Abstract
Although acute colonic pseudo-obstruction (ACPO), also known as Ogilvie syndrome, is a well-known clinical entity, in many respects it remains poorly understood and continues to challenge physicians and surgeons alike. Our understanding of ACPO continues to evolve and its epidemiology has changed as new conditions have been identified predisposing to ACPO with critical illness providing the common thread among them. A physician must keep ACPO high in the list of differential diagnoses when dealing with the patient experiencing abdominal distention, and one must be prepared to employ and interpret imaging studies to exclude mechanical obstruction. Rapid diagnosis is the key, and institution of conservative measures often will lead to resolution. Fortunately, when this fails pharmacologic intervention with neostigmine often proves effective. However, it is not a panacea: consensus on dosing does not exist, administration techniques vary and may impact efficacy, contraindications limit its use, and persistence and or recurrence of ACPO mandate continued search for additional medical therapies. When medical therapy fails or is contraindicated, endoscopy offers effective intervention with advanced techniques such as decompression tubes or percutaneous endoscopic cecostomy providing effective results. Operative intervention remains the treatment of last resort; surgical outcomes are associated with significant morbidity and mortality. Therefore, a surgeon should be aware of all options for decompression—conservative, pharmacologic, and endoscopic—and use them in best combination to the advantage of patients who often suffer from significant concurrent illnesses making them poor operative candidates.
Keywords: acute colonic pseudo-obstruction, ogilvie syndrome
Objectives: On completion of this article, the reader should be able to review diagnostic testing confirming diagnosis of acute colonic pseudo-obstruction, identify initial conservative treatment measures, and discuss pharmacologic and endoscopic options for decompression.
Acute colonic pseudo-obstruction (ACPO) is a syndrome of massive distension of colon without mechanical obstruction. Ischemia and perforation remain the endpoint of progressive untreated distension and early recognition and timely intervention is of utmost importance. The diagnosis of ACPO depends on excluding mechanical bowel obstruction and its subsequent management has evolved with improved understanding of its pathophysiology and pharmacologic and endoscopic treatment options.
Historical Background
ACPO is also known by the eponym Ogilvie syndrome. Sir William Heneage Ogilvie described two patients diagnosed with colonic pseudo-obstruction in 1948.1 Both patients had retroperitoneal tumors invading the splanchnic plexus, which led to him conclude that “malignant infiltration surrounded, and may well have put out of action or even destroyed, the splanchnic nerves, the semilunar ganglia, and the coeliac plexus. ... so that the parasympathetic innervation, which in the distal colon comes from the second and third sacral nerves, was allowed to act unopposed.”1
The term pseudo-obstruction was proposed by Dudley to define functional obstruction of the colon.2,3 The term acute colonic pseudo-obstruction first appeared in the literature in 1982 in a review by Nanni et al.4 Rex used the acronym “ACPO” in his article in 1997.5 As there currently is a trend toward discouraging use of medical eponyms,6 the term acute colonic pseudo-obstruction is more prevalent in the recent literature to describe this clinical phenomenon.
Pathophysiology and Etiology of ACPO
ACPO is believed to be a functional disturbance in colonic motility. The pathophysiology is not entirely clear from the current understanding of gut motility, hence the lack of preventive strategies. Broadly speaking, the enteric nervous system remains the primary determinant of motility function in both the small and large intestine, while the central nervous system modulates motility patterns established by the “little brain” of the enteric nervous system.
Enteric nerves contain a variety of neurotransmitters responsible for smooth muscle contraction or relaxation. The major stimulatory neurotransmitters include acetylcholine, neurokinin A, and substance P, whereas the inhibitory nerves express vasoactive intestinal polypeptide and nitric oxide. The extrinsic influence of the sympathetic nerves from the thoracic and lumbar segments of the spinal cord tend to decrease motility, and parasympathetic nerves from the brainstem via the 10th cranial nerve (vagus) and from sacral spinal segments increase motility.7
The exact pathogenesis of ACPO remains unknown. The initial theory, as proposed by Ogilvie, to explain the acute colonic pseudo-obstruction was an imbalance in the activity of autonomic nervous system with parasympathetic overactivity leading to dilation of the colon.1 However, current evidence favors a relatively increased sympathetic tone and/or decreased parasympathetic tone leading to a functionally obstructing distal colon and a relaxed proximal colon (adynamic colon).8 The evidence in favor of this theory is the association of ACPO with diseases causing a disturbance in the autonomic flow to the gut and a remarkable response to pharmacologic therapy.
There are no animal models of ACPO. Current research on colonic motility is based on models of postoperative ileus and toxic megacolon. The interstitial cell of Cajal (ICC) was previously known as a type of mesenchymal cell present along the gastrointestinal tract in close association with smooth muscle cells and elements of the enteric nervous system. Recently, the ability to study ICCs was enhanced with immunohistochemical staining using c-kit (a tyrosine kinase receptor).9 ICCs are currently regarded as the source of the spontaneous slow waves of the gut musculature (pacemaker cells).9 ICCs were absent in patients with chronic intestinal pseudo-obstruction in a study performed by Jain et al.10 Certain groups of ICCs appear to be involved in the relaxation of smooth muscles triggered by nitric oxide.9 Nitric oxide generation was found to impair smooth muscle contractility in a rodent model of toxic megacolon.11 Cytokines also alter intestinal motility particularly in an inflammatory state.12 As the understanding of intestinal motility progresses, it will open avenues for novel therapeutic targets to treat ACPO and other motility disorders.
Epidemiology
Unfortunately, the incidence rate of ACPO is not known. Most studies indicate that elderly patients are at greatest risk for ACPO. Many conditions are associated with ACPO. A review by Vanek et al13 of 400 cases compiled a list of such conditions: obstetric, gynecologic, or pelvic operation (19%); trauma/orthopedic procedure (18%); infection (10%); cardiac events (10%); and neurologic events (9%). Other conditions leading to ACPO include electrolyte imbalance, certain medications (i.e., opioids, antidepressants), solid organ transplant, debilitated state, and connective tissue disorder (see Table 1).14
Table 1. Associations Underlying Acute Colonic Pseudo-Obstruction.
| Medications: Narcotics, anticholinergics, phenothiazines, laxative abuse, benzodiazepines, calcium channel blockers, clonidine, vincristine, interleukin, amphetamine overdose, cytotoxic drugs, antiparkinsonian agents |
| Medical: |
| • Cardiopulmonary – Mechanical ventilation, myocardial infarction, pneumonia, congestive heart failure, chronic obstructive pulmonary disease |
| • Infectious – Sepsis, herpes zoster, cytomegalovirus |
| • Metabolic – Hypokalemia, hyponatremia, hypocalcemia, hypercalcemia, diabetes, hypothyroidism |
| • Neurologic – Dementia, multiple sclerosis, Parkinson disease, spinal cord disease |
| • Oncologic – Small cell lung cancer, multiple myeloma, acute myeloid leukemia, disseminated cancer, pelvic irradiation, retroperitoneal invasion of lumbar sympathetic nervous system |
| • Miscellaneous – Organ failure, amyloidosis, physical exertion, idiopathic, alcoholism |
| Surgical: |
| • Inflammation – Appendicitis, cholecystitis, pancreatitis, gastritis, abscess |
| • Obstetric – Normal pregnancy, normal delivery, cesarean section, hysterectomy, placenta previa |
| • Organ transplantation – Liver, kidney, heart, lung |
| • Trauma and Orthopedic – Pelvic trauma, pelvic, hip fracture, pelvic, hip surgery, joint arthroplasty, spine surgery, burns |
| • Urologic – Ethanol ablation of renal cancer, nephrolithiasis |
| • Others – Gastrointestinal bleeding, retroperitoneal hematoma, mesenteric thrombosis, craniotomy, aortic aneurysms, thoracotomy |
Clinical Presentation and Natural History
Although symptoms and signs of a large bowel obstruction commonly occur, ACPO can have a variable clinical presentation. A high index of suspicion is necessary by the treating physician. Common symptoms are acute massive abdominal distention and pain. Nausea, vomiting, and constipation are not consistently present. Signs of systemic toxicity do not appear until catastrophic complications have occurred. If left unrecognized, progressive dilation of the colon can result in mural ischemia, perforation, and increased mortality. In their review, Vanek et al reported surgical mortality increased from 26% in cases with viable bowel to 36 to 44% in cases with perforated or ischemic bowel.13 In addition, the age of the patient, maximal cecal diameter, and delay in colonic decompression have a significant direct correlation to mortality.13 High mortality in this patient population is also a reflection of underlying physiologic derangement from the associated acute medical/surgical illness.
According to Laplace's Law, the pressure required to stretch the walls of a hollow viscus decreases in inverse proportion to the diameter. Progressive colonic distention causes highest tension in the wall of cecum that has the largest diameter. An increase in intramural pressure leads to ischemia with longitudinal splitting of the serosa and tenia, and herniation of the mucosa. Further distention results in worsening ischemia and perforation of the mucosa.8,13,15
The actual diameter at which perforation of the cecum occurs remains debatable. All agree that larger diameter is a predictor for perforation. In one study, all patients with cecal diameter > 9 cm were believed to have impending perforation or had perforated; hence, 9 cm was considered the threshold.16 Interestingly, Vanek found a cecal diameter of 12 cm or less is rarely associated with perforation whereas a cecal diameter of 14 cm has a 23% incidence of perforation.13 A range in cecal diameter of 9 to 12 cm has thus been suggested as a sign of impending perforation. Along with absolute diameter, the duration of distension may also be predictive of perforation. Saunders et al found higher risk of perforation if distension was present for more than 6 days.17
Diagnosis
The critical aspect in the management of ACPO is exclusion of mechanical obstruction. The clinical presentation is similar in both; therefore, diagnostic imaging for differentiation is required. Plain radiography can diagnose perforation and serial films are used to monitor measurement of colonic distension. However, the distinction between mechanical and functional obstruction cannot be made with plain radiography alone. A contrast enema and computed tomography (CT) scan are used to detect mechanical obstruction. A contrast enema has a sensitivity and specificity of 80% and 100%, respectively, in the diagnosis of large bowel obstruction.18 A water-soluble contrast agent is employed without bowel prep.19 Contrast is instilled until it reaches the dilated segment. Excess contrast use should be avoided. Although barium has a similar sensitivity to diagnose obstruction,18 in general, a water-soluble contrast is preferred given the small risk of extravasation of barium and more importantly, the potential therapeutic effect of water-soluble contrast leading to decompression of colon.20 In fact, a success rate of 78% has been reported with a single enema.21 The challenge of contrast enema testing is that it is staffing dependent and labor intensive. This is particularly true when performed after normal hours or on weekends or holidays. Contrast retention may be difficult in an elderly, frail, and critically ill patient. In addition, hypertonic contrast can further exacerbate dehydration and electrolyte imbalance.22
CT has largely replaced the contrast enema for the diagnosis of a large bowel obstruction.19 In contrast to fluoroscopic exams, access to CT generally is less problematic. The scanning protocol for bowel obstruction requires intravenous contrast and the patient in the supine position; images can be acquired quickly in a single breathholding time. A retrospective study over a 7-year period found an increasing trend toward the use of CT for large bowel obstruction compared with a contrast enema.19 The sensitivity and specificity of CT is 96% and 93%, respectively, to diagnose large bowel obstruction.18 Importantly, CT can diagnose complications including bowel ischemia and contained perforation, as well as the condition of pericolic structures.22 The common CT findings in ACPO is proximal colonic dilatation with an intermediate transitional zone at or adjacent to the splenic flexure. In distinction to mechanical large bowel obstruction, structural obstructing lesions are not visualized.23
Treatment Options
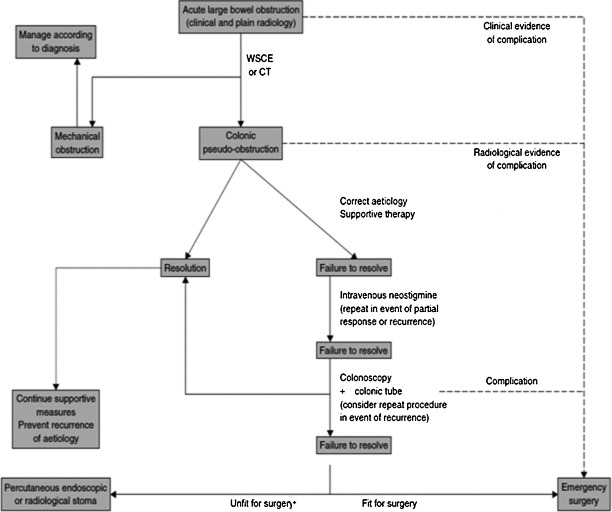
Timely recognition and close monitoring cannot be overemphasized in the management of ACPO. Perforation, ischemia, and peritonitis necessitate urgent surgical intervention. In uncomplicated cases, nonoperative management must be initiated immediately. Close clinical monitoring should include serial physical exams, abdominal radiograph, and laboratory studies every 12 to 24 hours. Deterioration or nonresolution despite maximal medical therapy within 48 to 72 hours of initiating therapy should prompt reconsideration of the management plan.24 Again, persistent colonic distention for > 6 days portends a higher risk of perforation (see treatment algorithm, Fig. 1).17
Figure 1.
Treatment algorithm. WSCE, water-soluble contrast enema. +Limited evidence available for percutaneous interventions. From De Giorgio, Knowles.24 With kind permission of John Wiley and Sons.
Nonoperative Management
The majority of patients with ACPO are successfully managed with conservative measures. Patients are placed nil per os, a nasogastric tube should be inserted, and aggressive fluid and electrolyte resuscitation initiated. Appropriate antibiotics are started if underlying infection is suspected. Any offending medication should be stopped. Osmotic laxatives lead to increased gas formation in the colon and should be avoided.24 The prone position with hips elevated on a pillow or the knee–chest position with the hips held high often aids the spontaneous evacuation of flatus. These positions should be alternated with right and left lateral decubitus positions regularly every hour when feasible.25 The usual duration of response to these measures is 3 to 5 days.13,17 Wegener et al reviewed 1027 case reports of ACPO and presented efficacy of various therapeutic modalities. In this review, 70% of patients responded to supportive management alone with a complication rate of 6% and mortality approximating 10%.21
Pharmacologic Management
Historically, patients not responding to supportive management were offered endoscopic decompression or surgery. In fact, a review published in 1982 advised against using pharmacologic agents citing increased perforation risk with inducing colonic contraction.4 Case reports using prokinetic agents erythromycin,26 metoclopramide,27 and cisapride revealed inconsistent response in the acute setting.28 Emmanuel et al reported a success rate of 40% with erythromycin and a recurrence rate of 50% in treatment of chronic pseudo-obstruction.28 Cisapride was withdrawn from clinical use in 2000 due to serious cardiac arrhythmia.
Based upon the presumed pathophysiology of ACPO, Hutchinson and Griffith used neostigmine and guanethidine for treating ACPO.29 Neostigmine acts as a reversible acetylcholinesterase inhibitor, thereby increasing acetylcholine and promoting colonic motor activity. Ponec et al performed a prospective randomized trial proving the efficacy of intravenous neostigmine compared with nonpharmacologic management.30 In this trial 10 out of 11 patients (91%) responded to a single dose of 2 mg neostigmine given intravenously over 3 to 5 minutes. The placebo group receiving supportive management only had no resolution. Subsequently, multiple prospective studies have validated the use of neostigmine in ACPO.31,32,33,34,35
The optimal dose of neostigmine and administration technique remains debatable. The commonly employed dose is 2 or 2.5 mg bolus administered over 3 to 5 minutes with success above 80% after the first dose.24 The onset on action of intravenous neostigmine is 20 to 30 minutes. A repeat dose can be given to a suggested limit of two to three doses17,36 if satisfactory response is not seen after the first dose within 3 hours.30,37 However, as opposed to a single or discrete dose, continuous infusion has been effective as well. White et al described such a protocol in a patient refractory to three doses of neostigmine bolus. They mixed 5 mg of neostigmine in 50 mL of normal saline solution for infusion at a dose of 0.4 mg per hour.36 van der Spoel et al performed a prospective crossover randomized trial using continuous 0.4 to 0.8 mg/h neostigmine infusion over 24 hours with overall response in 19 of 24 patients.31
Oral administration of neostigmine is not recommended in ACPO because of its erratic absorption in the gastrointestinal tract.24 Adverse effects of neostigmine are attributed to parasympathetic overactivity and include bradycardia, hypotension, asystole, seizures, restlessness, tremor, miosis, bronchoconstriction, hyperperistalsis, nausea, vomiting, salivation, diarrhea, sweating, and abdominal cramps. Patients receiving neostigmine should be monitored in a telemetry unit for cardiac arrhythmia.24 Atropine must be available at the bedside to treat severe bradycardia. Premedication with glycopyrrolate can prevent hypersalivation. Relative contraindications limiting its use include recent myocardial infarction, acidosis, asthma, chronic obstructive pulmonary disease, bradycardia, peptic ulcer disease, renal insufficiency, and therapy with β-blockers.24
Relapse after successful treatment with neostigmine is reported from 17 to 38% in different studies.30,33,34 Polyethylene glycol (PEG) has been shown to prevent recurrence. In a prospective, randomized, placebo controlled trial, a balanced solution of PEG was given orally after successful decompression with neostigmine or colonoscopy. Patients in the PEG group had no recurrence of distension versus 33% in the placebo group.38
Recently, there have been several studies exploring the novel pharmacologic treatment of ACPO. O’Dea et al described success with oral pyridostigmine, a long-acting acetylcholinesterase inhibitor, to treat recurrent pseudo-obstruction.39 In this prospective nonrandomized study, all seven patients who had failed previous treatment with neostigmine and endoscopic decompression received 10 to 30 mg pyridostigmine two times a day and had response to treatment. Side effects of pyridostigmine are generally felt to be less severe compared with neostigmine. Pyridostigmine is commonly used for the outpatient treatment of myasthenia gravis as a first-line agent and is considered very safe.40 Moreover, no side effects of pyridostigmine were seen in the study by O’ Dea et al.39 Further studies to define the role of pyridostigmine in recurrent ACPO and in patients unable to tolerate intravenous neostigmine are needed.
Narcotic use is commonly associated with ACPO as well as postoperative ileus. Recently, increased interest in opioid receptor antagonists in the treatment of postoperative ileus suggests a potential role in ACPO. Methylnaltrexone, a µ-opioid-receptor antagonist, has been effective in a double-blinded study in patients with opioid-induced constipation.41 Weinstock et al reported a case of ACPO in which brisk response to methylnaltrexone was seen after failed treatment with neostigmine.42 Recommended dose of methylnaltrexone is weight based. Adults weighing 62 to 114 kg receive 12 mg subcutaneous injection every other day to a maximum dose of one injection every day. Adverse effects include abdominal cramps, pain, nausea, diarrhea, and dizziness.
Another peripherally acting µ-opioid-receptor antagonist (PAMORA) is alvimopan, which is approved to prevent postoperative ileus following gastrointestinal surgery.43 There are no clinical studies reported to evaluate alvimopan and ACPO; however, it has been found to reverse the inhibitory effect of codeine on small bowel and colonic transit in healthy subjects.44 Further studies in this area are needed to establish efficacy and safety of these agents as they may prove beneficial in treatment and prophylaxis of opioid related ACPO.
As stated previously, most patients with ACPO respond to neostigmine after single or multiple doses. PEG, pyridostigmine, and methylnaltrexone may have a potential role in reducing recurrence or in treating nonresponders to neostigmine, but these agents are not yet adequately tested to know the role they may ultimately play in the management of ACPO. The treating clinician should be aware of all pharmacologic options and utilize them in the appropriate setting.
Any relapse after successful pharmacologic treatment may be managed as a new occurrence of dilatation; however, the threshold for intervention may become lower compared with the initial presentation of ACPO. In patients not responding to maximal supportive and pharmacologic therapy and without signs of ischemia or perforation, endoscopic decompression should be considered.
Table 2 summarizes pharmacologic treatment.
Table 2. Pharmacologic Management of Acute Colonic Pseudo-Obstruction.
| First line therapy | Neostigmine 2 or 2.5 mg IV bolus over 3–5 minutes. Repeat dose up to 3 times if no response in 3 hours. |
| Salvage therapy | Neostigmine 0.4–0.8 mg/hour infusion for 24 hours OR Pyridostigmine* 10–30 mg orally two times a day |
| Adjunct to prevent relapse | 29.5 g of Polyethylene glycol In 500 ml of water orally in two doses |
| Opioid related ACPO | Methylnaltrexone* 12 mg† sub cut injection every other day to every day |
| Historical drugs | Erythromycin, Metoclopromide, Cisapride‡ |
IV, intravenous; ACPO, acute colonic pseudo-obstruction.
Pyridostigmine and methylnaltrexone activity has been seen in case reports only.
Dose is weight based.
Withdrawn from market due to side effects.
Role of Endoscopy
Successful colonoscopic decompression was first described by Kukora and Dent in 1977.45 The Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy (ASGE) has issued guidelines for colonoscopy in ACPO.25 Colonoscopy for ACPO is performed without administration of oral laxatives or bowel preparation. In patients undergoing colonoscopy for decompression of ACPO, sedation with benzodiazepines alone is preferred because narcotics inhibit colonic motility. Cecal intubation is not required because decompression at the level of the proximal hepatic flexure is usually sufficient.25,46 Success at the initial procedure ranges from 61 to 95%, and ultimate clinical success after one or more procedures ranges from 73 to 88%.17,21,25 Recurrences after colonoscopic decompression remains problematic, however, and have been reported to occur in up to 40% of patients.5 As previously discussed, the use of a balanced PEG solution following colonoscopy may result in a lower incidence of relapse.38
Decompression tube insertion may provide benefit when treating ACPO.46 A guidewire is placed through the instrument channel, followed by colonoscope withdrawal with regular suction. Ultimately, under fluoroscopic guidance, a decompression tube is passed into the colon over the guidewire. The decompression tube should be placed to gravity drainage and flushed every 4 to 6 hours to prevent clogging. It may be sutured to the patient to ensure retention. In a retrospective review of 56 patients, Geller et al reported a success rate of 60 to 90% after colonoscopy and decompression tube placement and 25% when a decompression tube was not used. Harig et al conducted a nonrandomized study in which four out of nine patients had recurrence in the colonoscopy-only group versus none out of 11 in the colonoscopy plus decompression tube group.
There are no prospective randomized studies comparing the efficacy of colonoscopy in ACPO with other treatment methods and colonoscopy with or without a decompression tube. However, multiple case reviews have reported successful decompression using colonoscopy. There are fewer complications with colonoscopy, a perforation rate of 2%46 and mortality of 1%,14 compared with surgical decompression.
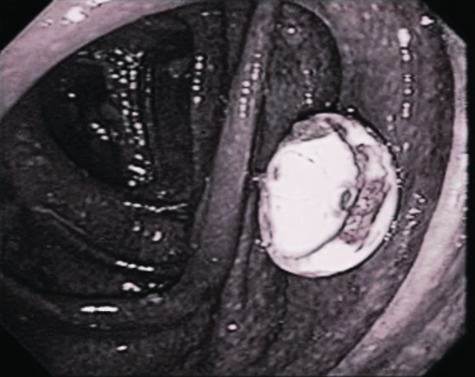
Another advanced endoscopic technique is percutaneous endoscopic colostomy (PEC) of the cecum, which has been described and can be performed either through a combined endoscopic and radiologic approach or in a manner analogous to placement of a percutaneous endoscopic gastrostomy tube.47,48 Once in place, the PEC tube is left open to vent. Tap water flushes are used to keep the tube patent. An antegrade enema with PEG solution also can be given.48 The PEC tube is removed once all symptoms have resolved and underlying disease leading to ACPO has been treated (see Fig. 2).
Figure 2.
Percutaneous endoscopic colostomy (PEC). From Google image search. Available at: http://media.daveproject.org/media/images/clip_img/fullsize/uvs070320-0051.JPG
PEC is proposed as a safe and effective procedure in the hands of an experienced endoscopist48; however, a complication rate of up to 42% has been reported with PEC including wound infection, bleeding or hematoma formation, perforation leading to peritonitis, granuloma, retraction of PEC and buried bumper.49 A technique called “introducer method” is proposed to reduce incidence of wound infection associated with the pull method.50 It uses T fasteners to secure cecum to the abdominal wall and is similar to the gastrostomy technique using a nylon T fastener.51 There are no studies to establish superiority of one method over another. The choice of technique depends on the preference of the endoscopist.
In a review, Bertolini et al49 found 60 patients who underwent PEC placement for various indications. Among these cases, nine patients from three case series had ACPO and all improved with PEC. The major advantage of PEC is the avoidance of general anesthesia. This approach may prove useful for patients who are not responding to maximal medical and endoscopic management and are poor surgical candidates. However, further studies are needed to define its safety and efficacy in comparison to surgical cecostomy for ACPO.
Image-guided percutaneous cecostomy (PCC) has also been reported in the literature. One can access the cecum under CT guidance and insert a decompression tube transperitoneally.52 One case of needle decompression via a retroperitoneal approach using a 22-gauge needle has also been reported.53 More studies are needed to assess the efficacy and safety of this procedure and to compare it with other interventions.
Endoscopy is the key intervention in the management of ACPO, which is refractory to medical management. It presents a unique challenge and requires special skills compared with routine screening colonoscopy. Placement of a decompression tube should be considered at the time of decompression colonoscopy. PEC may provide an alternative method of decompression in patients who will not tolerate general anesthesia. As mentioned above, colonoscopy and related interventions have a high success rate in treating ACPO, leaving surgery for the treatment of complicated ACPO.
Operative Intervention
Surgical intervention rarely is necessary and should be reserved for patients with ischemia, peritonitis, or ACPO refractory to decompression via pharmacologic or endoscopic therapy. Patients without any clinical concern for peritonitis and ischemia should be offered a full range of nonoperative management before resorting to operation. As previously mentioned, supportive management may take 3 to 5 days before an improvement is seen. If the condition deteriorates, then all pharmacologic options must be used followed by endoscopy. Patients who are refractory to these measures and with no clinical signs for ischemia/perforation may be considered for PEC placement.
Surgical options include cecostomy or colectomy. ACPO is one of very few conditions where cecostomy in indicated. Cecostomy, via a limited incision or laparoscopically, with or without ante grade lavage, provides effective decompression of colon.54 Laparoscopy has the potential additional benefit of visualization of the entire colon to diagnose unsuspected ischemia or infarction.55 However, laparoscopy can be challenging in the face of a massively dilated colon. A two-port technique using one umbilical 10-mm port and one 5-mm port in the right quadrant for the placement of a tube cecostomy is described.55 In this report, pneumoperitoneum was kept at 15 mmHg pressure and was decreased to 10 mmHg when cecum was drawn to the abdominal wall. Authors used a postoperative contrast study to confirm placement. Regardless of technique, cecostomy is associated with postoperative management challenges including tube and appliance management issues, the corrosive nature of the effluent, and catheter displacement.54
Laparotomy is indicated for ischemia, perforation, or if the diagnosis is not clear. The diagnosis of colonic ischemia is clinical as suggested by changes in perceived pain, physical exam findings, and laboratory and imaging data. Worsening abdominal pain, fever, leukocytosis, and lactic acidosis should raise suspicion of mucosal ischemia. Full-thickness ischemia presents with peritonitis.56 Plain films may reveal thumb printing, which results from mucosal edema and submucosal hemorrhage.56 CT often shows nonspecific colonic wall thickening and pericolic fat stranding.56 Pneumatosis and/or gas in the mesenteric veins are ominous signs when associated with bowel wall thickening and are due to bowel infarction.57
At laparotomy, the extent of colon resection is dictated by the extent of colon involvement. In the event a colectomy is needed, stoma creation and mucous fistula should be performed and anastomosis avoided.54 Complications and mortality from surgery are significant. In one retrospective series of 179 patients undergoing surgery for ACPO, the morbidity and mortality rates were 6% and 30%, respectively.13 High mortality is a reflection of the underlying disease process. Therefore, surgery is reserved for patients who fail pharmacologic and endoscopic attempts at decompression.
Conclusion
ACPO continues to complicate the hospital stay of acutely ill medical and surgical patients. ACPO carries a poor prognosis largely attributed to the underlying illness afflicting these patients. Exact pathophysiology remains a topic of investigation, and no effective prevention strategy is known. High clinical suspicion and appropriate use of diagnostic imaging differentiates ACPO from mechanical obstruction and prompts specific management. Advances in pharmacologic and endoscopic therapy reduce the need for surgical intervention. Initiation of conservative treatment successfully resolves ACPO in the majority of patients. Neostigmine remains the first-line pharmacologic treatment. Recent advances such as neostigmine infusion have improved overall efficacy of pharmacologic decompression. In addition, novel therapies such as oral pyridostigmine and µ-opioid antagonists are on the horizon to potentially treat neostigmine nonresponders. Colonoscopic decompression with decompression tube reliably provides relief of distension; it should be undertaken in patients not responding to maximal medical therapy. Advanced colonoscopic technique like PEC may provide alternative options of decompression when a cecostomy is needed for decompression. Further studies are needed to validate its safety and efficacy as an alternative to surgical cecostomy. Cecostomy remains effective albeit associated with significant morbidity. Recent advances in the management of ACPO—conservative, pharmacologic, or endoscopic—have a potential to reduce the need of emergency salvage surgery and may hopefully reduce the attendant morbidity and mortality of ACPO.
References
- 1.Ogilvie H. Large-intestine colic due to sympathetic deprivation; a new clinical syndrome. BMJ. 1948;2(4579):671–673. doi: 10.1136/bmj.2.4579.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley H AF, Paterson-Brown S. Pseudo-obstruction. Br Med J (Clin Res Ed) 1986;292(6529):1157–1158. doi: 10.1136/bmj.292.6529.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley H AF, Sinclair I S, McLaren I F, McNair T J, Newsam J E. Intestinal pseudo-obstruction. J R Coll Surg Edinb. 1958;3(3):206–217. [PubMed] [Google Scholar]
- 4.Nanni G, Garbini A, Luchetti P, Nanni G, Ronconi P, Castagneto M. Ogilvie's syndrome (acute colonic pseudo-obstruction): review of the literature (October 1948 to March 1980) and report of four additional cases. Dis Colon Rectum. 1982;25(2):157–166. doi: 10.1007/BF02553265. [DOI] [PubMed] [Google Scholar]
- 5.Rex D K. Colonoscopy and acute colonic pseudo-obstruction. Gastrointest Endosc Clin N Am. 1997;7(3):499–508. [PubMed] [Google Scholar]
- 6.Jeffcoate W J. Should eponyms be actively detached from diseases? Lancet. 2006;367(9519):1296–1297. doi: 10.1016/S0140-6736(06)68553-X. [DOI] [PubMed] [Google Scholar]
- 7.Barrett K E. New York, NY: McGraw-Hill; 2006. Intestinal motility; pp. 154–158. [Google Scholar]
- 8.Durai R. Colonic pseudo-obstruction. Singapore Med J. 2009;50(3):237–244. [PubMed] [Google Scholar]
- 9.Vanderwinden J-M. Role of interstitial cells of Cajal and their relationship with the enteric nervous system. Eur J Morphol. 1999;37(4-5):250–256. doi: 10.1076/ejom.37.4.250.4728. [DOI] [PubMed] [Google Scholar]
- 10.Jain D, Moussa K, Tandon M, Culpepper-Morgan J, Proctor D D. Role of interstitial cells of Cajal in motility disorders of the bowel. Am J Gastroenterol. 2003;98(3):618–624. doi: 10.1111/j.1572-0241.2003.07295.x. [DOI] [PubMed] [Google Scholar]
- 11.Mourelle M, Vilaseca J, Guarner F, Salas A, Malagelada J R. Toxic dilatation of colon in a rat model of colitis is linked to an inducible form of nitric oxide synthase. Am J Physiol. 1996;270(3 Pt 1):G425–G430. doi: 10.1152/ajpgi.1996.270.3.G425. [DOI] [PubMed] [Google Scholar]
- 12.Akiho H, Ihara E, Motomura Y, Nakamura K. Cytokine-induced alterations of gastrointestinal motility in gastrointestinal disorders. World J Gastrointest Pathophysiol. 2011;2(5):72–81. doi: 10.4291/wjgp.v2.i5.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanek V W, Al-Salti M. Acute pseudo-obstruction of the colon (Ogilvie's syndrome). An analysis of 400 cases. Dis Colon Rectum. 1986;29(3):203–210. doi: 10.1007/BF02555027. [DOI] [PubMed] [Google Scholar]
- 14.Kahi C J, Rex D K. Bowel obstruction and pseudo-obstruction. Gastroenterol Clin North Am. 2003;32(4):1229–1247. doi: 10.1016/s0889-8553(03)00091-8. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser A M. Ogilvie transition to colonic perforation. Am J Surg. 2010;200(1):e15–e16. doi: 10.1016/j.amjsurg.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Davis L, Lowman R M. An evaluation of cecal size in impending perforation of the cecum. Surg Gynecol Obstet. 1956;103(6):711–718. [PubMed] [Google Scholar]
- 17.Saunders M D, Kimmey M B. Systematic review: acute colonic pseudo-obstruction. Aliment Pharmacol Ther. 2005;22(10):917–925. doi: 10.1111/j.1365-2036.2005.02668.x. [DOI] [PubMed] [Google Scholar]
- 18.Godfrey E M, Addley H C, Shaw A S. The use of computed tomography in the detection and characterisation of large bowel obstruction. N Z Med J. 2009;122(1305):57–73. [PubMed] [Google Scholar]
- 19.Jacob S E, Lee S H, Hill J. The demise of the instant/unprepared contrast enema in large bowel obstruction. Colorectal Dis. 2008;10(7):729–731. doi: 10.1111/j.1463-1318.2007.01415.x. [DOI] [PubMed] [Google Scholar]
- 20.Schermer C R, Hanosh J J, Davis M, Pitcher D E. Ogilvie's syndrome in the surgical patient: a new therapeutic modality. J Gastrointest Surg. 1999;3(2):173–177. doi: 10.1016/s1091-255x(99)80029-8. [DOI] [PubMed] [Google Scholar]
- 21.Wegener M, Börsch G. Acute colonic pseudo-obstruction (Ogilvie's syndrome). Presentation of 14 of our own cases and analysis of 1027 cases reported in the literature. Surg Endosc. 1987;1(3):169–174. doi: 10.1007/BF00590926. [DOI] [PubMed] [Google Scholar]
- 22.Beattie G C, Peters R T, Guy S, Mendelson R M. Computed tomography in the assessment of suspected large bowel obstruction. ANZ J Surg. 2007;77(3):160–165. doi: 10.1111/j.1445-2197.2006.03998.x. [DOI] [PubMed] [Google Scholar]
- 23.Choi J S, Lim J S, Kim H. et al. Colonic pseudoobstruction: CT findings. AJR Am J Roentgenol. 2008;190(6):1521–1526. doi: 10.2214/AJR.07.3159. [DOI] [PubMed] [Google Scholar]
- 24.De Giorgio R, Knowles C H. Acute colonic pseudo-obstruction. Br J Surg. 2009;96(3):229–239. doi: 10.1002/bjs.6480. [DOI] [PubMed] [Google Scholar]
- 25.Harrison M E, Anderson M A, Appalaneni V. et al. The role of endoscopy in the management of patients with known and suspected colonic obstruction and pseudo-obstruction. Gastrointest Endosc. 2010;71(4):669–679. doi: 10.1016/j.gie.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 26.Bonacini M, Smith O J, Pritchard T. Erythromycin as therapy for acute colonic pseudo-obstruction (Ogilvie's syndrome) J Clin Gastroenterol. 1991;13(4):475–476. doi: 10.1097/00004836-199108000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Lipton A B, Knauer C M. Pseudo-obstruction of the bowel. Therapeutic trial of metoclopramide. Am J Dig Dis. 1977;22(3):263–265. doi: 10.1007/BF01072287. [DOI] [PubMed] [Google Scholar]
- 28.Emmanuel A V, Shand A G, Kamm M A. Erythromycin for the treatment of chronic intestinal pseudo-obstruction: description of six cases with a positive response. Aliment Pharmacol Ther. 2004;19(6):687–694. doi: 10.1111/j.1365-2036.2004.01900.x. [DOI] [PubMed] [Google Scholar]
- 29.Hutchinson R, Griffiths C. Acute colonic pseudo-obstruction: a pharmacological approach. Ann R Coll Surg Engl. 1992;74(5):364–367. [PMC free article] [PubMed] [Google Scholar]
- 30.Ponec R J, Saunders M D, Kimmey M B. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med. 1999;341(3):137–141. doi: 10.1056/NEJM199907153410301. [DOI] [PubMed] [Google Scholar]
- 31.der Spoel J I van, Oudemans-van Straaten H M, Stoutenbeek C P, Bosman R J, Zandstra D F. Neostigmine resolves critical illness-related colonic ileus in intensive care patients with multiple organ failure—a prospective, double-blind, placebo-controlled trial. Intensive Care Med. 2001;27(5):822–827. doi: 10.1007/s001340100926. [DOI] [PubMed] [Google Scholar]
- 32.Amaro R, Rogers A I. Neostigmine infusion: new standard of care for acute colonic pseudo-obstruction? Am J Gastroenterol. 2000;95(1):304–305. doi: 10.1111/j.1572-0241.2000.01737.x. [DOI] [PubMed] [Google Scholar]
- 33.Stephenson B M, Morgan A R, Salaman J R, Wheeler M H. Ogilvie's syndrome: a new approach to an old problem. Dis Colon Rectum. 1995;38(4):424–427. doi: 10.1007/BF02054234. [DOI] [PubMed] [Google Scholar]
- 34.Mehta R, John A, Nair P. et al. Factors predicting successful outcome following neostigmine therapy in acute colonic pseudo-obstruction: a prospective study. J Gastroenterol Hepatol. 2006;21(2):459–461. doi: 10.1111/j.1440-1746.2005.03994.x. [DOI] [PubMed] [Google Scholar]
- 35.Trevisani G T, Hyman N H, Church J M. Neostigmine: safe and effective treatment for acute colonic pseudo-obstruction. Dis Colon Rectum. 2000;43(5):599–603. doi: 10.1007/BF02235569. [DOI] [PubMed] [Google Scholar]
- 36.White L Sandhu G Continuous neostigmine infusion versus bolus neostigmine in refractory Ogilvie syndrome Am J Emerg Med 2011295576, e1–e3 [DOI] [PubMed] [Google Scholar]
- 37.Paran H, Silverberg D, Mayo A, Shwartz I, Neufeld D, Freund U. Treatment of acute colonic pseudo-obstruction with neostigmine. J Am Coll Surg. 2000;190(3):315–318. doi: 10.1016/s1072-7515(99)00273-2. [DOI] [PubMed] [Google Scholar]
- 38.Sgouros S N, Vlachogiannakos J, Vassiliadis K. et al. Effect of polyethylene glycol electrolyte balanced solution on patients with acute colonic pseudo obstruction after resolution of colonic dilation: a prospective, randomised, placebo controlled trial. Gut. 2006;55(5):638–642. doi: 10.1136/gut.2005.082099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Dea C J, Brookes J H, Wattchow D A. The efficacy of treatment of patients with severe constipation or recurrent pseudo-obstruction with pyridostigmine. Colorectal Dis. 2010;12(6):540–548. doi: 10.1111/j.1463-1318.2009.01838.x. [DOI] [PubMed] [Google Scholar]
- 40.Maggi L, Mantegazza R. Treatment of myasthenia gravis: focus on pyridostigmine. Clin Drug Investig. 2011;31(10):691–701. doi: 10.2165/11593300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Thomas J, Karver S, Cooney G A. et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;358(22):2332–2343. doi: 10.1056/NEJMoa0707377. [DOI] [PubMed] [Google Scholar]
- 42.Weinstock L B, Chang A C. Methylnaltrexone for treatment of acute colonic pseudo-obstruction. J Clin Gastroenterol. 2011;45(10):883–884. doi: 10.1097/MCG.0b013e31821100ab. [DOI] [PubMed] [Google Scholar]
- 43.Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23(5):616–622. doi: 10.1097/ACO.0b013e32833c3473. [DOI] [PubMed] [Google Scholar]
- 44.Gonenne J, Camilleri M, Ferber I. et al. Effect of alvimopan and codeine on gastrointestinal transit: a randomized controlled study. Clin Gastroenterol Hepatol. 2005;3(8):784–791. doi: 10.1016/s1542-3565(05)00434-9. [DOI] [PubMed] [Google Scholar]
- 45.Kukora J S, Dent T L. Colonoscopic decompression of massive nonobstructive cecal dilation. Arch Surg. 1977;112(4):512–517. doi: 10.1001/archsurg.1977.01370040164025. [DOI] [PubMed] [Google Scholar]
- 46.Geller A, Petersen B T, Gostout C J. Endoscopic decompression for acute colonic pseudo-obstruction. Gastrointest Endosc. 1996;44(2):144–150. doi: 10.1016/s0016-5107(96)70131-1. [DOI] [PubMed] [Google Scholar]
- 47.Baraza W, Brown S, McAlindon M, Hurlstone P. Prospective analysis of percutaneous endoscopic colostomy at a tertiary referral centre. Br J Surg. 2007;94(11):1415–1420. doi: 10.1002/bjs.5858. [DOI] [PubMed] [Google Scholar]
- 48.Lynch C R, Jones R G, Hilden K, Wills J C, Fang J C. Percutaneous endoscopic cecostomy in adults: a case series. Gastrointest Endosc. 2006;64(2):279–282. doi: 10.1016/j.gie.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 49.Bertolini D, De Saussure P, Chilcott M, Girardin M, Dumonceau J M. Severe delayed complication after percutaneous endoscopic colostomy for chronic intestinal pseudo-obstruction: a case report and review of the literature. World J Gastroenterol. 2007;13(15):2255–2257. doi: 10.3748/wjg.v13.i15.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uno Y. Introducer method of percutaneous endoscopic cecostomy and antegrade continence enema by use of the Chait Trapdoor cecostomy catheter in patients with adult neurogenic bowel. Gastrointest Endosc. 2006;63(4):666–673. doi: 10.1016/j.gie.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 51.Brown A S, Mueller P R, Ferrucci J T. Controlled percutaneous gastrostomy: nylon T-fastener for fixation of the anterior gastric wall. Radiology. 1986;158(2):543–545. doi: 10.1148/radiology.158.2.2934763. [DOI] [PubMed] [Google Scholar]
- 52.vanSonnenberg E, Varney R R, Casola G. et al. Percutaneous cecostomy for Ogilvie syndrome: laboratory observations and clinical experience. Radiology. 1990;175(3):679–682. doi: 10.1148/radiology.175.3.2343112. [DOI] [PubMed] [Google Scholar]
- 53.Crass J R, Simmons R L, Frick M P, Maile C W. Percutaneous decompression of the colon using CT guidance in Ogilvie syndrome. AJR Am J Roentgenol. 1985;144(3):475–476. doi: 10.2214/ajr.144.3.475. [DOI] [PubMed] [Google Scholar]
- 54.Maloney N, Vargas H D. Acute intestinal pseudo-obstruction (Ogilvie's syndrome) Clin Colon Rectal Surg. 2005;18(2):96–101. doi: 10.1055/s-2005-870890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duh Q-Y, Way L W. Diagnostic laparoscopy and laparoscopic cecostomy for colonic pseudo-obstruction. Dis Colon Rectum. 1993;36(1):65–70. doi: 10.1007/BF02050304. [DOI] [PubMed] [Google Scholar]
- 56.Bullard K M, Dunn R D. New York: McGraw-Hill; 2010. Colon, rectum, and anus; p. 1058. [Google Scholar]
- 57.Theodoropoulou A, Koutroubakis I E. Ischemic colitis: clinical practice in diagnosis and treatment. World J Gastroenterol. 2008;14(48):7302–7308. doi: 10.3748/wjg.14.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]