = 0.005). There were no significant positive or negative correlations between FTND and perfusion in any other regions. In 9-repeat carriers, FTND scores averaged 4.4 ± 0.4 and ranged from 1–7. There were no correlations between brain responses and FTND scores in 9-repeat carriers. In 10/10-repeats, FTND scores averaged 4.9 ± 0.5 and ranged from 1–9. In 10/10-repeats, brain correlates of nicotine dependence were observed in the insula, medial OFC, medial temporal cortex and superior frontal cortex with t-values ranging from 3.89–5.88. Correlations were significantly stronger in 10/10-repeats compared with all subjects. No significant decreases in CBF were observed.
Table 5.
Association between Brain Perfusion and FTND scores during exposure to smoking cues.
| All | 10-repeats | 9-repeat carriers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Increases | X | Y | Z | t Val | r2 | X | Y | Z | t Val | r2 | None observed |
| M D insula | −42 | 0 | 10 | 3.71 | 0.25 | −40 | −2 | 12 | 3.89 | 0.62 | |
| M OFC | 6 | 56 | −16 | 4.82 | 0.59 | ||||||
| M temporal G | 64 | −48 | 10 | 3.62 | 0.37 | 60 | −48 | 10 | 5.11 | 0.62 | |
| 54 | −72 | −14 | 4.93 | 0.60 | |||||||
| S frontal G | 4 | 38 | 50 | 3.67 | 0.33 | −4 | 52 | 44 | 5.88 | 0.68 | |
| Precuneus (S parietal G) | −4 | −56 | 64 | 3.56 | 0.37 | ||||||
| S temporal G | −56 | −24 | −6 | 3.47 | 0.25 | ||||||
| Decreases | None observed | None observed | None observed | ||||||||
D = dorsal; G = gyrus; M = medial; OFC = orbitofrontal cortex; S = superior. Coordinates are in MNI. t-Values are from the suprathreshold voxel in the cluster.
DISCUSSION
Major findings
Consistent with our previous studies, we observed significant SC-induced craving and increased brain perfusion in reward-related regions in a new cohort of smokers using a similar perfusion fMRI SC paradigm (Franklin et al. 2007, 2009). Brain activity during SC exposure was heightened bilaterally in the VS and mOFC in 9-repeat carriers compared with10/10-repeats.
We hypothesized that a less efficient or less functional DAT may lead to slower reuptake of DA so that it lingers longer in the synapse, prolonging the reward message and strengthening the associations between nicotine and the cues. As the incentive value of SCs is enhanced, so is the craving and associated brain activity triggered by their presence. In our earlier work, we found that 9-repeat carriers had greater brain responses than 10-repeats bilaterally in the inter-connected VS and medial OFC, possibly identifying a subgroup of smokers more vulnerable to relapse when confronted with SCs (Franklin et al. 2009). Here, in a similar paradigm, and in a new cohort of smokers, we confirm the finding that 9-repeat carriers have enhanced responses in reward-related brain regions compared with 10/10-repeats during SC exposure. These results provide further evidence that heterogeneity in SC responsivity is partially mediated by genetic variance in the DAT, and that 9-repeat carriers might represent a SC-vulnerable group.
As cigarette smoking is a complex and multifaceted behavior, the genetic contribution to smoking is most likely mediated by numerous genes in multiple transmitter systems that affect the rewarding, stimulating and conditioned effects of nicotine. Additionally, there are a host of non-genetic factors involved in the motivation to smoke and that promote relapse, including stress, peer pressure, availability, sex, menstrual cycle phase and weight management (Perkins 2001; Perkins et al. 2001; Franklin et al. 2004; Sinha & Li 2007; Franklin et al. 2008; Dagher et al. 2009). Thus, we did not expect or observe an exact replication of our earlier study. However, the strongest DAT genotype mediated effects in both this and the previous study are located in comparable a priori brain regions and demonstrate the same overall result: greater SC-induced neural activity in 9-repeat carriers compared to 10/10-repeats in the VS and mOFC. As in Franklin et al. 09, sample size was small; however, we feel this study, that confirms the robust effects of DAT genotype on SC reactivity observed in our earlier work, will stimulate further research into the role of the DAT in SC and other drug cue reactivity studies.
The role of genetic variance in the dopamine receptor 4 subtype (DRD4) in cigarette dependence, and SC reactivity is beginning to be elucidated. For example, the longer, 7 VNTR allele was shown to be associated with SC exposure (Hutchison et al. 2002). Greater craving, more arousal, less positive affect and more attention to the SCs were observed in smoker’s homo-or heterozygous for the longer allele compared with smokers homozygous for the shorter allele. The possibility of a role for the DRD4 is supported by a BOLD imaging study wherein the longer allele was associated with increased brain activity in the right superior frontal gyrus and right insula. No correlations with craving or other behavioral measures were reported (McClernon et al. 2007a). Our work and the work cited above suggest an interaction may exist between select polymorphisms of the DRD4 and the DAT that may predict even greater vulnerability to relapse in the presence of cues. Future studies within our own laboratory include continuing to acquire data sets to increase the sample size and examine the contributions and possible interactions between DAT and DRD4 polymorphisms.
As stated, the most outstanding responses to SC exposure were observed in the interconnected rostral medial portions of the OFC and the VS. Historically, the VS, and in particular its medial and rostral aspects, are important players in stimulus-reinforcement association learning (Di Chiara et al. 1999) and exert strong control over emotional and motivational behavior including craving (Cardinal et al. 2002). Several lines of evidence support the theory that the OFC is involved in assimilating information on the reward value of incoming stimuli to determine the appropriate course of action. Consistently, processing is prominent in the medial aspect of the OFC when reward value is high (Schultz, Tremblay & Hollerman 2000; Kringelbach 2005). Using a monetary decision-making task, Liu et al. 2007 reported that reward and the anticipation of reward, produce a pattern of activity in the mOFC and the rostral medial VS that is hard to distinguish from the findings reported here in smokers overall (Liu et al. 2007). However, the effects in the VS/mOFC in all subjects reported here, and in our earlier study appear to be carried solely by individuals possessing a 9-repeat allele of the DAT.
A potentially intriguing finding is that perfusion during SC exposure was significantly decreased bilaterally in the dorsal posterior insular cortex in all comparisons. McBride and colleagues also observed decreased activation during exposure to videos of SCs (versus non-SCs) in the same anatomically distinct subregion of this large and highly heterogeneous structure (McBride et al. 2006). Given that the dorsal posterior subregion of the insula was activated during stimulus independent thought processes (when individuals are asked to let their minds wander), its deactivation might be related to focused attention directed toward the smoking stimuli (Mason et al. 2007). This finding may provide hypothesis-generating data, which could be tested directly in future studies.
Associations between brain activity and subjective reports of craving
In all smokers and within each allelic group, correlations between subjective reports of craving and brain activity in reward-related brain regions were observed. In all subjects and in 9-repeat carriers, a region characterized as the SLEA was prominently and positively correlated with cue-elicited craving. The SLEA is anatomically defined as a subset of brain regions constrained by the bed nucleus of the stria terminalis on its medial side that includes the VS, the ventral pallidum and the central and medial amygdalar nuclei. The SLEA is featured in the animal literature as a pathway involved in responding to stimuli that predict biologically relevant outcomes, and dysfunction in this pathway is heavily implicated in psychological disorders, including addiction (Di Chiara et al. 1999; Heimer 2003). In 10/10-repeats, positive correlations were observed in the VS, and the precentral gyrus. In all subjects and in 10/10-repeats activity was positively correlated with SC-induced craving in the dorsal middle insula and the post central gyrus, and inverse correlations were observed in the middle temporal gyrus and the DLPFC. Notably, there were several correlative relationships in all subjects that overlapped with those observed in separate allelic groups, but there was no overlap observed between 9/9 and repeat carriers and 10/10-repeats. The associations between SC-induced craving and neural activity observed are in partial alignment with the existing literature (Smolka et al. 2006) (McClernon et al. 2005); however, there are numerous differences in study design between the published studies and the study here. For example, in Smolka et al. versus this study, subjects were 10 males versus 26 male/females, a BOLD fMRI block design was used versus perfusion fMRI, smoking behavior was ‘as usual’ versus smoking immediately before scanning, and change scores were calculated between craving from SC and neutral stimuli when shown again later after scanning versus change scores calculated from SC and nonSC cues from pre- to post-scanning. Smolka and colleagues observed activation in several structures including the amygdala, hippocampus and fusiform gyrus. Craving/brain correlations were also reported in McClernon et al. wherein the study design and sample was also considerably different than that used here. BOLD event-related design was used, smokers were abstinent or sated (no correlations were observed in the sated state), craving scores were reported as correlations between the effect of overnight abstinence on craving [abstinent session—satiated session] during scanning and the effect of abstinence on brain responses to smoking and control cues. Activation in McClernon et al. was observed in the inferior, middle and superior frontal gyri, and ventral and dorsal anterior cingulate. In the current study, we found that brain activity correlated with craving in both allelic groups in regions known to respond to reward and rewarding stimuli, but regional activations were dissimilar from our previous studies. As discussed above, studies demonstrating correlative relationships between craving induced by drug cue exposure and brain activity are often in opposition (Brody et al. 2002; Franklin et al. 2009) (McClernon et al. 2005) or have not been observed (Due et al. 2002; David et al. 2005; Okuyemi et al. 2006; McClernon et al. 2007b). These and other discordant findings both within our own studies and across previous studies of SC reactivity highlight the caveats associated with reliance on subjective measures and encourage the use of objective markers such as cognitive bias and/or attentional tasks to examine brain/behavioral relationships.
Subjective indices of craving and withdrawal
As discussed above, subjects were asked to rate their craving immediately prior to and following SC exposure. The SCs elicited craving for a cigarette in both 9-repeat carriers and 10/10-repeats. However, the reasons underlying craving between allelic groups diverged. Both 9-repeat carriers and 10/10-repeats desired to smoke a cigarette (item 3), but only 9-repeat carriers desired to smoke ‘just to smoke’ (item 4) and for pleasure (item 6) while 10/10-repeats desired to smoke because they were edgy (item 5) and for relief (item 7). One interpretation for the divergent cue-elicited motivations to smoke across groups might be due to group differences in appetitive versus aversive feelings about the SCs when sated. We base this interpretation on the combination of several mitigating factors. First, over 70% of smokers in the United States want to eventually quit (American Lung Association 2008), and greater than 80% of smokers participating in this study expressed a wish to quit. Second, the sample studied here smoked a cigarette immediately prior to the scanning session. Third, while 9-repeats had robust SC-elicited brain activity in the reward relevant VS/mOFC, this region did not respond to SCs in 10/10-repeats. Thus, we suggest that the cues may acquire aversive properties when sated in the 10/10-repeats: ‘I just had a cigarette, I smell it on my clothes and I taste the staleness in my mouth. … I despise that I smoke, I don’t like seeing cigarette reminders right now’. Conversely, satiation may not diminish appetitive craving, or affect unpleasant motivation to smoke in the presence of SCs in 9-repeat carriers. Again, larger sample sizes are necessary to draw any firm conclusions about this self-report measure that may reveal underlying reasons for craving a cigarette.
Associations between brain activity and nicotine dependence
In secondary analyses, we predicted that severity of nicotine dependence would be related to the degree of insula activation during exposure to SCs and that its activation would be DAT genotype dependent. One role of the anterior insula is to process interoceptive physiological information acquired during biologically salient situations, and relay the information to higher-level cognitive brain regions (Craig 2009). In light of recent findings, the insula is gaining considerable attention in the drug addiction field. Smokers who acquired lesions to the brain that included sizable portions of the insular cortex spontaneously quit smoking, while smoking addiction was not disrupted in smokers with minimal damage (Naqvi et al. 2007). Furthermore, the smokers with large insula lesions reported an absence of craving for cigarettes while craving for other naturally rewarding substances, such as food, was left intact. Our first study and this one suggest that 9-repeat carriers are more vulnerable to relapse in the presence of SCs (a cue-vulnerable endophenotype), although this was not a treatment study and that hypothesis remains untested. Given that cigarette smoking is prevalent in both allelic groups and that both genotypes experience difficulty quitting, we hypothesize that 10/10-repeat smoking behavior may be influenced more by pharmacological withdrawal from nicotine (nicotine dependent). Greater dependence on nicotine, as assessed by the FTND is associated with more extended and/or severe smoking histories. We posit that the repeated pairings of the smoking stimuli with nicotine would correlate with the degree of insula activation to SCs in 10/10-repeats, whereas a direct association would not be observed in more SC vulnerable 9-repeat smokers. Thus, we examined whether severity of nicotine dependence, as assessed by the FTND, was associated with neural responses to SCs, and whether the association was DAT genotype dependent.
Congruent with the hypothesized outcome, we observed associations between nicotine dependence and brain activity in smokers overall, and we found the association to be DAT-genotype dependent, such that no correlations were found in 9-repeat carriers while strong associations between nicotine dependence and brain activity were observed in the mOFC, ventral medial insula, and medial temporal and superior frontal gyri in 10/10-repeats. As shown previously, and in the present study, these regions are associated with SC-elicited brain activity specifically (Brody et al. 2002; McBride et al. 2006; Franklin et al. 2007; Dagher et al. 2009; Franklin et al. 2009), and in smoking behavior in general (Naqvi et al. 2007; Rose et al. 2007).
The finding of an association between FTND scores and neural activation during SC exposure has been observed in other studies. Both Smolka et al. 2006 and McClernon et al. 2008 utilized a BOLD fMRI block design, similar types of cues (pictures) and smokers with high nicotine dependence to examine neural activity with respect to FTND scores (Heinz & Smolka 2006; McClernon et al. 2008). However, there was only one region of overlap between the two studies in brain activity (anterior cingulate). Our perfusion fMRI results with minutes-long cue sets and moderately dependent smokers are also in disagreement with the BOLD studies. These contrasting findings across three different studies may at first appear disconcerting, especially the differences between the McClernon et al. 2008 and Smolka et al. 2006 studies, that utilized similar paradigms. However, these divergent findings in differing populations exemplify the substantial individual variability in the complex and multifaceted underlying motivations to continued smoking and relapse. Thus, we are obliged to continue to study, and to identify all possible relapse predictors; those that are mediated by genotype, those mediated by a host of other variables including sensitivity to withdrawal, race, sex, menstrual cycle phase and stress (Perkins, Donny & Caggiula 1999; Franklin et al. 2004; Okuyemi et al. 2006; Sinha & Li 2007; Franklin et al. 2008; Dagher et al. 2009), and their likely interaction.
Rationale for cue sequence
Cue sets were not counterbalanced, which in some paradigms may introduce interference in signal capture related to withdrawal. In Franklin et al. 2007 and 2009 we did counterbalance cue sets and allowed subjects to smoke a cigarette before each 10-minute cue set to minimize any withdrawal that may occur over the course of the scanning session. We demonstrated that withdrawal does not accrue over the course of a 40-minute scanning session in sated smokers and that no changes in subjective reports of craving occurred during presentation of the non-smoking video (Franklin et al. 2007; Franklin et al. 2009). We further substantiate our claim that smokers were not experiencing any withdrawal in the current study by demonstrating that pre- and post-scanning Shiffman–Jarvik scores did not differ in all subjects or in separate allelic groups. We conclude that counterbalancing to minimize withdrawal is unnecessary in our paradigm and in fact, may introduce order effects. It has been shown that ‘carry over’ arousal initiated when drug cues are shown first can potentially affect responses to non-drug cues (Waters et al. 2005; Wilson et al. 2007; Sharma & Money 2009).
Significance
The central ‘brain’ finding of enhanced responses to SCs in reward-relevant interconnected medial ventral aspects of the mesolimbic system is consistent across our three studies. These regions have also been consistently observed in cocaine, heroin (other) nicotine and sexual cues using a variety of imaging modalities (Brody et al. 2002; Childress et al. 2008; Langleben et al. 2008; McClernon et al. 2008; Dagher et al. 2009). This congruence across studies underscores the strength of direct neurobiological assays, such as fMRI, to study addiction processes.
There is a variable and underwhelming therapeutic response to existing medications such as bupropion, varenicline and nicotine replacement therapy (Anon 2000; Lancaster & Stead 2000; Biberman et al. 2003; Hughes et al. 2003; Coe et al. 2005; Jorenby et al. 2006; Wu et al. 2006; Rollema et al. 2007; Benowitz 2009). Issues related to tolerability also hinder medication effectiveness. The variable treatment response to existing medications, which act to reduce withdrawal and the reinforcement received from nicotine, may indicate heterogeneity in the relative contribution of SCs and withdrawal to the maintenance of dependence. Smokers whose smoking behavior is influenced more by pharmacological withdrawal from nicotine may be the subgroup that benefits from the existing nicotine replacement medications, while smokers whose relapse is influenced more by cue exposure may receive less benefit. There is a crucial need to develop pharmacotherapeutics that can treat the vulnerabilities associated with exposure to SCs, which is predictive of smoking cessation outcome (Waters et al. 2004; Payne et al. 2006). Knowledge of the underlying functional neurobiological consequences of genetic variance on SC reactivity using the combined tools of neuroimaging and genomics may offer the potential to develop predictive biomarkers of relapse vulnerability and identify novel targets for individualized treatment.
Acknowledgments
Work supported by NIH 5-P60-DA-005186-18, Pfizer Pharmaceuticals (an Investigator-initiated research grant) and The Alexander Foundation.
The authors would like to acknowledge the nursing staff at the University of Pennsylvania Addiction Treatment Research for conducting physical evaluations. We also would like to thank Anita Hole PhD for helping to conduct the psychological evaluations. And third, we take this opportunity to thank the MRI technicians at the Hospital of the University of Pennsylvania for conducting the scanning sessions.
Footnotes
DISCLOSURES
The following authors served as consultants within the past 2 years: TF (Pfizer, Abbott), CO (Abbott, Embera), AR (Abbott). JD has a patent for the perfusion fMRI technique.
Authors Contributions
TF, AR, CO and ZW were responsible for study concept, design and interpretation of findings. TF, JS, MG, JF, and WJ contributed to acquisition of imaging, genetic and behavioral data. ZW and JD optimized and monitored perfusion fMRI data acquisition. ZW, YL and TF analyzed imaging data. FL was the geneticist. KK, MG and JS determined physical and mental status of potential subjects. JC, RH and WJ screened, and consented subjects and ran the imaging sessions. TF, RH and JS contributed to analysis of behavioral data. All authors critically reviewed content and approved final version for publication.
References
- Anonymous. Bupropion to aid smoking cessation. Drug Ther Bull. 2000;38:73–75. doi: 10.1136/dtb.2000.381073. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Detre JA, Wang J. Perfusion fMRI for functional neuroimaging. Int Rev Neurobiol. 2005;66:213–236. doi: 10.1016/S0074-7742(05)66007-2. [DOI] [PubMed] [Google Scholar]
- American Lung Association. Helping Smokers Quit: State Cessation Coverage 2008. 2008:1–24. Available from http://www.lungusa.org.
- Baker TB, Morse E, Sherman JE. The motivation to use drugs: a psychobiological analysis of urges. Nebr Symp Motiv. 1986;34:257–323. [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberman R, Neumann R, Katzir I, Gerber Y. A randomized controlled trial of oral selegiline plus nicotine skin patch compared with placebo plus nicotine skin patch for smoking cessation. Addiction. 2003;98:1403–1407. doi: 10.1046/j.1360-0443.2003.00524.x. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S, Zamora-Paja E, Farahi J, Saxena S, London ED, McCracken JT. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch Gen Psychiatry. 2006;63:808–816. doi: 10.1001/archpsyc.63.7.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O’Brien CP. Prelude to passion: limbic activation by ‘unseen’ drug and sexual cues. Plos ONE. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dagher A, Tannenbaum B, Hayashi T, Pruessner JC, McBride D. An acute psychosocial stress enhances the neural response to smoking cues. Brain Res. 2009;1293:40–48. doi: 10.1016/j.brainres.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Wang J. Technical aspects and utility of fMRI using BOLD and ASL. Clin Neurophysiol. 2002;113:621–634. doi: 10.1016/s1388-2457(02)00038-x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann NY Acad Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Dijkstra A, Tromp D. Is the FTND a measure of physical as well as psychological tobacco dependence? J Subst Abuse Treat. 2002;23:367–374. doi: 10.1016/s0740-5472(02)00300-8. [DOI] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O’Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addict Behav. 1995;20:657–673. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- Drummond DC. What does cue-reactivity have to offer clinical research? Addiction. 2000;95 (Suppl 2):S129–S144. doi: 10.1080/09652140050111708. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain: Surface, Three-Dimensional Sectional Anatomy with Magnetic Resonance Imaging and Blood Supply. 2. New York: Springer-Verlag Wien; 1999. [Google Scholar]
- Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12:159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Floyd TF, Ratcliffe SJ, Wang J, Resch B, Detre JA. Precision of the CASL-perfusion MRI technique for the measurement of cerebral blood flow in whole brain and vascular territories. J Magn Reson Imaging. 2003;18:649–655. doi: 10.1002/jmri.10416. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Involvement of the nucleus accumbens and medial prefrontal cortex in the expression of conditioned hyperactivity to a cocaine-associated environment in rats. Neuropsychopharmacology. 2000;23:633–644. doi: 10.1016/S0893-133X(00)00162-7. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Ehrman R, Lynch KG, Harper D, Sciortino N, O’Brien CP, Childress AR. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: a retrospective analysis. J Womens Health (Larchmt) 2008;17:287–292. doi: 10.1089/jwh.2007.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Lohoff FW, Wang Z, Sciortino N, Harper D, Li Y, Jens W, Cruz J, Kampman K, Ehrman R, Berrettini W, Detre JA, O’Brien CP, Childress AR. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2009;34:717–728. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Napier K, Ehrman R, Gariti P, O’Brien CP, Childress AR. Retrospective study: influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob Res. 2004;6:171–175. doi: 10.1080/14622200310001656984. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J. 2001;1:152–156. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry. 2003;160:1726–1739. doi: 10.1176/appi.ajp.160.10.1726. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Heinz A, Smolka MN. The effects of catechol O-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks. Rev Neurosci. 2006;17:359–367. doi: 10.1515/revneuro.2006.17.3.359. [DOI] [PubMed] [Google Scholar]
- Ho MK, Tyndale RF. Overview of the pharmacogenomics of cigarette smoking. Pharmacogenomics J. 2007;7:81–98. doi: 10.1038/sj.tpj.6500436. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Shiffman S, Callas P, Zhang J. A meta-analysis of the efficacy of over-the-counter nicotine replacement. Tob Control. 2003;12:21–27. doi: 10.1136/tc.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, LaChance H, Niaura R, Bryan A, Smolen A. The DRD4 VNTR polymorphism influences reactivity to smoking cues. J Abnorm Psychol. 2002;111:134–143. doi: 10.1037//0021-843x.111.1.134. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alcohol Depend. 1994;34:211–216. doi: 10.1016/0376-8716(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster T, Stead LF. Mecamylamine (a nicotine antagonist) for smoking cessation. Cochrane Database Syst Rev. 2000:CD001009. doi: 10.1002/14651858.CD001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, O’Brien CP, Childress AR. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008;165:390–394. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Control of the reinforcing effects of nicotine by associated environmental stimuli in animals and humans. Trends Pharmacol Sci. 2005;26:287–293. doi: 10.1016/j.tips.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27:4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Liu J, Salley AN, Behm FM, Rose JE. Selectively reduced responses to smoking cues in amygdala following extinction-based smoking cessation: results of a preliminary functional magnetic resonance imaging study. Addict Biol. 2007a;12:503–512. doi: 10.1111/j.1369-1600.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hutchison KE, Rose JE, Kozink RV. DRD4 VNTR polymorphism is associated with transient fMRI-BOLD responses to smoking cues. Psychopharmacology (Berl) 2007b;194:433–441. doi: 10.1007/s00213-007-0860-6. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- Mai JK, Voss T, Paxinos G. Atlas of the Human Brain. 3. New York: Elsevier Academic Press; 2008. [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Powell JN, Savage CR, Hall SB, Nollen N, Holsen LM, McClernon FJ, Ahluwalia JS. Enhanced cue-elicited brain activation in African American compared with Caucasian smokers: an fMRI study. Addict Biol. 2006;11:97–106. doi: 10.1111/j.1369-1600.2006.00007.x. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, Adams SG, Diefenbach L. Pretreatment cue reactivity predicts end-of-treatment smoking. Addict Behav. 2006;31:702–710. doi: 10.1016/j.addbeh.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Marcus MD, Levine MD, D’Amico D, Miller A, Broge M, Ashcom J, Shiffman S. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. J Consult Clin Psychol. 2001;69:604–613. [PubMed] [Google Scholar]
- Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the fagerstrom tolerance questionnaire and the fagerstrom test for nicotine dependence. Addict Behav. 1994;19:33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Prevention CfDCa . Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. MMWR. 2008;57:1226–1228. [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl) 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Salley AN, Bates JE, Coleman RE, Hawk TC, Turkington TG. Regional brain activity correlates of nicotine dependence. Neuropsychopharmacology. 2007;32:2441–2452. doi: 10.1038/sj.npp.1301379. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Sharma D, Money S. Carryover effects to addiction-associated stimuli in a group of marijuana and cocaine users. J Psychopharmacol. 2009;24:1309–1316. doi: 10.1177/0269881109350079. [DOI] [PubMed] [Google Scholar]
- Sheehan B, Lecrubier Y, Sheehan K. The Mini International Neuropsychiatric Interview (MINI): the development and validation of structured diagnostic interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Shiffman S, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology. 1976;50:35–39. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus DF. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl) 2006;184:577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, Baldwin RM, Innis RB, Gelernter J. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med. 2005;46:745–751. [PubMed] [Google Scholar]
- Vandenbergh DJ, Bennett CJ, Grant MD, Strasser AA, O’Connor R, Stauffer RL, Vogler GP, Kozlowski LT. Smoking status and the human dopamine transporter variable number of tandem repeats (VNTR) polymorphism: failure to replicate and finding that never-smokers may be different. Nicotine Tob Res. 2002;4:333–340. doi: 10.1080/14622200210142689. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Aguirre GK, Rao H, Wang J, Fernandez-Seara MA, Childress AR, Detre JA. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26:261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Sayette MA, Franken IH, Schwartz JE. Generalizability of carry-over effects in the emotional Stroop task. Behav Res Ther. 2005;43:715–732. doi: 10.1016/j.brat.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J Consult Clin Psychol. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Reutenauer EL, Allen TM, Termine A, Vessicchio JC, Sacco KA, Easton CJ, McKee SA, George TP. Reliability of the fagerstrom test for nicotine dependence, minnesota nicotine withdrawal scale, and tiffany questionnaire for smoking urges in smokers with and without schizophrenia. Drug Alcohol Depend. 2007;86:278–282. doi: 10.1016/j.drugalcdep.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Weschler D. Wechsler Abbreviated Scale of Intelligence Manual. San Antonio, TX: Harcourt Brace & Company; 1999. [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA, Brough E. Carry-over effects of smoking cue exposure on working memory performance. Nicotine Tob Res. 2007;9:613–619. doi: 10.1080/14622200701243144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Wilson K, Dimoulas P, Mills EJ. Effectiveness of smoking cessation therapies: a systematic review and meta-analysis. BMC Public Health. 2006;6:300. doi: 10.1186/1471-2458-6-300. [DOI] [PMC free article] [PubMed] [Google Scholar]