ABSTRACT
Background:
Transcatheter arterial chemoembolization (TACE) improves survival in patients with unresectable hepatocellular carcinoma (HCC). Partial liver radiotherapy with modern techniques has been shown to be safe. The purpose of this study was to evaluate the survival value of external beam radiation therapy (EBRT) with concurrent chemotherapy combined with TACE.
Methods:
A University of Virginia Interventional Radiology patient log was used to identify patients treated with TACE ± another modality from 1999 through 2005. During this time, 44 patients received TACE for unresectable HCC, and 7 of these received adjuvant EBRT. Univariate analysis and multivariable proportional hazards survival modeling were used to identify factors impacting survival.
Results:
We compared 37 patients receiving TACE alone to 7 receiving TACE and EBRT (5 with concurrent capecitabine). Unadjusted mean transplant-free survival times were TACE only = 376 days (standard error [SE] = 63 days), TACE + EBRT = 879 days (SE = 100 days). EBRT, TNM stage, and MELD score were important predictors for survival on univariate analysis (p < .10). The adjusted hazard ratio for transplant or death in the TACE + EBRT group was 0.15 (0.02–0.95, p = .026).
Conclusion:
EBRT with concurrent chemotherapy following TACE is feasible and well tolerated with modern treatment techniques. Further research should be directed toward determining the potential overall survival benefit of adjuvant EBRT with chemotherapy following TACE for hepatocellular carcinoma.
Liver cancer ranks third in causes of death from cancer and is the fifth most common form of cancer in the world.1 Eighty-five to ninety percent of primary liver cancers are hepatocellular carcinomas (HCC).2 The incidence of HCC in the United States is increasing from 1.4 per 100,000 (1976–1980) to 2.4 per 100,000 (1991–1995).3
Patients with an early-stage single lesion who are not cirrhotic or are cirrhotic but with normal bilirubin and a hepatic vein pressure gradient less than 10 mm Hg may be considered for surgical resection. Patients with a single lesion ≤ 5 cm or up to three lesions < 3 cm may be offered either liver transplantation or percutaneous ablation with alcohol or radiofrequency based upon their physical status. Unfortunately, patients with large or multifocal HCC are left with noncurative options for treatment. In cases without vascular invasion or spread outside the liver, transarterial chemoembolization (TACE) may also be an option.4
The theoretical basis for embolization and chemoembolization rests on the finding that HCC tumors receive their blood supply almost exclusively from the hepatic artery.5 Several randomized controlled trials have shown a survival benefit with TACE over conservative management in patients with unresectable HCC.6,7 Llovet and Bruix performed a meta-analysis of six small randomized controlled trials from Europe and Asia comparing embolization (with or without chemotherapy) to conservative management or substandard therapies and found an improvement in 2-year survival with embolization.8
Due to advances in delivery technique, external beam radiation therapy (EBRT) is increasingly being recognized as a potential therapy for HCC.9 Three-dimensional conformal radiotherapy at a dose of 66 Gy has been shown to produce excellent imaging-based response rates with acceptably low toxicity in cirrhotic patients who have one tumor ≤ 5 cm or two tumors ≤3cm.10 Radiologic response rate increased with higher doses of radiation in a multivariate analysis.11 Higher radiation dose was also shown to be significantly associated with survival in multivariate analysis.12 EBRT has been used as a primary therapy, as an adjunct to other modalities, or as salvage therapy after failure of other treatments.10–13 Three-dimensional conformal EBRT combined with TACE has been shown to be a viable strategy in a dedicated study of patients with HCC complicated by portal vein tumor thrombosis.14
Currently there is insufficient evidence available, particularly in North American patients, to evaluate adequately the survival benefit of treating unresectable HCC with TACE combined with local radiotherapy. Our aim was to characterize the outcomes of HCC patients undergoing TACE and other modalities compared to those patients undergoing these targeted therapies accompanied by EBRT at our center and identify predictors of beneficial response to adjunctive EBRT. Our hypothesis is that EBRT provides additional transplant-free survival benefit when patients undergo TACE (with additional interventional modalities as clinically indicated) for HCC, particularly in unresectable tumors.
METHODS
This study is a retrospective, case-control study comparing outcomes of TACE + EBRT compared to TACE alone in patients with HCC. An Interventional Radiology patient log was used to identify patients treated with TACE at the University of Virginia from 1999 through 2005. Patients treated with another modality such as radiofrequency ablation (RFA) or ethanol injection were included. Thus, three patients in the TACE and EBRT group received adjunctive RFA. Similarly, in the TACE group, four patients received adjunctive RFA, and two patients received adjunctive alcohol injection. Demographic characteristics, laboratory values, tumor characteristics, treatment course, and outcome were extracted from the medical record. Patient characteristics are listed in Table 1.
Table 1.
Demographic and cancer-related characteristics of patients receiving TACE or TACE + EBRT for hepatocellular carcinoma
| Gender (F/M) | Race | Age | MELD | TBR (mg/dL) | Alb (g/dL) | INR | CPT score | No. lesions | Total size of lesions (cm) | |
|---|---|---|---|---|---|---|---|---|---|---|
| TACE | 9/28 | White, 29 | 58.3 (10.8) | 12.7 (3.4) | 2.2 (1.2) | 3.2 (0.5) | 1.4 (0.2) | 8 | 1 | 4.0 (1.6) |
| Black, 3 | (5–12) | (1–5) | ||||||||
| Asian, 3 | ||||||||||
| TACE + EBRT | 0/7 | White, 6 | 64.2 (9.1) | 9.4 | 1.0 (0.4) | 3.7 | 1.2 (0.2) | 5 | 1 | 13.4 |
| Black, 1 | (2.4) | (0.8) | (5–8) | (1–4) | (5.3) | |||||
Values are counts, mean (SD), or median (range).
Abbreviations: Alb = albumin; CPT = Child-Pugh-Turcotte; EBRT = conformal external beam radiation with concurrent capecitabine; INR = international normalized ratio; MELD = Model for End-stage Liver Disease; TACE = transarterial chemoembolization; TBR = total bilirubin.
To avoid possible confounding data from multiple sources of radiation, exclusion criteria included treatment with radioactive microspheres. Those who underwent resection were also excluded. The Social Security Master Death Registry was used to confirm and identify date of death for treated patients.
The typical chemotherapeutic agents used for TACE were cisplatin, doxorubicin, and mitomycin C. Mitomycin C was omitted in 6 patients receiving TACE alone and 1 patient receiving TACE and EBRT.
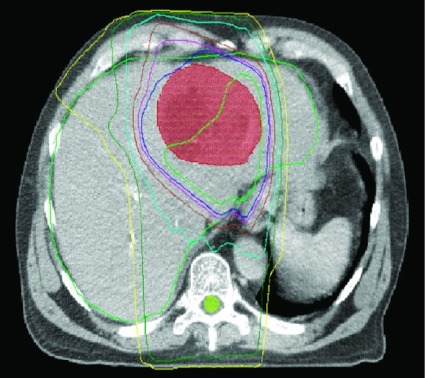
Radiation was delivered to a median dose of 30 Gy in 12 fractions (range from 20 Gy in 8 fractions to 64.8 Gy in 36 fractions). A representative treatment plan is shown in Figure 1. Treatment was delivered daily, 5 days a week. Concurrent capecitabine was used for 5 patients, delivered at fixed dose of 1 g in the morning and 2 g in the afternoon only on days patients underwent radiotherapy.
Figure 1.
Representative dose distribution for patient being treated with 3D conformal radiotherapy to a hepatoma (red zone) after TACE. The dark blue line represents the volume receiving 100% of the dose and the light blue line represents the volume receiving 70% of the dose.
Univariate analysis was performed to assess significance to p < .10 with regard to transplant-free survival for the following variables: age, gender, race, primary and secondary etiology, total bilirubin, creatinine, alpha feto-protein (AFP), Model for End-stage Liver Disease (MELD) score, presence of ascites, presence of encephalopathy, number of lesions, cumulative lesion size, tumor stage, and use of radiotherapy. Of these possible predictors, MELD, cumulative tumor size, and use of EBRT met the conditions of significance and were included in the final multivariable Cox proportional hazards survival model to determine their collective effect on transplant-free survival. SAS 9.2 was used for all data management and analyses. Results were considered statistically significant if p < .05.
RESULTS
Forty-four patients were treated with TACE at the University of Virginia from 1999 through 2005 and had adequate follow-up. Seven of 44 patients (15.9%) received EBRT after TACE failure. Five of the 7 were treated with EBRT and concurrent capecitabine.
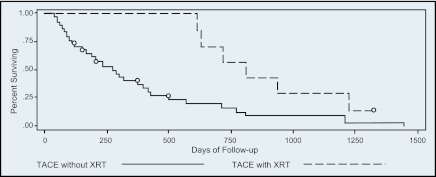
Thirteen patients ultimately underwent liver transplantation. Unadjusted transplant-free survival is depicted in Figure 2, which shows that patients undergoing adjunctive EBRT had better survival compared to those without EBRT as part of their therapy, though the result was not statistically significant (log-rank test, p = .94), most likely related to type II error due to small sample size.
Figure 2.
Unadjusted survival after TACE vs. TACE and EBRT.
Among the variables that were tested, treatment with EBRT, cumulative tumor burden, and MELD were associated with transplant-free survival in the univariate analysis. These factors along with age were then included in the Cox proportional hazards survival model, which demonstrated significantly improved transplant-free survival for patients receiving EBRT with concurrent capecitabine after TACE (adjusted hazard ratio [HR] 0.15, 95% confidence interval [CI] 0.02–0.95) in the adjusted model (Table 2).
Table 2.
Cox proportional hazards regression results
| Variable | Hazard ratio | 95% confidence interval | p value |
|---|---|---|---|
| EBRT | 0.15 | 0.02–0.95 | 0.44 |
| MELD (per 1 point) | 1.07 | 0.97–1.19 | 0.19 |
| Cumulative tumor burden (per linear cm diameter) | 1.09 | 0.93–1.28 | 0.29 |
Abbreviations: EBRT = conformal external beam radiation with concurrent capecitabine; MELD = Model for End-stage Liver Disease.
DISCUSSION
In this retrospective cohort study, we have shown that chemotherapy and conformal EBRT along with capecitabine after TACE improves transplant-free survival as compared to TACE alone. In particular, median survival increased from 270 days to 805 days. Although limited in size and by its retrospective nature, our results are comparable to Asian studies (Table 3) and support a role for EBRT in these patients. These results, taken with those from Asia and Europe, support systematic study of the use of adjuvant EBRT with other typical treatment modalities for HCC in a randomized controlled trial.
Table 3.
Comparison of trials evaluating transarterial chemoembolization (TACE) in combination with radiation therapy (RT) for hepatocellular carcinoma (HCC)
| Study | Size | Study type | Child class | Etiology | Mean tumor size (cm) | Median survival (months) |
|---|---|---|---|---|---|---|
| Yamada et al., | 19 (TACE + RT) | Prospective | A 13 | Unspecified | 5 | 7 |
| Japan | B 5 | |||||
| C 1 | ||||||
| Seong et al., | 27 (TACE + RT) | Prospective | A 17 | Viral 20 | 7 | 26 |
| Republic of Korea | B 10 | Other 7 | ||||
| Cupino et al., | 44 | Retrospective | A 17 | Viral 23 | 13 | 26 |
| Virginia | B 21 | Ethanol 13 | ||||
| C 6 | Other 12 | |||||
| Shim et al., | 105 | Retrospective | A 65 | Unspecified | 10 | 20 |
| China | B 8 | |||||
| Guo et al., Republic of Korea | 165 | Retrospective | A 137 | Unspecified | Unspecified | 19 |
| B 28 | ||||||
While North American publications on TACE combined with EBRT are limited only to our institution, a number of positive studies have been done in Asia. Although the techniques and doses vary, the results suggest TACE with EBRT provides superior results to TACE alone. Guo studied 76 patients treated with TACE and EBRT along with 89 patients from the same period treated with TACE alone. Survival rates at 1, 3, and 5 years were 64%, 28.6%, and 19.3%, respectively, for treatment with TACE and EBRT vs. 39.9%, 9.5%, and 7.2% for TACE alone (p = .0,001).15 Shim et al examined 73 patients treated incompletely with TACE. Thirty-five received repeat TACE, and 38 also received local radiotherapy. Two-year survival after TACE and radiotherapy was 36.8% vs. 14.3% for TACE alone (p = .001).16 Our data also support a survival benefit with the addition of EBRT in North American patients.
Meng performed a meta-analysis of 17 Asian clinical trials concentrating on tumor response and overall survival after TACE vs. TACE and EBRT. They found significant improvement with TACE and EBRT for complete response rate as well as overall survival at years 1 through 5.17 Moreover, they found no significant difference with respect to toxicities, namely, nausea, vomiting, leucopenia, alanine aminotransferase levels, and total bilirubin levels. The analysis is limited by unclear randomization techniques and a patient population dissimilar to those found in North America, particularly with respect to cirrhotic etiology. One common thread, though, is a response rate related to tumor size.
When patients in the Shim study were broken down by tumor size (5–7, 8–10, and >10 cm), there was a significant survival advantage for TACE and radiotherapy in the 8–10 cm group (50% vs. 0%, p = .03) and for the >10 cm group (17% vs. 0%, p = .0,002). Results for the 5-cm group, however, were not statistically significant (63% vs. 42%, p = .22).16 In a similar series from our institution, McIntosh and coworkers evaluated patients with HCC treated with accelerated intensity-modulated radiation therapy and concurrent capecitabine. Median tumor size in this group was 9.5 cm. While not all patients received TACE prior to radiation, the group demonstrated a median survival of 9.6 months after completion of radiation therapy and a 50% 2-year overall survival,18 which also supports the Asian data.
Song reported that patients with larger tumors were more likely to have post-TACE increases in IGF-2 levels.19 They found larger tumors were more likely to metastasize, and poor response to TACE was linked to posttreatment metastases. This propensity to increased growth factors after TACE enhances the argument for additional therapy. Indeed, in our patient population, retrospective analysis shows the patients selected for adjuvant radiotherapy were those with larger cumulative tumor size. Further investigation in this direction may allow us to select patients up front for whom adjuvant radiotherapy would be of most benefit.
Yao defined the University of California San Francisco criteria and showed a subset of patients with advanced HCC can be treated in the neoadjuvant setting to allow for liver transplantation.20 However, Mazzaferro found no difference in survival between transplantation alone and local therapy prior to transplant.21 As of yet, no level I evidence exists for defining a subgroup of patients eligible for down-staging prior to transplant. However, the combination of TACE and EBRT increase the number of patients who can be rendered eligible for transplantation.
Indeed, a Canadian trial has suggested EBRT is a safe and efficacious therapy for bridging patients to liver transplantation.22 Their success with radiation alone suggests the use of multimodality therapy, such as TACE and EBRT, may warrant further evaluation. Recent review articles and consensus statements suggest a rising interest in randomized trials to evaluate further the benefit of EBRT in combination with TACE.23–24
Level I evidence has emerged for the use of sorafenib in advanced HCC. Llovet demonstrated a 3-month increase in median survival for Child-Pugh class A cirrhotic patients with HCC.25 Though cumulative lesion size was not described in their paper, the target lesion diameter by Response Criteria in Solid Tumor (RECIST) could be as small as 10 mm. While the results of this trial have resulted in more patients with large tumors being treated with sorafenib, it should be recalled that advanced HCC was defined in part by failure of locoregional therapies.
While the role of biologic agents such as sorafenib remains to be fully defined in the spectrum of treatment alternatives in HCC, side effects25 and costs26 will affect their applicability. Small studies, including the current one, suggest TACE and EBRT should be considered as a locoregional therapeutic option.15–18 As with other single-institution retrospective analyses, the current study is limited due to bias in patient selection, small sample size, and heterogeneous treatment technique.
CONCLUSION
With close follow-up after TACE, local treatment failures can be treated by adding EBRT to improve local control. Modern radiation therapy techniques allow for liver sparing and safe administration of combination chemoradiotherapy with concurrent capecitabine following TACE. Larger randomized, controlled studies are needed to identify factors to delineate patient selection for postintervention EBRT plus capecitabine.
Footnotes
Disclosures of Potential Conflicts of Interest
Dr. Argo has served on an Advisory Board for Bayer/Onyx.
REFERENCES
- 1. Parkin D: Global cancer statistics in the year 2000. Lancet Oncol 2:533–543, 2001 [DOI] [PubMed] [Google Scholar]
- 2. El-Serag H, Rudolph K: Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132:2557–2576, 2007 [DOI] [PubMed] [Google Scholar]
- 3. El-Serag H, Mason A: Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 340:745–750, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Bruix J, Sherman M: AASLD practice guideline: management of hepatocellular carcinoma. Hepatology 42:1208–1236, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Breedis C, Young G: The blood supply of neoplasms of the liver. Am J Pathol 30:969–985, 1954 [PMC free article] [PubMed] [Google Scholar]
- 6. Lo C, Ngan H, Tso W, et al. : Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35:1164–1171, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Llovet J, Real M, Montana X, et al. : Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 359:1734–1739, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Llovet J, Bruix J: Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37:429–442, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Aebersold DM: Potential and future strategies for radiotherapy in hepatocellular carcinoma. Liver Int 29:145–146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mornex F, Girard N, Beziat C, et al. : Feasibility and efficacy of high-dose three-dimensional-conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies—mature results of the French phase II RTF-1 trial. Int J Radiat Oncol Biol Phys 66:1152–1158, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Park HC, Seong J, Han KH, et al. : Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 54:150–155, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Seong J, Park HC, Han KH, et al. : Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys 55:329–336, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Seong J, Park HC, Han KH, et al. : Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys 47:1331–1335, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Yamada K, Izaki K, Sugimoto K, et al. : Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombosis in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 57:113–119, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Guo W, Yu E, Liu L, et al. : Comparison between chemoembolization combined with radiotherapy and chemoembolization alone for large hepatocellular carcinoma. World J Gastroenterol 9:1697–1701, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shim S, Seong J, Han K, et al. : Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatcellular carcinoma. Liver Int 25:1189–1196, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Meng MB, Cui YL, Lu Y, et al. : Transcatheter arterial chemoembolization with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol 92:184–194, 2009 [DOI] [PubMed] [Google Scholar]
- 18. McIntosh A, Hagspiel K, Al-Osaimi AM, et al. : Accelerated treatment using intensity-modulated radiation therapy plus concurrent capecitabine for unresectable hepatocellular carcinoma. Cancer 115:5117–5125, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Song BC, Chung YH, Kim JA, et al. : Association between insulin-like growth factor-2 and metastases after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Cancer 91:2386–2393, 2001 [PubMed] [Google Scholar]
- 20. Yao FY, Kerlan RK, Hirose R, et al. : Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 48:819–827, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mazzaferro V, Regalia E, Doci R, et al. : Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334:693–699, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Sandroussi C, Dawson LA, Lee M, et al. : Radiotherapy as a bridge to liver transplantation for hepatocellular carcinoma. Transpl Int 23:299–306, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Dawson LA: Overview: where does radiation therapy fit in the spectrum of liver cancer local-regional therapies? Semin Radiat Oncol 21:241–246, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Schwarz RE, Abou-Alfa GK, Geschwind JF, et al. : Nonoperative therapies for combined modality treatment of hepatocellular cancer: expert consensus statement. HPB 12:313–320, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Llovet JM, Ricci S, Mazzaferro V, et al. : Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Muszbek N, Shah S, Carroll SM, et al. Economic evaluation of sorafenib vs. best supportive care in hepatocellular carcinoma. J Clin Oncol 26(15S):378–6527, 2008 [Google Scholar]