Abstract
While polyphenolic compounds have many health benefits, the potential development of polyphenols for the prevention/treatment of neurological disorders is largely hindered by their complexity as well as by limited knowledge regarding their bioavailability, metabolism, and bioactivity, especially in the brain. We recently demonstrated that dietary supplementation with a specific grape-derived polyphenolic preparation (GP) significantly improves cognitive function in a mouse model of Alzheimer's disease (AD). GP is comprised of the proanthocyanidin (PAC) catechin and epicatechin in monomeric (Mo), oligomeric, and polymeric forms. In this study, we report that following oral administration of the independent GP forms, only Mo is able to improve cognitive function and only Mo metabolites can selectively reach and accumulate in the brain at a concentration of ∼400 nm. Most importantly, we report for the first time that a biosynthetic epicatechin metabolite, 3′-O-methyl-epicatechin-5-O-β-glucuronide (3′-O-Me-EC-Gluc), one of the PAC metabolites identified in the brain following Mo treatment, promotes basal synaptic transmission and long-term potentiation at physiologically relevant concentrations in hippocampus slices through mechanisms associated with cAMP response element binding protein (CREB) signaling. Our studies suggest that select brain-targeted PAC metabolites benefit cognition by improving synaptic plasticity in the brain, and provide impetus to develop 3′-O-Me-EC-Gluc and other brain-targeted PAC metabolites to promote learning and memory in AD and other forms of dementia.
Introduction
Plant-derived polyphenolic compounds possess diverse biological activities, including strong antioxidant, anti-inflammatory, antimicrobial, and antitumorogenic activities. There is a growing interest in the development of polyphenolic compounds for preventing or treating chronic and degenerative diseases such as cardiovascular disorders, cancer, as well as neurological diseases including Alzheimer's disease (AD).
Our laboratory and others have shown that polyphenolic compounds from multiple dietary sources, including a specific grape-derived polyphenolic preparation (GP), are capable of attenuating cognitive deterioration and reducing brain neuropathology in animal models of AD (Rezai-Zadeh et al., 2005; Hartman et al., 2006; Wang et al., 2008). GP is a complex mixture of proanthocyanadins (PACs) consisting of flavan-3-ol units including catechin (C), epicatechin (EC), catechin gallate, and epicatechin gallate. These monomeric units form the basis of various types of oligomers and polymers through C4→C8 or C4→C6 interflavan bonds (Sharma et al., 2011). PACs are the most abundant and complex polyphenols in grapes and grape-derived products.
The application of phytotherapeutic agents, including GP and other PAC-rich natural compounds, in the treatment of neurological disorders is largely hindered by the limited knowledge of their metabolisms, bioactivities, and whether sufficient concentrations can reach and accumulate in the brain to exert biological activities.
Our studies were designed to explore the pharmacokinetics (PK) of specific GP components and the mechanistic basis of their bioactivities in the brain. Our studies also tested whether a biosynthetic brain-targeted PAC metabolite could recapitulate the biological activity of GP, and provide insight on the development of “phytodrugs” as novel therapeutic agents for the attenuation of cognitive deterioration in AD and other forms of dementia.
Materials and Methods
Chemicals and materials.
(+)-Catechin (C), (−)-epicatechin (EC), and gallic acid standards were purchased from Sigma-Aldrich. 3′-O-methyl-epicatechin (3′-O-Me-EC) was from Nacalai USA. All extraction and liquid chromatography (LC) solvents were of certified HPLC and American Chemical Society grade from J.T. Baker. GP was obtained from Polyphenolics.
Fractionation of GP.
GP were extracted in acetone:water (7:3) under N2 and dissolved in Milli-Q water (Millipore) and re-extracted three times with equal volumes of ethyl acetate. The organic phase was evaporated, redissolved, and applied to an solid phase extraction (SPE) Column (ENV1 18, 10 g; Supelco). The PAC monomer (Mo)-enriched fraction was eluted with diethyl ether, evaporated, redissolved in water, and freeze-dried. The aqueous layer containing PAC oligomers and polymers (Po) was evaporated, freeze-dried, and fractionated as described previously (Sharma et al., 2011).
AD mice and treatment.
Female Tg2576 AD transgenic mice were purchased from Taconic and all procedures were approved by the Mount Sinai School of Medicine Institutional Animal Care and Use Committee. Mice were randomized into the nontreated control group, the Mo-treated group, or the Po-treated group. Animals were treated for 5 months starting at 7 months of age before the development of AD neuropathology/cognitive deficits. Both Mo and Po were delivered through their drinking water (Wang et al., 2008). The Mo-treated group was treated with 80 mg/kg/d Mo, equivalent to the amount of monomer PACs in 200 mg/kg/d GP, which was used in our previous study (Wang et al., 2008). The Po-treated group was treated with 120 mg/kg/d Po, equivalent to the amount of polymer PACs from 200 mg/kg/d GP.
Behavioral assessment by the Morris water maze test.
Spatial learning memory was assessed by the Morris water maze behavioral test when the mice were ∼12 months of age following 5 months treatment, as previously described (Wang et al., 2007).
Assessment of AD-type amyloid neuropathology.
Total Aβ1–40 or Aβ1–42 in the brain were quantified by sandwich ELISA (BioSource), as previously described (Wang et al., 2005). The level of soluble Aβ oligomers was measured by a commercially available sandwich ELISA (Wang et al., 2008) according to the manufacturer's instruction. Specifically, soluble amyloid peptide was extracted in PBS and centrifuged at 78,500 × g for 1 h at 4°C, and the supernatant was quantified by ELISA to specifically detect aggregated Aβ (Invitrogen).
Bioavailability, metabolism, and brain penetration of GP and fractions.
Eight-week-old male Sprague Dawley (SD) rats were placed on a polyphenol-free AIN-93M diet. Doses of Mo and Po were designed to match monomer and polymer PAC doses from GP, respectively, which are 41 mg/kg body weight (BW) GP, 17 mg/kg BW Mo, and 28.3 mg/kg BW Po. Doses of GP and fractions were administered to rats over a 10 d period through gavage. On day 11, animals were administered a final dose and killed an hour later by compressed CO2. The brain was harvested following PBS perfusion and placed in saline (0.2% ascorbic acid) and stored at −80°C until analysis.
Extraction and analysis of C metabolites from brain tissues.
C and EC metabolites were extracted from brain tissues by solid phase extraction. Briefly, ∼500 mg of brain tissues were extracted with methanol, dried, and resolubilized in 1.5 m formic acid before loading onto preactivated Oasis HLB SPE cartridges (Waters). The C metabolites were eluted with acidified methanol, vacuum-dried, sonicated, and resolubilized in mobile phase before LC-MS analysis.
Analysis of Cs from the brain was performed using an Agilent 6400 Series QQQ in multiple reaction monitoring (MRM) mode using identical ionization conditions used on the time-of-flight (TOF) with 30 eV collision energy used for MS/MS experiments. C and EC metabolite quantifications were estimated using calibration curves from parent standard compounds.
Electrophysiological recordings.
Tg2576 mice aged 22–24 months were used to assess the effect of 3′-O-Me-EC-β-Gluc on basal synaptic transmission and long-term potentiation (LTP). This model of AD is known to exhibit significant synaptic impairments at this age (Chapman et al., 1999). Hippocampal slices (350 μm) were acclimated in oxygenated artificial CSF and treated with 300 nm 3′-O-Me-EC-β-Gluc for 4–5 h. The field EPSPs (fEPSPs) were recorded from the CA1 region as described previously (Gong et al., 2004). LTP was induced using theta-burst stimulation (four pulses at 100 Hz, with the bursts repeated at five Hz, and each tetanus including three 10-burst trains separated by 15 s).
Multipathway cell signaling assays and Western blot analysis.
Luminex xMAP multiplexed immunoassays (Millipore) were used to evaluate the levels of phosphorylated proteins: CREB (Ser133), Erk/MAP kinase 1/2 (Thr185/Tyr187), AKT (Ser473), JNK (Thr183/Tyr185), p70 S6 kinase (Thr412), MEK1 (Ser222), p38 (Thr189/Tyr182), and Rsk1 (Ser380) in brain slices treated with 300 nm 3′-O-Me-EC-Gluc. For Western blot, samples were separated by SDS-PAGE and antibodies specific for phosphor-CREB, total CREB (Millipore), phosphor-CaMKII, total CaMKII, and β-tubulin (Santa Cruz Biotechnology) were used as probes.
Statistical analysis.
Data were analyzed using Prism software (V4.03; GraphPad Software). Data are presented as mean ± SEM and analyzed using two-way ANOVA with repeated measure (RM), one-way ANOVA followed by Bonferroni's post hoc tests, or two-tailed Student's t test. In all analyses, the null hypothesis was rejected at the 0.05 level.
Results
Fractionation of GP
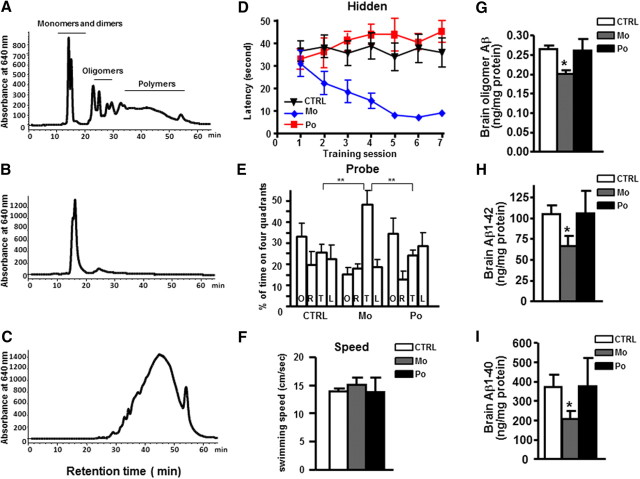
Based on reverse-phase HPLC analysis with detection by MS, or normal-phase HPLC analysis with detection by postcolumn derivatization, unfractionated intact GP is comprised of increasing proportions of monomeric, oligomeric, and polymeric proanthocyanidin components (Fig. 1A). In comparison, the Mo preparation is highly enriched in the monomeric components (Fig. 1B), whereas the Po preparation is comprised of predominantly polymeric components with traces (<1%) of Mo components (Fig. 1C).
Figure 1.
Fractionation of GP and in vivo efficacy of Mo and Po on Aβ-related neuropathology in Tg2576 mice. A–C, Normal phase HPLC chromatograms of GP fractions: GP (A), Mo-enriched fraction (B), Po-enriched fraction (C). D–F, The influence of chronic Mo or Po treatment on Aβ-related spatial memory in Tg2576 mice using MWM test. D, Hidden platform acquisition; latency score represents the time taken to escape to the platform. E, Probe trial. Percentage of time in four different quadrants (T, target; O, opposite; R, right; L, left). F, Swimming speed. G–I, Quantifications of oligomeric Aβ (G), total Aβ1–42 (H), and Aβ1–40 (I) in brains of Mo-treated, Po-treated, or control (CTRL) mice using ELISA assay. Data represents mean ± SEM, n = 8–10 mice per group. *p < 0.05, **p < 0.01.
In an in vitro analysis using the photo-induced cross-linking of unmodified proteins (PICUP) technique, we found that, similar to GP, both Mo and Po are capable of interfering with the initial protein–protein interaction of Aβ1–40 and Aβ1–42 that is necessary for the formation of neurotoxic oligomeric Aβ species (data not shown).
Dietary supplementation with Mo, not Po, improves cognitive function in AD mice
To explore the potential roles of specific components of GP in protection against AD-type cognitive deterioration, we treated Tg2576 mice with either Mo or Po. The Morris water maze (MWM) behavior test showed that Mo treatment significantly improved the cognitive behavioral performance of Tg2576 mice following 5 months of treatment, as reflected by a significant time-dependent decrease in the latency for finding the submerged escape platform compared with nontreated control Tg2576 mice (p < 0.01, two-way RM-ANOVA; Fig. 1D). In contrast, Po treatment did not lead to detectable improvements (Fig. 1D). In the probe trial phase of the MWM, Mo-treated Tg2576 mice, compared with Po-treated mice and nontreated control mice, spent significantly more time in the target quadrant area compared with the other quadrants (one-way ANOVA, p < 0.005; Fig. 1E), confirming that Mo treatment significantly improved spatial memory retention. The treatment did not affect nonspatial parameters such as swimming speed (Fig. 1F) that might interfere with MWM test.
In parallel control studies to test whether the cognitive improvement is associated with Aβ-mediated mechanism, we found that similar Mo treatment did not alter MWM performance in strain- and gender-matched wild-type mice (data not shown).
Neuropathology analysis also showed that compared with nontreated control Tg2576 mice, Mo treatment significantly reduced the content of oligomeric Aβ species (Fig. 1G) as well as the contents of Aβ1–42 (Fig. 1H) and Aβ1–42 (Fig. 1I) in the brain while no detectable changes were observed following Po treatments.
Plasma pharmacokinetic response for C and EC metabolites from GP and specified fractions
A major consideration for the potential development of polyphenolics for treating neurodegenerative disorders is bioavailability, particularly in the brain. In this study, we used SD rats. The choice of SD rats was based on their well established use as a model for bioavailability and metabolism of polyphenols in humans and based on the fact that both rats and mice possess similar xenobiotic enzyme systems, and similar metabolites of C and EC, namely methylated and glucuronidated metabolites, are observed in both species in previous studies (Feng, 2006).
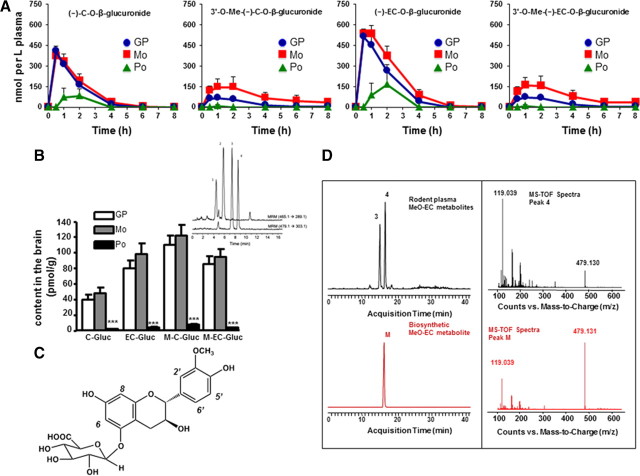
Polyphenol PK studies are traditionally conducted using a single, acute dose paradigm. However, a repeated dose paradigm more accurately reflects polyphenol PK in response to long-term application of polyphenols for clinical application. We found that metabolites of PAC monomers, specifically C and EC, were detected in rat plasma following 10 d of GP or Mo treatment (Fig. 2A). The predominant plasma metabolites of C and EC were identified by LC-MS-TOF as (−)-C-O-β-glucuronide, 3′-O-methyl-(±)-C-O-β-glucuronide, (−)-EC-O-β-glucuronide, and 3′-O-Me-EC-Gluc (Fig. 2A, from left to right). Characterization of these metabolites is consistent with previous reports (Roura et al., 2005; Tsang et al., 2005). Only small levels of the same C and EC methylated and glucuronidated metabolites were found in the plasma following Po treatment (Fig. 2A). There were no quantifiable levels of PAC dimers, trimers, or larger oligomeric PACs in the plasma following oral administration of GP, Mo, or Po.
Figure 2.
Plasma pharmacokinetics, brain levels of C and EC metabolites, and structural characterization of biosynthetic EC metabolite. A, Plasma pharmacokinetic profile of major C and EC metabolites following repeated dosing of rats by treatment with GP, Mo, and Po. B, Concentration of C and EC metabolites in brain tissue following 10 d of treatment. Inset, LC-MS/MS separation of major C and EC metabolites detected in extracts of rat brain tissue collected after 10 d of treatment. MRM trace is shown for C/EC-O-β-glucuronide (465.1→289.1 m/z) and MeO-C/EC-O-β-glucuronide (479.1→303.1 m/z). Peak identifications: peak 1: (±)-C-O-β-glucuronide; peak 2: (-)-EC-O-β-glucuronide; peak 3: 3′-O-Me-(±)-C-O-β-glucuronide; peak 4: 3′-O-Me-(-)-EC-O-β-glucuronide. ***p < 0.001, n = 5 per group. C, Proposed structure of the primary EC metabolite identified as 3′-O-Me-(-)-EC-5-O-β-glucuronide present in blood and brain tissues following repeated dosing of rats by treatment with GP or Mo fraction. D, LC-MS-TOF separation and online spectra of C and EC metabolites detected in extracts of rat plasma (black) and biosynthetic EC metabolite (red). Extracted ion chromatogram is shown for MeO-C/EC-O-β-glucuronide (479.13). Peak identifications: peak 3: 3′-O-Me-(±)-C-5-O-β-glucuronide; peak 4: 3′-O-Me-(-)-EC-5-O-β-glucuronide; peak M: 3′-O-Me-(-)-EC-5-O-β-glucuronide.
Compared with single acute dosage, bioavailability of C and EC metabolites was significantly higher following repeated GP or Mo dosing, as summarized in Table 1. Both area under the curve (AUC) and Cmax values were significantly higher (p < 0.01) for each of the C and EC metabolites following repeated dosing compared with single acute treatment with identical doses of intact GP or Mo (Table 1). AUC values for total C and EC glucuronides increased by ∼10- to 20-fold, with the largest increases observed for methylated C and EC metabolites from Mo. Increases in AUC values for methylated EC glucuronides were also significantly (p < 0.05) higher from Mo (216.0–952 nmol/L*h) compared with GP (187.7–241.7 nmol/L*h). Similar increases were noted for methylated C glucuronides (Table 1).
Table 1.
Eight-hour pharmacokinetic parameters from male Sprague Dawley rats (n = 5) treated with a single dose of GP (41 mg/kg BW), Mo (17 mg/kg BW), or Po (28.3 mg/kg BW) or after repeated exposure to extracts for 10 d
| Metabolite | Treatment | Single acute dose |
Acute dose following 10 d repeated exposure |
||||
|---|---|---|---|---|---|---|---|
| AUC(0–8 h) (nmol/L*h) | Cmax (nmol/L) | T1/2 (h) | AUC(0–8 h) (nmol/L*h) | Cmax (nmol/L) | T1/2 (h) | ||
| (−)-C-O-β-glucuronide | GP | 142.2 ± 33.9a | 65.8 ± 13.4a | 1.5 ± 0.4a | 711.8 ± 35.2*a | 407.8 ± 35.4*a | 0.9 ± 0.2a |
| Mo | 151.2 ± 24.1a | 70.8 ± 9.3a | 1.3 ± 0.2a | 807.4 ± 131.4*a | 394.6 ± 48.7*a | 0.9 ± 0.1a | |
| Po | ND | ND | 179.3 ± 73.9b | 154.5 ± 67.9b | ND | ||
| (−)-EC-O-β-glucuronide | GP | 223.9 ± 58.2a | 91.0 ± 17.5a | 1.3 ± 0.1a | 1063.4 ± 98.3*a | 532.9 ± 29.2*a | 1.1 ± 0.4a |
| Mo | 282.2 ± 31.2a | 110.8 ± 14.4a | 1.6 ± 0.3a | 1445.1 ± 237.4*a | 586.6 ± 78.8*a | 0.9 ± 0.2a | |
| Po | ND | ND | ND | 311.1 ± 138.5b | 251.9 ± 104.2b | ND | |
| 3′-O-Me-(−)-C-O-β-glucuronide | GP | 141.8 ± 31.6a | 44.8 ± 6.0a | 1.8 ± 0.2a | 220.3 ± 51.5*a | 68.0 ± 15.5*a | 1.3 ± 0.3a |
| Mo | 183.2 ± 25.3a | 42.8 ± 5.1a | 3.1 ± 0.3b | 938.5 ± 598.7*b | 177.4 ± 70.4*b | 2.4 ± 0.7b | |
| Po | ND | ND | ND | 6.5 ± 6.5c | 8.7 ± 8.7c | ND | |
| 3′-O-Me-(−)-EC-O-β-glucuronide | GP | 187.7 ± 42.5a | 51.9 ± 5.9a | 2.0 ± 0.2a | 241.7 ± 64.6*a | 72.4 ± 16.8*a | 1.4 ± 0.3a |
| Mo | 216.0 ± 32.2a | 46.7 ± 4.4a | 2.8 ± 0.2b | 952.0 ± 513.9*b | 184.2 ± 66.3*b | 2.4 ± 0.3b | |
| Po | ND | ND | ND | 7.3 ± 7.3c | 9.7 ± 9.7c | ND | |
Doses of GP, Mo and Po were administered as a single intragastric gavage as described in the Materials and Methods section. AUC(0–8 h), Plasma area under the curve; Cmax, maximum plasma concentration; T1/2, elimination half-life; ND, not determined.
a,b,cSignificant difference between single-dose treatments (p < 0.05).
*Significant difference (p < 0.01) between single acute dose and repeated dose parameters.
Assessment of metabolite accumulation in rat brain tissue following 10 d of repeated dosing
A central issue in drug development for the CNS is whether the drug crosses the blood–brain barrier and whether sufficient concentrations of the drug reach the brain. We found trace contents (below levels for quantification) of free C and EC, but much higher contents of C and EC phase II metabolites (both O-β-glucuronides and O-Me-β-glucuronides) in the brain following repeated dosing with GP or Mo. The calculated total C and EC metabolite levels in the brain are 316.7 and 363.5 pmol/g following GP or Mo treatment, respectively (Fig. 2B). Consistent with plasma PK data, we found much lower levels of total monomeric metabolites (∼19.2 pmol/g) in the brain following Po treatment (Fig. 2B). Figure 2B, inset, shows the MRM traces for C/EC-O-β-glucuronides (465.1→289.1 m/z) and Me-C/EC-O-β-glucuronides (479.1→303.1 m/z). Interestingly, higher concentrations of methylated C and EC compared with simple glucuronide derivatives were found in the brain following GP or Mo treatment, indicating either a preferential accumulation or metabolism of these methylated metabolites from the blood, or potential differences in the kinetics of transferring C and EC metabolite across the blood–brain barrier.
Biosynthetic 3′-O-Me-EC-Gluc improves basal synaptic transmission and LTP in hippocampal slices from AD mice
We next explored whether PAC metabolites that accumulated in the brain might contribute to the benefit of Mo treatment in terms of improving cognitive function in Tg2576 AD mice. We selected 3′-O-Me-EC-Gluc for initial bioactivity studies based on evidence suggesting a potential role of EC in promoting cognitive function (van Praag et al., 2007) and that high contents of 3′-O-Me-EC-Gluc are found in the brain following long-term treatment with Mo (Fig. 2B). We explored whether 3′-O-Me-EC-Gluc might modulate LTP, which is one of the major cellular mechanisms known to play a key role in learning and memory functions (Bliss and Collingridge, 1993).
We biosynthetically generated 3′-O-Me-EC-Gluc by incubating commercially available 3′-O-Me-EC with recombinant human uridine diphosphate (UDP) glucuronosyltransferase 1 (UGT1A9) in the presence of UDP-glucuronic acid, and then HPLC purified the resultant 3′-O-Me-EC-Gluc (Fig. 2C). The authenticity of our biosynthetic 3′-O-Me-EC-Gluc is validated by (1) its coelution with 3′-O-Me-EC-Gluc found in vivo (Fig. 2D, left), (2) matching in-line MS-TOF spectra to in vivo metabolites (Fig. 2D, right), and (3) NMR analysis confirming the molecular structure.
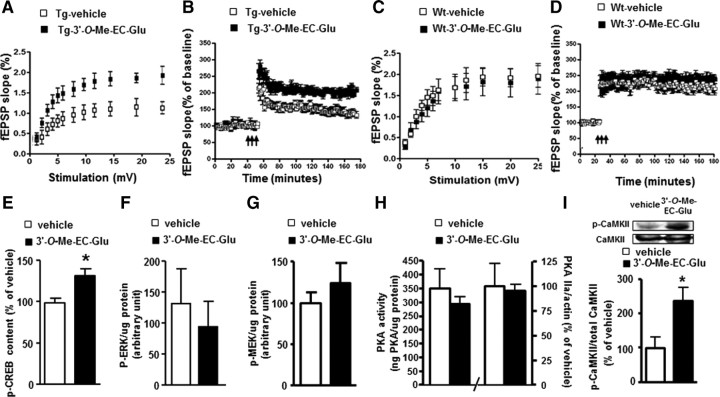
We treated hippocampal slices from old Tg2576 mice that had demonstrated deficits both in basal synaptic transmission and LTP with 300 nm concentration of the biosynthetic 3′-O-Me-EC-Gluc. We found 3′-O-Me-EC-Gluc treatment significantly increased basal synaptic transmission in the CA1 region of hippocampal slices compared with vehicle-treated control slices (p < 0.01; Fig. 3A). Treatment with 3′-O-Me-EC-Gluc resulted in a significantly increased LTP, expressed as a percentage of baseline fEPSP slope compared with the vehicle treatment (225 ± 15% vs 145 ± 12%, p < 0.01; Fig. 3B).
Figure 3.
Biosynthetic 3′-O-Me-EC-5-O-β-glucuronide improves basal synaptic transmission and long-term potentiation coinciding with increased CREB hyperphosphorylation. A–D, The effect of 300 nm 3′-O-Me-EC-Gluc treatment on basal neuronal transmission and LTP in hippocampal slices from AD (A, B) and wild-type (Wt) mice (C, D). Arrow indicate the beginning of tetanus to induce LTP. E–I, The effect of 3′-O-Me-(-)-EC-5-O-β-glucuronide on CREB signaling pathway in hippocampal slices from old Tg2576 mice following 5 h 300 nm 3′-O-Me-EC-Gluc treatment. The levels of phosphor-proteins of CREB phosphorylation at Ser133 (E), Erk1/2 phosphorylation at Thr185/Tyr187 (F), MEK phosphorylation at Ser222 (G). H, I, PKA activity and protein content of PKA IIa subunit expression (H) and phosphor-CaMKII and total CaMKII (I) in brain slices from Tg2576 mice. Inset, representative Western blot image of P-CaMKII and total CaMKII. *p < 0.05, n = 5 per group.
In parallel control studies using brain slices derived from age-matched wild-type mice, we found that treatment with 3′-O-Me-EC-Gluc did not have any effect on basal synaptic transmission or on LTP (Fig. 3C,D).
Our observations revealed, for the first time, that a brain-targeted PAC metabolite is capable of restoring the strength of synaptic transmission and synaptic plasticity in the hippocampal formation, a brain region that is central to normal cognitive function as well as cognitive impairments in AD.
3′-O-Me-EC-Gluc improves LTP through cAMP response element binding protein signaling
We continued to explore the molecular mechanism by which 3′-O-Me-EC-Gluc promotes LTP. Based on evidence that the cAMP response element binding protein (CREB) signaling pathway is critical for LTP and memory formation (Bartsch et al., 1998), we assessed the effect of 3′-O-Me-EC-Gluc on the regulation of the CREB signaling pathway. Using an ELISA-based assay, we quantified the level of phosphorylated [Ser133]-P-CREB as a direct reflection of CREB activation in the hippocampal formation. We found that treatment of hippocampal slices with 300 nm 3′-O-Me-EC-Gluc significantly increased levels of [Ser133]-phosphorylated active CREB levels compared with vehicle-treated control slices (p < 0.05; Fig. 3E). Increased phosphorylation and activation of CREB by 3′-O-Me-EC-Gluc was independently confirmed by Western blot analysis using an antibody specific to [Ser133]-P-CREB (data not shown).
Multiple pathways can lead to the phosphorylation and activation of CREB including extracellular signal-related protein kinase/mitogen-activated protein (Erk/MAP) kinase pathway, protein kinase A (PKA) and Ca2+/calmodulin-dependent protein kinases (CaMKs). Using a multiplex pathway signaling ELISA assay, we found that 3′-O-Me-EC-Gluc treatment did not modulate the phosphorylation status of Erk1/2 (Thr185/Tyr187) or MEK (Fig. 3F,G); nor did it affect PKA (both by PKA activity assay and PKA IIa protein content; Fig. 3H). However, examination of CaMKII by Western blot analysis showed that 3′-O-Me-EC-Gluc treatment significantly increased the level of [Thr286]-phosphorylated active CaMKII without influencing the level of total CaMKII (Fig. 3I), suggesting that 3′-O-Me-EC-Gluc-modulated CREB activation is, in part, mediated by the CaMKII signaling pathway.
Discussion
Age-related dementia, including AD, is one of the most persistent and devastating disabilities in the ever-aging population. In recent years, there has been an increasing interest in the potential value of polyphenolic compounds for preventing and/or treating AD. However, due to the complexity of these compounds and a limited understanding of their bioactivity, absorption, metabolism, and distribution to brain tissues, the development of effective polyphenolic compounds suitable for clinical application has been rather limited.
In this study, we showed that fractions of GP, namely Mo and Po, in vitro, interfere with the generation of soluble, neurotoxic Aβ oligomer species implicated in neuronal dysfunction in AD (Funke et al., 2007; Selkoe, 2008). However, in vivo studies revealed that only Mo is capable of improving spatial memory function and reducing Aβ-mediated neuropathology in the brain following oral administration. This is mainly due to the fact that polyphenolic components and metabolites from Mo are bioavailable, while these components from Po are largely not bioavailable.
Pharmacokinetic studies illustrated that the primary circulating forms of polyphenols from Mo are C and EC monomeric glucuronides and methylated glucuronide metabolites. We demonstrated that repeated dosing of Mo resulted in the accumulation of C and EC metabolites in the brain with concentrations reaching >300 pmol/g. Moreover, a biosynthetic brain-targeted PAC metabolite, 3′-O-Me-EC-Gluc, at a physiologically relevant concentration, can significantly improve basal synaptic transmission and maintenance of LTP through mechanisms associated with activation of CREB signaling, a pathway involved in synaptic plasticity essential for learning and memory (Chrivia et al., 1993; Bartsch et al., 1998).
Our observation of C/EC-glucuronide metabolites in both plasma and perfused brain tissues in this study is consistent with observations by Abd El Mohsen and colleagues (2002, 2006) and Milbury and Kalt (2010), who have previously identified flavonoid glucuronides in perfused brain tissues of rodents and pigs. It is also well known that catechins can be metabolized to sulfate conjugates, which are often seen in urine and to a limited extent in blood and select tissues. However, to date, catechin sulfate metabolites have not been reported in brain tissues. In the present study, sulfate derivatives were not observed as major metabolites in the brain but it is possible that a small amount of sulfanated metabolites present and contribute to the benefits we observed in our study. Moreover, C/EC-glucuronide metabolites in the plasma might also have some beneficial impact on the brain through peripheral mechanisms, e.g., increases blood flow. These areas merit further investigation.
In conclusion, our studies suggest that chronic Mo treatment results in the accumulation of bioactive metabolites in the brain that are capable of reducing pathology and restoring neuronal function associated with learning and memory in the AD brain. Furthermore, our studies provide the first experimental evidence that a biosynthetic PAC metabolite can restore basal synaptic transmission and LTP. Future studies will clarify the identity of other specific bioactive PAC metabolites and their mechanisms of action, and will establish a scientific basis for the development of novel phytotherapeutics in the treatment of AD and other forms of dementia.
Notes
Supplemental material for this article is available at http://www.mountsinai.org/static_files/MSMC/Files/Faculty%20Profile%20Pdfs/Wang%20et%20al%20supplementary%20figures.pdf. Supplemental data for in vitro PICUP assay, images of brain amyloid and NMR data for the epicatechin metabolite. This material has not been peer reviewed.
Footnotes
This work was supported by grants from the NIH (PO1AT004511) and Department of Veterans Affairs to G.M.P., the Jim Easton Consortium for Alzheimer's Drug Discovery and Biomarkers at University of California Los Angeles to D.B.T., NIH Grant AG027818 to D.B.T., and the Samuel Roberts Noble Foundation (R.A.D.).
References
- Abd El Mohsen MM, Kuhnle G, Rechner AR, Schroeter H, Rose S, Jenner P, Rice-Evans CA. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med. 2002;33:1693–1702. doi: 10.1016/s0891-5849(02)01137-1. [DOI] [PubMed] [Google Scholar]
- Abd El-Mohsen M, Bayele H, Kuhnle G, Gibson G, Debnam E, Kaila Srai S, Rice-Evans C, Spencer JP. Distribution of [3H]trans-resveratrol in rat tissues following oral administration. Br J Nutr. 2006;96:62–70. doi: 10.1079/bjn20061810. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Feng WY. Metabolism of green tea catechins: an overview. Curr Drug Metab. 2006;7:755–809. doi: 10.2174/138920006778520552. [DOI] [PubMed] [Google Scholar]
- Funke SA, Birkmann E, Henke F, Görtz P, Lange-Asschenfeldt C, Riesner D, Willbold D. Single particle detection of Abeta aggregates associated with Alzheimer's disease. Biochem Biophys Res Commun. 2007;364:902–907. doi: 10.1016/j.bbrc.2007.10.085. [DOI] [PubMed] [Google Scholar]
- Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004;114:1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RE, Shah A, Fagan AM, Schwetye KE, Parsadanian M, Schulman RN, Finn MB, Holtzman DM. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiol Dis. 2006;24:506–515. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Milbury PE, Kalt W. Xenobiotic metabolism and berry flavonoid transport across the blood–brain barrier. J Agric Food Chem. 2010;58:3950–3956. doi: 10.1021/jf903529m. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, Ehrhart J, Townsend K, Zeng J, Morgan D, Hardy J, Town T, Tan J. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura E, Andrés-Lacueva C, Jáuregui O, Badia E, Estruch R, Izquierdo-Pulido M, Lamuela-Raventós RM. Rapid liquid chromatography tandem mass spectrometry assay to quantify plasma (−)-epicatechin metabolites after ingestion of a standard portion of cocoa beverage in humans. J Agric Food Chem. 2005;53:6190–6194. doi: 10.1021/jf050377u. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Zhang C, Pasinetti G, Dixon R. Fractionation of grape seed proanthocyanidins for bioactivity assessment. In: Gang DR, editor. The biological activity of phytochemicals. NY: Springer; 2011. pp. 33–46. [Google Scholar]
- Tsang C, Auger C, Mullen W, Bornet A, Rouanet JM, Crozier A, Teissedre PL. The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of a grape seed extract by rats. Br J Nutr. 2005;94:170–181. doi: 10.1079/bjn20051480. [DOI] [PubMed] [Google Scholar]
- van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, Yip E, Afanador M, Schroeter H, Hammerstone J, Gage FH. Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci. 2007;27:5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, Maniar K, Dolios G, Wang R, Hof PR, Pasinetti GM. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer's disease. FASEB J. 2005;19:659–661. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Chen L, Zhao Z, Zhao W, Qian X, Humala N, Seror I, Bartholomew S, Rosendorff C, Pasinetti GM. Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J Clin Invest. 2007;117:3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ho L, Zhao W, Ono K, Rosensweig C, Chen L, Humala N, Teplow DB, Pasinetti GM. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer's disease. J Neurosci. 2008;28:6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]