Abstract
Aspergillus section Sparsi includes species which have large globose conidial heads with colours ranging from light grey to olive-buff. In this study, we examined isolates of species tentatively assigned to section Sparsi using a polyphasic approach. The characters examined include sequence analysis of partial β-tubulin, calmodulin and ITS sequences of the isolates, morphological and physiological tests, and examination of the extrolite profiles. Our data indicate that the revised section Sparsi includes 10 species: A. anthodesmis, A. biplanus, A. conjunctus, A. diversus, A. funiculosus, A. implicatus, A. panamensis, A. quitensis, A. sparsus, and the new taxon A. haitiensis. The recently described A. quitensis and A. ecuadorensis are synonyms of A. amazonicus based on both molecular and physiological data. The white-spored species A. implicatus has also been found to belong to this section. Aspergillus haitiensis sp. nov. is characterised by whitish colonies becoming reddish brown due to the production of conidial heads, and dark coloured smooth stipes. The taxon produces gregatins, siderin and several unknown but characteristic metabolites.
Keywords: Aspergillus section Sparsi, β-tubulin, calmodulin, Eurotiales, extrolites, ITS, polyphasic taxonomy
INTRODUCTION
The Aspergillus sparsus species group (Aspergillus section Sparsi; Gams et al. 1985) was established by Raper & Fennell (1965) to accommodate four species isolated from tropical or subtropical soils. Species assigned to this group have large globose conidial heads, which irregularly split with age, with colours ranging from light grey to olive-buff. Samson (1979) suggested that A. gorakhpurensis should also be placed to this section. However, phylogenetic analysis of parts of the ribosomal RNA gene cluster indicated that this species belongs to Aspergillus section Cremei (Peterson 1995, 2000). According to the recent data of Peterson et al. (2008) and Peterson (2008), the monophyletic section Sparsi belongs to subgenus Nidulantes, and in addition to A. sparsus, A. biplanus, A. diversus and A. funiculosus, originally placed to this section by Raper & Fennell (1965), it also includes A. panamensis and A. conjunctus previously assigned to section Usti, and A. anthodesmis which was previously placed in the A. wentii group (Raper & Fennell 1965).
In this study, we examined available isolates of the species proposed to belong to section Sparsi to clarify the taxonomic status of this section. The methods used include sequence analysis of the ITS region (including internal transcribed spacer regions 1 and 2, and the 5.8 S rRNA gene of the rRNA gene cluster), and parts of the β-tubulin and calmodulin genes, analysis of macro- and micromorphological characters and extrolite profiles.
MATERIALS AND METHODS
Morphological examinations
The strains examined are listed in Table 1. The strains were grown for 7 d as three-point inoculations on Czapek agar, Czapek yeast autolysate agar (CYA), malt extract agar (MEA), and oatmeal agar (OA) at 25 °C and 37 °C (medium compositions in Samson et al. 2010).
Table 1. The Aspergillus section Sparsi isolates examined in this study.
| Species | Strain No. | Origin |
|---|---|---|
| A. amazonicus | CBS 124228T = E19D | Soil, Makas, Ecuador |
| A. anthodesmis | CBS 552.77T = NRRL 22884 | Soil, Ivory Coast |
| A. biplanus | CBS 468.65T = NRRL 5071 | Soil, Tilaran, Costa Rica |
| A. biplanus | CBS 469.65 = NRRL 5073 | Soil, Tilaran, Costa Rica |
| A. biplanus | NRRL 5072 | Soil, Tilaran, Costa Rica |
| A. conjunctus | CBS 476.65T = NRRL 5080 | Forest soil, Palmar, Province of Punteras, Costa Rica |
| A. diversus | CBS 480.65T = NRRL 5074 | Soil, Esparta, Costa Rica |
| A. ecuadorensis | CBS 124229T =E19F | Soil, Makas, Ecuador |
| A. funiculosus | CBS 116.56T = NRRL 4744 | Soil, Ibadan, Nigeria |
| A. haitiensis | CBS 464.91T = NRRL 4569 | Soil under sage and cactus, Haiti |
| A. haitiensis | CBS 468.91 = NRRL 4568 | Desert soil, Haiti |
| A. implicatus | CBS 484.95T | Soil, Ivory Coast |
| A. panamensis | CBS 120.45T = NRRL 1785 | Soil, Panama |
| A. panamensis | NRRL 1786 | Soil, Panama |
| A. quitensis | CBS 124227T = E19C | Soil, Makas, Ecuador |
| A. sparsus | CBS 139.61T = NRRL 1933 | Soil, Costa Rica |
| A. sparsus | NRRL 1937 | Soil, San Antonio, Texas, USA |
Analysis for Extrolites
The cultures were analysed according to the HPLC-diode array detection method of Frisvad & Thrane (1987, 1993) as modified by Smedsgaard (1997). The isolates were analysed on CYA and YES agar using three agar plugs (Smedsgaard 1997). The secondary metabolite production was confirmed by identical UV spectra with those of standards and by comparison to retention indices and retention times for pure compound standards (Frisvad & Thrane 1993, Rahbaek et al. 2000).
Isolation and analysis of nucleic acids
The cultures used for the molecular studies were grown on malt peptone (MP) broth using 1 % (w/v) of malt extract (Oxoid) and 0.1 % (w/v) bacto peptone (Difco), 2 mL of medium in 15 mL tubes. The cultures were incubated at 25 °C for 7 d. DNA was extracted from the cells using the Masterpure™ yeast DNA purification kit (Epicentre Biotechnologies) according to the instructions of the manufacturer. Fragments containing the ITS region were amplified using primers ITS1 and ITS4 as described previously (White et al. 1990). Amplification of part of the β-tubulin gene was performed using the primers Bt2a and Bt2b (Glass & Donaldson 1995). Amplifications of the partial calmodulin gene were set up as described previously (Hong et al. 2005). Sequence analysis was performed with the Big Dye Terminator Cycle Sequencing Ready Reaction Kit for both strands, and the sequences were aligned with the MT Navigator software (Applied Biosystems). All the sequencing reactions were purified by gel filtration through Sephadex G-50 (Amersham Pharmacia Biotech, Piscataway, NJ) equilibrated in double-distilled water and analyzed on the ABI PRISM 310 Genetic Analyzer (Applied Biosystems). The unique ITS, β-tubulin, and calmodulin sequences were deposited at the GenBank nucleotide sequence database under accession numbers FJ491645–FJ491675, and FJ943936–FJ943941.
Data analysis
The sequence data was optimised using the software package Seqman from DNAStar Inc. Sequence alignments were performed by MEGA v. 4.0 (Tamura et al. 2007) and improved manually. For parsimony analysis, the PAUP v. 4.0 software was used (Swofford 2002). Alignment gaps were treated as a fifth character state and all characters were unordered and of equal weight. Maximum parsimony analysis was performed for all data sets using the heuristic search option with 100 random taxa additions and tree bisection and reconstruction (TBR) as the branch-swapping algorithm. Branches of zero length were collapsed and all multiple, equally parsimonious trees were saved. The robustness of the trees obtained was evaluated by 1000 bootstrap replications (Hillis & Bull 1993). An A. ochraceoroseus isolate belonging to section Ochraceorosei of subgenus Nidulantes (Peterson et al. 2008) was used as outgroup in these experiments. The alignments were deposited in TreeBASE (<treebase.org/treebase-web/home.html>) under accession number S11028.
RESULTS AND DISCUSSION
Phylogeny
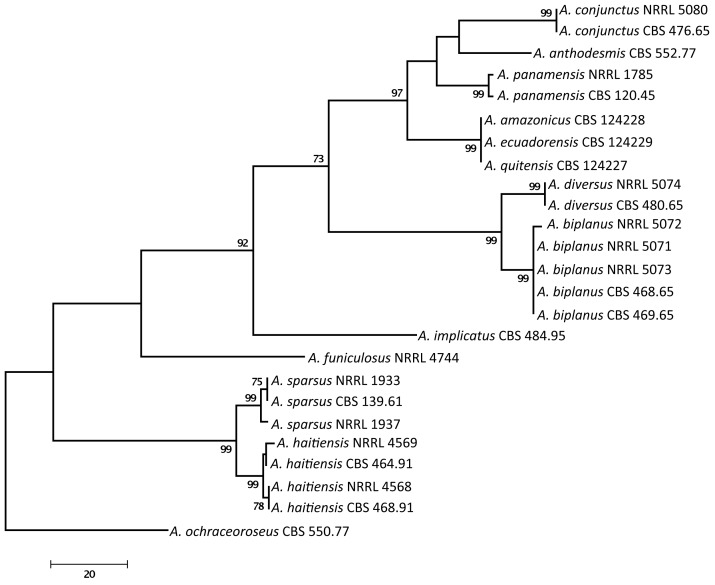
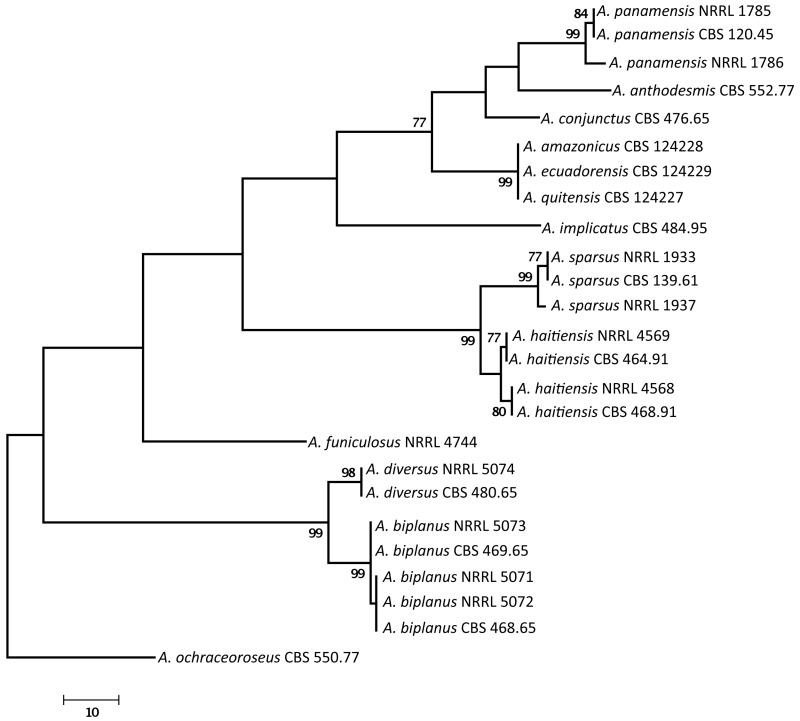
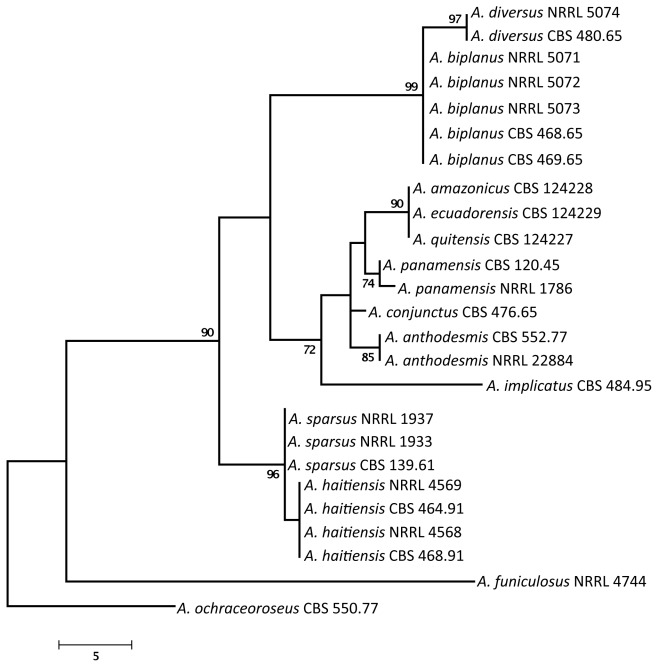
We examined the genetic relatedness of section Sparsi isolates using sequence analysis of the ITS region of the ribosomal RNA gene cluster, and parts of the calmodulin and β-tubulin genes. The calmodulin data set included 566 characters, with 288 parsimony informative characters. One of the 56 MP trees is shown in Fig. 1 (tree length: 741, consistency index: 0.7247, retention index: 0.8903). During analysis of a part of the β-tubulin gene, 494 characters were analysed, among which 196 were found to be parsimony informative. The single MP tree based on partial β-tubulin genes sequences is shown in Fig. 2 (length: 507 steps, consistency index: 0.7179, retention index: 0.8938). The ITS data set included 559 characters with 58 parsimony informative characters. One of the 702 MP trees is presented in Fig. 3 (tree length: 180, consistency index: 0.8056, retention index: 0.8763).
Fig. 1.
One of the MP trees obtained based on phylogenetic analysis of calmodulin sequence data of Aspergillus section Sparsi. Numbers above branches are bootstrap values. Only values above 70 % are indicated.
Fig. 2.
The single MP tree obtained based on phylogenetic analysis of β-tubulin sequence data of Aspergillus section Sparsi. Numbers above branches are bootstrap values. Only values above 70 % are indicated.
Fig. 3.
One of the MP trees obtained based on phylogenetic analysis of ITS sequence data of Aspergillus section Sparsi. Numbers above branches are bootstrap values. Only values above 70 % are indicated.
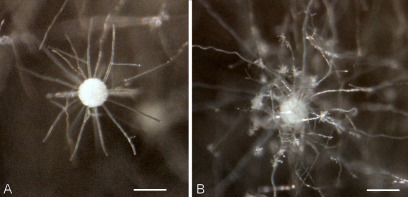
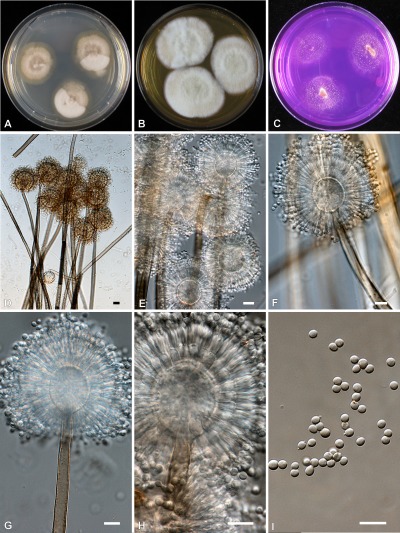
Phylogenetic analysis of β-tubulin, calmodulin and ITS sequence data indicated that Aspergillus section Sparsi includes 10 species. Aspergillus biplanus and A. diversus are closely related to each other on all trees, while another clade includes A. panamensis, A. anthodesmis, A. conjunctus, and the recently described A. amazonicus, A. quitensis and A. ecuadorensis isolates on the trees based on β-tubulin and ITS sequence data (Figs 2, 3; Mares et al. 2008). Although Mares et al. (2008) found that these three isolates have identical ITS sequences, they were suggested to represent distinct species based on morphological data (length of talks, diameter of vesicles, morphology of conidia and number of phialides), and were placed in Aspergillus section Wentii. However, these three isolates could not be distinguished from each other based on molecular, morphological or extrolite data in our study, and clearly belong to section Sparsi (Figs 1–4). Aspergillus amazonicus is chosen as the correct name for the taxon and A. quitensis and A. ecuadorensis are considered synonyms. Aspergillus implicatus, a white-spored species originally assigned to Aspergillus section Candidi (Maggi & Persiani 1994), also belongs to this section. This species was described to produce conidiophores surrounded by sterile hyphae, not yet seen in any other species of the Aspergillus genus. Unfortunately the ex-type culture showed only poor sporulation and only a few conidiophores with sterile outgrowth could be observed (Fig. 5).
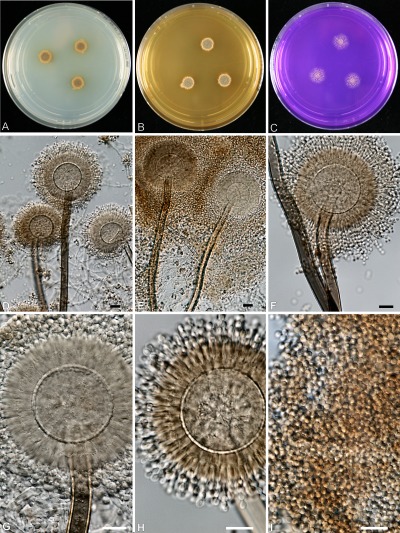
Fig. 4.
Aspergillus amazonicus (CBS 124228). A–C. Colonies of 7 d grown at 25 °C; A on CYA, B on MEA, C on CREA. D–I. Conidiophores and conidia. Bars = 10 μm.
Fig. 5.
Aspergillus implicatus (CBS 484.95). A–B. Conidal heads showing sterile outgrowths. Bars = 100 μm.
Phylogenetic analysis of sequence data indicated that the four examined A. sparsus isolates fall into two closely related clades. The three phylogenies were concordant, with no conflict between the topologies of the gene trees, in accordance with the phylogenetic species recognition concept detailed by Taylor et al. (2000). The ex-type strain of A. sparsus (CBS 139.61T) together with an isolate from Texas, USA form one clade, while two isolates came from soil from Haiti form another clade on all trees (Figs 1–3). Both of the latter isolates were found by Raper & Fennell (1965) to differ from the ex-type strain of A. sparsus in producing more restrictedly growing colonies in shades of reddish brown on MEA plates, while one of the isolates (CBS 464.91 = NRRL 4569) also produced “small fragmentary sporulating structures adjacent to the agar surface that bear conidia similar to those of normal heads” (Raper & Fennell 1965). Here we describe this new species as Aspergillus haitiensis.
Regarding the value of the different loci for species delimitation in section Sparsi, all species could be distinguished using either ITS, β-tubulin or calmodulin sequence data. However, the resolving power was much higher for the protein coding genes than for the ITS region. The situation is more difficult in other sections of Aspergilli, including for example sections Nigri (Samson et al. 2007), Clavati (Varga et al. 2007), and Cervini (J. Varga, unpubl. observ.), where the ITS region cannot be used reliably to distinguish all species assigned to the given section.
Extrolite profiles
Among the species assigned to Aspergillus section Sparsi, A. panamensis produces cyclogregatin and gregatins (also called graminins or aspertetronins; Anke et al. 1980a, b, 1988), while A. funiculosus has been found to produce ethericin A (also called violaceol I or aspermutarubrol), and ethericin B (or funicin; König et al. 1978, 1980, Nakamura et al. 1983) (Table 2). Ethericin A was first isolated and called aspermutarubrol from A. sydowii, causing the red colouration of the medium, as this unstable compound will turn into a red dye by oxidation (Shibata et al. 1978). The ethericins (or violaceols) are also produced by A. versicolor and several Emericella species (Fremlin et al. 2009). Gregatins are also produced by A. anthodesmis and one of the A. haitiensis isolates (Table 2). Siderin is related to kotanins produced by some black Aspergilli and A. clavatus (Samson et al. 2007, Varga et al. 2007), and is also produced by A. panamensis, A. anthodesmis, A. conjunctus and by an A. haitiensis isolate (NRRL 4569). Auraglaucin production is shared by A. biplanus, A. conjunctus and A. diversus, and is also produced by some Eurotium species (Gould & Raistrick 1934, Quilico et al. 1949). Aspergillus implicatus (Fig. 5) has been found to produce a versicolorin derivative. The two A. haitiensis isolates produced quite distinct extrolite profiles, but shared the production of several unknown compounds including those tentatively named tidmyco1-3. Several of the other extrolites produced by species assigned to Aspergillus section Sparsi have also been detected in other species assigned to sections Nidulantes, Usti and Versicolores, justifying the assignment of section Sparsi to Aspergillus subgenus Nidulantes (Peterson et al. 2008).
Table 2. Extrolites produced by species of Aspergillus section Sparsi. The structures of the extrolites in brackets have not yet been elucidated.
| Species | Extrolites |
|---|---|
| A. amazonicus | an aszonalenin, (dob-indol, fot, Vurs1, vurs2, stan) |
| A. anthodesmis | gregatins, siderin (alk-769gl; AMF1, AMF2, AMF3, ANTW, kota, met k, tidmyco1, tidmyco2, senmyco1, senmyco2, senmyco3, UNTW) |
| A. biplanus | auroglaucin, (BLØDO, CUR-678, KONI, OKSI-1121, RAI-701, RAI-843, SKOT, VERN-652, VERN-655, VERN-661, VERN-673, vers-965, vers-979, vers-1049, vers-1107) |
| A. conjunctus | auroglaucin, siderin?, (alk-1538, alk-1756, blæam, CONJ1, CONJ2, CONJ3, DUTS, INSUX,JON1, JON2, JON3, JON4, kola, kola2, SVIF1, SVIF2, UT, verruc1, verruc2, vers-1049, vers-1107), a falconensin (? by A. conjunctus SRRC 423) |
| A. diversus | auroglaucin, mycophenolic acid?, (alka-704, CONJ1, kola2, OKSI-1, OKSI-2, vers-965, vers-979, vers-1049, vers-1107; OKSI-3, OKSI-4, OKSI-5, OKSI-6 by NRRL 5075) |
| A. funiculosus | arugosin E, ethericin A, funicin = ethericin B, terrein?, (AQ-798, AQ-1456, bianthron-1396, DERH, DRI, emon, hæms, NOL, RAI-921, RAI-972, storå, SULTI-1, SULTI-2, vers-818, vers-856) |
| A. haitiensis NRRL 4568 | (ATROV, GYLA, NIDU, tidmyco1, tidmyco2, tidmyco3, spar1, spar2, spar3) |
| A. haitiensis NRRL 4569 | gregatins, siderin, (AMF1, AMF2, AMF3, senmyco1, senmyco2, senmyco3, tidmyco1, tidmyco2, tidmyco3) |
| A. implicatus | a versicolorin, an austalide derivative (?) |
| A. panamensis | gregatins, siderin, (AQ-1456, OTTO), |
| A. sparsus | (NIDU, senmyco1, senmyco2, senmyco3, spar1, spar2) |
Taxonomy
Aspergillus haitiensis Varga, Frisvad & Samson, sp. nov.
MycoBank MB517384
(Fig. 6)
Fig. 6.
Aspergillus haitiensis (CBS 464.91). A–C. Colonies of 7 d grown at 25 °C; A on CYA, B on MEA, C on CREA. D–I. Conidiophores and conidia. Bars = 10 μm.
Speciebus Aspergilli sect. Sparsi similes, sed coloniis porphyreis et stipitibus fuscatis, laevibus distinguitur.
Typus: Haiti: isolated from soil under sage and cactus, W. Scott (as 113a) (CBS H-20503 -- holotypus, cultures ex-holotype CBS 464.91 = NRRL 4569).
Colonies on MEA 50–60 mm, on CYA 30–35 mm, after 14 d at 25 °C, moderate growth on MEA after 7 d at 37 °C. Conidial heads produced sparsely on CYA, colony colour first white then reddish brown, colony texture floccose, reverse creamish to light brown. Conidial heads radiate; stipes 5–9 μm, thick-walled, dark brown in colour; vesicles 10–25 μm wide, biseriate; metulae covering the whole vesicle, measuring 2.5–4 × 5–7 μm. Conidiogenous cells (phialides) 2–2.5 × 7–8 μm. Conidia globose to ellipsoidal 4–5.6 × 5–6 μm, smooth. Fragmentary sporulating structures in addition to the normal conidial heads are also present.
Additional isolate studied: Haiti: Port de Paix, from desert soil, W. Scott (as 103b) (CBS 468.91 = NRRL 4568).
Diagnostic features: Thin whitish colonies turning to reddish brown colour on CYA, brown-coloured smooth stipes, and production of unknown extrolites tentatively called tidmyco1-3.
Acknowledgments
We are grateful to Tineke van Doorn who helped with the morphological data, Uwe Braun with the Latin diagnosis, and our referees.
REFERENCES
- Anke H, Casser I, Schrage M, Steglich W. (1988) Cyclogregatin, a new metabolite from Aspergillus panamensis. Journal of Antibiotics 41: 1681–1684 [DOI] [PubMed] [Google Scholar]
- Anke H, Schwab H, Achenbach H. (1980a) Tetronic acid derivatives from Aspergillus panamensis. Planta Medica 39: 195 [DOI] [PubMed] [Google Scholar]
- Anke H, Schwab H, Achenbach H. (1980b) Tetronic acid derivatives from Aspergillus panamensis. Production, isolation, characterization and biological activity. Journal of Antibiotics 33: 931–939 [DOI] [PubMed] [Google Scholar]
- Fremlin LJ, Piggott AM, Lacey E, Cappon RJ. (2009) Cottequinazoline, A and cotteslosins A and B, metabolites from an Austsralian marine-derived strain of Aspergillus versicolor. Journal of Natural Products 72: 666–670 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Thrane U. (1987) Standardized high performance liquid chromatography of 182 mycotoxins and other fungal metabolites based on alkylphenone retention indices and UV-VIS spectra (diode array detection). Journal of Chromatography A 404: 195–214 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Thrane U. (1993) Liquid chromatography of mycotoxins. Journal of Chromatography Library 54:253–372 [Google Scholar]
- Gams W, Christensen M, Onions AH, Pitt JI, Samson RA. (1985) Infrageneric taxa of Aspergillus. In: Samson RA, Pitt JI, (eds), Advances in Penicillium and Aspergillus Systematics: 55–62 New York: Plenum Press; [Google Scholar]
- Glass NL, Donaldson GC. (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould A, Raistrick H. (1934) The biochemistry of microrganisms. XL. The crystalline pigments of species in the Aspergillus glaucus series. Biochemical Journal 28: 1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki T, Kimura Y, Hatsuda Y, Sugawara S. (1980) Isolation and structure of funicin, antimicrobial substance, produced by Aspergillus funiculosus. Agricultural and Biological Chemistry 44: 1357–1360 [Google Scholar]
- Hillis DM, Bull JJ. (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182–192 [Google Scholar]
- Hong SB, Cho HS, Shin HD, Frisvad JC, Samson RA. (2006) Novel Neosartorya species isolated from soil in Korea. International Journal of Systematic and Evolutionary Microbiology 56: 477–486 [DOI] [PubMed] [Google Scholar]
- Johnson GT, Gould BS. (1953) Pigment production in certain of the Aspergillus glaucus group. Mycologia 45: 172–193 [Google Scholar]
- König WA, Krause R, Loeffler W, Schanz D. (1980) Metabolic products of microorganisms. 196. The structure of ethericin B, a new diphenylether antibiotic. Journal of Antibiotics 33: 1270–1273 [DOI] [PubMed] [Google Scholar]
- König WA, Pfaff KP, Loeffler W, Schanz D, Zähner H. (1978) Stoffwechselprodukte von Mikroorganismen, 171. Ethericin A; Isolierung, Charakterisierung und Strukturaufklärung eines neuen, antibiotisch wirksamen Diphenylethers. Justus Liebigs Annalen der Chemie 1978: 1289–1296 [Google Scholar]
- Maggi O, Persiani M. (1994) Aspergillus implicatus, a new species isolated from Ivory Coast forest soil. Mycological Research 98: 869–873 [Google Scholar]
- Mares D, Andreotti E, Maldonado ME, Pedrini P, Colalongo C, Romagnoli C. (2008) Three new species of Aspergillus from Amazonian forest soil (Ecuador). Current Microbiology 57: 222–229 [DOI] [PubMed] [Google Scholar]
- Nakamura M, Fukuyama K, Tsukihara T, Katsube Y, Hamasaki T. (1983) Structure of funicin, antimicrobial substance from Aspergillus funiculosus, C17H18O5. Acta Crystallographica Section C. Crystal Structure Communications 39: 268–270 [Google Scholar]
- Peterson SW. (1995) Phylogenetic analysis of Aspergillus sections Cremei and Wentii, based on ribosomal DNA sequences. Mycological Research 99: 1349–1355 [Google Scholar]
- Peterson SW. (2000) Phylogenetic relationships in Aspergillus based on rDNA sequence analysis. In: Samson RA, Pitt JI. (eds), Integration of modern taxonomic methods for Penicillium and Aspergillus classification: 323–355 Amsterdam: Harwood Academic Publishers; [Google Scholar]
- Peterson SW. (2008) Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 100: 205–226 [DOI] [PubMed] [Google Scholar]
- Peterson SW, Varga J, Frisvad JC, Samson RA. (2008) Phylogeny and subgeneric taxonomy of Aspergillus. In: Varga J, Samson RA. (eds), Aspergillus in the Genomic Era: 33–56 Wageningen: Wageningen Academic Publishers; [Google Scholar]
- Quilico A, Panizzi L, Mugnaini E. (1949) Structure of flavoglaucin and auroglaucin. Nature 164: 26 [DOI] [PubMed] [Google Scholar]
- Raper KB, Fennell DI. (1965) The genus Aspergillus. Williams & Wilkins, Baltimore, USA: [Google Scholar]
- Samson RA. (1979) A compilation of the Aspergilli described since 1965. Studies in Mycology 18: 1–38 [Google Scholar]
- Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B. (2010) Food and Airborne Fungi. [CBS Laboratory Manual Series no. 2.] Utrecht: CBS-KNAW Fungal Biodiversity Centre; [Google Scholar]
- Samson RA, Noonim P, Meijer M, Houbraken J, Frisvad JV, Varga J. (2007) Diagnostic tools to identify black Aspergilli. Studies in Mycology 59: 129–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Kamihawa T, Kaneda N, Taniguchi M. (1978) New metabolite aspermutarubrol, from Aspergillus sydowii. Chemistry Letters 1978: 797–798 [Google Scholar]
- Smedsgaard J. (1997) Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. Journal of Chromatography A 760: 264–270 [DOI] [PubMed] [Google Scholar]
- Swofford T. (2000) PAUP*: phylogenetic analysis using parsimony. Version 4.0. Sunderland, MA: Sinauer Associates; [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. (2007) MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC. (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31: 21–32 [DOI] [PubMed] [Google Scholar]
- Varga J, Due M, Frisvad JC, Samson RA. (2007) Taxonomic revision of Aspergillus section Clavati based on molecular, morphological and physiological data. Studies in Mycology 59: 89–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR Protocols: a guide to methods and applications: 315–322 New York: Academic Press; [Google Scholar]