Abstract
In 1961, the satellite cell was first identified when electron microscopic examination of skeletal muscle demonstrated a cell wedged between the plasma membrane of the muscle fiber and the basement membrane. In recent years it has been conclusively demonstrated that the satellite cell is the primary cellular source for muscle regeneration and is equipped with the potential to self renew, thus functioning as a bone fide skeletal muscle stem cell (MuSC). As we move past the 50th anniversary of the satellite cell, we take this opportunity to discuss the current state of the art and dissect the unknowns in the MuSC field.
Introduction
The observation that skeletal muscle has the capacity to regenerate following injury was well documented by microscopic examination in the mid 19th century, primarily in German literature (Scharner and Zammit, 2011). Still, the cellular basis of this regenerative potential remained elusive for a century until 50 years ago when Alexander Mauro detected a mononucleated cell, which he termed a “satellite cell”, closely apposed to mature myofibers in electron micrographs of skeletal muscle (Mauro, 1961). Without any functional evidence, he hypothesized that this could represent a kind of muscle progenitor cell akin to those present in the developing embryo, capable of forming new muscle in response to injury. This turned out to be a very accurate prediction, as five decades of research on satellite cells have demonstrated that they have the characteristics that Mauro surmised. In modern parlance, the satellite cell is considered a muscle stem cell distinguished from the plethora of adult tissue-specific stem cells that have been described by the fact that it was identified anatomically before it was characterized functionally; most adult stem cells have been first demonstrated to exist in functional assays which are then followed by a hunt for the cells histologically.
The history of the satellite cell has been the subject of several recent reviews (Scharner and Zammit, 2011; Yablonka-Reuveni, 2011) and the regulation and contribution of satellite cell progenitors during lineage progression, differentiation, and contribution to muscle repair has also been extensively documented (Charge and Rudnicki, 2004; Wang and Rudnicki, 2011; Zammit et al., 2006). In this review, we focus on the current status of satellite cell research through the lens of stem cell biology. We highlight recent studies illustrating that satellite cells are essential for maintenance of the stem cell pool and repair of the differentiated muscle tissue in which they reside. In addition, we discuss the properties that satellite cells possess in common with other stem cell populations and the mechanisms that regulate satellite cell functions.
Satellite Cell Identification and Stem Cell Properties
After anatomical identification of satellite cells in 1961, their behavior in response to growth and regeneration was investigated. It was noted that in regenerating muscle, undifferentiated cells increase in abundance and align with the periphery of damaged fibers. As regeneration progresses, immature myogenic progenitors are replaced with more mature myoblasts (Allbrook, 1962). At later stages of repair, undifferentiated cells begin to appear in association with the regenerated fibers (Shafiq and Gorycki, 1965). A series of studies using tritiated thymidine confirmed that satellite cells were mitotically dormant in mature muscle and the source for regenerating muscle (Reznik, 1969; Schultz et al., 1978; Snow, 1977) and that daughters of satellite cells contributed to both the satellite cell compartment and differentiated nuclei in growing muscle (Lipton and Schultz, 1979; Moss and Leblond, 1970, 1971; Schultz, 1996). Thus with the evidence that satellite cells were capable of asymmetric divisions and endowed with self-renewal properties, a new era was born, in which satellite cells were considered as bona fide MuSCs.
Cell transplantation was becoming more commonplace in regenerative biology to test cellular contribution to tissue repair and renewal of progenitor populations. The grafting of committed satellite cell progeny (myoblasts) between mice with different isoenzyme subtypes confirmed that donor cells could fuse with host cells or myofibers (Partridge et al., 1978; Watt et al., 1982), providing evidence of a renewable cell source with regenerative capacity. However, identification and isolation of the self-renewing cells, MuSCs, remained elusive for many years.
Eventually, immunotypic analysis identified the paired box 7 transcription factor, Pax7, as a uniform marker of satellite cells (Seale et al., 2000). In response to injury, Pax7+ satellite cells enter cycle and differentiate, a subset returns back to quiescence to replenish the dormant satellite cell pool (Abou-Khalil and Brack, 2010). A body of work during the late 1980s and early 1990’s, discovered the family of myogenic regulatory factors (MRFs), including Myod, Myf5, MRF4 and Myogenin, genes that coordinate developmental myogenesis (Bentzinger et al., 2012). In their quiescent state, adult satellite cells express, along with Pax7, the Myf5 transcript but lack Myod. During activation and lineage progression, satellite cells turn on Myod and Myf5 protein, followed by the marker of terminal differentiation, Myogenin (Charge and Rudnicki, 2004).
Demonstration of a tractable muscle resident cell that contributed to muscle fiber repair and repopulation of the niche was achieved through transplantation of a FACS isolated subset of muscle resident cells selected via cell surface receptor expression (such as CD31−, CD45−, Sca1−, CXCR4+, CD31+, Integrin B1+) (Montarras et al., 2005; Sherwood et al., 2004). Engrafting a single myofiber with its satellite cells marked by a nuclear Myf5lacZ reporter provided the first definitive demonstration that satellite cells possess stem cell activity (Collins et al., 2005). In recent years, satellite cells have been proven to self-renew at the single cell level (Sacco et al., 2008) and to retain stem cell function over seven rounds of serial transplantation, and therefore function as MuSCs (Rocheteau et al., 2012).
With the advent of conditional Cre/lox systems, lineage tracing of satellite cells has demonstrated that adult Pax7+ cells self-renew and differentiate in their endogenous environment in response to injury (Lepper et al., 2009; Shea et al., 2010), suggesting that adult Pax7+ satellite cells are sufficient for both MuSC pool maintenance and myofiber repair. Moreover, studies using inducible genetic strategies to ablate adult Pax7+ cells have demonstrated that satellite cells are essential for muscle repair and replenishment of the MuSC pool (Lepper et al., 2011; McCarthy et al., 2011; Murphy et al., 2011; Sambasivan et al., 2011). Cre/lox technology has also enabled the assessment of lineage potential of satellite cells. Based on studies using Pax7CreER™ and MyodCre, it appears that most, if not all satellite cells are restricted to the myogenic lineage, arguing in favor of unipotency of adult satellite cells (Lepper et al., 2009; Shea et al., 2010; Starkey et al., 2011). However, it appears that at least a subset of satellite cells are driven out of lineage, becoming fibrogenic in pathological conditions or during aging (Amini-Nik et al., 2011; Brack et al., 2007).
While the contribution of satellite cells to tissue repair is indisputable, their role in other aspects of skeletal muscle biology is less certain (Figure 1). During normal daily activity, muscle fiber size and myonuclear content remain relatively constant throughout most of adult life (Hughes and Schiaffino, 1999), and satellite cells may exhibit a modest decline depending upon the specific muscle and species examined (Brack and Rando, 2007). Are satellite cells participating to maintain muscle fibers during homeostasis? Indelible-marked adult satellite cells chased over 6 weeks, do not appear to undergo proliferation or contribute to muscle fibers (Lepper et al., 2009; Shea et al., 2010). Moreover, two weeks after ablation of the satellite cell pool using a genetic strategy, muscle fiber size appears unaffected (Lepper et al., 2011). Even six months after depletion of satellite cells from a muscle, gross histological appearance is normal and fiber size changes negligibly (Cheung et al., 2012). Together, the data suggest that satellite cell contribution to myofiber maintenance is minimal during homeostasis. It would be informative to determine whether ablation of the adult pool would exacerbate muscle atrophy in contexts of higher cellular demand such as exercise or aging.
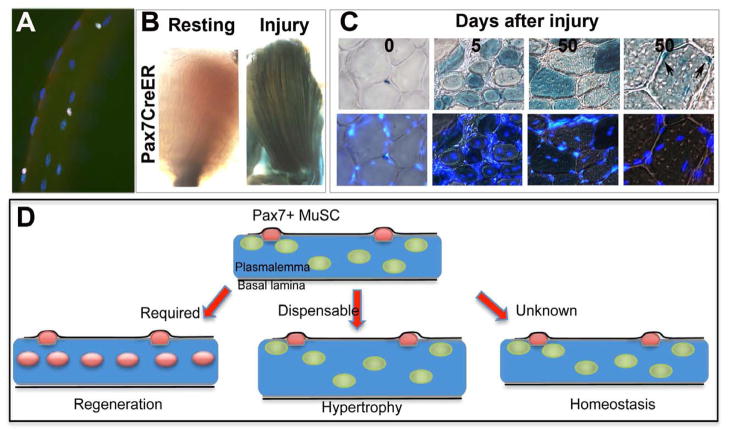
Figure 1. Satellite cell functions.
(A) Adult single muscle fiber showing Pax7+ satellite cells (pink) located on individual multinucleated adult muscle fibers, myonuclei stained with DAPI (blue). (B) An example of satellite cell contribution to muscle repair using Pax7CreER™.R26Rβ-gal is shown by X-gal reaction on an adult lower limb muscle prior to and after injury (reproduced from Shea et al, 2010). Note the many X-gal+ muscle fibers running throughout the regenerating muscle. (C) Pax7+ cells located in satellite cell position in uninjured muscle and their contribution to both myofiber repair and repopulation in the satellite cell position after regeneration (reproduced from Shea et al., 2010). (D) A schematic showing the participating roles of satellite cells. Pax7 expressing satellite cells (red) reside between the plasmalemma (brown line) of the muscle fiber and the basal lamina (black line). A mature adult muscle contains thousands of differentiated nuclei (green) and ~10 SCs (this number is highly dependent on age, muscle length and metabolic character). In response to injury, Pax7+ cells proliferate, most differentiate and fuse to regenerate myofibers (blue), while a minority self renew, return back to quiescence to replenish the satellite cell pool (left). After genetic ablation of satellite cells, muscle regeneration is prevented. During rapid hypertrophic stimuli, satellite cells fuse to add differentiated myonuclei. After genetic ablation of satellite cells, muscle hypertrophy occurs without addition of myonuclei, suggesting satellite cells are dispensable for protein accumulation and hypertrophy (center). During normal tissue homeostasis the contribution of satellite cells to maintenance of tissue or the satellite cell pool remains unknown (right).
Adult skeletal muscle is capable of hypertrophy, and the question as to satellite cell participation in that process has been debated for many years. Recent studies have provided direct evidence of hypertrophy without any satellite cell contribution. Using a genetic strategy to ablate the adult Pax7+ cell pool, mice deficient in satellite cells where able to rapidly increase muscle fiber size during a 2 week hypertrophic stimulus (McCarthy et al., 2011). This occurred in the absence of satellite cell-derived myonuclear accretion, suggesting that satellite cells are dispensable for increase in muscle mass. However, it is possible that over longer periods of time, additional differentiated nuclei, and hence a maintained myonuclear domain size in hypertrophic muscle, would be required to maintain a larger muscle fiber (Hughes and Schiaffino, 1999). This is supported by the fact that loss of differentiated nuclei in aged muscle fibers precedes muscle fiber atrophy (Brack et al., 2005).
Stem Cell Characteristics
Developmental Origins
Based on anatomical position and expression of Pax7, satellite cell precursors first appear during late fetal stage (Relaix et al., 2004, 2005). Lineage tracing approaches demonstrated that the majority of adult Pax7+ satellite cells are formed from a somitic origin of cells that transition through a Pax7+/Myod+ state (Kanisicak et al., 2009; Lepper and Fan, 2010; Schienda et al., 2006). It is clear that the number of Pax7+ cells declines during development and postnatal growth (Schultz, 1989; White et al., 2010). Therefore not all embryonic Pax7+ cells will form the self-renewing adult pool, but instead will primarily contribute to the growing myofiber. This suggests that some fetal Pax7+/Myod+ cells differentiate and other Pax7+/Myod+ cells form the adult satellite cell pool. Myod is considered a master regulator of myogenesis (Weintraub et al., 1991). Based on germline mutants, Myod is required for satellite cell differentiation but not for the formation of the satellite cell pool (Charge et al., 2008; Rudnicki et al., 1992). In vitro experiments demonstrate that subsets of myogenic progenitors lose Myod and return to a quiescent state (Halevy et al., 2004; Olguin and Olwin, 2004; Yoshida et al., 1998; Zammit et al., 2004). Moreover, self-renewing adult satellite cells transiently express Myod prior to niche repopulation during repair (Shea et al., 2010). Therefore, Pax7+/Myod+ precursors are able to form the satellite cell pool providing that Myod is repressed. Based on studies using a Myf5CreYFP reporter, it was demonstrated that the majority (90%) of the adult satellite cell pool is formed from a Myf5+ precursor (Kuang et al., 2007). However, the formation of the satellite cell pool in germline Myf5 nulls suggests that Myf5 is not required for satellite cell formation; compensation by Myod cannot be excluded (Gensch et al., 2008; Haldar et al., 2008). While there are multiple caveats to consider when interpreting these studies, including the contribution of non-Cre-recombined cell populations, these results suggest that the adult satellite cell pool is formed from precursors that are under different transcriptional control. Contribution from the extrinsic environment may also participate in differential regulation of satellite cell precursors.
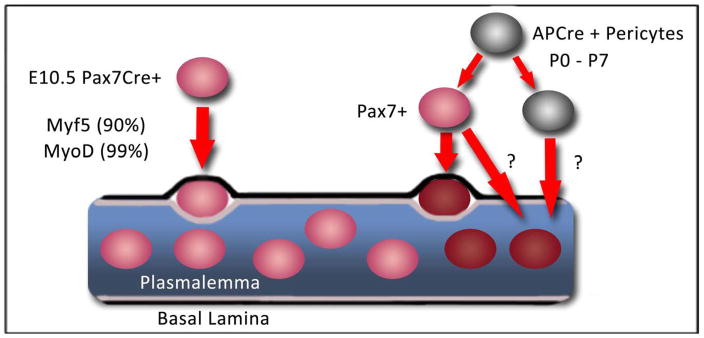
While the adult satellite cell pool is formed from Pax7+ embryonic precursors, this does not preclude a model whereby an alternative source of cells express Pax7 and home to the satellite cell niche. Using immunotypic markers (CD34+/Sca1+) in combination with donor cell marking for transplantation studies, it has been demonstrated that a subset of PICs (PW1+ Pax7− interstitial cells) self-renew and contribute to myofibers and Pax7+ satellite cells in injured adult muscle (Mitchell et al., 2010). Moreover, myogenic contribution of PICs was Pax7-dependent and temporally restricted to postnatal growing muscle. Based on lineage tracking, a subset of Alkaline Phosphatase (AP)-expressing pericytes indelibly marked in postnatal mice contributed to the formation of the adult Pax7+ satellite cell compartment (Dellavalle et al., 2011). Therefore the origin of the adult satellite cell pool may be heterogeneous (Figure 2). In the future, it will be important to dissect the unique properties and lineage relationship between pericytes and PICs that express Pax7 relative to Pax7-derived satellite cells.
Figure 2. MuSC heterogeneity.
Origins of the MuSC pool. SC precursors (Pax7CreER+) located in the myotome begin expressing Pax7 (red) at ~E10.5, most express Myf5+ and Myod+ before occupancy into the niche. During early postnatal growth (P0–P7), a subset of Alkaline Phosphatase (AP+) derived pericytes as detected with APCre (grey) express Pax7 (red) and occupy the MuSC niche (dark red). A subset of AP+ pericytes is capable of contributing to myofiber differentiation (dark red myonuclei). Whether perictyes that commit to myogenic fusion express Pax7 during their lineage progression is unknown (thick red arrows).
Asymmetric cell divisions
While asymmetric cell divisions are one of the fundamental characteristics of stem cells underlying the process of self-renewal, stem cells may undergo self-renewal by virtue of symmetric divisions (Tajbakhsh et al., 2009). As long as only one daughter of a symmetric cell division is instructed to differentiate, the stem cell pool can be maintained while differentiated progeny are produced. Historically, there were no seminal observations that provided evidence to either support or refute asymmetric cell divisions in the satellite cell lineage, and it was only in the past decade that this has been the focus of studies in the MuSC field.
The first evidence of asymmetric cell divisions came from studies of the role of Notch signaling during satellite cell activation and proliferative amplification. It was found that the Notch inhibitory protein, Numb, was asymmetrically segregated in progenitors undergoing cell division, and that Numb was localized to one pole of dividing cells, perpendicular to the plane of cell division (Conboy and Rando, 2002). This asymmetric Numb localization is reminiscent of what had been previously described in specific lineages during Drosophila development (Petersen et al., 2002; Zhong et al., 1996). The result of such an asymmetric segregation in the satellite cell lineage is the inheritance of Numb by one daughter and not the other, the former being destined to differentiate and the latter remaining an undifferentiated progenitor (Conboy and Rando, 2002). The asymmetric segregation of Numb was subsequently investigated during satellite cell activation, and asymmetric segregation of Numb was also detected in cells prior to the onset of differentiation (Shinin et al., 2009). Other proteins, such as Myod (Zammit et al., 2004) and Dek (Cheung et al., 2012), are asymmetrically segregated in activated satellite cells, and those proteins are more highly expressed in cells that have progressed further along the myogenic lineage.
Asymmetric cell divisions have also been suggested in relation to the aforementioned distinction of satellite cell subsets based on developmental history of Myf5 expression (Kuang et al., 2007). In this model, satellite cells undergo asymmetric cell divisions, giving rise to two daughters, one being another stem cell (still with no history of Myf5 gene expression) and the other becoming part of the larger pool of satellite cells (and expressing the Myf5 transcript). In this case, there is no direct evidence as yet of an asymmetric segregation of any specific protein or transcript in the mother cell, and the asymmetry may instead be a divergent cell fate dictated by the different environments of the two daughters. This is particularly likely given the observation that daughters exhibiting divergent fates, one self-renewing and the other committing toward differentiation, were much more frequent when the plane of division was perpendicular to the axis of the myofiber (Kuang et al., 2007). By contrast, divisions that occurred parallel to the myofiber axis were much more likely to give rise to two daughters with identical characteristics as opposed to divergent fates, suggesting that the local environment of the satellite cell niche might be dictating the fate of satellite cell progeny.
Yet another asymmetry that has been described is the asymmetric segregation of sister chromatids as satellite cells divide and the population expands (Conboy et al., 2007; Shinin et al., 2006). Recent evidence suggests that a subset of satellite cells, in fact those with characteristics of long-term stem cells, are more likely to exhibit non-random sister chromatid segregation (Rocheteau et al., 2012). This further supports the notion that asymmetric segregation of sister chromatids might be a property of stem cells as originally proposed (Cairns, 1975) (Figure 3). Although the functional significance of this remains theoretical, the proposed function is to segregate DNA strands that have been damaged during replication to the more differentiated daughter and reserve for the self-renewing stem cells those strands that served as templates during the previous round of cell division, thus acquiring few replication-induced errors (Charville and Rando, 2011). While this teleological function has not been demonstrated and there may be detrimental consequences of template strand segregation during aging (Charville and Rando, 2011), it is supported by the fact that the oldest template strands segregate with the less differentiated satellite cell daughter (Conboy et al., 2007; Shinin et al., 2006).
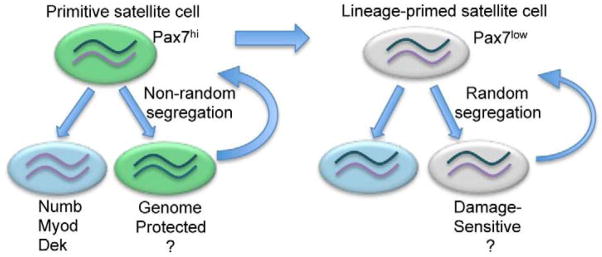
Figure 3. Non-random segregation of chromosomes and asymmetric fate determination.
The satellite cell pool is composed of primitive (Pax7hi, green) and lineage primed (Pax7low, grey) subsets. Pax7hi cells are in a dormant (less metabolically active) state. Pax7low satellite cells are lineage-primed. Following cytokinesis, satellite cell daughters can each inherit some chromosomes bearing newer (purple) or older template strand (dark green). Pax7hi subsets undergo non-random segregation of chromosomes, whereby one daughter contains exclusively chromosomes bearing older template strands, while its sister contains only chromosomes bearing newer template strands. In contrast, Pax7low cells undergo random segregation of chromosomes ensuring each daughter cell inherits an older and newer template strand. Subsets of Pax7hi and Pax7low are capable of multiple rounds of self-renewal (curved arrows) and differentiation (blue cells), however Pax7hi cells are endowed with enhanced self-renewal capability. Daughter cells containing newer template strands express high levels of Numb, Myod and Dek1 and are biased to differentiate (blue cell). Pax7hi cells can give rise to Pax7low, but not vice versa, suggesting a hierarchical relationship in the satellite cell pool based on Pax7 expression. In addition to cell fate determination, non-random segregation of chromosomes may act to protect genome integrity during cell proliferation. Due to low metabolic output of primitive satellite cells they may be protected from oxidative damage. Lineage-primed satellite cells are capable of self-renewal but may be acutely sensitive to proliferative demands and other forms of genotoxic insults.
Satellite Cell Heterogeneity
It is becoming more apparent that adult stem cell populations are heterogeneous. Heterogeneity in the adult satellite cell pool has been demonstrated based on multiple criteria, such as expression profile (Beauchamp et al., 2000; Rocheteau et al., 2012), proliferation kinetics in vitro (Day et al., 2009), self renewal potential (Kuang et al., 2007), and molecular regulation (Kitamoto and Hanaoka, 2010; Kuang et al., 2007; Shea et al., 2010). Functional heterogeneity within a pool of adult stem cells can arise from differentially specified subsets that retain distinct properties during cellular division, or evolution of a subset of cells derived from a single homogeneous cell population that will emerge during cellular division.
It is widely accepted that Pax7 expression declines in differentiating progenitors (Zammit et al., 2004). Evidence for varied Pax7 levels within the satellite cell pool was recently demonstrated using a transgenic GFP reporter of Pax7 transcript (Rocheteau et al., 2012). Functional analysis of satellite cells at extreme ends of the Pax7 expression distribution demonstrated that Pax7Hi cells had reduced metabolic activity and slower cell cycle entry kinetics, whereas Pax7Lo cells were primed to differentiate in vitro. While cells with lower Pax7 expression appeared to be more prone to differentiation, all Pax7+ cells could support over 6 serial transplantations, demonstrating that both subsets of satellite cells possess remarkable regenerative capacity and the ability to self-renew. Using an inducible Cre/lox system, Pax7 was deleted from satellite cells in growing postnatal muscle and adult muscle prior to injury. The results demonstrated that Pax7 is essential for satellite cell function during early postnatal growth and dispensable for adult muscle tissue repair (Lepper et al., 2009). Given that a subset of postnatal Pax7+ cells seeds the adult Pax7+ pool, it supports the hypothesis that there is a small subset of postnatal Pax7+ cells that evolve during the selective pressure of muscle growth to propagate satellite cells that function independently of Pax7. It raises the possibility that functionally distinct satellite cell subsets can interconvert for selective advantage under conditions of high cellular demand as observed in spermatogonial stem cells (Nakagawa et al., 2007; Nakagawa et al., 2010).
Adult stem cells with limited proliferative history are endowed with greater stem cell capability than more frequently dividing counterparts (Foudi et al., 2009; Wilson et al., 2008), suggesting that stem cell self-renewal capacity may decline in proportion with the number of divisions a stem cell has undergone in its history. Interestingly, subsets of presumptive satellite cells in growing postnatal muscle divide with slower proliferation kinetics, based on tritiated thymidine uptake (Schultz, 1996). This data suggests that one level of functional heterogeneity in the satellite cell pool may exist based on their proliferative history. In dystrophic muscle, disease progression correlates with proliferative capacity of satellite cells, suggesting that restricting proliferative output of stem cells relative to their downstream progeny may extend self-renewal capacity and ameliorate disease pathogenesis (Sacco et al., 2010). Whether functional heterogeneity across a pool of satellite cells is stable once specified or adaptive based on cellular demand, it is clear that the satellite cell pool is functionally heterogeneous. The challenge in the future is to determine how molecular heterogeneity at the cell intrinsic and extrinsic level, instructs functional diversity within the satellite cell pool.
Regulation of Satellite Cell Function
Stem Cell Niche
The stem cell niche refers to the microenvironment that maintains ‘stemness’ (Schofield, 1978). The attributes of the niche as originally conceptualized were, a) a defined anatomical site, b) a location where stem cells could reproduce, c) a place where differentiation is inhibited, and d) a space that also limits the numbers of stem cells. Therefore the niche is a protector of stem cell number and function, restraining proliferation and differentiation of stem cells, and maintaining a quiescent phenotype.
The location of the satellite cell, i.e. residing in a depression in the plasmalemma and beneath the basal lamina of the muscle fiber, provided a defined anatomical site of the putative MuSC (Mauro, 1961). The hypothesis that the muscle fiber is a satellite cell niche is supported by evidence generated from single muscle fiber experiments. Removal of the myofiber plasmalemma drives quiescent satellite cells into cycle, suggesting a role in inhibition of mitogen-induced cell cycle entry (Bischoff, 1986a). Quiescence is a conserved property of stem cells mediated by the niche (Orford and Scadden, 2008). Studies on freshly isolated single muscle fibers have shown that a subset of quiescent satellite cells can proliferate and return back to quiescence when in contact with the single muscle fiber, whereas a larger subset commits to differentiation but their fusion is inhibited (Bischoff, 1986a; Olguin and Olwin, 2004; Zammit et al., 2004). Therefore the muscle fiber fulfills three of Schoffield’s criteria of a stem cell niche. Whether the muscle fiber constrains stem cell numbers is to be determined.
In response to injury, the muscle fiber degenerates, which likely leads to niche destruction and a loss of inhibitory signals. Besides losing inhibitory factors that restrict proliferation and differentiation from the degenerated niche, muscle injury also promotes the release of stimulatory factors present at the basal lamina of the muscle fiber that drive proliferation and differentiation (Bischoff, 1986b; Sheehan and Allen, 1999). The observation that microenvironment stiffness is a regulator of stem cell potential and fate decisions suggests that maintenance of stemness from the niche is regulated at many levels (Engler et al., 2006; Gilbert et al., 2010). Resolving the signals that drive self-renewal versus differentiation will require a careful characterization of the basal and apical aspects of the niche (Figure 4A).
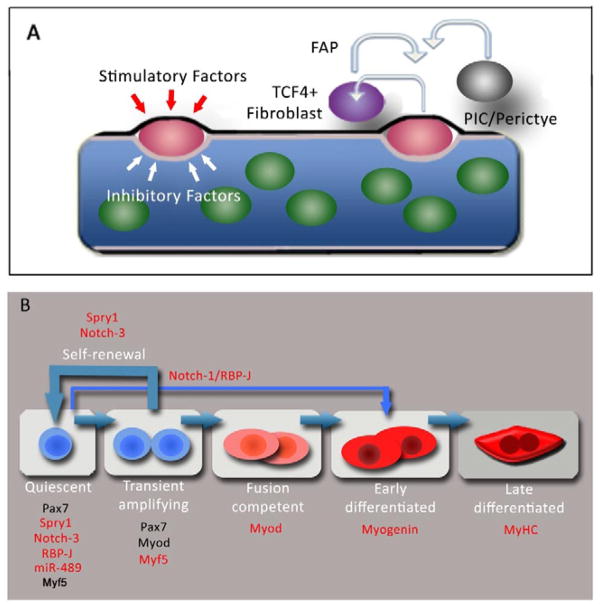
Figure 4. Regulation of MuSC functions in satellite cells.
(A) The MuSC niche. The satellite cell (red) resides in a quiescent state in contact with the plasmalemma (brown) of the myofiber and basal lamina (black) in a milieu of inhibitory (white arrows) and stimulatory factors (red arrows) that retain satellite cells in a stem cell state and inhibit their differentiation. Other cells in close proximity to satellite cells in vivo such as TCF4+ fibroblasts, FAPs, PICs and pericytes may constitute components of the MuSC niche and signal to the satellite cell and vice versa. (B) Schematic of MuSC regulation. Satellite cells transition from quiescence into a transient amplification stage most progress along a myogenic lineage to differentiate while a subset self renew and return back to quiescence (thick blue arrows). Markers denoting each stage of lineage progression are shown in black, molecules required for distinct satellite cell functions are shown in red. Quiescent satellite cells express Spry1, Notch-3 and miR-489. RBP-J and Myf5 is expressed in quiescent and cycling satellite cells. RBP-J and miR-489 (red) are required to retain satellite cells in quiescence. Spry1 and Notch-3 are required in cycling satellite cells for their return to quiescence and homeostasis of satellite cell pool after injury. Myf5 is required for normal transient amplification of progenitors. Myod, Myogenin and Myosin Heavy Chain (MyHC) are required for differentiation. Normal step-wise lineage progression of satellite cells depends on RBP-J (thin blue arrow), possibly through modulation of Notch-1 signaling.
The satellite cell niche may be composed of different cell types, as observed for other stem cell compartments (Morrison and Spradling, 2008). Besides the muscle fiber, there are other muscle-resident cells in close proximity to the satellite cell, such as Ang1-expresssing endothelial cells (Abou-Khalil et al., 2009; Christov et al., 2007). It is becoming clear that other cell types, such as fibro-adipogenic progenitors (FAPs) and TCF4+ fibroblasts, influence satellite cell behavior in contexts of regeneration and growth (Joe et al., 2010; Murphy et al., 2011). It remains to be determined whether such cell types constitute the niche and therefore regulate stemness or function as paracrine agents involved in proliferation and differentiation of satellite cell progeny (Figure 4A). As cell-specific Cre drivers become more readily available, it will be possible and essential to determine the cell types and mode of regulation that contribute to a functional satellite cell niche.
Signaling pathway regulation of satellite cell function
A balance between extrinsic cues and intracellular signals converge to preserve stem cell function. Over the past 50 years, many excellent studies have demonstrated that multiple extrinsic signaling pathways, such as IGF, FGF, Wnt, Notch, BMP, and TGFβ, function in the activation of satellite cells, their downstream progeny and their lineage progression (Kuang et al., 2008). More recently, the signaling pathways that modulate functions specific to stem cells, such as maintenance of quiescence during homeostasis, reversible quiescence and self-renewal after proliferation, asymmetric fate decisions, and symmetric expansion of stem cells are becoming more defined (Figure 4B).
It was recently shown that active Notch signaling is required to maintain satellite cells in the quiescent state, implying a role of niche-derived Notch ligand that binds to a Notch receptor on the quiescent satellite cell (Bjornson et al., 2011; Mourikis et al., 2011). In these studies, RBP-J, the downstream transcriptional co-activator in the Notch pathways, was deleted from adult satellite cells in uninjured muscle. This led to activation and ectopic differentiation of satellite cells, possibly in the absence of cell cycle entry and therefore bypassing the transient amplifying (TA) progenitor stage (Bjornson et al., 2011; Mourikis et al., 2011). Therefore, not only is RBP-J important for restricting cell cycle entry, it also argues that in non-quiescent satellite cells RBP-J, possibly as a mediator of Notch1 signaling, may be required for normal step-wise lineage progression (Conboy and Rando, 2002). That the satellite cell pool is lost when the TA stage is bypassed has implications for understanding the relationship between self-renewal and differentiation potential of downstream progenitors within the stem cell hierarchy.
To replenish the satellite cell pool after injury, stem cells return back to quiescence after proliferating. Our understanding of reversion back to quiescence in vivo is limited. Based on in vitro experiments it is becoming apparent that multiple signaling pathways are actively involved in promoting satellite cell quiescence. Activation of the Ang1/Tie2 signaling complex promotes the return to quiescence of myoblasts in vitro (Abou-Khalil et al., 2009). P38/MAPK pathway has also been implicated in regulating quiescence of satellite cells (Jones et al., 2005). Finally, Myostatin, a secreted growth factor belonging to the TFGβ family, was shown to negatively regulate quiescence and the return to quiescence of satellite cells in vitro (McCroskery et al., 2003; McFarlane et al., 2008). Together these data illustrate a critical role of growth factor signaling in the return of cycling satellite cell progenitors back to a quiescent state. Further in vivo analysis and genetic approaches that directly target satellite cells will clarify the signaling cascades that regulate the maintenance of quiescence and return to quiescence after proliferation.
Recently, signaling through Notch-3 was found to negatively regulate satellite cell pool size in regenerating muscle (Kitamoto and Hanaoka, 2010). Whether this is regulated at the cell-autonomous level of the satellite cell or by a niche-based mechanism will require cell-specific genetic mutants. Using a satellite cell-specific mutant for Spry1, it was demonstrated that Spry1, an inhibitor of growth factor signaling, is required to restore satellite cell pool size after injury (Shea et al., 2010). This suggests that a balance between inhibitory and stimulatory cues from the microenvironment is required for quiescence to be achieved. One can theorize that Spry1 and other intracellular growth factor inhibitors help to dampen the stimulatory factors at the basal lamina to direct fate towards quiescence and the repression of differentiation. The challenge in the future is to identify niche-derived inhibitory signals that promote and retain stemness after injury.
Stem cells possess a unique ability to divide symmetrically or asymmetrically depending on tissue requirements (Tajbakhsh et al., 2009). Non-canonical Wnt signaling through the Wnt7A-Frz7- Vangl2 cascade was recently implicated in driving symmetric expansion of satellite cells located on single muscle fibers in vitro (Le Grand et al., 2009). It will be interesting to unravel the relationship between asymmetric divisions via Numb and symmetric divisions via Wnt7A-Vangl2 that occur to control stem cell expansion and differentiation.
In general, the cellular output within a pool of cells in response to manipulation of signaling cascades is not equivalent. Through loss-of-function approaches, it has been demonstrated that satellite cells differentially respond to manipulation of signaling molecules such as Spry1 and Notch-3 (Kitamoto and Hanaoka, 2010; Shea et al., 2010). It will be important to decipher whether heterogeneous cell signaling requirements are due to extrinsic differences, such as localization of satellite cells in discrete niches, or cell intrinsic differences, based on lineage relationships.
While knowledge of the signaling cascades that regulate stem cell properties of satellite cellss is progressing, it is likely that more pathways participate than have been identified. During cell division and lineage progression, signaling molecules are used reiteratively. For example, Numb is partitioned to initially allow asymmetric fate of neural precursors and subsequently to maintain progenitors (Petersen et al., 2004). Likewise, Numb may be important for satellite cell self-renewal and also, at a later stage of myogenic lineage progression, for regulating the divergent fates of proliferating progenitors (Conboy and Rando, 2002; Jory et al., 2009; Shinin et al., 2006). Therefore while canonical Wnt, FGF and BMP signaling cascades participate in myogenic lineage progression and differentiation (Kuang et al., 2008), it may be that they are also deployed in some fashion to direct satellite cell properties.
Epigenetic regulation of satellite cell function
Based on what we know about extrinsic cues controlling stem cell function, the role of the stem cell niche cannot be underestimated. However, more stable modes of regulation may also maintain key functions of satellite cells. Control of the function of stem cells, particularly embryonic stem cells (ESCs), has focused on regulatory mechanisms at the level of epigenetics, namely the ensemble of heritable changes in gene function that occur without modifications of the primary DNA sequence (Bird, 2007). Such changes include DNA methylation and chromatin structural modifications mediated by histone modifications and nucleosome positioning. Not too long after the identification of the satellite cell, electron micrographs demonstrated that there are changes in chromatin organization in satellite cells from adult muscle and growing muscle consistent with their transition from a quiescent to a proliferative state (Church, 1969). As our knowledge in the field of epigenetics becomes more sophisticated, it is becoming appreciated that the regulators of epigenetic states in stem cells include mediators of DNA methylation and demethylation, microRNAs, and other non-coding RNAs, as well as modifiers of histones, including acetylases, deacetylases, methylases, and demethylases. This field is redefining cellular states at a molecular level that are much more varied and complex and likely reflect the dynamic interaction of stem cells with their environment resulting in greater functional heterogeneity than was previously envisioned.
It is likely that epigenetic states will better define such key satellite cell features as prolonged quiescence and lineage fidelity. It is also likely that DNA and histone modifications will underlie many of the changes in aged satellite cells that account for age-related declines in functionality and rejuvenation through exposure to the systemic environment (Brack et al., 2007; Conboy et al., 2005). Although there has been extensive research on the epigenetic control of myogenesis, primarily using the C2C12 myoblast cell line (Juan et al., 2011), studies in satellite cells have been limited because of the need for large numbers of cells for such analysis. Advances in both satellite cell purification and methodologies for genome and epigenome-wide analyses of limited cell numbers will allow for rapid advances in these areas in the coming years.
There have, however, been studies of regulators of epigenetic states in satellite cells. It was shown that Pax7 functions with the Wdr5-Ash2L-MLL2 histone methyltransferase (HMT) complex to direct methylation of histone H3 lysine 4 (H3K4). Binding of the Pax7-HMTcomplex resulted in H3K4 trimethylation of chromatin at the Myf5 locus (McKinnell et al., 2008), suggesting that Pax7 may act through chromatin modifications to stably and hereditably maintain the myogenic program. Conversely, the regulation of Pax7 expression may be determined by the regulation of the repressive Polycomb repressive complex 2 (PRC2) and its enzymatic component, EZH2 (Palacios et al., 2010), suggesting a network providing stability of the myogenic program related to Pax7 expression.
The regulation of myogenesis by microRNAs has likewise focused mainly on developmental myogenesis and the regulation of myogenic differentiation (Braun and Gautel, 2011). Recent studies have also examined the role of miRNAs in the regulation of quiescent satellite cells. miR-206 was recently shown to target Pax3 in quiescent satellite cells, accounting for the differential expression in Pax3 in cellular subsets (Boutet et al., 2012). This study also revealed that alternate polyadenylation of the Pax3 transcript is an important determinant, not only of satellite cell heterogeneity, but also of the susceptibility of the Pax3 transcript to regulation by any miRNA (Boutet et al., 2012). Another microRNA, miR-489, was recently shown to be highly expressed in quiescent satellite cells and to regulate specifically the quiescent state by targeting the transcript of the Dek gene, a gene known to be important for cell cycle regulation and alternative splicing (Cheung et al., 2012). Knockdown of miR-489 in vivo led to spontaneous activation of quiescent satellite cells, thus demonstrating the importance of a stable miRNA network for maintaining the quiescent state.
Conclusions and Perspectives
The last fifty years have led to a remarkable understanding of the satellite cell. It is widely accepted that the satellite cell is essential for regenerative myogenesis and can maintain itself after injury. It has been demonstrated that the muscle fiber constitutes a major functional component of the satellite cell niche. More recently, experiments have illustrated the functional heterogeneity within the satellite cell pool. As we move forward, what will the next fifty years bring?
Remarkably, we do not know whether satellite cells maintain themselves or differentiate to maintain muscle tissue during normal daily wear-and-tear. This information is critical as biologists devise strategies to harness skeletal muscle strength of the ever-expanding human aged population.
To date, direct experimental evidence is lacking to determine whether stem cell number is limiting. If MuSCs have a finite capacity, then a quorum will be required to functionally repair muscle tissue and maintain homeostasis. In the context of regenerating a tissue, such as skeletal muscle, it may be too simplistic to consider MuSC number and function as independent entities. Rather, there is likely a cooperative relationship between MuSC number and function in response to a regenerative insult. For example, in the presence of few MuSCs, a greater functional demand will be imposed on those rare cells, leading to their exhaustion. In contrast, increasing the number of MuSCs in a muscle will lessen the burden on each cell imposed by the regenerative insult. In scenarios of limiting satellite cell number, other MuSCs may participate, contributing as facultative stem cells. Other cell types such as PICs, mesangioblasts and pericytes are able to contribute to muscle tissue repair (Peault et al., 2007). Further analysis is needed to determine whether such cell types function as facultative stem cells; that are dependent on cellular context. Understanding whether their contribution is dependent on the presence of a functional pool of satellite cells may reveal further complexities of the regulation of skeletal muscle repair. Development of genetic tools to ablate fractions of satellite cells will illustrate the intimate cooperative relationship between satellite cell number and function and their interdependency with other contributing cell types.
Technological advances in recent years have provided greater resolution of stem cell heterogeneity. With the advent of fluorescent reporters of proliferative history it is now possible to isolate and compare such heterogeneous cells to dissect their function and molecular regulation (Foudi et al., 2009; Tumbar et al., 2004). Marking satellite cells based on Cre drivers has proven invaluable for tracking them and their contribution to growth and repair (Lepper and Fan, 2010; Lepper et al., 2011; Shea et al., 2010). More recent advances through the use of multicolor Cre reporters or genetic barcoding utilized in other stem cell compartments have provided improved cellular resolution that facilitates the tracking of clonally diverse cells in a population to be studied (Lu et al., 2011; Snippert et al., 2010). Such techniques reveal that the level of stem cell heterogeneity is more dynamic and context dependent than previously appreciated (Lu et al., 2011). Information gleaned from such approaches will allow the relationship between molecular and functional heterogeneity to be resolved.
The anatomical location of the satellite cell provides a landmark for the satellite cell niche. Based on its conceptual framework, although the niche impacts stem cell properties of the satellite cell, the mechanism by which this is achieved remains a mystery. In the future, functional components of the satellite cell niche will be identified through technological advances in live imaging, micro-dissection and biochemical analysis. As our understanding of satellite cell heterogeneity evolves, it will be interesting to identify whether there are distinct niches that, in some way, establish satellite cell heterogeneity. Advances in our knowledge of cell intrinsic mechanisms that regulate stem cell function, such as epigenetic regulation of satellite cells, will illustrate the communication between the extrinsic environment and intrinsic effectors to specify and maintain stem cell states.
In conclusion, the seminal observations of the satellite cell in its niche made by Alexander Mauro almost fifty years ago have spawned many great advances in our understanding of this tissue-specific stem cell. It is apparent that there is greater heterogeneity in the satellite cell pool in terms of cellular subsets that specify the pool, their function and the signaling cascades that regulate them. We are just beginning to unravel the microenvironmental influences that mediate stem cell properties and how epigenetic regulation governs stable properties of satellite cells during homeostasis and repair.
Acknowledgments
This work was supported by grants from the NIH (R01 AR060868) to A.S.B and (P01 AG036695, R37 AG23806 (MERIT Award) and R01 AR056849) to T.A.R. We thank Jenna Norton for artwork and Josef Christensen for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Khalil R, Brack AS. Muscle stem cells and reversible quiescence: the role of sprouty. Cell Cycle. 2010;9:2575–2580. doi: 10.4161/cc.9.13.12149. [DOI] [PubMed] [Google Scholar]
- Abou-Khalil R, Le GF, Pallafacchina G, Valable S, Authier FJ, Rudnicki MA, Gherardi RK, Germain S, Chretien F, Sotiropoulos A, et al. Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell. 2009;5:298–309. doi: 10.1016/j.stem.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allbrook D. An electron microscopic study of regenerating skeletal muscle. Journal of anatomy. 1962;96:137–152. [PMC free article] [PubMed] [Google Scholar]
- Amini-Nik S, Glancy D, Boimer C, Whetstone H, Keller C, Alman BA. Pax7 expressing cells contribute to dermal wound repair, regulating scar size through a beta-catenin mediated process. Stem Cells. 2011;29:1371–1379. doi: 10.1002/stem.688. [DOI] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. JCell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, Rudnicki MA. Building muscle: molecular regulation of myogenesis. Cold Spring Harbor perspectives in biology. 2012:4. doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Proliferation of muscle satellite cells on intact myofibers in culture. DevBiol. 1986a;115:129–139. doi: 10.1016/0012-1606(86)90234-4. [DOI] [PubMed] [Google Scholar]
- Bischoff R. A satellite cell mitogen from crushed adult muscle. DevBiol. 1986b;115:140–147. doi: 10.1016/0012-1606(86)90235-6. [DOI] [PubMed] [Google Scholar]
- Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch Signaling is Necessary to Maintain Quiescence in Adult Muscle Stem Cells. Stem Cells. 2011 doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutet SC, Cheung TH, Quach NL, Liu L, Prescott S, Edalati A, Iori K, Rando TA. Alternative polyadenylation mediates microRNA regulation of muscle stem cell function. Cell Stem Cell. 2012;10:231–346. doi: 10.1016/j.stem.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Bildsoe H, Hughes SM. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. JCell Sci. 2005;118:4813–4821. doi: 10.1242/jcs.02602. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Brack AS, Rando TA. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem cell reviews. 2007;3:226–237. doi: 10.1007/s12015-007-9000-2. [DOI] [PubMed] [Google Scholar]
- Braun T, Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nature reviews Molecular cell biology. 2011;12:349–361. doi: 10.1038/nrm3118. [DOI] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Charge SB, Brack AS, Bayol SA, Hughes SM. MyoD- and nerve-dependent maintenance of MyoD expression in mature muscle fibres acts through the DRR/PRR element. BMCDevBiol. 2008;8:5. doi: 10.1186/1471-213X-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Charville GW, Rando TA. Stem cell ageing and non-random chromosome segregation. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2011;366:85–93. doi: 10.1098/rstb.2010.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TH, Quach NL, Liu L, Charville GW, Park L, Edalati A, Yoo B, Hoang P, Rando TA. Maintenance of muscle stem cell quiescence by microRNA-489. Nature. 2012;482:524–528. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov C, Chretien F, bou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. MolBiolCell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JC. Satellite cells and myogenesis; a study in the fruit-bat web. Journal of anatomy. 1969;105:419–438. [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. DevCell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Conboy MJ, Karasov AO, Rando TA. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoSBiol. 2007;5:e102. doi: 10.1371/journal.pbio.0050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day K, Paterson B, Yablonka-Reuveni Z. A distinct profile of myogenic regulatory factor detection within Pax7+ cells at S phase supports a unique role of Myf5 during posthatch chicken myogenesis 1. DevDyn. 2009;238:1001–1009. doi: 10.1002/dvdy.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nature communications. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Foudi A, Hochedlinger K, Van BD, Schindler JW, Jaenisch R, Carey V, Hock H. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. NatBiotechnol. 2009;27:84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensch N, Borchardt T, Schneider A, Riethmacher D, Braun T. Different autonomous myogenic cell populations revealed by ablation of Myf5-expressing cells during mouse embryogenesis. Development. 2008;135:1597–1604. doi: 10.1242/dev.019331. [DOI] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar M, Karan G, Tvrdik P, Capecchi MR. Two cell lineages, myf5 and myf5-independent, participate in mouse skeletal myogenesis. Developmental cell. 2008;14:437–445. doi: 10.1016/j.devcel.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O, Piestun Y, Allouh MZ, Rosser BW, Rinkevich Y, Reshef R, Rozenboim I, Wleklinski-Lee M, Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. DevDyn. 2004;231:489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Schiaffino S. Control of muscle fibre size: a crucial factor in ageing. Acta physiologica Scandinavica. 1999;167:307–312. doi: 10.1046/j.1365-201x.1999.00622.x. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nature cell biology. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, Tyner KJ, Nibarger L, Stanley HM, Cornelison DD, Fedorov YV, Olwin BB. The p38alpha/beta MAPK functions as a molecular switch to activate the quiescent satellite cell. JCell Biol. 2005;169:105–116. doi: 10.1083/jcb.200408066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jory A, Le Roux I, Gayraud-Morel B, Rocheteau P, Cohen-Tannoudji M, Cumano A, Tajbakhsh S. Numb promotes an increase in skeletal muscle progenitor cells in the embryonic somite. Stem Cells. 2009;27:2769–2780. doi: 10.1002/stem.220. [DOI] [PubMed] [Google Scholar]
- Juan AH, Derfoul A, Feng X, Ryall JG, Dell’Orso S, Pasut A, Zare H, Simone JM, Rudnicki MA, Sartorelli V. Polycomb EZH2 controls self-renewal and safeguards the transcriptional identity of skeletal muscle stem cells. Genes & development. 2011;25:789–794. doi: 10.1101/gad.2027911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanisicak O, Mendez JJ, Yamamoto S, Yamamoto M, Goldhamer DJ. Progenitors of skeletal muscle satellite cells express the muscle determination gene. MyoD DevBiol. 2009 doi: 10.1016/j.ydbio.2009.05.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T, Hanaoka K. Notch3 null mutation in mice causes muscle hyperplasia by repetitive muscle regeneration. Stem Cells. 2010;28:2205–2216. doi: 10.1002/stem.547. [DOI] [PubMed] [Google Scholar]
- Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le GF, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand F, Jones AE, Seale V, Scime A, Rudnicki MA. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4:535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements 2. Nature. 2009;460:627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells 1. Genesis. 2010;48:424–436. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton BH, Schultz E. Developmental fate of skeletal muscle satellite cells. Science. 1979;205:1292–1294. doi: 10.1126/science.472747. [DOI] [PubMed] [Google Scholar]
- Lu R, Neff NF, Quake SR, Weissman IL. Tracking single hematopoietic stem cellsin vivo using high-throughput sequencing in conjunction with viral genetic barcoding. Nature biotechnology. 2011;29:928–933. doi: 10.1038/nbt.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. The Journal of biophysical and biochemical cytology. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. The Journal of cell biology. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane C, Hennebry A, Thomas M, Plummer E, Ling N, Sharma M, Kambadur R. Myostatin signals through Pax7 to regulate satellite cell self-renewal. Experimental cell research. 2008;314:317–329. doi: 10.1016/j.yexcr.2007.09.012. [DOI] [PubMed] [Google Scholar]
- McKinnell IW, Ishibashi J, Le Grand F, Punch VG, Addicks GC, Greenblatt JF, Dilworth FJ, Rudnicki MA. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nature cell biology. 2008;10:77–84. doi: 10.1038/ncb1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Pannerec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nature cell biology. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Nature of dividing nuclei in skeletal muscle of growing rats. The Journal of cell biology. 1970;44:459–462. doi: 10.1083/jcb.44.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. The Anatomical record. 1971;170:421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S. A Critical Requirement for Notch Signaling in Maintenance of the Quiescent Skeletal Muscle Stem Cell State. Stem Cells. 2011 doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. DevCell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. DevBiol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. NatRevGenet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- Palacios D, Mozzetta C, Consalvi S, Caretti G, Saccone V, Proserpio V, Marquez VE, Valente S, Mai A, Forcales SV, et al. TNF/p38alpha/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell. 2010;7:455–469. doi: 10.1016/j.stem.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge TA, Grounds M, Sloper JC. Evidence of fusion between host and donor myoblasts in skeletal muscle grafts. Nature. 1978;273:306–308. doi: 10.1038/273306a0. [DOI] [PubMed] [Google Scholar]
- Peault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, Gussoni E, Kunkel LM, Huard J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. MolTher. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- Petersen PH, Zou K, Hwang JK, Jan YN, Zhong W. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419:929–934. doi: 10.1038/nature01124. [DOI] [PubMed] [Google Scholar]
- Petersen PH, Zou K, Krauss S, Zhong W. Continuing role for mouse Numb and Numbl in maintaining progenitor cells during cortical neurogenesis. Nature neuroscience. 2004;7:803–811. doi: 10.1038/nn1289. [DOI] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev. 2004;18:1088–1105. doi: 10.1101/gad.301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Reznik M. Thymidine-3H uptake by satellite cells of regenerating skeletal muscle. The Journal of cell biology. 1969;40:568–571. doi: 10.1083/jcb.40.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012;148:112–125. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Braun T, Hinuma S, Jaenisch R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992;71:383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice 5. Cell. 2010;143:1059–1071. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- Scharner J, Zammit PS. The muscle satellite cell at 50: the formative years. Skeletal muscle. 2011;1:28. doi: 10.1186/2044-5040-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienda J, Engleka KA, Jun S, Hansen MS, Epstein JA, Tabin CJ, Kunkel LM, Kardon G. Somitic origin of limb muscle satellite and side population cells. ProcNatlAcadSciUSA. 2006;103:945–950. doi: 10.1073/pnas.0510164103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Schultz E. Satellite cell behavior during skeletal muscle growth and regeneration. MedSciSports Exerc. 1989;21:S181–S186. [PubMed] [Google Scholar]
- Schultz E. Satellite cell proliferative compartments in growing skeletal muscles. DevBiol. 1996;175:84–94. doi: 10.1006/dbio.1996.0097. [DOI] [PubMed] [Google Scholar]
- Schultz E, Gibson MC, Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. JExpZool. 1978;206:451–456. doi: 10.1002/jez.1402060314. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Shafiq SA, Gorycki MA. Regeneration in skeletal muscle of mouse: some electron-microscope observations. The Journal of pathology and bacteriology. 1965;90:123–127. doi: 10.1002/path.1700900113. [DOI] [PubMed] [Google Scholar]
- Shea KL, Xiang W, Laporta VS, Licht JD, Keller C, Basson MA, Brack AS. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell. 2010;6:117–129. doi: 10.1016/j.stem.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan SM, Allen RE. Skeletal muscle satellite cell proliferation in response to members of the fibroblast growth factor family and hepatocyte growth factor. JCell Physiol. 1999;181:499–506. doi: 10.1002/(SICI)1097-4652(199912)181:3<499::AID-JCP14>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, Wagers AJ. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Shinin V, Gayraud-Morel B, Gomes D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nature cell biology. 2006;8:677–687. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- Shinin V, Gayraud-Morel B, Tajbakhsh S. Template DNA-strand co-segregation and asymmetric cell division in skeletal muscle stem cells. Methods MolBiol. 2009;482:295–317. doi: 10.1007/978-1-59745-060-7_19. [DOI] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den BM, Kroon-Veenboer C, Barker N, Klein AM, van RJ, Simons BD, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells 22. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Snow MH. Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. II. An autoradiographic study. AnatRec. 1977;188:201–217. doi: 10.1002/ar.1091880206. [DOI] [PubMed] [Google Scholar]
- Starkey JD, Yamamoto M, Yamamoto S, Goldhamer DJ. Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2011;59:33–46. doi: 10.1369/jhc.2010.956995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocheteau P, Le Roux I. Asymmetric cell divisions and asymmetric cell fates. Annual review of cell and developmental biology. 2009;25:671–699. doi: 10.1146/annurev.cellbio.24.110707.175415. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Rudnicki MA. Satellite cells, the engines of muscle repair. Nature reviews Molecular cell biology. 2011;13:127–133. doi: 10.1038/nrm3265. [DOI] [PubMed] [Google Scholar]
- Watt DJ, Lambert K, Morgan JE, Partridge TA, Sloper JC. Incorporation of donor muscle precursor cells into an area of muscle regeneration in the host mouse. Journal of the neurological sciences. 1982;57:319–331. doi: 10.1016/0022-510x(82)90038-7. [DOI] [PubMed] [Google Scholar]
- Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- White RB, Bierinx AS, Gnocchi VF, Zammit PS. Dynamics of muscle fibre growth during postnatal mouse development. BMC developmental biology. 2010;10:21. doi: 10.1186/1471-213X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. The skeletal muscle satellite cell: still young and fascinating at 50. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2011;59:1041–1059. doi: 10.1369/0022155411426780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N, Yoshida S, Koishi K, Masuda K, Nabeshima Y. Cell heterogeneity upon myogenic differentiation: down-regulation of MyoD and Myf-5 generates ‘reserve cells’. JCell Sci. 1998;111(Pt 6):769–779. doi: 10.1242/jcs.111.6.769. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? JCell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. JHistochemCytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- Zhong W, Feder JN, Jiang MM, Jan LY, Jan YN. Asymmetric localization of a mammalian numb homolog during mouse cortical neurogenesis. Neuron. 1996;17:43–53. doi: 10.1016/s0896-6273(00)80279-2. [DOI] [PubMed] [Google Scholar]