Abstract
Evidence suggests that recombinant human bone morphogenetic protein 2 (rhBMP-2) increases the mechanical integrity of callus tissue during bone healing. This effect may be either explained by an increase of callus formation or a modification of the trabecular microarchitecture. Therefore the purpose of the study was to evaluate the potential benefit of rhBMP-2 on the trabecular microarchitecture and on multidirectional callus stiffness. Further we asked, whether microarchitecture changes correlate with optimized callus stiffness. In this study a tibial distraction osteogenesis (DO) model in 12 sheep was used to determine, whether percutaneous injection of rhBMP-2 into the distraction zone influences the microarchitecture of the bone regenerate. After a latency period of 4 days, the tibiae were distracted at a rate of 1.25 mm/day over a period of 20 days, resulting in total lengthening of 25 mm. The operated limbs were randomly assigned to one treatment groups and one control group: (A) triple injection of rhBMP-2 (4 mg rhBMP-2/injection) and (B) no injection. The tibiae were harvested after 74 days and scanned by µCT (90 µm/voxel). In addition, we conducted a multidirectional mechanical testing of the tibiae by using a material testing system to assess the multidirectional strength. The distraction zones were tested for torsional stiffness and bending stiffness antero-posterior (AP) and medio-lateral (ML) direction, compression strength and maximum axial torsion. Statistical analysis was performed using multivariate analysis of variance (ANOVA) followed by student's t-test and Regression analysis using power functions with a significance level of P<0.05. Triple injections of rhBMP-2 induced significant changes in the trabecular architecture of the regenerate compared with the control: increased trabecular number (Tb.N.) (treatment group 1.73 mm/1 vs. control group 1.2 mm/1), increased cortical bone volume fraction (BV/TV) (treatment group 0.68 vs. control group 0.47), and decreased trabecular separation (Tb.Sp.) (treatment group 0.18 mm vs. control group 0.43 mm).
The analyses of the mechanical strength of regenerated bone showed significant differences between treatment group (A) and the control group (B). The bending stiffness anterior-posterior (treatment group 17.48 Nm vs. control group 8.3 Nm), medial-lateral (treatment group 18,9 Nm vs. control group 7.92 Nm) and the torsional stiffness (treatment group 41.17N/° vs. control group 16.41N/°) are significantly higher in the treatment group than in the control group. The regression analyses revealed significant non-linear relationships between BV/TV, TB.N., Tb.Sp. and all mechanical properties. Maximal correlation coefficients were found for the Tb.Sp. vs. the bending stiffness AP and ML with R2=0.69 and R2=0.70 (P<0.0001). There was no significant relation between Connectivity and the compression strength and the maximum axial torque. This study suggests that rhBMP-2 optimizes the trabecular microarchitecture of the regenerate, which might explain the advanced mechanical integrity of newly formed bone under rhBMP-2 treatment.
Key words: bone morphogenetic protein (BMP), Modeling and remodeling, Microcomputed Tomography (µCT), load bearing capacity.
Introduction
Recombinant human bone morphogenetic protein 2 (rhBMP-2) has been shown to enhance bone healing1–10 and thereby to improve the mechanical integrity11,12 of bone regenerate at a distinct time point. However, it remains unclear whether rhBMP-2 application just accelerates bone consolidation resulting in terms of a time lead of healing or even induces a microstructural optimized trabecular microarchitecture in long bone healing. To investigate this question we used a sheep distraction osteogenesis (DO) model as a model of controlled bone healing.
Specifically we asked, whether rhBMP-2 influences the bone consolidation process. During consolidation the bone remodeling of the callus tissue due to osteoblast and osteoclast activation and the mineralisation of the bone matrix are the main processes. While BMP-2 has been shown to be a potential osteoinductive factor13,14 in the first instance, it has also been shown to stimulate osteoblast and osteoclast differentiation,15–20 and to be involved in late stages of osteoblast development and matrix mineralisation.16,21,22 Therefore a remodeling effect during bone consolidation may be supposed.
Further evidence suggests that rhBMP-2 increases the mechanical integrity of the callus, but the responsible mechanism remains unclear since there are conflicting results about the rhBMP-2-effect on the total amount of callus and the callus mineralisation.1–3,6,7,11 For trabecular bone the strength not only depends on the amount of calcified tissue present, but primarily on its spatial distribution.23 Therefore the strength and the fracture resistance of bone are determined by the architecture of its trabecular network and its cortical shell.24,25 However, a potential modification of the trabecular microarchitecture by rhBMP-2 application during DO has been investigated by Ming and Zhu et al., but these studies examined rhBMP-2 in combination with other biological agents. Other studies investigated the effect of rhBMP-2 on bone microarchitecture in anterior lumbar interbody fusion.26,27
In this study the microarchitecture of the bone regenerate was assessed using 3D microcomputed tomography (µCT). We hypothesized that repetitive rhBMP 2-injections improve the microarchitecture of the bone regenerate and thereby increase its mechanical resistance. A second hypothesis was that changes in the trabecular microarchitecture would correlate with the mechanical stiffness of the callus tissue.
Materials and Methods
Animals
Twelve skeletally mature female sheep with a mean presurgical weight of 81 kg±13.6 kg were used. Animals were housed indoors in boxes during the experimentation period and fed a standard cereal foodstuff. Animal experiments were conducted under an ethic commission approved protocol according to German federal animal welfare legislation.
Surgery and distraction
For preanesthetic sedation animals received 5–10 mg propofol intravenous. The sheep were intubated and fixed in a left lateral position. Inhalation anaesthesia was performed with Isofluran for the duration of surgery. A modified external half ring fixator,28 consisting of four half-rings, connected by threaded rods, was applied to the right tibia under radiographic control. Six 5 mm Schanz-screws were inserted in the tibia in a defined order. A middiaphyseal transversal osteotomy was created over a medial approach using an oscillating saw. The periosteum was reapproximated and the wound closed. A postoperative antimicrobial prophylaxis, consisting of penicillin and streptomycin (0.1 mL/kg, Tardomycel, Bayer GmbH, Leverkusen) was applied by subcutaneous injection. To protect the sheep from hurting themselves and each other, a soft plastic cover was fixed on the fixator frame.
Unrestricted weight bearing and activity were allowed postoperatively. After a latency period of 4 days the tibiae were distracted at a rate of 1.25 mm daily in two increments over a period of 20 days. The distraction resulted in total lengthening of 25 mm. The distraction period was followed by a consolidation period of 50 days, during which the fixator was hold in place with no distraction. Throughout the study sheep were examined daily for signs of pin-infection and pain. Antero-posterior x-ray views of the lengthened tibiae were performed weekly.
Injection protocol
The operated sheep were randomly assigned to the treatment group or the control group resulting in 6 animals per group. Treatment groups included: (A) triple injection of 1 mL rhBMP-2/NaCl (4 mg rhBMP-2/ml) (Genetics Institute, MA). The control group had no injection (B). The sheep in treatment group (A) were injected on day 3, 10 and 17. During the injection procedure the sheep were fixed in a dorsal position. Injections were performed at the center of the distraction zone (0.4 mL solution) and 1 cm distal and proximal from the central point (0.2 mL solution each) under fluoroscopic control.
Sheep were then sacrificed after 74 days by intravenous injection of propofol and pentobarbital (Eutha 77, Essex Pharma, Germany). Afterwards the external fixator was removed and the right tibiae was resected and frozen at −20°C until µCT analysis were performed.
Stereological analysis
To determine 3D stereological histomorphometric parameters indices a complete three-dimensional digitized image of each distraction zone was generated using a microcomputed tomography system (µCT). The system is equipped with a microfocus x-ray tube with a focal spot of 10 mm, producing a cone beam that is detected by a charge-couple device (CCD) 512×512 pixel array. The tibiae were positioned in custom-made fixation device in air for scanning. The whole distraction zone with a diameter of approximately 2,5 cm was scanned. All distraction zones were reconstructed on a 120 µm mesh, so that each 3D data point of the reconstruction (voxel) represented a subvolume of the actual specimen of (90×90×90 µm3). The distraction callus was analyzed for bone volume/total volume (BV/TV), trabecular number (Tb.N.: mm−1), trabecular thickness (Tb.Th.: mm), trabecular separation (Tb.Sp.: mm) and Connectivity, as defined by Parfitt and Odgaard.29;30 The region of interest (ROI) was fit into the distraction zone (1.) as a sphere with the diameter of the distraction zone: total volume; (2.) as two spheres with half of the diameter of the distraction zone: cortical weighted subvolume (CWS) (Figure 1). Both ROI methods were used to determine BV/TV. All other parameters were measured in the cortical weighted subvolume (method 2). A modified algorithm of the Hipp and Simmons method of directed secants was used to compute the structural parameters defined above.31
Figure 1.
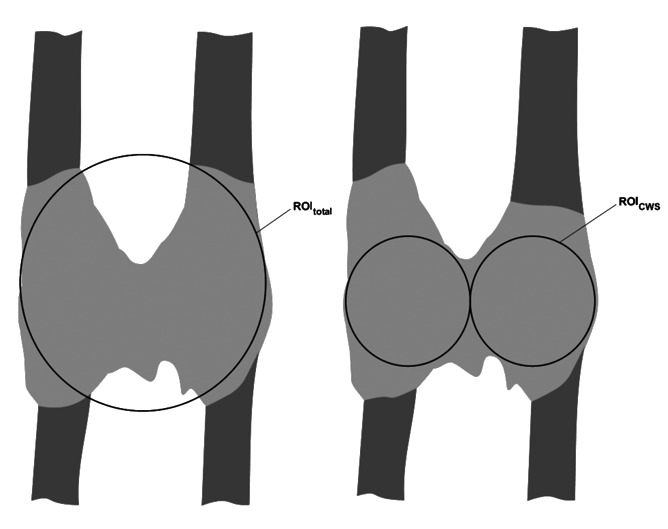
Model of region of interest (ROI): total volume and region of interest: cortical weighted subvolume.
Mechanical test
Before biomechanical testing at day 74, the frozen tibiae were thawed at room temperature and kept moist using gauze soaked in 0.9% NaCl solution during the entire test period. The embedded specimens were mounted in specially constructed jigs for compressive, 4-point-bending, or torsional testing in a Material Testing System (MTS [Model 858, MTS Corp., Minneapolis, USA]) (Figure 2). The order of stiffness testing was randomised and measurements were performed on each specimen by using compressive, bending (anteroposterior and mediolateral) and torsional stiffness. The resulting deformation was detected by custom-made compression, torsion and deflection sensors (LVTD and precision potentiometer). For each stiffness testing-procedure, a preconditioning of 10 cycles was conducted before the actual testing in order to assure repeatability. The callus tissue within the specimens was loaded during the different types of testing up to 15 Nm for torsional, to 750 N for compressive and to 6.5 Nm for bending load. During testing, load and deformation were simultaneously recorded in order to determine stiffness, which is defined as the slope of the load-deformation curve. In a final experiment, the specimens were loaded in torsion until failure to determine the load-bearing capacity. The detailed testing protocol was performed as previously described.32
Figure 2.
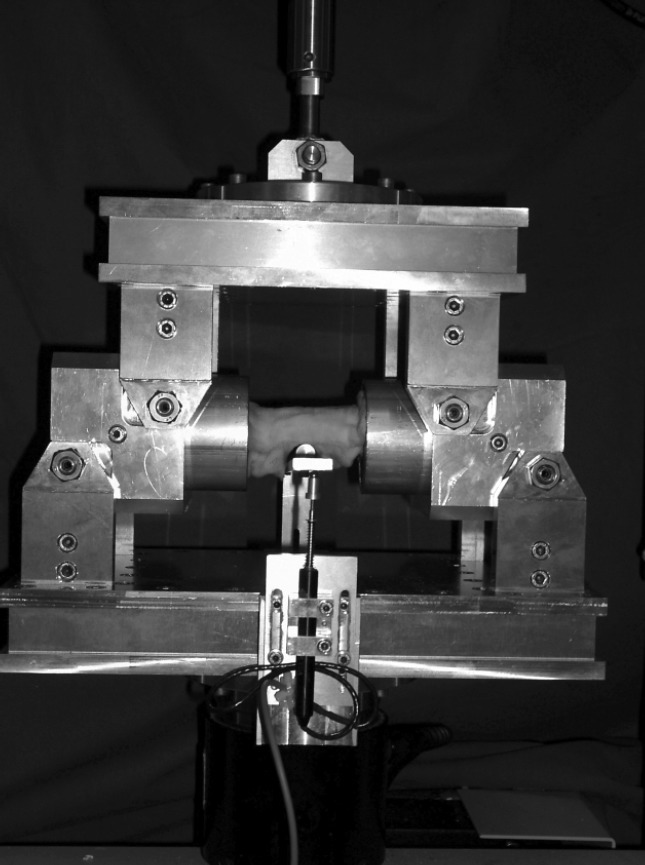
Photography of the custom-made jig for bending testing including the specially constructed deflection sensor.
Statistics
The results are presented as mean and SD. For comparison of the structural parameters obtained from the treatment group (A) and the control group (B) data were analyzed using multivariate analysis of variance (ANOVA) followed by student's t-test. The mechanical parameters (ordinate) and the different types of bone parameters as BV/TV, Tb.N. and Tb.Sp. were plotted in a diagram. For each measured parameter an analysis of variance (ANOVA) was performed with SPSS Software (SPSS, SPSS Inc., Chicago, USA). Regression analyses were performed using power functions. A P value of less than 0.05 was considered significant.
Results
The stereological evaluation revealed a significantly increased higher cortical BV/TV in the treatment group A compared the control group (B). The increase was given by 45% (P=0.001). Measured for the total volume of the distraction zone the BV/TV showed no significant difference between the two groups (Figure 3). The trabecular number (Tb.N.) in the treatment group (A) was significantly increased compared with the control group (B). The percentage difference was 46% (P=0.004), which corresponds to an absolute increase of the mean trabecular number (Tb.N.) by 5–6 trabeculae per millimeter. Regarding the trabecular spacing (Th.Sp.), there were significant differences between the treatment group (A) and the control group (B). Treatment group (A) compared with control group (B) had a lower mean trabecular spacing (Tb.Sp.) by 58% (P=0.022), representing an absolute difference of 0.25 mm. For the trabecular thickness (Tb.Th.), there were no significant differences between the treatment group (A) and the control group (B). The maximum trabecular thickness (Tb.Th.) in treatment group (A) reached 0.39 mm compared to 0.35 mm in the control group (Figure 4).
Figure 3.
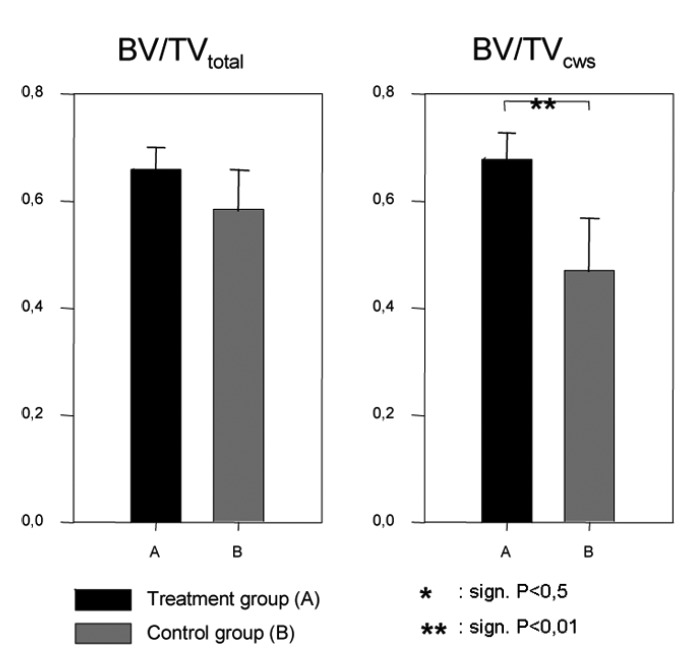
Comparison of the bone volume fraction (BV/TV) in the total volume and the cortical weighted subvolume.
Figure 4.
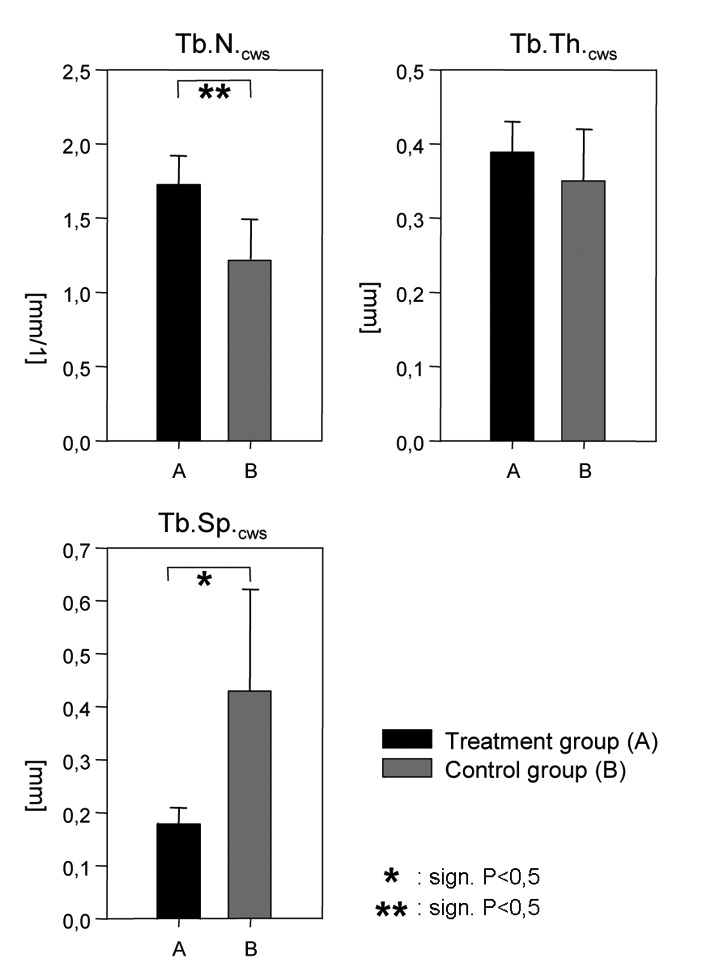
Comparison of the trabecular number (Tb.N), trabecular spacing (Tb.Sp.) and trabecular thickness (Tb.Th.) in the cortical weighted subvolume.
The analyses of the mechanical strength of the distraction zones indicated significant differences between treatment (A) and control group (B). The comparison showed an increase in torsional stiffness of the treatment group of 248% to controls (P=0.012). The anterior-posterior bending stiffness revealed an increase of 210% (P=0.007) and the medial-lateral bending stiffness showed an increase of 239% (P=0.002). There were no significant differences between the treatment group and the control group in compression and maximum axial torque (Figure 5).
Figure 5.
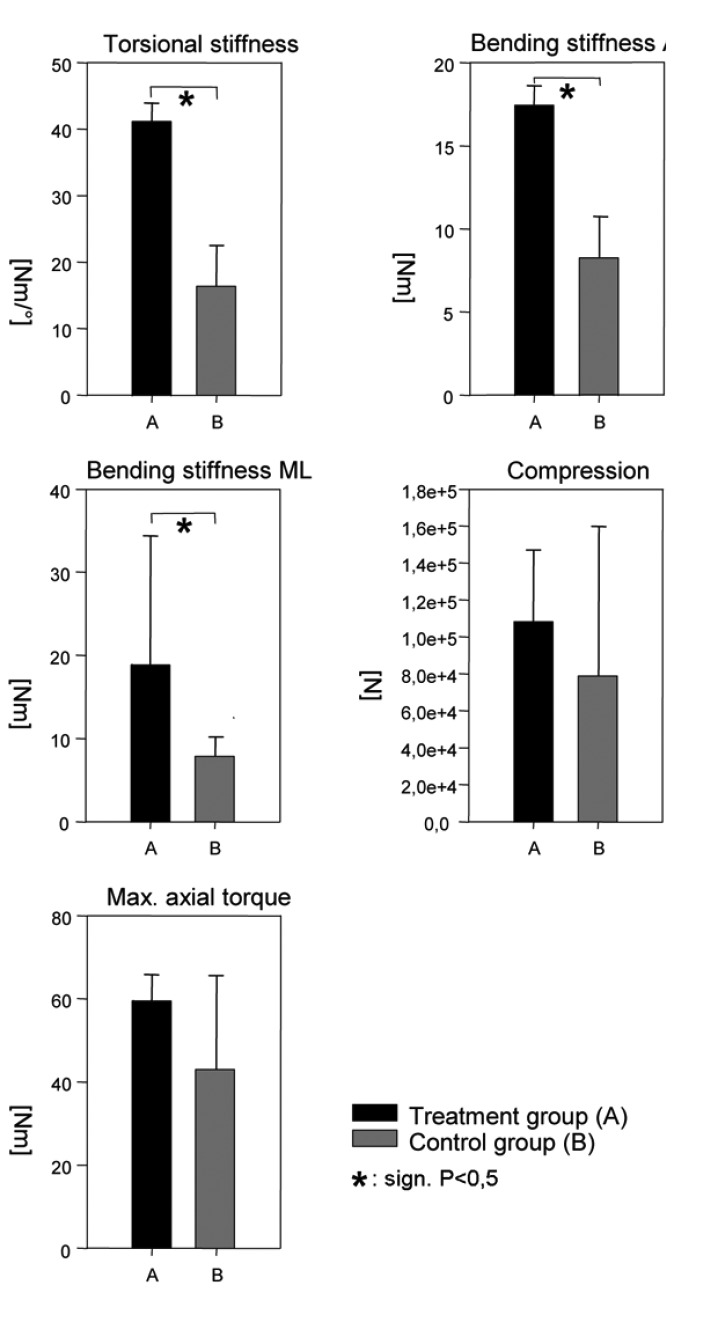
Comparison of the bending stiffness anterior-posterior and medial-lateral, compression, torsional stiffness and maximum axial torque.
The regression analyses revealed significant non-linear relationships between BV/TV, Tb.N., Tb.Sp. and all mechanical properties. The Tb.Sp. showed the highest correlation witch each of the stiffness parameters. Maximal correlation coefficients were found for the Tb.Sp. vs. the bending stiffness AP and ML with R2=0.69 and R2=0.70 (P<0.0001; n=12) (Figure 6). Thereby relationship between trabecular spacing (Tb.Sp.) and maximal torsional stiffness was found to be inversely proportional. Poor correlations (R2 <0.4) were found for Connectivity with all parameters. There was no significant correlation between Connectivity and the compression strength and the maximum axial torque (P>0.05) (Table 1).
Figure 6.
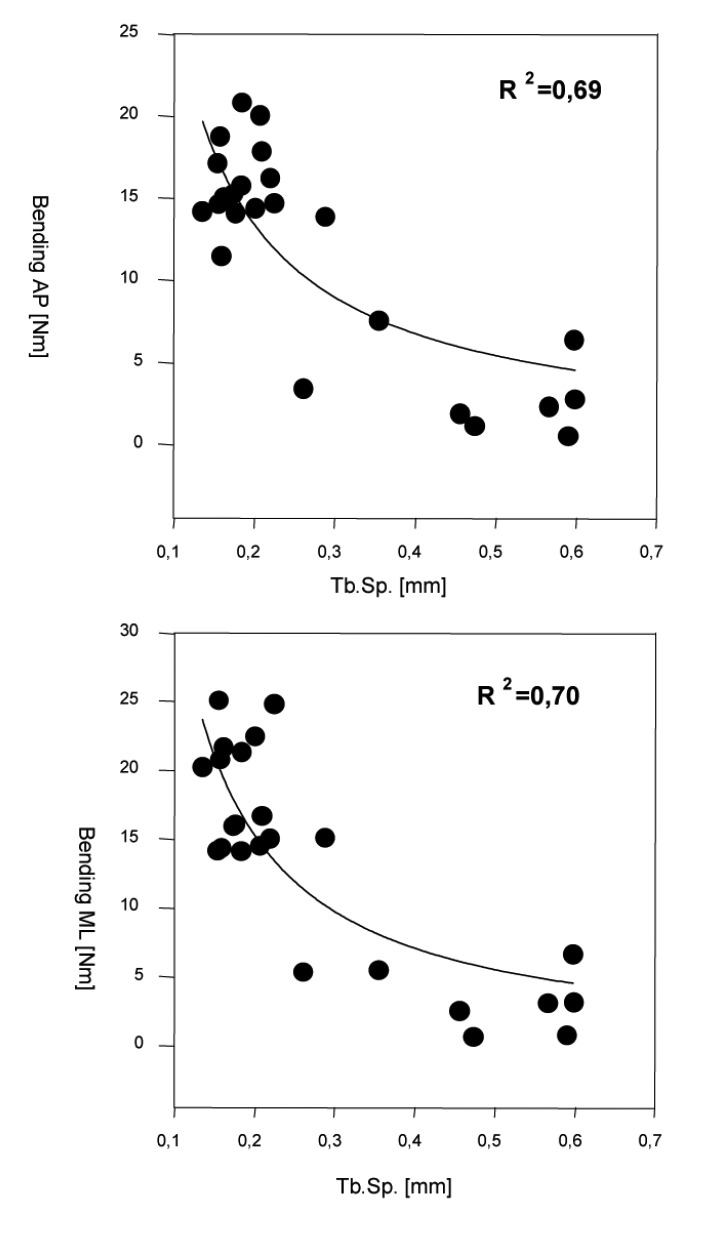
Regression of trabecular separation (Tb.Sp.) vs. bending stiffness in anterior-posterior (AP) and medio-posterior (ML) direction.
Table 1. Regression (R2) values of the microarchitectural parameters to the multiaxial Mechanical stiffness.
| Trabecular Spacing (Tb.Sp.) | trabecular number (Tb.N) | connectivity | |
|---|---|---|---|
| bending AP | 0.69 | 0.53 | 0.31 |
| Bending ML | 0.70 | 0.59 | 0.26 |
| Compression | 0.53 | 0.35 | n.sign. |
| Torsion | 0.66 | 0.50 | 0.39 |
| Max. torque | 0.59 | 0.41 | n.sign. |
AP, anterior-posterior; ML, and medio-posterior
Discussion
Our results indicate that local rhBMP-2 application induces significant changes of the regenerate microarchitecture in a sheep tibial DO model. The µCT measurements indicated that the trabecular microstructure of the rhBMP-2 treated distraction zones were characterized by an increased cortical bone volume fraction (BV/TV), an increased Tb.N and a decreased Tb.Sp. There were no significant differences found for the Tb.Th. between the rhBMP-2 injected group and the control. While the BV/TV in the cortical weighted ROI is increased for treatment group (A), there were no significant differences found for the BV/TV in the total ROI (Figure 3). As these results suggest, rhBMP-2 does not increase the total amount of newly formed bone, but it enhances the reformation of the cortex and the marrow cavity.
These findings were confirmed by previous studies, which investigated the effect of rhBMP-2 on the rate of callus formation and mineralisation.1,5 The authors argue that the amount of bone and cartilage in fracture callus, and therefore its size, is primarily influenced by mechanical factors, while rhBMP-2 accelerates the rate of development of the callus and the cortical union. But this effect may be time dependent. Li et al. investigated the effect of rhBMP-2 on bone consolidation in an rabbit DO model and found a significant higher bone mineral content (BMC) in the rhBMP-2 treated groups 14 days after surgery compared with the control.5 However at 28 days the BMC of the regenerate was similar in all groups. The assimilation of the BMC between control and treatment groups at 28 days was explained by an advanced remodeling of the bone regenerate in the treatment groups, which included a redistribution of mineralized bone tissue from the central region of the distraction zone to the newly formed cortex. Our results have been strengthened by Zhu et al., who found an increased BV/TV, Tb.Th. and Tb.N. and significantly decreased Tb.Sp. in the rhBMP-2 group compared to their control group in a rabbit model. These authors investigated the combined effects of rhBMP-2 and NEL-like molecular-1 (NELL-1) on bone regeneration in rapid distraction osteogenesis.33 Moreover, they showed that the combination of rhBMP-2 and NELL-1 reached the highest value in BV/TV, Tb.Th. and Tb.N. and the lowest value at Th.Sp. in comparison to the control group. This provides an indication that the osteogenesis can be positively influenced by other biological agents. Nevertheless, rhBMP-2 takes an important role, because the trabecular microarchitecture parameters were significant higher in the rhBMP-2-only-treated group than in the NELL-1 group. The accelerating effect on the cortex regeneration as found in the current study may be explained by the fact, that BMP is primarily expressed by chondrocytes during the consolidation, which are typically located in external and periosteal callus.34 A more rapid chondral ossification of the external and periosteal callus, which is induced by external application rhBMP-2, is likely to explain the enhanced cortical bone formation in this study. Box et al. stated that rhBMP-2 does not increase the size of the callus significantly but enhances the callus maturation so that the rhBMP-2 effects during distraction osteogenesis are characterized to be qualitative, not quantitative.1 Some studies have been demonstrated the potential positive influence of rhBMP-2 on the trabecular microarchitecture of bone, however, in conjunction with other biological agents or other body parts. In this study a local application of rhBMP-2 has been shown to induce significant changes in the callus microarchitecture.
The microstructural changes in the cortical subvolume: increase of Tb.N and decrease of Tb.Sp. are discussed to be biomechanically highly relevant.23,35 Our results were confirmed by Foldager et al., who investigated the difference in early osteogenesis and bone microarchitecture in anterior lumbar interbody fusion with rhBMP-2, equine bone protein extract and autograft.26 They found that BV/TV and Tb.N. are increased in rhBMP-2 treatment group, but Tb.Th. significantly decreased as in osteoporotic bone morphology after 8 weeks. However, the connectivity density was significantly higher in the rhBMP-2 treated group, suggesting that the trabecular number is more important for the bone strength than the trabecular thickness.
Therefore the rhBMP-2 induced microstructural changes, characterized in the present study: increased BV/TV, increased Tb.N. and decreased Tb.Sp. correspond to the microstructural pattern formulated for a biomechanically optimized trabecular microarchitecture. These results coincide with the results of the current study as we found significant correlations between BV/TV, Tb.N., Tb.Sp. and the mechanical parameters like bending stiffness, torsional stiffness, compression and maximum axial torque measured by mechanical testing. These findings were confirmed by Ni et al. who found a higher torsional stiffness and higher maximum torque in the investigation of rabbit DO model with hydroxyapatite/tri-calcium phosphates (HA-TCP biomaterial), rhBMP-2 and alendronate.36 Moreover Sandhu et al. support our findings regarding the increased stiffness in the treatment group when they could show that the rhBMP-2 treated group had a significantly increased bending stiffness in al directions compared to the control group. These 3D structural indices Tb.N and Tb.Sp were shown to have a high ability to reflect the bone strength.37
Our assumption, that repetitive injections of rhBMP-2 may optimize the trabecular microstructure due to an increase of Tb.N. and decrease of Tb.Sp. resulting in an increased bone stiffness is supported by other authors.27
In our own regression analyses we found a strong correlation between microstructure parameters (BV/TV, Tb.N., Tb.Sp.) and the multiaxial stiffness in callus tissue. Thereby the trabecular separation (Tb.Sp.) was observed to be the best predictor of callus stiffness.
On the other hand we found a poor correlation between the connectivity in our 3D analysis and the stiffness parameters. This observation may be explained by the presence of isolated bone particles in the distraction zones, which may be interpreted unconnected trabeculae, as they have been characterized during DO(38). Isolated particles in the 3D dataset have been reported to produce negative values of Connectivity.39 Thereby its obvious that isolated bone particles will contribute to the bone volume fraction of the regenerate (BV/TV) but not to its mechanical competence.
We are aware of several limitations of our study. First, we were unable to measure weight bearing in the operated and limbs during consolidation, which may influence healing and ultimately, the microarchitecture of the regenerate. Second, the microarchitecture was only assessed in vitro at one timepoint (day 74), so that we ware not able to evaluate the microarchitectural development during the healing process. Nevertheless our data strongly support an effect use of a triple injection of rhBMP-2 to accelerate the consolidation period during.
In conclusion this study suggests that a triple injection of rhBMP-2 optimizes the trabecular microarchitecture in the distraction zone, which might explain the advanced mechanical integrity of newly formed bone under rhBMP-2 treatment as found in our study for bending and torsional stiffness.
References
- 1.Bax BE, Wozney JM, Ashhurst DE. Bone morphogenetic protein-2 increases the rate of callus formation after fracture of the rabbit tibia. Calcif Tissue Int. 1999;65:83–9. doi: 10.1007/s002239900662. [DOI] [PubMed] [Google Scholar]
- 2.Bouxsein ML, Turek TJ, Blake CA, et al. Recombinant human bone morphogenetic protein-2 accelerates healing in a rabbit ulnar osteotomy model. J Bone Joint Surg Am. 2001;83A:1219–30. doi: 10.2106/00004623-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Lane JM, Yasko AW, Tomin E, et al. Bone marrow and recombinant human bone morphogenetic protein-2 in osseous repair. Clin Orthop. 1999;361:216–27. doi: 10.1097/00003086-199904000-00028. [DOI] [PubMed] [Google Scholar]
- 4.Lee FY, Storer S, Hazan EJ, et al. Repair of bone allograft fracture using bone morphogenetic protein-2. Clin Orthop. 2002;397:119–26. doi: 10.1097/00003086-200204000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Li G, Bouxsein ML, Luppen C, et al. Bone consolidation is enhanced by rhBMP-2 in a rabbit model of distraction osteogenesis. J Orthop Res. 2002;20:779–88. doi: 10.1016/S0736-0266(01)00166-8. [DOI] [PubMed] [Google Scholar]
- 6.Luppen CA, Blake CA, Ammirati KM, et al. Recombinant human bone morphogenetic protein-2 enhances osteotomy healing in glucocorticoid-treated rabbits. J Bone Miner Res. 2002;17:301–10. doi: 10.1359/jbmr.2002.17.2.301. [DOI] [PubMed] [Google Scholar]
- 7.Welch RD, Jones AL, Bucholz RW, et al. Effect of recombinant human bone morphogenetic protein-2 on fracture healing in a goat tibial fracture model WELCH1998. J Bone Miner Res. 1998;139:1483–90. doi: 10.1359/jbmr.1998.13.9.1483. [DOI] [PubMed] [Google Scholar]
- 8.Bishop GB, Einhorn TA. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;31:721–7. doi: 10.1007/s00264-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Einhorn TA, Majeska RJ, Mohaideen A, et al. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J Bone Joint Surg Am. 2003;85A:1425–35. doi: 10.2106/00004623-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Azad V, Breitbart E, Al-Zube L, et al. rhBMP-2 enhances the bone healing response in a diabetic rat segmental defect model. J Orthop Trauma. 2009;23:267–76. doi: 10.1097/BOT.0b013e31819f290e. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler DL, Chamberland DL, Schmitt JM, et al. Radiomorphometry and biomechanical assessment of recombinant human bone morphogenetic protein 2 and polymer in rabbit radius ostectomy model. J Biomed Mater Res. 1998;43:365–73. doi: 10.1002/(sici)1097-4636(199824)43:4<365::aid-jbm4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Yasko AW, Lane JM, Fellinger EJ, et al. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg Am. 1992;74:659–70. [PubMed] [Google Scholar]
- 13.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 14.Wozney JM. Bone morphogenetic proteins. Prog Growth Factor Res. 1989;1:267–80. doi: 10.1016/0955-2235(89)90015-x. [DOI] [PubMed] [Google Scholar]
- 15.Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002;87:305–12. doi: 10.1002/jcb.10309. [DOI] [PubMed] [Google Scholar]
- 16.Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8:147–59. doi: 10.1034/j.1601-0825.2002.01829.x. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto M, Murai J, Yoshikawa H, Tsumaki N. Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J Bone Miner Res. 2006;21:1022–33. doi: 10.1359/jbmr.060411. [DOI] [PubMed] [Google Scholar]
- 18.Reddi AH. Initiation of fracture repair by bone morphogenetic proteins. Clin Orthop. 1998;355(Suppl):S66–S72. doi: 10.1097/00003086-199810001-00008. [DOI] [PubMed] [Google Scholar]
- 19.Abe E, Yamamoto M, Taguchi Y, et al. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J Bone Miner Res. 2000;15:663–73. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- 20.Giannoudis PV, Kanakaris NK, Einhorn TA. Interaction of bone morphogenetic proteins with cells of the osteoclast lineage: review of the existing evidence. Osteoporos Int. 2007;18:1565–81. doi: 10.1007/s00198-007-0441-x. [DOI] [PubMed] [Google Scholar]
- 21.Luppen CA, Smith E, Spevak L, et al. Bone morphogenetic protein-2 restores mineralization in glucocorticoid-inhibited MC3T3-E1 osteoblast cultures. J Bone Miner Res. 2003;18:1186–97. doi: 10.1359/jbmr.2003.18.7.1186. [DOI] [PubMed] [Google Scholar]
- 22.van der HG, van Bezooijen RL, Deckers MM, et al. Differentiation of murine preosteoblastic KS483 cells depends on autocrine bone morphogenetic protein signaling during all phases of osteoblast formation. Bone. 2002;31:661–9. doi: 10.1016/s8756-3282(02)00903-1. [DOI] [PubMed] [Google Scholar]
- 23.Kleerekoper M, Villanueva AR, Stanciu J, et al. The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int. 1985;37:594–7. doi: 10.1007/BF02554913. [DOI] [PubMed] [Google Scholar]
- 24.Engelke K, Karolczak M, Lutz A, et al. [Micro-CT. Technology and application for assessing bone structure] Radiologe. 1999;39:203–12. doi: 10.1007/s001170050497. [Article in German] [DOI] [PubMed] [Google Scholar]
- 25.Parfitt AM. Implications of architecture for the pathogenesis and prevention of vertebral fracture. Bone. 1992;13(Suppl2):S41–S47. doi: 10.1016/8756-3282(92)90196-4. [DOI] [PubMed] [Google Scholar]
- 26.Foldager C, Bendtsen M, Nygaard JV, et al. Differences in early osteogenesis and bone micro-architecture in anterior lumbar interbody fusion with rhBMP-2, equine bone protein extract, and autograft. Bone. 2009;45:267–73. doi: 10.1016/j.bone.2009.04.240. [DOI] [PubMed] [Google Scholar]
- 27.Sandhu HS, Toth JM, Diwan AD, S, et al. Histologic evaluation of the efficacy of rhBMP-2 compared with autograft bone in sheep spinal anterior interbody fusion. Spine (Phila Pa 1976 ) 2002;27:567–75. doi: 10.1097/00007632-200203150-00003. [DOI] [PubMed] [Google Scholar]
- 28.Windhagen H, Kolbeck S, Bail H, et al. Quantitative assessment of in vivo bone regeneration consolidation in distraction osteogenesis. J Orthop Res. 2000;18:912–9. doi: 10.1002/jor.1100180610. [DOI] [PubMed] [Google Scholar]
- 29.Odgaard A, Gundersen HJ. Quantification of connectivity in cancellous bone, with special emphasis on 3-D reconstructions. Bone. 1993;14:173–82. doi: 10.1016/8756-3282(93)90245-6. [DOI] [PubMed] [Google Scholar]
- 30.Parfitt AM. Bone histomorphometry: proposed system for standardization of nomenclature, symbols, and units. Calcif Tissue Int. 1988;42:284–6. doi: 10.1007/BF02556360. [DOI] [PubMed] [Google Scholar]
- 31.Hipp JA, Jansujwicz A, Simmons CA, Snyder BD. Trabecular bone morphology from micro-magnetic resonance imaging. J Bone Miner Res. 1996;11:286–97. doi: 10.1002/jbmr.5650110218. [DOI] [PubMed] [Google Scholar]
- 32.Floerkemeier T, Hurschler C, Witte F, et al. Comparison of various types of stiffness as predictors of the load-bearing capacity of callus tissue. J Bone Joint Surg Br. 2005;87:1694–9. doi: 10.1302/0301-620X.87B12.16247. [DOI] [PubMed] [Google Scholar]
- 33.Zhu S, Song D, Jiang X, et al. Combined effects of recombinant human BMP-2 and Nell-1 on bone regeneration in rapid distraction osteogenesis of rabbit tibia. Injury. 2011;42:1467–73. doi: 10.1016/j.injury.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 34.Rauch F, Lauzier D, Croteau S, et al. Temporal and spatial expression of bone morphogenetic protein-2, -4, and -7 during distraction osteogenesis in rabbits. Bone. 2000;27:453–9. doi: 10.1016/s8756-3282(00)00337-9. [DOI] [PubMed] [Google Scholar]
- 35.Ito M, Nakamura T, Matsumoto T, et al. Analysis of trabecular microarchitecture of human iliac bone using microcomputed tomography in patients with hip arthrosis with or without vertebral fracture. Bone. 1998;23:163–9. doi: 10.1016/s8756-3282(98)00083-0. [DOI] [PubMed] [Google Scholar]
- 36.Ni M, Li G, Tang PF, et al. rhBMP-2 not alendronate combined with HA-TCP biomaterial and distraction osteogenesis enhance bone formation. Arch Orthop Trauma Surg. 2011;131:1469–76. doi: 10.1007/s00402-011-1357-7. [DOI] [PubMed] [Google Scholar]
- 37.Ito M, Koga A, Nishida A, et al. Evaluation of mechanical properties of trabecular and cortical bone. Adv Exp Med Biol. 2001;496:47–56. doi: 10.1007/978-1-4615-0651-5_6. [DOI] [PubMed] [Google Scholar]
- 38.Fink B, Pollnau C, Vogel M, et al. Histomorphometry of distraction osteogenesis during experimental tibial lengthening. J Orthop Trauma. 2003;17:113–8. doi: 10.1097/00005131-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Kinney JH, Lane NE, Haupt DL. In vivo, three-dimensional microscopy of trabecular bone. J Bone Miner Res. 1995;10:264–70. doi: 10.1002/jbmr.5650100213. [DOI] [PubMed] [Google Scholar]


