Abstract
The mid-winter development of refractoriness to melatonin (Mel) triggers recrudescence of the atrophied reproductive apparatus of rodents. As a consequence, over-wintering animals become reproductively competent just before the onset of spring conditions favorable for breeding. The neural target tissues that cease to respond to winter Mel signals have not been identified. We now report that the suprachiasmatic nucleus of the hypothalamus, which contains the principal circadian clock, and the reuniens and paraventricular nuclei of the thalamus, each independently becomes refractory to melatonin. Small implants of Mel that were left in place for 40 wk and that act locally on these brain nuclei, induced testicular regression within 6 wk in male Siberian hamsters; 12 wk later Mel implants no longer suppressed reproduction and gonadal recrudescence ensued. Hamsters that were then given a systemic Mel infusion s.c. immediately initiated a second gonadal regression, implying that neurons at each site become refractory to Mel without compromising responsiveness of other Mel target tissues. Refractoriness occurs locally and independently at each neural target tissue, rather than in a separate “refractoriness” substrate. Restricted, target-specific actions of Mel are consistent with the independent regulation by day length of the several behavioral and physiological traits that vary seasonally in mammals.
Most temperate-zone mammals synchronize reproductive effort with the external environment such that young are born during the spring and summer months when conditions are most favorable for the survival of offspring (1). Exposure to short or decreasing day lengths and long durations of nocturnal melatonin (Mel) secretion (also referred to as Mel “signals”) in late summer initiate the transition to the winter phenotype, culminating in suppressed reproduction, molting to a winter pelage, and a loss of body mass in Siberian hamsters (for review see ref. 2). After ≈18–24 wk, despite continued maintenance in short day lengths, hamsters spontaneously revert to the long day phenotype (3–6). Thereafter, exposure to short day lengths or long Mel signals is ineffective in eliciting short day responses (4, 7, 8); this defines the refractory state. To regain responsiveness to short day lengths (breaking of refractoriness), hamsters must be exposed for ≥11 wk to long day lengths or short Mel signals (9).
The suprachiasmatic nucleus (SCN) of the hypothalamus, the nucleus reuniens (NRE), the paraventricular nucleus (PVT) of the thalamus, as well as the pars tuberalis of the pituitary gland and several other brain regions contain high affinity binding sites for Mel (10–12). Microdialysis of Mel for 10 h/day in the SCN, PVT, or NRE prevents gonadal maturation in photo-inhibited juvenile male Siberian hamsters housed in constant light (13). Constant release implants or timed injections of Mel in the vicinity of the SCN also cause gonadal inhibition in female white-footed mice (14, 15). Because the SCN responds to lower concentrations of Mel (7.5 and 20 pg/infusion) than the PVT or NRE (75 pg/infusion), one can infer that this tissue is particularly sensitive to the hormone (13). Infusions of low doses of Mel into the SCN, but not NRE or PVT, also resulted in decreased serum concentrations of prolactin (13). Furthermore, ablation of the SCN, but not the NRE or PVT, eliminated the ability of Mel infusions or short day lengths to inhibit reproduction in Siberian hamsters (16, 17). Destruction of the NRE, but not the PVT, influenced the photoperiodic body mass response (17). Thus, the SCN may be necessary and sufficient for gonadal responsiveness to Mel whereas the NRE and PVT are not necessary but may be sufficient to mediate effects of day length on reproduction.
Several components of the system by which ambient day length and Mel control seasonal adaptations are well specified. The major exception is the neuroendocrine basis for the development of refractoriness to day length, which remains largely unknown, despite its universal presence in photoperiodic mammals. In sheep, refractoriness occurs at the level of the pituitary gland, at least for photoperiodic control of prolactin secretion (18). In Syrian hamsters, the firing rates of SCN neurons in response to Mel treatment were lower in males refractory to short day lengths than in photoresponsive animals (19).
We consider four ways in which refractoriness to Mel may develop in the central nervous system: (i) Each neural target tissue initially responsive to Mel may with continued exposure independently become refractory to the hormone without affecting responsiveness at other Mel-binding sites. (ii) Development of refractoriness at one critical Mel target tissue may render the entire system unresponsive to Mel perhaps because this substrate induces loss of responsiveness to the hormone in all other Mel-binding sites; alternatively, the critical neurons could be part of the final common pathway by which Mel influences the several effector systems that control secretion of gonadotropins and prolactin. (iii) Refractoriness may require action of Mel at multiple brain sites, several of which must necessarily be engaged for the system to lose its ability to respond to previously effective Mel signals. (iv) Refractoriness in one target tissue may induce refractoriness in a subset of other Mel target tissues. It remains possible that refractoriness is mediated by Mel target tissues distinct from those responsible for initial responses to the hormone.
To discriminate among these hypotheses, hamsters were pinealectomized to remove the endogenous source of circulating Mel and were implanted with a Mel-containing cannula in one of three nuclei: the SCN, NRE, or PVT. We established that localized exposure to Mel at each site induced gonadal regression and then determined whether spontaneous recrudescence eventually occurred; the latter is indicative of refractoriness to Mel. We then administered daily Mel infusions systemically for 6 wk so as to provide all target tissues with long duration, inhibitory Mel signals (20). In this way, we assessed whether refractoriness is limited to those tissues directly exposed to Mel. If Mel actions localized to one brain site render the entire system refractory to the hormone, then animals previously implanted with neural Mel implants will remain unresponsive to the s.c. infusion. Alternatively, if refractoriness at one neural site spares responsiveness to the hormone at one or more separate Mel target tissues, then animals should undergo gonadal regression in response to the s.c. Mel infusion.
Methods
Animals.
Male Siberian hamsters (Phodopus sungorus) from our breeding colony were maintained on a 16 h light:8 h dark (16L) photoperiod at 22 ± 1°C. The daily dark phase began at 1800 h, Pacific Standard Time. Hamsters had ad libitum access to food (mouse chow No. 5015, Purina) and tap water.
Surgery and Cannulation.
At 2–3 mo of age (week 0) under ketamine mixture anesthesia (21.0 mg of ketamine/2.4 mg of xyalzine/0.3 mg of acepromazine/ml; 0.34 ml/100 g of body mass), hamsters were pinealectomized as described by Carter and Goldman (21). At the same time, stainless steel guide cannulas (Plastics One, Roanoke, VA) were stereotaxically implanted into one of three neural sites: the left SCN (n = 19) (with head level; 0.1 mm anterior to bregma; +0.3 mm from midline; the guide cannula was lowered 6.1 mm below dura), the NRE (n = 14) (with incisor bar set at + 0.1 mm; 0.1 mm anterior to bregma; 0.0 from midline and lowered 3.1 mm below the dura), or the PVT (n = 14) (as for NRE but lowered 4.5 mm below the dura). The guide cannula consisted of a threaded cylindrical plastic pedestal molded around a piece of stainless steel (22 gauge) hypodermic tubing that extends below the pedestal. This guide cannula was cemented in place with dental acrylic and anchored to the skull with three stainless steel screws. The inner cannula (28 gauge) locked onto the threaded plastic top of the guide cannula and extended 0.5 mm from the tip of the guide cannula into the brain tissue. Cannulas contained either Mel mixed with beeswax (5-methoxytryptamine; Sigma, 1:4 ratio of Mel:beeswax; cf. ref. 14) or beeswax alone. The internal cannulas were removed beginning on week 4 and every other week thereafter and repacked with fresh hormone or beeswax before being reinserted into the animal.
Mel Implants.
Mel for implants was prepared by kneading 50 mg of finely ground Mel powder uniformly into 200 mg of beeswax. The internal cannulas were then tamped into this slab several times to form a pellet inside the tubing. The external surface of the tubing was cleaned with 70% ethanol before insertion into the brain.
Testis Measurements.
At week 0, in animals lightly anesthetized with methoxyflurane vapors (Metofane, Pitman–Moore, Washington Crossing, NJ), the length and width of the left testis (±0.01 mm) were measured with analog calipers. The product (testis width2) × (testis length) yields an estimated testis volume (ETV), which is highly correlated (R > 0.95) with paired testis weight (4). ETV and body mass were recorded every other week for 40 consecutive weeks. Testicular regression was considered to have occurred when ETV was reduced by >30% compared to the week 0 value and sustained for at least two consecutive measures. The onset of gonadal recrudescence was defined by the first of four consecutive measurements during which ETV increased. Brain Mel implants were removed at week 40 and replaced with beeswax-filled cannulas. s.c. polyethylene infusion catheters were implanted as described previously (21), and hamsters were provided with timed daily 12-h infusions of Mel (100 ng/infusion) for 6 consecutive weeks. ETV, paired testis weights, and body mass were obtained at the end of treatment.
An additional control group consisted of hamsters that were pinealectomized and implanted s.c. at week 0 with Silastic capsules (Dow-Corning, i.d. 1.47 mm; o.d. 1.96 mm, length 15 mm) filled with crystalline Mel to a length of 10 mm (n = 9) or left empty (n = 7). ETV and body mass were obtained every 4 wk for this group, at which time the implants also were replaced. Testis regression and recrudescence were defined as above. At week 40 the implants were removed, hamsters fitted with polyethylene catheters, and infused as above.
At the conclusion of the infusions, the animals were deeply anesthetized (pentobarbital sodium 50 mg/ml, 0.3 ml/animal). Testes were removed and weighed and the hamsters perfused transcardially with 10% formalin. Brains were postfixed for 2–7 days before cryoprotection with sucrose- (10%) buffered fixative for ≈2–4 wk. Frozen coronal sections (40 μm) were cut and mounted on slides for histological verification of the implant location. The sections were stained with Cresyl violet-Nissl stain and observed under a light microscope. All procedures were approved by the Animal Care and Use Committee of the University of California at Berkeley.
Statistics.
A two-factor repeated measures ANOVA was used to analyze ETVs over the 46 wk; posthoc comparisons were done by Fisher's Protected Least Significant Difference test where appropriate (statview 5; SAS Institute, Cary, NC). A one-way ANOVA was used to analyze the timing of the onset of testicular recrudescence and the final testis response (ETV and paired testes weight) to the s.c. infusion. χ2 and Fisher's Exact probability tests were used where appropriate to compare proportions of animals that were refractory in the several groups. Differences were considered significant if P < 0.05. Animals bearing intracranial beeswax implants and empty s.c. implants did not differ on any dependent measure at week 46 and thus were combined to form a single control group (control) for purposes of statistical analyses of week 46 measures (ANOVA; P > 0.9).
Results
Localization of Brain Implants.
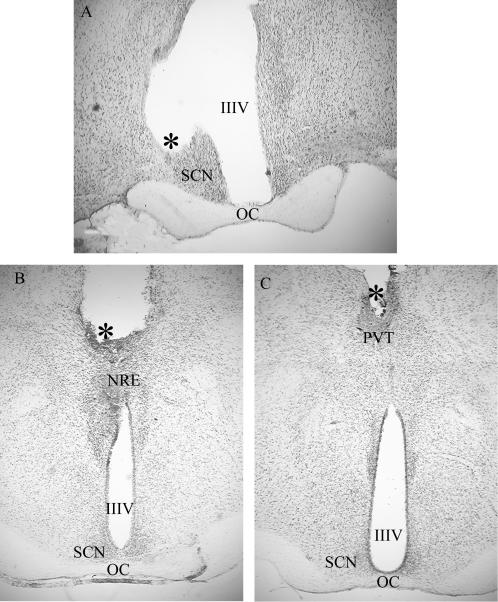
Representative cannula placements are illustrated in Fig. 1. Six animals were classified as having misplaced Mel cannulas based on either a distance of ≥500 μm of the cannula tip from the target site or cannula placement in a ventricle. Of these six “misses” two were in the third ventricle and one each in the fourth ventricle, optic chiasm, lateral hypothalamus, and the central medial thalamic nucleus posterior to the NRE. The six animals with misplaced cannulas constitute a control group for spread of Mel from the implant sites. The distance of spread observed from similar sized implants in previous experiments was 0.2 mm (14). The resulting number of animals per group were as follows; SCN: n = 13 Mel, three misplaced Mel and three beeswax implants; NRE: n = 10 Mel, one misplaced Mel, and three beeswax; PVT: n = 8 Mel, two misplaced Mel, and four beeswax. None of the beeswax implants qualified as misplaced (i.e., they were all <0.5 mm from the target site). The animals implanted with beeswax at different sites were combined to form a single group (n = 10) for purposes of statistical analyses from week 0 to week 40 because no differences were observed among these animals on any measure (P > 0.9).
Figure 1.
Photomicrographs of representative cannula placements for each of the three target sites. SCN (A), NRE (B), and PVT (C). IIIV, third ventricle; *, tip of cannula; OC, optic chiasm.
Testicular Response to Implants.
A two-factor repeated measures ANOVA indicated a significant treatment effect (Mel vs. beeswax; P ≤ 0.005) but no significant effect of implant site (P > 0.90) and no significant interaction of treatment by site (P > 0.60) on ETV. Repeated measures ANOVA indicated significant changes in testis size over time (P ≤ 0.001) and a significant interaction between treatment and time (P ≤ 0.001). Thus, ETV varied significantly over time depending on the presence of Mel; hormone-treated animals exhibited significant testicular regression whereas hamsters treated with beeswax did not. Implants at all three sites tested (NRE, PVT, and SCN) were equally effective in inducing gonadal regression (Fig. 2).
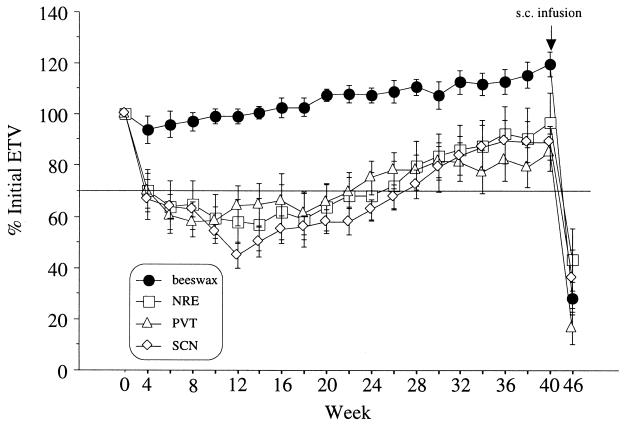
Figure 2.
Percentage change (± SEM) in estimated testis volume over the 46-wk experiment for hamsters that had been treated with Mel- or beeswax-containing cannulas in the NRE, PVT, or SCN. All Mel-implanted groups exhibited at least a 30% reduction in ETV after week 6 and differed significantly from controls from week 4 to week 40 (P ≤ 0.001). Between week 22 and 28, testes of all Mel-implanted groups had increased in volume well above the minimum values attained ≈10 wk earlier. At week 40, all brain Mel implants were removed and replaced with beeswax implants. The arrow at week 40 depicts the onset of the s.c. Mel infusion. At week 46, after 6 wk of s.c. Mel infusions, all groups had similarly regressed gonads (P > 0.5).
The majority of animals implanted with Mel in each site underwent gonadal regression by week 6 (SCN, 9/13; NRE, 8/10; PVT, 7/8) (Fig. 2), whereas none of the 10 hamsters treated with the beeswax vehicle, nor any of those with misplaced cannulas did so. The latter two groups did not differ significantly over the 46-wk experiment (P > 0.90; Fig. 3). The proportion of animals undergoing regression did not differ significantly among animals implanted with Mel in the three neural sites (P > 0.80; df = 2) nor did these groups differ when compared to the hamsters with s.c. Mel implants (5/9; P > 0.70; df = 3). Timing of the onset of gonadal recrudescence did not differ among groups (P > 0.10). The onset of spontaneous testicular recrudescence occurred after 18.9 wk in the SCN-implanted hamsters and began on weeks 15 and 16.6 in the NRE- and PVT-implanted groups, respectively. Hamsters bearing constant release s.c. implants initiated testicular regression by week 4 and recrudescence at week 14.4. Neither the magnitude of decreases in ETV nor the timing of recrudescence differed among the several groups (P > 0.5).
Figure 3.
Percentage change (± SEM) in ETV in hamsters with Mel-containing cannula that deviated from designated target sites by ≥0.5 mm (misplaced; n = 6) and in control hamsters treated with beeswax (n = 10). Groups were not significantly different (P > 0.9).
Testicular Response to s.c. Mel Infusions.
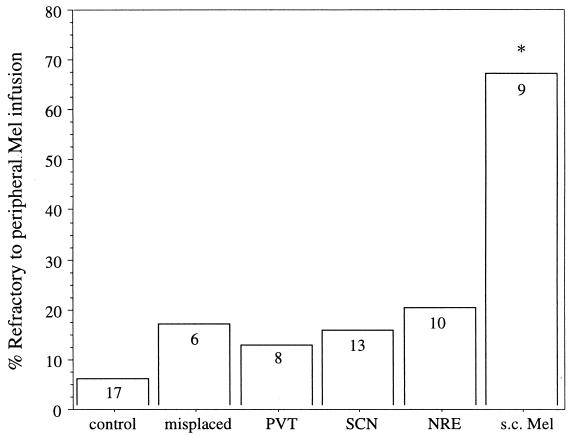
After infusions with Mel that began at week 40, a significant majority of hamsters (83.9%) previously bearing neural Mel implants were responsive to the s.c. infusions (i.e., a reduction in ETV ≥30%), whereas most of the hamsters previously treated with s.c. Mel implants were unresponsive (67%), i.e., refractory; (P ≤ 0.03; Fig. 4). There were no significant differences among the three brain-implanted groups in the proportion that were refractory (P > 0.90; Fig. 4). A one-way ANOVA on the percentage of reduction in ETV in response to the s.c. Mel infusion revealed a significant effect of previous treatment (P ≤ 0.004); hamsters previously treated with s.c Mel implants exhibited a significantly smaller reduction (30.7 ± 11.5%) in ETV than all other groups [P ≤ 0.02 vs. NRE (59.7 ± 9.4%); P ≤ 0.001 vs. PVT (75.2 ± 8.4%); P ≤ 0.008 vs. SCN (62.3 ± 7.8%); P ≤ 0.001 vs. control (77.1 ± 4.2%)]. Animals with neural Mel implants did not, however, differ from each other or from controls in the degree of gonadal regression (P > 0.2). Among animals who did respond to the s.c. Mel-infusion, the degree of gonadal regression did not differ between groups (mean paired testes weight = 211.4 ± 25.0 mg, P > 0.50). The only hamster in the PVT group that was unresponsive to the s.c. infusion also had failed to respond to the initial Mel implant. Only one of the three s.c. Mel-implanted hamsters that was not refractory to the s.c. Mel infusion had undergone testicular regression in response to the initial implant.
Figure 4.
Percentage of each group that was refractory to the s.c. Mel infusions. Sample size is indicated within each bar. Control group combines animals that received beeswax brain implants and those implanted with an empty s.c. capsule. *, Significantly different from all other groups (P ≤ 0.03).
Body Mass and Pelage Responses to Mel Treatments.
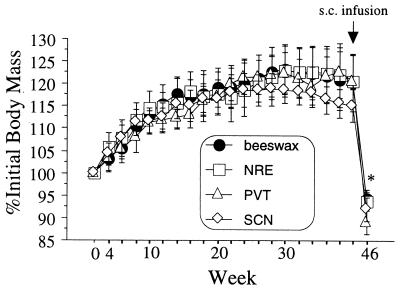
None of the central or s.c. Mel implants effected a significant reduction of body mass (Fig. 5) or a molt to winter pelage (data not illustrated). Body mass declined in all groups in response to the s.c. Mel infusion, irrespective of the gonadal response (Fig. 5).
Figure 5.
Body mass as a percentage (± SEM) of initial values of hamsters bearing Mel- or beeswax-containing cannulas in the NRE, PVT, or SCN. On week 40 (arrow), all brain cannulas were filled with beeswax and animals infused s.c. with Mel for 6 wk. *, Significantly different from week 40 value for all groups (P ≤ 0.001).
Discussion
Mel implants located in the SCN, NRE, or PVT induced testicular regression, which was followed several weeks later by recrudescence but did not induce refractoriness in other Mel-target tissues. Each of the three central Mel-binding sites mediates gonadal regression in sexually mature hamsters, extending previous observations (13) that Mel delivered to these targets prevents gonadal development in prepubertal male hamsters. The present findings also suggest that Mel restricted to any one of the three sites, after first inducing gonadal involution, eventually loses the ability to suppress gonadotropin secretion, thereby initiating subsequent gonadal recrudescence. Presumably each of these targets contains an interval timer that limits the duration of responsiveness to Mel and thereby the timing of recrudescence.
Loss of responsiveness to Mel may be common to all Mel-responsive tissues exposed to long duration Mel signals for a sufficient interval. Each Mel target tissue may cease to inhibit gonadotropin secretion at approximately the same time. An alternative hypothesis, that a systemic signal triggered by Mel action at a single locus renders all Mel targets unresponsive after 12–15 wk of long duration Mel signals, is incompatible with the present data. Mel restricted to one of the three sites probed in our study may render only that site refractory to the hormone. We cannot on the basis of the present results, however, discount the possibility that Mel acting on a subset of target tissues (>1) can render the remainder refractory to the hormone. Also, refractoriness in one target tissue (e.g., SCN) may cause some other target tissues (e.g., PVT and NRE) to become refractory, even as others remain responsive to Mel.
Each of several Mel-responsive neural substrates apparently undergoes a seasonal cycle of responsiveness to Mel. Different neural tissues may control follicle-stimulating hormone and prolactin secretion, each of which changes markedly from summer to winter under the influence of Mel (2). This arrangement would be parsimonious in short day breeders such as sheep in which the seasonal suppression of prolactin secretion coincides with enhanced secretion of the gonadotropins (22, 23). Refractoriness of separate Mel target tissues could mediate the transition to the spring phenotype independently for separate traits, e.g., reproduction and pelage. Such a contention is compatible with findings in sheep (18) and Syrian hamsters (24). In the latter species, elimination of photoperiodic control of gonadotropin but not prolactin secretion is observed in males that have sustained damage to the dorsomedial hypothalamus (24).
The present results suggest a reinterpretation of the role of the SCN in photoperiod responses of Siberian hamsters (16). Males in which the SCN has been ablated fail to undergo gonadal regression in response to short day lengths or long duration Mel infusions (25). Animals rendered reproductively refractory to Mel by an implant localized to the SCN do, however, retain the ability to undergo gonadal regression in response to systemic Mel infusions. This suggests that endocrine or neuroendocrine sequelae of SCN damage, other than elimination of Mel targets in the SCN, account for the loss of responsiveness to Mel. This conclusion must be tempered, however, because in the present study only unilateral SCN implants were used, so it remains possible that the contralateral SCN never develops refractoriness to Mel and mediates gonadal regression to the s.c. Mel infusion. Because implants of the type and size used in the present experiment typically allow Mel to diffuse ≈200–750 μm (13, 14), we presume that sufficient amounts of Mel reached the contralateral SCN to render it refractory as well. This issue can best be resolved by using bilateral implants in future work.
The lack of a typical short day body mass or pelage response to centrally administered Mel implies either that none of the sites exposed to hormone is involved in controlling these traits, or that concurrent exposure of several Mel targets is required to elicit changes in body mass and pelage. Alternatively, the use of constant release implants may be responsible for this pattern; decreases in body weight and a pelage molt were also absent in animals with s.c. Mel implants. Although the majority of hamsters treated s.c. with Mel for 40 wk became refractory to the anti-gonadal effects of the hormone, a greater percentage of animals becomes refractory after exposure to short day lengths for 40 wk (8). Constant release implants may be less effective signals for the induction of refractoriness than physiological short day Mel signals of 10–12 h duration each night (26). Technical limitations prevent the use of timed daily infusions of Mel over the 40-wk interval required by the present protocol.
Diffusion of Mel from the implant site to distant targets is a minor concern in the present study given that cannulas, which deviated from the target sites by as little as ≈0.5 mm, failed to elicit gonadal regression. Hormone diffusion is also less problematic because spread of Mel to additional target sites would be expected to bias the outcome in the direction of an increased incidence of refractoriness to subsequent Mel challenges. This did not materialize. The limited diffusion of functionally significant amounts of Mel from the implant site is also indicated by the failure of Mel implants centered in the ventricles (n = 3) to induce either gonadal regression or refractoriness.
In summary, responses to Mel implants at any of the three Mel-target tissues induced gonadal regression followed by recrudescence, indicative of refractoriness to Mel. Hamsters exhibiting refractoriness in response to a neural Mel implant were not refractory to peripheral Mel infusions, indicating that this refractoriness was site specific and did not extend to all other Mel-responsive tissues. These results are consistent with the hypothesis that evolution has independently modified Mel-sensitive neural circuits to permit species- and trait-specific timing of photoperiod responses (27).
Acknowledgments
We thank Christiana Tuthill and Daniel Lewis for expert technical assistance and Brian Prendergast, Mark Rosenzweig, and three anonymous reviewers for comments on the manuscript. This research was supported by National Institutes of Health Grants MH61171 and MH11655.
Abbreviations
- Mel
melatonin
- ETV
estimated testis volume
- NRE
nucleus reuniens
- PVT
paraventricular nucleus of the thalamus
- SCN
suprachiasmatic nucleus
References
- 1.Bronson F H. Mammalian Reproductive Biology. Chicago: Univ. of Chicago; 1989. [Google Scholar]
- 2.Bartness T J, Powers J B, Hastings M H, Bittman E L, Goldman B D. J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 3.Reiter R J. Anat Rec. 1972;173:365–372. doi: 10.1002/ar.1091730311. [DOI] [PubMed] [Google Scholar]
- 4.Gorman M R, Zucker I. Am J Physiol. 1995;269:R800–R806. doi: 10.1152/ajpregu.1995.269.4.R800. [DOI] [PubMed] [Google Scholar]
- 5.Schlatt S, DeGeyter M, Kliesch S, Nieschlag E, Bergmann M. Biol Reprod. 1995;53:1169–1177. doi: 10.1095/biolreprod53.5.1169. [DOI] [PubMed] [Google Scholar]
- 6.Anchordoquy H C, Lynch G R. J Biol Rhythms. 2000;15:406–416. doi: 10.1177/074873000129001495. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann K. J Reprod Fertil. 1978;54:29–35. doi: 10.1530/jrf.0.0540029. [DOI] [PubMed] [Google Scholar]
- 8.Prendergast B J, Flynn A K, Zucker I. J Neuroendocrinol. 2000;12:303–310. doi: 10.1046/j.1365-2826.2000.00452.x. [DOI] [PubMed] [Google Scholar]
- 9.Watson-Whitmyre M, Stetson M H. In: Processing of Environmental Information in Vertebrates. Stetson M H, editor. Berlin: Springer; 1988. pp. 219–249. [Google Scholar]
- 10.Weaver D R, Rivkees S A, Reppert S M. J Neurosci. 1989;9:2581–2590. doi: 10.1523/JNEUROSCI.09-07-02581.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams L M, Lincoln G A, Mercer J G, Barrett P, Morgan P J, Clarke I J. J Neuroendocrinol. 1997;9:639–643. doi: 10.1046/j.1365-2826.1997.00625.x. [DOI] [PubMed] [Google Scholar]
- 12.Bittman E L. Am Zool. 1993;33:200–211. [Google Scholar]
- 13.Badura L L, Goldman B D. Brain Res. 1992;598:98–106. doi: 10.1016/0006-8993(92)90172-6. [DOI] [PubMed] [Google Scholar]
- 14.Glass J D, Lynch G R. Neuroendocrinology. 1982;34:1–6. doi: 10.1159/000123269. [DOI] [PubMed] [Google Scholar]
- 15.Glass J D, Lynch G R. Neuroendocrinology. 1982;35:117–122. doi: 10.1159/000123365. [DOI] [PubMed] [Google Scholar]
- 16.Bartness T J, Goldman B D, Bittman E L. Am J Physiol. 1991;260:R102–R112. doi: 10.1152/ajpregu.1991.260.1.R102. [DOI] [PubMed] [Google Scholar]
- 17.Purvis C C, Duncan M J. Am J Physiol. 1997;273:R226–R235. doi: 10.1152/ajpregu.1997.273.1.R226. [DOI] [PubMed] [Google Scholar]
- 18.Lincoln G A, Clarke I J. Biol Reprod. 2000;62:432–438. doi: 10.1095/biolreprod62.2.432. [DOI] [PubMed] [Google Scholar]
- 19.Mason R, Rusak B. Brain Res. 1990;533:15–19. doi: 10.1016/0006-8993(90)91789-j. [DOI] [PubMed] [Google Scholar]
- 20.Bartness T J, Goldman B D. Am J Physiol. 1988;255:R812–R822. doi: 10.1152/ajpregu.1988.255.5.R812. [DOI] [PubMed] [Google Scholar]
- 21.Carter D S, Goldman B D. Endocrinology. 1982;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- 22.Lincoln G A, Richardson M. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119:283–294. doi: 10.1016/s0742-8413(98)00017-6. [DOI] [PubMed] [Google Scholar]
- 23.Langford G A, Ainsworth L, Marcus G J, Shresthsa J N. Biol Reprod. 1987;37:489–499. doi: 10.1095/biolreprod37.2.489. [DOI] [PubMed] [Google Scholar]
- 24.Maywood E S, Hastings M H. Endocrinology. 1995;136:144–153. doi: 10.1210/endo.136.1.7828525. [DOI] [PubMed] [Google Scholar]
- 25.Song C K, Bartness T J. J Biol Rhythms. 1996;11:14–26. doi: 10.1177/074873049601100102. [DOI] [PubMed] [Google Scholar]
- 26.Darrow J M, Goldman B D. J Biol Rhythms. 1986;1:39–54. doi: 10.1177/074873048600100106. [DOI] [PubMed] [Google Scholar]
- 27.Lincoln G A. Adv Exp Med Biol. 1999;460:137–153. doi: 10.1007/0-306-46814-x_16. [DOI] [PubMed] [Google Scholar]