Abstract
Reconstruction of complex defects of the anterior abdomen is both challenging and technically demanding for reconstructive surgeons. Advancements in the use of pedicle and free tissue transfer along with the use of bioprosthetic and synthetic meshes have provided for novel approaches to these complex defects. Accordingly, detailed knowledge of abdominal wall and lower extremity anatomy in combination with insight into the design, implementation, and limitations of various flaps is essential to solve these complex clinical problems. Although these defects can be attributed to a myriad of etiologic factors, the objectives in abdominal wall reconstruction are consistent and include the restoration of abdominal wall integrity, protection of intraabdominal viscera, and the prevention of herniation. In this article, the authors review pertinent anatomy and the various local, regional, and distant flaps that can be utilized in the reconstruction of these complex clinical cases of the anterior abdomen.
Keywords: complex defects anterior abdominal wall, restoration abdominal wall integrity, locoregional flaps, distant flaps, bioprosthetic mesh, synthetic mesh
Reconstruction of the Anterior Abdominal Wall
Classification of Defect
Defects of the abdominal wall can be characterized as partial thickness or full thickness based upon the anatomic components of the defect. A partial-thickness defect describes a wound where either the skin or the subcutaneous tissue is resected. Full-thickness defects encompass a loss of both superficial soft tissue and the deeper musculofascial layers. This distinction is important when considering reconstructive options. It follows that partial-thickness defects are more amendable to primary closure, negative-pressure assisted closure, and skin grafting. Full-thickness defects often require musculofascial reinforcement and therefore need composite flap coverage with or without mesh to prevent herniation and bulge formation.
Surgical Reconstructive Options
Primary Closure
Primary closure is the preferred method of closing smaller, partial-thickness defects of the anterior abdominal wall. The presence of significant excess abdominal tissue, such as that seen in obese patients, can facilitate primary closure by providing available vascularized tissue for coverage. Accordingly, one should attempt primary closure of these partial thickness defects of the anterior abdominal wall when the size of the defect is less than 5 cm.1 Raising fasciocutaneous flaps off the abdominal wall fascia can help facilitate this closure. However, care should be taken to provide adequate laxity in an effort to prevent ischemia, potential dehiscence, or abdominal compartment syndrome.2
Skin Grafting
Skin grafting can provide cutaneous coverage of abdominal wall defects that are not amendable to primary closure. Given that skin grafts provide little musculofascial support, they are generally not used when there is loss of abdominal wall musculature; therefore, they are relatively contraindicated in larger, complete defects. At times, they can be used to cover exposed bowel in situations where it is clinically inappropriate to close the abdominal wall. Advantages of skin grafting include relative abundance of donor tissue and the ability to graft on top of viscera. Disadvantages include the aforementioned relative lack of structural support, donor site morbidity, and poor aesthetic outcomes.
Tissue Expansion
Tissue expansion can provide superficial as well as full-thickness coverage for abdominal wall defects depending upon the needs for reconstruction and the suitability of the surrounding tissues. Although tissue expansion is a delayed procedure that requires serial expansions, it can provide well-perfused, autologous tissue with excellent aesthetic outcomes. Given that infection of the implant invariably leads to explantation of the prosthesis, care must be taken to ensure sterility and a surgically sound operation.
Negative Pressure-Assisted Closure
Negative pressure-assisted closure can provide for temporary coverage in anterior abdominal wall defects when definitive reconstruction is delayed. When utilized correctly this device can promote vascularization, decrease edema, and increase local granulation tissue.3 Although this modality can be used to prepare the wound bed for definitive reconstruction with flaps, it can also be used to promote healing by secondary intention in partial-thickness defects.
Locoregional Flaps
Component separation with or without the use of mesh has revolutionized the treatment of complex anterior abdominal wall defects.4 Although these operations address many of the challenges encountered with providing underlying tissue strength, the reconstruction of the more superficial fascial layers of the abdominal wall remains a challenge. To address the soft tissue coverage of these defects, most reconstructive surgeons advocate using flaps from the local or regional vicinity. As such, the reconstruction is typically performed in a pedicled fashion with the choice of flap depending on the location and size of the defect. Commonly utilized locoregional flap options include, but are not limited to, the external oblique muscle, tensor fascia lata, rectus abdominis muscle, rectus femoris muscle, anterolateral thigh with or without a portion of vastus lateralis muscle, latissimus dorsi muscle, and omental flaps. Combining soft tissue reconstruction with bioprosthetic or synthetic mesh allows the reconstructive surgeon the ability to recapitulate abdominal wall form and function.
The external oblique muscle provides a local option in the correction of abdominal wall defects and can be used anywhere in the abdominal wall. Given the limited arc of rotation, some authors recommend that the flap's usage is limited to the upper two thirds of the abdominal wall.5 Additionally, its usage can be limited due to concerns pertaining to the amount and viability of the underlying skin that can safely be transferred with this flap.
The tensor fascia lata flap is generally regarded as one of the more useful regional flaps used in reconstruction of anterior abdominal wall defects that are located in the lower two thirds of the abdomen.6 The thickness of the overlying fascia lata provides the much needed strength required for reconstruction in this area and often precludes the need for underlying mesh placement. This flap can be harvested with or without an overlying skin paddle. However, this flap should be used with caution in the reconstruction of defects of the upper one third of the abdomen due to the relative unreliability of the distal one third of the skin paddle.
The rectus abdominis muscle flap provides a local option for defects anywhere in the anterior abdominal wall. The pedicles on this flap can be manipulated to provide cranial coverage based upon the superior epigastric artery or caudal coverage based upon the deep inferior epigastric artery. Care must be taken when closing the donor site to prevent future bulge and hernia formation. As such, some authors advocate closing the defects with mesh to prevent these complications.7
The rectus femoris muscle flap provides another option in reconstruction of anterior defects of the lower two thirds of the abdominal wall. This flap contains a large arc of rotation, albeit not as great as that of the tensor fascia lata flap. However, due to the important function of the rectus femoris muscle in ambulation, this flap can be associated with donor site morbidity due to weakening of the quadriceps function.8
The vastus lateralis muscle flap is another viable option in the reconstruction of anterior portions of the lower one third of the abdominal wall. The vastus lateralis muscle can be harvested with a skin island in the form of an anterolateral thigh (ALT) myocutaneous flap. The utility of this flap is that it can be harvested with variable amounts of skin, fascia, and muscle. The abdominal wall defect will dictate the soft tissue requirements from the thigh. This flap can be harvested as either a pedicle or free flap. When using the pedicle option, it is important to tunnel the flap below the rectus femoris muscle proximally and to create a large subcutaneous tunnel into the abdomen so as not to compress the flap or pedicle. This flap can easily reach defects of the anterior abdomen and higher based on length of the donor thigh and the amount of skin taken with the flap. When large skin paddles are taken with the ALT, the donor site is closed with a skin graft. Donor site morbidity is low as most patients ambulate without any difficulty postoperatively.9
The latissimus dorsi muscle flap is an option for regional flap reconstruction of anterior defects of the upper one third of the abdominal wall. The major potential limitation of this flap is the ability to reach the upper abdomen in patients with a longer torso. Another potential limitation of this flap is the donor site morbidity of harvesting a large skin paddle and the potential need for skin graft coverage of the back. Additionally, the latissimus dorsi harvest can limit upper extremity motion in some patients; however, compensation by the rotator cuff muscles is typically seen.10
The omentum, though not the first choice in regional flap coverage, can prove excellent protection of the underlying viscera in situations where options are limited. It derives a reliable blood supply from the right and left gastroepiploic arteries. This flap can provide protection of the entire abdominal wall and perineal areas and has the advantage of being highly vascular while providing substantial soft tissue volume for transfer. Unfortunately, the omentum requires skin for grafting and repair of the resultant hernia with mesh. Thus, omental flaps can be considered an option in abdominal wall reconstruction when all other regional sources are exhausted or free tissue transfer is not available.
Free Tissue Transfer
Free tissue transfer for abdominal wall defects is considered by some to be a last resort in abdominal wall reconstruction. However, free tissue transfer can present distinct advantages over regional flaps because they provide a larger volume of tissue, augment local blood supply to promote rapid healing, and do not incur donor morbidity in the adjacent abdominal wall. They should be considered when potential regional flaps are not available, are not within reach of the defect, or are of insufficient size to safely cover the defect. Potential difficulties in free tissue transfer to this region are the lack of a suitable recipient vessel, as the etiology of the defect may have rendered surrounding tissue nonviable. Recipient vessels within this region include the inferior and superior epigastric arteries, the deep circumflex iliac artery, and internal thoracic artery. Vein grafts can be used to augment the reach of free tissue transfers when recipient vessels are not close or pedicle length is short. Specifically, arteriovenous flaps can be brought off of the femoral vessels using a saphenous vein graft.11
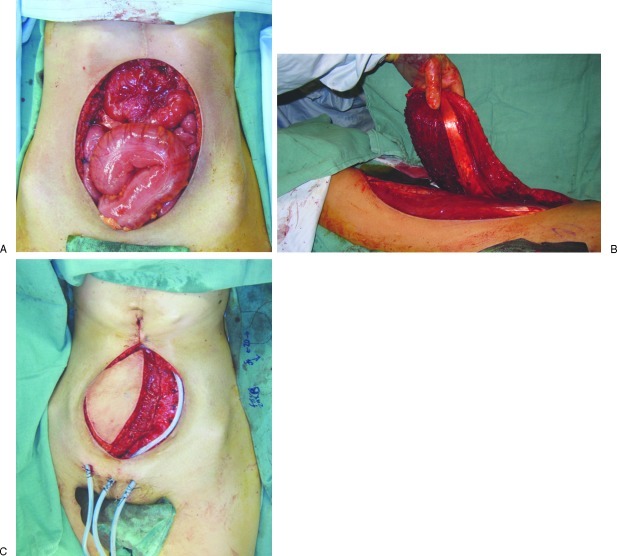
Commonly utilized free flaps for reconstruction of this region include the ALT and the tensor fasciae lata flaps. These flaps are based upon the lateral circumflex femoral system. The abdominal wall defect will dictate the appropriate flap based on the need for fasciocutaneous and musculocutaneous elements. The ALT myocutaneous flap provides an increased amount of muscle and fascia and can be utilized in larger abdominal wall defects. This tissue also contains some of the resilient deep fascia of the lateral thigh and can provide for strong coverage of abdominal wall defects. This flap can be combined with bioprosthetic mesh to reconstruct large sections of the anterior abdominal wall (Fig. 1).12
Figure 1.
(A) An intraoperative photograph showing a full-thickness defect of the anterior abdominal wall with exposed viscera due to resection of gastric tumor involving the abdominal wall. (B) Intraoperative photograph showing elevation of the pedicled anterolateral thigh (ALT) myocutaneous flap. (C) Intraoperative photograph showing inset of pedicled ALT myocutaneous flap.
The free tensor fascia lata flap of the lateral thigh provides an ideal flap for smaller abdominal wall defects. The deep fascia of the lateral thigh includes the iliotibial tract and fascia lata and provides strong and tough tissue, which can aid in wall strength and potentially prevent postoperative abdominal wall laxity. Although there can be many different flaps utilized in free tissue transfer, it is recommended that the fascia of the resultant flap be sutured into the native abdominal wall in an underlay or inlay fashion to prevent herniation. This repair should have some tension, but effort should be taken as to not make the fascia exceptionally tight to prevent ischemia of local tissues.
Postoperative Care
Postoperative care of the patient undergoing abdominal wall reconstruction is similar to that of any patient with major abdominal surgery. These patients will typically have their bowel manipulated, which can lead to an ileus. Standard advancement of diet with the resumption of bowel activity must be performed. The patient can be placed in an abdominal binder postoperatively in an effort to reduce strain on the fascial repair. In addition, antiemetic therapy should be given aggressively to these patients postoperatively in an effort to prevent vomiting, which can increase intraabdominal pressures that can further complicate repair. These patients should have judicious pulmonary toilet and monitoring of pulmonary function as abdominal distention can compromise respiratory function. Finally, patients should be instructed to avoid strenuous activity for upwards of 3 to 6 months to optimize healing and prevent bulge or hernia formation. When reconstructing the abdominal wall using either pedicle or free flaps, it is critical to place liberal amounts of closed self-suction drains. Placement of drains will help limit the potential for dead-space secondary to hematoma and seroma formation. Fluid collections in the abdominal wall have the potential to lead to poor wound healing and infectious complications.
Conclusions
Reconstruction of complex anterior abdominal wall defects presents a unique reconstructive challenge to physicians. With the myriad of techniques available today, it is possible to reconstruct the entire abdomen safely. The use of autologous tissue in the form of pedicle and free flaps provides for optimal results in the majority of these cases and can be safely performed with knowledge of the benefits and potential limitations of their usage. Bioprosthetic and synthetic meshes offer the reconstructive surgeon additional options to combine with soft tissue reconstructive techniques. Careful assessment of the defect combined with sound preoperative planning and meticulous surgical execution allows the reconstructive surgeon the ability to close complex anterior abdominal wall defects with confidence.
References
- 1.Rohrich R J, Lowe J B, Hackney F L, Bowman J L, Hobar P C. An algorithm for abdominal wall reconstruction. Plast Reconstr Surg. 2000;105(1):202–216, quiz 217. doi: 10.1097/00006534-200001000-00036. [DOI] [PubMed] [Google Scholar]
- 2.Diebel L N, Wilson R F, Dulchavsky S A, Saxe J. Effect of increased intra-abdominal pressure on hepatic arterial, portal venous, and hepatic microcirculatory blood flow. J Trauma. 1992;33(2):279–282, discussion 282–283. doi: 10.1097/00005373-199208000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Morykwas M J, Argenta L C, Shelton-Brown E I, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38(6):553–562. doi: 10.1097/00000637-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez O M, Ruas E, Dellon A L. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86(3):519–526. doi: 10.1097/00006534-199009000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Spear S L, Walker R K. The external oblique flap for reconstruction of the rectus sheath. Plast Reconstr Surg. 1992;90(4):608–613. doi: 10.1097/00006534-199210000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Huwitz D J, Hollins R R. Vol. 1994. Boston: Little Brown; Reconstruction of the abdominal wall and groin; p. 1357. [Google Scholar]
- 7.Wan D C, Tseng C Y, Anderson-Dam J, Dalio A L, Crisera C A, Festekjian J H. Inclusion of mesh in donor-site repair of free TRAM and muscle-sparing free TRAM flaps yields rates of abdominal complications comparable to those of DIEP flap reconstruction. Plast Reconstr Surg. 2010;126(2):367–374. doi: 10.1097/PRS.0b013e3181de1b7e. [DOI] [PubMed] [Google Scholar]
- 8.Caulfield W H, Curtsinger L, Powell G, Pederson W C. Donor leg morbidity after pedicled rectus femoris muscle flap transfer for abdominal wall and pelvic reconstruction. Ann Plast Surg. 1994;32(4):377–382. doi: 10.1097/00000637-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Hanasono M M, Skoracki R J, Yu P. A prospective study of donor-site morbidity after anterolateral thigh fasciocutaneous and myocutaneous free flap harvest in 220 patients. Plast Reconstr Surg. 2010;125(1):209–214. doi: 10.1097/PRS.0b013e3181c495ed. [DOI] [PubMed] [Google Scholar]
- 10.Koh C E, Morrison W A. Functional impairment after latissimus dorsi flap. ANZ J Surg. 2009;79(1-2):42–47. doi: 10.1111/j.1445-2197.2008.04797.x. [DOI] [PubMed] [Google Scholar]
- 11.Giovanoli P, Meyer V E. Use of vein loops in reconstructive procedures. Microsurgery. 1998;18(4):242–245. doi: 10.1002/(sici)1098-2752(1998)18:4<242::aid-micr5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Wong C H, Lin C H, Fu B, Fang J F. Reconstruction of complex abdominal wall defects with free flaps: indications and clinical outcome. Plast Reconstr Surg. 2009;124(2):500–509. doi: 10.1097/PRS.0b013e3181addb11. [DOI] [PubMed] [Google Scholar]