Abstract
The new species Aspergillus karnatakaensis sp. nov. is described and illustrated. All three isolates of this species were isolated from Indian soil; two from soil under a coconut palm in a coffee plantation in Karnataka, and one from soil in the Machrar river bed in Bansa district. This species is closely related to, but clearly distinct, from A. aeneus based on β-tubulin or calmodulin sequence data. Sequences of the ITS region of these two species are identical. Aspergillus karnatakaensis produced terrein, gregatins, asteltoxin, karnatakafurans A and B and the unknown metabolite, provisionally named NIDU. Aspergillus karnatakaensis belongs to a well-defined clade within Aspergillus subgenus Nidulantes together with eight other species including A. aeneus, A. crustosus, A. eburneocremeus, A. heyangensis, and the teleomorph producing-species Emericella bicolor, E. discophora, E. spectabilis, and E. foeniculicola. This clade is placed in a new section, Aspergillus sect. Aenei sect. nov. All teleomorph species assigned to this section are able to produce sterigmatocystin.
Keywords: Aspergillus subgen. Nidulantes, β-tubulin, calmodulin, Eurotiales, extrolites, ITS, polyphasic taxonomy
INTRODUCTION
Aspergillus subgenus Nidulantes is one of the largest subgenera of the genus Aspergillus, including about 80 species (Peterson 2008, Peterson et al. 2008). Several species of this subgenus have a teleomorph assigned to Emericella (Pitt et al. 2000, Samson 2000, Frisvad & Samson 2004). Species of subgenus Nidulantes are important as opportunistic human pathogens (Verweij et al. 2008, Varga et al. 2008), as producers of various secondary metabolites which are useful for the pharmaceutical industry (e.g. penicillin, echinocandins, ophiobolins), and mycotoxins which are harmful to animals and humans (e.g. aflatoxins, sterigmatocystin; Frisvad et al. 2004, 2005, Frisvad & Samson 2004, Zalar et al. 2008).
During surveys of Aspergillus isolates from soil samples from subtropical regions, two interesting isolates were recovered which did not match any known species of the genus. We used the polyphasic approach, including sequence analysis of parts of the β-tubulin and calmodulin genes and the ITS nrDNA region, macro- and micromorphological analyses, and examination of the extrolite profiles of the isolates to differentiate the new species Aspergillus karnatakaensis sp. nov. We also analysed strains of species which appeared to be closely related to the new species for the production of extrolites and found sterigmatocystin in all species with a teleomorphic state studied and also in Aspergillus ebureocremeus.
MATERIALS AND METHODS
Isolates
The strains used in this study are listed in Table 1.
Table 1. Isolates of Aspergillus and Emericella spp. examined in this study.
| Species | Strain No.a | Substratum, country, location |
GenBank No. |
||
|---|---|---|---|---|---|
| β-tubulin | calmodulin | ITS | |||
| A. aeneus | CBS 128.54T = NRRL 4769 | Forest soil, Modilen, Somalia | EF652298 | EF652386 | EF652474 |
| A. crustosus | CBS 478.65T = NRRL 4988 | Skin scrapings, man, Kankakee, Illinois, USA | EF652313 | EF652401 | EF652489 |
| A. eburneocremeus | CBS 130.54T = NRRL 4773 | Forest soil, Modien Forest, Somalia | EF652300 | EF652388 | EF652476 |
| A. heyangensis | CBS 101751T | Placentae of Gossypium sp., Heyang, Shaanxi Province, China | FJ491520 | FJ491521 | FJ491522 |
| A. karnatakaensis | CBS 102800T = IBT 22153 | Soil under coconut palm in coffee plantation, India, Karnataka | EU482438 | EU482431 | EU482441 |
| A. karnatakaensis | CBS 102799 = IBT 22154 | Soil under coconut palm in coffee plantation, India, Karnataka | EU482436 | EU482430 | EU482443 |
| A. karnatakaensis | NRRL 4649 | Soil in the Machrar river bed located in district Bansa, state Madhya Pradesh, India | EF652292 | EF652380 | EF652468 |
| E. bicolor | CBS 425.77T | Soil from Artemisia grassland, USA, Wyoming, Teton Basin | EF652335 | EF652423 | EF652511 |
| E. discophora | CBS 469.88T = IBT 21910 | Soil, Spain | AY339999 | EU443970 | EU448272 |
| E. discophora | CBS 470.88 = IBT 21911 | Forest soil, Spain | AY340000 | EU443969 | EU448266 |
| E. foeniculicola | CBS 156.80T | Foeniculum vulgare seed, China | EU443990 | EU443968 | EU448274 |
| E. heterothallica | CBS 489.65T | Soil, Costa Rica | EU076369 | EU076361 | AB248987 |
| E. spectabilis | CBS 429.77T | Coal mine spoil material, Wyoming, USA, Seminole no. 1 mine | EU482437 | EU482429 | EU482442 |
aCultures are deposited in/were obtained from the following collections: CBS, CBS-KNAW Fungal Biodiversity Centre, Utrecht, the Netherlands; IBT, Culture Collection of Fungi, Mycology Group, BioCentrum-DTU, Technical University of Denmark, Lyngby, Denmark; NRRL, Agricultural Research Service Culture Collection, Peoria, IL, USA.
Morphological analysis
For macromorphological observations, Czapek yeast autolysate (CYA), malt extract autolysate (MEA) agar, Yeast Extract Sucrose agar (YES), creatine sucrose agar (CREA), and oatmeal agar (OA) were used (Samson et al. 2010). The isolates were inoculated at three points on each plate of each medium and incubated at 25 °C and 37 °C in the dark for 7 d. For micromorphological observations, microscopic mounts were made in lactic acid from MEA and OA colonies and a drop of alcohol was added to remove air bubbles and excess conidia.
Extrolite analysis
The isolates were grown on CYA and YES at 25 °C for 7 d. Extrolites were extracted after incubation. Five 6 mm plugs of each agar medium were taken and pooled together into the same vial for extraction with 0.75 mL of a mixture of ethyl acetate/dichloromethane/methanol (3:2:1) (v/v/v) with 1 % (v/v) formic acid. The extracts were filtered and analyzed by HPLC using alkylphenone retention indices and diode array UV-VIS detection as described by Frisvad & Thrane (1987, 1993), with minor modifications as described by Smedsgaard (1997). The column used was a 50 × 2 mm Luna C-18 (II) reversed phase column (Phenomenex, CA, USA) fitted with a 2 × 2 mm guard column.
Genotypic analysis
The cultures used for the molecular studies were grown on malt peptone (MP) broth using 1 % (w/v) of malt extract (Brix 10) and 0.1 % (w/v) bacto peptone (Difco), 2 mL of medium in 15 mL tubes. The cultures were incubated at 25 °C for 7 d. DNA was extracted from the cells using the Masterpure™ yeast DNA purification kit (Epicentre Biotechnology.) according to the instructions of the manufacturer. The ITS region and parts of the β-tubulin and calmodulin genes were amplified and sequenced as described previously (Varga et al. 2007a–c).
Data analysis
The sequence data was optimised using the software package Seqman from DNAStar Inc. Sequence alignments were performed by MEGA v. 4.0 (Tamura et al. 2007) and improved manually. For parsimony analysis, PAUP v. 4.0b10 software was used (Swofford 2003). Alignment gaps were treated as a fifth character state and all characters were unordered and of equal weight. Maximum parsimony analysis was performed for all data sets individually using the heuristic search option with 100 random taxa additions and tree bisection and reconstruction (TBR) as the branch-swapping algorithm. Branches of zero length were collapsed and all multiple, equally parsimonious trees were saved. The robustness of the trees obtained was evaluated by 1000 bootstrap replications (Hillis & Bull 1993). Eurotium heterothallica was used as outgroup in these analyses (Houbraken et al. 2007). The alignments were deposited in TreeBASE (<treebase.org/treebase-web/home.html>) under accession number S11027.
RESULTS AND DISCUSSION
Phylogeny
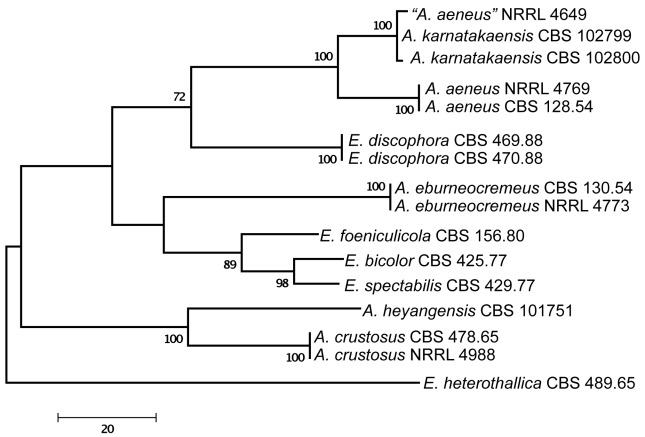
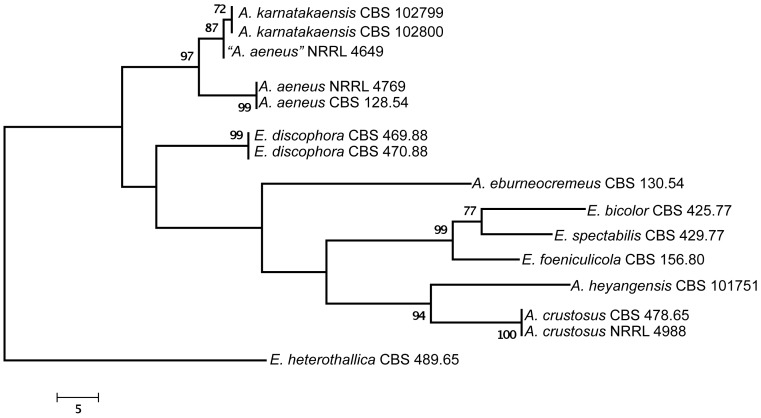
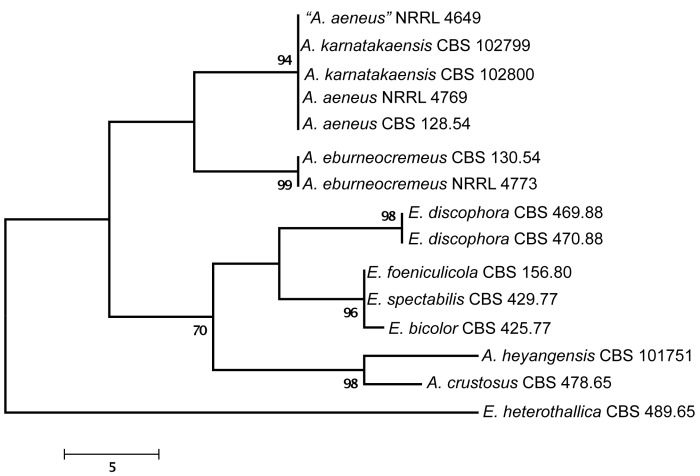
Of the aligned β-tubulin sequences, a portion with 438 positions, including 107 parsimony informative characters, was selected for the analysis; MP analysis of the sequence data resulted in two similar, equally most parsimonious trees (tree length = 289 steps, consistency index = 0.7855, retention index = 0.7919), one of which is shown in Fig. 1. The calmodulin data set consisted of 492 characters, including 188 parsimony informative sites; MP analysis resulted in a single most parsimonious tree (length = 485, consistency index = 0.7402, retention index = 0.8040), which is presented in Fig. 2. The ITS data set consisted of 451 characters, including 43 parsimony informative sites; MP analysis resulted in four equally most parsimonious trees (length = 105, consistency index = 0.8190, retention index = 0.8541), one of which is presented in Fig. 3.
Fig. 1.
One of the two equally MP trees obtained based on phylogenetic analysis of β-tubulin sequence data of Aspergillus sect. Aenei. Numbers above branches are bootstrap support values. Only values above 70 % are indicated.
Fig. 2.
The single MP tree obtained based on phylogenetic analysis of calmodulin sequence data of Aspergillus sect. Aenei. Numbers above branches are bootstrap support values. Only values above 70 % are indicated.
Fig. 3.
One of four equally MP trees obtained based on phylogenetic analysis of ITS sequence data of Aspergillus sect. Aenei. Numbers above branches are bootstrap support values. Only values above 70 % are indicated.
The two isolates from Karnataka, India were found to be closely related to Aspergillus aeneus based on phylogenetic analysis of protein coding sequences (Fig. 1, 2), and had identical ITS sequences to A. aeneus (Fig. 3). One additional isolate also from India, “A. aeneus” NRRL 4649 (= IMI 086833) was found to be conspecific with these two isolates. This isolate was obtained from soil of the Machrar river bed in the district of Bansa, Madhya Pradesh (Rai et al. 1964), and is morphologically similar to the other two Indian isolates. The three isolates are described here as a new taxon, A. karnatakaensis sp. nov. A typical characteristic is the formation of a crust of Hülle cells. The strains were incubated on various media for ascoma production, but in none of the strains were ascomata or ascospores found. Also, a mating experiment with the three strains did not induce ascoma production.
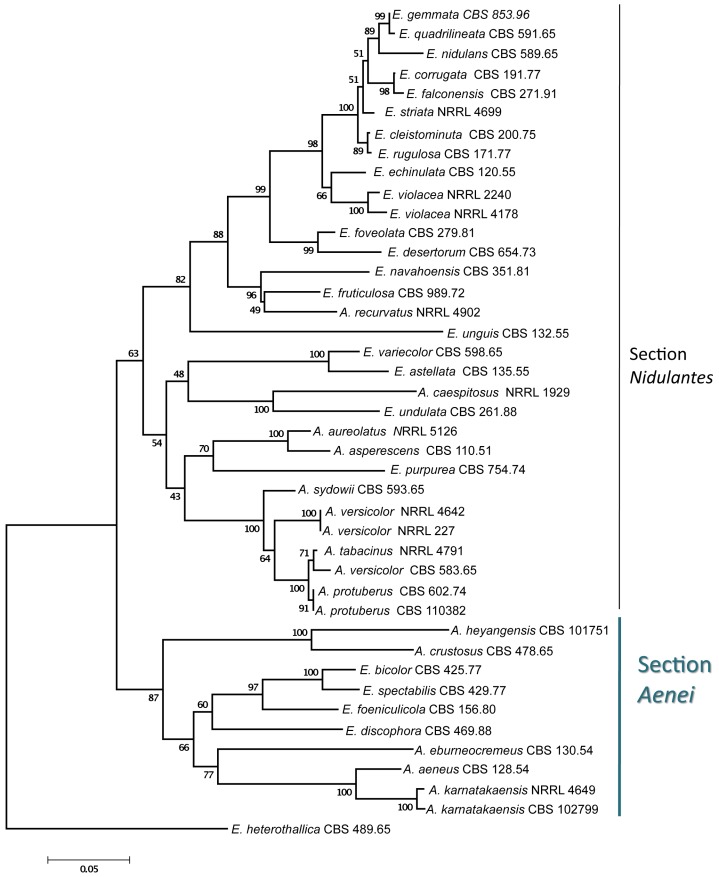
Aspergillus karnatakaensis formed a well-supported clade together with four Emericella species, E. foeniculicola, E. bicolor, E. spectabilis and E. discophora, and four species known to reproduce only asexually, including A. aeneus, A. eburneocremeus, A. crustosus and A. heyangensis on the trees based on calmodulin (Fig. 4), β-tubulin, and ITS sequence data (data not shown). Based on these observations, we describe Aspergillus sect. Aenei sect. nov. to accommodate these species within subgenus Nidulantes. This group of species was originally assigned to section Nidulantes (Raper & Fennell 1965, Christensen et al. 1978, Samson 1979, Udagawa & Muroi 1979, Sun & Qi 1994).
Fig. 4.
Phylogenetic affinities of Aspergillus section Aenei to section Nidulantes based on neighbor-joining analysis of calmodulin sequence data of selected species assigned to these sections. Numbers above branches are bootstrap values. Only values above 70 % are indicated.
Extrolites
Aspergillus karnatakaensis isolates were found to produce karnatakafurans A and B (Manniche et al. 2004), terrein, gregatins, asteltoxin (until now only detected in CBS 102799) and the partially characterised metabolite NIDU. Both gregatins and NIDU are also produced by A. granulosus, while karnatakafurans are produced in common with A. aeneus and A. multicolor. However, phylogenetic analysis of sequence data of A. multicolor (Peterson 2008) and A. granulosus (Houbraken et al. 2007) indicated that they are not closely related to A. karnatakaensis, while A. aeneus is.
Among the other species found to belong to the same clade as A. karnatakaensis, Emericella bicolor produces sterigmatocystin, versicolorins, some anthraquinones, and a polar extrolite with end-absorption; E. foeniculicola produces sterigmatocystin (and many other sterigmatocystin and versicolorin-related compounds), xanthocillin derivatives, and the partially characterized (but common) metabolite DRI; E. spectabilis produces two members of the shamixanthone biosynthetic family (both more polar than shamixanthone itself) and a member of the sterigmatocystin biosynthetic family; A. heyangensis produces a decaturin in common with A. aeneus and A. karnatakaensis and NIDU, while E. discophora produces sterigmatocystin and versicolorins (Zalar et al. 2008). Decaturins are antiinsectan metabolites which have previously been identified in Penicillium species including P. thiersii and P. decaturense (Zhang et al. 2003, Li et al. 2005). Aspergillus eburneocremeus has both sterigmatocystin and mer NF-8054X in common with E. heterothallica. Aspergillus crustosus is different from all these species in producing only PR-toxin and related mycotoxins, and has no extrolites in common with the other species in sect. Aenei. All Emericella species in sect. Aenei produce sterigmatocystin, while the Aspergillus species without a known teleomorph apparently cannot produce it, with the exception of A. eburneocremeus. However, sterigmatocystin is common throughout the different sections of subgenus Nidulantes, and has even been found in sections Ochraceorosei and Flavi (Frisvad et al. 2005). Other extrolites such as shamixanthones, mer NF-8054X and the related emesterones, and terrein have also been found in other species in section Nidulantes. Aspergillus heyangensis is only known from ex-type cultures and re-examination of the cultures showed that the taxon has great similarities with the species mentioned above, including its inability to grow at 37 °C, and the shape of the conidial heads and vesicles, although this species does not produce Hülle cells (Fig. 5). That species also produces the unknown metabolite NIDU, as do A. karnatakaensis and E. discophora (Table 2).
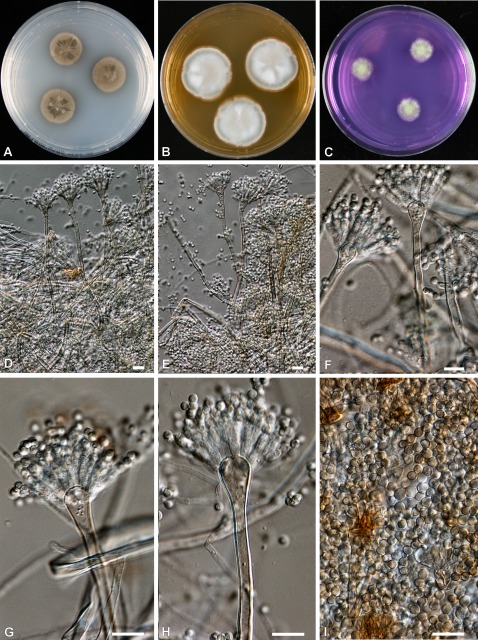
Fig. 5.
Aspergillus heyangensis (CBS 101751). A–C. Colonies incubated at 25 °C for 7 d; A on CYA, B on MEA, C on CREA. D–I. Conidiophores and conidia. Bars = 10 μm.
Table 2. Extrolites produced by members of Aspergillus section Aenei.
| Species | Culture collection number | Extrolites |
|---|---|---|
| A. aeneus | IMI 069855ii = CBS 128.54 | asteltoxin, fumitremorgin B, karnatakafurans, a decaturin, GUUM* |
| A. crustosus | IMI 135819 = CBS 478.65 | PR-toxin |
| A. eburneocremeus | IMI 069856 = CBS 130.54 | mer-NF 8054X, sterigmatocystin |
| A. heyangensis | CBS 101751 = IBT 29634 | a decaturin, NIDU* |
| A. karnatakaensis | IBT 22154 = CBS 102799 | asteltoxin, gregatins, karnatakafuran A and B, quinolactacin, terrein, NIDU*, GUUM* |
| A. karnatakaensis | IBT 22153 = CBS 102800 | asteltoxin, gregatins, karnatakafuran A and B, physcion, quinolactacin, terrein, NIDU*, GUUM* |
| A. karnatakaensis | IMI 086833ii = WB 4649 | a decaturin, karnatakafuran A and B, terrein, GUUM* |
| E. bicolor | CBS 425.77 = IBT 22833 | sterigmatocystin |
| E. discophora | CBS 469.88 = IBT 21910 | sterigmatocystin |
| E. discophora | CBS 470.88 = IBT 21911 | sterigmatocystin, NIDU* |
| E. foeniculicola | CBS 156.80 = IBT 22831 | DRI, sterigmatocystin, xanthocillin FA |
| E. heterothallica | WB 5097 = IBT 22604 | DRI, emeheteron, sterigmatocystin, mer-NF 8054X, stellatin |
| E. heterothallica | CBS 489.65 = WB 5096 = IBT 22607 | DRI*, NIDU*, versicolorins, mer-NF 8054X |
| E. heterothallica | WB 4981 = IBT 22605 | DRI*, sterigmatocystin, mer-NF 8054X |
| E. heterothallica | WB 4983 = IBT 22606 | DRI*, sterigmatocystin, mer-NF 8054X |
| E. spectabilis | CBS 429.77 = IBT 22891 | extrolites with shamixanthone chromophore, trace of sterigmatocystin |
*NIDU, GUUM, and DRI are common extrolites with a characteristic UV chromophore. Their structure has not been elucidated yet.
Aspergillus karnatakaensis Varga, Frisvad & Samson, sp. nov.
MycoBank MB517549
(Fig. 6)
Fig. 6.
Aspergillus karnatakaensis (CBS 102800). A, B. Colonies incubated at 25 °C for 7 d, A on CYA, B on MEA. C, Crusts of Hülle cells,. D, E, and G–I. Conidiophores and conidia. F. Hülle cells. Bars = 10 μm, except F = 100 μm.
Coloniis Emericellae similibus. Conidiophoris cum stipitibus laevibus, conidiis subglobosis vel late ellipsoideis. Aggregationibus insignibus cum tegumento ex cellulis globosis efferentibus.
Typus: India: Karnataka, near Chickmagalur, Netraconda Estate, isolated from soil under coconut palm (Cocos nucifera) in coffee plantation, 20 Dec. 1996, J.C. Frisvad (CBS H-20502 -- holotypus, culture ex-holotype CBS 102800).
Colonies on CYA, at 25 °C: 31–37 mm diam after 7 d, reverse orange; on MEA, at 25 °C: 12–19 mm, reverse yellow; on YES, at 25 °C: 33–45 mm, reverse pink to raspberry-red reverse; on OAT, at 25 °C: 16–23 mm, Hülle cells present; on CYA, at 37 °C: no growth to micro-colony (<1 mm); on CREA: weak to moderate growth, no acid production. Conidial heads reddish brown, yellow exudate droplets on CYA colonies. Conidiophores biseriate, smooth, light brown stipes, 2.5–4 μm wide; vesicles subglobose to subclavate, 5–8 μm diam. Conidiogenous cells (phialides) 2–2.5 × 4–5 μm, metulae, 2–3 × 4–6 μm. Conidia globose or rarely subglobose, smooth to finely roughened, Hülle cells produced in crusts, globose to ellipsoidal, thick-walled, hyaline, 75–200 μm diam.
Diagnostic features: Apart from producing gregatins, terrein, and karnatakafuran A and B, isolates of this species produce a series of fluorescing extrolites (more than 33) with characteristic UV spectra. Also distinguished by producing Hülle cells in crusts.
Aspergillus sect. Aenei Varga & Samson, sect. nov.
MycoBank MB517672
Sectionis Nidulantium similis, sed taxis cum conidiophoris brunneolis, vesiculis ampulliformibus et capitulis conidiorum biserialibus; statu anamorphoso cum ascosporis laevibus, convexis, aequatorialiter bicristatis; in cultura ad 40 °C haud crescenti.
Typus: Aspergillus aeneus Sappa. 1954.
Species assigned to Aspergillus sect. Aenei form a well-supported clade, basal to section Nidulantes sensu Peterson (2008) based on ITS, β-tubulin, calmodulin, and RNA polymerase 2 sequences (see Fig. 11 in Peterson 2008). The section includes four species able to reproduce both sexually and asexually (Emericella discophora, E. bicolor, E. spectabilis, E. foeniculicola), and five species for which the teleomorph is unknown (A. aeneus, A. eburneocremeus, A. crustosus, A. heyangensis, and A. karnatakaensis). All species are characterised by brownish conidiophores, flask-shaped vesicles, and biseriate conidial heads. Several species produce Hülle cells abundantly in masses (except for A. heyangensis, which does not produce Hülle cells at all). The teleomorph-producing species assigned to this section all have smooth convex ascospores with two equatorial crests. None of the species assigned to this section are able to grow at or above 40 °C. All teleomorph species, together with A. eburneocremeus, are able to produce sterigmatocystin. The relationship of E. spectabilis to A. crustosus has already been suggested by Christensen et al. (1978), while E. discophora was found to be related to E. foeniculicola (Zalar et al. 2008).
Acknowledgments
We are grateful to Dr R Naidu for permission to sample soil for mycological examinations in the Coffee Research Station and associated estates near Chickmagalur, Karnataka, India. Tineke van Doorn helped with the morphological data and Uwe Braun kindly provided the Latin diagnosis. We are also indebted to our referees.
REFERENCES
- Christensen M, Raper KB, States JS. (1978) Two new Aspergillus nidulans group members from Wyoming soils. Mycologia 70: 332–342 [Google Scholar]
- Frisvad JC. (1985) Secondary metabolites as an aid to Emericella classification. In: Samson RA, Pitt JI. (eds), Advances in Penicillium and Aspergillus Systematics: 437–443 New York: [Google Scholar]
- Frisvad JC, Samson RA. (2004) Emericella venezuelensis, a new species with stellate ascospores producing sterigmatocystin and aflatoxin B1. Systematic and Applied Microbiology 27: 672–680 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Samson RA, Smedsgaard J. (2004) Emericella astellata, a new producer of aflatoxin B1, B2 and sterigmatocystin. Letters in Applied Microbiology 38: 440–445 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Skouboe P, Samson RA. (2005) Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-O-methylsterigmatocystin, Aspergillus rambellii sp. nov. Systematic and Applied Microbiology 28: 442–453 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Thrane U. (1993) Liquid chromatography of mycotoxins. Journal of Chromatography Library 54: 253–372 [Google Scholar]
- Hillis DM, Bull JJ. (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182–192 [Google Scholar]
- Houbraken J, Due M, Varga J, Meijer M, Frisvad JC, Samson RA. (2007) Polyphasic taxonomy of Aspergillus section Usti. Studies in Mycology 59: 107–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Gloer JB, Wicklow DT, Dowd PF. (2005) Antiinsectan decaturin and oxalicine analogues from Penicillium thiersii. Journal of Natural Products 68: 319–322 [DOI] [PubMed] [Google Scholar]
- Manniche S, Sprogoe K, Dalsgaard PW, Christophersen C, Larsen TO. (2004) Karnatakafurans A and B: Two dibenzofurans isolated from the fungus Aspergillus karnatakaensis. Journal of Natural Products 67: 2111–2112 [DOI] [PubMed] [Google Scholar]
- Peterson SW. (2008) Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 100: 205–226 [DOI] [PubMed] [Google Scholar]
- Peterson SW, Varga J, Frisvad JC, Samson RA. (2008) Phylogeny and subgeneric taxonomy of Aspergillus. In: Varga J, Samson RA. (eds), Aspergillus in the genomic era: 33–56 Wageningen, Wageningen Academic Publishers; [Google Scholar]
- Pitt JI, Samson RA, Frisvad JC. (2000) List of accepted species and their synonyms in the family Trichocomaceae. In: Samson RA, Pitt JI. (eds), Integration of Modern Taxonomic Methods for Penicillium and Aspergillus Classification: 9–49 Amsterdam: Harwood Academic Publishers; [Google Scholar]
- Rai JN, Tewari JP, Murekji KG. (1964) Cultural and taxonomic studies on two rare species of Aspergillus – A. paradoxus and A. aeneus, and an interesting strain of A. variecolor from Indian soils. Mycopathologia et Mycologia Applicata 24: 369–376 [DOI] [PubMed] [Google Scholar]
- Raper KB, Fennell DI. (1965) The genus Aspergillus. Williams & Wilkins, Baltimore, USA: [Google Scholar]
- Samson RA. (2000) List of names of Trichocomaceae published between 1992 and 1999. In: Samson RA, Pitt JI. (eds). Integration of Modern Taxonomic Methods for Penicillium and Aspergillus Classification: 73–79 Amsterdam: Harwood Academic Publishers; [Google Scholar]
- Samson RA, Houbraken J, Frisvad JC, Thrane U, Andersen B. (2010) Food and Indoor fungi. [CBS Laboratory Manual no. 2.] Utrecht: CBS-KNAW Fungal Diversity Centre; [Google Scholar]
- Smedsgaard J. (1997) Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. Journal of Chromatography A 760: 264–270 [DOI] [PubMed] [Google Scholar]
- Sun ZM, Qi ZT. (1994) New taxa and a new record of Aspergillus and Eurotium. Acta Mycologica Sinica 13: 81–87 [Google Scholar]
- Swofford T. (2003) PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4.0. Sunderland, MA: Sinauer Associates; [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Udagawa S, Muroi T. (1979) Some interesting species of ascomycetes from imported spices. Transactions of the Mycological Society of Japan 20: 13–22 [Google Scholar]
- Varga J, Due M, Frisvad JC, Samson RA. (2007c) Taxonomic revision of Aspergillus section Clavati based on molecular, morphological and physiological data. Studies in Mycology 59: 89–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J, Frisvad JC, Samson RA. (2007b) Polyphasic taxonomy of Aspergillus section Candidi based on molecular, morphological and physiological data. Studies in Mycology 59: 75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J, Houbraken J, Lee HAL van der, Verweij PE, Samson RA. (2008) Aspergillus calidoustus sp. nov., causative agent of human infections previously assigned to Aspergillus ustus. Eukaryotic Cell 7: 630–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J, Kocsubé S, Tóth B, Frisvad JC, Perrone G, Susca A, Meijer M, Samson RA. (2007a) Aspergillus brasiliensis sp. nov., a biseriate black Aspergillus species with world-wide distribution. International Journal of Systematic and Evolutionary Microbiology 57:1925–1932 [DOI] [PubMed] [Google Scholar]
- Verweij PE, Varga J, Houbraken J, Rijs AJMM, Verduynlunel FM, Blijlevens NMA, Shea YR, Holland SM, Warris A, Melchers WJG, Samson RA. (2008) Emericella quadrilineata as cause of invasive aspergillosis. Emerging Infectious Diseases 14: 566–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalar P, Frisvad JC, Gunde-Cimerman N, Varga J, Samson RA. (2008) Four new species of Emericella from the Mediterranean region of Europe. Mycologia 100: 779–795 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li C, Swenson DC, Gloer JB, Wicklow DT, Dowd PF. (2003) Novel antiinsectan oxalicine alkaloids from two undescribed fungicolous Penicillium spp. Organic Letters 5: 773–776 [DOI] [PubMed] [Google Scholar]