Abstract
Various fungi were isolated during the course of a survey in a cold-store of apples in the Netherlands. One of these fungi belongs to the genus Penicillium and produces cleistothecia at 9 and 15 °C. A detailed study using a combination of phenotypic characters, sequences and extrolite patterns showed that these isolates belong to a new species within the series Roqueforti. The formation of cleistothecia at low temperatures and the inability to produce roquefortine C, together with a unique phylogenetic placement, make these isolates a novel entity in the Roqueforti series. The name Penicillium psychrosexualis sp. nov. (CBS 128137T) is proposed here for these isolates.
Keywords: Penicillium, roqueforti, P. carneum, P. paneum, taxonomy, phylogeny
INTRODUCTION
Penicillium species are commonly occurring worldwide, and have been isolated from various substrates including air, soil, various food and feed products and indoor environments (Pitt 1979, Samson et al. 2010, Houbraken et al. 2010). Penicillium roqueforti is a member of this genus is and this species has both adverse and beneficial properties. The main beneficial property of this species is its role in the production of blue-veined cheeses, such as Roquefort, Danish blue, and Gorgonzola (Nichol 2000). However, this species is also frequently encountered as a spoilage organism, and is able to damage a vast array of food and feed products, due to its ability to grow under harsh conditions. These conditions include growth at low oxygen and high carbon dioxide levels, in the presence of preservatives and/or at low temperatures (Samson et al. 2010).
The taxonomy of series Roqueforti was studied by Samson & Frisvad (2004) using a polyphasic approach, combining partial β-tubulin sequences, extrolite patterns, phenotypic and physiological data. They showed that P. paneum and P. carneum are closely related to P. roqueforti, together forming the series Roqueforti. This series shares certain characters, such as a fast growth rate on agar media, the ability to grow on malt extract agar supplemented with acetic acid and the production of the extrolite roquefortine C. Despite the various shared characters, also various features are known to differentiate between these species (Frisvad & Samson 2004, Karlshøj & Larsen 2005, O’Brien et al. 2008). These include the growth rate at 30 °C, reverse colours on Czapek yeast agar and yeast extract agar, extrolite patterns and Ehrlich reaction (Samson & Frisvad 2004, Samson et al. 2010).
Various fungi were isolated during the course of a survey in a cold-store of apples in The Netherlands. The apples were stored in wooden crates, which were covered by a white fungal growth of Fubulorhizoctonia psychrophila. The latter species only grows at temperatures below 20 °C, and during the isolation of this species growth of an ascospore-forming Penicillium species was detected. This species appeared to be related to the series Roqueforti and a detailed study was performed on these isolates using a polyphasic approach. For the phylogenetic analysis, ITS, partial β-tubulin and calmodulin sequences were used, and these data were combined with extrolite analysis and macro- and microscopical characteristics. The combination of these datasets show that this species is new and is here described as Penicillium psychrosexualis.
MATERIAL AND METHODS
Strains and morphological examination
All examined strains belong to the Penicillium series Roqueforti. The strains (Table 1) were grown for 7 d as three point inoculations on Czapek yeast agar (CYA), malt extract agar, yeast extract sucrose agar (YES), creatine sucrose agar (CREA) and oatmeal agar (OA). The effect of various incubation temperatures (9–36 °C with intervals of 3 °C) on the growth was studied on CYA and OA.
Table 1. Overview of Penicillium strains used in this study.
| CBS no. | Other no. | Name | Substrate, locality |
|---|---|---|---|
| 449.78 | IBT 21509=IBT 3473=IBT 6753 | P. carneum | Cheddar cheese |
| 466.95 | ATCC 46837=IBT 6885 | P. carneum | Cured meat, Germany |
| 467.95 | IBT 3466 | P. carneum | Hotwater tank, North Sealand, Denmark |
| 112297T | IBT 6884 | P. carneum | Type, mouldy rye bread, Denmark |
| 463.95 | IBT 12392 | P. paneum | Chocolate sauce, Norway |
| 464.95 | IBT 11839 | P. paneum | Rye bread (non preserved), Odense, Denmark |
| 465.95 | IBT 13929 | P. paneum | Mouldy baker’s yeast, Vangede, Denmark |
| 101032T | IBT 21541=IBT 12407 | P. paneum | Type, mouldy rye bread, Denmark |
| 112296 | IBT 21729 | P. paneum | Cassava chips, Africa |
| 112294 | IBT 16402=NRRL 1168 | P. paneum | Unknown substrate, Ottawa , Canada |
| 128137T | DTO 70G9 = IBT 29551 | P. psychrosexualis | Type, wooden crate in cold-store of apples, the Netherlands |
| 128136 | DTO 70H7 | P. psychrosexualis | Wooden crate in cold-store of apples, the Netherlands |
| 128035 | DTO 70H4 | P. psychrosexualis | Wooden crate in cold-store of apples, the Netherlands |
| 128036 | DTO 70H9 | P. psychrosexualis | Wooden crate in cold-store of apples, the Netherlands |
| 135.67 | IBT 19475=MUCL 8491 | P. roqueforti | Blue veined cheese, Germany |
| 221.30NT | ATCC 10110=ATCC 1129=CECT 2905=IBT 6754=IFO 5459=IMI 024313=NRRL 849 | P. roqueforti | Neotype, French Roquefort cheese, USA |
| 234.38 | IBT 19781=IMI 291202 | P. roqueforti | Blue Cheshire cheese |
| 479.84 | IBT 21543 | P. roqueforti | Mouldy baker’s yeast, Denmark |
| 498.73 | ATCC 24720=FRR 1480=IBT 19476=IMI 174718=IMI 291199=VKM F-1748 | P. roqueforti | Fruit of Malus sylvestris (apple), Russia |
Molecular analysis
Genomic DNA was isolated using the Ultraclean™ Microbial DNA Isolation Kit (MoBio, Solana Beach, CA, USA) according the manufacturer’s instructions. The ITS regions (ITS), a part of the β-tubulin (BenA) or calmodulin (Cmd) gene were amplified and sequenced according the method described in Houbraken et al. (2007). Each dataset was aligned using the Clustal W program in MEGA5 (Tamura et al. 2007), and subsequently manually optimised. The evolutionary history was inferred by using the Maximum Likelihood (ML) method based on the Tamura-Nei model (Tamura & Nei 1993). The bootstrap consensus tree inferred from 1 000 replicates is taken to represent the evolutionary history of the taxa analysed (Tamura et al. 2007). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1 000 replicates) is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically as follows. When the number of common sites is < 100 or less than one fourth of the total number of sites, the maximum parsimony method was used; otherwise BIONJ method with MCL distance matrix was used. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. All positions containing gaps and missing data were eliminated. Evolutionary analyses were conducted in MEGA5 (Felsenstein 1985, Tamura et al. 2007). All phylograms were rooted with Penicillium egyptiacum CBS 244.32NT. The newly obtained sequences were deposited in GenBank under accession numbers HQ442319–HQ442359.
Extrolite analysis
Plugs with mycelium and agar were extracted from 7 d old cultures grown on CYA and YES. Extracts were prepared using the method described by Smedsgaard (1997). Each extract was filtrated through a 0.45 PTFE filter and subsequently analysed using HPLC with diode array detection (DAD) detection. The UV spectrum and the RI value, and comparison with authentic chemical standards, were used to characterise the extrolites produced (Frisvad & Thrane 1987).
RESULTS
Phylogeny
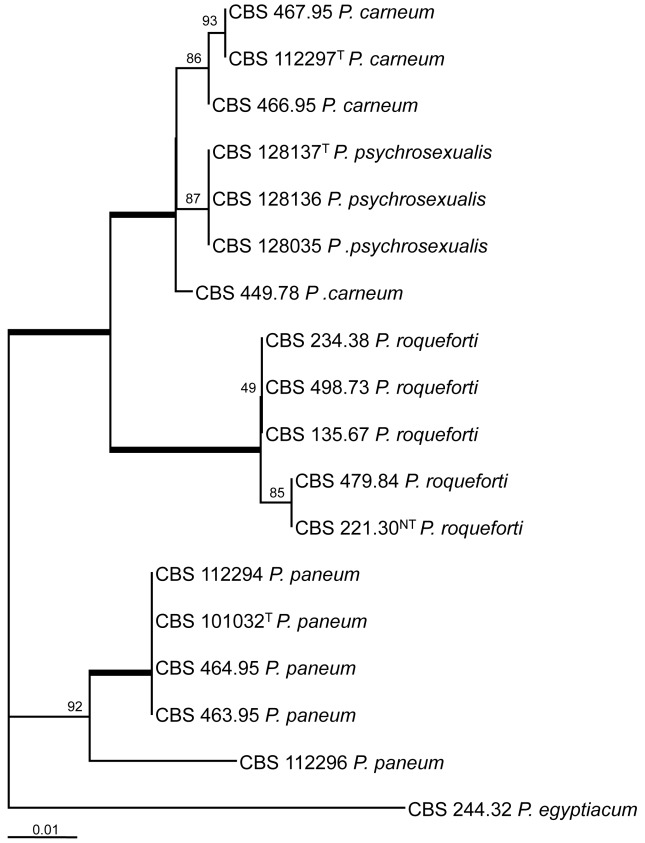
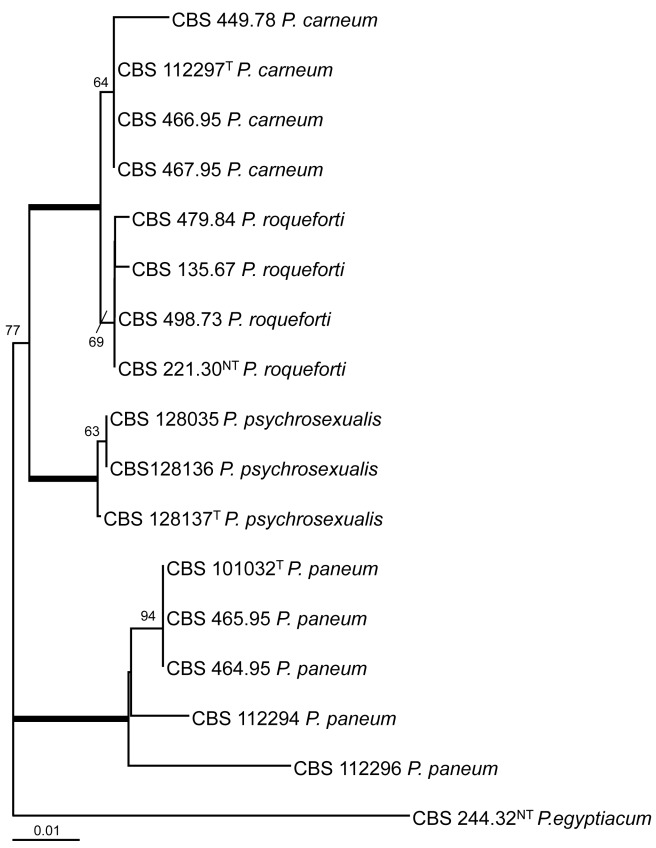
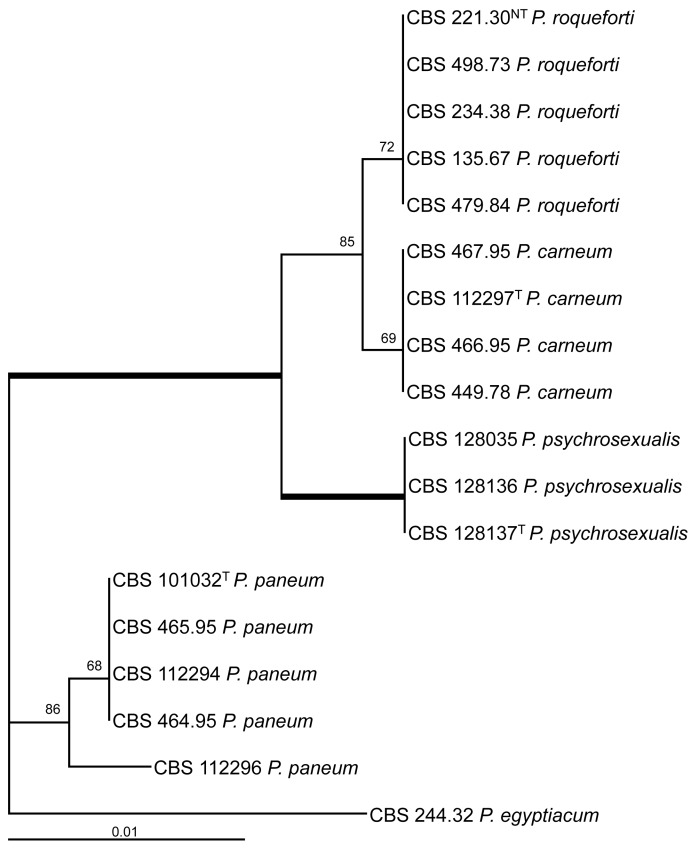
The ITS regions and parts of the β-tubulin (BenA) and calmodulin (Cmd) gene were sequenced and analysed. The BenA alignment included 432 positions, and 35 positions were parsimony informative. The bootstrap consensus tree based on the results of the maximum likelihood analysis of this alignment is shown in Fig. 1. The total length of the calmodulin alignment was 500 positions long, and 27 sites were parsimony informative. The bootstrap consensus tree derived from the maximum likelihood analysis is shown in Fig. 2. The phylogram in Fig. 3 is based on the ITS regions (incl 5.8S rDNA), and 585 bases were used in the maximum likelihood analysis. Of these 585 characters, 16 were parsimony informative (including alignment gaps).
Fig. 1.
Bootstrap consensus tree from a maximum likelihood analysis of partial β-tubulin sequences. The bootstrap values from 1 000 replicates are shown at the nodes, the branches in bold have a bootstrap support higher than 95 %. The tree was rooted with Penicillium egyptiacum CBS 244.32NT.
Fig. 2.
Bootstrap consensus tree from a maximum likelihood analysis of partial calmodulin sequences. The bootstrap values from 1 000 replicates are shown at the nodes, the branches in bold have a bootstrap support higher than 95 %. The tree was rooted with Penicillium egyptiacum CBS 244.32NT.
Fig. 3.
Bootstrap consensus tree from a maximum likelihood analysis of ITS sequences. The bootstrap values from 1 000 replicates are shown at the nodes, the branches in bold have a bootstrap support higher than 95 %. The tree was rooted with Penicillium egyptiacum CBS 244.32NT.
The result of the analysis of the three datasets shows that P. psychrosexualis belongs to the series Roqueforti. The species is related to P. carneum and P. roqueforti in all three analysed loci, and P. paneum is basal to these three species. Penicillium carneum is the closest relative of P. psychrosexualis in the tubulin phylogram (99 %, Fig. 1), and P. roqueforti is basal to these two species. However, this relationship is not supported in the phylograms based on the calmodulin and ITS sequences. In these datasets, P. carneum and P. roqueforti are sister species and in both cases P. psychrosexualis is basal to these two species. Two isolates (CBS 449.78 and CBS 112296) warrant further attention. Penicillium carneum CBS 449.78, an isolate from cheddar cheese, has a unique position in the tubulin and calmodulin phylograms (Fig. 1, 2). In addition, this strain is morphologically slightly deviating from the majority of examined P. carneum isolates. Isolate CBS 449.78 is cream-brown in reverse on CYA, more restricted colonies on creatine agar and slightly slower growth rate at 30 °C. The other isolate which warrants attention is P. paneum CBS 112296. This strain has a unique β-tubulin, calmodulin and ITS sequence. However, extrolite analysis shows that this strain produces a typical array of P. paneum extrolites. More strains of these two types should be collected and examined to determine whether these strains should be raised to species level.
Taxonomy
Penicillium psychrosexualis Houbraken & Samson, sp. nov.
MycoBank MB519086
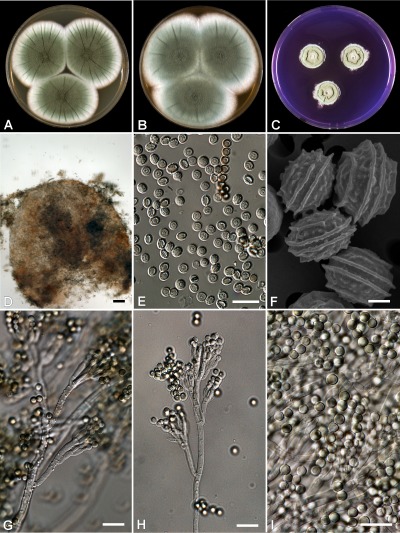
(Fig. 4)
Fig. 4.
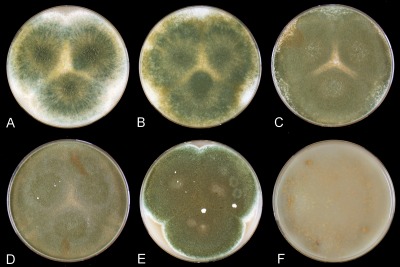
Penicillium psychrosexualis (CBS 128036 , ex wooden crate in cold-store of apples, the Netherlands). (A–C) Colonies grown at 25 °C for 7 d on (A) CYA, (B) MEA, and (C) YES; (D) cleistothecium; (E–F) ascospores; (G) conidiophores on DG18 with warted stipes; (H) conidiophore with smooth stipe; (I) conidia. Bar = 10 μm, except (F) = 1 μm.
In Penicillium subgenus Penicillium sect. Roqueforti ser. Roqueforti
Coloniis in MEA cum 0.5 % acore acetica crescentibus et item in agaro MEA, CYS et YES celeriter crescentibus, et formatione cleistotheciorum ad temperationem exiguam. Roquefortino C haud producenti.
Typus: The Netherlands: wooden crate in cold-store of apples covered by growth of Fubulorhizoctonia psychrophila, 3 Apr. 2008, J. Houbraken & F. van der Geijn (CBS H-20501 holotype; cultures ex type – CBS 128137 = IBT 29551 = DTO 70G9).
Colony diameter at 7 d (in mm): CYA, 25 °C, 47–55; CYA, 15 °C, 35–46; CYA, 30 °C, 14–27; no growth on CYA at 37 °C; MEA >60; YES >60; DG18, 40–50; ratio CYAS : CYA 1.2–1.4; creatine agar 15–25, good growth and no or weak acid production (under colony), delayed base production.
Strong sporulation on CYA, velvety, slightly floccose in centre, dull green or dark dull green conidia, mycelium inconspicuous, exudates absent, soluble pigment absent, radial sulcate, reverse warm brown. Good sporulation on YES, conidia dull-green, soluble pigments absent, reverse mustard-yellow, none sporulating edge 6–10 mm. Good sporulation on DG18, conidia dull-green, reverse pale. Colonies on MEA dull-green towards pure-green, velvety, soluble pigments absent. No reaction with an Ehrlich test.
Cleistothecia on OA at 25 °C sparsely produced and not visible due to the presence of a layer of conidia, formation of cleistothecia induced and sporulation reduced at low temperatures (9–15 °C, Fig. 5), cleistothecia white, soft and sterile when young, maturing slowly and becoming pale orange-brown after 3–4 mo of incubation, (50–)100–175 μm diam. Ascospores ellipsoidal, 4–5 × 3–4 μm, with two distinct equatorial ridges, often with additional secondary ridges, one on either side of the main ones, suggesting the presence of four ridges when observed with light microscopy, valves slightly roughened when viewed with SEM. Conidiophores terverticillate, slightly reduced conidiophores with smooth walled stipes on MEA and other agar media (PDA, PCA), on DG18 robust conidiophores with warted stipes, 3–4 μm. Metulae 10–15 × 3–4 μm. Conidiogenous cells (phialides) ampulliform, 8–10 × 3–4 μm. Conidia globose, smooth, 3.5–4 μm.
Fig. 5.
Growth of Penicillium psychrosexualis CBS 128036 on oatmeal agar at various incubation temperatures. A–F: 9, 12, 18, 24, 27 and 33 °C.
Extrolites: Penicillium psychrosexualis produces the extrolites andrastin A, mycophenolic, patulin, roquefortine C and the uncharacterized extrolite tentatively named “fumu”. Furthermore, P. psychrosexualis produces the same odour as P. roqueforti.
Diagnostic features: The growth on MEA containing 0.5 % acetic acid, the formation of cleistothecia at relatively low temperatures for the genus (9 °C) and fast growth rate on MEA, CYA and YES are diagnostic features of P. psychrosexualis. An overview of characteristics of P. psychrosexualis in comparison with other members of the series Roqueforti is shown in Table 2.
Table 2. Overview of selected characters of members of Penicillium series Roqueforti (after Frisvad & Samson 2004, Sumarah et al. 2005, Nielsen et al. 2006, O’Brien et al. 2006, Månsson et al. 2010). See Karlshøj & Larsen (2005) for further differences in volatiles.
| Species | Ehrlich reaction | Reverse on YES | Cleistothecia/sclerotia | Growth rate on CYA30 °C (mm) | Extrolites** |
|---|---|---|---|---|---|
| P. carneum | Violet | Cream-beige | - | 15–30 | Roquefortine C, isofumigaclavine A&B, mycophenolic acid, patulin, cyclopaldic acid, penitrem A, andrastin A, (penicillic acid in CBS 449.78) |
| P. paneum | Negative | Cream yellow/beige* | - | 30–45 | Roquefortine C, marcfortin A, patulin, andrastin A, citreoisocoumarin, (botryodiploidin) |
| P. psychrosexualis | Negative | Mustard-yellow | + | 15–25 | Andrastin A, mycophenolic, patulin and roquefortine C and the uncharacterized extrolite tentatively named “fumu” |
| P. roqueforti | Violet | Blackish green | -/(+) | (0–)5–15 | Roquefortine C, isofumigaclavine A&B, PR-toxin, andrastin A, citreoisocoumarin, (mycophenolic acid) |
*Often turning strawberry-red with age; with colour diffusing into the medium.
**The extrolites mentioned between brackets are not produced by the majority of isolates.
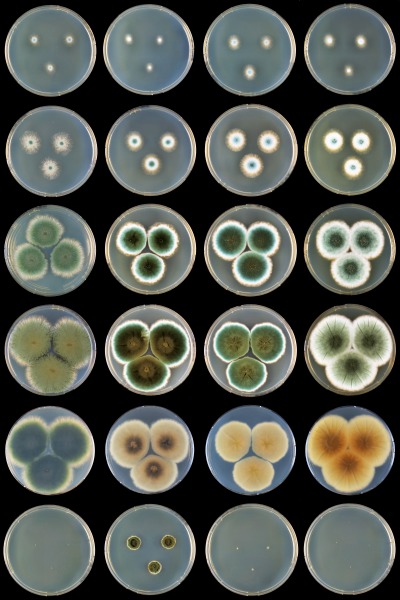
Similar species and taxonomy: Phylogenetically P. psychrosexualis belongs to series Roqueforti. This species shares a fast growth rate on agar media, the ability to grow on MEA supplemented with 0.5 % acetic acid and forms conidiophores with warted stipes on DG18. This species produces the extrolites andrastin A, mycophenolic, patulin and roquefortine C and is chemically close to P. carneum. However, P. carneum also produces penitrem A, isofumigaclavine A and cyclopaldic aicd, while P. psychrosexualis produces the uncharacterised extrolite “fumu”. Penicillium psychrosexualis produces the same odour as P. roqueforti, and is thus very different from the strong odour of P. carneum. Another difference between P. psychrosexualis and the other members of the Roqueforti series is the production of cleistothecia by the former species. The growth rate on CYA at 30 °C is a diagnostic tool to differentiate between P. roqueforti and P. carneum on one hand and P. paneum on the other. Penicillium psychrosexualis has similar growth rates at 30 °C as P. roqueforti and P. carneum. This observation is concordant with the phylogeny, which also shows that these three species are closely related and that P. paneum is basal to these species. An overview of growth rates on CYA at various temperatures is shown in Fig. 6.
Fig. 6.
Overview of growth rates of the members of Penicillium series Roqueforti on CYA at various temperatures. Row, top to bottom: 9, 12, 18, 24, 24 (reverse), 30 °C; columns, left to right: P. roqueforti DTO 81D6, P. paneum DTO 28G8, P. carneum DTO 128A9 and P. psychrosexualis CBS 128036.
Nomenclature: Although the new species produces cleistothecia, we decided to describe the taxon in Penicillium rather than Eupenicillium in accordance with the recommendations of Hawksworth (2010) on best-practice in such instances in a period when the rules of nomenclature that permit the dual naming of pleomorphic fungi are under revision.
Distribution and ecology: This species has been isolated from wood and apples (Elstar) stored in a cold-store in the Netherlands. The conditions in the cold-store were 1.5–2.0 °C in combination with an oxygen level of 1.0–1.5 %, a carbon dioxide level of 2.0 % and a relative humidity of 92–95 %. These conditions strongly inhibit the growth of most fungi; however, a low temperature and microaerophilic conditions do not prevent growth of members of the Roqueforti series (Samson et al. 2010).
DISCUSSION
The taxonomy of Penicillium series Roqueforti has been studied extensively in the past, mainly due to its role in cheese manufacture. These studies were based on phenotypic characters (Thom 1906, 1910, Raper & Thom 1949, Pitt 1980, Samson et al. 1977), extrolite patterns (Frisvad & Filtenborg 1989, Boysen et al. 1996, Samson & Frisvad 2004, Smedsgaard et al. 2004) and/or molecules (Boysen et al. 1996, Skouboe et al. 1999, Samson et al. 2004). This is the first study using a multigene approach to determine the relationship of species belonging to the Roqueforti series. All three studied loci are suitable for species recognition. Even the ITS regions, normally not recommended for species identification in Penicillium, have enough variation in this series (Skouboe et al. 1999, Houbraken et al. 2010, Samson et al. 2010). Incongruence was detected during the phylogenetic analysis of the calmodulin, β-tubulin and ITS loci. Penicillium psychrosexualis was, with high bootstrap support, basal to P. carneum and P. roqueforti in the ITS and calmodulin dataset, while P. roqueforti was basal to P. carneum and P. psychrosexualis in the β-tubulin dataset. The use of β-tubulin in taxonomy was debated by Peterson (2008) and he excluded this locus in his study due to his doubt about the homology of this locus between members of sections in Aspergillus. Furthermore, Hubka & Kolařík (2010) showed that the commonly used primers Bt2a and Bt2b could amplify the β-tubulin paralog tubC in Aspergilli. The interpretation of paralogous genes with non-homologous function in the same phylogenetic analysis posses a great risk and might create incongruence within and between datasets (Hubka & Kolařík 2010).
A limited number of penicillia are able to produce cleistothecia and ascospores, and these species were referred to the genus Eupenicillium in a number of studies. Only a limited number of penicillia known to reproduce sexually belong in subgenus Penicillium. Samson & Frisvad (2004) omitted these species in their monograph of this subgenus, and they recommended that a multigene study needs to be conducted to resolve the placement of these teleomorphic penicillia within the subgenus Penicillium. Peterson (2000) included various Eupenicillium species in his phylogenetic study of Penicillium, and showed that E. crustaceum, E. egyptiacum, E. baarnense, E. tularense, and Hemicarpenteles paradoxus belonged to Group 6. This group largely corresponds with the subgenus Penicillium as circumscribed by Samson & Frisvad (2004). Until now, only homothallic species are described in this subgenus; however, recent studies indicated that various species belonging to this subgenus are heterothallic. Hoff et al. (2008) showed that P. chrysogenum is heterothallic, and analysis of 12 P. chrysogenum isolates showed an equal mating type distribution, indicating the potential of this species to reproduce sexually. In addition, Eagle (2009) detected either MAT1-1-1 or MAT1-2-1 gene fragments in isolates of P. camemberti, P. roqueforti and P. verrucosum, also indicating heterothallism. Although various trials were undertaken to inducing mating in P. chrysogenum (Hoff et al. 2008, Eagle 2009, Houbraken unpubl. data) none of them have been successful. In addition, mating trials with P. roqueforti under conditions known to induce sex in Aspergillus fumigatus were unsuccessful and no cleistothecia were detected after 6 mo of incubation (Eagle 2009). Various growth factors induce formation of cleistothecia, such as temperature, light, nutrients and oxygen levels (Han et al. 2003). In this study, we show that P. psychrosexualis, a species related to P. roqueforti, produces cleistothecia abundantly at 9 °C. The production of a sexual stage at low temperatures might be more widespread in Penicillium, and mating experiments with P. roqueforti at this temperature might result in a sexual stage. Furthermore, P. psychrosexualis might be a good model species for comparison purposes in sex induction experiments or expression studies of genes required for sex in P. roqueforti. There are also indications of a sexual stage in P. roqueforti. Sclerotia were observed in cultures in P. roqueforti (Samson et al. 1977, Shimada & Ichinoe 1998) and it was postulated that similar structures have a dual function in the life-cycle in Aspergillus sect. Flavi. Survival of adverse conditions is one of them; the other is providing genetic variation in populations through sexual reproduction as a cleistothecium (McAlpin & Wicklow 2005, Horn et al. 2009). The possible discovery of the sexual stage in P. roqueforti could have consequences for the stability of starter cultures and might have advantages in strain improvement programs using conventional genetical approaches.
The effect of temperature on sexual reproduction in species belonging to the subgenus Penicillium is poorly studied. Many of these species are capable to grow at low temperatures and are therefore common spoilage organisms in refrigerators. McCulloch & Cain (1928) found an effect of the temperature on the formation of sclerotia of Penicillium gladioli. This species produces blue-green conidial structures abundantly when incubated at 14–15 °C, but produced comparatively a high number of sclerotia and only a few conidial structures, when incubated at 22 °C or higher. This observation is opposite to the results reported here, if the assumption is followed that sclerotia are immature cleistothecia. On the other hand, large white sclerotia are occasionally seen in P. italicum, a species related to P. psychrosexualis and also belonging to the subgenus Penicillium. These structures have been observed in cultures incubated in darkness at 0 °C for 3 mo (Raper & Thom 1949, Samson & Frisvad 2004), also suggesting the induction of a sexual cycle at low temperatures.
Members of series Roqueforti have a worldwide distribution, mainly related to human environments, and occur on various substrates. Penicillium roqueforti, P. paneum, and P. carneum occur on (preserved) food and silage, and only P. roqueforti has been frequently isolated as a saprobe in nature. Reports of the occurrence of P. carneum and P. paneum in nature are rare, and recently P. paneum has been found in stone tombs in Japan (An et al. 2009). Penicillium psychrosexualis is the second saprobic species in this series and has also been isolated from wood. Several reports are made on the occurrence of P. roqueforti on woods such as sawn wood (logs), wood stakes in soil, wood in sea, cut lumber, Quercus robur, and very wet wood in indoor environments (Picci 1966, Pitt 1980, Land et al. 1985, Kubátová 2000, Seifert & Frisvad 2000, Sumarah et al. 2005).
Acknowledgments
We thank Frank van de Geijn (Agrotechnology & Food Innovations BV, Wageningen, the Netherlands) for collecting the wood and apples samples. Dae-Hoo Kim is greatly acknowledged for the preparations of the SEM images of the ascospores, and we thank Uwe Braun for providing the Latin diagnosis.
REFERENCES
- An K-D, Kiyuna T, Kigawa R, Sano C, Miura S, Sygiyama J. (2009) The identity of Penicillium sp. 1, a major contaminant of the stone chambers in the Takamatsuzuka and Kitora Tumuli in Japan, is Penicillium paneum. Antonie van Leeuwenhoek 96: 579–592 [DOI] [PubMed] [Google Scholar]
- Boysen M, Skouboe P, Frisvad J, Rossen L. (1996) Reclassification of the Penicillium roqueforti group into three species on the basis of molecular genetic and biochemical profiles. Microbiology 142: 541–549 [DOI] [PubMed] [Google Scholar]
- Eagle CE. (2009) Mating-type genes and sexual potential in the Ascomycete genera Aspergillus and Penicillium. PhD thesis, University of Nottingham; [Google Scholar]
- Felsenstein J. (1985) Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783–791 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Filtenborg O. (1989) Terverticillate penicillia: chemotaxonomy and mycotoxin production. Mycologia 81: 837–861 [Google Scholar]
- Frisvad JC, Samson RA. (2004) Polyphasic taxonomy of Penicillium subgenus Penicillium: a guide to identification of food and air-borne terverticillate penicillia and their mycotoxins. Studies in Mycology 49: 1–173 [Google Scholar]
- Frisvad JC, Thrane U. (1987) Standardized High‐Performance Liquid Chromatography of 182 mycotoxins and other fungal metabolites based on alkylphenone indices and UV‐VIS spectra (diode‐array detection). Journal of Chromatography 404: 195–214 [DOI] [PubMed] [Google Scholar]
- Han K-H, Lee D-B, Kim J-H, Kim M-S, Han K-Y, Kim W-S, Park Y-S, Kim H-B, Han D-M. (2003) Environmental factors affecting development of Aspergillus nidulans. Journal of Microbiology 41: 34–40 [Google Scholar]
- Hawksworth DL. (2010) Naming Aspergillus species: progress towards one name for each species. Medical Mycology: DOI: 10.3109/13693786.2010.504753. [DOI] [PubMed] [Google Scholar]
- Hoff B, Pöggeler S, Kück U. (2008) Eighty years after its discovery, Fleming’s Penicillium strain discloses the secret of its sex. Eukaryotic Cell 7: 465–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn BW, Ramirez-Prado JH, Carbone I. (2009) The sexual state of Aspergillus parasiticus. Mycologia 101: 275–280 [DOI] [PubMed] [Google Scholar]
- Houbraken J, Due M, Varga J, Meijer M, Frisvad JC, Samson RA. (2007) Polyphasic taxonomy of Aspergillus section Usti. Studies in Mycology 59: 107–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J, Frisvad JC, Samson RA. (2010) Taxonomy of Penicillium citrinum and related species. Fungal Diversity 44: 117–133 [Google Scholar]
- Hubka V, Kolařík M. (2010). Beta-tubulin paralog tubC – risk of taxonomy? - In: The Biology of Fungi, 9th International Mycological Congress (IMC9), Edinburgh, UK: [Google Scholar]
- Karlshøj K, Larsen TO. (2005) Differentiation of species from the Penicillium roqueforti group by volatile metabolite profiling. Journal of Agricultural and Food Chemistry 53: 708–715 [DOI] [PubMed] [Google Scholar]
- Kubátová A. (2000) Neglected Penicillium spp. associated with declining trees. – In: Samson RA, Pitt JI. (eds), Integration of Modern Taxonomic Methods for Penicillium and Aspergillus Classification: 299–307 Amsterdam: Harwood Academic Publishers; [Google Scholar]
- Land CJ, Banhidi ZG, Albertson AC. (1985) Surface discoloring and blue stain in cold-tolerant filamentous fungi on outdoor softwood in Sweden. Material und Organismen 20: 133–156 [Google Scholar]
- Månsson M, Phipps RK, Gram L, Munro MHG, Larsen TO, Nielsen KF. (2010) Explorative solid-phase extraction (E-SPE) for accelerated microbial natural product discovery, dereplication, and purification. Journal of Natural Products 73: 1126–1132 [DOI] [PubMed] [Google Scholar]
- McAlpin CE, Wicklow DT. (2005) Culture media and sources of nitrogen promoting the formation of stromata and ascocarps in Petromyces alliaceus (Aspergillus section Flavi). Canadian Journal Microbiology 51: 765–771 [DOI] [PubMed] [Google Scholar]
- McCulloch L, Thom C. (1928) A corm rot of gladiolus caused by a Penicillium. Science 67: 216–217 [DOI] [PubMed] [Google Scholar]
- Nichol AW. (2000) Cheese/mould-ripened varieties. In: Robinson RK. (ed.), Encyclopedia of Food Microbiology: 387–393 San Diego: Academic Press; [Google Scholar]
- Nielsen KF, Sumarah MW, Frisvad JC, Miller JD. (2006) Production of metabolites from the Penicillium roqueforti complex. Journal of Agricultural and Food Chemistry 54: 3756–3763 [DOI] [PubMed] [Google Scholar]
- O’Brien M, Egan D, O’Kiely P, Forristal PD, Doohan FM, Fuller HT. (2008) Morphological and molecular characterisation of Penicillium roqueforti and P. paneum isolated from baled grass silage. Mycological Research 112: 921–932 [DOI] [PubMed] [Google Scholar]
- O’Brien M, Nielsen KF, O’Kiely P, Forristal PD, Fuller HT, Frisvad JC. (2006) Mycotoxins and other secondary metabolites produced in vitro by Penicillium paneum Frisvad and Penicillium roqueforti Thom isolated from baled grass silage in Ireland. Journal of Agricultural and Food Chemistry 54: 9268–9276 [DOI] [PubMed] [Google Scholar]
- Peterson SW. (2000) Phylogenetic analysis of Penicillium species based on ITS and LSU-rDNA nucleotide sequences. In: Samson RA, Pitt JI. (eds), Integration of modern taxonomic methods for Penicillium and Aspergillus classification: 163–178 Plenum Press, New York: [Google Scholar]
- Peterson SW. (2008) Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 100: 205–226 [DOI] [PubMed] [Google Scholar]
- Picci G. (1966) Sulla microflora presente nelle strutture in legno soggette all’azione dell’acqua de mare. La Ricera scientifica 36: 153–157 [Google Scholar]
- Pitt JI. (1980) [‘1979’] The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, London: [Google Scholar]
- Raper KB, Thom C. (1949) Manual of the Penicillia. Baltimore: Williams & Wilkins; [Google Scholar]
- Samson RA, Eckardt C, Orth R. (1977) The taxonomy of Penicillium species from fermented cheeses. Antonie van Leeuwenhoek 43: 341–350 [DOI] [PubMed] [Google Scholar]
- Samson RA, Frisvad JC. (2004) Penicillium subgenus Penicillium: new taxonomic schemes and mycotoxins and other extrolites. Studies in Mycology 49: 1–266 [Google Scholar]
- Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B. (2010) Food and Indoor Fungi. CBS Laboratory Manual Series 2. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands: [Google Scholar]
- Samson RA, Seifert KA, Kuijpers AFA, Houbraken JAMP, Frisvad JC. (2004) Phylogenetic analysis of Penicillium subgenus Penicillium using partial β-tubulin sequences. Studies in Mycology 49: 175–200 [Google Scholar]
- Seifert KA, Frisvad JC. (2000) Penicillium on solid wood products. In: Samson RA, Pitt JI. (eds), Integration of Modern Taxonomic Methods for Penicillium and Aspergillus Classification: 285–292 Amsterdam: Harwood Academic Publishers; [Google Scholar]
- Shimada T, Ichinoe M. (1998) Fungal species from imported and domestic mold-ripened cheese. Journal of the Food Hygienic Society of Japan 39: 350–356 [Google Scholar]
- Skouboe P, Frisvad JC, Taylor JW, Lauritsen D, Boysen M, Rossen L. (1999) Phylogenetic analysis of nucleotide sequences from the ITS region of terverticillate Penicillium species. Mycological Research 103: 873–881 [Google Scholar]
- Smedsgaard J. (1997) Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. Journal of Chromatography A 760: 264–270 [DOI] [PubMed] [Google Scholar]
- Smedsgaard J, Hansen ME, Frisvad JC. (2004) Classification of terverticillate Penicillia by electrospray mass spectrometric profiling. Studies in Mycology 49: 235–251 [Google Scholar]
- Sumarah MW, Miller JD, Blackwell BA. (2005) Isolation and metabolite production by Penicillium roqueforti, P. paneum and P. crustosum isolated in Canada. Mycopathologia 159: 571–577 [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Tamura K, Nei M. (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10: 512–526 [DOI] [PubMed] [Google Scholar]
- Thom C. (1906) Fungi in cheese ripening: Camembert and Roquefort. USDA Bureau of Animal Industry Bulletin 82: 1–39 [Google Scholar]
- Thom C. (1910) Cultural studies of species of Penicillium. USDA Bureau of Animal Industry Bulletin 118: 1–109 [Google Scholar]