Abstract
Staphylococcus aureus and other staphylococci cause severe human disease, and there are currently no vaccines available. We evaluated whether manganese transport protein C (MntC), which is conserved across the staphylococcal species group, could confer protection against S. aureus and Staphylococcus epidermidis. In vivo analysis of S. aureus MntC expression revealed that expression occurs very early during the infectious cycle. Active immunization with MntC was effective at reducing the bacterial load associated with S. aureus and S. epidermidis infection in an acute murine bacteremia model. Anti-MntC monoclonal antibodies have been identified that can bind S. aureus and S. epidermidis cells and are protective in an infant rat passive protection model and induce neutrophil respiratory burst activity. This is the first description of a protein that has the potential to provide protection across the staphylococcal species group.
Staphylococci cause diseases ranging from relatively mild superficial skin infections to life-threatening conditions, including sepsis and endocarditis. Increasingly, staphylococci have developed antibiotic resistance, reducing treatment options and highlighting the need for an effective prophylactic vaccine. Preclinical studies have assessed several antigens that, either alone [1, 2] or in combination [3], have the ability to reduce staphylococcal disease in preclinical models. Staphylococcal strains are known for the phenotypic plasticity of their antigenic repertoire, which provides mechanisms for both survival in diverse host niches and for immune evasion. Thus the development of a broadly protective vaccine that can prevent different diseases caused by diverse strains from within the species group poses challenges.
S. aureus distinguishes itself from other staphylococci by an impressive array of virulence factors [4] and the production of coagulase. S. epidermidis also causes disease with associated mortality [5, 6]. S. aureus and S. epidermidis share a core genome representing approximatly two thirds of their genes; the proteins encoded by the core genesaverage 70% amino acid sequence identity between the two organisms [4]. We were interested in evaluating proteins with the potential to prevent staphylococcal disease caused by either S. aureus or S. epidermidis.
Effective bacterial vaccine components should be conserved and perform important functions during infection. Initial attempts to identify S. aureus vaccine candidates came from assessment of surface antigens from bacteria grown in vitro [7]. Advances in in vivo technology have led to a better understanding of bacterial antigen expression during infection, enhancing our knowledge of host-pathogen relationships. When S. aureus and other pathogenic bacteria invade, they initiate expression of multiple virulence pathways. S. aureus produces capsular polysaccharide to avoid attack from the innate immune system. It also expresses various ligand-binding proteins with roles ranging from immune cloaking [8] to scavenging essential ions [9]. One group of proteins associated with in vivo survival of S. aureus are the manganese transport (Mnt) proteins. The Mnt complex is an ABC transporter composed of an ATP-binding protein (MntA), an integral membrane transporter (MntB), and a manganese binding surface lipoprotein (MntC) [10]. Recently MntC was identified as being expressed on the cell surface of S. aureus in biofilms generated in in vivo models of infection [11]. The orthologous protein in S. epidermidis is staphylococcal iron transport C (SitC) [12], which was detected in convalescent-phase serum from rabbits infected with S. epidermidis and found to be protective in a S. epidermidis murine kidney abscess model [13].
A critical attribute for an effective staphylococcal vaccine antigen is early expression during infection, which provides an opportunity for vaccine-induced antibodies to inactivate bacteria before infection is firmly established. We therefore evaluated S. aureus MntC temporal expression in vivo. S. aureus MntC and S. epidermidis SitC have a high level of sequence identity (72%), and polyclonal antisera have been demonstrated to be cross-reactive [13]. We explored whether S. aureus MntC could produce cross-protective antibodies against both S. aureus and S. epidermidis infection.
MATERIALS AND METHODS
Bacterial Strains
S. aureus strains Mu50 and Newman were used for cloning and MntC flow cytometry studies, respectively. The S. aureus strains used for in vivo evaluations are listed in Table 1. S. aureus clinical isolates described in [14] were used to determine mntC sequence variability. S. epidermidis strain 0–47 [15] was used to assess cross protection. Staphylococcal cultures were grown in a Chelex-treated TSB medium for MntC/SitC in vitro expression.
Table 1.
Characteristics of Staphyloccocus aureus Isolates Used for This Investigation and Detection of Manganese Transport Protein C (MntC), IsdA, and IsdB During Bacteremia
| CP8 strain, methicillin resistance, ST/CC |
CP5 strain, methicillin resistance, ST/CC |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0003 | 0182 | 0101 | 0186 | 0155 | 0210 | 0158 | 0140 | Reynolds | 0212 | ||
| Yes | No | Yes | No | No | Yes | No | No | No | Yes | ||
| Antigen | Time (h) | 88/78 | 51/51 | 255/78 | 57/30 | 26/25 | 231/5 | 28/25 | 9/9 | 25/25 | 235/235 |
| MntC | 0 | − | − | − | − | − | − | + | − | − | + |
| 1 | + | − | + | + | + | + | − | + | + | − | |
| 4 | + | + | + | + | + | + | + | + | + | + | |
| 6 | + | + | − | + | + | − | + | + | + | + | |
| IsdB | 0 | − | − | − | − | − | − | − | − | − | − |
| 1 | − | − | − | − | − | + | − | − | − | − | |
| 4 | − | − | − | − | − | − | + | − | − | − | |
| 6 | + | + | + | + | + | + | + | + | + | − | |
| IsdA | 0 | − | − | − | − | − | − | − | − | − | − |
| 1 | − | − | − | − | − | − | − | − | − | − | |
| 4 | − | + | + | − | + | − | − | − | + | − | |
| 6 | + | + | − | − | − | − | − | − | − | + | |
Each S. aureus isolate was used to infect 9 mice. At each time point (1, 4, and 6 hours) after infection, 3 mice were sacrificed, the blood pooled, and the bacteria isolated. The bacteria at the time of challenge (in vitro: T0) and after isolation from the bloodstream (in vivo: T1, T4, and T6) were analyzed by immunofluorescence microscopy. The experiments for each isolate were repeated 3 times.
Abbreviations: CC, clonal complex; ST, sequence type; +, antigen was expressed by majority of the bacterial cells; −, undetectable antigen expression on bacterial cells.
Cloning of Recombinant Antigens
mntC (SAV0631) from S. aureus Mu50 chromosomal DNA was amplified by polymerase chain reaction (PCR), using primers 5′-TTTCTTCCATGGGTACTGGTGGTAAACAAAGCAG-3′ and 5′-TTTCTTGCTCAGCATTATTTCATGCTTCCGTGTACAG-3′ containing NcoI and BlpI sites, respectively. The PCR product was cloned into NcoI- and BlpI-cleaved pET28a vector (Novagen) to create pLP1215, which was transformed into Escherichia coli strain BLR(DE3) (Novagen) to generate strain PVPR-161, used for recombinant protein expression. IsdB and IsdA were cloned and expressed as described previously [1, 16].
Nucleotide Sequencing
Isolates were grown in tryptic soy broth (5 mL) for 3–5 hours. Pelleted bacteria were resuspended in 200 µL lysis buffer (50 mM Tris-HCl pH 7.5, 145 mM NaCl, 0.1 mg/mL lysostaphin), and genomic DNA was purified using DNeasy Blood and Tissue kit (QIAGEN). Sequencing of mntC was performed using the following primers: 5′-CACAAAATTTACGAATAGAAAGAAACGAG-3′ and 5′-AAAATATTGGAGATACCAATATTTTAGGTTG-3′ (PCR) and 5′-ACAAACATTTATCGATAACGAC-3′ and 5′-GAGCAATGTATTTGTTACCTTG-3′ (sequencing). SAV0631 was used for the reference sequence.
Expression and Purification of Recombinant MntC
Recombinant MntC was produced in E. coli by fed batch fermentation in minimal medium at 35°C. Protein expression was induced by the addition of 0.1 mM IPTG. Cells were harvested by centrifugation and disrupted by microfluidization at 15 000 psi. The soluble fraction was clarified by polyethyleneimine flocculation. Protein was purified using a sulfopropyl sepharose column followed by a phenyl sepharose column. The resulting protein was >95% pure.
Generation of Rabbit Immune Sera
New Zealand White female rabbits (Charles River Canada) were vaccinated intramuscularly at weeks 0, 4, and 9 with 20 μg MntC, IsdA, or IsdB formulated with AlPO4. Serum samples were collected at weeks 0 and 10.
Monoclonal Antibodies
Hybridomas were generated from a fusion of a nonsecreting myeloma cell line (X63Ag8.653, ATCC) with splenocytes that were harvested from mice immunized with purified recombinant MntC. Anti-MntC–secreting hybridomas were selected by MntC protein and SitC protein enzyme-linked immunosorbant assays, using standard procedures, and flow cytometry.
Flow Cytometry
S. aureus strain Newman was grown in Chelexed TSB medium for 18 hours. Bacteria were killed (60°C, one hour), then fixed in 1% paraformaldehyde. Bacteria were then blocked in heat-inactivated porcine sera (10%)(PS)/Phosphate buffered saline (PBS) (JR Scientific Inc), then transferred to a 96-well U-bottomed polystyrene plate (100 µL per well). Cells were washed with porcine serum in phosphate-buffered saline (PBS) prior to adding anti-MntC monoclonal antibodies or an isotype control (Jackson Immunoresearch). Cells were then washed in PBS and incubated on ice with biotinylated goat anti-mouse immunoglobulin G (IgG; Jackson Immunoresearch). Cells were washed with PBS and incubated on ice with streptavidin-phycoerythrin (BD Biosciences). After a final wash step, the cell pellets were resuspended in 1% paraformaldehyde. A total of 20 000 events/well were acquired on a BD LSR II flow cytometer and analyzed using FlowJo software (Treestar). The mean fluorescence intensity (MFI) was determined for each sample after gating on bacteria. An MFI was considered positive if it was ≥3 times that of the control antibody MFI and >100.
Epitope Mapping by Surface Plasmon Resonance
Interference epitope mapping by surface plasmon resonance with a BIACore instrument was performed as described by Johne et al [17]. Briefly, rat anti-mouse Fc antibodies (Thermo Scientific) were immobilized to CM5 Series S sensor chips (GE Healthcare) with NHS/EDC (GE Healthcare). All monoclonal antibodies (mAbs) and MntC were diluted in HBS-EP (GE Healthcare). After rat anti-mouse IgG immobilization, a 2-μM solution of primary test mAb was injected. Each flow cell bound at least 300 RU of mAbs. Once all 4 flow cells were populated by a specific mAb, unoccupied rat-anti-mouse Fc was blocked using normal mouse serum. MntC (2 μM) was injected into the flow cells and allowed to form a stable MntC-primary mAb complex for 4 minutes. The 4 test secondary mAbs were added to the mAb-MntC complexes, 1 per flow cell, and the level of binding measured in a BIACore T100 (GE Healthcare) and analyzed using BIAevaluation version 4.1 software (GE Healthcare). Monoclonal antibodies were classified as noncompetitive if they yielded an RU of >99.
Monitoring In Vivo Expression During Murine Bacteremia
CD1 mice (Charles River Laboratories, Wilmington, MA) were infected by intraperitoneal injection of approximately 2 × 108 colony-forming units (CFU) of S. aureus grown to OD600 of approximately 2.5 in tryptic soy broth (Sigma Chemical Company, St. Louis, MO). Mice were exsanguinated at 1, 4, and 6 hours after infection, and blood from 3 mice was pooled into ice-cold 0.4% sodium citrate, pH 7.0. Eukaryotic cells were lysed by sonication. The bacteria were washed with PBS and were deposited on microscope slides. Slides were incubated overnight at 4°C with primary rabbit antibodies at 5 µg/mL and detected with ALEXA488-conjugated goat-α-rabbit antibody (1:250, Invitrogen, Carlsbad, CA). A coverslip was then mounted with VectaShield HardSet medium (Vector Laboratories, Burlingame, CA). Images were obtained using an Olympus BX60 light/fluorescent microscope with a 60× oil immersion objective and EM-CCD Hamamatsu camera mounted in the optical path of the microscope (Olympus, Center Valley, PA) for both DIC and fluorescence. MetaMorph Software (Universal Imaging, Sunnyvale, CA) was used to acquire images.
Murine Bacteremia Model
Groups of 10 CD-1 mice (Charles River Laboratories, Wilmington, MA) were vaccinated at weeks 0, 3, and 6 with 10 µg MntC + 22 µg AlPO4, followed by intraperitoneal challenge on week 8 with 5 × 108 CFU S. aureus Reynolds or S. epidermidis O-47. Animals were exsanguinated 3 hours after challenge, and serial dilutions of blood were plated to enumerate recovered bacteria. Statistical significance was determined via the Student t test, and a P value of ≤.05 was considered significant.
Respiratory Burst Assay
HL-60 cells (40 000) were differentiated with dimethylformamide and mixed with S. aureus PFESA PFESA0186 or S. epidermidis 0–47 cells (2000 CFU), rabbit complement (Pel-Freez, Rogers, AZ), and test mAb (10 µg). For the specificity test, recombinant MntC (20 µg) was also added. Kinetic respiratory burst activity was measured every 5 minutes for up to 90 minutes by luminol (TCI America, Portland, OR) chemiluminescence in a Luminoskan Ascent (Thermo Scientific, Waltham, MA).
Infant Rat Passive Immunization Model
Groups of Sprague-Dawley infant rats (Charles River Laboratories, Wilmington MA) were immunized intraperitoneally with 0.4 mg of either test mAb or isotype control mAb. Sixteen hours after immunization, rats were challenged intraperitoneally with 1 × 108 CFU of a S. aureus clinical isolate, PFESA0140. Four hours later, blood was collected, and serial dilutions were plated to enumerate recovered bacteria. Statistical significance was determined via the Student t test, and a P value of ≤.05 was considered significant.
All animal studies were conducted according to Institutional Animal Care and Use Committee guidelines.
RESULTS
MntC Is Highly Conserved Among Diverse S. aureus Clinical Isolates
We evaluated the MntC distribution and sequence heterogeneity from 3 collections of S. aureus clinical isolates (n = 289) that define the breadth of S. aureus disease-causing strains. The first collection represented the diversity of both current and historical S. aureus isolates, with an emphasis on maximizing the diversity of multilocus sequence typing (MLST) lineages; the second collection represented a prevalence set of recently circulating clinical isolates from the Centers for Disease Control and Prevention [14, 18]; an additional 65 strains from Pfizer's collection were also included. Overall, 90 different sequence types in >25 MLST clonal complexes were represented. MntC was found to be highly conserved: only 8 different protein sequence variants were identified, and a single variant (MntC_001, the vaccine antigen) was found in 88% of these strains. One isolate (NCTC8325) had an insertion of 3 amino acids at residue 190, and the other 6 sequences differed from the reference sequence by ≤2 amino acids. We also investigated the distribution and divergence of MntC orthologs among other Staphylococcus species (Table 2). The MntC orthologs were somewhat conserved, with an average protein pairwise identity of 75%, ranging from 66% to 94%. The 2 closest orthologs were present in Staphyloccocus hominis and Staphyloccocus haemolyticus, the most distant was in Staphylococcus pseudintermedius. SitC, the S. epidermidis ortholog of MntC, was 77% identical to S. aureus MntC.
Table 2.
Comparison of Sequence Divergence for Manganese Transport Protein C (MntC) Orthologs in Staphyloccocus Species
| S. aureus (MntC) | S. carnosus | S. saprophyticus | S. capitus | S. caprae | S. epidermidis (SitC) | S. haemolyticus | S. hominis | S. lugdunensis | S. pseudintermedius | S. warneri | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus (MntC) | 100 | 79 | 75 | 76 | 76 | 77 | 76 | 76 | 76 | 66 | 78 |
| S. carnosus | … | 100 | 75 | 73 | 73 | 72 | 72 | 71 | 68 | 69 | 73 |
| S. saprophyticus | … | … | 100 | 75 | 75 | 73 | 72 | 72 | 70 | 65 | 72 |
| S. capitus | … | … | … | 100 | 100 | 84 | 75 | 77 | 75 | 67 | 76 |
| S. caprae | … | … | … | … | 100 | 84 | 75 | 77 | 75 | 67 | 76 |
| S. epidermidis (SitC) | … | … | … | … | … | 100 | 75 | 75 | 72 | 67 | 76 |
| S. haemolyticus | … | … | … | … | … | … | 100 | 94 | 76 | 68 | 77 |
| S. hominis | … | … | … | … | … | … | … | 100 | 75 | 67 | 77 |
| S. lugdunensis | … | … | … | … | … | … | … | … | 100 | 64 | 72 |
| S. pseudintermedius | … | … | … | … | … | … | … | … | … | 100 | 68 |
| S. warneri | … | … | … | … | … | … | … | … | … | … | 100 |
Data are pair-wise percentage identities between MntC orthologs and the reference sequence SAV0631 from S. aureus Mu50. Proteins used were ZP_04677758.1 (S. warneri L37603), ZP_03613094.1 (S. capitis SK14), YP_002633371.1 (S. carnosus subspecies carnosus TM300), YP_252056.1 (S. haemolyticus JCSC1435), ZP_04060318.1 (S. hominis SK119), YP_302178.1 (S. saprophyticus subspecies saprophyticus ATCC 15305), YP_003472410.1 (S. lugdunensis HKU09-01), ADX77369.1 (S. pseudintermedius ED99) and ZP_07840309.1 (S. caprae C87).
Abbreviation: SitC, staphylococcal iron transport C.
MntC Is Expressed on the Cell Surface In Vivo Early During Infection
The in vivo expression of S. aureus ion-scavenging proteins MntC, IsdA, and IsdB was investigated in a murine bacteremia model, using 10 S. aureus isolates. Bacteria were harvested at various time points after infection, and antigen expression was assessed without further culture by immunofluorescence microscopy. The antigens were sparsely expressed, if at all, by the in vitro grown cells used to infect the mice. Analysis of the temporal in vivo antigen expression revealed that MntC was rapidly upregulated in vivo during bacteremia, with 7 of 10 strains expressing MntC on the surface by 1 hour and all strains expressing MntC by 4 hours. In contrast, only 1 strain expressed IsdB 1 hour after infection, and no strains tested expressed IsdA at this time point (Table 1; Figure 1).
Figure 1.
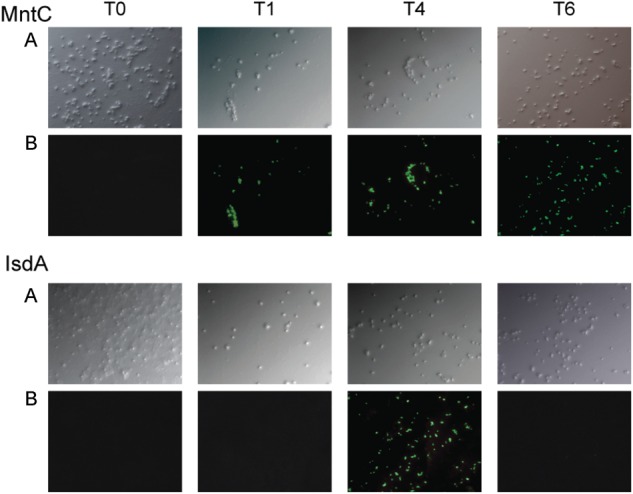
Visualization of antigen expression by Staphylococcus aureus PFESA0155 during bacteremia. In this strain, manganese transport protein C (MntC) is expressed rapidly after infection and remains expressed throughout the experiment. In contrast, IsdA expression is delayed and not continuous throughout the experiment. The expression profiles for additional clinical isolates are given in Table 1. Three groups of 3 mice each were infected by intraperitoneal injection of 5 × 108 colony-forming units of the S. aureus clinical isolate PFESA0155. At 1, 4, and 6 hours after infection, one group was sacrificed, blood from the animals was pooled, and the bacteria were isolated. Bacteria at the time of challenge (T0) and bacteria isolated from the bloodstream (T1, T4, and T6) were stained with affinity-purified rabbit anti-MntC, anti-IsdA, or control immunoglobulin G and visualized with a fluorescent microscope. A, Differential interference contrast (DIC) images of anti-MntC smears. B, Fluorescence microscopy images of MntC expression. C, DIC images of anti-IsdA smears. D, Fluorescence microscopy images of IsdA expression.
Active Vaccination With MntC Reduces S. aureus and S. epidermidis Bacteremia in a Murine Bacteremia Model
Active immunization experiments were conducted to determine whether a MntC vaccine could reduce S. aureus bacteremia in mice. Mice were vaccinated 3 times with MntC and then challenged with S. aureus Reynolds. A significant reduction in the number of bacteria recovered from the blood 3 hours after challenge was observed. The experiment was repeated an additional 9 times. In all experiments, fewer bacteria were recovered from the blood of MntC-vaccinated animals than from the blood of controls (Table 3). For 6 of the 10 experiments, this reduction was statistically significant. Meta-analysis of all 10 experiments demonstrated that vaccination with MntC significantly reduced bacteremia, compared with observations for controls (P < .0001).
Table 3.
Immunization With Manganese Transport Protein C Reduces Recovered Colony-Forming Units (CFU) in a Murine Bacteremia Model
| Challenge Staphylococcal Organism, Experiment | No. of Animals | Log CFU Reduction of Mean vs Control Mean | P |
|---|---|---|---|
| S. aureus | |||
| 1 | 10 | 1.2 | .0056 |
| 2 | 10 | 1.6 | .0002 |
| 3 | 10 | 0.81 | .0544 |
| 4 | 10 | 1.38 | .0055 |
| 5 | 10 | 0.47 | .1367 |
| 6 | 10 | 0.83 | .0090 |
| 7 | 10 | 0.74 | .0444 |
| 8 | 10 | 0.75 | .0180 |
| 9 | 10 | 0.41 | .3066 |
| 10 | 10 | 0.59 | .0882 |
| Meta-analysis | 100 | 0.955 | <.0001 |
| S. epidermidis | |||
| 1 | 10 | 0.87 | .0291 |
| 2 | 10 | 1.02 | .0229 |
| Meta-analysis | 20 | 0.95 | .0013 |
Groups of female (5–6-week-old) CD1 mice were vaccinated by subcutaneous injection with either saline or 10 µg MntC in 22 μg AlPO4 as adjuvant. Two weeks after the last immunization, mice were challenged by intraperitoneal injection of approximately 5 × 108 CFU of either S. aureus strain Reynolds or S. epidermidis 0-47. Three hours after challenge, animals were sacrificed and the bacteria in the blood enumerated. Experiments with statistically significant results are in bold.
MntC and its S. epidermidis ortholog SitC are 77% identical, and sera against SitC can cross-react with MntC [13]. Therefore, the effect of immunization with MntC against heterologous S. epidermidis challenge was also explored in the bacteremia model. Mice were vaccinated 3 times with MntC and then challenged intraperitoneally with S. epidermidis 0–47. Immunization with MntC resulted in a significant reduction in bacterial blood levels for 2 independent studies (Table 3). This demonstrates that MntC could potentially cross-protect against S. epidermidis infection.
Demonstration of MntC Antibody-Mediated Protection
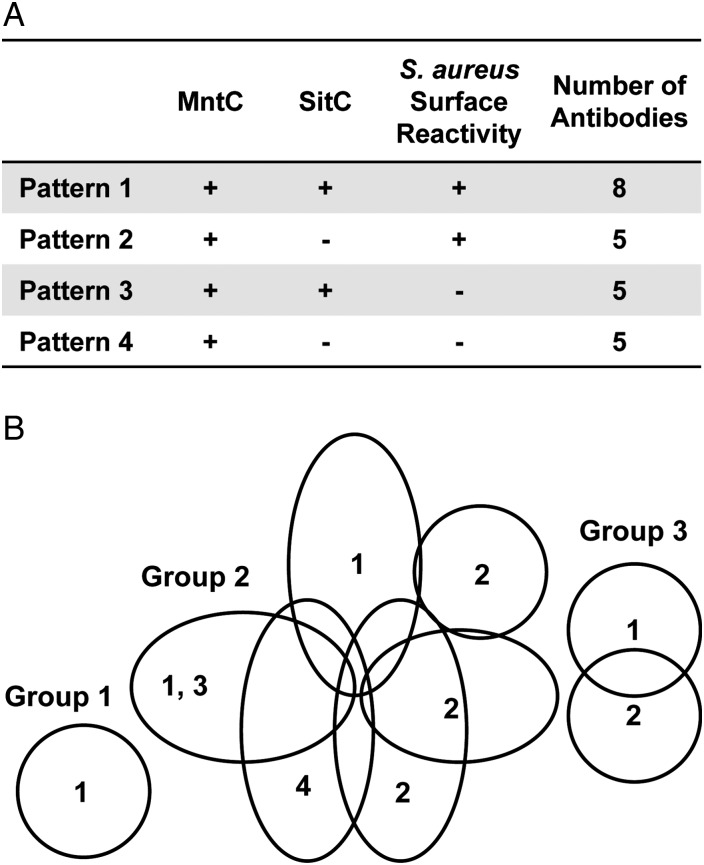
Murine anti-MntC mAbs were made using the MntC vaccine. These antibodies fell into 1 of 4 patterns: (1) cross-reactive to both MntC and SitC protein and binding to native protein on the cell surface, (2) reactive to MntC only and binding to native protein on the cell surface, (3) cross-reactive to both MntC and SitC, with no binding to surface-exposed epitopes, and (4) reactive to MntC only with no binding to surface-exposed epitopes (Figure 2). A representative selection of mAbs from each of the 4 patterns was evaluated by BIACore to determine antigen-binding interference groups (Figure 2). A sandwich approach was used, in which the primary test mAb was attached to the chip and used to capture MntC and then a secondary mAb was applied. This established whether the 2 mAbs recognized the same or different regions of the protein. The antibodies were grouped into 3 interference groups representing 3 independent mAb-binding regions. These interference groups all contained mAbs from pattern 1 that could recognize MntC on the cell surface of S. aureus and cross-reacted with the S. epidermidis ortholog SitC. Monoclonal antibodies that did not recognize the native MntC antigen on the cell surface of S. aureus and therefore did not recognize relevant epitopes could not be mapped by Biacore because they were unable to capture the protein when presented in a soluble matrix, underscoring the importance of monitoring antibody binding to native antigens on the bacterial cell surface.
Figure 2.
Characterization of anti–manganese transport protein C (MntC) monoclonal antibodies (mAbs). Reactivity patterns of anti-MntC mAbs (A) and binding interference patterns detected by BIAcore (B). A, The 23 mAbs generated were grouped into 4 distinct patterns, by reactivity to MntC and SitC protein in enzyme-linked immunosorbant assays, and to MntC on the cell surface of Staphyloccocus aureus, by flow cytometry. B, The overlapping ellipses display interference by BIACore analysis. Pattern numbers of the mAbs are indicated within the ellipses, which represent antibody interference space and are arbitrary in size. The 3 interference groups are identified.
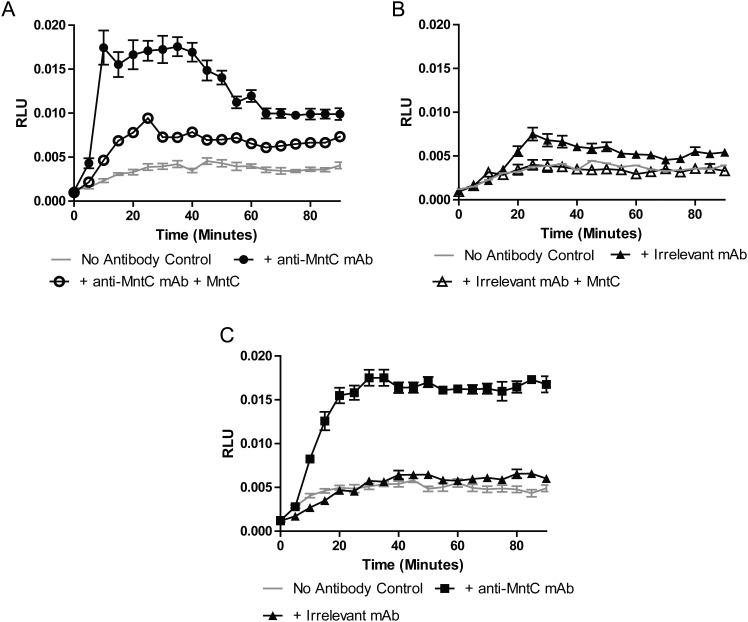
Both an in vivo passive protection model and an in vitro respiratory burst assay were used to assess the functionality of anti-MntC antibodies. Antibodies from all 3 binning groups were tested for their ability to be protective in the infant rat model and were able to reduce the bacteremia, compared observations for controls (Table 4; data not shown for interference group1). Enhanced neutrophil respiratory burst activity was observed for the anti-MntC mAbs using both S. aureus and S. epidermidis strains; this was abrogated by preincubation with MntC, indicating that the response is specific to antibody binding to MntC (Figure 3).
Table 4.
Passive Immunization With Anti–Manganese Transport Protein C Monoclonal Antibodies Significantly Reduces Recovered Colony-Forming Unites (CFU) in an Infant Rat Model of Infection
| Passive Immunization (Interference Group) | No. of Animals | Mean (95% CI) Log CFU/mL Blood | P vs IgG1 Control |
|---|---|---|---|
| 305-78-7 (2) | 10 | 3.40 (2.73–4.05) | .0053 |
| 305-101-8 (3) | 10 | 3.64 (3.13–4.14) | .0082 |
| IgG1 control | 10 | 4.40 (4.12–4.67) | |
| PBS | 10 | 4.40 (4.04–4.76) | NS |
The rats were passively immunized by intraperitoneal injection with either saline or 0.4 mg of monoclonal antibodies 16 hours before they were challenged by intraperitoneal injection with approximately 1 × 108 CFU of Staphyloccocus aureus PFESA0140. At 4 hours after challenge, the animals were sacrificed and the bacteria in the blood enumerated. The antibody mAB interference groups as identified by BIACore are noted.
Abbreviations: CI, confidence interval; IgG1, immunoglobulin G1; NS, not significant; PBS, phosphate-buffered saline.
Figure 3.
Anti–manganese transport protein C (MntC) antibody 305-78-7 induces neutrophil respiratory burst activity against both Staphyloccocus aureus and Staphyloccocus epidermidis. HL-60 neutrophil-like cells were incubated with bacteria and a source of complement alone, or with bacteria, complement, and anti-MntC antibodies (closed symbols). Respiratory burst activity was measured by chemiluminescence in a kinetic manner, with reads every 5 minutes. A, The anti-MntC antibody 305-78-7 was able to elicit a robust respiratory burst against S. aureus (closed symbols), which was abrogated by preincubation with MntC protein (open symbols). B, An irrelevant isotype controlled antibody was not able to elicit a robust respiratory burst against S. aureus (closed symbols), and preincubation with MntC protein demonstrated only a minor reduction of respiratory burst activity (open symbols). C, The anti-MntC monoclonal antibody (mAb) 305-78-7 was also able to elicit a respiratory burst against S. epidermidis, compared with an irrelevant control antibody. Abbreviation: RLU, relative light units.
DISCUSSION
Many antigens have been evaluated in the search to find a vaccine with the potential to protect against staphylococcal disease [7]. In this study, we provide preclinical evidence for the inclusion of MntC as a component of a multiantigen prophylactic antistaphylococcal vaccine. Staphylococci are renowned for their ability to evade immune responses, which is attributable to the expression of virulence factors such as polysaccharide capsules that enhance their survival and to the formation of biofilms. Seminal studies by Burke [19] demonstrated that presurgical antibiotic prophylaxis is only effective when administered in a time frame that maintains the drug in the bloodstream prior to and during surgery, the time at which the patient becomes infected with the bacteria. Antibiotic prophylaxis administered after this critical period is less effective in preventing infection. Therefore, we hypothesize that a vaccine will have the highest probability of success if antibodies can engage and eliminate the bacteria during this early phase of infection. The identification of an antigen that induces a protective effect and is expressed early during infection is a critical attribute for an antistaphylococcal vaccine in order to prevent a full-blown infection and bacterial persistence. We have generated evidence that the manganese transporter MntC is such an antigen. Staphylococci require trace elements such as manganese for growth. However, such ions become toxic when present in excess [20], so manganese uptake is highly regulated. While these uptake systems are not expressed during in vitro growth in nutrient-rich conditions, they are rapidly upregulated to aid bacterial survival in vivo. In contrast to MntC, IsdA and IsdB, which are also under evaluation for vaccine development [16, 21, 22], were detected on the cell surface much later in vivo, if at all (Table 1; Figure 1). This is in agreement with the findings of Cheng et al [23], who demonstrated, using isogenic strain sets carrying IsdA or IsdB deletions, that neither protein was required for early infection events but that both were required for events occurring later in infection, such as abscesses formation.
Manganese may also play a role for S. aureus in immune evasion by protecting the bacteria from neutrophil attack, which will occur once antibodies interact with the bacteria in the bloodstream. A primary mechanism for bacterial killing within neutrophils is the generation of superoxides that kill the ingested pathogen. In vitro assays have demonstrated that S. aureus can neutralize these agents with either manganese alone [10] or with superoxide dismutases that require manganese as a cofactor [24, 25]. It is therefore plausible that S. aureus uses these mechanisms to evade killing in the phagosome, upregulating MntC and acquiring manganese early during infection. Anti-MntC antibodies therefore may have the potential to interfere with the bacteria's ability to evade this critical immune defense mechanism, an aspect that warrants further investigation.
Both the MntC vaccine and anti-MntC mAbs were protective in several animal models of infection. To investigate whether the mechanism was related to opsonophagocitic killing, we developed a respiratory burst assay and demonstrated that anti-MntC antibodies bound to S. aureus and S. epidermidis cells can induce a neutrophil respiratory burst that is an important indicator of neutrophil activation and initiation of the bacterial killing cascade. Specificity of the reaction was demonstrated by the addition of recombinant MntC to the reaction, which prevented the binding of the MntC antibodies to the bacteria. The respiratory burst assay was conducted in place of an opsonophagocytic assay (OPA), which measures antibody- and complement-mediated bacterial killing. OPAs require in vitro antigen expression, and MntC is elaborated only under manganese-free conditions that render the cells fragile and susceptible to nonspecific kill, thus precluding the OPA to monitor antibody function. The neutrophil respiratory burst is recognized as an important mechanism for bacterial killing, as evidenced in patients with chronic granulomatous disease, who lack neutrophil respiratory burst activity and are at increased risk of infection [26].
Previous studies had demonstrated that anti MntC antibodies are cross-reactive across different staphylococcal species [13]. The highly conserved nature of this protein family (Table 2) led us to examine the potential for S. aureus protein antigens to cross-protect against S. epidermidis (Table 4). This is the first demonstration of such cross-protection in the literature and offers exciting prospects for future vaccine development for the prevention of staphylococcal diseases.
In recent years, 2 vaccines designed to prevent S. aureus infections have not progressed past phase III clinical trials [7]. The first was a bivalent conjugate polysaccharide conjugate vaccine [27], and the second contained the iron-binding protein IsdB [22]. Both vaccines presented preclinical and early clinical data that suggested efficacy but were not substantiated during phase III studies. A drawback for both of these approaches was that the vaccines were based on a single component (capsular polysaccharide or IsdB), and in vivo expression data have shown that single antigens may not be expressed by all strains [28] or early enough during infection (Table 2) to eliminate bacteria early in the infection cycle. Furthermore, in a preclinical model of infection, Stranger-Jones et al [3] demonstrated that indeed multiple antigens are required for more robust protection. Taking all data together, a vaccine will need to include multiple bacterial antigens to be effective against these challenging pathogens, and antigen expression will need to occur early during infection. We have described the potential usefulness of MntC as a vaccine component on the basis of 4 major attributes of critical importance: early expression during infection, an essential role for the protein in vivo, protection in preclinical models of infection, and potential cross-protection against other staphylococci. MntC is currently in clinical trials as a component of a multiantigen vaccine that includes capsule polysaccharide conjugates and clumping factor A (ClinicalTrials.gov registry number NCT01364571) and has the potential to contribute to the prevention of S. aureus disease, thus fulfilling a significant unmet medical need.
Notes
Acknowledgments. We thank Mark Ruppen (Pfizer) and the Pfizer Vaccines Early Development Group, for providing the protein for immunological evaluation; Alita Miller (Pfizer), for scientific input during the preparation of the manuscript; Jane Broughan (Pfizer), Edward Zito (Pfizer), and Bruce Green (Pfizer), for helpful review; Art Illenburger (Pfizer), for assistance with in vivo studies; and Bret Sellman (formerly employed by Wyeth before Pfizer's acquisition of Wyeth), for his contributions in identifying the vaccine potential of SitC.
Financial support. This work was supported by Pfizer Vaccine Research.
Potential conflicts of interest. All the authors were employees of Pfizer when this work was conducted and as such may be shareholders of company stock.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kuklin NA, Clark DJ, Secore S, et al. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect Immun. 2006;74:2215–23. doi: 10.1128/IAI.74.4.2215-2223.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Josefsson E, Hartford O, O'Brien L, Patti JM, Foster T. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J Infect Dis. 2001;184:1572–80. doi: 10.1086/324430. [DOI] [PubMed] [Google Scholar]
- 3.Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A. 2006;103:16942–7. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gill SR, Fouts DE, Archer GL, et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005;187:2426–38. doi: 10.1128/JB.187.7.2426-2438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piette A, Verschraegen G. Role of coagulase-negative staphylococci in human disease. Vet Microbiol. 2009;134:45–54. doi: 10.1016/j.vetmic.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 6.von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect Dis. 2002;2:677–85. doi: 10.1016/s1473-3099(02)00438-3. [DOI] [PubMed] [Google Scholar]
- 7.Broughan J, Anderson R, Anderson AS. Strategies for and advances in the development of Staphylococcus aureus prophylactic vaccines. Expert Rev Vaccines. 2011;10:695–708. doi: 10.1586/erv.11.54. [DOI] [PubMed] [Google Scholar]
- 8.Burman JD, Leung E, Atkins KL, et al. Interaction of human complement with Sbi, a staphylococcal immunoglobulin-binding protein: indications of a novel mechanism of complement evasion by Staphylococcus aureus. J Biol Chem. 2008;283:17579–93. doi: 10.1074/jbc.M800265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stauff DL, Skaar EP. The heme sensor system of Staphylococcus aureus. Contrib Microbiol. 2009;16:120–35. doi: 10.1159/000219376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horsburgh MJ, Wharton SJ, Cox AG, Ingham E, Peacock S, Foster SJ. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol Microbiol. 2002;44:1269–86. doi: 10.1046/j.1365-2958.2002.02944.x. [DOI] [PubMed] [Google Scholar]
- 11.Brady RA, Leid JG, Camper AK, Costerton JW, Shirtliff ME. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect Immun. 2006;74:3415–26. doi: 10.1128/IAI.00392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cockayne A, Hill PJ, Powell NB, Bishop K, Sims C, Williams P. Molecular cloning of a 32-kilodalton lipoprotein component of a novel iron-regulated Staphylococcus epidermidis ABC transporter. Infect Immun. 1998;66:3767–74. doi: 10.1128/iai.66.8.3767-3774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sellman BR, Howell AP, Kelly-Boyd C, Baker SM. Identification of immunogenic and serum binding proteins of Staphylococcus epidermidis. Infect Immun. 2005;73:6591–600. doi: 10.1128/IAI.73.10.6591-6600.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy E, Lin SL, Nunez L, et al. Challenges for the evaluation of Staphylococcus aureus protein based vaccines: monitoring antigenic diversity. Hum Vaccin. 2011;7(Suppl):51–9. doi: 10.4161/hv.7.0.14562. [DOI] [PubMed] [Google Scholar]
- 15.Heilmann C, Gerke C, Perdreau-Remington F, Gotz F. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect Immun. 1996;64:277–82. doi: 10.1128/iai.64.1.277-282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke SR, Brummell KJ, Horsburgh MJ, et al. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J Infect Dis. 2006;193:1098–108. doi: 10.1086/501471. [DOI] [PubMed] [Google Scholar]
- 17.Johne B. Epitope mapping by surface plasmon resonance in the BIAcore. Mol Biotechnol. 1998;9:65–71. doi: 10.1007/BF02752698. [DOI] [PubMed] [Google Scholar]
- 18.Limbago B, Fosheim GE, Schoonover V, et al. Characterization of methicillin-resistant Staphylococcus aureus isolates collected in 2005 and 2006 from patients with invasive disease: a population-based analysis. J Clin Microbiol. 2009;47:1344–51. doi: 10.1128/JCM.02264-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161–8. [PubMed] [Google Scholar]
- 20.Jakubovics NS, Jenkinson HF. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology. 2001;147:1709–18. doi: 10.1099/00221287-147-7-1709. [DOI] [PubMed] [Google Scholar]
- 21.Raedler MD, Heyne S, Wagner E, et al. Serologic assay to quantify human immunoglobulin G antibodies to the Staphylococcus aureus iron surface determinant B antigen. Clin Vaccine Immunol. 2009;16:739–48. doi: 10.1128/CVI.00478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harro C, Betts R, Orenstein W, et al. Safety and immunogenicity of a novel Staphylococcus aureus vaccine: results from the first study of the vaccine dose range in humans. Clin Vaccine Immunol. 2010;17:1868–74. doi: 10.1128/CVI.00356-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23:3393–404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karavolos MH, Horsburgh MJ, Ingham E, Foster SJ. Role and regulation of the superoxide dismutases of Staphylococcus aureus. Microbiology. 2003;149:2749–58. doi: 10.1099/mic.0.26353-0. [DOI] [PubMed] [Google Scholar]
- 25.Tseng HJ, Srikhanta Y, McEwan AG, Jennings MP. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol Microbiol. 2001;40:1175–86. doi: 10.1046/j.1365-2958.2001.02460.x. [DOI] [PubMed] [Google Scholar]
- 26.Johnston RB, Jr, Keele BB, Jr, Misra HP, et al. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975;55:1357–72. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinefield H, Black S, Fattom A, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–6. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 28.Nanra JS, Timofeyeva Y, Buitrago SM, et al. Heterogeneous in vivo expression of clumping factor A and capsular polysaccharide by Staphylococcus aureus: implications for vaccine design. Vaccine. 2009;27:3276–80. doi: 10.1016/j.vaccine.2009.01.062. [DOI] [PubMed] [Google Scholar]