Abstract
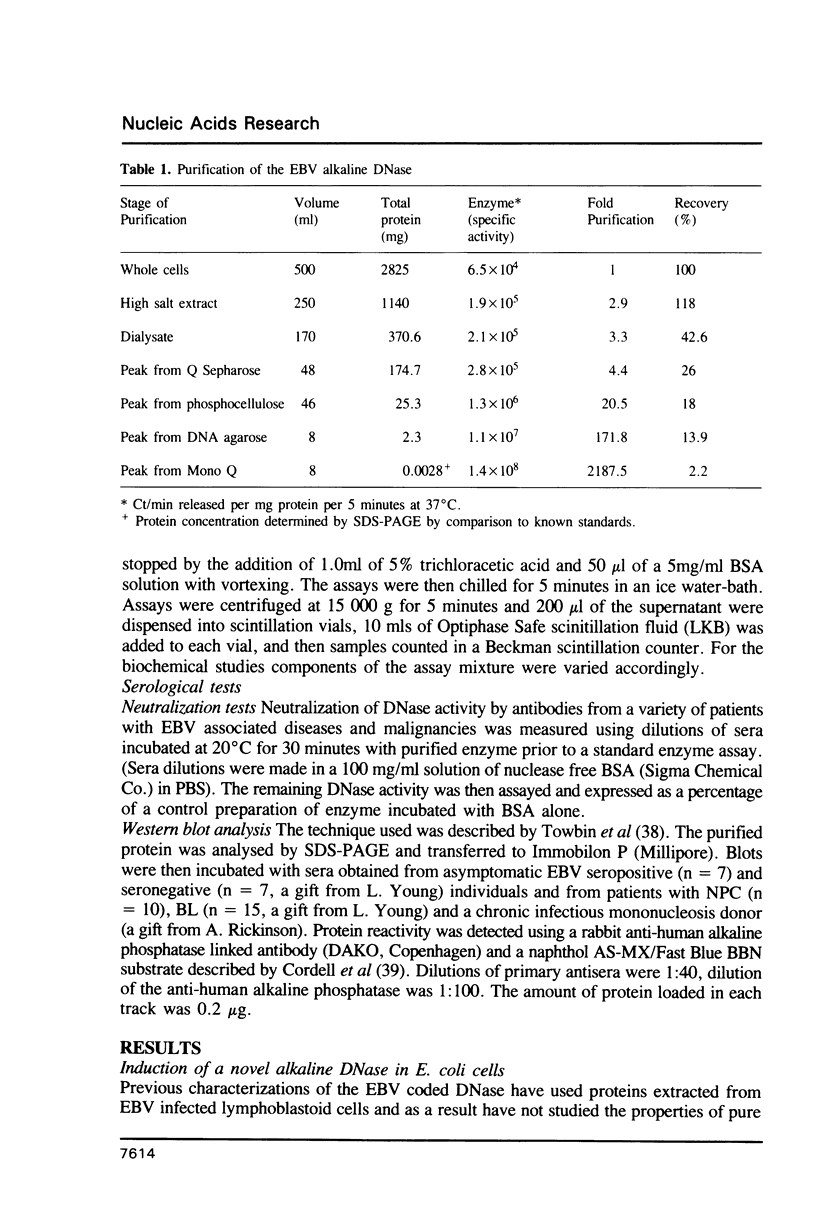
Studies of nucleic acid homology suggest the BGLF5 open reading frame of Epstein-Barr virus (EBV) encodes an alkaline deoxyribonuclease (DNase) sharing some homology with that of herpes simplex virus. We report here the expression of the BGLF5 open reading frame in E. coli and the expression of high levels of a novel alkaline DNase activity in induced cells. This alkaline DNase has been purified to apparent homogeneity as a single protein species. This is the first report of the expression of a herpesvirus coded DNase in a prokaryotic system and of the purification of the EBV DNase to demonstrable purity. It has the biochemical characteristics of a typical herpesvirus alkaline exonuclease showing a high pH optimum, an absolute requirement for Mg2+ for activity and sensitivity to high salt concentrations and polyamines. The enzyme activity was neutralized by sera from patients with nasopharyngeal carcinoma and was reactive with these sera in Western blot analysis. Thus the prokaryotic expression system described here provides an economical and efficient source of the EBV DNase for biochemical and seroepidemiological analysis.
Full text
PDF

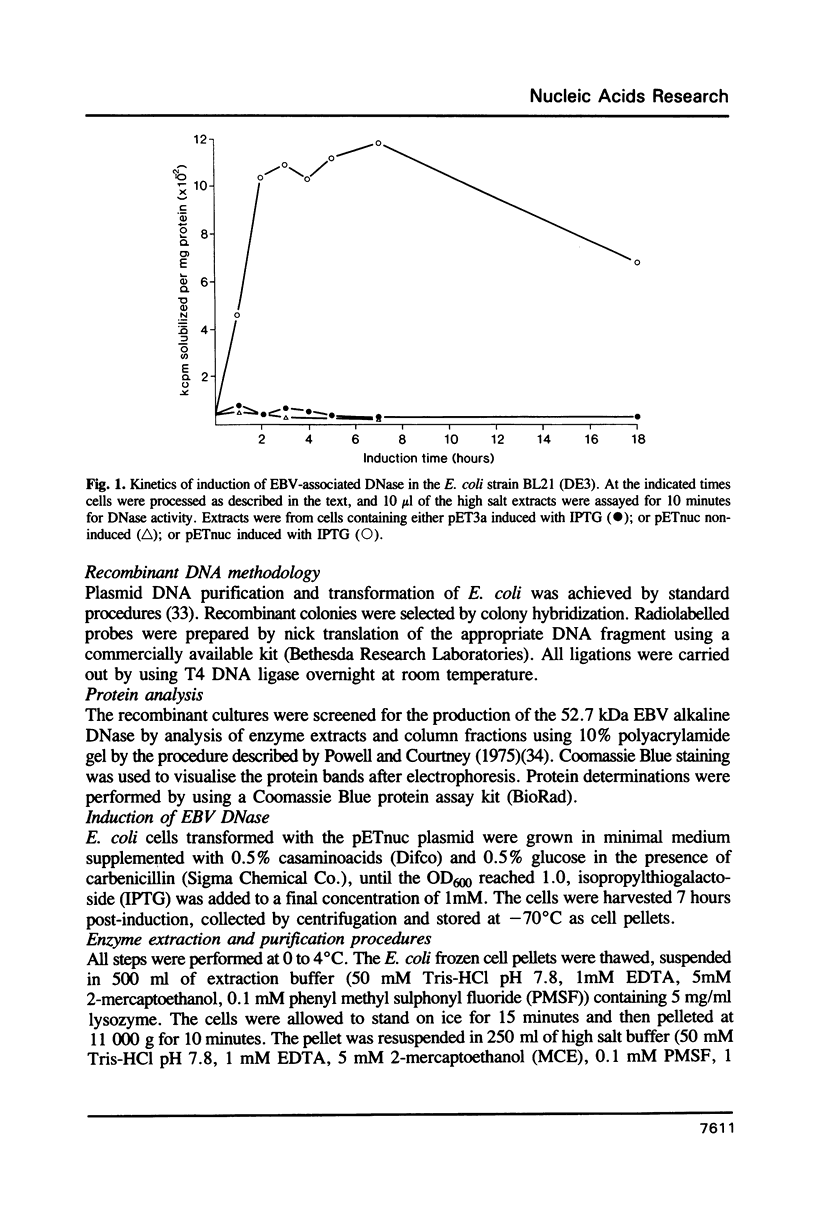
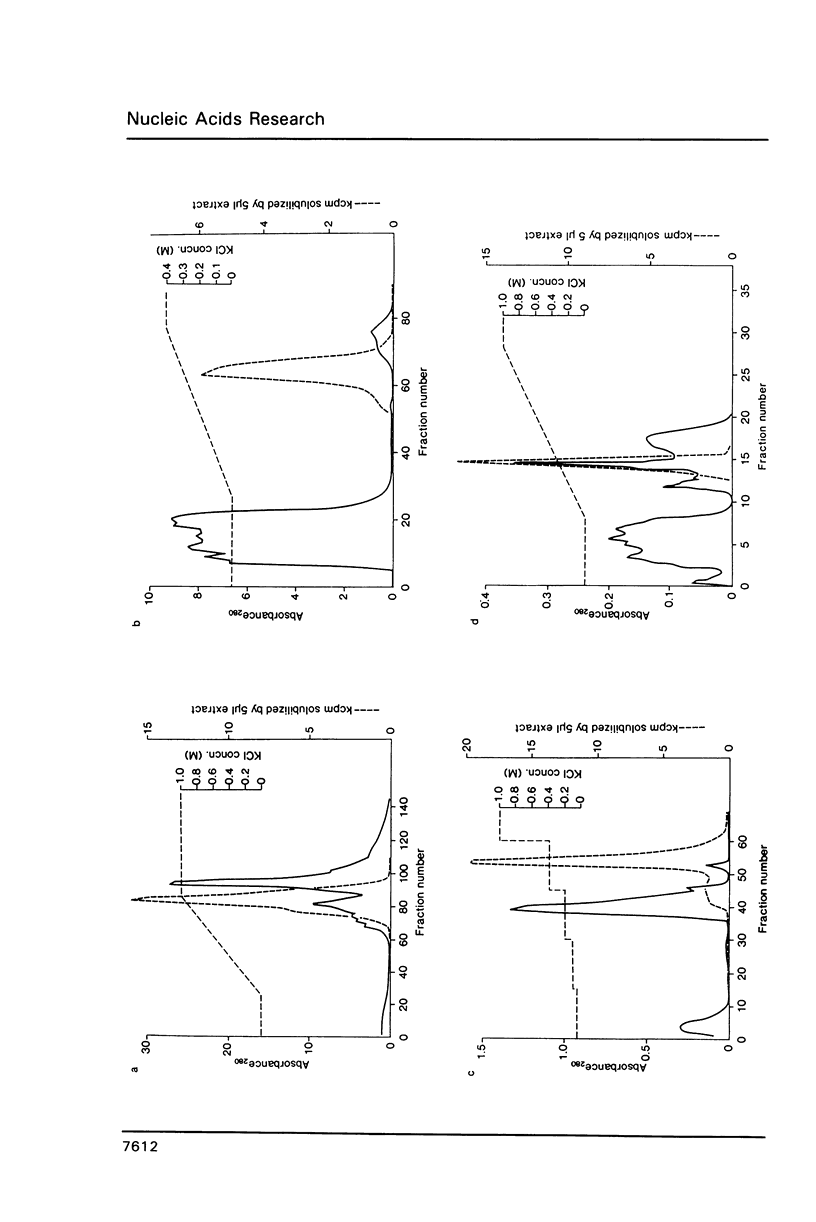


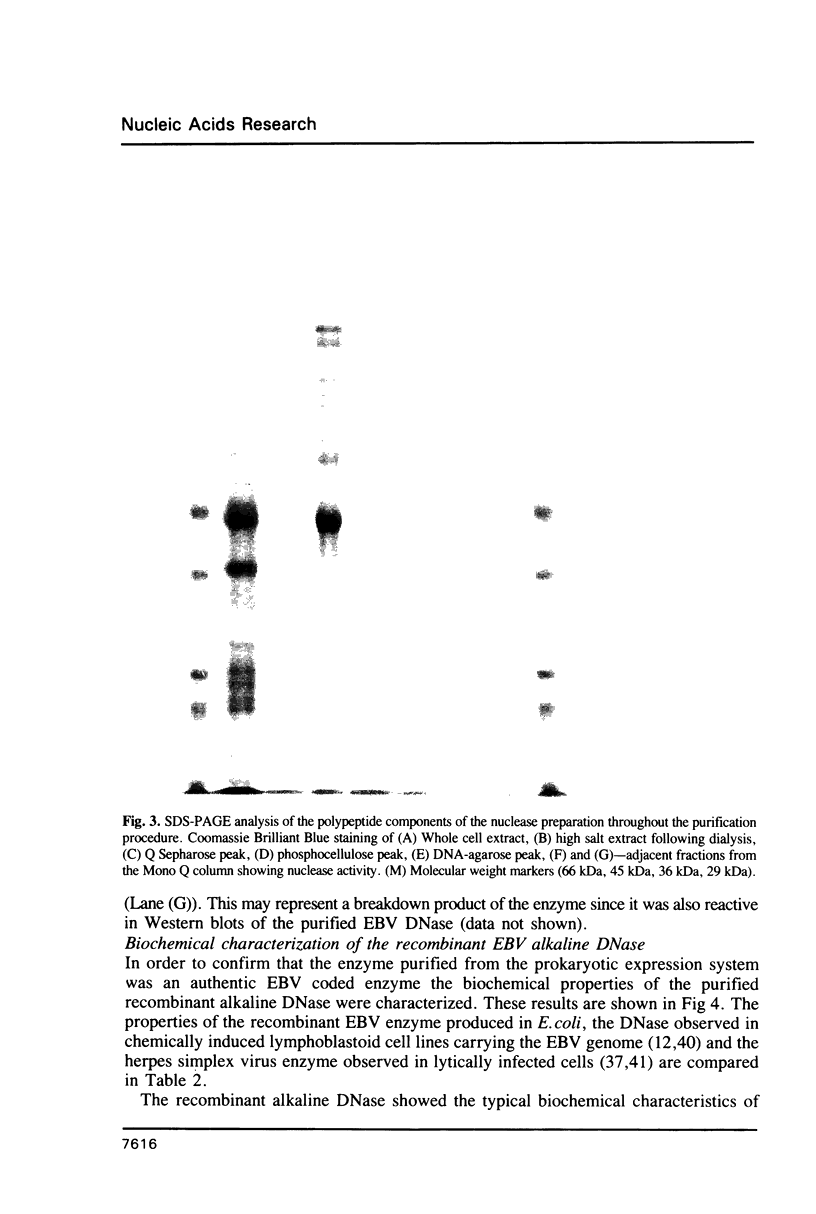
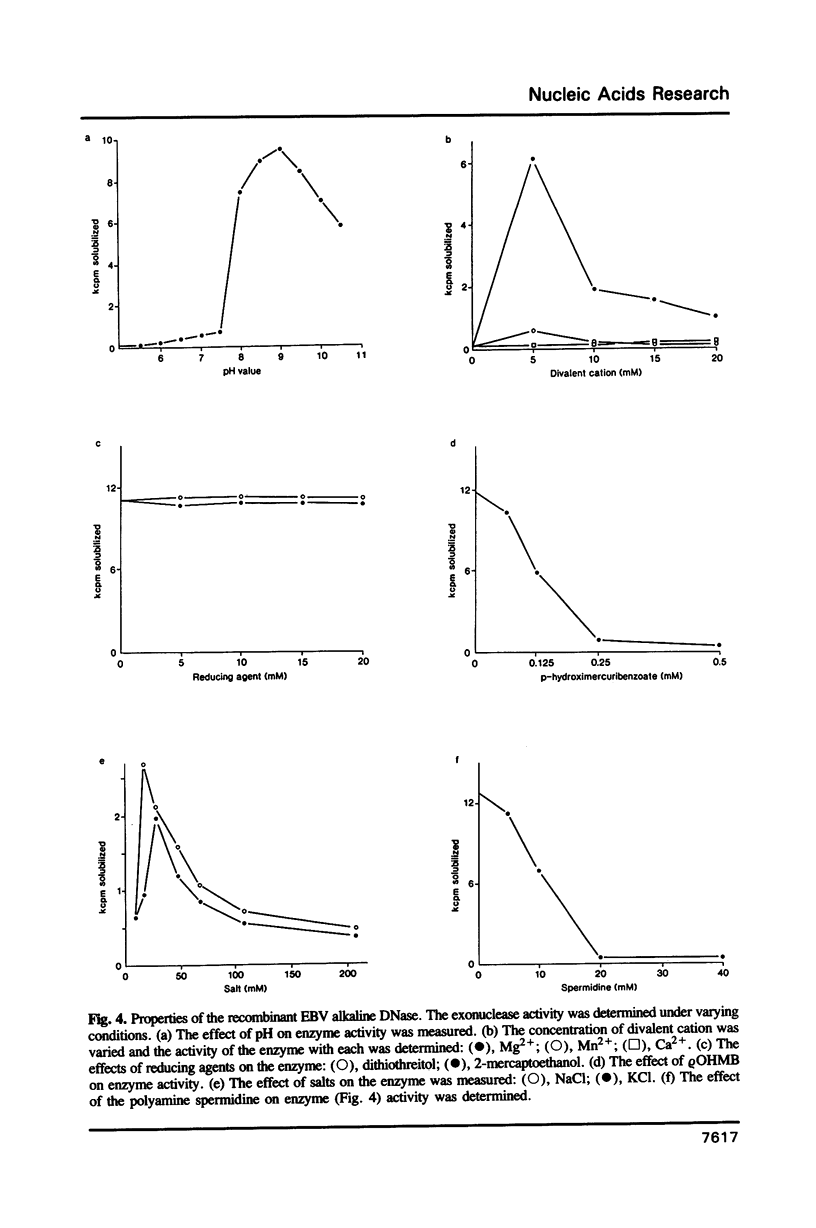

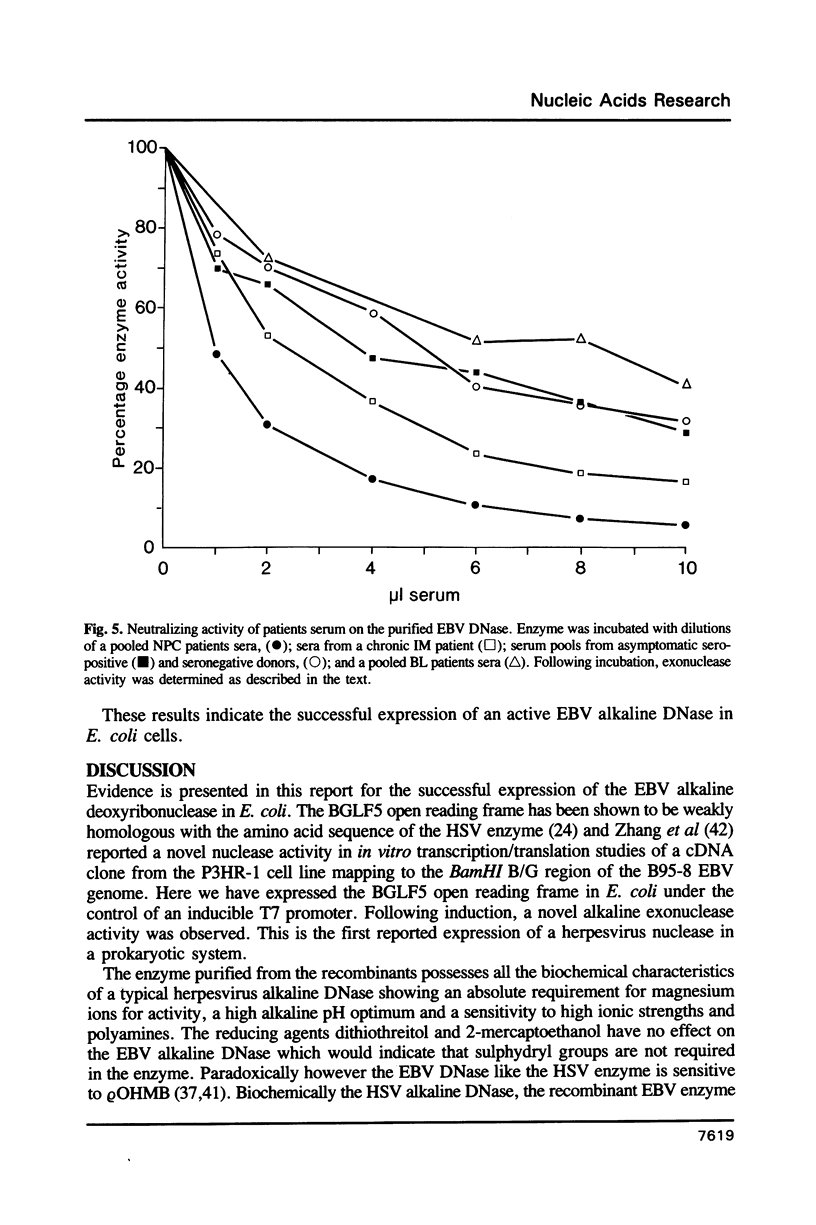



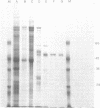
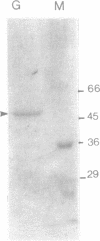
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaudeen H. S., Bertino J. R. Isolation of a herpesvirus-specific DNA polymerase from tissues of an American patient with Burkitt lymphoma. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4504–4508. doi: 10.1073/pnas.75.9.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrand J. R., Rymo L., Walsh J. E., Björck E., Lindahl T., Griffin B. E. Molecular cloning of the complete Epstein-Barr virus genome as a set of overlapping restriction endonuclease fragments. Nucleic Acids Res. 1981 Jul 10;9(13):2999–3014. doi: 10.1093/nar/9.13.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Chartrand P., Timbury M. C., Hay J., Moss H. Mutant of herpes simplex virus type 2 with temperature-sensitive lesions affecting virion thermostability and DNase activity: identification of the lethal mutation and physical mapping of the nuc-lesion. J Virol. 1979 Oct;32(1):140–146. doi: 10.1128/jvi.32.1.140-146.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Y., Chen C. J., Liu M. Y., Cho S. M., Hsu M. M., Lynn T. C., Shieh T., Tu S. M., Lee H. H., Kuo S. L. Antibodies to Epstein-Barr virus-specific DNase in patients with nasopharyngeal carcinoma and control groups. J Med Virol. 1987 Sep;23(1):11–21. doi: 10.1002/jmv.1890230103. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Chen J. Y., Glaser R., Henle W. Frequency and levels of antibodies to Epstein-Barr virus-specific DNase are elevated in patients with nasopharyngeal carcinoma. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6162–6165. doi: 10.1073/pnas.77.10.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Roizman B. Characterization of DNA sequence-common and sequence-specific proteins binding to cis-acting sites for cleavage of the terminal a sequence of the herpes simplex virus 1 genome. J Virol. 1989 Mar;63(3):1059–1068. doi: 10.1128/jvi.63.3.1059-1068.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough W. Deoxyribonuclease activity found in Epstein--Barr virus producing lymphoblastoid cells. Biochemistry. 1979 Oct 16;18(21):4517–4521. doi: 10.1021/bi00588a009. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A., ACHONG B. G., BARR Y. M. VIRUS PARTICLES IN CULTURED LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. Lancet. 1964 Mar 28;1(7335):702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- Francke B., Garrett B. The effect of a temperature-sensitive lesion in the alkaline DNase of herpes simplex virus type 2 on the synthesis of viral DNA. Virology. 1982 Jan 15;116(1):116–127. doi: 10.1016/0042-6822(82)90407-x. [DOI] [PubMed] [Google Scholar]
- Francke B., Moss H., Timbury M. C., Hay J. Alkaline DNase activity in cells infected with a temperature-sensitive mutant of herpes simplex virus type 2. J Virol. 1978 May;26(2):209–213. doi: 10.1128/jvi.26.2.209-213.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Ho H. C., Burtin P., Cachin Y., Clifford P., de Schryver A., de-Thé G., Diehl V., Klein G. Antibodies to Epstein-Barr virus in nasopharyngeal carcinoma, other head and neck neoplasms, and control groups. J Natl Cancer Inst. 1970 Jan;44(1):225–231. [PubMed] [Google Scholar]
- Littler E., Halliburton I. W., Powell K. L., Snowden B. W., Arrand J. R. Immunological conservation between Epstein-Barr virus and herpes simplex virus. J Gen Virol. 1988 Aug;69(Pt 8):2021–2031. doi: 10.1099/0022-1317-69-8-2021. [DOI] [PubMed] [Google Scholar]
- Liu M. Y., Chen J. Y., Yang C. S. Activity of DNase associated with replication of Epstein-Barr virus in NPC-204 cells. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi. 1984 Aug;17(3):121–130. [PubMed] [Google Scholar]
- McGeoch D. J., Davison A. J. DNA sequence of the herpes simplex virus type 1 gene encoding glycoprotein gH, and identification of homologues in the genomes of varicella-zoster virus and Epstein-Barr virus. Nucleic Acids Res. 1986 May 27;14(10):4281–4292. doi: 10.1093/nar/14.10.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss H. The herpes simplex virus type 2 alkaline DNase activity is essential for replication and growth. J Gen Virol. 1986 Jun;67(Pt 6):1173–1178. doi: 10.1099/0022-1317-67-6-1173. [DOI] [PubMed] [Google Scholar]
- Ooka T., Lenoir G., Daillie J. Characterization of an Epstein-Barr virus-induced DNA polymerase. J Virol. 1979 Jan;29(1):1–10. doi: 10.1128/jvi.29.1.1-10.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett P. E., Biggin M. D., Barrell B., Roizman B. Epstein-Barr virus genome may encode a protein showing significant amino acid and predicted secondary structure homology with glycoprotein B of herpes simplex virus 1. J Virol. 1985 Dec;56(3):807–813. doi: 10.1128/jvi.56.3.807-813.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Tan R. S., Datta A. K., Cheng Y. C. Identification and characterization of a DNase induced by Epstein-Barr virus. J Virol. 1982 Dec;44(3):893–899. doi: 10.1128/jvi.44.3.893-899.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. V., Boak A., Glaser R. Antigenic variation in alkaline deoxyribonuclease induced by three different strains of Epstein-Barr virus. J Med Virol. 1988 Oct;26(2):207–215. doi: 10.1002/jmv.1890260212. [DOI] [PubMed] [Google Scholar]