ABSTRACT
Colorectal cancer (CRC) is the third most common cancer in men and the second most common cancer in women worldwide. Both genetic and epigenetic alterations are common in CRC and are the driving force of tumorigenesis. The adenoma-carcinoma sequence was proposed in the 1980s that described transformation of normal colorectal epithelium to an adenoma and ultimately to an invasive and metastatic tumor. Initial genetic changes start in an early adenoma and accumulate as it transforms to carcinoma. Chromosomal instability, microsatellite instability and CpG island methylator phenotype pathways are responsible for genetic instability in colorectal cancer. Chromosomal instability pathway consist of activation of proto-oncogenes (KRAS) and inactivation of at least three tumor suppression genes, namely loss of APC, p53 and loss of heterozogosity (LOH) of long arm of chromosome 18. Mutations of TGFBR and PIK3CA genes have also been recently described. Herein we briefly discuss the basic concepts of genetic integrity and the consequences of defects in the DNA repair relevant to CRC. Epigenetic alterations, essential in CRC tumorigenesis, are also reviewed alongside clinical information relevant to CRC.
Colorectal cancer (CRC) is the third most common cancer in men and the second most common cancer in women worldwide. More than 1.2 million new cases of colorectal cancers are diagnosed globally, with more than 600,000 related deaths in 2008.1 Both genomic and epigenetic alterations are common in CRC and are the driving forces of tumorigenesis. The chromosomal instability pathway (CIN) and microsatellite instability pathway (MSI) are the two recognized pathways of carcinogenesis in CRC. In the 1980s researchers proposed a four-step progression of gene alterations in colonic epithelium. This phenomenon starts from transformation of normal epithelium to an adenoma, proceeding to in situ carcinoma, and ultimately to invasive and metastatic tumor.
In 1990 Fearon and Vogelstein elucidated specific pathways essential to the development of CRC, consisting of accumulated mutations in multiple genes that regulate cell growth and differentiation.2 Both genetic and epigenetic alterations, the latter leading to aberrant methylation of tumor suppressor genes, result in inactivation of these genes and subsequent promotion of neoplasia. Sporadic CRCs share major genetic abnormalities with their inherited counterparts. Hence, the study of inherited familial CRC syndromes such as Familial Adenomatous Polyposis (FAP) and Lynch Syndrome (Hereditary Non-Polyposis Colorectal Cancer [HNPCC]) has greatly assisted understanding of the molecular pathogenesis underlying sporadic CRC. Herein we briefly discuss the basic concepts of genetic integrity and the consequences of defects in the DNA repair relevant to CRC. Epigenetic alterations, essential in CRC tumorigenesis, are also reviewed alongside clinical information relevant to CRC.
GENOME INTEGRITY
The human genome employs various strategies to protect colonic epithelial stem cells from accumulating genomic errors.3 Stem cells are quiescent and rarely replicate. They are positioned in areas throughout tissues with low exposure to environmental toxins and mutagens. Colonic epithelial stem cells reside deep in the bottom of crypts where they are covered with thick mucin produced by neighboring cells. If damaged, they initiate apoptosis rather than repair.
Stem cells pump out potential mutagenic molecules from the cell via a plasma membrane protein called Mdr1 (multidrug resistance 1). A high level of Mdr1 expression occurs on the surface of stem cells, and it is believed that the resistance of cancer stem cells to chemotherapeutic agents is partly due to the action of this protein pump.
Asymmetric DNA strand allocation is a strategy to preserve DNA integrity of the colonic epithelium. When stem cells divide, only one of two daughter cells proceeds further to become the “transit-amplifying cell” and undergo subsequent differentiation. These cells live for 5–7 days and are then sloughed from the surface of the intestinal mucosa, thereby losing the opportunity to become cancerous. The other daughter cell allocated to remain a stem cell is protected from further mutation and damage. The same daughter cell may serve as the template to decrease the chances of DNA error. (conserved-stand model). Approximately 30% of genes in the human genome encode for proteins that regulate DNA fidelity.
GENOMIC INSTABILITY
In colon cancer, three distinct pathways of genomic instability have been recognized: the (1) Chromosomal Instability, (2) Microsatellite Instability, and (3) CpG Island Methylator Phenotype pathways.4
The Chromosomal Instability Pathway
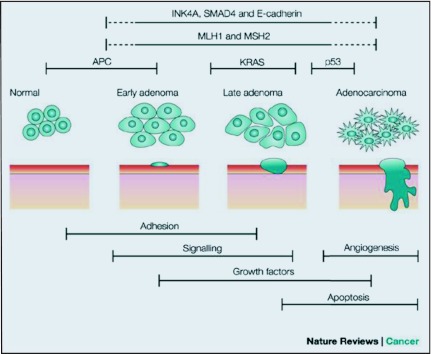
This Chromosomal Instability (CIN) pathway is also known as the adenoma-carcinoma sequence (Figure 1), and it follows a predictable progression of genetic and corresponding histologic changes. The genomic changes include activation of proto-oncogenes (K-Ras) and inactivation of at least three tumor suppression genes, namely, loss of APC (chromosome region 5q21), loss of p53 (chromosome region 17p13), and loss of heterozygosity for the long arm of chromosome 18 (18q LOH).3 Recently mutations involving other genes have been described, such as the TGFBR and PIK3CA, that are required for the adenoma-carcinoma sequence model.
Figure 1.
Adenoma-carcinoma sequence.
APC is the most common initial gene mutated in familial/inherited and sporadic colon cancer. Controversy exists as to whether genomic instability initiates the adenoma-carcinoma sequence or whether it arises during the process and facilitates evolution to CRC.5 Chromosomal instability or microsatellite instability (MSI) can be observed in adenomas.6 Thus, genetic instability appears to be present during the initiation of adenoma, before APC gene mutation and progression to frank malignancy.7
Adenomatous Polyposis Coli (APC) Gene and Wnt Signaling Pathway
APC-associated polyposis conditions include Familial Adenomatous Polyposis (FAP), attenuated FAP, Gardner syndrome, and Turcot syndrome. FAP is a CRC-predisposition syndrome in which hundreds to thousands of precancerous colon polyps develop, beginning in the second decade of life. It accounts for less than 1% of all colon cancers. Carcinoma develops a decade after the appearance of the polyps. Without prophylactic colectomy, colon cancer will occur in 100% of these patients.
Patients with attenuated FAP have significantly fewer polyps; the location of the polyps is in the more proximal part of the colon, and colon cancer develops at a later age. Gardner syndrome is a subtype of FAP associated with osteomas and soft tissue tumors. Turcot syndrome manifests as colonic polyps along with central nervous system tumors.
The APC/Wnt/β-catenin pathway plays a major role in CRC carcinogenesis in both sporadic and hereditary CRC. The majority (98%) of APC mutations are either frameshift or nonsense mutations leading to the synthesis of a truncated protein. This mutation is found in approximately 30%–70% of sporadic adenomas and sporadic CRCs.8–10
The APC tumor suppressor gene normally blocks transition from G1 to S phase of the cell cycle. The Wnt signaling pathway maintains native stem cells in their undifferentiated state in the base of the colonic crypts, which contributes not only to the survival of normal stem cells but also to the survival of cancer cell stem cells. β-Catenin is a major player in the Wnt signaling pathway. Unmutated APC induces degradation of β-catenin and therefore functions as a negative regulator of the Wnt signaling pathway.11,12 Sustained levels of intracellular β-catenin result in prolonged activation of the Wnt pathway in APC mutated colorectal cancer cells.
In Sporadic CRC cases with wild-type APC gene, APC gene promoter hypermethylation or point mutation in the β-catenin structure has been described to explain the sustained activation of the Wnt signaling pathway.13 In normal colonic mucosa, stem cells migrate out of the epithelial crypts as they differentiate and are subsequently sloughed off 3–7 days post-apoptosis. β-Catenin controls this migratory behavior. In otherwise healthy people, many cells acquire various mutations during replication and differentiation. However, since they are normally shed in less than a week, they do not have the opportunity to induce cancer. Accumulation of β-catenin in enterocyte precursors due to APC inactivation leads to retention of a stem cell phenotype, which prevents them from migrating to the surface to be sloughed off. The accumulation of undifferentiated cells in the colonic crypts eventually leads to the formation of a polyp. As proposed in the adenoma-carcinoma sequence model, accumulation of subsequent additional mutations, involving genes, such as K-RAS and TP53, may eventually lead to carcinoma.
TP53 Mutation
The TP53 gene is significantly involved in the control of the cell cycle and apoptosis and is commonly mutated in CRC.14,15 The p53 protein induces G1 cell-cycle arrest and facilitates DNA repair prior to a cell committing to the process of DNA replication. If DNA repair is unsuccessful, p53 induces cell death (apoptosis). TP53 mutation is generally believed to occur at the time of transition from adenoma to cancer.
Numerous studies have attempted to elaborate on the prognostic significance of TP53 mutation in CRC, with conflicting results. Zeng studied the prognostic significance of TP53 gene overexpression in node-positive CRC patients. The 5-year disease-free survival for p53-positive patients was almost double the p53-negative group (60% vs. 35%).16 Adrover concluded that overexpression of p53 in stage III CRC carries a better overall survival in CRC patients.17 Popat et al prospectively studied the prognostic significance of p53 and thymidine synthase status as a marker for overall survival in the adjuvant treatment of CRC in approximately 1000 patients. In their study, 60% of patients had rectal cancer and 90% received adjuvant chemotherapy. P53 was overexpressed in 60% of the tumors, and there was no significant prognostic value in the adjuvant setting of CRC.18
Inherited or germline mutations in TP53 are the cause of Li-Fraumeni syndrome, a cancer predisposition syndrome associated with a variety of neoplasms, including soft tissue sarcoma, osteosarcoma, premenopausal breast cancer, brain tumors, and adrenocortical carcinoma.
18q Loss of Heterozygosity (LOH)
LOH in the region of 18q21 is frequently seen in advanced colorectal cancer. LOH is defined as loss of one of the two copies or alleles of a gene. Often the remaining allele is affected by a mutation. The DCC (Deleted in Colorectal Carcinoma) gene is located on the long arm of chromosome 18 (18q21.3). It encodes the transmembrane protein DCC. DCC is a “conditional tumor suppressor gene.”
Contrary to the common types of transmembrane receptors, DCC blocks cell growth in the absence of its ligand, netrin-1. Approximately 70% of CRCs show LOH in the DCC gene region. Netrin-1 is produced deep in the crypts of the colorectal mucosa. As epithelial cells differentiate and move toward the surface, the concentration of netrin-1 decreases. This concentration gradient is felt to contribute to the normal process of apoptosis and sloughing of epithelial cells. When the DCC gene is mutated, netrin-1 will not bind to DCC transmembrane protein, resulting in abnormal cell survival.
Netrin-1 overexpression has also been reported in patients with advanced CRC and can overcome the DCC apoptotic effect.19 Some, but not all, studies have found an inverse relationship between CRC patient survival and 18qLOH.20,21 A meta-analysis of 17 studies comprising more than 2000 patients found significantly worse overall survival in patients with 18q allelic imbalance and loss of DCC (hazard ratio [HR] = 2, 95% confidence interval [CI] 1.49–2.69). However, there was evidence of heterogeneity and publication bias.22 Ogino prospectively evaluated the effect of 18q LOH on 532 non–MSI-high, stage I–IV CRC tumors.
In patients with non-MSI-high colorectal cancer, 18q LOH or allelic imbalances were not significantly associated with a difference in survival. The 5-year overall survival was 70% among patients with 18q LOH-positive tumors, and 68% among those with 18q LOH-negative tumors. This study is in agreement with studies, by Halling et al (n = 432 non-MSI-high tumors)23 and Barratt et al (n = 279 non-MSI-high tumors).24 On the other hand, Watanabe found a significant survival difference with 18qLOH and non-MSI-high groups. The group with stage III colon cancer had poor survival. This study identified the 5-year disease-free survival of high-risk stage II colon cancer to be 64% with retained 18q compared to 44% among those with LOH at 18q (p = .002). The corresponding 5-year overall survival rates were 69% with retention of 18q alleles and 50% with allelic loss at 18q (p = .005).20 MSI-high tumors rarely exhibit 18qLOH. The American Society of Clinical Oncology does not recommend testing for DCC due to the conflicting data at this time.
Microsatellite Instability (MSI) and Mismatch Repair Pathways (MMR)
DNA nucleotides are altered by environmental mutagens and spontaneous errors. During cell replication, DNA polymerase “reads” an intact DNA strand as a template and uses it to synthesize an identical copy (semiconservative replication). However, DNA polymerase is not perfect, and errors occur during DNA replication. While DNA polymerase is assembling nucleotides in a 5′ to 3′ direction, it continuously looks backward scanning the assembled strand for errors. When an error is detected DNA polymerase moves backwards and utilizes its endogenous exonuclease activity to remove the erroneous section. This proofreading function is not perfect. The mismatch repair (MMR) system checks and repairs defects that were overlooked by DNA polymerase.
MSI is the hallmark of Lynch syndrome (Hereditary Non-Polyposis Colorectal Cancer [HNPCC]) and is seen in more than 95% of these patients.25 In contrast, for most sporadic colorectal cancers, the mechanisms responsible for chromosomal instability remain elusive, and MSI is responsible for only 15%–20% of the cases.25
Lynch syndrome, which accounts for 3%–5% of all colorectal cancers, is an autosomal dominant cancer-susceptibility disorder caused by germline mutations in one of the several MMR genes. It is characterized by an increased risk of colon cancer and cancers of the endometrium, ovary, stomach, small intestine, hepatobiliary tract, urinary tract, brain, and skin. The lifetime risk of colorectal cancer and endometrial cancer in Lynch syndrome is 60%–80% and 40%–60%.26
Short tandem repeats (STRs), also known as microsatellites, are small stretches of repetitive DNA, composed of mono-, di-,tri-, and tetranucleotides repeats on the order of hundreds of nucleotides in each block scattered throughout the genome.27 STRs occur frequently in humans, with dinucleotide repeats such as (CA)n or (CACACACACAC … ) being found on average every 30–60 kilobases.28 Microsatellite alleles are present in two copies in most individuals. During cell replication, strand slippage occasionally generates DNA polymerase stutter. This phenomenon is seen more frequently in areas of microsatellites.
The MMR enzymes correct errors that are missed by the proofreading function of DNA polymerase and act as an additional system to preserve genomic integrity.29 A defective MMR system will leave the genome with microsatellites that are either longer or shorter than the parent cell and this phenomenon is termed microsatellite instability (MSI). Using molecular testing, Lynch syndrome can be diagnosed when a mutation is found in one of the four mismatch repair genes. These genes are MLH1, MSH2, MSH6, and PMS2. MSI serves as a marker for the loss of DNA MMR activity. If the mismatch occurs in the coding region of a gene, the newly introduced point mutation may affect the expression or function of that gene.30
Inactivation of the MMR enzymes can occur either through aberrant methylation of promoter CpG islands (discussed further below) of MLH1 gene or via point mutations in a member of the MMR family. “Microsatellite high” (MSI-H) is defined as the presence of instability in ≥30% of the markers. “Microsatellite low” (MSI-L) is defined as the presences of instability in 10%–29% of markers, and “microsatellite stable” (MSS) is defined as no unstable markers.20 Immunohistochemical staining of formalin-fixed paraffin embedded colorectal carcinoma tissue for MLH1, MSH2, MSH6, and PMS2 is helpful in identifying which specific MMR protein is deficient.
Patients with germline loss of DNA mismatch repair capability typically develop CRC by age 40 in 80% of cases.31 The majority of MMR defects in sporadic CRC are due to epigenetic silencing of MLH1 gene expression by promoter hypermethylation.32–34 Both MMR gene alleles have to be affected in order to lose MMR function, since one wild-type allele is sufficient for an MSS genotype in HNPCC as well as in sporadic CRC.35 MSI tumors typically arise in the proximal colon and display mucinous histology as well as Crohn's disease-like lymphocytic infiltration. Although MSI-H tumors are frequently poorly differentiated, this feature is not considered to be a high-risk category. Patients with MSI-associated colorectal cancer are usually younger than 50 years old, and, interestingly, their survival is better than patients who have other types of chromosomal alterations.
The MMR system is also effective in the correction of DNA damage due to certain drugs such as alkylators and intercalating agents that generate a similar structural damage as mismatches resulting in apoptosis. Thus an intact MMR system may contribute to chemoresistance, while MMR defective tumors may exhibit chemosensitivity.36 The MMR proteins also recognize intra- and interstrand crosslinks induced by cisplatin or carboplatin but not with oxaliplatin.37 It is well known that carboplatin and cisplatin, as opposed to oxaliplatin, do not have activity in CRC. On the other hand, oxaliplatin resistance is not due to MMR defect. 5-Fluorouracil (5-F) is recognized by the MMR system. Cells deficient in MMR proteins are resistant to 5-FU in retrospective and prospective studies. By the same principle, patients with Lynch syndrome also are resistant to 5-FU.
Testing for MMR is currently recommended by the National Comprehensive Cancer Network (NCCN) for all patients younger than 50 years old with newly diagnosed CRC with stage II (T3–4N0M0) disease based on the increased likelihood of Lynch syndrome in this population (NCCN guidelines version 1.2012). Stage II MSH-high patients may have a good prognosis and do not benefit from 5-FU treatment.38 Conventional risk factors are currently used in a standard fashion to justify chemotherapy benefit from 5-FU-based chemotherapy in stage II CRC patients. These risk factors are grade 3 and 4 tumors on histology, lymphatic and vascular invasion, localized perforation or bowel obstruction, less than 12 lymph nodes examined, perineural invasion, and indeterminate or positive surgical margins.
Epigenetic Instability and CpG Methylation
Multiple epigenetic regulatory mechanisms regulate DNA expression without altering the nucleotide sequence. Aberrant epigenetic regulation via inappropriate methylation of gene promoter regions is common in CRC and is as significant as DNA mutation in inactivating tumor suppressor genes. Aberrant hypermethylation involves the covalent attachment of a methyl group to the 5′ position of cytosine and takes place in repetitive CG dinucleotides or CpG-rich stretches of DNA within the promoter region. CpG indicates cytosine (C) followed by guanosine (G), with an intervening phosphodiester (p) bond.
In normal cells, CpG islands are usually maintained in an unmethylated state. In the absence of methylation, the gene is expressed normally. In the presence of promoter methylation, the gene is transcriptionally downregulated (ie silenced). Silencing of tumor suppressor gene function can result from promoter hypermethylation involving both copies of a tumor suppressor gene, or a combination of loss of one allele via deletion or mutation combined with silencing of the other allele via promoter hypermethylation.
Aberrant methylation of MLH1 occurs in 80% of sporadic MSI colorectal cancers. CpG island methylator phenotype (CIMP) is a subclass of colorectal cancers with a high proportion of the gene being hypermethylated. These subclasses of tumors commonly have BRAFV600E mutations.39 CIMP subclassification has also been suggested (eg CIMP2, CIMP-low, CIMP-high), raising the possibility of classifying tumors not only by genomic instability but also through epigenetic instability.40,41
EGFR (endothelial growth factor receptor) is a transmembrane tyrosine kinase that transduces signals through two parallel intracellular pathways to activate cellular proliferation and survival. EGF (endothelial growth factor), the ligand to the EGFR, binds to the extracellular domain of EGFR leading to receptor dimerization. Following dimerization, the intracellular domain of EGFR is autophosphorylated and activates multiple downstream proteins of the RAS/RAF/MAPK and PI3K/AKT pathways (Figure 2). This signaling cascade reaches cell DNA within the nucleus to induce cell proliferation, angiogenesis, cell motility, and metastasis.
Figure 2.
EGFR KRAS and BRAF and Ras-Raf-MAPK family.
Cetuximab and panitumumab are anti-EGFR monoclonal antibodies that are engineered to block the EGFR signaling pathway at the extracelluar domain of the EGFR receptor. These two drugs are used in the treatment of metastatic CRC in combination with conventional chemotherapy or as single agents. The FDA approved cetuximab after the pivotal BOND 1 trial in 2004. Patients with metastatic CRC who progressed on irinotecan-based therapy were randomized to irinotecan plus cetuximab alone vs. irinotecan alone. The response rate, time to progression, and overall survival was 23% vs. 11%, 4 months vs. 1.4 months, 8.6 vs. 8.6 months, respectively, all in favor of the combination arm.42
Clinical studies have shown that not all patients with EGFR overexpression respond to monoclonal antibodies mentioned above. Mutations in oncogenes coding for the downstream proteins are partially responsible for this resistance. When the downstream effectors of EGFR signaling, KRAS, BRAF PI3K, and PTEN, are all unmutated (quadruple negative) the anti-EGFR monoclonal antibodies show the best response in CRC.
KRAS and BRAF are members of the MAP kinase (MAPK) pathway. The RAS/RAF/MAPK pathway regulates cell proliferation, differentiation, senescence, and apoptosis. The RAS oncogenes include HRAS, NRAS, and KRAS. KRAS is the most commonly mutated RAS family member in CRC and is mutated in 40% of sporadic CRCs. KRAS is not seen as a germline mutation. It is a small protein that transduces signals from the EGFR family.
The mitogen-activated protein kinase pathway (MAPK) is a major cell proliferation signal transduction pathway from the cell surface to the nucleus. This activation uses a series of intermediate proteins including RAS, RAF, and MEK. Binding of ligand to EGFR results in receptor dimerization and phosphorylation. RAS activates the cascade through phosphoinositol kinases (PI3K) as well as RAF and thus acts as a central distributor of the signal. Activation of PI3K inhibits apoptosis, whereas RAF activation stimulates cellular proliferation. This cascade is involved in the control of growth signals, cell survival, and invasion in cancer. KRAS mutations lead to constitutive changes and promote cell proliferation and survival independent of the EGFR receptor. Hence, therapeutic EGFR inhibition is rendered ineffective, since KRAS is located downstream from EGFR. RAS mutations involve codons 12 and 13 on exon 2 and codon 61 on exon 3. Codon 12 is the most common codon affected, usually by a missense mutation.
KRAS mutations are felt to occur after APC mutations in the adenoma-carcinoma sequence model. The current developed monoclonal antibodies against the extracellular domain of EGFR, namely, cetuximab and panitumumab, are ineffective in patients with KRAS mutations in codons 12, 13, and should not be used. Livre identified KRAS mutations in 27% of patients. Patients with KRAS mutations showed a response rate of 0% to cetuximab vs. 40% in wild-type tumors, and a median overall survival of 10.1 vs. 14.3 months, respectively.43
KRAS has been specifically targeted in some clinical trials, with insignificant activity shown thus far. Cunningham et al showed tipifarnib, a farnesyl transferase, did not give a statistically significant overall survival benefit compared to best supportive care in a phase III double blinded placebo controlled trial in patients with refractory advanced colorectal cancer.44 Farnesyl transferase inhibitors target the enzyme protein farnesyl transferase, which catalyzes the addition of a farnesyl isoprenoid moiety to the ras proteins. Some studies suggest that not only do EGFR monoclonal antibodies not have efficacy in mCRC patient with KRAS mutation, it may have detrimental effects with worsening overall survival.45,46
Further studies have recently shown that all KRAS mutations are not the same. Patients with mutation in the Kras G13D codon on exon 2 show response to EGFR monoclonal antibodies. The response is not robust as in patients with wild-type KRAS, but patients had a better overall and progression-free survival.47 NCCN currently recommends checking KRAS mutation routinely before using cetuximab or panitumumab in the treatment of patients with mCRC.
BRAF is a member of RAF family of serine/threonine kinases and mediates cellular responses to growth signals through the RAS-RAF-MAP kinase pathway. Activating mutations in BRAF have recently been found in about 10% of sporadic CRC and are rare in familial Lynch syndrome CRC. BRAF mutations were identified in 4% of MSI-low and 40% in MSI-high tumors.48 The vast majority of these were represented by the V600E (Val600Glu) hotspot mutation. As it is rare for patients with Lynch syndrome-associated CRC to have BRAF mutations, the identification of a BRAF V60OE mutation in the setting of MSI tumors is helpful in separating familial CRC (Lynch syndrome) from sporadic CRC.
Patients with BRAF V600E mutation appear to have a poorer prognosis. BRAF mutations are, for all practical purposes, limited to those tumors that do not have KRAS exon 2 mutations. As BRAF is downstream of activated KRAS protein in the EGFR pathway, constitutive activation of BRAF by V600E mutation renders therapeutic inhibition of EGFR by cetuximab or panitumumab ineffective. Therefore, NCCN currently recommends BRAF mutational analysis as “optional” in CRC without mutations in KRAS to assist in determining whether or not to include EGFR monoclonal antibodies as a part of the patient's treatment regimen.
PI3K/AKT Pathway, PTEN, and TGFβRII
Wild-type KRAS is required but not sufficient to confer sensitivity to EGFR inhibitors in colorectal cancer. Cetuximab and panitumumab have a 40%43 and 17% response rate in wild-type KRAS CRCs.49 As a consequence, it has been a challenge to identify other molecular markers for the effectiveness of these monoclonal antibodies in these patients. The phosphoinositide 3-kinases (PI3K)/AKT/mammalian target of rapamycin (mTOR) is an alternative EGFR-mediated signaling pathway.50 PI3Ks are a family of lipid kinases that that activate AKT through phosphorylation. Once activated, phospho-AKT phosphorylates up to 100 other proteins, including mTOR.
Activating mutations in PIK3CA, the gene encoding the catalytic subunit of PI3K, are identified as novel mechanisms of inducing oncogenic PI3K signaling. PIK3CA is somatically mutated in over 25% of colorectal cancer.51 Among patients with wild-type KRAS CRC, the presence of PIK3CA mutations was recently correlated with a significant increase in colon cancer-specific mortality. In contrast, the activation of the PI3K/AKT pathway apparently does not play an important role in tumor aggressiveness on a background where the KRAS oncogene is not activated.52
PTEN is a phosphatase that negatively regulates the PI3K/AKT signaling pathway by dephosphorylating PIP3 to inhibit activation of AKT via hyperactivation of PI3K signaling.53 PTEN protects the genome from instability. The PTEN gene is activated in cancer. Several small clinical studies have shown resistance to monoclonal EGFR inhibitors, cetuximab, or panitumumab with PI3KCA mutation. Others have not confirmed this effect. In addition, loss of PTEN was associated with either lack of tumor response or worse overall survival. Overall, these data indicated that the concomitant evaluation of the molecular status of the PIK3CA/PTEN and KRAS pathways is able to identify up to 70% of mCRC patients unlikely to respond to anti-EGFR moAbs.
TGFβ (tumor growth factor beta) is a group of multifunctional proteins that regulate many cellular processes through binding to TGFβ receptors. Three types of TGFβ receptors (type I [I], type II [RII], and type III [RIII]) are identified in most cells.43 TGFβRII (transforming growth factor-β receptor type II) is mutated in up to 90% of colon cancer tumors with MSI.54 TGFβRII appears to function in two ways during tumorogenesis. In early stages of tumorigenesis, TGFβRII mediates tumor-suppressive effects, but in late stages it enhances tumor progression by inhibiting tumor cell death and immune repression. It also induces epithelial to mesenchymal transformation (EMT) known to induce tumor progression, invasion, and metastasis.55 Mutation of TGFβRII will interfere with EMT and reduce the invasiveness and metastatic capability of the tumors. EMT has been shown to be impaired in MSI colon cancer cells. Tumors with MSI but without TGFβRII mutations can undergo EMT in vitro in response to TGFβRII, which suggests that TGFβRII and not MSI status, per se, may be the key determinant of invasiveness and metastasis and prognosis.56,57
It has been shown by Liu that TGFβRII activates downstream PI3K/AKT, and this causes resistance to growth factor deprivation and stress-induced apoptosis and promotes cell motility in vitro. LY294002, a potent PI3K inhibitor, prevented TGFB promotility and survival effect in this study.56 Watanabe reported that mutation in TGFβRII is marginally associated with improved 5-year overall survival (p = .06) in stage III colon cancer. Among stage III colon cancer patients who had both TGFβRII mutation and MSI, the rate of disease-free survival at 5 years was 79% as compared to 40% among those whose tumors had high levels of MSI but no mutation of the TGFβR2 gene.21 Currently there is no established clinical utility in measuring TGFβRII in the management of CRC, and data from studies of drugs that target mutated PI3K and inactivated PTEN are currently in early stages.
GENETIC SEQUENCING OF CRC
The identification and utility of somatic chromosomal rearrangements has revolutionized monitoring of disease relapse, residual disease, and disease progression in lymphoma and leukemias. A classic example is monitoring copy numbers of BCR-ABL by PCR (polymerase chain reaction) in chronic myelogenous leukemia. Similar and recurrent genetic alterations do not occur in most of the solid tumors of the same tumor type; however, each individual solid tumor has a set of unique genetic alterations that may serve as a tool to monitor the disease. Leary58 have identified patient-specific rearrangements in four CRC and two breast cancers with massive parallel sequencing by PARE (personalized analysis of rearranged ends). These alterations were used to develop PCR-based quantitative analyses for personalized tumor monitoring of plasma samples or bodily fluids. PCR identified mutant circulating DNA in patient plasma samples at levels lower than 0.001%. Testing on the plasma of one patient after surgery, chemotherapy, and liver metastasectomy showed a decrease in the mutant DNA fragment compared with before each intervention. The PCR level did not reach zero in this patient, which suggested residual disease (most likely in the liver).
Bass59 reported whole-genome sequencing from nine patients with colorectal cancer. An average of 75 somatic rearrangements per tumor was identified, including complex networks of translocations between pairs of chromosomes. Eleven rearrangements encode predicted in-frame fusion proteins, including a fusion of VTI1A and TCF7L2 found in 3 out of 97 colorectal cancers.
SUMMARY
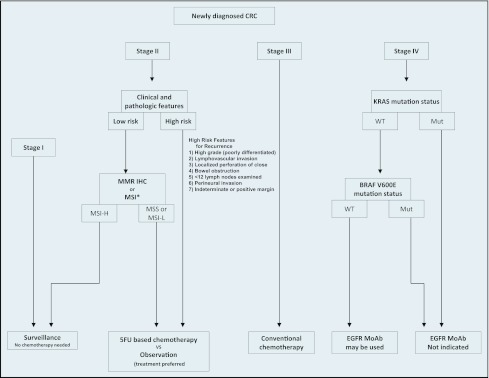
Genetic alteration in CRC tumors has been extensively studied, and it continues to evolve. The deeper understanding of the mechanisms of colorectal cancer cell genetic alteration and related consequences as well as the corresponding epigenetic phenomenon are the mainstay of drug development for the predication of efficacy of treatment and in some instances defining prognosis of this common cancer. Risk stratification algorithms for genetic testing and clinical decision making for CRC are shown in Figures 3 and 4.
Figure 3.
Algorithm in approaching CRC treatment based on conventional risk factors and molecular markers. *MSI-high tumor with poorly differentiated features is not considered a high-risk category.
Figure 4.
Approach to treating inherited CRC based on MSI status.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. Jemal A, Bray F, Center MM, et al. : Global cancer statistics. CA Cancer J Clin 61(2):69–90, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Fearon ER, Vogelstein B: A genetic model for colorectal tumorigenesis. Cell 1;61(5):759–767, 1990 [DOI] [PubMed] [Google Scholar]
- 3. Weinberg RA: The Biology of Cancer. Baltimore, MD, Garland Science, 2006 [Google Scholar]
- 4. Pino MS, Chung DC: The chromosomal instability pathway in colon cancer. Gastroenterology 138(6):2059–2072, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sieber OM, Heinimann K, Tomlinson IPM: Genomic instability: the engine of tumorigenesis? Nat Rev Cancer 3(9):701–708, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Shih IM, Zhou W, Goodman SN, et al. : Evidence that genetic instability occurs at an early stage of colorectal tumorigenesis. Cancer Res 61(3):818–822, 2001 [PubMed] [Google Scholar]
- 7. Michor F, Iwasa Y, Vogelstein B, et al. : Can chromosomal instability initiate tumorigenesis? Semin Cancer Biol 15(1):43–49, 2005 [DOI] [PubMed] [Google Scholar]
- 8. De Filippo C, Luceri C, Caderni G, et al. : Mutations of the APC gene in human sporadic colorectal cancers. Scand J Gastroenterol 37(9):1048–1053, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Powell SM, Zilz N, Beazer-Barclay Y, et al. : APC mutations occur early during colorectal tumorigenesis. Nature 359(6392):235–237, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Cottrell S, Bicknell D, Kaklamanis L, et al. : Molecular analysis of APC mutations in familial adenomatous polyposis and sporadic colon carcinomas. Lancet 340(8820):626–630, 1992 [DOI] [PubMed] [Google Scholar]
- 11. Polakis P: The adenomatous polyposis coli (APC) tumor suppressor. Biochim Biophys Acta 1332(3):F127–147, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Kinzler KW, Vogelstein B: Lessons from hereditary colorectal cancer. Cell 87(2):159–170, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Morin PJ, Sparks AB, Korinek V, et al. : Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275(5307):1787–1790, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Lane DP, Benchimol S: p53: oncogene or anti-oncogene? Genes Dev 4(1):1–8, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Vogelstein B, Fearon ER, Hamilton SR, et al. : Genetic alterations during colorectal-tumor development. N Engl J Med 319(9):525–532, 1988 [DOI] [PubMed] [Google Scholar]
- 16. Zeng ZS, Sarkis AS, Zhang ZF, et al. : p53 nuclear overexpression: an independent predictor of survival in lymph node—positive colorectal cancer patients. J Clin Oncol 12(10):2043–2050, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Adrover E, Maestro ML, Sanz-Casla MT, et al. : Expression of high p53 levels in colorectal cancer: a favourable prognostic factor. Br J Cancer 81(1):122–126, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Popat S, Chen Z, Zhao D, et al. : A prospective, blinded analysis of thymidylate synthase and p53 expression as prognostic markers in the adjuvant treatment of colorectal cancer. Ann Oncol 17(12):1810–1817, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Forcet C, Ye X, Granger L, et al. : The dependence receptor DCC (deleted in colorectal cancer) defines an alternative mechanism for caspase activation. Proc Natl Acad Sci USA 98(6):3416–3421, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ogino S, Nosho K, Irahara N, et al. : Prognostic significance and molecular associations of 18q loss of heterozygosity: a cohort study of microsatellite stable colorectal cancers. J Clin Oncol 27(27):4591–4598, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watanabe T, Wu TT, Catalano PJ, et al. : Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med 344(16):1196–1206, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Popat S, Houlston RS: A systematic review and meta-analysis of the relationship between chromosome 18q genotype, DCC status and colorectal cancer prognosis. Eur J Cancer 41(14):2060–2070, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Halling KC, French AJ, McDonnell SK, et al. : Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst 91(15):1295–1303, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Barratt PL, Seymour MT, Stenning SP, et al. : DNA markers predicting benefit from adjuvant fluorouracil in patients with colon cancer: a molecular study. Lancet 360(9343):1381–1391, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Geiersbach KB, Samowitz WS:Microsatellite instability and colorectal cancer. Arch Pathol Lab Med 135(10):1269–1277, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Meyer LA, Broaddus RR, Lu KH: Endometrial cancer and Lynch syndrome: clinical and pathologic considerations. Cancer Control 16(1):14–22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abeloff MD: Clinical Oncology. Sutton, UK, Churchill Livingstone, 1995 [Google Scholar]
- 28. Kwiatkowski DJ, Henske EP, Weimer K, et al. : Construction of a GT polymorphism map of human 9q. Genomics 12(2):229–240, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Ionov Y, Peinado MA, Malkhosyan S, et al. : Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 363(6429):558–561, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Jung B, Doctolero RT, Tajima A, et al. : Loss of activin receptor type 2 protein expression in microsatellite unstable colon cancers. Gastroenterology 126(3):654–659, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Grady WM, Carethers JM: Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology 135(4):1079–1099, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakagawa H, Nuovo GJ, Zervos EE, et al. : Age-related hypermethylation of the 5′ region of MLH1 in normal colonic mucosa is associated with microsatellite-unstable colorectal cancer development. Cancer Res 61(19):6991–6995, 2001 [PubMed] [Google Scholar]
- 33. Hemminki A, Mecklin JP, Järvinen H, et al. : Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology 119(4):921–928, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Samowitz WS, Curtin K, Ma KN, et al. : Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev 10(9):917–923, 2001 [PubMed] [Google Scholar]
- 35. Veigl ML, Kasturi L, Olechnowicz J, et al. : Biallelic inactivation of hMLH1 by epigenetic gene silencing, a novel mechanism causing human MSI cancers. Proc Natl Acad Sci USA 95(15):8698–8702, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karran P, Bignami M: DNA damage tolerance, mismatch repair and genome instability. Bioessays 16(11):833–839, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Mishima M, Samimi G, Kondo A, et al. : The cellular pharmacology of oxaliplatin resistance. Eur J Cancer 38(10):1405–1412, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Sargent DJ, Marsoni S, Monges G, et al. : Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 28(20):3219–3226, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weisenberger DJ, Siegmund KD, Campan M, et al. : CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet 38(7):787–793, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Ogino S, Kawasaki T, Kirkner GJ, et al. : Molecular correlates with MGMT promoter methylation and silencing support CpG island methylator phenotype-low (CIMP-low) in colorectal cancer. Gut 56(11):1564–1571, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shen L, Toyota M, Kondo Y, et al. : Integrated genetic and epigenetic analysis identifies three different subclasses of colon cancer. Proc Natl Acad Sci USA 104(47):18654–18659, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cunningham D, Humblet Y, Siena S, et al. : Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351(4):337–345, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Lièvre A, Bachet J-B, Boige V, et al. : KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26(3):374–379, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Rao S, Cunningham D, de Gramont A, et al. : Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. J Clin Oncol 22(19):3950–3957, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Douillard J-Y, Siena S, Cassidy J, et al. : Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28(31):4697–4705, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Van Cutsem E, Köhne C-H, Láng I, et al. : Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29(15):2011–2019, 2011 [DOI] [PubMed] [Google Scholar]
- 47. Tejpar S., Bokemeyer C., Celik I., et al. : Influence of KRAS G13D mutations on outcome in patients with metastatic colorectal cancer (mCRC) treated with first-line chemotherapy with or without cetuximab. J Clin Oncol 29: (suppl; abstr 3511), 2011 [DOI] [PubMed] [Google Scholar]
- 48. Iacopetta B, Li WQ, Grieu F, et al. : BRAF mutation and gene methylation frequencies of colorectal tumours with microsatellite instability increase markedly with patient age. Gut 55(8):1213–1214, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Amado RG, Wolf M, Peeters M, et al. : Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26(10):1626–1634, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Carracedo A, Pandolfi PP: The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene 27(41):5527–5541, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Samuels Y, Wang Z, Bardelli A, et al. : High frequency of mutations of the PIK3CA gene in human cancers. Science 304(5670):5527–554, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Ogino S, Nosho K, Kirkner GJ, et al. : PIK3CA mutation is associated with poor prognosis among patients with curatively resected colon cancer. J Clin Oncol 27(9):1477–1484, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yin Y, Shen WH: PTEN: a new guardian of the genome. Oncogene 27(41):5443–5453, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Markowitz S, Wang J, Myeroff L, et al. : Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science 268(5215):1336–1338, 1995 [DOI] [PubMed] [Google Scholar]
- 55. Thiery JP: Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2(6):442–454, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Liu X-Q, Rajput A, Geng L, et al. : Restoration of transforming growth factor-beta receptor II expression in colon cancer cells with microsatellite instability increases metastatic potential in vivo. J Biol Chem 286(18):16082–16090, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57. Pino MS, Kikuchi H, Zeng M, et al. : Epithelial to mesenchymal transition is impaired in colon cancer cells with microsatellite instability. Gastroenterology 138(4):1406–1417, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leary RJ, Kinde I, Diehl F, et al. : Development of personalized tumor biomarkers using massively parallel sequencing. Sci Transl Med 2(20):1–15, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bass AJ, Lawrence MS, Brace LE, et al. : Genomic sequencing of colorectal adenocarcinomas identifies a recurrent VTI1A-TCF7L2 fusion. Nature Genet 43:964–968, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]