Abstract
During May 2010, sporocarps of what appeared to be an Armillaria sp. were found in large clumps in historic Kirstenbosch Botanical Gardens on the foot of Table Mountain, Cape Town, South Africa. These sporocarps could be physically linked to the roots of unidentified dead trees and Protea spp. The aim of this study was to identify the Armillaria sp. found fruiting in Kirstenbosch. To achieve this goal isolates were made from the mycelium under the bark of dead roots linked to sporocarps. The ITS and IGS-1 regions were sequenced and compared to sequences of Armillaria spp. available on GenBank. Cladograms were generated using ITS sequences to determine the phylogenetic relationship of the isolates with other Armillaria spp. Sequence comparisons and phylogenetic analyses showed that the isolates represented A. mellea. They were also identical to isolates of this species previously discovered in the Company Gardens in South Africa and introduced from Europe apparently by the early Dutch Settlers. Armillaria mellea is alien and apparently invasive in Cape Town, fruits profusely and has the potential to spread to sensitive native forests on the foothills of the City.
Keywords: Armillaria mellea, Armillaria root rot, fungal introduction, Proteaceae
INTRODUCTION
Species of Armillaria are some of the most important pathogens of woody plants in the world. These fungi have been known as tree pathogens since their first discovery by Danish botanist Martin Vahl. While the taxonomy of these Armillaria spp. has been controversial and widely debated over an extended period of time, application of the biological species concept (Korhonen 1978, Anderson & Ullrich 1979, Ota et al. 1998, Qin et al. 2007) and more recently DNA sequence comparisons (Coetzee et al. 2000a, 2003a, 2005, Gezahgne et al. 2004, Keča et al. 2006, Mwenje et al. 2006, Hasegawa et al. 2010) have resolved many problems relating to the delineation of species. At least 40 species are now recognised in Armillaria (Volk & Burdsall 1995, Lima et al. 2008, Pildain et al. 2010) and it is likely that other species will emerge from under-sampled areas in the future.
Armillaria root rot, the disease caused by pathogenic Armillaria spp. can result in serious losses to productivity in tree plantations, fruit tree orchards and in gardens (Gregory et al. 1991, Hood et al. 1991). In native forests, Armillaria spp. cause disease but this is most typically a natural process (Kile et al. 1991). Interestingly, species of these fungi exist as clones covering huge areas of land and in these situations they are considered to be amongst the largest and oldest living organisms (Gould 1992, Smith et al. 1992).
Only a single native Armillaria sp. occurs in South Africa (Coetzee et al. 2000a). This fungus, A. fuscipes is occasionally found on native trees (Kotzé 1935, referred to as A. mellea). In contrast, it can be a serious pathogen in plantations of non-native Pinus spp. and on fruit trees planted in moist areas that have been cleared of native forest (Lundquist 1986, 1987, Coetzee et al. 2000a). A more intriguing Armillaria sp. in South Africa is A. mellea that was discovered in the Company (Dutch East India Company) Gardens in the centre of Cape Town (Coetzee et al. 2001). The fungus in that situation represents a single genetic entity that was shown to be at least 358 years old. It was most likely introduced into the city when gardens were established to provide sailors travelling to the East with fresh produce (Coetzee et al. 2001).
Some years after the discovery of the A. mellea clone in Cape Town, Armillaria root rot was found killing Protea plants in the historic Kirstenbosch Botanical Gardens (<sanbi.org>) on the foothills of South Africa’s iconic Table Mountain (Coetzee et al. 2003b). The fungus in that situation was never seen fruiting but isolates were identified as those of A. gallica, and it was suggested that the fungus was introduced into the gardens with plants brought from Asia (Coetzee et al. 2003b). A few of the isolates collected on the Protea plants were also thought to represent A. mellea, but the identification was tentative and based only on RFLP comparisons, without comparison of DNA sequence data against other Armillaria spp.
During May 2010, sporocarps of what appeared to be an Armillaria sp. were found in large clumps in the upper corner of Kirstenbosch Botanical Gardens and close to Rycroft’s Gate. These sporocarps could be physically linked to the roots of unidentified dead trees and Protea spp. (Fig. 1B, C). Upon removal of the bark from the dead roots, sheets of white mycelium typical of Armillaria root rot were found. The aim of this study was to identify the Armillaria sp. found fruiting in Kirstenbosch using data that were not available at the time of the discovery of Armillaria root rot in Cape Town (Coetzee et al. 2003b).
Fig. 1.
Armillaria root rot in Kirstenbosch Botanical Gardens. A. Native woody shrubs and forest deeper in the valleys common on the Cape Peninsula. B, C. Clusters of fruiting bodies found on a stump. D–G. Robust fruiting bodies of Armillaria sp. showing a yellow cap, prominent annulus and stipe tapering down to the base. H. Rhizomorphs produced in culture.
MATERIALS AND METHODS
Isolates
Isolation and purification of isolates followed the methods outlined in Coetzee et al. (2003). Cultures were maintained on malt extract yeast agar (MYA) (15 g/L malt extract, 2 g/L yeast extract, 15 g/L agar). Cultures are stored in the culture collection (CMW) of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria and with the Centraalbureau voor Schimmelcultures (CBS), Utrecht, Netherlands.
Molecular methods
Isolates for DNA extractions were grown at 25 °C in the dark for 3 wk in conical flasks containing liquid malt extract yeast (MY). Mycelium was harvested using a tea strainer, freeze-dried and lyophilised. DNA extractions followed the protocol of Coetzee et al. (2000b). A NanoDrop spectrophotometer (Thermo Fisher Scientific, USA) was used to quantify the DNA. The IGS-1 and ITS regions of the rDNA operon were amplified using primer pairs P-1 / O-1 and ITS-1 / ITS-4, respectively. PCR reaction mixture and conditions were the same as those published by Coetzee et al. (2000b), except that FastStart Tag DNA polymerase was used instead of an Expand High Fidelity PCR System. The PCR products were purified prior to sequencing using a MSB® Spin PCRapace kit (Invitek, Germany). DNA sequences for the IGS-1 and ITS-1 regions were obtained in both directions using the same primers employed for their amplification. The sequence reactions were carried out using an ABI PRISM Dye Terminator Cycle Sequencing Ready kit with AmpliTaq DNA Polymerases FS (Applied Biosystems) following the manufacturer’s instructions. Chromatographs were analysed and contigs assembled in CLC Main Workbench v. 5.7 (CLC bio, Denmark).
DNA sequence comparisons and phylogenetic analyses
DNA sequences were compared against those available in the NCBI GenBank database using a BlastN search. IGS-1 and ITS sequences generated in this study were aligned against those of isolates CMW 3975 and CMW 3978 available on GenBank and originating from the Company Gardens in Cape Town. This was done to determine nucleotide variation between the isolates from the Company Gardens and those from Kirstenbosch Botanical Gardens.
Phylogenetic analysis was conducted with a sub-set of the ITS-1 dataset generated by Coetzee et al. (2003). The dataset was amended with DNA sequences for A. fuscipes from South Africa and A. mellea from Europe, Asia, western USA, eastern USA and the Company Gardens, South Africa. Sequences were re-aligned using MAFFT v. 6 (Katoh & Toh 2008). Cladograms were generated using a heuristic tree search algorithm in PAUP v. 4 with branch swapping set to TBR and random addition of sequences (10 replicates). Trees were rooted to A. fuscipes. Bootstrap analysis (1000 replicates) was done to gain support for the grouping of taxa using the same settings as above but with addition of sequences set to nearest.
RESULTS
Isolates
The macro-morphology of basidiocarps produced by the fungus was similar to that described for A. mellea (Watling et al. 1982) (Fig. 1D–G). The cap colour of the basidiocarps was distinctly yellow and they had thick annuli and stipes tapering towards the base. The basidiocarps also had a caespitose growth habit typical of A. mellea.
Two isolates (CMW 36264 and CMW 36265) were retrieved from infected roots and these produced rhizomorphs typical of Armillaria spp. in culture (Fig. 1H). The rhizomorphs displayed a dichotomous growth habit and were produced in abundance. White aerial mycelium was observed on the surface of the rhizomorphs at areas that had grown out of the medium.
DNA sequence comparisons and phylogenetic analysis
The IGS-1 and ITS DNA sequences of isolates from Kirstenbosch were most similar to sequences of A. mellea in GenBank. Comparisons of IGS-1 and ITS sequences revealed the absence of nucleotide variation between isolates from Kirstenbosch and A. mellea from the Company Gardens.
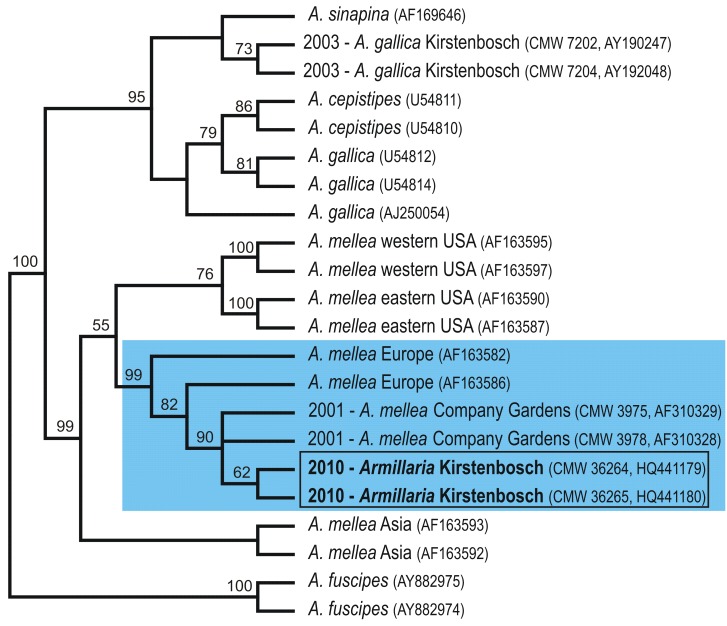
The ITS dataset included 925 characters of which 183 characters were parsimoniously informative. A heuristic search generated 6 trees with tree lengths of 250 steps (Fig. 2). The consistency index was 0.864, and retention index 0.941. The isolates from Kirstenbosch formed a monophyletic group with A. mellea from the Company Gardens with strong bootstrap support and together these were placed in a clade that included sequences of A. mellea from Europe (99 % bootstrap support).
Fig. 2.
Cladogram generated from ITS DNA sequence data. Bootstrap values are indicated above the tree branches. The year during which isolates from Kirstenbosch and the Company Gardens were reported are indicated next to the taxon name. Isolates obtained during this study are shown in the rectangle.
DISCUSSION
Sporocarps linked to infected roots from which cultures were made in this study were morphologically similar to those of A. mellea previously found in the Cape Town city centre. DNA sequence comparisons also showed that the cultures were those of A. mellea and the sequences were identical to those from the Company Gardens. Although vegetative compatibility tests were not done to test whether these represent the same clone as those in the City Centre, there was no IGS-1 or ITS nucleotide variation between isolates from the two locations and they most likely are the same.
There are three possible means of introduction of A. mellea into Kirstenbosch Botanical Gardens, via air-dispersed basidiospores, on infected plant material or on infested wood mulch. Armillaria mellea in the Company Gardens fruits profusely every year at the onset of the first rains in autumn. Although the fungus clone is entirely surrounded by roads and buildings, the basidospore cloud is likely to easily spread within the city and at least up the foothills of Table Mountain, on which Kirstenbosch is situated. While A. mellea might have been introduced into Kirstenbosch separately to that of the clone found in the Company Gardens and as A. gallica must have been, it would perhaps more easily have spread to this nearby location via basidiospores. One further possible route of introduction to consider relates to the cultivation practices used in the garden. Flowerbeds and paths are frequently covered with wood and bark mulch. As Armillaria spp. are common wood rotting fungi, it is possible that A. mellea was introduced into the Gardens through this substrate.
Armillaria sporocarps have not previously been found in Kirstenbosch. This may simply be related to the fact that they are ephemeral and have not been present when mycologists or plant pathologists might have been visiting the botanical garden. When these sporocarps were discovered, they were relatively widespread and all were morphologically similar. The infected roots from which isolates were made were also from a number of locations, none of which had been associated with the infection by A. gallica. It is possible that A. gallica also fruits in the garden, but at a different time to A. mellea, or it is less prone to fruiting. Regular observations will be needed to resolve this question.
Peripheral surveys of the native forest on the foothills of Table Mountain and that extending out of the Kirstenbosch Botanical Gardens have not revealed evidence of Armillaria root rot. The fact that A. mellea is able to fruit profusely in the gardens suggests that it may spread to native forests in the vicinity (Fig. 1A) and more careful surveys should be undertaken to determine whether this is already occurring. Certainly this invasive alien fungus has the capacity to result in serious disease problems in the native environment as has been true with the introduced invasive Phytophthora cinnamomi on Leucodendron argenteum (Silver Trees) in Kirstenbosch (van Wyk 1973, Linde et al. 1997). This potential risk to Kirstenbosch and the native forest associated with it deserve consideration.
The Dutchman Jan Van Riebeeck was the founder and first commander of Cape Town between 1652 and 1662. One of his tasks was to establish a vegetable and fruit garden to provide ships of the Dutch East India Company sailing between The Netherlands and East Asia with fresh produce and to offset serious problems due to vitamin C deficiency [for a fascinating account of the ship’s surgeons of the Dutch East India Company see Bruijn (2009)]. This is the origin of the Company Gardens and the historic avenue of oak (Quercus robur) trees that line Government Avenue, the death of which prompted the discovery of A. mellea in Cape Town (Coetzee et al. 2001). At the time of this discovery, popular press took an interest in the problem (<fabinet.up.ac.za/tpcp/news>) and referred to the tragic death of historic trees as “Van Riebeeck’s curse”. The appearance of A. mellea fruiting profusely in Kirstenbosch, another historic garden of great national importance, suggests that the fungal “Van Riebeeck’s curse” remains not only present but is growing in importance. It further illustrates the devastating impact that invasive alien pathogens can have on natural woody ecosystems many years after their introduction.
Acknowledgments
We thank the Department of Science and Technology (DST)/ National Research Foundation (NRF) Centre of Excellence in Tree Health Biotechnology (CTHB) for funding that made this study possible.
REFERENCES
- Anderson JB, Ullrich RC. (1979) Biological species of Armillaria mellea in North America. Mycologia 71: 402–414 [Google Scholar]
- Bruijn I. (2009) Ship’s surgeons of the Dutch East India Company. Leiden University Press, The Netherlands: [Google Scholar]
- Coetzee MPA, Wingfield BD, Coutinho TA, Wingfield MJ. (2000a) Identification of the causal agent of Armillaria root rot of Pinus species in South Africa. Mycologia 92: 777–785 [Google Scholar]
- Coetzee MPA, Wingfield BD, Harrington TC, Dalevi D, Coutinho TA, Wingfield MJ. (2000b) Geographical diversity of Armillaria mellea s. s. based on phylogenetic analysis. Mycologia 92: 105–113 [Google Scholar]
- Coetzee MPA, Wingfield BD, Harrington TC, Steimel J, Coutinho TA, Wingfield MJ. (2001) The root rot fungus Armillaria mellea introduced into South Africa by early Dutch settlers. Molecular Ecology 10: 387–396 [DOI] [PubMed] [Google Scholar]
- Coetzee MPA, Wingfield BD, Bloomer P, Ridley GS, Wingfield MJ. (2003a) Molecular identification and phylogeny of Armillaria isolates from South America and Indo-Malaysia. Mycologia 95: 285–293 [PubMed] [Google Scholar]
- Coetzee MPA, Wingfield BD, Roux J, Crous PW, Denman S, Wingfield MJ. (2003b) Discovery of two northern hemisphere Armillaria species on Proteaceae in South Africa. Plant Pathology 52: 604–612 [Google Scholar]
- Coetzee MPA, Wingfield BD, Kirisits T, Chhetri DB, Bloomer P, Wingfield MJ. (2005) Identification of Armillaria isolates from Bhutan based on DNA sequence comparisons. Plant Pathology 54: 36–45 [Google Scholar]
- Gezahgne A, Coetzee MPA, Wingfield BD, Wingfield MJ, Roux J. (2004) Identification of the Armillaria root rot pathogen in Ethiopian plantations. Forest Pathology 34: 133–145 [Google Scholar]
- Gould SJ. (1992) A humongous fungus among us. Natural History 7: 10–16 [Google Scholar]
- Gregory SC, Rishbeth J, Shaw CG. (1991) Pathogenicity and virulence. In: Armillaria Root Disease. (Shaw CG, Kile GA, eds): 76–87 Forest Service United States, Department of Agriculture, USA: [Google Scholar]
- Hasegawa E, Ota Y, Hattori T, Kikuchi T. (2010) Sequence-based identification of Japanese Armillaria species using the elongation factor-1 alpha gene. Mycologia 102: 898–910 [DOI] [PubMed] [Google Scholar]
- Hood IA, Redfern DB, Kile GA. (1991) Armillaria in planted hosts. In: Armillaria Root Disease. (Shaw CG, Kile GA, eds): 122–149 Forest Service United States, Department of Agriculture, USA: [Google Scholar]
- Katoh K, Toh H. (2008) Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics 9: 286–298 [DOI] [PubMed] [Google Scholar]
- Keča N, Bodles WJA, Woodward S, Karadzic D, Bojovic S. (2006) Molecular-based identification and phylogeny of Armillaria species from Serbia and Montenegro. Forest Pathology 36: 41–57 [Google Scholar]
- Kile GA, McDonald GI, Byler JW. (1991) Ecology and disease in natural forests. In: Armillaria Root Disease. (Shaw CG, Kile GA, eds): 102–121 Forest Service United States, Department of Agriculture, USA: [Google Scholar]
- Korhonen K. (1978) Interfertility and clonal size in the Armillariella mellea complex. Karstenia 18: 31–42 [Google Scholar]
- Kotzé JJ. (1935) Forest fungi: The position in South Africa. In: Papers and statements on exotics. 4th British Empire Forestry Conference: 12. South Africa. [Google Scholar]
- Lima MLA, Asai T, Capelari M. (2008) Armillaria paulensis: a new South American species. Mycological Research 112: 1122–1128 [DOI] [PubMed] [Google Scholar]
- Linde C, Drenth A, Wingfield MJ, Broembsen SL von. (1997) Population structure of Phytophthora cinnamomi in South Africa. Phytopathology 87: 822–827 [DOI] [PubMed] [Google Scholar]
- Lundquist JE. (1986) Fungi associated with Pinus in South Africa. Part I. The Transvaal. South African Forestry Journal 138: 1–14 [Google Scholar]
- Lundquist JE. (1987) Fungi associated with Pinus in South Africa, Part III, Natal, the Orange Free State and the Republic of Transkei. South African Forestry Journal 143: 11–19 [Google Scholar]
- Mwenje E, Wingfield BD, Coetzee MPA, Nemato H, Wingfield MJ. (2006) Armillaria species on tea in Kenya identified using isozyme and DNA sequence comparisons. Plant Pathology 55: 343–350 [Google Scholar]
- Ota Y, Matsushita N, Nagasawa E, Terashita T, Fukuda K, Suzuki K. (1998) Biological species of Armillaria in Japan. Plant Disease 82: 537–543 [DOI] [PubMed] [Google Scholar]
- Pildain MB, Coetzee MPA, Wingfield BD, Wingfield MJ, Rajchenberg M. (2010) Taxonomy of Armillaria in the Patagonian forests of Argentina. Mycologia 102: 392–403 [DOI] [PubMed] [Google Scholar]
- Qin GF, Zhao J, Korhonen K. (2007) A study on intersterility groups of Armillaria in China. Mycologia 99: 430–441 [DOI] [PubMed] [Google Scholar]
- Smith ML, Bruhn JN, Anderson JB. (1992) The fungus Armillaria bulbosa is among the largest and oldest living organisms. Nature 356: 428–431 [Google Scholar]
- Volk TJ, Burdsall HH. (1995) A nomenclatural study of Armillaria and Armillariella species (Basidiomycotina, Tricholomataceae). Fungiflora, Norway: [Google Scholar]
- Watling R, Kile GA, Gregory NM. (1982) The genus Armillaria - nomenclature, typification, the identity of Armillaria mellea and species differentiation. Transactions of the British Mycological Society 78: 271–285 [Google Scholar]
- Wyk PS van. (1973) Root and crown rot of silver trees. South African Journal of Botany 39: 255–260 [Google Scholar]