Abstract
Objectives
The aim of this study was to validate a novel, angle-independent, feature-tracking method for the echocardiographic quantitation of regional function.
Background
A new echocardiographic method, Velocity Vector Imaging (VVI) (syngo Velocity Vector Imaging technology, Siemens Medical Solutions, Ultrasound Division, Mountain View, California), has been introduced, based on feature tracking—incorporating speckle and endocardial border tracking, that allows the quantitation of endocardial strain, strain rate (SR), and velocity.
Methods
Seven dogs were studied during baseline, and various interventions causing alterations in regional function: dobutamine, 5-min coronary occlusion with reperfusion up to 1 h, followed by dobutamine and esmolol infusions. Echocardiographic images were acquired from short- and long-axis views of the left ventricle. Segment-length sonomicrometry crystals were used as the reference method.
Results
Changes in systolic strain in ischemic segments were tracked well with VVI during the different states of regional function. There was a good correlation between circumferential and longitudinal systolic strain by VVI and sonomicrometry (r = 0.88 and r = 0.83, respectively, p < 0.001). Strain measurements in the nonischemic basal segments also demonstrated a significant correlation between the 2 methods (r = 0.65, p < 0.001). Similarly, a significant relation was observed for circumferential and longitudinal SR between the 2 methods (r = 0.94, p < 0.001 and r = 0.90, p < 0.001, respectively). The endocardial velocity relation to changes in strain by sonomicrometry was weaker owing to significant cardiac translation.
Conclusions
Velocity Vector Imaging, a new feature-tracking method, can accurately assess regional myocardial function at the endocardial level and is a promising clinical tool for the simultaneous quantification of regional and global myocardial function.
Quantification of regional myocardial function is crucial in the diagnosis of ischemic heart disease. Myocardial strain and strain rate (SR) derived from tissue Doppler imaging are valuable tools in the assessment of left ventricular (LV) regional contraction (1). It has been shown that myocardial strain is more sensitive and accurate than tissue velocities in the assessment of regional myocardial function, owing to lesser effects of tethering and translational motion of the heart (2,3).
However, Doppler-derived strain imaging is limited by angle dependency. Recently, 2-dimensional (2D) strain and SR measurements from grayscale images have been introduced based on speckle tracking. Speckle tracking is a method in which ultrasound speckles within the image are tracked and strain is determined from the displacement of speckles in relation to each other, therefore, providing an angle-independent parameter of myocardial function. A new feature-tracking echocardiographic method, which incorporates speckle and endocardial contour tracking has been developed, which can measure myocardial strain, SR, and velocity of the regional endocardium, thus providing quantification of regional and global ventricular function. The aim of this study was to validate the regional function parameters obtained with this novel method against sonomicrometry in an animal model of ischemiareperfusion and pharmacologic interventions.
Methods
Animal preparation
The protocol was approved by the Baylor College of Medicine Animal Review Committee and was conducted in accordance with institutional guidelines. Seven mongrel dogs of either gender were pre-anesthetized with xylazine (0.75 to 1.5 mg/kg), atropine (0.02 to 0.06 mg/kg), and propofol (5 mg/kg), and endotracheally intu bated and ventilated using an external respirator. Anesthesia was maintained by 2% isoflurane inhalation for the remainder of the study. The heart was exposed through a median sternotomy and suspended in a pericardial cradle. A calibrated high-fidelity pressure catheter (5-F, SPC-350, Millar Instruments, Houston, Texas) was advanced into the LV through a purse string (3 mm in extent) in the apex for measurement of LV pressure and peak negative dP/dt (–dP/dt) continuously. A rectal heat probe was inserted, and the temperature was maintained around 37°C.
Two pairs of ultrasonic sonomicrometer crystals were inserted in the basal and apical regions of the LV antero-lateral wall. Crystal pairs of 2-mm diameter were inserted 1 to 1.5 cm apart to a depth of 4 to 8 mm and oriented in a diamond pattern to measure longitudinal and circumferential dimensions and shortening (4–7). Crystals in the apical regions yielded displacement data from the ischemic segments, whereas basal crystals represented nonischemic segments of the LV.
The midleft anterior descending artery after the first diagonal branch was dissected free from surrounding tissue and encircled with a snare for transient occlusion of the left anterior descending coronary artery (LAD).
Experimental protocol
To effect different changes in global and regional myocardial function, pharmacologic interventions with different inotropic effects as well as transient coronary occlusion with reperfusion to cause regional myocardial stunning were undertaken as follows. After acquiring baseline images, intravenous dobutamine was infused at a dose of 2.5 μg/kg–1/min–1 and titrated gradually to achieve close to 100% increase in dP/dt relative to baseline.
Transient regional myocardial ischemia was then induced by ligating the LAD for 5 min followed by reperfusion up to 1 h. After the reperfusion period, a second similar dose of dobutamine was infused to alter the inotropic state, as the stunned myocardium responds to inotropic stimulation. Lastly, after a wash-out period for dobutamine of 10 min, intravenous esmolol (100 μg/kg–1/min–1) was infused to achieve close to 50% reduction in dP/dt relative to baseline. Hemodynamic, sonomicrometry, and echocardiographic data were obtained at baseline; during maximal dobutamine (before ischemia); during coronary occlusion; at 15, 30, and 60 min after reperfusion period; during maximal dobutamine; post-reperfusion; and during esmolol. Thus, a total of 8 datasets were obtained for each animal.
Sonomicrometry measurements
Circumferential and longitudinal myocardial strain by sonomicrometry was measured in the basal and apical regions of the LV anterolateral wall. Each pair of ultrasonic crystals was attached to a sonomicrometer module in a 6-channel ultrasonic flow-dimension mainframe, designed and constructed in our laboratory (8). Thus, in each region we could evaluate myocardial strain in both orthogonal directions. The tracings obtained from the pair of crystals were analyzed by data acquisition software (AcqKnowledge 3.8.2, BioPac System, Inc., Goleta, California). Strain was calculated as: (L–L0)/L0, where L0 is the segment length at the onset of QRS and L is the segment length at the time of peak –dP/dt. Strain rate was computed by taking the time derivative of displacement data using the same software.
Echocardiographic image acquisition
An ACUSON Sequoia 512 (Siemens, Mountain View, California) ultra-sound system with a 5-MHz transducer was used to acquire images by placing the transducer directly on the epicardium, separated by a small layer of ultrasonic gel inside a rubber condom. Echocardiographic images were acquired from both short- and long-axis views of the LV at the times of the protocol noted in the preceding text. Short-axis views were acquired at the basal and apical levels where sonomicrometry crystals were located, corresponding to control and ischemic regions. Echocardiographic image sections were kept at the same plane of the crystals either by observing the brightness of the crystal on the image when possible, or by manually indenting the myocardium in the region of the crystals. For each image, 2 to 3 cardiac cycles were acquired at a frame rate of 60 to 70 Hz. Images were stored and transferred to a computer for off-line analysis. Owing to the interference between the sonomicrometers and the echocardiographic image, crystal data were acquired immediately before the echocardiographic image acquisition and switched off during image acquisition.
Velocity vector imaging (VVI)
The new off-line software (syngo Velocity Vector Imaging technology [VVI], Siemens) provides angle-independent 2D velocity, strain, and SR. “Feature tracking” of the myocardium with VVI is achieved through the combination of speckle tracking, mitral annulus motion, tissue-blood border detection, and the periodicity of the cardiac cycle using R-R intervals. In order to improve the tracking results, the algorithm applies a carefully designed sequence of intermediate passages to accurately follow myocardial motion. All these passages are performed with the aid of Fourier techniques that ensure a higher accuracy using the periodicity of the heart motion. From the multiple 1-dimensional interrogations, a 2D solution is achieved and the displacement information of the tracked points is obtained. The endocardial border is visually identified by the user and manually outlined. The manual placement of an endocardial tracing over 1 frame is then automatically tracked throughout the cardiac cycle. The software allows editing of the initial trace if the resulting tracking is assessed as suboptimal. The endocardial velocity is derived as the ratio between frame-to-frame displacement and the time interval. The velocity vectors in the 2D plane are displayed throughout the cardiac cycle, representing both the magnitude of the velocity and the direction of the motion (Fig. 1). Strain and SR are obtained by comparing displacement of the speckles in relation to each other along the endocardial contour throughout the cardiac cycle.
Figure 1. Short-Axis Views During Baseline, Dobutamine Infusion, and LAD Occlusion, and the Corresponding Velocity, Strain, and SR Curves Obtained by VVI.
Note the increased amplitude of the velocity vectors and velocity, strain, and strain rate (SR) values during dobutamine infusion and the dyskinetic motion, positive strain, and SR pattern during left anterior descending coronary artery (LAD) occlusion. End-diastole and end-systole (time of peak –dP/dt) are shown in blue lines. Values of the regional function parameters are shown. VVI = Velocity Vector Imaging.
Longitudinal and circumferential strain, SR, and velocity were computed from long-axis and short-axis views, respectively, in the ischemic and nonischemic zones. Since ultrasonic crystals were located in the myocardium and not at the endocardial order, and the reported myocardial strain by the crystals may thus be slightly different, additional analyses were performed by tracing the myocardium at the level of the crystals in a subset of dogs where crystal pairs could be identified visually on the 2D images. In order to eliminate the errors arising from postsystolic thickening of the ischemic segments, –dP/dt was used as a reference point for strain measurements for both VVI and sonomicrometry. For SR and velocity, maximal values during systole (between QRS onset and –dP/dt) by both methods were obtained for comparison. The average time needed for the analysis by VVI was 4 to 5 min for each acquisition.
Reproducibility of the VVI method
For intraobserver variability, the same observer reviewed the echocardiographic images and repeated VVI measurements several weeks later, during various hemodynamic states. To obtain interobserver variability, another observer who was blinded to VVI and sonomicrometry data repeated the VVI measurements.
Statistical analysis
Data are expressed as mean ± standard deviation. Regional function parameters during different hemodynamic states were compared with 1-way analysis of variance using Holm-Sidak method for post-hoc correction. Relations between strain and SR values obtained by VVI and by sonomicrometry were tested using linear regression analysis. Random effects generalized least squares regression (STATA version 9.0, STATA Corporation, College Station, Texas) was used to account for repeated measures in each dog. Intra- and interobserver variability were reported as the correlation coefficient between measurements as well as the mean difference between respective measurements. Statistical significance was set at a value of p < 0.05.
Results
Eight different hemodynamic states were tested in 7 animals (weight 30 ± 5 kg), except 1 that died during coronary occlusion due to ventricular fibrillation. From a total of 152 measurements from all sites and hemodynamic manipulations, 17 echocardiographic and 26 sonomicrometry acquisitions were excluded from the study. This was mainly due to suboptimal tracking by VVI due to papillary muscles and noisy signals from the sonomicrometry crystals.
Strain and SR in ischemic segments by VVI and sonomicrometry
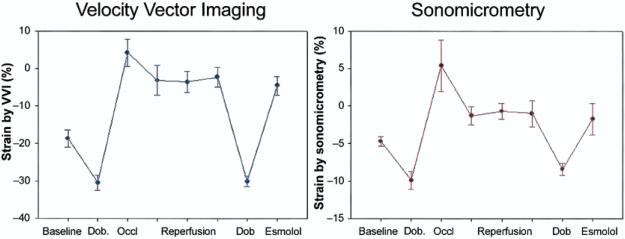
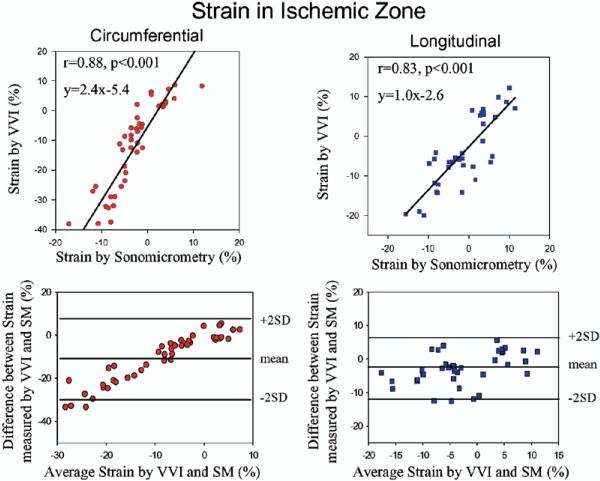
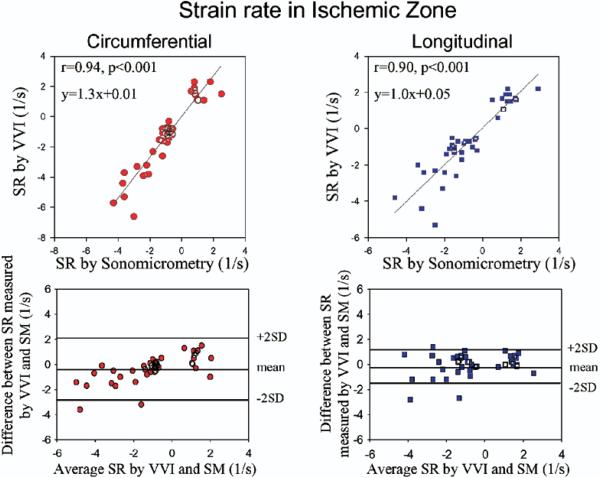
The hemodynamic changes observed during the protocol and corresponding changes in strain and SR by sonomicrometry and VVI are detailed in Tables 1 and 2. Before coronary occlusion, dobutamine infusion resulted in an increase in strain measured by both methods. After coronary occlusion, worsening of strain and SR were induced as expected, which were maintained during reperfusion. With subsequent dobutamine challenge, regional function improved and then worsened again after esmolol. Changes in regional function by sonomicrometry were tracked well with VVI with a parallel trend during the various hemodynamic manipulations (Fig. 2, Table 1). An example of changes in hemodynamics and strain by both methods from baseline to dobutamine and after coronary occlusion is shown on Figure 3. Circumferential and longitudinal strain by VVI for all hemodynamic states in the ischemic zone correlated well with sonomicrometry (r = 0.88, p < 0.001 and r = 0.83, p < 0.001, respectively) (Fig. 4). The results for these and subsequent correlations were essentially unchanged after accounting for repeated measures in each dog. A good correlation was also observed for systolic SR by VVI and sonomicrometry in the ischemic zone (Fig. 5).
Table 1.
SBP, HR, and Strain Values by VVI and Sonomicrometry From Ischemic Segment and Nonischemic Segment During Different Hemodynamic Conditions
| Strain by VVI (%) |
Strain by SM (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SBP (mm Hg) | HR (beats/min) | Isc Seg SAX | Isc Seg LAX | Non-Isc Seg SAX | Non-Isc Seg LAX | Isc Seg SAX | Isc Seg LAX | Non-Isc Seg SAX | Non-Isc Seg LAX | |
| Baseline | 91.5 ± 24.5 | 123.5 ± 7.4 | –18.4 ± 6.6 | –7.4 ± 3.4 | –6.8 ± 8.4 | –5.7 ± 2.3 | –4.8 ± 0.6 | –3.5 ± 1.8 | –8.4 ± 2.6 | –1.4 ± 4.6 |
| Dobutamine | 149.9 ± 56.9* | 148.7 ± 8.1† | –33.9 ± 6.7† | –16.2 ± 3.3† | –29.2 ± 7.2† | –12.4 ± 4.6‡ | –10.8 ± 3.3* | –7.6 ± 6.4‡ | –12.8 ± 3.2 | –4.1 ± 5.5‡ |
| Occlusion | 72.6 ± 17.8 | 117.0 ± 19.3 | 3.5 ± 5.4† | 4.7 ± 2.1† | –10.2 ± 6.4 | –2.8 ± 4.0 | 7.1 ± 6.4† | 6.5 ± 5.6† | –7.9 ± 1.6 | –1.7 ± 3.4 |
| Rep. 1 | 81.7 ± 15.2 | 120.8 ± 7.0 | –2.9 ± 8.4* | 0.5 ± 5.5* | –9.2 ± 12.7 | –6.2 ± 3.0 | –0.7 ± 3.1‡ | 0.3 ± 4.6 | –6.5 ± 3.1 | –1.4 ± 2.7 |
| Rep. 2 | 83.8 ± 16.7 | 123.3 ± 5.1 | –3.0 ± 7.0* | –0.3 ± 5.0 | –12.7 ± 3.1 | –3.3 ± 5.8 | –0.5 ± 2.9‡ | 0.7 ± 5.1 | –8.1 ± 6.1 | –1.8 ± 3.4 |
| Rep. 3 | 100.2 ± 17.3 | 122.6 ± 6.6 | –2.3 ± 6.0* | –2.7 ± 5.3 | –11.8 ± 4.7 | –9.8 ± 4.0 | –1.0 ± 3.7‡ | 0.6 ± 5.2 | –7.0 ± 4.6 | –3.1 ± 2.8 |
| Dobutamine | 144.3 ± 32.0* | 140.8 ± 14.7‡ | –34.8 ± 7.0* | –15.8 ± 3.3* | –25.2 ± 7.3* | –14.2 ± 5.8* | –8.5 ± 2.0* | –3.1 ± 6.7 | –16.9 ± 0.5 | –5.2 ± 4.9‡ |
| Esmolol | 63.4 ± 21.1 | 101.5 ± 5.3† | –4.2 ± 6.0* | –0.2 ± 5.8‡ | –9.4 ± 4.1 | –2.6 ± 6.5 | –1.2 ± 3.5‡ | 0.8 ± 4.5‡ | –7.3 ± 3.7 | –2.0 ± 3.8 |
p < 0.01
p < 0.001
p < 0.05 when compared with baseline.
HR = heart rate; Isc Seg = ischemic segment; LAX = long axis, longitudinal; Non-Isc Seg = nonischemic segment; Rep. = reperfusion; SAX = short axis, circumferential; SBP = systolic blood pressure; SM = sonomicrometry; VVI = Velocity Vector Imaging.
Table 2.
Systolic Strain Rate Values by VVI and Sonomicrometry From Ischemic Segment and Nonischemic Segment During Different Hemodynamic Conditions
| Strain Rate by VVI (s–1) |
Strain Rate by SM (s–1) |
|||||
|---|---|---|---|---|---|---|
| Isc Seg SAX | Isc Seg LAX | Non-Isc Seg LAX | Isc Seg SAX | Isc Seg LAX | Non-Isc Seg LAX | |
| Baseline | –2.0 ± 1.1 | –0.9 ± 0.3 | –0.4 ± 1.4 | –1.3 ± 0.7 | –1.0 ± 0.5 | –0.4 ± 0.8 |
| Dobutamine | –4.9 ± 1.2* | –3.2 ± 0.7† | –2.5 ± 0.9* | –3.2 ± 0.8* | –3.2 ± 1.0* | –2.2 ± 1.5‡ |
| Occlusion | 0.8 ± 1.3† | 1.5 ± 1.0† | –0.5 ± 1.1 | 0.8 ± 1.2* | 1.4 ± 1.0† | –0.7 ± 0.2 |
| Rep. 1 | 0.1 ± 1.7‡ | –0.07 ± 1.1 | –0.8 ± 0.3 | –0.1 ± 1.0‡ | –0.3 ± 1.1 | –0.6 ± 0.1 |
| Rep. 2 | –0.1 ± 1.0‡ | –0.1 ± 1.6 | –0.3 ± 0.8 | –0.4 ± 0.8 | –0.3 ± 1.4 | –0.3 ± 0.6 |
| Rep. 3 | 0.3 ± 1.7‡ | –0.4 ± 1.3 | –1.1 ± 0.5 | –0.1 ± 1.1 | –0.7 ± 1.3 | –0.6 ± 0.2 |
| Dobutamine | –4.0 ± 0.9* | –2.9 ± 1.2* | –2.5 ± 0.9* | –2.7 ± 1.0‡ | –2.3 ± 0.7‡ | –2.6 ± 1.5‡ |
| Esmolol | –0.4 ± 0.9‡ | 0.9 ± 1.2‡ | –0.5 ± 0.8 | –0.8 ± 0.2 | 0.5 ± 1.2‡ | –0.6 ± 0.1 |
Figure 2. Changes in Strain During Occlusion-Reperfusion and Pharmacologic Manipulation.
Circumferential strain measured by Velocity Vector Imaging (VVI) and sonomicrometry in the ischemic zone during various hemodynamic states. Dob. = dobutamine; Occl = occlusion.
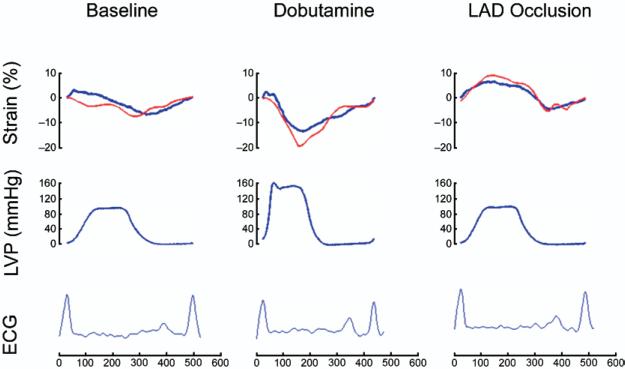
Figure 3. Example of Changes in Hemodymanic and Regional Function During the Protocol.
Recordings of strain (top), left ventricular pressure (LVP) (middle), and electrocardiogram (ECG) (bottom) from a single experiment during baseline, dobutamine infusion, and left anterior descending coronary artery (LAD) occlusion. Blue line = sonomicrometry; red line = Velocity Vector Imaging.
Figure 4. Relation of Strain by VVI to SM in the Ischemic Zone.
Plots showing the relation between circumferential and longitudinal strain values by Velocity Vector Imaging (VVI) and sonomicrometry (SM) in the ischemic zone and corresponding Bland-Altman plots.
Figure 5. Relation of SR by VVI to SM in the Ischemic Zone.
Plots showing the relation between circumferential and longitudinal strain rate (SR) by Velocity Vector Imaging (VVI) and sonomicrometry (SM) in the ischemic segments and corresponding Bland-Altman plots.
Strain and SR in nonischemic zone by VVI and sonomicrometry
Endocardial strain and SR in nonischemic basal segments demonstrated an increase by dobutamine infusion and remained unchanged compared with baseline during LAD occlusion (Table 2). Velocity Vector Imaging yielded strain and SR values that tracked well the corresponding data obtained by sonomicrometry during various hemodynamic states (Fig. 6, Table 1). Regression analysis for the measurement of strain at basal levels of long-axis views indicated a significant correlation between the 2 methods (r = 0.65, p < 0.001).
Figure 6. Changes in Strain During Occlusion-Reperfusion and Pharmacologic Manipulation in the Nonischemic Zone.
Circumferential strain measured by VVI and sonomicrometry in the nonischemic zone during various hemodynamic states. Abbreviations as in Figure 2.
Relation between strain values obtained by VVI at myocardial level and sonomicrometry
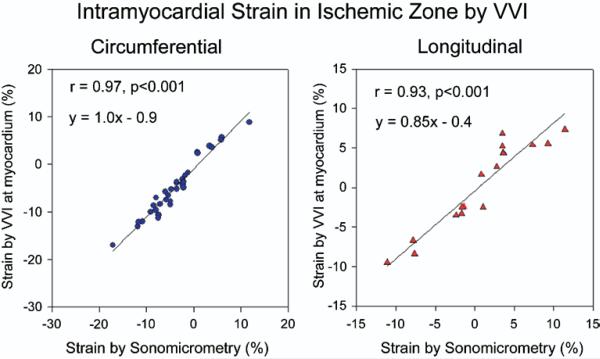
Strain and SR in the myocardium by VVI were recorded at the exact site (intramyocardial) where the crystals were located in a subset of animals where the crystals could be clearly visualized. Strain at midmyocardium by VVI and sonomicrometry were –6.5 ± 2.1% and –4.8 ± 1.7% (p = NS) from short axis at the apical level, and –3.5 ± 1.8% and –3.4 ± 1.8% (p = NS) from long axis at basal level, respectively, during baseline. Changes in strain and SR correlated strongly with sonomicrometry data. In general, VVI at the intramyocardial level yielded lower absolute values of strain than at the endocardial level, with a regression equation closer to strain values obtained by sonomicrometry (Fig. 7).
Figure 7. Circumferential and Longitudinal Strain by VVI at the Exact Intramyocardial Level of the Ultrasound Crystals.
Relation of strain by Velocity Vector Imaging (VVI) to strain by sonomicrometry in ischemic segments at the level of intramyocardial crystals.
Endocardial velocity data by VVI
Endocardial velocity of segments to be rendered ischemic and nonischemic segments imaged from short-axis apical and basal levels increased significantly with dobutamine when compared with that seen at baseline (from 2.6 ± 0.7 cm/s to 7.0 ± 1.8 cm/s, p < 0.001 for segments to be rendered ischemic and from 3.6 ± 2.0 cm/s to 8.3 ± 3.3 cm/s, p = 0.007 for nonischemic segment). During LAD occlusion, velocity decreased significantly in ischemic segment (2.6 ± 0.7 cm/s vs. –2.6 ± 2.4 cm/s, p < 0.001), whereas it remained unchanged in nonischemic segment (3.6 ± 2.0 cm/s vs. 2.08 ± 2.1 cm/s, p > 0.05). There was a significant inverse relation between strain and velocity both at apical and basal levels from short-axis views (r =–0.73, p < 0.001 and r = –0.79, p < 0.001, respectively) (Fig. 8). No significant relation was demonstrated between the parameters mentioned in the preceding text from long-axis views, where long-axis translation was noted to be excessive.
Figure 8. Endocardial Velocity Versus Strain Measurements.
Relation of circumferential endocardial velocity by Velocity Vector Imaging to strain measurement by sonomicrometry in the ischemic and nonischemic zones.
Reproducibility of the VVI method
There was a significant correlation for both longitudinal and circumferential strain between the strain measurements of the same observer (r = 0.94, p = 0.001, mean difference –2.2 ± 1.7%, for ischemic region; r = 0.96, p < 0.001, mean difference –1.7 ± 0.7 %, for nonischemic region). There was a good correlation between the strain values obtained by 2 independent observers (r = 0.98, p < 0.001, mean difference –3.1 ± 1.5%, for ischemic region; and r = 0.92, p = 0.003, mean difference –2.9 ± 2.1%, for nonischemic region).
Discussion
The present study demonstrates that a new feature-tracking echocardiographic method, VVI, can accurately assess regional myocardial systolic function over a wide range of physiologic conditions compared with that assessed by the segment-length sonomicrometry reference. The method provides a measure of innermost endocardial function by tracking endocardial motion through a new algorithm, combining tracking of ultrasound speckle, myocardial blood interface, and myocardial shape.
Assessment of strain and SR by VVI
Myocardial strain, SR, and velocity have significantly improved the quantitation of regional function with echocardiography. Doppler-derived myocardial strain and SR imaging have been shown to be superior to tissue velocity in the evaluation of regional function because of the lesser effects of tethering of adjacent myocardial segments, cardiac translation, and rotation (2,3). Recently, new speckle tracking echocardiographic methods have been developed and validated that forgo the limitations of Doppler angulation (9–11) . These methods evaluate strain and SR over a certain thickness of the myocardium and not only at the endocardial border. Velocity Vector Imaging is a novel feature-tracking method that provides a measure of innermost endocardial function by tracking endocardial motion through a new algorithm that combines ultrasound speckle and myocardial structure. The present study is the first study to validate VVI in the quantitation of regional function. A strong correlation was observed between VVI and sonomicrometry for both longitudinal and circumferential strain and SR. Changes in regional myocar-dial function determined by sonomicrometry during ischemia-reperfusion and pharmacologic manipulations were tracked well by VVI under various hemodynamic conditions. Endocardial velocity also related well with regional function changes in the short-axis views. No relation was observed from the apical window for longitudinal velocity and is most likely due to the open-chest, open-pericardium animal model that demonstrated exaggerated translation and apical indentation when imaging from the apical window. The high correlation of strain and SR with sonomicrometry in this situation highlights again the robustness of strain and SR compared with velocity under more difficult situations of cardiac translation and excessive motion. In the clinic however, this should be less of a problem unless excessive cardiac motion is present.
Of interest is that while the directional changes of strain and SR with VVI were similar to the invasive reference and the relations were strong, the absolute values determined by the 2 methods were not similar and were lower for sonomicrometry, particularly from the short axis. This is most likely due to the fact that strain was measured at different depths of the myocardium: for VVI analysis, the values reflect endocardial parameters as the endocardial border is tracked whereas for sonomicrometry, the crystal pairs are implanted into the myocardium. This is supported by the additional VVI acquisition and processing at the exact level of crystals in the myocardium in the subset of animals where the crystals could be visualized, where VVI yielded lower absolute values of strain, similar to sonomicrometry. Hashimoto et al. (12) had recently reported a significant difference in strain values between the 3 layers of the LV (endocardial, midmyocardial, and epicardial) in baseline conditions and in response to hemodynamic alterations. Similarly, a study with magnetic resonance imaging also showed that myocardial deformation is largest in the endocardium and verified the endo-epicardial gradient of strain in normal ventricles (13).
Clinical applications
Some clinical applications of VVI have already been reported. We have demonstrated that right ventricular systolic function can be estimated by VVI in patients with pulmonary hypertension (14). Right ventricular peak systolic strain determined by VVI was the best predictor of pulmonary artery pressure. Cannesson et al. (15) have used VVI to quantify LV mechanical dyssynchrony and predict response to resynchronization therapy. Importantly, the availability of automated endocardial contours that track the endocardium throughout the cardiac cycle can simultaneously be used for the quantitation of global function in addition to regional function, by the derivation of cardiac chamber volumes and ejection fraction that can be applied to the LV and conceivably to other cardiac chambers (14). We have previously demonstrated that endocardial borders tracked with VVI relate very closely to those traced manually (14).
Study limitations
Two-dimensional speckle tracking algorithms are inherently dependent on image quality and endocardial border definition. We had to exclude some data due to suboptimal tracking by VVI. This was mainly due to poor endocardial definition throughout the cardiac cycle because of prominent papillary muscles. There were variations between 2 methods with respect to absolute strain values. Endocardial versus myocardial motion tracking with 2 methods, possible misalignment between the ultrasound plane and the crystals are the most probable sources of the variations. In the present study, only the longitudinal and circumferential strain was assessed. Radial strain needs to be further validated. It is crucial to determine post-systolic thickening in acute ischemia. In this study, we have derived peak –dP/dt from LV pressure tracings to determine end systole. Strain values both by VVI and sonomicrometry were measured at the time where peak –dP/dt occurred, decreasing errors that might result from timing due to post-systolic thickening.
Conclusions
The present study demonstrates that a novel feature-tracking echocardiographic method, VVI, can quantify regional myocardial function at the endocardial level. Velocity Vector Imaging provides accurate and angle-independent measurements of strain and SR and has the potential to be a clinical tool for the simultaneous quantitation of regional as well as global function.
Acknowledgments
This research was sponsored by an investigator-initiated grant from Siemens Medical Solutions, Ultrasound Division, Mountain View, California.
Abbreviations and Acronyms
- LAD
left anterior descending coronary artery
- LV
left ventricle/ventricular
- SR
strain rate
- VVI
Velocity Vector Imaging
- 2D
2-dimensional
Footnotes
This study has been presented as an abstract at the annual scientific sessions of the American Heart Association, Chicago, Illinois, November 15, 2006.
REFERENCES
- 1.Sutherland GR, Di Salvo SG, Claus P, D'hooge J, Bijnens B. Strain and strain rate imaging: a new clinical approach to quantifying regional myocardial function. J Am Soc Echocardiogr. 2004;17:788–802. doi: 10.1016/j.echo.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg NL, Firstenberg MS, Castro PL, et al. Doppler-derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation. 2002;105:99–105. doi: 10.1161/hc0102.101396. [DOI] [PubMed] [Google Scholar]
- 3.Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation. 2000;102:1158–64. doi: 10.1161/01.cir.102.10.1158. [DOI] [PubMed] [Google Scholar]
- 4.Hanley HG, Lewis RM, Hartley CJ, Franklin D, Schwartz A. Effects of an inotropic agent, RO 2-2985 (X-537A), on regional blood flow and myocardial function in chronically instrumented conscious dogs and anesthetized dogs. Circ Res. 1975;37:215–25. doi: 10.1161/01.res.37.2.215. [DOI] [PubMed] [Google Scholar]
- 5.Pinsky WW, Lewis RM, Hartley CJ, Entman ML. Permanent changes of ventricular contractility and compliance in chronic volume overload. Am J Physiol. 1979;237:H575–83. doi: 10.1152/ajpheart.1979.237.5.H575. [DOI] [PubMed] [Google Scholar]
- 6.Theroux P, Franklin D, Ross J, Jr., Kemper WS. Regional myocardial function during acute coronary artery occlusion and its modification by pharmacologic agents in the dog. Circ Res. 1974;35:896–908. doi: 10.1161/01.res.35.6.896. [DOI] [PubMed] [Google Scholar]
- 7.Wood JM, Hanley HG, Entman ML, et al. Biochemical and morphological correlates of acute experimental myocardial ischemia in the dog. IV. Energy mechanisms during very early ischemia. Circ Res. 1979;44:52–61. doi: 10.1161/01.res.44.1.52. [DOI] [PubMed] [Google Scholar]
- 8.Hartley CJ, Hanley HG, Lewis RM, Cole JS. Synchronized pulsed Doppler blood flow and ultrasonic dimension measurement in conscious dogs. Ultrasound Med Biol. 1978;4:99–110. doi: 10.1016/0301-5629(78)90035-2. [DOI] [PubMed] [Google Scholar]
- 9.Langeland S, D'hooge J, Wouters PF, et al. Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation. 2005;112:2157–62. doi: 10.1161/CIRCULATIONAHA.105.554006. [DOI] [PubMed] [Google Scholar]
- 10.Amundsen BH, Helle-Valle T, Edvardsen T, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006;47:789–93. doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Korinek J, Wang J, Sengupta PP, et al. Two-dimensional strain—a Doppler-independent ultrasound method for quantitation of regional deformation: validation in vitro and in vivo. J Am Soc Echocardiogr. 2005;18:1247–53. doi: 10.1016/j.echo.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto I, Li X, Hejmadi BA, Jones M, Zetts AD, Sahn DJ. Myocardial strain rate is a superior method for evaluation of left ventricular subendocardial function compared with tissue Doppler imaging. J Am Coll Cardiol. 2003;42:1574–83. doi: 10.1016/j.jacc.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Prinzen FW, Hunter WC, Wyman BT, McVeigh ER. Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol. 1999;33:1735–42. doi: 10.1016/s0735-1097(99)00068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pirat B, McCulloch ML, Zoghbi WA. Evaluation of global and regional right ventricular systolic function in patients with pulmonary hypertension using a novel speckle tracking method. Am J Cardiol. 2006;98:699–704. doi: 10.1016/j.amjcard.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 15.Cannesson M, Tanabe M, Suffoletto MS, Schwartzman D, Gorcsan J., III Velocity vector imaging to quantify ventricular dyssynchrony and predict response to cardiac resynchronization therapy. Am J Cardiol. 2006;98:949–53. doi: 10.1016/j.amjcard.2006.04.045. [DOI] [PubMed] [Google Scholar]