Abstract
Inflammation is a complex and potentially life-threatening condition that involves the participation of a variety of chemical mediators, signaling pathways, and cell types. The microcirculation, which is critical for the initiation and perpetuation of an inflammatory response, exhibits several characteristic functional and structural changes in response to inflammation. These include vasomotor dysfunction (impaired vessel dilation and constriction), the adhesion and transendothelial migration of leukocytes, endothelial barrier dysfunction (increased vascular permeability), blood vessel proliferation (angiogenesis), and enhanced thrombus formation. These diverse responses of the microvasculature largely reflect the endothelial cell dysfunction that accompanies inflammation and the central role of these cells in modulating processes as varied as blood flow regulation, angiogenesis, and thrombogenesis. The importance of endothelial cells in inflammation-induced vascular dysfunction is also predicated on the ability of these cells to produce and respond to reactive oxygen and nitrogen species. Inflammation seems to upset the balance between nitric oxide and superoxide within (and surrounding) endothelial cells, which is necessary for normal vessel function. This review is focused on defining the molecular targets in the vessel wall that interact with reactive oxygen species and nitric oxide to produce the characteristic functional and structural changes that occur in response to inflammation. This analysis of the literature is consistent with the view that reactive oxygen and nitrogen species contribute significantly to the diverse vascular responses in inflammation and supports efforts that are directed at targeting these highly reactive species to maintain normal vascular health in pathological conditions that are associated with acute or chronic inflammation.
Keywords: Vasomotor dysfunction, Leukocyte–endothelial cell adhesion, Angiogenesis, Vascular permeability, Coagulation, Thrombosis, Free radicals
Introduction
Inflammation is a manifestation of immune system function that is triggered by microbial invasion and/or tissue injury. Accordingly, an inflammatory response is directed toward isolating and destroying invading microorganisms and injured cells and preparing the tissue for eventual repair and regeneration. Both the induction and the resolution phases of the inflammatory response are critically dependent on functional and structural changes in the microcirculation. These changes include impaired vasomotor function, the recruitment of leukocytes, diminished endothelial barrier function, angiogenesis, and enhanced thrombosis [1–6]. A variety of seemingly unrelated diseases that have been linked to inflammation, including cancer, obesity, diabetes, hypertension, ulcerative colitis, and Alzheimer disease, appear to exhibit all or most of these abnormalities in microvascular function. Given the growing recognition that inflammation is an underlying mechanism in many diseases associated with significant morbidity and mortality, much attention has been devoted to defining the mechanisms that link inflammation to microvascular dysfunction.
Two major effector systems that are frequently implicated in the vascular alterations associated with inflammation involve the generation of reactive oxygen and nitrogen species. The literature in this area is generally consistent with the view that the enhanced production of reactive oxygen species (ROS) and diminished bioavailability of nitric oxide (NO) that accompany an inflammatory response play a pivotal role in mediating the microvascular dysfunction and that restoration of the normal balance between ROS and NO will return vascular function to a normal state [7–12]. Although it remains unclear how the imbalance between ROS and NO levels caused by inflammation can exert an influence on responses as diverse as impaired vasomotor function, angiogenesis, endothelial barrier dysfunction, and thrombogenesis, the dual role of endothelial cells as a source of ROS and NO and as a major target of the signaling mechanisms activated by these reactive species may be important in this regard.
The objective of this article is to describe the contributions of ROS and NO to the microvascular alterations that are characteristic of an inflammatory response. The focus here is less on whether ROS and NO are involved in the vascular dysfunction of inflammation but more directed toward how these reactive species exert the vascular changes by interacting with specific targets in cells that either comprise or surround the blood vessel wall. For each of the five characteristic vascular abnormalities associated with inflammation (impaired vasomotor function, leukocyte recruitment, endothelial barrier dysfunction, angiogenesis, and enhanced thrombus formation) we provide background information on the physiological process and follow this with an analysis of the molecular signals that are targeted by ROS or NO to yield the observed vascular abnormality.
Vasomotor dysfunction
Inflammation is generally associated with an altered capacity of resistance vessels to respond to endothelium-dependent vasodilators and vasoconstrictors [1]. It is generally accepted that vascular smooth muscle tone can be regulated by adjacent endothelial cells. Arteriolar endothelium is separated from the underlying vascular smooth muscle by the internal elastic lamina, the mean thickness of which is approximately 1.5 µm [13]. Endothelial cell projections through the internal elastic lamina bring the membranes of endothelial cells and myocytes into even closer apposition (0–200 nm) [14]. At points of contact, myoendothelial gap junctions (MEGJs) are present, with a greater number of MEGJs in the distal than in the proximal arteriolar segments. The MEGJs allow for direct bidirectional communication between the two cell types as evidenced by the use of gap junction inhibitors in isolated arterial segments [15] or coculture of endothelial and vascular smooth muscle cells [16].
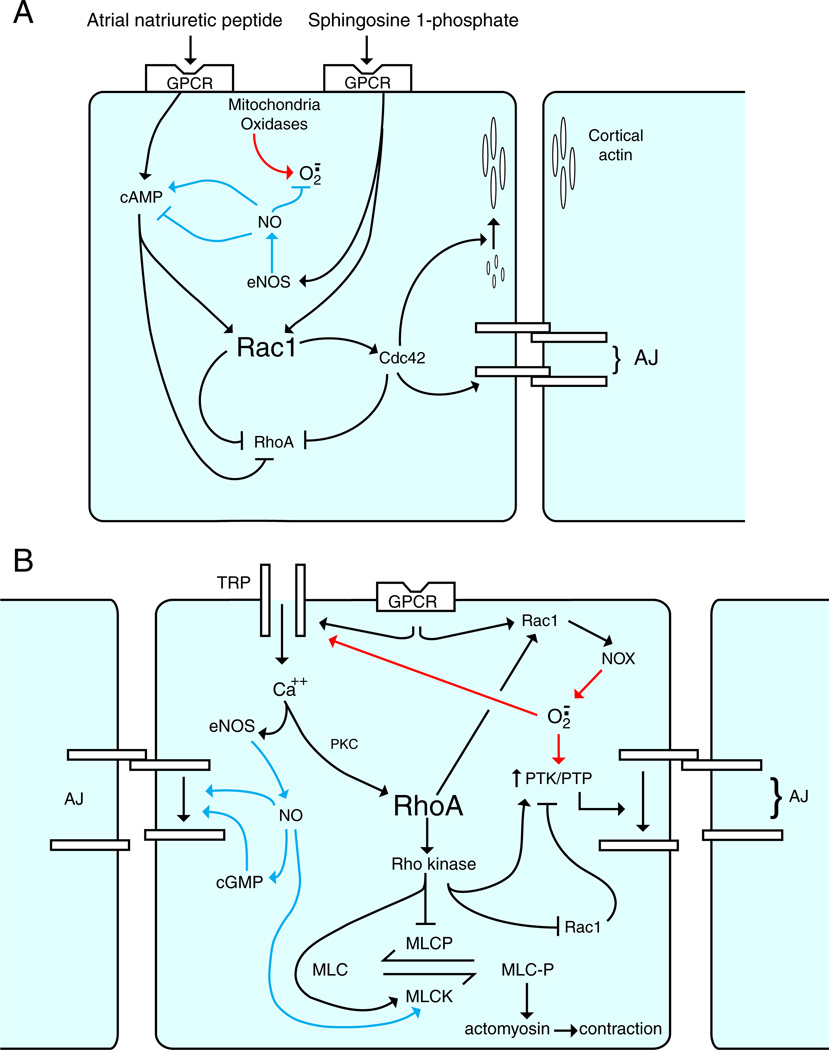
Endothelium-dependent vasodilation (Fig. 1)
Fig. 1.
Representative endothelial-dependent relaxing factors (EDRFs) and endothelial-dependent hypopolarizing factors (EDHFs). Endothelial activation by shear stress or ligands of G-protein-coupled receptors (GPCRs) increases intracellular levels of Ca2+, which is the initial event in the generation of EDRFs and EDHFs. EDRFs (red pathways): elevated endothelial Ca2+ levels activate at least two enzymes that generate EDRFs, nitric oxide synthase (eNOS) and cyclo-oxygenase (COX). NO (or HNO) derived from eNOS diffuses to smooth muscle and activates cGMP. The prostanoid PGI2 derived from COX interacts with its receptor (IPR) and activates cAMP. These second-messenger systems, in turn, activate Ca2+-dependent K+ channels (BKCa++) in smooth muscle resulting in inhibition of voltage-gated Ca2+ channels (Ca++V). The resultant decrease in intracellular Ca2+ leads to smooth muscle relaxation. EDHFs (green pathways): elevated endothelial Ca2+ levels also result in smooth muscle hyperpolarization by either activating Ca2+-dependent K+ channels or generating H2O2. Increased K+ efflux (via SKCa++ or IKCa++) results in endothelial membrane hyperpolarization (MHP), which can be transmitted to smooth muscle via myoendothelial gap junctions. Alternatively, the K+ ions entering the internal elastic lamina can cause smooth muscle hyperpolarization. In either case, Ca++V are inhibited and smooth muscle Ca2+ levels decrease, resulting in smooth muscle relaxation. Finally, various oxidases (e.g., NADPH oxidase, COX) as well as eNOS (e.g., uncoupled) can generate superoxide, which is rapidly converted to H2O2 by Cu,Zn-SOD. H2O2 can then diffuse to the smooth muscle where it activates BKCa++ and inhibits Ca++V.
Interest in endothelium-dependent vasodilation stemmed from the observation that removal of the endothelial cells from isolated arteries prevented the vasodilator response to acetylcholine [17]. Since then, numerous other vasoactive substances, such as G-protein-coupled receptor (GPCR) ligands, as well as shear stress, have been shown to exhibit a similar dependence on an intact endothelium for induction of vasodilation [18]. Based on pharmacologic blockade and bioassay approaches, endothelium-dependent vasodilation has been attributed to the release of prostacylin (PGI2) and NO, which are referred to as endothelium-derived relaxing factors (EDRFs) [17,19,20]. Based on the relative impact of pharmacologic inhibitors of nitric oxide synthases (NOSs) and cyclo-oxygenases (COXs) on endothelium-dependent vasodilation, NO seems to be the dominant EDRF and therefore it has received the most attention.
The residual vasodilation after pharmacologic blockade of cyclooxygenase and NOS is (1) associated with vascular smooth muscle hyperpolarization and (2) sensitive to blockade of Ca2+-activated K+ channels. This endothelium-dependent vasodilation has been attributed to some endothelium-derived hyperpolarizing factor or factors (EDHFs) [21,22]. In general, EDHF-induced vasodilation is more prevalent in distal than in proximal arterial segments [22,23], correlating with the relative distribution of MEGJs [14]. A number of candidate EDHFs have been proposed, including cytochrome P450 epoxygenase-derived epoxyeicosatrienoic acids (EETs) [24], mitochondrial- or oxidase-derived H2O2 [25–27], and potassium ions/channels or direct electrical coupling via MEGJs [22,23,28].
Because the EDRFs NO and PGI2 can also hyperpolarize vascular smooth muscle [22], it has been suggested that the term EDHF should refer only to myocyte hyperpolarization as a result of the spread of endothelial hyperpolarization to vascular smooth muscle by direct electrical coupling via MEGJs and/or accumulation of K+ ions in the intercellular space between them [22,23,29]. Two distinct types of EDHF pathways have been proposed to deal with this issue: one involving endothelial cell hyperpolarization with spread of the hyperpolarization to the smooth muscle and another involving endothelial release of synthesized chemical mediators that activate K+ channels on smooth muscle [30]. The contributions of EDRFs and EDHFs to endothelium-dependent vasodilation vary depending on species, vascular bed, and segment (proximal versus distal) of arteriole being assayed [22,23,31–34]. Further, there are interactions between EDRFs as well as between EDHFs and EDRFs [35–37].
An initial event in endothelial cell activation in response to ligation of GPCR or shear stress is an increase in intracellular Ca2+. Ca2+ influx occurs via receptor-operated channels (ROCs) and/or store-operated channels (SOCs) [38,39]. ROC activation occurs in response to external stimuli (e.g., GPCR ligation), which stimulate phospholipase C/inositol-3-phosphate (PLC/IP3) signaling. SOC activation is mediated by intracellular signals generated when Ca2+ stores are depleted (e.g., endoplasmic reticulum). The molecular identity of the various Ca2+ channels remains unclear. Of relevance to endothelial-dependent vasoreactivity, some members of the transient receptor proteins (TRPs) have been shown to be major components of Ca2+ channel complexes [38,40]. For example, TRPC1 is believed to be an important ROC, as well as a SOC component, depending on whether it is incorporated into lipid rafts [41]. Transient receptor potential vallinoid type 4 (TRPV4) also plays an important role in Ca2+ influx [40]. Both GPCR- and shear-stress-mediated vasodilation are significantly blunted after genetic blockade of TRPV4 [42–44]. Interestingly, TRPV4 and TRPC1 are associated with caveolae [40] and can form a complex to more efficiently promote Ca2+ influx [45]. Despite the ambiguity surrounding the molecular composition of the various endothelial Ca2+ channels, an increase in intracellular Ca2+ drives both EDRF and EDHF pathways of vascular smooth muscle relaxation.
In the EDRF pathway, an increased intracellular Ca2+ activates endothelial (e) NOS, which generates NO during the conversion of l-arginine to l-citrulline. NO diffuses to the myocyte where it binds to the heme moiety of soluble guanylate cyclase (sGC) and displaces iron from its usual position in the porphyrin ring allowing sGC to catalyze the formation of cGMP [20]. Increased levels of myocyte cGMP initiate signaling cascades that result in a decrease in intracellular Ca2+ and subsequent smooth muscle relaxation. The decrease in myocyte intracellular Ca2+ is a result of efflux of K+ due to activation of large conductance channels (BKCa) resulting in hyperpolarization of the myocyte membrane and reduced influx of Ca2+ [28,46]. Alternatively, NO can induce relaxation of smooth muscle via sGC-independent mechanisms (e.g., S-nitrosothiols) [20,47]. By contrast, PGI2 ligation of the prostacyclin receptor, IP, results in the generation of cAMP, which initiates a signaling cascade leading to a decrease in myocyte Ca2+ levels via activation of BKCa [28,46].
With respect to the EDHF pathway, the increase in intracellular Ca2+ results in activation of small and intermediate conductance Ca2+-activated K+ channels (SKCa and IKCa) located in endothelial caveolae and cell projections [22,23,29]. Genetic blockade approaches have revealed that functional SKCa and IKCa channels are critical for both agonist and shear-stress-induced EDHF pathways of vasodilation [32]. Movement of K+ out of the cell (due to activation of SKCa and IKCa) results in hyperpolarization of the endothelial cells, which can spread to the myocyte via current conduction and/or movement of signaling molecules through the MEGJs. In addition, the extracellular accumulation of endothelial-derived K+ can hyperpolarize adjacent myocytes via stimulation of Na+/K+-ATPase and/or inward rectifier K+ channels [22]. Finally, vascular smooth muscle hyperpolarization/relaxation induced by synthesized and diffusible EDHFs (e.g., H2O2, EETs) is a result of activation of BKCa in vascular smooth muscle [30].
Role of ROS and NO (Fig. 1)
NO derived from eNOS is an EDRF whose mechanism of action has classically been attributed to activation of sGC in vascular smooth muscle [20]. NO can also undergo interconversion to nitroxyl anion (NO−), which exists as HNO in an aqueous milieu [31,48]. HNO seems to contribute equally with NO to the acetylcholine-induced, sGC-dependent vasodilation in rat and mouse mesenteric arteries [30], indicating that HNO can also be considered an EDRF. Further complicating matters, NO may also substantially contribute to the EDHF-mediated vasodilation. For example, acetylcholine-induced, EDHF-mediated vasodilation (i.e., eNOS and COX independent) can be prevented by scavenging NO [31]. This can be attributed to NO released from endothelial stores independent of eNOS activation, with nitrites and/or S-nitrosothiols as the most likely storage pool of NO [20]. Alternatively, activation of endothelial SKCa and IKCa channels, a hallmark feature of the EDHF pathway, also enhances NO synthesis via eNOS and contributes to acetylcholine-induced vasodilation [49,50]. Endothelium-derived H2O2 has been proposed to be an EDHF based on the following lines of evidence. Endothelial superoxide and H2O2 production is increased in mesenteric and coronary arteries in which endothelium-dependent vasodilation is resistant to pharmacologic blockade of NOS and cyclo-oxygenase [34,51]. Catalase can inhibit both agonist- and flow-induced endothelium-dependent vasodilation in isolated arteries [26,35,51]. Catalase can also prevent acetylcholine-induced smooth muscle hyperpolarization and relaxation, whereas exogenous H2O2 can induce hyperpolarization and relaxation of denuded arteries [25,52]. The endothelial component of the H2O2-mediated response has been attributed to enhanced Ca2+ release from cellular stores (e.g., endothelium reticulum) and the secondary activation of SKCa and IKCa channels [35,53]. There is evidence that H2O2 can interact with smooth muscle after extracellular release from endothelial cells [51] or by intracellular communication via MEGJs [52,53].
Endothelial generation of H2O2 requires the presence of Cu,Zn-superoxide dismutase (SOD) [54,55], supporting the contention that H2O2 is derived from the dismutation of superoxide. The endothelial sources of superoxide generation in response to ligation of GPCR or shear stress are not entirely clear but may be species, vascular bed, and/or stimulus specific. In human coronary arteries, flow-induced vasodilation and H2O2 production have been attributed to mitochondrial respiration; neither NOS nor NADPH oxidase seems to be involved [56]. However, in the same vascular bed, bradykinin-induced vasodilation and the associated endothelial production of H2O2 are dependent on NADPH oxidase [57]. Acetylcholine-induced H2O2 production and vasodilation in mesenteric arteries have been attributed to NOS; neither mitochondrial respiration nor other oxidases (e.g., xanthine oxidase, NADPH oxidase) have been implicated in this response [58].
There are significant antagonistic interactions between endothelial-derived ROS and NO, which can have an impact on the endothelium-dependent vasodilation induced by agonists or shear stress. Superoxide can interfere with NO-induced activation of sGC by interacting with NO, an event ameliorated by Cu,Zn-SOD [7]. Conversely, the direct interaction between superoxide and NO can diminish the ROS component of endothelium-dependent vasodilation. In addition, oxidant stress can lead to direct inhibition of eNOS by inducing phosphorylation of Tyr657 [59] or the uncoupling of eNOS via oxidation of the cofactor tetrahydrobiopterin (BH4) [60,61]. Another interesting interaction between NO and ROS has been uncovered in endothelial cells exposed to shear stress for up to 24 h [62]. Short durations of shear stress (2 h) increase both ROS and NO production by endothelial cells and, as the duration of shear stress increases, endothelial ROS production declines, whereas NO production continues to increase. The decline in ROS production has been attributed to NO-induced downregulation of NADPH oxidase. Finally, if an agonist can interact with GPCRs on endothelial cells and smooth muscle cells, the end result may not be predictable. For example, there is evidence that smooth-muscle-derived superoxide can negatively modulate endothelial-dependent, NO-mediated vasodilation [63]. In this scenario, ligation of a GPCR (e.g., 5-HT) on smooth muscle cells generates superoxide, which can traverse MEGJs to interact with eNOS-derived NO, resulting in diminished NO bioavailability for activation of myocyte sGC.
ROS and NO may act cooperatively in the endothelium-dependent vasodilation induced by agonists or shear stress. NOS-derived superoxide may be required for the generation of H2O2 implicated in the EDHF-mediated, endothelium-dependent vasodilation. Genetic deletion of all three isoforms of NOS results in the abolition of acetylcholine-induced H2O2 production and vasodilation in mesenteric arteries [34]. Because BH4 bioavailability was not affected, it was assumed that NOS uncoupling was not involved in the generation of superoxide and subsequent formation of H2O2. However, oxidation of BH4, rather than BH4 depletion per se, seems to be a prerequisite for eNOS uncoupling [64]. Furthermore, limiting concentrations of other factors that regulate eNOS activity (e.g., substrate) can also lead to eNOS uncoupling [65]. Finally, the validity of these observations has been questioned based on the rather severe phenotype of the triple-NOS knockout mouse [22]. Irrespective, blockade of NOS via pharmacologic or knockdown approaches supports an NOS/H2O2 pathway in acetylcholine-induced vasodilation [66]. It has been proposed that the NOS/NO EDRF pathway is dominant in arteries and the NOS/H2O2 EDHF pathway is dominant in arterioles [27].
On the other hand, exogenous H2O2 can induce endothelium-dependent vasodilation that is blocked by inhibition of NOS, indicating that H2O2 can activate eNOS [60]. However, this phenomenon appears to be dose dependent. Low concentrations (≈50 µM) of H2O2 induced eNOS phosphorylation on Tyr657 (inhibitory site) while not affecting eNOS phosphorylation on Ser1177 (stimulatory site) [59]. These phosphorylation events diminished eNOS activation by bradykinin and inhibited acetylcholine-induced vasodilation. On the other hand, high concentrations of H2O2 (≈500 µM) can induce eNOS activation via phosphorylation of Ser1177 and dephosphorylation of Thr495 (inhibitory site) [67]. The ability of H2O2 and NO to regulate each other's bioavailability argues in favor of endothelial compartmentation of the systems involved in ROS and NO generation [68].
Shear-induced vasodilation: endothelial mechanosensing/transduction
The vasodilation induced by shear stress is dependent on endothelial sensing/transduction of the shear induced by flowing blood. The proposed mechanosensors on the luminal surface of the endothelium include components of the glycocalyx (glycoproteins and proteoglycans), caveolae, and ion channels [28,40,69–73]. The major intracellular mechanotransduction element seems to be the endothelial cytoskeleton [70,74].
Functional components of mechanosensing/transduction
The glycocalyx decorating the endothelial cell surface consists of proteoglycans and associated glycosaminoglycan (heparan sulfates, chondroitin sulfates, and hyaluronan) side chains and terminal sialic acid [75,76]. Two of the major proteoglycan core proteins of the glycocalyx are syndecan and glypican; syndecan is a transmembrane proteoglycan and glypican is anchored to membrane lipid rafts/caveolae via glycosylphosphatidylinositol. Enzymatic destruction of the glycosaminoglycans (GAGs) can severely blunt shear-induced vasodilation [77,78]. The sensitivity of the glycocalyx to proteolytic degradation may result in a less impressive structure when endothelial cells are enzymatically isolated and cultured [79]. However, this may be a fixation artifact, because rapid freezing of the cells reveals a glycocalyx comparable to that noted in vivo [80]. Whether or not the glycocalyx is compromised during the isolation and culture of endothelial cells, there is a sufficient structural integrity to allow the cells to respond to shear stress in a manner analogous to the in vivo situation [79]. Platelet-endothelial cell adhesion molecule-1 (PECAM-1), a transmembrane glycoprotein that serves as one of the core proteins of the glycocalyx [75,76], can be activated (phosphorylated) by shear stress [81] or mechanical “tugging” with magnetic beads [72]. Flow-induced vasodilation is blunted in arterioles from PECAM-1-deficient mice [82].
Caveolae are flask-shaped invaginations in the endothelial membrane that are enriched in cholesterol and sphingolipids and provide a microdomain for a variety of signaling complexes (e.g., Ca2+-handling proteins) [40,83,84]. Caveolin-1, a protein constituent of caveolae, plays a role in caveola assembly [85] and, more importantly, caveolin-1 interacts with various signaling components to regulate flow-induced vasodilation [86]. Caveolin-1-deficient arteries exhibit a marked reduction in flow-induced dilation that is rescued by selectively reconstituting caveolin-1 expression in endothelium [85].
Calcium channel activation and endothelial Ca2+ influx are two of the earliest events in shear-induced endothelial activation [70]. For example, shear stress activates TRPV4 in endothelial cells, resulting in Ca2+ influx [43]. Furthermore, flow-mediated vasodilation is inhibited by interfering with TRPV4 activity (genetic, pharmacologic, and siRNA blockade).
The endothelial cytoskeleton, an intracellular network of actin microfilaments, microtubules, and intermediate filaments, plays an important role in shear-induced alterations in endothelial cell morphology and flow-induced vasodilation [74]. Pharmacologic depolymerization or stabilization of the microfilament/microtubule network impairs flow-induced vasodilation without affecting agonist-mediated vasodilation [74].
A unifying hypothesis
The various mechanosensor/transducers implicated in the vasodilation induced by shear stress most probably do not operate independently, but rather work in concert as interconnected networks [70]. Individual blockade of the various proposed mechanosensors/transducers does not distinguish whether they are involved in the sensing versus the transduction of the shear stress. It has been predicted that fluid drag within the glycocalyx reduces the shear stress to negligible levels at the endothelial cell membrane proper [76]. Thus, it seems unlikely that membrane structures (e.g., caveolae and associated receptors, enzymes, or ion channels) play an important direct role in the detection of shear by endothelial cells with an intact glycocalyx. However, the GAGs (e.g., hyaluronan) and core proteins (e.g., PECAM-1) of the glycocalyx that readily respond to shear stress can interact with the cytoskeleton [70,74] and signaling components of lipid rafts/caveolae [87,88]. Many of the relevant ion (K+ and Ca2+) channels in the endothelial cell membrane proper are associated with lipid rafts/caveolae [28,39,40,68,89]. For example, the endothelial Ca2+ influx induced by shear stress can be prevented by enzymatic disruption of the glycocalyx [70]. Collectively, the literature is consistent with a unified hypothesis encompassing an initial sensing of shear stress by glycocalyx components that ultimately activate signaling complexes located in lipid rafts/caveolae. This hypothetical scenario has been alluded to in the past with respect to flow-induced arterial remodeling [74] and warrants further attention, specifically to address the sequence in which the various mechanosensors/transducers operate to elicit the vasodilation associated with shear stress.
Linkage to ROS and NO
Endothelial production of NO induced by shear stress is inhibited by enzymatic degradation of GAGs in vivo and in vitro [73,87]. Selective enzymatic degradation of various GAGs indicates that heparan sulfates (HS), hyaluronan (HA), and sialic acid (SA) constituents, but not chondroitin sulfate, are critical for the shear-induced increase in endothelial NO (nitrite) [87,90]. Interestingly, the decline in endothelial NO associated with degradation of HS and SA, but not HA, can be prevented by scavenging ROS [90]. These findings indicate that only HA is directly involved in endothelial NO production; the role of HS and SA is indirect, serving to limit ROS generation and increase NO bioavailability. It has been proposed that HA can activate eNOS either through direct binding to CD44 in caveolae, which contain eNOS, or indirectly via the HA-rich glypican binding to caveolae (glypican/caveolae/eNOS axis) [87,90].
As mentioned above, degradation of the HS and SA constituents of the glycocalyx results in increased ROS production; consequently the inability to detect endothelial NO production probably reflects reduced NO bioavailability [90]. This is in contrast to observations that, in endothelial cells exposed to shear stress, H2O2 activates eNOS (Ser1177 phosphorylation) and increases NO production, an event associated with a decrease in catalase activity initiated by a protein kinase Cγ (PKCγ)-mediated phosphorylation event [91]. Interestingly, PKCs are enriched in caveolae [68], but whether and how the glycocalyx is linked to PKCγ in caveolae remain unclear.
The glycocalyx-associated glycoprotein PECAM-1 plays an important role in flow-induced vasodilation, but its direct relationship with eNOS activation is controversial [70]. For example, PECAM-1 association with eNOS has been shown to increase [81] or decrease [88] with application of shear to the endothelium. Flow-induced vasodilation is reduced in PECAM-1-deficient coronary arteries, but the portion of the dilation attributable to NO is not affected and, indeed, eNOS Ser1177 phosphorylation and NO production are still noted in these arteries [92]. One issue worth exploring is the role of the endothelial mechanosensory PECAM-1/vascular endothelial growth factor receptor 2 (VEGFR2) signalosome (or complex) [93] in flow-induced vasodilation. The components of the complex are associated with caveolae [68] and VEGFR2 can activate eNOS in a ligand-independent manner and plays a role in flow-induced vasodilation [94]. Thus, blockade (genetic or otherwise) of either one of the components of the PECAM-1/VEGFR2 signalosome would more than likely render it nonfunctional and inhibit flow-induced vasodilation.
Caveolin-1 binding to eNOS is important in maintaining eNOS within the caveolae while inhibiting its enzymatic activity [68,70]. An increase in shear stress enhances phosphorylation of both caveolin-1 and eNOS (Ser1177) [91,95]. Subsequently, caveolin-1 disassociates from eNOS leaving eNOS free to interact with calmodulin, resulting in increased eNOS activity [68,70,84]. As mentioned above, flow-induced vasodilation is impaired in caveolin-1-deficient mice and this impairment can be rescued by selectively overexpressing caveolin-1 in the endothelium [85,86].
Although shear stress has been shown to increase ROS production by endothelial cells [62,90], little is known about the potential links between mechanosensory/transduction components of endothelial cells and ROS-generating systems. Enzymatic degradation of HS or SA, but not HA, prevents shear-induced ROS production [90]. These findings indicate that shear-induced ROS production is dependent on HS and SA components of the glycocalyx. This differs from shear-induced NO production, which seems to be primarily dependent on HA [90].
Flow-induced ROS production is more likely to be demonstrated in arteries derived from animals or humans with pathologies associated with cardiovascular complications. The impaired flow-induced vasodilation in experimental hypertension (SHR) has been attributed to NADPH oxidase activation [96]. Angiotensin II, which is known to activate endothelial cell NADPH oxidase, has been implicated in the impaired vasodilatory response associated with hypertension [97]. In coronary arteries from patients with coronary artery disease, the impaired flow-induced vasodilation has been attributed to mitochondrial-derived superoxide and H2O2 [98].
Endothelium-dependent vasoconstriction
In addition to mediating vasodilation via EDRFs and EDHFs, the endothelium can mediate vasoconstriction via release of factors that elicit contraction of the surrounding smooth muscle. These endothelium-derived contracting factors (EDCFs) were identified using approaches similar to those utilized to characterize EDRFs and EDHFs, i.e., endothelial denudation of blood vessels, pharmacologic blockade, and bioassays [99,100]. In general, EDCFs are uncovered (1) after pharmacologic blockade of EDRFS and/or EDHFs (e.g., NO) [100] or (2) during pathogenesis of cardiovascular disorders (e.g., hypertension) in which NO bioavailability is compromised [101,102]. The decreased NO bioavailability in various pathologic conditions seems to result from enhanced ROS production via cyclo-oxygenase [103] or NADPH oxidase [104]. Endothelium-dependent contractions can be enhanced by compromising NO bioavailability or amplifying ROS bioavailability [105].
The major candidate EDCFs are COX-derived prostanoids such as thromboxane, prostaglandin F2α, and PGI2, with PGI2 receiving the most experimental support [99–101,106,107]. As mentioned above PGI2 is considered an EDRF that induces smooth muscle relaxation by activating IP receptors on myocytes. The PGI2-mediated vasoconstriction is a result of PGI2 ligation of TP receptors, which induce smooth muscle contraction via either Ca2+ release from intracellular stores [99,105] or inhibition of the cGMP/cAMP vasodilator pathway [108]. Although both IP and TP receptors are expressed on smooth muscle, in the SHR model of hypertension, the IP receptors are dysfunctional [109], whereas the TP receptors are hyperresponsive [105].
Role of ROS and NO
EDCFs were uncovered at around the same time as EDRFs [101], yet other than the interaction between NO and superoxide, little is known about the potential direct contribution of endothelial ROS or NO to EDCF-induced vasoconstriction. This presumably results from the complexities inherent in pathologic conditions (e.g., hypertension) wherein the mechanisms underlying the oxidative stress and decreased NO availability remain undefined. In one model (rat renal artery), with acetylcholine inducing an endothelium-dependent vasoconstriction under conditions of normal NO bioavailability, endothelial NADPH oxidase-derived H2O2 was shown to behave as an EDCF [110]. Nonetheless, the current consensus holds that ROS contribute to EDCF-mediated vasoconstriction under certain pathologic conditions. For example, acetylcholine increases endothelial oxidative stress in aortic endothelial cells from hypertensive (SHR) rats, but not in endothelial cells from normotensive (WKY) rats [103].
The following has been proposed to explain the role of ROS in EDCF-mediated vasoconstriction in SHR: upon ligation of GPCR by acetylcholine, there is excessive accumulation of intracellular Ca2+ in endothelial cells, which results in enhanced COX activation and ROS production [103]. ROS can further activate COX in endothelial cells and/or diffuse to the neighboring vascular smooth muscle to activate COX and produce more prostanoids. This positive feedback mechanism leads to excessive prostanoid generation that activates hyperresponsive TP receptors and induces smooth muscle contraction [101,105]. Furthermore, ligation of TP receptors on vascular smooth muscle enhances their stability (less internalization) via a ROS-dependent mechanism [111].
Leukocyte recruitment
The major function of the inflammatory response is to clear invading pathogens or damaged tissue/cells and initiate repair [112,113]. Signals derived from injured tissue or invading microbes activate resident sentinel cells, such as macrophages and/or mast cells. If the episode is minor, the macrophages/mast cells clear the interstitial debris/microbes. If the stress is more severe, the sentinel cells recruit circulating neutrophils (polymorphonuclear cells, or PMNs), and later monocytes, to the affected site to aid in the clearing process [112,114]. This sequential recruitment of PMNs and monocytes is believed to result from progressive activation of the endothelium and/or the ability of emigrated PMNs to facilitate monocyte recruitment [115]. To reach the site of injury/infection, leukocytes must first be captured by the endothelium via a coordinated series of adhesive interactions referred to as rolling, adhesion, and emigration [116–119].
Detection of tissue injury/infection by resident sentinel cells (Fig. 2A)
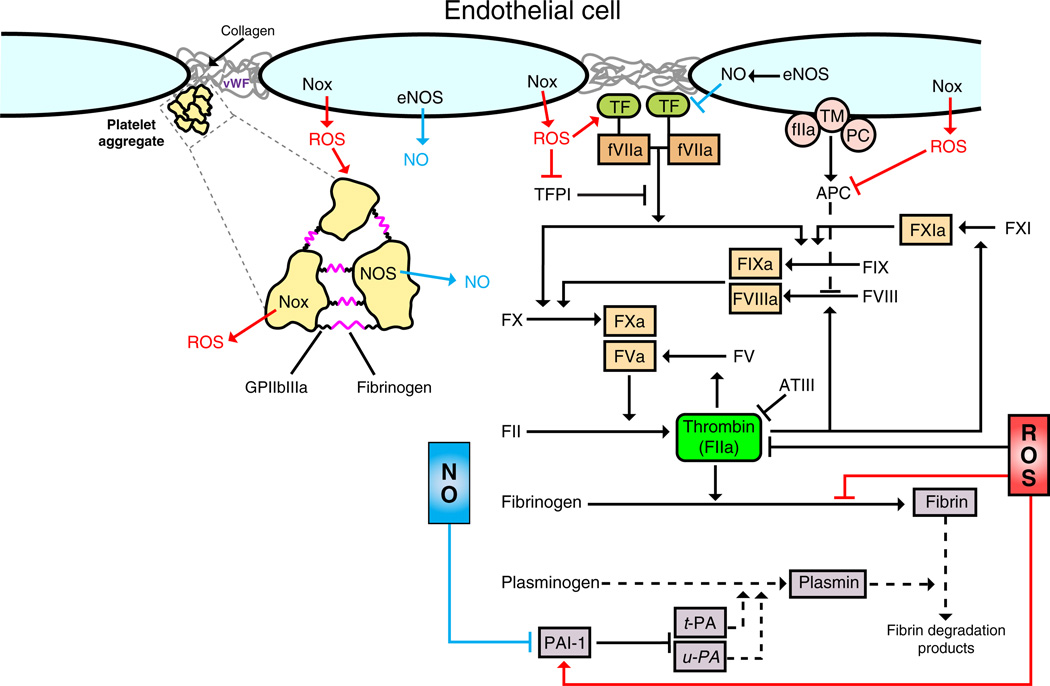
Fig. 2.
Leukocyte recruitment to sites of injury/infection: dominant role of ROS. (A) Activation of macrophages by infection/injury. PAMPs (e.g., LPS) generated by infection and DAMPs (e.g., HMGB1) generated by stressed/necrotic cells serve as ligands for TLR and RAGE on interstitial sentinel cells (e.g., macrophages). Ligation of these receptors results in activation of NADPH oxidase (NOX), which generates ROS (superoxide and its dismutation product H2O2) (red pathway). ROS can be (1) exported into the interstitium to affect adjacent endothelial cells and (2) further activate NOX (e.g., via an NF-κB pathway) leading to the generation of additional ROS (feed-forward mechanism). Activation of NF-κB can also increase iNOS levels, which results in NO generation (blue pathway). NO can dampen the inflammatory response by interacting with superoxide within macrophages or adjacent cells. (B) Rapid and delayed phases of endothelial activation. Endothelial cells are activated by the proinflammatory milieu (chemokines, cytokines, ROS, LPS, HMGB1). Rapid activation (NF-κB independent) of endothelial cells by chemokines and ROS results in further ROS generation via NOX (red pathways). Endothelial ROS contribute to adhesion molecule expression (P-selectin), which facilitates leukocyte rolling. ROS have also been implicated in endothelial cell generation of leukocyte activators (e.g., PAF and CXCL8), which are sequestered within the glycocalyx and facilitate leukocyte adhesion to the endothelium. Leukocyte adhesion to endothelium results in the clustering of endothelial adhesion molecules (docking structures). The resultant cell signaling disrupts adherens junctions (AJ) via NOX-derived ROS and facilitates leukocyte TEM. Delayed activation (NF-κB dependent) of endothelium reinforces the leukocyte–endothelial adhesive interactions via continued and amplified generation of ROS, chemokines, and cytokines via the NF-κB pathway.
The initiation of an inflammatory response is dependent on the recognition of invading microbes and/or damaged tissue by resident immune cells. The major sentinel cells involved in this innate immune response are the macrophages and mast cells [120–124].With microbial invasion, these cells recognize highly conserved components of microbes termed pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS) [125].With sterile tissue injury, the sentinel cells detect substances released by damaged cells and/or the extracellular matrix (ECM), referred to as damage-associated molecular patterns (DAMPs), such as heat shock proteins, high mobility group protein-B1 (HMGB1), and hyaluronan [126,127]. Both macrophages and mast cells can be activated by PAMPs and DAMPs. Although mast cells seem to be capable of phagocytosis [128], clearance of bacteria and/or cellular debris is generally the responsibility of macrophages [114]. Upon activation, both mast cells and macrophages release various inflammatory mediators (e.g., platelet activating factor (PAF)), chemokines (interleukin- 8 (CXCL8)), and cytokines (e.g., tumor necrosis factor (TNF), interleukin-1 (IL-1)) [129,130].
A major class of membrane receptors for PAMPs and DAMPs are the Toll-like receptors (TLRs) [125,127]. During infection, various components of bacterial cell membranes interact with different homodimers or heterodimers of TLRs on the cell membranes of sentinel cells [131–133]. A classic example is the interaction of LPS with macrophage TLR4. During sterile tissue injury, proteins (e.g., HMGB1) released from damaged cells or breakdown products of disrupted ECM (e.g., hyaluronan) can also interact with TLRs (e.g., TLR4, TLR2) [127,131]. Some diversity in the system exists, because HMGB1 can also interact with RAGE (receptor for advanced glycation end products) [134]. There is also evidence that IL-1α released from necrotic cells utilizes the IL-1R on sentinel cells to initiate an inflammatory response [135]. Activation of TLRs, RAGE, or IL-1R results in the activation/nuclear translocation of transcription factors involved in the inflammatory response (e.g., NF-κB) [131,136–138]. Collectively, most of the available information indicates that TLRs are the major receptors for PAMPs and DAMPs and that there is a convergence of molecular pathways at the level of NF-κB. However, it has been proposed that the intensity of the response induced by PAMPs may be greater than that induced by DAMPs, i.e., the DAMP response can be downregulated at the postreceptor level by concurrent activation of a CD24–Siglec pathway [139].
Although cells of the innate immune system are generally believed to be the major sentinel cells, there is evidence indicating that parenchymal cells can serve in this capacity [140,141]. Cardiac myocytes, renal mesangial cells, alveolar epithelial cells, and endothelial cells also possess TLRs and can respond to DAMPs and PAMPs [142–147]. These cells can be converted to a proinflammatory phenotype when activated via TLRs. For example, cardiac myocytes can generate cytokines/chemokines and endothelial cells can increase adhesion molecule expression in response to the TLR4 ligand, LPS [143,145,146].
Tissue injury is often associated with infection, which makes an accurate assessment of the relative roles of PAMPs and DAMPs to the overall inflammatory response rather difficult [112]. Specific experimental models have been used to isolate the inflammatory response to sterile injury (e.g., ischemia/reperfusion; I/R) from that due to infection (e.g., LPS) [112,113]. Based on these approaches, it is well accepted that TLR4 plays an important role in PAMP (LPS)-induced responses [143,145], whereas both TLR4 and RAGE play important roles in sterile (I/R) injury and inflammatory responses [127,134,141]. However, even under these well-defined experimental conditions the potential for interactions between PAMPs and DAMPS should be kept in mind. For example, PAMPs can activate adjacent cells to release DAMPs [127,144], as well as interacting with each other (e.g., HMGB1 binds LPS) [148–151].
Role of ROS and NO (Fig. 2A)
As discussed above, DAMPs and PAMPs generated during injury/infection can activate perivascular (e.g., macrophages, mast cells) and intravascular (e.g., leukocytes) immune cells, as well as endothelial cells; all of which subsequently generate ROS at an accelerated rate. The increased ROS production can also generate oxidation products from host-derived cells/debris, which also can serve as DAMPs and propagate the inflammatory response [152].
Activation of mast cells increases intracellular ROS, which may be involved in the intracellular signaling that leads to secretion of inflammatory mediators [153,154]. For example, NADPH oxidase-derived ROS have been implicated in the generation of IL-8 production by IL-1β-activated mast cells [155]. Although mast cells contain NOS and can generate NO [156], the role of NO in mast cell activation by inflammatory stimuli is equivocal [157,158]. In short, the available information, albeit limited, indicates that mast-cell-derived ROS and NO are used for intracellular signaling purposes, rather than being exported. By contrast, activation of macrophages results in substantial ROS production via NADPH oxidase and NO production via inducible (i) NOS, both of which are involved in the killing of pathogens [159–162].
Activation of macrophages by LPS, a TLR4 ligand, induces ROS generation both intracellularly and extracellularly via NADPH oxidase [163,164], with intracellular ROS serving a signaling function (e.g., potentiation of TNFα secretion) and extracellular ROS affecting adjacent cells (e.g., cytotoxicity) [163]. With respect to intracellular signaling, activation of the TLR/IRAK-1 pathway in macrophages can (1) rapidly activate NADPH oxidase via the small GTPase Rac1 and (2) induce transcriptional events (e.g., NF-κB) to increase the expression of a NADPH oxidase subunit, Nox1 [165]. In a cell-free system, IRAK-4 has been shown to interact with and phosphorylate p47phox, leading to rapid activation of NADPH oxidase; a result confirmed in LPS-activated PMNs [166]. Whether a similar role for IRAK-4 is operative in macrophages awaits confirmation. The NADPH oxidase pathway is also critical for the LPS-induced conversion of endothelial cells to a proinflammatory phenotype, e.g., generation of chemokines [146]. Of particular interest with respect to pathology is the proposed propagation of ROS production, i.e., H2O2 can activate NADPH oxidase to generate further ROS [167]. Once generated extracellularly, ROS can activate both mast cells and macrophages [153,168,169], thereby amplifying the inflammatory response. Coculture approaches indicate that transfer of oxidants (e.g., H2O2) generated by immune cells (high output) can have an impact on adjacent nonimmune cells (neurons, endothelial cells) [163,169,170]. For example, challenge of macrophages with H2O2 results in an intracellular oxidant stress and CXCL8 production, events mediated by TLR2 [169]. On the other hand, H2O2 can elicit oxidant stress in HEK293 cells, which are devoid of TLRs [169]. The relative importance of receptor-mediated transfer versus passive permeation of H2O2 in the transfer of oxidant stress between cells warrants further attention.
Activation of macrophages with LPS also increases iNOS expression, a response that seems to be mediated via the NADPH oxidase/NF-κB pathway [171]. Intracellular NO may directly interact with ROS (superoxide) and thereby dampen the inflammatory response [160,161,172]. In addition, NO may interfere with the generation of ROS by NADPH oxidase. NO can inhibit Nox1 expression in IL-1-activated mesangial cells via a cGMP signaling mechanism [173]. Alternatively, NO can suppress endothelial cell NADPH oxidase activity by S-nitrosylation of p47phox, rather than via a cGMP pathway [174]. Regardless of the mechanisms involved, NO appears to dampen intracellular oxidative stress/signaling. Indeed, NO is generally considered anti-inflammatory particularly with respect to recruiting PMNs to affected sites [175,176]. Finally, macrophage-derived NO has been shown to dampen mast cell activity [153].
Role of interstitial sentinel cells in leukocyte recruitment
The role of interstitial sentinel cells in promoting leukocyte emigration to sites of tissue injury/infection is well documented in a variety of organ systems [145,177–181]. The primary interstitial immune cells involved in inflammation are the mast cells [182] and macrophages [122]. With respect to mast cells, induction of mast cell degranulation in vivo results in leukocyte emigration across postcapillary venules and their subsequent interstitial migration toward the activated mast cells [177]. Furthermore, the leukocyte emigration elicited by either sterile injury (I/R) or infectious challenge (Helicobacter pylori extract or Clostridium difficile toxin) is prevented by mast cell stabilizers [177]. In vitro, co-incubation of activated mast cells with endothelial cells increases PMN adhesion to the endothelial cells, whereas co-incubation with nonactivated mast cells does not [158]. With respect to macrophages, depletion of regional macrophages in vivo prevents leukocyte infiltration into the colon in response to local mucosal injury [180], as well as the lung leukocyte infiltration in response to sepsis induced by peritonitis [183]. In vitro, activated macrophages promote PMN migration across endothelial monolayers [170].
As mentioned above, in addition to interstitial immune cells, parenchymal cells can function as sentinel cells. For example, alveolar type II cells can respond to TLR ligands by generating cytokines/chemokines and promoting leukocyte emigration, i.e., they can be converted to a proinflammatory phenotype [184]. Similar phenotypic changes in cardiac myocytes can be induced by challenge with septic plasma [185] or anoxia/reoxygenation (A/R; in vitro counterpart to I/R) [186]. These activated cardiomyocytes can promote leukocyte transendothelial migration (TEM) by releasing inflammatory mediators. Interestingly, activation of endothelial cells with TLR ligands can convert them to a proinflammatory phenotype both in vivo [145] and in vitro [187]. However, if the only responding sentinel cell to PAMPs or DAMPs is the endothelial cell, it is difficult to envision how the leukocytes reach the affected site without additional cues from other interstitial cells. This issue is underscored by the observation that systemic administration of LPS in mice expressing TLR exclusively in endothelial cells induces an increase in leukocyte adhesion to brain endothelium, but the leukocytes fail to emigrate into the brain parenchyma [145].
Role of ROS and NO
It has been proposed that NO derived from eNOS keeps vascular endothelium in a quiescent state and that inhibition or removal of this source of NO upregulates the inflammatory pathway [188]. Studies using intravital microscopy to assess leukocyte interactions with venular endothelium indicate that pharmacologic blockade of NOS leads to oxidative stress within the venular wall and adjacent interstitium. This is accompanied by a rapid (within 30 min) increase in leukocyte adhesion to and migration across venules [175,189]. Moreover, basal leukocyte adhesion is increased in eNOS-deficient mice [176]. Based on these in vivo studies, it has been proposed that eNOS-derived NO is anti-inflammatory with respect to leukocyte adhesion to endothelium, presumably because of its ability to neutralize superoxide [188,190].
Qualitatively consistent with in vivo studies, pharmacologic inhibition of NOS in endothelial cell monolayers results in an intracellular oxidant stress and increased adhesivity for PMN and monocytes [191,192]. However, although the oxidant stress is noted within 30 min after inhibition of NOS, the increase in PMN or monocyte adhesion is not apparent until 2–4 h later and is not as robust as that noted in vivo. If endothelial cells are cocultured with mast cells, PMN adhesion to endothelial cells increases within 30 min after blockade of NO, a response more consistent with in vivo studies [158,175]. The increase in PMN–endothelial cell interactions was attributed to mast cell activation (e.g., oxidant stress, PAF generation). Because the PMN adhesion to endothelium in the coculture setting was prevented by extracellular SOD, it was proposed that limiting NO bioavailability resulted in an increase in endothelial production of ROS, which activated the adjacent mast cells [158].
Challenge of cardiac myocytes with A/R (sterile stress) results in an oxidant stress (linked to increased H2O2 production) and induction of a proinflammatory phenotype, i.e., it promotes PMN TEM [186,193]. Similarly, macrophage activation during sepsis (infectious stress) can induce oxidant stress in adjacent endothelial cells with a resultant increase in PMN TEM [170]. This response was dependent on the activation of endothelial cell NADPH oxidase because genetic blockade of NADPH oxidase prevented the PMN TEM [170]. An interesting study in zebrafish larvae indicates that epithelial cells at a sterile wound edge (tail-fin tip amputation) generates a H2O2 gradient via Duox (a member of the NADPH oxidase family) to recruit neutrophils to the wound [194]. It was proposed that H2O2 could be directly chemotactic for PMNs or that PMN migration toward the wound was due to chemotactic factors released from cells activated by H2O2. Collectively, these observations indicate that interstitial sentinel cells (e.g., myocytes, macrophages) that incur an oxidant stress upon activation can “transfer” ROS (probably as H2O2) to other cells (e.g., endothelial cells) and thereby facilitate PMN recruitment.
Endothelial cell capture of leukocytes (Fig. 2B)
To reach the site of injury/infection, circulating leukocytes must first be captured by the endothelium, i.e., adhere to endothelium. The capture of leukocytes by vascular endothelium is a highly regulated process involving sequential engagement of various families of endothelial and leukocyte adhesion molecules [116,117,119]. In general, leukocyte–endothelial cell adhesive interactions occur in postcapillary venules. As leukocytes leave the small-caliber capillaries and enter the larger postcapillary venules, they are forced toward the endothelial lining by hydrodynamic forces, thereby allowing them to interact with the endothelium [195]. If an inflammatory focus is present locally, the leukocytes form weak adhesive interactions with the endothelium, referred to as tethering or rolling. These initial adhesive interactions involve P-selectin and E-selectin on the endothelium and PSGL-1 and ESL-1 on neutrophils. Monocytes can roll on endothelial selectins and on the cell adhesion molecule VCAM-1. Leukocyte arrest during rolling is attributed to activation of leukocyte integrins by chemokines or other activators (e.g., PAF) present on the surface of the endothelium. Activated CD11/CD18 integrins on neutrophils interact with ICAM-1 on endothelial cells, whereas activated VLA4 on monocytes interacts with VCAM-1 on endothelial cells. After adhering to the endothelium, leukocytes change shape and crawl to a preferred site for emigration. Although the adhesion cascade described is generally accepted as representative, there are numerous overlaps with respect to the roles of various adhesion molecules in the successful capture of PMN and monocytes, as well as some notable variations related to specific organ systems [116,117,119,196–198].
Leukocyte–endothelial cell interactions occur despite the presence of an extensive filamentous network covering the surface of the endothelium, i.e., the glycocalyx. The glycocalyx decorating the endothelial cell surface consists of proteoglycans and associated glycosaminoglycan (heparan sulfates, chondroitin sulfates, and hyaluronan) side chains [75,76]. The glycocalyx extends for approximately 500 nm from the surface of the endothelial cell. This compares to the 10- to 30-nm length of the endothelial adhesion molecules that are responsible for capturing circulating leukocytes [116,199]. Although it would seem that interactions between leukocyte adhesion molecules and their counterparts on endothelial cells would be severely hindered by the glycocalyx, the steric hindrance is overcome somewhat by the localization of PSGL-1 (ligand for endothelial selectins) on PMN microvilli, which are approximately 300 nm in length [200]. Furthermore, the PMN-derived cationic protein myeloperoxidase may interact with negatively charged heparin sulfates and allow PMNs to overcome the electrostatic hindrance of the glycocalyx and penetrate it sufficiently to interact with endothelial adhesion molecules [201]. Finally, leukocyte capture by endothelial cells during inflammation is facilitated by partial degradation of the glycocalyx and shedding of its constituents (e.g., heparan sulfate), thereby exposing relevant adhesion molecules [199,202–204]. The degradation and shedding of the glycocalyx is likely to play an important role in leukocyte recruitment because heparan sulfates localized within this structure appear to regulate the presentation of chemokines on the endothelial cell surface, which is critical for the activation and capture of leukocytes [79,205].
The endothelial lining of the microvasculature is not simply a passive barrier to leukocyte emigration, but an active participant in the inflammatory response [117,206–208]. Endothelial cell activation has been conveniently subdivided into two types of functional responses: a rapid response, which is transcription independent, and a more delayed response, which is transcription dependent [206]. According to this paradigm, the rapid response is generally initiated by ligands of GPCRs, such as histamine, PAF, LTB4, and CXCL8. Activation of GPCRs results in three major functional events of relevance to endothelial cell–PMN interactions. First, activation of endothelial matrix metalloproteases (MMPs) results in local degradation of the glycocalyx, which facilitates leukocyte–endothelial cell adhesive interactions [204]. Second, activation of myosin light-chain kinase induces mobilization of Weibel-Palade bodies, which transport P-selectin [206] to the endothelial cell membrane and CXCL8 to the endothelial glycocalyx [209]. Third, activation of phospholipase A2 ultimately results in the generation of PAF from phosphatidylcholine, which is subsequently secreted and binds to the endothelial glycocalyx [206]. The rapid response is short-lived (<30 min) and serves to tether leukocytes (via P-selectin) and activate them (via PAF and CXCL8). The delayed response is generally elicited by LPS or cytokines, such as IL-1 and TNFα [206]. Activation of their respective receptors results in an intracellular signaling cascade culminating in NF-κB/AP-1-mediated transcription of genes encoding adhesion molecules (E-selectin, ICAM-1, VCAM-1) and chemokines (e.g., CXCL8). The adhesion molecules are expressed on the endothelial cell membrane, whereas the chemokines are bound to the glycocalyx. The delayed response is long-lived (> 12–24 h) and allows for further activation of neutrophils and monocytes (via CXCL8 for neutrophils and CCL2 for monocytes) and promotes firm adhesion to the endothelium (via ICAM-1 for neutrophils and VCAM-1 for monocytes).
During inflammation, the rapid and delayed functional responses of activated endothelial cells are not discrete. For example, inflammatory agents that elicit rapid responses can also elicit delayed responses, i.e., histamine can elicit an NF-κB-dependent increase in VCAM-1 expression [210]. Thus, in vivo, where the interstitial milieu consists of a variety of inflammatory mediators (cytokines/chemokines), the rapid and delayed phases of functional activation of the endothelium most probably occur as a continuum. A good example to illustrate this point is I/R-induced inflammation. In an in vitro model of I/R (A/R) in which only endothelial cell monolayers are exposed to A/R, there are two phases of PMN adhesion to the monolayers, a rapid phase occurring within 30 min (NF-κB independent) and a delayed phase occurring at 120 min (NF-κB dependent) [211–213]. However, in vivo the two phases of I/R-induced leukocyte adhesion to postcapillary venules are not as distinct, but appear to be a continuum. Leukocyte adhesion gradually increases over the first 1–2 h and subsequently increases more rapidly over the next 2 h [214].
Role of ROS and NO (Fig. 2B)
Under basal conditions, endothelial cell ROS production is generally attributed to superoxide generation during mitochondrial respiration [215], although NADPH oxidase may also contribute [216]. No appreciable oxidant stress is incurred under basal conditions, because ROS accumulation is limited by the scavenging ability of endogenous antioxidant molecules (e.g., SOD, glutathione, catalase) [217]. However, the endothelial cell activation that accompanies inflammation generally results in a large increase in ROS production that has been largely attributed to cytosolic and/or membrane-bound enzymes such as NADPH oxidase and xanthine oxidase [218]. Of these, NADPH oxidase is considered to be the most critical [167,218–220], in part because ROS production by NADPH oxidase can influence other ROS-generating sources, such as mitochondria (oxidative damage) and xanthine oxidase (oxidative activation), to enhance their production of ROS [167,218,221]. Although enzymatic generation of ROS usually involves an initial production of superoxide, it is rapidly converted to H2O2, which is the most important ROS involved in intracellular signaling [215].
Activation of endothelial cells with some GPCR ligands results in an intracellular oxidant stress leading to rapid conversion to a proinflammatory phenotype [220]. For example, challenge of endothelial cells with thrombin or CXCL8 results in a rapid (30–60 min), but transient (<2 h), increase in ROS production, which is mediated by NADPH oxidase [222,223]. The thrombin-induced ROS production is associated with P-selectin expression (rapid functional response) [223]. Activation of endothelial cells with either LPS or TNFα also results in a rapid (30 min) generation of ROS in endothelial cells, which is primarily dependent on NADPH oxidase and to a lesser extent mitochondria; xanthine oxidase and NOS (uncoupled) do not seem to be involved [164,224]. The LPS-induced ROS production by endothelial cells is more robust and prolonged than noted with the GPCR ligand CXCL8 [222]. The increase in oxidant stress induced by LPS and TNFα has been implicated in NF-κB-mediated transcription of adhesion molecules and chemokines that are involved in leukocyte capture by endothelial cells (delayed functional response) [146,206]. Collectively, these observations support a continuum of endothelial cell activation characterized by the induction of an initial oxidant-mediated (NF-κB-independent) proadhesive phenotype, followed by a subsequent, more prolonged (NF-κB-dependent) response.
As mentioned above, the source and roles of ROS in mediating the rapid and delayed activation of endothelial cells may not be readily differentiated during an inflammatory response. For example, histamine (GPCR ligand) induces a ROS-mediated (NADPH oxidase-derived), NF-κB-dependent increase in endothelial VCAM-1 mRNA within 60 min [210]. Conversely, LPS-induced ROS production is mediated by a CXCL8/NADPH oxidase pathway [222]. In both instances, endothelial cell ROS play an important role in the induction of the proadhesive phenotype. A more direct (receptor-independent) method of inducing an oxidant stress in endothelial cells is to challenge them with either A/R or H2O2 [212,225]. This approach results in an initial (30 min) phase of PMN adhesion to endothelium (oxidant-induced PAF generation) followed by a delayed (4 h) phase of PMN adhesion (e.g., NF-κB-mediated adhesion molecule expression). The sources of ROS after A/R include mitochondria, xanthine oxidase, and NADPH oxidase [212,226]. Once generated, ROS can be self-perpetuating. For example, the H2O2 generated via NADPH oxidase can further activate the enzyme resulting in propagation of the H2O2 signal [167,221]. Left unchecked, such a “feed-forward” mechanism would be detrimental to cell viability. However, increases in endothelial oxidant production result in increases in the production of antioxidant enzymes (e.g., SOD) via NF-κB [225] and Nrf2 [216]. The antioxidants minimize the oxidant stress and blunt the conversion to a proadhesive phenotype.
GPCR activation can also generate NO within endothelial cells. An interesting example is the bradykinin receptors, B2R (constitutive) and B1R (inducible) [227]. B2R activation results in eNOS activation and the transient production of NO. The role of NO in the development of a proadhesive phenotype in endothelial cells is unclear. As mentioned above, inhibition of endogenous NO production results in an NF-κB-mediated increase in endothelial surface expression of adhesion molecules and capture of leukocytes [192]. However, eNOS deficiency, per se, does not affect cytokine-induced increases in adhesion molecule expression or leukocyte adhesion to the endothelium [228]. It has been proposed that eNOS-derived NO, per se, does not directly modulate endothelial cell conversion to a proadhesive phenotype, but rather serves to dampen the impact of ROS by interacting with superoxide and preventing further ROS generation. In an oxidative environment eNOS can be “uncoupled,” i.e., converted from an NO-producing enzyme to a ROS-producing one [190,229]. The uncoupling of eNOS is generally attributed to a deficiency in BH4 due to its oxidation by NADPH oxidase-derived ROS. However, the relative importance of BH4 levels on eNOS uncoupling has been questioned [230]. Regardless, eNOS uncoupling during oxidative stress would be predicted to exacerbate endothelial cell activation (conversion to a proinflammatory phenotype).
Endothelial NADPH oxidase-derived ROS can induce the expression of iNOS [231] as can activation of the B2R on endothelial cells [227]. How this may influence the overall activation of the endothelium is not entirely clear. Although iNOS uncoupling can occur in an oxidative environment, iNOS activity is relatively resistant to H2O2 in this respect [232]. Alternatively, the high output of NO from iNOS may serve to inhibit NADPH oxidase activity via S-nitrosylation of p47phox, thereby inhibiting ROS production by NADPH oxidase [174] and limiting endothelial cell activation. Clearly, the precise role of NO generated via either eNOS or iNOS in the capture of leukocytes by endothelium remains unclear and additional work is warranted.
Both ROS and NO can have an impact on the integrity of the glycocalyx. In an I/R model of inflammation, ROS generated by endothelial cell membrane-bound xanthine oxidase was implicated in the local degradation of the glycocalyx [233]. Furthermore, exogenous administration of aqueous NO has been shown to prevent the shedding of the endothelial glycocalyx induced by I/R [234]. A causal link between either ROS or NO and glycocalyx integrity and leukocyte adhesion to endothelium seems likely [235]; however, it has not been firmly established.
Endothelial regulation of leukocyte transendothelial migration
Once captured by ICAM-1 and VCAM-1, the leukocyte adhesive interactions are strengthened by enrichment (clustering) of these adhesion molecules at the base of the adherent leukocytes, forming “docking structures” [118,208]. In addition, adhesion molecules implicated in TEM, such as PECAM-1 and CD99, are recruited to the docking structures via membrane recycling that involves vesicle-like trafficking to the site of leukocyte adhesion [118,208,236]. Under some experimental conditions (e.g., excessive activation of leukocytes [118]), endothelial cell projections have been observed enveloping the adherent leukocyte [237,238]. Leukocyte TEM can occur via either a paracellular or a transcellular pathway [116,118,119,196,239]. Regardless of the pathway utilized, it is currently accepted that active participation of the endothelium is critical for leukocyte TEM [207,240–242]. Although the membrane dynamics and adhesion molecules involved may be very similar for the para- and transcellular pathways [118,236,243], the endothelial cell signaling mechanisms involved in leukocyte TEM have been more extensively characterized for the paracellular pathway.
An obvious assumption regarding paracellular TEM is that it requires separation of adjoining endothelial cells. There are two major interendothelial adhesion junctions (IEJs) that directly interact with the actin cytoskeleton to maintain endothelial barrier integrity, i.e., adherens and tight junctions. The adherens junction (AJs) complex consists of vascular endothelial cadherin (VE-cadherin), whose extracellular domain forms homotypic adhesion interactions with VE-cadherin on adjacent endothelial cells [244]. The intracellular domain forms complexes with α-, β-, and γ-catenin, which allows for an interaction with the actin cytoskeleton. Within tight junctions (TJs) occludins/claudins form homotypic bonds with their counterparts on adjacent endothelial cells [240,245]. Their cytoplasmic domains form complexes with ZO-1/ZO-2 to allow for interaction with actin. The stability of the AJ and TJ complexes is primarily regulated by the balance of activities of protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs) [246]. In general, phosphorylation of AJ and TJ components loosens junctions, whereas dephosphorylation stabilizes the junctions. For example, the PTP, VE-PTP, is associated with VE-cadherin and keeps the cadherin in a dephosphorylated state, favoring AJ stability [244]. By contrast, increased activity of PTKs can phosphorylate components of both adherent and tight junctions, leading to their disassembly, e.g., Rho kinase can phosphorylate occludin [247] and pyk2 can phosphorylate β-catenins [248].
Endothelial cell-associated MMPs have been shown to play an important role in leukocyte TEM, i.e., inhibition of MMP activity blunts leukocyte TEM [241,249,250]. Because MMPs are capable of degrading both VE-cadherin [251] and occludin [252], as well as inducing gap formation between endothelial cells, it has been proposed that endothelial cell MMPs contribute to the disassembly of the adherens and tight junctions during leukocyte paracellular migration [241]. However, the role of MMPs in loosening IEJs is controversial. VE-cadherin is generally believed to be internalized rather than degraded [118] and the role of MMPs in the degradation of occludin is equivocal [250,253]. Further, the direct addition of MMP-2 to naïve (nonactivated) endothelial cells does not induce occludin cleavage or gap formation [252]. Interestingly, inhibition of MMPs has been shown to prevent formyl methionine leucine phenylalanine (fMLP)-induced (1) degradation of the glycocalyx and (2) PMN adhesive interactions with endothelium [204]. The possibility that endothelial-derived MMPs may modulate leukocyte TEM via localized degradation of the glycocalyx warrants further attention. It is conceivable that MMPs facilitate leukocyte migration via degradation of key components of both IEJs and the glycocalyx.
Role of ROS and NO
There is evidence that ROS and/or NO may be involved in the signaling of ICAM-1- and VCAM-1-dependent leukocyte TEM across paracellular junctions. Ligation of ICAM-1 on cytokine-activated endothelial cells with PMNs or antibodies results in endothelial cytoskeleton reorganization, an effect attributed to ROS generation by xanthine oxidase [254]. Ligation of endothelial cell ICAM-1 on nonactivated endothelium with leukocytes or antibodies has also been shown to activate eNOS (phosphorylation) and generate NO [255]. Pharmacologic blockade of eNOS or sGC, but not xanthine oxidase nor NADPH oxidase, inhibits leukocyte TEM. Blockade of eNOS also prevents VE-cadherin phosphorylation. Based on these and previous studies [256], it is proposed that eNOS-derived NO is critical for VE-cadherin phosphorylation and leukocyte TEM. Because of the paucity of information on the role of ROS and NO in ICAM-1 signaling that leads to leukocyte TEM, it is difficult to reconcile with any certainty why some studies implicate ROS and others support a role for NO. It has been proposed that ligation of ICAM-1 on nonactivated endothelium may favor NO generation, whereas ligation of ICAM-1 on cytokine endothelium would favor ROS generation [255].
VCAM-1 engagement by leukocytes or antibodies results in activation of NADPH oxidase and the resultant ROS generation is believed to facilitate leukocyte paracellular TEM via two potential mechanisms: phosphorylation of VE-cadherin [240,245] and activation of MMPs [241,242]. The current consensus is that VCAM-1 clustering at the docking structure results in Rac1 activation, which leads to activation of NADPH oxidase and the generation of ROS [118,240,245]. Other sources of ROS do not seem to be involved because only blockade of NADPH oxidase interferes with leukocyte TEM; blockade of xanthine oxidase or NOS does not [242].
NADPH oxidase-derived ROS are believed to activate the PTKs and inhibit the PTPs, thereby altering the PTK/PTP balance in favor of PTKs and facilitating VE-cadherin/catenin phosphorylation [240]. For example, in the context of leukocyte TEM, NADPH oxidase-derived ROS has been shown to activate pyk2 (kinase), which subsequently phosphorylates β-catenin and results in the disassembly of the VE-cadherin/catenin complex [248]. Furthermore, ROS generation by VCAM-1 ligation can activate kinase cascades (e.g., PKC/Src kinase pathway) [257,258] leading to VE-cadherin phosphorylation [259]. NADPH oxidase-derived ROS can also activate a phosphatase, PTP1B, the activity of which seems to be critical for leukocyte TEM [260]. The activation of PTP1B is a result of ROS-induced activation of the kinase PKCα. This observation underscores the complexity of ROS-mediated modulation of the PTK/PTP balance with respect to leukocyte TEM.
The enhanced production of NADPH oxidase-derived ROS elicited by VCAM-1 ligation has also been implicated in the activation of endothelial-cell-associated MMP-2 and MMP-9 [249]. A comparable level of MMP activation results after endothelial cell exposure to H2O2, at concentrations detected after VCAM-1 ligation. MMP activation occurs within minutes and pharmacologic blockade of MMP activity substantially blunts leukocyte TEM. Components of adherens and tight junction complexes are considered the likely targets of the activated MMP [241,251]. As discussed above, NADPH oxidase-derived ROS have also been implicated in the disassembly of endothelial cell junctions via phosphorylation-mediated events. The question therefore arises as to whether phosphorylation of junction components and MMP-mediated degradation of these components occurs simultaneously to ensure gap formation and facilitate leukocyte TEM. One possibility is that phosphorylation events occur with a mild inflammatory stimulus and as stimulus intensity increases proteolytic events begin to contribute. This possibility is supported by the observation that low concentrations of H2O2 induce occludin phosphorylation, whereas higher concentrations result in occludin proteolysis [252].
Vascular permeability
Under resting conditions, the endothelial lining of the microvasculature is fairly permeable to small-molecule solutes (e.g., glucose, <3-nm radius) yet it significantly restricts the movement of plasma proteins (e.g., albumin; ≈6-nm radius). Transendothelial movement of protein and fluid can be envisaged as involving either paracellular (between cells) or transcellular (through cells) pathways or some combination of both [261–263]. During inflammation, chemical cues released by sentinel cells (e.g., macrophages, mast cells) activate endothelial cell signaling pathways, which target structural elements (e.g., actin/myosin) that regulate vascular permeability. Widening of the interendothelial junctions and frank separation of endothelial cells (gaps) results in microvascular protein (and fluid) leakage into the interstitium (edema). Alterations in other endothelial structural elements (e.g., intracellular vesicles, glycocalyx, basement membrane) can also contribute to the barrier dysfunction that accompanies inflammation [261–263].
Structural determinants: endothelium
Ultramicroscopic studies (e.g., electron microscopy) have identified several structural elements in the endothelial lining of the microcirculation that can potentially serve as paracellular pathways for solute and fluid exchange [264]. Interendothelial junctions consist of protein complexes that couple adjacent endothelial cells and maintain them in close apposition. Within the IEJs there are two major complexes relevant to paracellular permeability: TJs and AJs. The paracellular width imposed by the adhesive interactions of AJ complexes is ≈15–20 nm, whereas that imparted by TJ complexes is only ≈5 nm [265,266], TJs being further subdivided into closed (<3 nm) or open (>3 nm) [266]. The density (and/or ratio) of TJs and AJs in the IEJs seems to determine their restrictive properties [261]. In most vascular beds, water and small hydrophilic solutes (<3 nm in diameter) are believed to freely traverse the capillary endothelium via IEJs, the exception being the brain, where TJ density is relatively high and their width may be <1 nm (blood–brain barrier) [261,267,268].Within a vascular bed, the venular end of the microcirculation has a lower TJ/AJ ratio and the TJ are less well developed than at the arterial end. Furthermore, in regions where three venular endothelial cells converge (tricellular region) the IEJ width can be as great as 30 nm [269]. Because the diameter of albumin is ≈6–7 nm, the venular end of the microcirculation is the major site of albumin leakage[1,270,271].
At the molecular level, TJs contain occludin and claudin, whose extracellular domains allow for homotypic interactions between endothelial cells, whereas within the AJs the extracellular domains of VE-cadherin allow for such interactions [270,272,273]. Their respective intracellular domains interact with proteins (e.g., VE-cadherin with catenins, occludin with ZO-1) that create structural/functional linkages with the actin cytoskeleton (e.g., cortical actin). Loss of function studies indicate that (1) claudin-5 plays a role in permselectivity of brain capillaries to small molecules (<0.8 kDa) [274] and (2) VE-cadherin is important in restricting albumin leakage from heart and lung microvessels [275]. Although TJs and AJs are generally considered independent entities there is significant “cross talk” between these IEJ complexes, which could have an impact on endothelial barrier function [276–279].
In addition to the paracellular exchange pathway, macromolecular transport can occur via a transcellular pathway. Transcellular exchange involves specialized endothelial organelles such as fenestrae or vesicle-like structures (e.g., caveolae) [271,280–283]. Fenestrae (20–30 nm) are generally covered with a semipermeable diaphragm, except in the liver, where they are not [261,264,284]. Fenestrae appear to allow the free and rapid passage of water and small solutes, yet their diaphragms offer restriction to the movement of proteins (behaving as 5-nm filters) [285]. Vesicles or caveolae (20–30 nm) associated with abluminal and luminal endothelial membranes have stomata that are generally covered by a diaphragm. It has been proposed that the caveolae can “shuttle” engulfed material (e.g., proteins) across the endothelium [261,264,286]. Alternatively, the vesicles may coalesce, forming transendothelial channels through which plasma constituents may traverse the endothelium. A unique potential transendothelial channel, the vesiculovacuolar organelle (VVO), has also been noted in close proximity to IEJs, predominantly in postcapillary venules [261,264,280]. The VVOs are grape-like clusters of vesicle-like structures (20–30 nm or multiples thereof) linked to one another via diaphragmed stomata and generally extending from the abluminal to the luminal aspect of the endothelium.
Although there is general agreement that both paracellular and transcellular pathways contribute to transendothelial protein and fluid exchange, the relative contributions of the two pathways are controversial. This issue may prove to be difficult to resolve, because there are indications that there may be structural and functional linkages between the two pathways. For example, the frequency of vesicle-like structures (e.g., VVOs) is much higher in the vicinity of IEJs and frequently the VVOs coalesce with the IEJs at various points [286]. Furthermore, caveolin-1 protein levels can influence the relative distribution and/or density of molecular constituents of IEJs [287]. Indeed, an attempt has been made to address a similar controversy regarding leukocyte movement across endothelium (paracellular vs transcellular) by proposing that there is an interaction between IEJ components with vesicle-like structures [236,288].
Structural determinants: glycocalyx and basement membrane
Endothelial cell-associated structures on both the luminal and the abluminal aspect of the cell have also been implicated in the regulation of vascular permeability [199]. The luminal surface of the endothelium is covered by a glycocalyx (200–500 nm in thickness) consisting of proteoglycans with GAG side chains (e.g., heparan sulfates) [76,289]. Enzymatic degradation of the glycocalyx components reduces its thickness and increases transendothelial albumin flux [290]. Conversely, stabilizing the glycocalyx (e.g., angiopoietin-1) decreases albumin permeability [291]. The overall negative charge of the GAGs is believed to impose a significant barrier to protein movement through the glycocalyx, but poses little hindrance to the movement of water [283,290].
The basement membrane (BM) adjacent to the abluminal aspect of the endothelium may also contribute to the microvascular permeability. Although the BM contains GAGs, in addition to collagen IV and laminin [283,292], the density of negative charges is too low to confer any significant charge selectivity with respect to albumin movement [293]. Overall, the limited amount of studies on the permeability properties of the BM suggest that it has minimal impact on protein flux across the microvasculature [283].
Functional assessments
Various in vivo and in vitro approaches have been used to assess the restrictive properties of the endothelial barrier [294–297]. In vivo approaches include whole-organ estimates of the osmotic reflection coefficient for plasma proteins (lymphatic flux analysis), microvascular bed protein leakage, in situ single-vessel estimates of hydraulic conductivity (Lp) or solute permeability (Ps), and discrete estimates (e.g., electron microscopy (EM)) of transcapillary protein movement. In vitro approaches have involved ex vivo single-vessel estimates of Ps or an assessment of endothelial cell monolayer Lp, electrical resistance (TEER), or transendothelial movement of protein. All of these approaches have their advantages and limitations. In vivo approaches allow for the assessment of barrier integrity in the context of a physiologic milieu, which also includes the contribution of various accessory structures (glycocalyx) and cells (e.g., leukocytes) [294,297]. However, the possibility that changes in solute flux may be a result of convective (filtration-dependent) and/or diffusive (surface area-dependent) movement, rather than alterations in the restrictive properties of the endothelium, must be taken into consideration. In vitro approaches allow for the assessment of various intracellular signaling pathways and their impact on the endothelial structures relevant to microvascular protein exchange under rigorously controlled conditions [294]. However, endothelial cell monolayers do not completely recapitulate the structural and functional properties of microvessels in vivo [294,296,298], i.e., endothelial monolayer permeability is generally higher than postcapillary permeability. Despite their limitations, both in vivo and in vitro approaches have yielded unique, yet complementary, information that has provided the basis for current concepts regarding the regulation of microvascular permeability.
Basal vascular permeability: role of ROS and NO
“Loss-of-function” approaches (pharmacologic and genetic blockade) have implicated NO in the regulation of basal vascular permeability. Pharmacologic blockade of NO production (exogenous and endogenous NOS inhibitors) increases basal microvascular permeability in a variety of vascular beds in vivo [299–303]. In addition to increasing vascular protein leakage, NO inhibitors also promote leukocyte– endothelial cell adhesion [175,300], and when the leukocyte adhesion is prevented (e.g., by immunoblockade of adhesion glycoproteins) the increased vascular protein leakage is also prevented [175,300]. However, efforts to “flush out” leukocytes from the microvasculature (e.g., blood-free perfusion of microvessels) have yielded equivocal results; blockade of NOS either did [302,304] or did not increase permeability [301,303]. This inconsistency may reflect the influence of perivascular interstitial cells on the vascular permeability response to alterations in NO bioavailability. For example, in blood-free preparations, the increase in albumin leakage and associated endothelial actin reorganization induced by NOS inhibitors are associated with mast cell degranulation [304]. Indeed, a role for both circulating leukocytes and interstitial mast cells has been proposed in modulating basal vascular permeability [1,305]. Consequently, although local inhibition of NOS in a vascular bed can increase microvascular permeability, it is difficult to differentiate the direct effects of NOS inhibitors on the endothelium per se from those resulting from inhibition of NO production by local circulating or interstitial accessory cells.
Time-course studies in blood-perfused mesenteric preparations have revealed an early (leukocyte-independent) and late (leukocyte- dependent) phase of albumin leakage in response to an NO inhibitor [300]. The late phase of protein leakage was directly related to the extent of leukocyte–endothelial adhesive interactions (adhesion and emigration). The early phase was attributed to a direct action on the endothelial cell, which is consistent with reports that NOS inhibitors can increase the flux of macromolecules across isolated glomeruli [306,307] or endothelial cell monolayers in vitro [308–310]. The endothelial barrier dysfunction detected in monolayer preparations is associated with the formation of stress fibers and disruption of adherens junctions [310]. In both in vivo and in vitro models, a cyclic GMP analogue can reverse the functional/structural effects of NOS inhibition [300,310]. This observation implicates an NO/cGMP pathway in microvascular barrier stabilization under basal conditions.
Genetic blockade of eNOS has also yielded inconclusive results regarding the role of NO in the regulation of vascular permeability. In one study, basal albumin permeability was increased in multiple vascular beds of eNOS-deficient mice, a result confirmed in wild-type mice after long-term treatment with an NOS inhibitor [302]. The increase in transvascular albumin flux was attributed to open IEJs in all vascular beds examined, i.e., lung, heart, gut, and muscle. Transendothelial trafficking of caveolae was not affected. However, in another study it was noted that basal vascular albumin flux in the mesentery does not differ between eNOS and wild-type mice [311]. Mice deficient (heterozygous) in DDAH (dimethylarginine dimethylaminohydrolase) exhibit an increased basal albumin leak across pulmonary capillaries [310]. Because DDAHs metabolize the endogenous NOS inhibitor asymmetric methylarginine (ADMA) [312], this finding was attributed to inhibition of NOS. Furthermore, endothelial cell monolayers derived from DDAH1-deficient mice also exhibited an enhanced permeability, which could be restored by a cGMP analogue [310]. Collectively, the genetic blockade approaches targeting eNOS provide support for the contention that the NO/cGMP pathway plays a role in stabilizing microvascular permeability in the basal state.
By contrast, genetic approaches designed to enhance eNOS activity suggest that eNOS-derived NO may destabilize the endothelial barrier. Under basal conditions, most of the intracellular pool of eNOS is associated with the scaffolding domain of caveolin-1, which prevents eNOS activation and function [313,314]. Therefore, it would be predicted that removal of this inhibitory function of caveolin-1 would result in increased NO production and a strengthening of the endothelial barrier against protein extravasation. However, caveolin-1- deficient mice are “hyperpermeable” as evidenced by an enhanced escape of plasma protein from the circulation and a corresponding accumulation of protein in a variety of organs [315]. It is corrected, however, by an inhibitor of NOS activity. The hyperpermeable state noted in caveolin-1-deficient animals was attributed to loosening of endothelial cells from the basement membrane and an increased permeability of IEJs [315]. Downregulation of caveolin-1 (siRNA for caveolin-1) in endothelial cell monolayers results in loss of both TJ (occludin and ZO-1) and AJ (VE-cadherin and catenin) proteins from the IEJ [316]. Another approach resulting in enhanced eNOS activity and NO production by endothelial cells is deletion of CEACAM-1 [317]. CEACAM-1-deficient mice are also hyperpermeable, as evidenced by increased basal protein accumulation in various tissues and ultrastructural evidence of disorganized IEJs.
The discordant results regarding the role of eNOS-derived NO on basal microvascular permeability are somewhat paradoxical. It has been suggested that in in vivo studies alterations in hemodynamic parameters (e.g., capillary surface area, hydrostatic pressure) can influence transvascular protein movement [295–297,318]. Of note is the argument that the enhanced vascular protein leakage noted in caveolin-1-deficient mice may not be due to alterations in microvascular permeability per se, but rather due to the NO-induced vasodilation. In this scenario, the vasodilation results in an increase in capillary hydrostatic pressure, which, in turn, simply increases the filtration of plasma proteins across an intact endothelial barrier [319,320]. Whereas hemodynamic alterations may be a confounding variable in states that increase endogenous NO production, they are unlikely to account for the increased vascular permeability noted during conditions that inhibit NO production [299].
Less attention has been devoted to the role of ROS in regulating basal endothelial barrier function. There is no published information on the effects of blockade of production or bioactivity of endogenous oxidants on basal microvascular permeability, as is the case for endogenous NO (e.g., NOS inhibitors). However, there is considerable evidence that exogenous oxidants increase permeability [321,322]. Based on the ability of superoxide to interact rapidly with NO, the overall consensus is that, in the basal state, the hyperpermeability associated with oxidant production is neutralized by the continuous endogenous NO production. This contention is supported by the following lines of evidence. In blood-perfused mesenteric venules, the early (leukocyte-independent) phase of albumin leakage induced by inhibition of endogenous NO production is associated with an increase in perivenular oxidant stress, which is blunted, along with albumin leakage, by treatment with antioxidants [189]. In in vitro studies, enzymatically generated superoxide can increase glomerular permeability to albumin, an effect prevented by an NO donor [306]. Finally, the increase in permeability of isolated glomeruli [306,307] or endothelial monolayers [308] induced by NO inhibitors can be rescued by inhibitors of NADPH oxidase or antioxidants.
Increased vascular permeability
Structural basis for hyperpermeability states
With respect to the paracellular pathway, disruption of IEJ complexes and widening of the interendothelial junctions may account for the enhanced vascular protein leakage in hyperpermeability states [261,262]. In general, alterations of the local PTK/PTP ratio in favor of PTKs promote phosphorylation of junctional proteins leading to their internalization and ultimately disassembly of the junctional complex [272,273]. In addition, the cortical actin adjacent to the IEJ is destabilized (F-actin converted to G-actin) [323], decreasing peripheral tension [324]. Finally, endothelial actomyosin-mediated contraction and central tension development pull the IEJ apart, creating “gaps” [6,324,325]. Actomyosin-mediated contraction is dependent on myosin light chain (MLC) phosphorylation, which, in turn, is regulated by the MLC kinase/MLC phosphatase balance [325]. In some hyperpermeability states, alterations in junctional complexes occur independent of alterations in cytoskeletal elements, i.e., blockade of actin/myosin interactions does not affect the disassembly of IEJ [296] or even gap formation [323]. It has been proposed that weak stimulation leads to junctional disruption of IEJ and stronger stimulation reinforces this response by initiating actin–myosin-based endothelial cell retraction [270,273].
With respect to the contribution of the transcellular pathway to hyperpermeability states, loss of diaphragms and widening of the VVO stomata [286] and an increase in the diameter of nondiaphragmed fenestrae have been implicated [326]. Little is known about the mechanisms involved, but it has been proposed that actin–myosin contractions contribute to these ultrastructural readjustments [280,286,327]. Increased vesicle trafficking has also been proposed to explain the increased albumin leakage that occurs during an inflammatory response [328].
Cellular and chemical mediators
Specific signals generated during injury/infection (PAMPs and DAMPs) can activate resident interstitial immune cells to initiate the inflammatory response. Perivascular mast cells have been implicated in the microvascular protein leakage associated with the inflammatory response induced by either infectious [329,330] or noninfectious insults [331,332]. Pharmacologic stabilization or genetic deletion of mast cells has been shown to blunt the leukocyte–endothelial cell adhesive interactions and vascular protein leakage in several experimental models of inflammation [1,177,333]. Furthermore pharmacologic activation of mast cells promotes leukocyte adhesion and protein leakage [177,334]. Analogous experimental approaches have also implicated resident macrophages in the microvascular protein leakage associated with leukocyte invasion of inflamed tissue. For example, pharmacologic depletion of macrophages or blockade of macrophage-derived chemokines reduces PMN emigration and vascular protein leakage [335,336].
Impact of circulating immune cells
Pharmacologic or genetic blockade of neutrophil–endothelial adhesive interactions can blunt the leakage response in experimental models of inflammation [1]. Furthermore, the magnitude of the albumin leakage is directly correlated with the number of leukocytes adhering to or migrating across the endothelium in vivo (mesenteric venules) [300,332] and in vitro (endothelial monolayers) [337]. PMN-mediated increases in vascular permeability occur even though the underlying endothelium extends projections to envelop the adherent/migrating PMNs, forming endothelial domes; the leakage response is presumably due to the transfer of entrapped plasma proteins within the “dome” [237,325].
The PMN-induced leakage response occurs whether PMN are utilizing the paracellular or the transcellular pathway [237]. There is evidence that PMN-derived proteases (e.g., elastase) can disrupt IEJ complexes and induce endothelial cell retraction, thereby increasing paracellular permeability [294,338]. Alternatively, PMN ligation of endothelial adhesion molecules can elicit both actin–myosin-based endothelial cell tension development and IEJ disassembly, resulting in widening of the IEJ or gap formation [245,325]. Endothelial cell adhesion molecule-dependent signaling can also increase transendothelial albumin movement via a caveola transport pathway, without affecting the paracellular pathway [339,340].
While there is a large body of evidence supporting a role for neutrophil-mediated increases in vascular permeability during an inflammatory response [297], there is also evidence indicating endothelial barrier dysfunction can be uncoupled from neutrophil–endothelial cell adhesion [305]. In blood-perfused vascular beds, induction of infectious [329] or sterile [214] inflammation increases both vascular protein leakage and neutrophil–endothelial adhesive interactions [329,332]. However, the protein leakage response can be subdivided into an early and a late phase, the early phase being PMN independent and the later phase being PMN dependent. Furthermore, the increase in transendothelial albumin flux associated with macrophage/mast cell activation is noted in both blood-free preparations in vivo and endothelial monolayers sans neutrophils in vitro [304,341–344]. Finally, histamine, a mast-cell-derived mediator, can increase vascular albumin leak without affecting leukocyte–endothelial adhesive interactions [345]. Collectively, the data are consistent with a direct impact of inflammatory mediators on endothelial permeability, which is further reinforced by PMN–endothelial cell interactions.
Although T-lymphocytes (CD4+ and CD8+) are classically viewed as components of the adaptive immune system, they have been implicated in vascular responses to acute inflammation (innate immune system) [297]. Genetic and pharmacologic depletion of T-cells appears to blunt leukocyte–endothelial cell adhesion and the associated increase in vascular protein leakage in various models of inflammation [346–348]. Because T-cells can enhance neutrophil-mediated endothelial dysfunction [349], it is generally held that the role of T-cells in inflammation-induced protein leakage is a result of their ability to enhance neutrophil reactivity [297].
Circulating platelets have also been implicated as modulators of vascular permeability. Platelets can ameliorate the hyperpermeability response to inflammatory mediators in vascular beds supplied with a blood-free perfusate [297], but contribute to the hyperpermeability noted in experimental inflammation in vivo (blood perfused) [350]. The protective effect of platelets has been attributed to platelet-derived sphingosine (S1P) [297,351,352], whereas their hyperpermeability effects have been attributed to the formation of platelet–leukocyte aggregates. Interestingly, another source of circulating S1P, a substance noted for its stabilizing effect on the endothelial barrier [353], is red blood cells. Vascular albumin leak and the formation of interendothelial gaps are noted in the lungs of mice genetically deficient in plasma S1P, a situation rectified by transfusion with wild-type erythrocytes [354].
Role of ROS and NO
Both xanthine oxidase- and NADPH oxidase-derived ROS have been implicated in the vascular permeability associated with inflammation [355]. In I/R models, both the increase in venular/perivenular oxidant stress and the albumin leakage can be prevented by antioxidants, inhibitors of xanthine oxidase, or mast cell stabilization [356,357]. A proposed scenario is that oxidant production by endothelial xanthine oxidase results in activation of perivenular mast cells that, in turn, contribute to the inflammatory response, i.e., leukocyte recruitment and vascular protein leakage [297]. However, in other models of oxidant stress (systemic hypoxia), mast cells seem to be the primary producers of ROS that elicit the leukocyte recruitment and subsequent vascular protein leakage [358]. Alveolar macrophages (AM), which seem to be critical for I/R- or sepsis-induced pulmonary vascular leakage [183,335], can be activated by oxidant stress and also generate more ROS [359]. ROS generated by LPS-activated AMs have been implicated in endothelial barrier dysfunction. The AM-induced reduction in endothelial monolayer TEER is associated with a shift in the localization of the TJ components (occludin, ZO-1, claudin-5) from cell borders to cytoplasm(not loss thereof), an effect prevented by inhibition of NADPH oxidase [360]. Moreover, the increased transendothelial albumin movement induced by cytokine-activated AMs is abolished when NADPH oxidase-deficient AMs are used in the assay [342]. Circulating leukocytes can also contribute to perivascular oxidant stress and increase microvascular permeability [321,361]. PMN NADPH oxidase has been implicated in the pulmonary hyperpermeability induced by LPS [362].
The use of in vitro approaches utilizing endothelial cell monolayers indicates that endothelial cells can generate enough ROS to mediate increased monolayer permeability in the absence of any auxiliary vascular or perivascular immune cells [321]. Challenge of endothelial cells with anoxia/reoxygenation increases monolayer permeability, a response blocked by antioxidants or inhibitors of xanthine oxidase [294,363]. Direct exposure of endothelial cells to exogenous xanthine/xanthine oxidase or H2O2 can increase monolayer permeability, an effect blocked by intracellular delivery of catalase (conjugated to PECAM-1 antibody) [364]. Many agents (e.g., LPS, histamine, bradykinin, thrombin, VEGF) shown to increase endothelial monolayer permeability also increase endothelial ROS production via NADPH oxidase [57,365–368]. Although in some cases the mediator-induced increase in monolayer permeability can be ameliorated by blockade of NADPH oxidase or ROS scavenging [364,367,368], in other cases, a cause–effect relationship may not be apparent. For example, thrombin-induced endothelial monolayer permeability is not prevented by intracellular delivery of antioxidant enzymes, catalase, or SOD [364]. Nonetheless, the literature is generally consistent with the view that accelerated intracellular ROS generation is an important underlying cause of the endothelial hyperpermeability induced by inflammatory mediators.
Delineation of the role of NO in the vascular permeability response associated with inflammation has been fraught with controversy [318,321,369]. As mentioned above, the general consensus is that NO serves to stabilize microvascular barrier function under basal conditions. However, during an inflammatory response, there is compelling evidence supporting a role for NO as both a mediator and an inhibitor of endothelial barrier dysfunction. In I/R-induced inflammation, the increase in vascular albumin leakage is associated with a reduction in local levels of NO (nitrate/nitrite levels), and the hyperpermeability response is diminished by local administration of NO donors [332]. The protective effect of NO donors has been attributed to the ability of NO to interfere with auxiliary cell function, e.g., platelet–neutrophil interactions and/or mast cell degranulation [1,297,334]. Genetic blockade approaches seem to be divided on the role of auxiliary cell iNOS in the hyperpermeability response. LPS-induced pulmonary hyperpermeability is exacerbated in iNOS-deficient mice, a response attributed to macrophage iNOS [370]. In contrast, an in vitro coculture approach indicates that cytokine-activated AMs increase pulmonary endothelial monolayer permeability to albumin, a response not noted with iNOS-deficient AMs [371].
Genetic blockade approaches have also implicated eNOS in various in vivo models of acute inflammation. The hyperpermeability response observed in carrageenan- or zymosan-induced acute inflammation is largely blocked in eNOS-deficient mice [372]. Interestingly, the leukocyte extravasation noted in these models was not affected by genetic blockade of eNOS, suggesting an uncoupling between leukocyte adhesion and permeability responses. PAF-induced hyperpermeability in hamster cheek pouch and mesentery are (1) directly related to endothelial generation of NO [373] and (2) blunted in eNOS- but not iNOS-deficient mice [311]. Based on data generated from bone marrow chimeras, a vascular, rather than auxiliary, cell Akt/eNOS pathway has been implicated in the eNOS-induced hyperpermeability associated with acute inflammation in peripheral vascular beds [374]. In the pulmonary vascular bed, however, eNOS does not seem to contribute to the inflammation-induced hyperpermeability. For example, LPS-induced pulmonary hyperpermeability is not altered in eNOS-deficient mice [370]. Furthermore, the hyperpermeability associated with ventilator-induced lung injury is exaggerated in eNOS-deficient mice, but prevented in iNOS-deficient mice, with the latter response attributed to increased eNOS activity [375,376].
Some interesting explanations have been proposed to address the contradictory observations regarding the role of NO in modulating vascular permeability in vivo. The effects of NO may be tissue specific. For example, PAF-induced hyperpermeability in hamster cheek pouch is associated with enhanced NO generation, whereas the hyperpermeability response in lung is associated with a reduction in NO generation [377]. Furthermore, the PAF-induced hyperpermeability in systemic vascular beds seems to be driven by eNOS-derived NO [311,378], whereas the same response in the lungs seems to be dampened by eNOS-derived NO [376,379]. A second level of complication results from the relative roles of iNOS and eNOS in various experimental models. For example, LPS-induced vascular leakage in the mesentery seems to be mediated by induction of iNOS, and pharmacologic elevation of eNOS and/or prevention of iNOS induction alleviates the hyperpermeability response [380,381]. This phenomenon is consistent with the view that the effects of NO on barrier function may be concentration dependent; low levels serve to stabilize and high levels serve to destabilize [321,377].
In vitro approaches have specifically targeted the role of endothelial-derived NO on monolayer hyperpermeability, again with conflicting results. Exposure of endothelial monolayers to hypoxia induces an oxidant stress and increases monolayer permeability, events prevented by an NO donor and an analogue of cGMP [382,383]. Endothelial monolayer permeability (TEER) induced by LPS or cytokines can also be reversed by NO donors or cGMP analogues [384]. In contrast, challenge of endothelial cell monolayers with histamine or PAF results in increased NO production and monolayer permeability, and the hyperpermeability response is prevented by inhibition or depletion of eNOS [374,385]. Administration of NO (NO donors) has yielded equivocal results with respect to microvascular barrier function. Although NO donors elicit dose-dependent effects on the permeability of endothelial cell monolayers, some donors increase monolayer permeability [310,386], whereas others decrease monolayer permeability [309,384]; in both instances, the effects of the NO donors involve the cGMP pathway.
The discordant results derived from in vitro models exposed to NO donors may reflect the somewhat artificial situation created by this mode of NO delivery to cells. It is likely that the quality (species) and quantity (rate, duration) of NO produced by intact cells differ from those derived from NO donors [387]. Further, exogenous application of NO donors subjects the entire intracellular environment to NO, whereas endogenous NO generation is likely to be compartmentalized [369]. For example, the PAF-induced increases in vascular permeability are dependent on eNOS internalization (via caveolae) and targeting to cytoplasmic locations [385]. On the other hand, acetylcholine, which does not alter barrier function, triggers internalization and compartmentalization of eNOS to the Golgi region [388]. Finally, the use of cyclic GMP analogues is also not without its own draw-backs. Moderate increases in intracellular cGMP serve to stabilize endothelial monolayer permeability, whereas higher levels diminish barrier function [389].
There is also evidence (albeit limited) that NO may play a role in the postinflammation recovery of endothelial barrier function. For example, the increase in paracellular gap formation and albumin leak induced by inflammatory mediators (e.g., PAF, bradykinin, histamine) is a transient phenomenon, lasting 30 min or less. The resealing of the endothelial barrier (gap closure and decreased protein leak) is inhibited by pharmacologic inhibition of eNOS [390]. S1P has also been implicated in barrier restoration after a hyperpermeability response (resealing of junctions and strengthening of cortical actin) via either endothelial cell receptor activation or an intracellular pathway [351,353,354]. Although S1P is a well-known activator of eNOS [391], the specific linkages between S1P, eNOS, and barrier resealing have not been firmly established.
Endothelial cell signaling (Fig. 3)
Fig. 3.
Role of Rac1/RhoA balance in endothelial barrier integrity: impact of ROS and NO. (A) Basal permeability. In quiescent endothelial cells, cytosolic Rac1 is dominant and inhibits RhoA activation. Rac1/Cdc42 stabilizes both the cortical actin and the adherens junction components (VE-cadherin/β-catenin). Basal eNOS activity generates NO (blue pathway), which dampens any superoxide production within endothelial cells. In addition, low levels of NO increase cAMP, whereas high levels decrease cAMP. Agents that tend to strengthen barrier integrity can either increase cAMP, leading to an increase in the Rac1/RhoA ratio (e.g., ANP), or increase eNOS activity (e.g., S1P). (B) Increased permeability. In response to proinflammatory mediators (e.g., VEGF, thrombin, histamine), RhoA becomes dominant and inhibits cytosolic Rac1. Activation of GPCRs increases intracellular Ca2+ levels (via TRP channels) and activates RhoA. RhoA (via Rho kinase) (1) increases the PTK/PTP ratio, leading to disorganization of adherens junctions, and (2) activates MLCK and inhibits MLCP, leading to actomyosin-mediated contraction. Paradoxically Rho kinase activates Rac1 at the membrane, leading to activation of NADPH oxidase and superoxide production (red pathway), which in turn increases the PTK/PTP ratio. The increased intracellular Ca2+ also activates eNOS. Although low levels of NO (not shown) tend to stabilize adherens junctions, higher levels of NO (shown) favor disruption of the junctions as well as increasing MLCK activity (blue pathways).
The increase in vascular permeability during inflammation requires activation of endothelial cells, such that they can make appropriate ultrastructural alterations favoring solute and fluid exchange. Endothelial activation is initiated either directly (via DAMPs and/or PAMPs) or indirectly (via release of mediators from auxiliary cells). In either case, endothelial activation results in cell signaling, which ultimately leads to an increase in the dimensions of the paracellular pathway, i.e., IEJ disassembly, loosening of cortical actin, and actomyosin contraction leading to interendothelial gap formation. Although endothelial activation can also lead to an increase in the dimensions of the transcellular pathway (VVOs) [280,286,327], less information is available regarding the cell signaling pathways involved in that process.
A multitude of signaling pathways have been implicated in the regulation of endothelial paracellular permeability. These pathways are to a large extent interrelated and a dominant feature is the role of the Rho GTPase family, specifically RhoA, Rac1, and Cdc42 [262,392,393]. In general, endothelial barrier stability is driven by Rac1/Cdc42, and endothelial barrier instability is driven by RhoA. Rac1/Cdc42 recruits IQGAP1 and inhibits its interaction with β-catenin, thereby strengthening AJ interaction with cortical actin; inhibition of Rac1/Cdc42 disassociates IQGAP1 from the complex and destabilizes AJs [393]. Furthermore, Rac1/Cdc42 recruits cortactin to strengthen cortical actin. Finally, Rac1/Cdc42 inhibits RhoA via several downstream targets. In response to ligation of GPCR (e.g., thrombin), PLC generates IP3 and diacylglycerol (DAG). IP3 releases Ca2+ from the endoplasmic reticulum to activate MLC kinase (MLCK) which, in turn, phosphorylates MLC to promote actomyosin-mediated endothelial contraction. DAG activates a transient receptor potential (TRP) channel to allow Ca2+ influx into the cell and activates PKCγ, which in turn activates RhoA. RhoA (via Rho kinase) inhibits MLC phosphatase (MLCP) activity, thereby further enhancing actomyosin contraction [325]. Finally, Rho kinase inhibits Rac1 activation.
The increase in permeability may be self-limiting or can be resolved by activation of Rac1/Cdc42 and inhibition of RhoA. For example, GPCR ligation by thrombin causes a delayed activation of Rac1/Cdc42 via PKCγ activation of sphingosine kinase and generation of S1P [394]. Cdc42, at the level of the Golgi, enhances transport of junctional proteins toward the membrane [392]. Restabilization of the AJ complex results in further inactivation of RhoA via a VE-cadherin/p120 pathway. In addition, exogenous agents that increase intracellular cAMP (e.g., atrial natriuretic peptide) can inhibit RhoA and activate Rac1 [389,393].
The balance between Rac1/Cdc42 and RhoA dominance represents a generalized framework for understanding some of the fundamental signaling pathways involved in the regulation of endothelial barrier function. However, the in vivo situation is much more complex. For example, RhoA can also stabilize AJ complexes via Dia1, and Rac1 can lead to actomyosin contractions via PAK [393,395,396]. The specific effectors targeted by the Rho kinases can elicit different responses and it has been proposed that the effectors targeted by Rho kinases (e.g., Rac1) may be determined by different guanine nucleotide exchange factors [397].
Role of ROS and NO (Fig. 3)
Exposure of endothelial cells to oxidants (e.g., H2O2) or endogenous generation of oxidants in response to endothelial activation (e.g., VEGF) results in disruption of the IEJ, actomyosin contractions, gap formation, and an increase in endothelial permeability [252,321,368,398]. Extracellular H2O2 (from auxiliary cells) can convert endothelial xanthine dehydrogenase to xanthine oxidase [399] and can activate NADPH oxidase to generate oxidants intracellularly [167], thereby creating positive feedback loops perpetuating oxidant generation. Because the sole physiologic function of NADPH oxidase is the generation of superoxide (and H2O2) and the oxidase can be activated via a variety of inflammatory mediators [219], it has received the greatest attention.
Generation of oxidants by NADPH oxidase is facilitated by Rac1 binding to the enzyme [397]. For example, the H/R-induced increase in NADPH oxidase activity, ROS generation, and endothelial monolayer permeability is abolished in Rac1-deficient cells [400]. Activation of NADPH oxidase by Rac1 seems to be incongruous with the proposed role for Rac1 in endothelial barrier stabilization. Based on studies using thrombin as an agonist, it has been proposed that cytosolic Rac1 is responsible for the barrier-stabilizing effect, whereas membrane-associated Rac1 is involved in NADPH oxidase activation [367]. In VEGF-challenged endothelial cells, NADPH oxidase-derived ROS have been implicated in the phosphorylation of AJ proteins (VE-cadherin, β-catenin) and increased endothelial monolayer permeability [368]. The phosphorylation status of AJ proteins is dependent on the PTK/PTP balance [246]. ROS can either inactivate PTP or activate PTK via oxidation of critical cysteine residues, leading to phosphorylation of VE-cadherin and β-catenin and compromising IEJ integrity [246,398]. A similar mechanism may be operative at the level of actomyosin-mediated contraction, because NADPH oxidase-derived ROS have been implicated in MLC phosphorylation and the increase in endothelial permeability in response to hypoxia [401]. Furthermore, in an in vitro stroke model, the endothelial hyperpermeability induced by the Rac1/NADPH oxidase pathway was attributed to activation of RhoA [402]. The RhoA/Rho kinase may increase endothelial permeability via either phosphorylation (and internalization) of junctional proteins [247] or MLC-induced actomyosin contractions [402], or both. ROS can also activate TRP channels to allow intracellular entry of Ca2+ [403], which would also facilitate actomyosin contraction via activation of MLCK and phosphorylation of MLC [325].
Activation of endothelial cells by GPCR ligation or nonreceptor mechanisms (e.g., H2O2) leads to Ca2+ entry into endothelial cells and binding of Ca2+ to calmodulin, as well as activation of various kinases (e.g., PKC, PI3-kinase) [6,314,404,405]. Under basal conditions, eNOS is concentrated in caveolae, where its activity is inhibited by interaction with caveolin-1 and constitutive phosphorylation at Thr95. Upon agonist activation, caveolin-1 is displaced from eNOS and the Thr95 residue is dephosphorylated, whereas Ser1177 is phosphorylated. These events facilitate Ca2+/calmodulin interactions with eNOS resulting in NO generation. The major downstream signaling target is sGC, which increases intracellular levels of cGMP.
The NO/cGMP pathway has been implicated as both a negative and a positive modulator of endothelial barrier integrity [325,406,407]. With respect to its role in hyperpermeability responses, scission of eNOS-containing caveolae and delivery of eNOS to the endothelial cytoplasm via endocytosis seem to be a prerequisite [369,385]. NO can activate MLCK and induce actomyosin contraction via the sGC/cGMP/PKG pathway [325]. In addition, NO can nitrosylate β-catenin (cGMP independent), leading to its disassociation from VE-cadherin and IEJ disruption [408]. By contrast, the eNOS/cGMP pathway has been implicated in the attenuation of the cytokine-induced increase in endothelial monolayer permeability [409]. The NO/cGMP pathway can also ameliorate the thrombin-induced hyperpermeability response by increasing cAMP levels [389]. One possible explanation for the discordant effects of the NO/cGMP pathway is centered on the dose-dependent effects of cGMP on cAMP-hydrolyzing phosphodiesterases, PDE2 and PDE3 [389]. In this scenario, low levels of NO and cGMP inhibit PDE3 activity and increase cAMP levels (enhance barrier stability), whereas high concentrations of NO and cGMP stimulate PDE2 activity and decrease cAMP levels (diminish barrier stability). In addition, it was proposed that any other mediators (e.g., TNF) with the potential to influence endothelial PDE activity could alter the cell's responses to activation of the NO/cGMP pathway.
An issue that has been understudied is the precise linkage between NO and the Rho GTPases. One specific point worth pursuing is the linkage between specific Rho GTPases and isoforms of NOS. There is evidence for a relationship between Rac1 and eNOS [409,410], as well as RhoA (Rho kinase) and iNOS [381]. Additional work is warranted to resolve this issue relative to the endothelial cell signaling mechanisms that regulate endothelial barrier function.
Angiogenesis
One of the consequences of inflammation is tissue hypoxia due to a combination of increased metabolic demand of infiltrating cells (both pathogenic and immune) and decreased O2 delivery (vascular damage and edema) [411]. Hypoxia is the primary stimulus for the formation of new blood vessels (angiogenesis) to restore O2 delivery to the affected site [412]. Angiogenesis can be broadly divided into two phases: an initial destabilization of the microvasculature, followed by a restabilization of newly formed microvessels. Destabilization of the endothelium (e.g., pericyte dropout and weakening of the interendothelial junctions) allows for migration of endothelial cells to create new blood vessels. A selected endothelial cell (referred to as a “tip cell”) begins to extend lamellipodia and degrade the basement membrane and ECM. The tip cell is followed by proliferating and migrating endothelial cells (referred to as “stalk cells”). Initially, the endothelial sprout of tip and stalk cells is relatively leaky and does not restrict the movement of macromolecules. During the restabilization phase, mural cells (e.g., pericytes) are recruited to the developing sprout and the interendothelial junctions of these neovessels become strengthened and less permeable to macromolecules.
Sentinel cells: signaling pathways (Fig. 4A)
Fig. 4.
Cooperative role of ROS and NO in angiogenesis. (A) Generation of VEGF by macrophages. Both ROS (red pathways) and NO (blue pathways) generated during hypoxia/inflammation can decrease PHD activity, which activates the HIF pathway as well as the NF-κB pathway. The HIF pathway leads to transcription of VEGF. The NF-κB pathway leads to transcription of inflammatory cytokines. During the inflammatory response the NF-κB predominates, and during the resolution of the inflammatory response the HIF pathway predominates. NO modulates both pathways at several points, being stimulatory in the HIF pathway and inhibitory in the NF-κB pathway. (B) Initiation of angiogenesis: endothelial destabilization. VEGF derived from macrophages or recruited endothelial progenitor cells (EPC) interacts with VEGF receptor (VEGFR) on selected endothelial “tip” cells to initiate cell migration. VEGFR ligation activates both eNOS and NOX to generate NO and superoxide, respectively. NO (blue pathways) is involved in (1) loosening of the adherens junctions and (2) MMP activation and TIMP inhibition to facilitate degradation of the ECM. Superoxide (red pathways) is involved in (1) formation of lamellipodia and (2) cyclic alterations in the strength of focal adhesion complexes (FACs). The migratory tip cells are followed by hyperpermeable (destabilized junctions) stalk cells.
The macrophage is also a pivotal effector cell in the initiation of angiogenesis [122,413,414]. Two major signaling pathways have been implicated in macrophage-induced angiogenesis: the hypoxia-inducible factor (HIF) and NF-κB pathways [415,416]. Both the HIFα and the NF-κB pathways can be activated by either inflammatory cytokines or hypoxia, or both [414,416,417]. The HIFα pathway leads to the transcriptional generation of the proangiogenic agent VEGF. The NF-κB pathway leads to transcription of various cytokines (e.g., TNFα, IL-1β). Of particular relevance to angiogenesis, NF-κB binding sites are present on the HIFα promoter and NF-κB can increase HIFα levels [416]. Thus, activation of both pathways within macrophages can lead to the generation of VEGF and the initiation of angiogenesis.
In the NF-κB pathway, the IKK complex phosphorylates IκB and targets it for proteasomal degradation, thereby freeing NF-κB to translocate to the nucleus and transcribe relevant genes. It is readily accepted that inflammatory mediators (e.g., TNFα, IL-1β) can activate NF-κB via the IKK-dependent “canonical pathway” by phosphorylation of serine residues (Ser32 and Ser36) on IκB [418]. On the other hand, hypoxia can activate NF-κB via an IKK-independent “atypical pathway” via tyrosine kinase (e.g., c-Src kinase) phosphorylation of tyrosine 42 on IκB [418].
In the HIF pathway, HIFα forms a heterodimer with HIFβ, which can transactivate target genes, e.g., VEGF. The HIF pathway is regulated primarily by prolyl hydroxylase domain-containing enzymes (PHDs) [415,419,420]. PHDs are members of the 2-oxyglutarate-dependent ferrous iron (Fe2+) dioxygenase family that hydroxylate HIFα and target it for proteasomal degradation. The activity of PHDs is dependent on the O2 concentration [415,420]. Under normoxic conditions PHDs are partially active, but any further decreases in local O2 suppress PHD activity [415]. Thus, under normoxic conditions there is minimal, if any, active HIFα because of proteasomal degradation. However, under hypoxic conditions HIFα is stabilized (not degraded) and is free to interact with HIFβ and transcribe relevant genes.
Interestingly, both the HIF and the NF-κB pathways can be activated by decreasing the activity of PHDs. As mentioned above, PHDs hydroxylate HIFα, thereby routing it to the proteasome [415,419,420]. In the NF-κB pathway, it has been proposed that PHDs hydroxylate the catalytic subunits of the IKK complex (at a target motif for hydroxylation similar to HIFα), thereby inhibiting IκB phosphorylation and degradation [416,421]. In an inflammatory/hypoxic milieu, PHD activity is suppressed and both the HIF and the NF-κB pathways are activated. Hypoxia, per se, is thought to be a stronger activator of the HIF pathway [416]. Thus, it is readily apparent that in an inflammatory/ hypoxic environment robust activation of both pathways would occur. As the inflammation resolves there would be a shift to a predominant role of the HIFα pathway favoring VEGF generation and angiogenesis.
Negative feedback loops exist for these two pathways; the HIF pathway leads to transcriptional increases in PHD levels and the NF-κB pathway leads to increases in IκB. Moreover, these two pathways are not mutually exclusive, but rather there is a great deal of cross talk at various levels of the two pathways [416,422–425]. There is also evidence that effective transcription of some genes may even require the binding of both transcription factors to their promoter elements [426]. Thus, although the salient features of the HIFα and NF-κB pathways of gene expression are depicted in Fig. 4, their relative contributions, interactions, and modulation by additional signaling pathways during angiogenesis are clearly much more complex.
The macrophage is believed to be a key effector cell in the initiation of angiogenesis via generation of VEGF [413,414,427,428]. Macrophages are adapted to functioning in a hypoxic environment and hypoxia alone can result in the induction of an angiogenic phenotype in these cells, e.g., upregulation of the VEGF gene [427]. However, VEGF can be generated not only by macrophages, but also by endothelial cells [429], mural cells [430], or epithelial cells [429,431]. The likelihood that endothelial-derived VEGF via a HIF or NF-κB signaling pathway contributes to the initiation of angiogenesis seems unlikely, because endothelial cells with a PHD deficiency adopt a quiescent stable phenotype rather than the migratory phenotype required for angiogenesis [432]. An additional caveat to consider is whether resident or newly recruited monocytes/macrophages are the major contributors to neovascularization. During hypoxia or wounding, resident macrophage-like cells exposed to hypoxia can generate chemoattractants via the HIF pathway, which can recruit additional monocyte/macrophage populations to the affected site; these recruited accessory cells play an important role in new vessel formation [433,434]. Indeed, macrophages recruited to developing tumors are believed to be the initiators of angiogenesis in that environment [413,428].
Role of ROS and NO (Fig. 4A)
The inflammatory cytokines TNFα and IL-1β and hypoxia can induce an intracellular oxidant stress via generation of ROS [219,435]. Current consensus holds that hypoxia or an oxidative stress induced by ROS activates the NF-κB pathway via either the IKK-dependent or an IKK-independent pathway [435]. Similarly, although it is accepted that ROS play an important role in stabilizing HIFα, most probably via a MAP kinase pathway, the signaling pathways may be indirect (e.g., activation of NF-κB pathway) [436–439].
An interesting integration of ROS-induced activation of both HIFα and NF-κB centers on the ability of redox-sensitive PHDs to regulate both NF-κB activation and HIFα stabilization [416,421]. Because inflammation and/or hypoxia can lead to oxidant stress, it is not surprising that ROS have been implicated in the regulation of PHD activity. Mitochondria-derived ROS appear to decrease PHD activity and increase HIFα availability in a variety of cell types [439,440], including macrophages [438]. NADPH oxidase-derived ROS have also been implicated in stabilizing HIFα via inhibition of PHDs [414,441]. Of note, mitochondrial ROS generation may be a prerequisite for NADPH oxidase activation [442–445]. A similar mechanism of action centered on regulation of PHDs has been proposed for the activation of the NF-κB pathway [416].
As mentioned above PHDs are members of the 2-oxyglutaratedependent Fe2+ dioxygenase family, which are highly sensitive to prevailing O2 [414,415,420]. It is generally believed that ROS exert their effects on PHD activity by oxidizing ferrous to ferric (Fe3+) iron [440,441]. Alternatively, ROS can reduce ascorbate levels, thereby increasing the ratio of Fe3+/Fe2+ [415]. Both of these ROS-mediated effects would render the enzyme inactive and promote stabilization of HIFα and activation of NF-κB.
NO can also modulate both the HIFα and the NF-κB pathways [446,447]. Most of the studies addressing this issue have made use of various NO donors. Generally, NO-mediated signaling involves chemical interactions with either transition metals (e.g., Fe-heme of sGC leading to increased cGMP) or protein thiols (e.g., cysteine nitrosylation) [446,448,449]. The latter mechanism seems to be operative in the HIFα and NF-κB pathway in the context of hypoxia/inflammation. S-nitrosylation is reversible within cells by “denitrosylases” such as S-nitrosoglutathione reductase or thioredoxin, thereby making this signaling system analogous to the kinase/phosphorylation system [448,449]. It has been proposed that S-nitrosylation is favored (over sGC activation) at high NO concentrations (micromolar) [446,450]. However, in the presence of an intracellular pool of chelatable iron, S-nitrosylation reactions have been demonstrated in macrophages even at low concentrations of NO (nanomolar), especially under hypoxic conditions (O2<1 µM) [451].
NO donors inhibit NF-κB binding to DNA via S-nitrosylation of Cys62 in the p50 subunit of NF-κB in both cell-free systems and various cells [447,452–454]. In addition, NO can nitrosylate Cys179 in IKKβ and, thereby, prevent phosphorylation and subsequent degradation of IκB [455]. Finally, NO has been shown to directly inhibit NF-κB activity by nitration of the tyrosine residues (Tyr66 and Tyr152) on p65 [456]. Thus, the available evidence favors inhibition of the NF-κB pathway by NO-mediated S-nitrosylation reactions. With respect to the HIF pathway, S-nitrosylation chemistry favors HIF stabilization. Under normoxic conditions NO can S-nitrosylate HIFα and promote stabilization of HIFα (i.e., Cys533 and possibly others) [457,458] as well as enhancing its transcriptional activity (Cys800) [459]. Finally, NO donors can stabilize hypoxia-induced HIFα by preventing HIF ubiquitination [460], perhaps via S-nitrosylation of the ubiquitin ligase [448]. Of particular relevance to angiogenesis is the observation that S-nitrosylated HIFα binds more avidly to the VEGF promoter region in vitro and increases capillary density in vivo under normoxic conditions [461]. Collectively, the S-nitrosylation chemistry of NO is stimulatory in the HIFα pathway, as opposed to playing an inhibitory role in the NF-κB pathway. The net result of increased NO generation is a general shift in macrophage signaling from pathways supporting inflammation to those favoring angiogenesis.
Another mechanism by which NO can modulate the HIFα pathway is via interactions with mitochondria and subsequent regulation of PHD activity [420,442,446]. In this scenario, NO competes with O2 for cytochrome c oxidase, leaving more O2 available for use by PHD and resulting in destabilization of HIFα, particularly under hypoxic conditions [462]. However, the use of NO donors has led to equivocal results, their effects being O2, time, and dose dependent [414,420]. For example, when NO is generated at high concentrations (micromolar) it promotes stabilization of HIFα under both hypoxic and normoxic conditions; when NO is produced at low concentrations (nanomolar) it promotes destruction of HIFα only under hypoxic conditions [419,420,447]. A working hypothesis has been proposed that attempts to integrate the confounding effects of NO donors on the PHD/HIFα pathway [420,463]. The pharmacologic release of large amounts of NO would directly inhibit PHD activity and stabilize HIFα. The inhibitory effect of NO donors on PHD mimics hypoxia, i.e., NO competes with O2 binding to the Fe2+ of PHD. The resultant decrease in proteasomal degradation of HIFα allows for an increased HIFα/HIFβ-mediated transcriptional generation of PHD. When the pharmacologic release of NO wanes the increased pool of PHDs rapidly shuttles HIFα to the degradation pathway, particularly under hypoxic conditions. Thus, because of the negative feedback loop, transient pharmacologic generation of NO initially increases HIFα, followed, at a later time point, by a decrease in HIFα.
Functional destabilization of microvessels (Fig. 4B)
In the adult, angiogenesis usually occurs by sprouting of new vessels from existing functional capillaries or microvessels [432,464,465]. This requires destabilization of the existing microvascular structure, i.e., pericyte dropout, alterations in endothelial cell phenotype, and breakdown of the basement membrane. Initially, an endothelial cell (tip cell) leaves the endothelial lining, penetrates the basement membrane, and invades the interstitium. The tip cell has a specialized phenotype, such as lamellipodia and filopodia to detect environmental cues for appropriately directed migration. The tip cell is followed by migratory/proliferative cells (trunk or stalk cells), which allow for extension of the sprouting vessel and lumen formation [465,466]. VEGF, generated by the HIF pathway, is believed to be the major growth factor involved in the initiation of angiogenic sprouting [467–472]. In addition, the cytokines TNFα and IL-1β, generated by the NF-κB pathway, seem to play an important accessory role in angiogenesis [473,474].
Secreted VEGF can bind to the ECM, thereby creating a gradient of VEGF from the source (e.g., macrophage) to the endothelium [471]. Although many endothelial cells are subjected to the VEGF gradient, only a minor proportion of them develop a tip cell phenotype. The selection process seems to be dependent on Delta-like 4 (Dll4)/Notch signaling in some of the endothelial cells [464,465,471]. In some cells, VEGFR (primarily VEGFR2 or KDR/Flk1) signaling increases VEGFR expression and induces the expression of Dll4; these cells will develop into tip cells. The enhanced Dll4/Notch signaling to adjacent endothelial cells suppresses VEGFR signaling via the Notch intracellular domain [471]. Thus the relatively lower Dll4/Notch signaling in the tip cell favors lamellipodia and filopodia development and migration, whereas the increased Dll4/Notch signaling in adjacent endothelial cells inhibits filopodia development and drives the cells into a stalk phenotype [465,471].
Angiogenesis is facilitated by the expression of MMPs, which are capable of local degradation of basement membrane and ECM components [465,475]. MMP activity is limited to the tips of the sprouting neovessels [476]. The degradation of the ECM is highly regulated (both spatially and temporally), because, in addition to providing an impediment to cell migration, various ECM components serve as a scaffold for endothelial cell migration and regulate growth factor bioavailability [475,477,478].
VEGF also influences the dynamics of endothelial cell–cell interactions in the angiogenic sprout. VEGFR is closely associated with VEcadherin and upon VEGFR ligation VE-cadherin is phosphorylated and internalized [272,479,480]. Loss of VE-cadherin from the AJ contributes to destabilization of endothelial cell–cell contacts, thus accounting for the high permeability of angiogenic sprouts [481–484].
Macrophage-derived VEGF may also contribute to the angiogenic process by increasing mobilization of myeloid cells and endothelial progenitor cells (EPCs) to the site of angiogenesis [434,474,485,486]. Most of the myeloid cells (e.g., macrophages or macrophage precursors) seem to be derived from the bone marrow [474], whereas the EPCs are recruited primarily from local sites (e.g., blood vessel wall) [474,486]. Of interest is the observation that a subpopulation of macrophages (M2; involved in wound healing and angiogenesis) can accumulate in the affected area via proliferation rather than recruitment from the vasculature [487]. The localization of VEGF-recruited accessory cells to the affected area is believed to be due to perivascular expression of SDF1 (CXCL12) to attract CXCR4-bearing myeloid and endothelial progenitor cells [434,488]. There is evidence to indicate that these recruited accessory cells are the major driving force for angiogenesis [434,474]. For example, macrophage-induced angiogenesis is dependent on VEGF secretion by EPCs [474]. The issue of whether recruited EPCs actually become incorporated into the angiogenic stalk remains controversial [434,486,488].
Activated macrophages also release the cytokines TNFα and IL-1β, which contribute to angiogenesis in the inflammatory/hypoxic setting. TNFα is believed to “prime” endothelial tip cells during the inflammatory phase for eventual migration induced by VEGF during the resolution phase [473]. IL-1β plays an important role in the recruitment of accessory cells, e.g., EPCs and myeloid cells [474].
Role of ROS and NO (Fig. 4B)
VEGF-induced angiogenesis is associated with increased endothelial cell production of both ROS [219,489,490] and NO [491–493]. Genetic blockade approaches indicate that a major source of ROS is NADPH oxidase (NOX) [219], whereas eNOS is the source of NO [494]. At the receptor level, VEGFR signaling induces activation and translocation of the small GTPase Rac1 into the plasma membrane where it activates NOX [490]. The ROS generated by NOX, in turn, oxidize PTP (shifting the PTK/PTP balance in favor of PTK), which allows for autophosphorylation (activation) of VEGFR. Thus, a positive feedback system is initiated that amplifies VEGFR signaling via NOX. VEGFR signaling also activates eNOS via a PI3-kinase/Akt or AMPK pathway [493], although the role of the latter pathway has been questioned [495]. VEGF-induced increases in Rac1 activity can enhance eNOS activity as well as NADPH oxidase activity; both NO and superoxide production can occur concurrently [410,496]. Interestingly, VEGFR, eNOS, and NOX are compartmentalized within endothelial lipid rafts or caveolae [68,405,480,497]. This compartmentalization may provide an important signaling platform for efficient angiogenesis, because caveolae-deficient endothelial cells cannot migrate [491].
Endothelial tip cell migration toward a VEGF gradient involves penetration of the ECM, a process facilitated by endothelial MMPs [465,475,491]. MT1–MMP is localized within caveolae, and caveolamediated trafficking of the MMP within the endothelial cells ensures appropriate localization and activity [498]. eNOS-derived NO promotes MT1–MMP clustering and activation at the migrating front of endothelial cells, a critical event in endothelial migration and tube formation [499]. At low concentrations, NO can inhibit tissue inhibitor of metalloproteinase (TIMP) via a cGMP pathway, thereby altering the TIMP/MMP balance in favor of MMP activity [500]. At high concentrations, NO may also interact with the cysteine–zinc moiety of MMPs, thereby enhancing their activity [500]. Endothelial cell migration requires a cycling of adhesive interactions to support directed movement, a process requiring ROS signaling [490]. As mentioned previously, NOX-derived ROS can alter the PTK/PTP balance in favor of PTK (oxidation of critical residues in PTPs), which results in phosphorylation of VE-cadherin/β-catenin and IEJ disassembly [246,398]. In addition, NOX is localized to the lamellipodia of migrating endothelial cells by polarized migration of lipid rafts [501]. Specific scaffolding proteins localize NOX signaling to focal adhesion complexes of lamellipodia and the leading “ruffled” edge of the migrating endothelium [502]. A ROS-induced PTK/PTP imbalance is believed to modulate focal complex turnover [501,502].
VEGF-induced destabilization of microvessels renders the developing microvasculature more permeable to macromolecules (vascular hyperpermeability) [368,481,483]. The VEGF-induced generation of NO and ROS is believed to play an important role in vascular hyperpermeability [368,503]. NOX-derived ROS have been implicated in the VEGF-induced hyperpermeability via phosphorylation of VEcadherin [368], leading to VE-cadherin disassociation from the junctions and internalization [484]. The phosphorylation of VE-cadherin has been attributed to the nonreceptor tyrosine kinases of the Src family [483,484]. VEGF-induced activation of eNOS also results in endothelial production of NO and increased endothelial monolayer permeability [503]. The NO-mediated hyperpermeability requires eNOS endocytosis to subcellular compartments [504] and may involve the sGC/cGMP pathway [505]. Alternatively, NO-mediated S-nitrosylation of β-catenin (Cys619) and AJ disassembly can contribute to the VEGF-induced hyperpermeability [408].
Functional restabilization of microvessels
As angiogenic sprouting progresses, cell-specific signals ensure appropriate maturation of the nascent blood vessel, i.e., vessel wall maturation/stabilization and lumen formation. Endothelial sprout maturation involves the recruitment of mural cells and strengthening of the barrier function of the neovessel [466,506,507]. Platelet-derived growth factor β (PDGFβ) secreted by tip cells is primarily responsible for the recruitment of mural cells, i.e., pericytes and vascular smooth muscle cells [467,468]. PDGFβ binds to local ECM components, thereby creating a gradient for recruitment of mural cells. Mural cells express the PDGFβ receptor, whereas endothelial cells do not [468]. PDGFβ-dependent recruitment of mural cells is complex and involves stimulation of mural cell proliferation and migration to and adhesive interactions with the sprouting endothelium [506].
The newly recruited mural cells play a pivotal role in stabilization of the neovessel by generating angiopoietin-1 (Ang-1), which interacts with Tie2 receptors on endothelial cells [466]. Ang-1 plays a role in ensuring that MMP activity is confined to the tip cells and inhibits MMP activity in the stalk cells [476]. Ang-1/Tie2 signaling serves to stabilize the endothelial lining by strengthening cell–cell interactions [481,506,508]. Ang-1 ensures Tie2 localization to cell–cell contacts, where it forms complexes with VE–PTP and serves to stabilize IEJs, presumably via prevention of VE-cadherin phosphorylation [508]. Ang-1/Tie2 signaling also inhibits VEGFR-mediated activation of Src kinase and subsequent VE-cadherin phosphorylation and internalization [509]. This may represent a major mechanism by which Ang-1/Tie2 signaling antagonizes VEGF-induced hyperpermeability [507]. As a caveat, Ang-1/Tie2 signaling can also contribute to the destabilization phase of angiogenesis, an effect observed primarily when cell–cell contacts are disrupted and involve endothelial cell– ECM Ang-1/Tie2 complex formation [508,509]. Ang-2, which is generated by endothelial cells in response to VEGF, can serve as an antagonist of Tie2 activation and support the destabilization phase [466,509]. Collectively, the available information indicates that Ang- 1 serves to stabilize the VEGF-induced angiogenic sprout, thereby contributing to the development of functional neovessels [510].
The mechanisms involved in lumen formation are poorly understood. It has been proposed that it occurs either by extensive coalescence of endothelial vesicles forming a space between adjacent endothelial cells or by invagination of endothelial cells around a preexisting space [465,511,512]. The Ang-1/Tie2 signaling pathway has been implicated in increasing the caliber of the sprouting vessels via apelin interactions with its receptor APJ [511,513].
Transforming growth factor β (TGFβ) and S1P signaling has also been implicated in the final maturation of neovessels. TGFβ secreted by both endothelial and mural cells is present in a latent form in the ECM [478].When activated by MMP degradation of ECM components, TGFβ induces differentiation and maturation of mural cells [506], inhibits endothelial cell proliferation [514], and contributes to endothelial cell synthesis of basement membrane components (e.g., collagen IV, fibronectin) [471]. Coculture systems highlight the cooperative interaction between endothelial cells and mural cells in (1) activating latent TGFβ [514,515] and (2) appropriate development of an endothelial basement membrane via regulation of the TIMP/MMP balance [516]. S1P, derived primarily from blood cells (red blood cells, platelets) [354,466,506] and also generated by endothelial cells [481], interacts with its receptor (S1P1) on endothelial cells to further strengthen both endothelial cell adhesive interactions (VE-cadherin) and endothelial cell–mural cell adhesive interactions (N-cadherin) [481]. In addition, S1P derived from recruited progenitor cells has been shown to be critical for endothelial adherens junction assembly [517]. There may also be significant cross talk between S1P and TGFβ, i.e., SIP can activate TGFβ receptors and activation of TGFβ receptors can generate S1P [518].
Role of ROS and NO
Although NADPH oxidase-derived ROS have been shown to play a role in PDGFβ-induced smooth muscle chemotaxis [519,520], a direct link to mural cell coverage of sprouting endothelium is lacking. Moreover, there is relatively little information regarding the role of ROS in other phases of blood vessel stabilization, e.g., strengthening of inter-endothelial cell junctions. Indeed, based on the development of the vasculature in mice with endothelial-specific deletion of Rac1, it has been proposed that NADPH oxidase is more important in the initiation of angiogenesis than in the development of mature vessels [521].
NO derived from eNOS has been shown to play an important role in vessel maturation and mural cell coverage of developing vessels in several models of angiogenesis. Mural cell recruitment is impaired in eNOS-deficient mice [522] and the adenoviral delivery of eNOS promotes pericyte coverage of neovessels [523]. However, the precise signaling mechanisms have not been addressed.
Ang-1 can reverse the VEGF-induced hyperpermeability of endothelial cell monolayers by phosphorylating the inhibitory Thr497 residue on eNOS, thereby decreasing NO production [503]. Alternatively, it has been proposed that Ang-1/Tie2 signaling can increase the expression of eNOS (via KLF2) and enhance IEJ stability [481]. The apelin/APJ pathway can also increase eNOS via the KLF2 pathway [524], but a direct linkage between NO generation and neovessel development has not been established.
Pharmacologic blockade of NO has been shown to enhance TGFβ production in wounds, implying a role for NO in limiting TGFβ production and excessive deposition of ECM (fibrosis) [525]. However, neither the source/isoform of NOS nor the mechanisms involved in eliciting this response are clear at present. S1P can also lead to eNOS activation [526], but whether NO is involved in the S1Pinduced strengthening of endothelial barrier function has not been directly assessed.
In summary, there is a paucity of information on the roles of ROS and NO in the stabilization phase of angiogenesis and further work in this understudied area is warranted.
Coagulation and thrombosis
It is now well recognized that inflammation and coagulation are intimately linked processes that can elicit a vicious cycle because activation of one process leads to activation of the other [527–529]. This interdependence of inflammation and coagulation is evident in a variety of acute (e.g., sepsis) and chronic (e.g., colitis) inflammatory diseases wherein changes in hemostatic biomarkers suggest subclinical activation of the coagulation system and there is an increased incidence of thromboembolic events [10,530–537]. Whereas the chemical mediators that link inflammation to coagulation and thrombosis remain poorly understood, the activation products of the various cell populations (platelets, leukocytes, endothelial cells, macrophages) that drive the inflammatory response have received the most attention. Reactive oxygen and nitrogen species are considered likely candidates because of their ability to alter platelet function and to influence the balance between procoagulant, anticoagulant, and fibrinolytic systems [538]. This section summarizes evidence that implicates ROS and reactive nitric oxide species (RNOS) as potential mediators of the procoagulant, prothrombotic phenotype that is assumed during inflammation.
The coagulation cascade (Fig. 5)
Fig. 5.
Role of ROS and NO in coagulation and platelet aggregation. Thrombus formation involves the adhesion, activation, and aggregation of platelets as well as activation of the coagulation cascade. With vessel injury, platelets bind to exposed collagen and von Willebrand factor (vWF). Upon activation, platelets bind to one another using fibrinogen and GPIIb/IIIa to form an aggregate. Tissue factor (TF) triggers coagulation by binding to activated factor VII (fVII), which ultimately leads to activation of other coagulation factors and the conversion of prothrombin to thrombin. Thrombin cleaves fibrinogen to generate fibrin monomers, which polymerize to form a stable clot. Fibrinolysis (proteolytic degradation of fibrin), which prevents excess thrombus growth, is mediated by plasminogen and its activators (t-PA and u-PA) and controlled by plasminogen activator inhibitor-1 (PAI-1), which inhibits t-PA and u-PA. ROS and NO are known to modulate the coagulation pathway and fibrinolysis by interacting with multiple components of this cascade. ROS (red pathways) can promote the initiation of coagulation by targeting the TF–fVII complex as well as tissue factor protein inhibitor (TFPI). ROS also promote coagulation and thrombus formation by inhibiting the production of activated protein C (APC), enhancing the conversion of fibrinogen to thrombin and enhancing PAI-1 activity. NO (blue pathways) tends to exert an opposite effect on the coagulation cascade. In addition, NO targets the platelets to inhibit aggregation, whereas ROS promote this process. NADPH oxidase (Nox) seems to be a major endothelial cell source of the ROS that modulate platelet aggregation.
The antithrombogenic properties of the vascular system can be attributed to normal endothelial cell function [539–541]. These cells produce (e.g., prostacyclin and nitric oxide) or limit the accumulation of (e.g., the ectonucleotidase CD39 degrades ATP and ADP) substances that inhibit the adhesion and aggregation of platelets [542,543]. Endothelial cells also facilitate the production of anticoagulants (activated protein C, or APC) that prevent the activation/ deposition of coagulation factors on the vessel wall. Another important function of endothelial cells is the formation of a barrier that physically separates blood elements from the subendothelial matrix [1]. When the endothelial lining is disrupted and the underlying matrix is accessible to blood, platelets will rapidly adhere to exposed collagen and von Willebrand factor in the subendothelial space (via an interaction between α2β1 and glycoprotein (GP) Ib/IX). This is followed by platelet aggregation (via fibrinogen and GPIIb/IIIa) and the expression of cell-surface phospholipids, on which clotting factors in blood can assemble. During inflammation, the blood vessel wall does not exhibit endothelial damage or exposure of collagen and matrix material. In this setting, endothelial cell activation can result in the binding of platelets, with or without attached leukocytes, which can also result in platelet aggregation, activation of the coagulation system, and thrombus development [1]. Tissue factor (TF), which is expressed by endothelial cells, monocytes, platelets, and other cells in response to inflammatory mediators (e.g., TNFα, IL-1β, CD40L), initiates coagulation when exposed to blood [544]. TF triggers coagulation by binding to activated factor VII, which ultimately leads to activation of other coagulation factors and the conversion of prothrombin to thrombin. Thrombin cleaves fibrinogen to generate fibrin monomers, which polymerize to form a stable clot. Thrombin also amplifies the coagulation process by inducing TF expression on the vessel wall, via feedback activation of cofactors VIII and V, and by activating platelets through engagement of thrombin receptors [545]. Fibrinolysis (proteolytic degradation of fibrin), which prevents excess thrombus growth, is mediated by plasminogen and its activators (t-PA and u-PA) and controlled by plasminogen activator inhibitor-1 (PAI-1), which inhibits t-PA and u-PA [546].
Endogenous anticoagulant mechanisms, including tissue factor pathway inhibitor (TFPI), heparin–antithrombin, and the protein C pathway, serve to either inhibit the generation of thrombin or inactivate it [527,547]. TFPI inactivates factor VIIa bound to TF, whereas the heparin–antithrombin mechanism neutralizes factor Xa, thrombin, and factor IXa, as well as factor VIIa bound to TF [527,547]. The protein C pathway is initiated by the binding of thrombin to thrombomodulin on the surface of endothelial cells. The thrombin– thrombomodulin complex activates protein C, but this activation proceeds more efficiently when protein C is bound to the endothelial protein C receptor (EPCR). Although APC retains its affinity to EPCR, upon dissociation from EPCR it binds to protein S, which enables it to inactivate factors Va and VIIIa. Collectively, the anticoagulant mechanisms serve to effectively shut off coagulation; however, chemical signals that downregulate these mechanisms can initiate coagulation [527,528,539,541].
ROS and coagulation
The tight control of the coagulation system observed in healthy tissue can be disrupted by ROS, which can alter the expression and/or activity of key components of the coagulation cascade [534]. Redox-sensitive regulatory mechanisms have been implicated in the production and activation of tissue factor. The gene encoding TF is redox sensitive [548]. Exposure of endothelial cells or monocytes to ROS (e.g., H2O2 or xanthine–xanthine oxidase) results in increased TF mRNA and protein expression as well as enhanced TF procoagulant activity [549,550]. The action of ROS on endothelial cell TF expression is probably amplified by a corresponding inhibitory effect of ROS on tissue factor pathway inhibitor production by endothelial cells [551]. Endogenous ROS exert a similar influence on TF expression because the increased TF expression elicited in endothelial cells in response to cytokines (e.g., TNFα) and on vascular smooth muscle or platelets activated by thrombin is blunted by antioxidant treatment [552–555]. The redox-sensitive expression of TF has also been demonstrated in the heart after exposure to I/R. The I/R-induced TF expression was abolished by treatment with oxygen radical scavengers [549]. Several studies have linked ROS-dependent induction/activation of TF to NADPH oxidase in endothelial cells, platelets, and neutrophils [553,556]. A role for NADPH oxidase-derived ROS in thrombin-induced TF expression is supported by studies employing p47phox−/− mice, which exhibit less TF expression after thrombin challenge than their wild-type counterparts [226].
Other components of the coagulation pathway are also influenced by ROS to promote a procoagulant environment. The protein C pathway is altered in several ways by ROS. The shedding of EPCR by endothelial cells can be induced by ROS and antioxidants have been shown to blunt the EPCR shedding elicited by cytokines [557]. APC, a serine protease, can be directly inactivated by oxidants, which induce changes in the structure of the active site of APC, in part via the oxidation of methionine [558]. Thrombomodulin, which also participates in the activation of protein C, is inactivated by neutrophil-derived oxidants via a mechanism that involves the oxidation of specific methionine residues [559]. Whereas ROS can act in multiple ways to curtail the generation of APC, it is interesting to note that this potent endogenous anticoagulant has been shown to possess intrinsic antioxidant properties [560], which has led to the proposal that the APC may exert its anti-inflammatory actions via this mechanism.
Thrombin and the heparin–antithrombin complex are also vulnerable to ROS-mediated functional alterations. Neutrophil-derived HOCl, but not H2O2, has been shown to oxidize and functionally impair human thrombin [561]. The oxidized thrombin exhibits a diminished capacity to interact with thrombomodulin, protein C, and the antithrombin III–heparin complex, as well as a reduction in its ability to activate platelets. Oxidation of antithrombin by hydrogen peroxide appears to minimally alter thrombin-binding activity and heparin binding of the anticoagulant [562]. However, lipid peroxides have been shown to significantly reduce antithrombin activity by targeting the heparin-binding site [563].
The procoagulant, prothrombotic effects of ROS are also the result of effects on key components of the fibrinolytic system. Oxidatively modified fibrinogen forms fibrin at an accelerated rate and has a reduced capacity to stimulate tissue plasminogen activator to convert plasminogen to plasmin, compared to nonoxidized fibrinogen [564]. ROS also enhance fibrinolysis by inhibiting plasminogen activator [565] and by increasing the transcription of PAI-1 in endothelial cells, vascular smooth muscle, and other cells [566,567]. The contention that PAI-1 expression is redox sensitive is supported by reports describing the inhibition of PAI-1 expression in vascular smooth muscle cells by treatment with antioxidants or depletion of the p22 subunit of NADPH oxidase [568]. The oxidant-sensitive transcription factor AP-1 has been implicated in ROS-induced upregulation of PAI-1 [569].
NO and coagulation
Whereas superoxide and hydrogen peroxide tend to activate the coagulation cascade, nitric oxide exerts an opposite effect. Endogenous NO appears to prevent the development of a prothrombotic phenotype in endothelial cells. l-Arginine- but not d-arginine-supplemented endothelial cells exhibit a blunted expression of TF in response to challenge with either LPS or interleukin-1β [570]. A similar effect on endothelial cell TF expression (induced by LPS) is noted on human umbilical vein endothelial cell monolayers exposed to the NO donor SNAP, whereas the NO synthase inhibitor L-NAME enhances the expression of TF [571]. The role of NO as a regulator of TF expression on endothelial cells has also been demonstrated in murine models of eNOS deficiency or overexpression [572].
Although endothelial cells have received considerable attention regarding the modulatory influence of NO on TF expression, there is also evidence implicating NO as a regulator of TF expression on monocytes. For example, the endogenous NO synthase inhibitor ADMA has been shown to elicit a dose-dependent increase in TF expression on the monocytic cell line THP-1 [573]. The effect of ADMA is NF-κB dependent, suggesting that NO mediates TF expression at the level of transcription. This differs mechanistically from the direct actions of peroxynitrite (ONOO), a product of the reaction of NO with superoxide, on TF activity. The extracellular domain of TF is rich in tyrosine, an amino acid that is vulnerable to attack by ONOO. Consequently, recombinant TF exposed to ONOO has more nitrotyrosine residues and exhibits reduced procoagulant activity [574]. The effects of ONOO on coagulation do not seem to be limited to TF. Exposure of human plasma to ONOO in vitro impairs hemostatic function and this is accompanied by decreased activities of factors VII and X and the factor VIII complex, as well as antithrombin and protein C activity [575].
The fibrinolytic system is also a target for NO and its products. In vitro studies indicate that NO donors decrease the expression and release of PAI-1 [576], whereas NOS inhibitors induce vascular PAI-1 expression [577,578]. The actions of NO on PAI-1 seem to be mediated via a cGMP-dependent mechanism. The net effect of NO on fibrinolytic balance remains unclear because the gaseous oxide also appears to reduce the expression and release of t-PA [579]. The influence of NO on fibrinolysis is evident in NO-deficient mice, which are characterized by increased t-PA levels, enhanced fibrinolysis, and decelerated thrombosis. The enhanced fibrinolysis has been attributed to a lack of NO-mediated Weibel-Palade release of t-PA (which is stored in these endothelial cell granules) [580].
Nitrosative stress has been implicated in the modulation of fibrinolysis. Some reports describe time- and dose-dependent plasminogen inactivation after exposure to the peroxynitrite donor 3-morpholinosydnonimine [581]. ONOO has also been reported to decrease fibrinogen function and inactivate t-PA [582]. It has been suggested that nitrating oxidants significantly accelerate clot formation and factor VIII cross-linking, whereas nonnitrating oxidants decelerate clot formation [583]. The results of this study also suggest the clots formed with fibrinogen exposed to nitrating oxidants may be more readily deformed by mechanical stress, which may have some bearing on the process of atherothrombosis.
Platelets: a source of ROS/RNOS
The activation, adhesion, and aggregation of platelets, a critical component of thrombus formation, represent the primary response to vascular injury (with activation of the coagulation cascade and fibrin formation as secondary responses). Upon exposure to activating signals (e.g., collagen), platelets rapidly adhere to the vessel wall, release the contents of their granules, undergo shape changes that allow them to spread and flatten along the vessel surface, and interact (aggregate) with one another to form a plug that seals the injured vessel surface [540,541,544]. Another consequence of platelet activation and aggregation is an enhanced ROS production, which is accompanied by increased oxygen consumption and elevated glutathione disulfide levels [584,585]. Stimulated platelets have been shown to produce superoxide, hydrogen peroxide, and hydroxyl radicals [586–589]. The quantity (flux) of superoxide produced by activated platelets is in the nanomolar range and comparable to that generated by endothelial cells, but is a small fraction of the amount (micromolar range) generated by activated neutrophils [590,591]. Consequently, it has been suggested that platelet-derived ROS probably play a more important role in autocrine or paracrine signaling, rather than a phagocytic function [586,592].
Although platelets have the potential to produce ROS from several enzymatic sources, most reports have attributed platelet-derived ROS to NADPH oxidase [586,592,593]. The isoform of NADPH oxidase expressed by platelets is similar to that found in neutrophils, with subunits for gp91phox, p47phox, and p22phox [590,594,595]. The limited capacity of platelets from patients with gp91phox deficiency to produce superoxide further highlights the importance of this enzymatic source [596]. Platelet agonists (e.g., collagen) increase NADPH oxidase-dependent ROS production via a mechanism that involves PI3-kinase- and PKCζ-mediated translocation of the p47phox subunit to the plasma membrane [597]. Although collagen-induced GPIIb/IIIa-mediated platelet aggregation is associated with enhanced superoxide production via NADPH oxidase [538], the time course of superoxide generation lags behind the aggregation response, suggesting that superoxide is not critical for the initiation of aggregation [592]. Nonetheless, both NADPH oxidase inhibitors and superoxide scavengers have been reported to blunt platelet aggregation and thrombus formation on collagen [598].
Platelets and megakaryocytes also express the same isoform (NOSIII) of nitric oxide synthase that is detected in endothelial cells [599]. Platelet-associated NOS normally generates nitric oxide, but when cofactor (e.g., BH4) levels are limited, the enzyme can also generate superoxide [8]. “Uncoupled” NOS has been implicated in platelet superoxide production because platelets derived from eNOS-deficient mice exhibit a marked attenuation of superoxide flux [600]. More attention has been devoted, however, to the nitric oxide-generating function of platelet-associated NOS. Both resting and aggregating platelets generate NO, but the levels of NO produced by platelets is much lower than reported for endothelial cells [601,602]. As described for endothelial cells, the activity of platelet-associated NOS is regulated by Ca2+/calmodulin and requires NADPH [601,603]. The dependence of platelet NO production on NOS is evidenced by the absence of NO generation in wild-type platelets treated with NOS inhibitors and in eNOS-deficient platelets [604]. Platelet agonists, such as collagen and ADP, activate platelet NOS, which leads to increased intracellular levels of cGMP [599]. The effect of collagen on platelet NOS is mediated through engagement of the GPVI receptor and involves both the PI3-kinase/Akt pathway and protein kinase C [600]. CD40L, which is largely derived from platelets, has been shown to mediate agonist-dependent production of NO as well as superoxide in platelets through activation of the Akt and MAP kinase signaling pathways [605].
Platelets: a target for ROS/RNOS
Both superoxide and nitric oxide have been implicated in the regulation of platelet function, with the former inducing cell activation and aggregation and the latter exerting an inhibitory effect. A variety of mechanisms have been implicated in the proaggregation actions of ROS, including the inactivation of NO [534,606], inhibition of redox-sensitive ecto-ADPases [534,590,606], and enhanced reactivity of platelets to agonists, such as thrombin, ADP, and collagen [607]. Activation of platelet NADPH oxidase results in an increased release of CD40L from platelets (a indicator of platelet activation) [608] and elicits a concomitant activation of glycoprotein IIb/IIIa and αIIbβ3 integrin, which mediate platelet aggregation and adhesion [594], suggesting that the low levels of ROS produced by platelets are sufficient to modulate platelet function. Whereas both intra- and extracellular appear to modulate CD40L expression and release, studies performed with extracellular antioxidants suggest that P-selectin expression is regulated by extracellular , and αIIbβ3 integrin activation is regulated by intracellular superoxide [609].
Although there is evidence that exogenous ROS can alter platelet function, the responses of platelets to various ROS species have been inconsistent and seem to result from differences in experimental protocols, including whether ROS exposure occurs in the presence of plasma. For example, superoxide is known to enhance platelet aggregation [610], but not in the presence of plasma [611–613]. The effect of superoxide is generally attributed to inactivation of NO; however, it has also been proposed that superoxide may indirectly promote platelet aggregation by inactivating PAF-acetylhydrolase, the enzyme that degrades PAF, a potent platelet agonist [614]. H2O2 can either stimulate or inhibit platelet aggregation [612], even in the presence of plasma [615–617]. The inhibitory effect of H2O2 seems to result from stimulation of guanylate cyclase [611], whereas the stimulatory effect has been attributed to Ca2+ release from the sarcoplasmic reticulum as a result of oxidation of sulfhydryl groups in the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase and enhanced Ca2+ release from mitochondria [618]. Oxidized low-density lipoprotein (oxLDL) can also stimulate or inhibit platelet function in a concentration-dependent manner [619]. Whether the levels of H2O2 and oxLDL shown to alter platelet function are pathophysiologically relevant remains unclear.
It is now widely accepted that NO is an important regulator of platelet function, with NO derived from either endothelial cells or platelets exerting an inhibitory influence on platelet adhesion and aggregation [593,599]. Nitric oxide is also able to disaggregate preformed platelet aggregates [620] and to inhibit the recruitment of platelets into aggregates [621]. NO is considered to have an autoregulatory function in modulating platelet reactivity, because the NO produced by platelets upon activation serves to limit further adhesion and aggregation [621]. The inhibitory effects of NO on platelet function are counteracted by superoxide, which inactivates NO, and stabilized by superoxide dismutase. NOS inhibitors promote platelet adhesion and aggregation in vivo, suggesting that the basal level of NO produced by endothelial cells and platelets is sufficient to prevent these responses. NO donors or l-arginine inhibit platelet adhesion and aggregation in vivo [622,623]. The effects of NO on platelet function are largely related to stimulation of sGC and the generation of cyclic GMP. Several signaling pathways have been implicated in NO-mediated, cGMP-dependent modulation of platelet function, including cyclic nucleotide-gated cation channels, cGMP-dependent protein receptors, cGMP-regulated phosphodiesterases, and cGMP-dependent protein kinases [624]. The net effect of cGMP-mediated activation of these pathways is a reduction in intracellular calcium, which inhibits the conformation change of GPIIb/IIIa into its active form [625]. Some actions of NO on platelets have also been attributed to a cGMP-independent mechanism [625]. For example, S-nitrosylation of N-ethylmaleimide-sensitive factor has been implicated in NO-mediated inhibition of platelet granule exocytosis and this effect is not prevented by sGC inhibition [626].
Thrombus formation: role of ROS and RNOS
Based on the known actions of ROS and NO on the coagulation cascade and platelet function, it can be predicted that ROS promote thrombus development and NO is antithrombotic. Studies that directly examine thrombus formation after endothelial injury in macroscopic or microscopic blood vessels are generally consistent with this prediction. Superoxide dismutase, catalase, and less specific free radical scavengers have been shown to blunt thrombus development in a variety of in vivo models [627,628]. The protection against thrombosis afforded by SOD has been attributed to preservation of NO bioavailability [629]. Iron chelation with desferrioxamine has also proven effective in blunting thrombosis [630], whereas chronic iron administration appears to accelerate arterial thrombosis [631], suggesting that iron-catalyzed radicals derived from superoxide and hydrogen peroxide contribute to thrombogenesis. There is evidence implicating both NADPH oxidase [598] and xanthine oxidase [632] as sources of the ROS involved in thrombus development.
The contribution of endogenous NO to thrombus formation is less clear. Pharmacological [633] inhibition of NOS appears to enhance thrombus development, an effect that can be reversed by coadministration of l-arginine or an NO donor [627,634,635]. This protective effect of endogenous NO is more evident in venules than in arterioles in otherwise healthy animals [635]. The prothrombotic effects of hypercholesterolemia and hyperhomocysteinemia have also been linked to reduced NO bioavailability [531,636]. Hypercholesterolemia elicits a more profound acceleration of thrombosis in arterioles than in venules, and the arteriolar response is reversed by l-arginine treatment [531]. eNOS-deficient mice, on the other hand, do not exhibit spontaneous thrombosis and some studies reveal a minor role for NO in the inhibition of arterial thrombosis [604,633]. Other studies, however, indicate that eNOS deficiency potentiates thrombus development and the hemodynamic abnormalities associated with thrombosis [637,638]. The inconsistent results generated in eNOS-deficient mice have been attributed to compensatory mechanisms that oppose the effects of eNOS gene deletion [580] as well as the observation that the platelet phenotype of eNOS-deficient mice is apparent only after moderate to high thrombotic stimulation [637]. The relative contributions of platelets, endothelial cells, and other cells (e.g., leukocytes, macrophages) to the ROS and NO that modulate thrombus formation remain unknown.
Epilogue
The literature is generally consistent with the view that alterations in ROS and NO contribute to the five characteristic microvascular responses to inflammation, i.e., vasomotor dysfunction, leukocyte recruitment, increased vascular permeability, angiogenesis, and thrombosis. Although the relative contributions of ROS and NO to the inflammation-induced changes in vascular function and structure remain unclear, it is evident that the highly reactive nature of NO and ROS with specific cellular and molecular targets within and surrounding the blood vessel wall enables these mediators to elicit the diverse microvascular responses to inflammation. Of equal importance is the role of endothelial cells, not only as a source and target of ROS and NO, but also as critical players in each of the five vascular responses to inflammation. Endothelial cells seem to serve as gatekeepers in the modulation of vascular smooth muscle tone and for the adhesion and transmigration of leukocytes and the proliferation of blood vessels, and they provide the interface for the activation and deposition of coagulation factors and the binding of platelets during thrombo-genesis. These unique properties of endothelial cells justify the inordinate attention that has been devoted to studying their production of, and responses to, reactive oxygen and nitrogen species.
Although much has been learned about the roles of NO and ROS in regulating the growth and function of blood vessels in health and disease, there are several areas of uncertainty and controversy in this field that warrant more attention. These include the interplay between the ROS- and the NO-generating systems, compartmentalization of ROS and NO generation, and the contribution of the endothelial glycocalyx as a source and target of ROS and NO.
A classic example of the interplay between ROS- and NO-generating systems is the uncoupling of eNOS by ROS, which converts eNOS from an NO-generating enzyme to one that generates superoxide [65]. The increased generation of ROS (e.g., NADPH oxidase) can result in BH4 oxidation [64] and the depletion of NADPH [216], which results in the uncoupling of eNOS. However, it is also becoming apparent that, under certain conditions, endogenous ROS (e.g., NADPH oxidase-derived H2O2) can prime/activate eNOS to generate increased levels of NO [639–641]. This phenomenon highlights the unpredictable nature of the interplay between ROS and NO and should serve as a warning about data interpretation when there is a potential for both systems to be activated.
The multiple sources of NO (NOS isoforms) and ROS (mitochondria, oxidases), as well as the proximity of these sources to their molecular targets, necessitate a mechanism to ensure specificity of signaling. It has been proposed that signaling specificity of ROS and NO is achieved by subcellular compartmentalization of the generating systems and the relevant effector molecules [97,369,405,641–643]. In endothelial cells, NOS has been localized to the Golgi apparatus, cytosol, cytoskeleton, or lipid rafts/caveolae of the plasma membrane. Similarly, NADPH oxidase has been localized to the perinuclear endoplasmic reticulum, cytoskeletal elements, and lipid rafts/caveolae of the plasma membrane. This differential localization of ROS- and NO-generating systems should allow for selective activation of the NO- and ROS-producing enzymes and localized production of these reactive species. Furthermore, the localization of the ROS- and NO-generating systems is not fixed; both eNOS and NADPH oxidase can be translocated to other compartments to induce functionally distinct microvascular responses. For example, acetylcholine-induced vasodilation is associated with translocation of eNOS from the plasma membrane to the Golgi, whereas PAF-induced increases in vascular permeability are associated with translocation of eNOS to a cytosolic compartment [369,644]. During angiogenesis NADPH oxidase is translocated to the lamellipodia of the migrating endothelial cells [97,502]. The mobile nature of the intracellular compartments housing NO and ROS generators is an exciting aspect of ROS and NO signaling that deserves more attention.
An interesting and potentially important revelation of our examination of this literature is the possible contribution of the endothelial glycocalyx to the diverse microvascular responses to inflammation. The endothelial glycocalyx is a dynamic lining of the luminal surface of endothelial cells whose composition varies based on the rate of shedding and biosynthesis of its components [75,76]. This network of carbohydrate-rich proteoglycans, glycoproteins, and associated GAGs exhibits a high binding affinity for a variety of endothelial-cell-derived and circulating molecules that act as generators (xanthine oxidase, myeloperoxidase) or detoxifiers (ecSOD) of ROS [75]. The net effect of this binding property of the glycocalyx is to concentrate molecules on the endothelial cell surface that can alter cell function (e.g., chemokines) and/or the quality and density of the glycocalyx itself (e.g., ROS generators). It is not unexpected that the glycocalyx exerts an influence on the microvascular response to inflammation because the partial dissolution of the glycocalyx that accompanies this condition can: (1) affect the attachment of transmembrane components of the glycocalyx to caveolae, thereby disrupting its function as a mechanosensor for shear-induced vasodilation [75,76]; (2) promote leukocyte–endothelial cell adhesive interactions; (3) diminish the charge selectivity of the endothelial barrier, thereby promoting protein extravasation; and (4) reduce the capacity of the glycocalyx to bind anticoagulant molecules (e.g., anti-thrombin, TFPI) and diminish the thrombo-resistance of the endothelial cell surface [75]. The functional relevance of the glycocalyx to inflammation-induced angiogenesis remains unclear and warrants exploration.
Future development of technology that provides a minimally invasive means for monitoring vascular dysfunction and for the detection, quantification, and selective ablation of ROS and NO in the living animal is critical for advancement of this field of inquiry. In this regard, substantial progress is evident, in terms of both the approaches being applied currently and those with potential for future applications. For example, a fluorescent H2O2 sensor (HyPer) is available to monitor H2O2 levels in vitro [640,645] and in vivo [194]. It is specific for H2O2 (does not respond to superoxide or NO) and allows for compartmental quantification of H2O2 in real time, e.g., up to hours after injury and for a distance of 100–200 mm from the site of injury [194]. The burgeoning area of posttranslational regulation of gene products by microRNAs (miR's) also holds promise in the field of ROS and NO research. Several miR's have been identified that can regulate the inflammatory response [646–648], including miR's that can exert an influence, either directly or indirectly, on endothelial NADPH oxidase [649] and NOS [650,651]. Future advances in this area should lead to the availability of specific miR's and antagomirs to more comprehensively assess the roles of ROS and NO in the microcirculatory responses to inflammation. Epigenetics [652] and gastrointestinal biota [653] are two of many additional factors that warrant consideration in future studies that address the roles of ROS and NO in mediating the microvascular dysfunction that is associated with inflammation.
The quantitative significance of the balance between NO and ROS to the microvascular dysfunction in inflammation remains uncertain. Hence, there is a clear need for more research on, and an improved understanding of, the involvement of reactive oxygen and nitrogen species in the vascular responses that accompany different acute and chronic inflammatory diseases. Future development of technology that provides a noninvasive means for monitoring vascular dysfunction and the detection, quantification, and selective ablation of ROS and NO in the living animal is critical for advancement of this field of inquiry. The information that would result from this effort should provide novel and potentially useful insights into the signals that drive inflammation and other microvascular diseases (e.g., diabetes) and ultimately lead to the development of novel therapeutic strategies that diminish the morbidity and mortality associated with these diseases.
Acknowledgments
D.N.G. was supported by grants from the National Heart, Lung, and Blood Institute (R01-HL26441) and the National Institutes of Diabetes and Digestive and Kidney Diseases (P01-DK43785).
References
- 1.Granger DN, Senchenkova E. Inflammation and the microcirculation. In: Granger DN, Granger JP, editors. Colloquium Series in Integrated Systems Physiology: from Molecules to Function. Princeton, NJ: Morgan-Claypool Life. Sci; 2010. [PubMed] [Google Scholar]
- 2.Ley K. The microcirculation in inflammation. In: Tuma RDW, Ley K, editors. Handbook of Physiology: Microcirculation. San Diego: Academic Press; 2008. pp. 387–448. [Google Scholar]
- 3.Majno G. Role of inflammatory mediators in angiogenesis. Am. J. Pathol. 1998;153:1035–1039. doi: 10.1016/S0002-9440(10)65648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan GB, Majno G. Acute inflammation: a review. Am. J. Pathol. 1977;86:183–276. [PMC free article] [PubMed] [Google Scholar]
- 5.Esmon C. Crosstalk between inflammation and thrombosis. Maturitas. 2004;47:305–314. doi: 10.1016/j.maturitas.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev. Mol. Med. 2009;11:e19. doi: 10.1017/S1462399409001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolin MS. Reactive oxygen species and the control of vascular function. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H539–H549. doi: 10.1152/ajpheart.01167.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolin MS, Gupte SA, Oeckler RA. Superoxide in the vascular system. J. Vasc. Res. 2002;39:191–207. doi: 10.1159/000063685. [DOI] [PubMed] [Google Scholar]
- 9.Tyml K. Critical role for oxidative stress, platelets, and coagulation in capillary blood flow impairment in sepsis. Microcirculation. 2011;18:152–162. doi: 10.1111/j.1549-8719.2010.00080.x. [DOI] [PubMed] [Google Scholar]
- 10.Tailor A, Cooper D, Granger DN. Platelet–vessel wall interactions in the microcirculation. Microcirculation. 2005;12:275–285. doi: 10.1080/10739680590925691. [DOI] [PubMed] [Google Scholar]
- 11.Granger DN, Kubes P. Nitric oxide as antiinflammatory agent. Methods Enzymol. 1996;269:434–442. doi: 10.1016/s0076-6879(96)69044-2. [DOI] [PubMed] [Google Scholar]
- 12.Suematsu M, Suzuki H, Delano FA, Schmid-Schonbein GW. The inflammatory aspect of the microcirculation in hypertension: oxidative stress, leukocytes/endothelial interaction, apoptosis. Microcirculation. 2002;9:259–276. doi: 10.1038/sj.mn.7800141. [DOI] [PubMed] [Google Scholar]
- 13.Aiello VD, Gutierrez PS, Chaves MJ, Lopes AA, Higuchi ML, Ramires JA. Morphology of the internal elastic lamina in arteries from pulmonary hypertensive patients: a confocal laser microscopy study. Mod. Pathol. 2003;16:411–416. doi: 10.1097/01.MP.0000067685.57858.D7. [DOI] [PubMed] [Google Scholar]
- 14.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ. Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 15.Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium. 2008;44:135–146. doi: 10.1016/j.ceca.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ. Res. 2007;100:246–254. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- 17.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 18.Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol. (Oxford) 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 19.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- 20.Bryan NS, Bian K, Murad F. Discovery of the nitric oxide signaling pathway and targets for drug development. Front. Biosci. 2009;14:1–18. doi: 10.2741/3228. [DOI] [PubMed] [Google Scholar]
- 21.Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br. J. Pharmacol. 1988;93:515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feletou M, Vanhoutte PM. EDHF: an update. Clin. Sci. (London) 2009;117:139–155. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- 23.Dora KA. Coordination of vasomotor responses by the endothelium. Circ. J. 2010;74:226–232. doi: 10.1253/circj.cj-09-0879. [DOI] [PubMed] [Google Scholar]
- 24.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- 25.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J. Clin. Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matoba T, Shimokawa H. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J. Pharmacol. Sci. 2003;92:1–6. doi: 10.1254/jphs.92.1. [DOI] [PubMed] [Google Scholar]
- 27.Shimokawa H. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflugers Arch. 2010;459:915–922. doi: 10.1007/s00424-010-0790-8. [DOI] [PubMed] [Google Scholar]
- 28.Feletou M. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br. J. Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ. Res. 2008;102:1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 31.Chauhan S, Rahman A, Nilsson H, Clapp L, MacAllister R, Ahluwalia A. NO contributes to EDHF-like responses in rat small arteries: a role for NO stores. Cardiovasc. Res. 2003;57:207–216. doi: 10.1016/s0008-6363(02)00611-9. [DOI] [PubMed] [Google Scholar]
- 32.Brahler S, Kaistha A, Schmidt VJ, Wolfle SE, Busch C, Kaistha BP, Kacik M, Hasenau AL, Grgic I, Si H, Bond CT, Adelman JP, Wulff H, de. Wit C, Hoyer J, Kohler R. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- 33.Andrews KL, Irvine JC, Tare M, Apostolopoulos J, Favaloro JL, Triggle CR, Kemp-Harper BK. A role for nitroxyl (HNO) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. Br. J. Pharmacol. 2009;157:540–550. doi: 10.1111/j.1476-5381.2009.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takaki A, Morikawa K, Tsutsui M, Murayama Y, Tekes E, Yamagishi H, Ohashi J, Yada T, Yanagihara N, Shimokawa H. Crucial role of nitric oxide synthases system in endothelium-dependent hyperpolarization in mice. J. Exp. Med. 2008;205:2053–2063. doi: 10.1084/jem.20080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, da Costa Goncalves A, Huang Y, Luft FC, Gollasch M. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler. Thromb. Vasc. Biol. 2009;29:54–60. doi: 10.1161/ATVBAHA.108.171298. [DOI] [PubMed] [Google Scholar]
- 36.Sun D, Liu H, Yan C, Jacobson A, Ojaimi C, Huang A, Kaley G. COX-2 contributes to the maintenance of flow-induced dilation in arterioles of eNOS-knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1429–H1435. doi: 10.1152/ajpheart.01130.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ. Res. 2008;102:59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cioffi DL, Lowe K, Alvarez DF, Barry C, Stevens T. TRPing on the lung endothelium: calcium channels that regulate barrier function. Antioxid. Redox Signaling. 2009;11:765–776. doi: 10.1089/ars.2008.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pani B, Singh BB. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium. 2009;45:625–633. doi: 10.1016/j.ceca.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rath G, Dessy C, Feron O. Caveolae, caveolin and control of vascular tone: nitric oxide (NO) and endothelium derived hyperpolarizing factor (EDHF) regulation. J. Physiol. Pharmacol. 2009;60(Suppl. 4):105–109. [PubMed] [Google Scholar]
- 41.Alicia S, Angelica Z, Carlos S, Alfonso S, Vaca L. STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: moving TRPC1 in and out of lipid rafts. Cell Calcium. 2008;44:479–491. doi: 10.1016/j.ceca.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension. 2009;53:532–538. doi: 10.1161/HYPERTENSIONAHA.108.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H466–H476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc. Res. 2008;80:445–452. doi: 10.1093/cvr/cvn207. [DOI] [PubMed] [Google Scholar]
- 45.Ma X, Qiu S, Luo J, Ma Y, Ngai CY, Shen B, Wong CO, Huang Y, Yao X. Functional role of vanilloid transient receptor potential 4-canonical transient receptor potential 1 complex in flow-induced Ca2+ influx. Arterioscler. Thromb. Vasc. Biol. 2010;30:851–858. doi: 10.1161/ATVBAHA.109.196584. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka Y, Koike K, Toro L. MaxiK channel roles in blood vessel relaxations induced by endothelium-derived relaxing factors and their molecular mechanisms. J. Smooth Muscle Res. 2004;40:125–153. doi: 10.1540/jsmr.40.125. [DOI] [PubMed] [Google Scholar]
- 47.Nimmegeers S, Sips P, Buys E, Brouckaert P, Van de Voorde J. Functional role of the soluble guanylyl cyclase alpha(1) subunit in vascular smooth muscle relaxation. Cardiovasc. Res. 2007;76:149–159. doi: 10.1016/j.cardiores.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Martin W. Nitroxyl anion—the universal signalling partner of endogenously produced nitric oxide? Br. J. Pharmacol. 2009;157:537–539. doi: 10.1111/j.1476-5381.2009.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheng JZ, Ella S, Davis MJ, Hill MA, Braun AP. Openers of SKCa and IKCa channels enhance agonist-evoked endothelial nitric oxide synthesis and arteriolar vasodilation. FASEB J. 2009;23:1138–1145. doi: 10.1096/fj.08-120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dalsgaard T, Kroigaard C, Misfeldt M, Bek T, Simonsen U. Openers of small conductance calcium-activated potassium channels selectively enhance NO-mediated bradykinin vasodilatation in porcine retinal arterioles. Br. J. Pharmacol. 2010;160:1496–1508. doi: 10.1111/j.1476-5381.2010.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ. Res. 2011;108:566–573. doi: 10.1161/CIRCRESAHA.110.237636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garry A, Edwards DH, Fallis IF, Jenkins RL, Griffith TM. Ascorbic acid and tetrahydrobiopterin potentiate the EDHF phenomenon by generating hydrogen peroxide. Cardiovasc. Res. 2009;84:218–226. doi: 10.1093/cvr/cvp235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edwards DH, Li Y, Griffith TM. Hydrogen peroxide potentiates the EDHF phenomenon by promoting endothelial Ca2+ mobilization. Arterioscler. Thromb. Vasc. Biol. 2008;28:1774–1781. doi: 10.1161/ATVBAHA.108.172692. [DOI] [PubMed] [Google Scholar]
- 54.Morikawa K, Shimokawa H, Matoba T, Kubota H, Akaike T, Talukder MA, Hatanaka M, Fujiki T, Maeda H, Takahashi S, Takeshita A. Pivotal role of Cu. Zn-superoxide dismutase in endothelium-dependent hyperpolarization. J. Clin. Invest. 2003;112:1871–1879. doi: 10.1172/JCI19351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yada T, Shimokawa H, Morikawa K, Takaki A, Shinozaki Y, Mori H, Goto M, Ogasawara Y, Kajiya F. Role of Cu. Zn-SOD in the synthesis of endogenous vasodilator hydrogen peroxide during reactive hyperemia in mouse mesenteric microcirculation in vivo. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H441–H448. doi: 10.1152/ajpheart.01021.2007. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ. Res. 2003;93:573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 57.Larsen BT, Bubolz AH, Mendoza SA, Pritchard KA, Jr, Gutterman DD. Bradykinin-induced dilation of human coronary arterioles requires NADPH oxidase-derived reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 2009;29:739–745. doi: 10.1161/ATVBAHA.108.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takaki A, Morikawa K, Murayama Y, Yamagishi H, Hosoya M, Ohashi J, Shimokawa H. Roles of endothelial oxidases in endothelium-derived hyperpolarizing factor responses in mice. J. Cardiovasc. Pharmacol. 2008;52:510–517. doi: 10.1097/FJC.0b013e318190358b. [DOI] [PubMed] [Google Scholar]
- 59.Loot AE, Schreiber JG, Fisslthaler B, Fleming I. Angiotensin II impairs endothelial function via tyrosine phosphorylation of the endothelial nitric oxide synthase. J. Exp. Med. 2009;206:2889–2896. doi: 10.1084/jem.20090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc. Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 61.Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid. Redox Signaling. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 62.Duerrschmidt N, Stielow, C.;Muller G, Pagano PJ, Morawietz H. NO-mediated regulation of NAD(P)H oxidase by laminar shear stress in human endothelial cells. J. Physiol. 2006;576:557–567. doi: 10.1113/jphysiol.2006.111070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Billaud M, Marthan R, Savineau JP, Guibert C. Vascular smooth muscle modulates endothelial control of vasoreactivity via reactive oxygen species production through myoendothelial communications. PLoS One. 2009;4:e6432. doi: 10.1371/journal.pone.0006432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karuppiah K, Druhan L, Chen CA, Smith T, Zweier JL, Sessa WC, Cardounel AJ. Suppression of eNOS-derived superoxide by caveolin-1: a biopterin-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H903–H911. doi: 10.1152/ajpheart.00936.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rabelink TJ, van Zonneveld AJ. Coupling eNOS uncoupling to the innate immune response. Arterioscler. Thromb. Vasc. Biol. 2006;26:2585–2587. doi: 10.1161/01.ATV.0000250932.24151.50. [DOI] [PubMed] [Google Scholar]
- 66.Capettini LS, Cortes SF, Lemos VS. Relative contribution of eNOS and nNOS to endothelium-dependent vasodilation in the mouse aorta. Eur. J. Pharmacol. 2010;643:260–266. doi: 10.1016/j.ejphar.2010.06.066. [DOI] [PubMed] [Google Scholar]
- 67.Dossumbekova A, Berdyshev EV, Gorshkova I, Shao Z, Li C, Long P, Joshi A, Natarajan V, Vanden Hoek TL. Akt activates NOS3 and separately restores barrier integrity in H2O2-stressed human cardiac microvascular endothelium. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2417–H2426. doi: 10.1152/ajpheart.00501.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel HH, Insel PA. Lipid rafts and caveolae and their role in compartmentation of redox signaling. Antioxid. Redox Signaling. 2009;11:1357–1372. doi: 10.1089/ars.2008.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barakat AI. Dragging along: the glycocalyx and vascular endothelial cell mechanotransduction. Circ. Res. 2008;102:747–748. doi: 10.1161/CIRCRESAHA.108.174839. [DOI] [PubMed] [Google Scholar]
- 70.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol. Rev. 2009;89:481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 71.Dahl KN, Kalinowski A, Pekkan K. Mechanobiology and the microcirculation: cellular, nuclear and fluid mechanics. Microcirculation. 2010;17:179–191. doi: 10.1111/j.1549-8719.2009.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujiwara K. Platelet endothelial cell adhesion molecule-1 and mechanotransduction in vascular endothelial cells. J. Intern. Med. 2006;259:373–380. doi: 10.1111/j.1365-2796.2006.01623.x. [DOI] [PubMed] [Google Scholar]
- 73.Tarbell JM, Ebong EE. The endothelial glycocalyx: a mechano-sensor and -transducer. Sci. Signaling. 2008;1(pt8) doi: 10.1126/scisignal.140pt8. [DOI] [PubMed] [Google Scholar]
- 74.Loufrani L, Henrion D. Role of the cytoskeleton in flow (shear stress)-induced dilation and remodeling in resistance arteries. Med. Biol. Eng. Comput. 2008;46:451–460. doi: 10.1007/s11517-008-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 77.Pohl U, Herlan K, Huang A, Bassenge E. EDRF-mediated shear-induced dilation opposes myogenic vasoconstriction in small rabbit arteries. Am. J. Physiol. 1991;261:H2016–H2023. doi: 10.1152/ajpheart.1991.261.6.H2016. [DOI] [PubMed] [Google Scholar]
- 78.VanTeeffelen JW, Brands J, Jansen C, Spaan JA, Vink H. Heparin impairs glycocalyx barrier properties and attenuates shear dependent vasodilation in mice. Hypertension. 2007;50:261–267. doi: 10.1161/HYPERTENSIONAHA.107.089250. [DOI] [PubMed] [Google Scholar]
- 79.Chappell D, Jacob M, Paul O, Rehm M, Welsch U, Stoeckelhuber M, Conzen P, Becker BF. The glycocalyx of the human umbilical vein endothelial cell: an impressive structure ex vivo but not in culture. Circ. Res. 2009;104:1313–1317. doi: 10.1161/CIRCRESAHA.108.187831. [DOI] [PubMed] [Google Scholar]
- 80.Ebong EE, Macaluso FP, Spray DC, Tarbell JM. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arterioscler. Thromb. Vasc. Biol. 2011;31:1908–1915. doi: 10.1161/ATVBAHA.111.225268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J. Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 82.Bagi Z, Frangos JA, Yeh JC, White CR, Kaley G, Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler. Thromb. Vasc. Biol. 2005;25:1590–1595. doi: 10.1161/01.ATV.0000170136.71970.5f. [DOI] [PubMed] [Google Scholar]
- 83.Sundivakkam PC, Kwiatek AM, Sharma TT, Minshall RD, Malik AB, Tiruppathi C. Caveolin-1 scaffold domain interacts with TRPC1 and IP3R3 to regulate Ca2+ store release-induced Ca2+ entry in endothelial cells. Am. J. Physiol. Cell Physiol. 2009;296:C403–C413. doi: 10.1152/ajpcell.00470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chidlow JH, Jr, Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc. Res. 2010;86:219–225. doi: 10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J. Clin. Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van der Meer AD, Kamphuis MM, Poot AA, Feijen J, Vermes I. Lowering caveolin-1 expression in human vascular endothelial cells inhibits signal transduction in response to shear stress. Int. J. Cell Biol. 2009;2009:532432. doi: 10.1155/2009/532432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem. Biophys. Res. Commun. 2007;355:228–233. doi: 10.1016/j.bbrc.2007.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dusserre N, L'Heureux N, Bell KS, Stevens HY, Yeh J, Otte LA, Loufrani L, Frangos JA. PECAM-1 interacts with nitric oxide synthase in human endothelial cells: implication for flow-induced nitric oxide synthase activation. Arterioscler. Thromb. Vasc. Biol. 2004;24:1796–1802. doi: 10.1161/01.ATV.0000141133.32496.41. [DOI] [PubMed] [Google Scholar]
- 89.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation. 2008;117:1065–1074. doi: 10.1161/CIRCULATIONAHA.107.731679. [DOI] [PubMed] [Google Scholar]
- 90.Kumagai R, Lu X, Kassab GS. Role of glycocalyx in flow-induced production of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2009;47:600–607. doi: 10.1016/j.freeradbiomed.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kumar S, Sud N, Fonseca FV, Hou Y, Black SM. Shear stress stimulates nitric oxide signaling in pulmonary arterial endothelial cells via a reduction in catalase activity: role of protein kinase C delta. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;298:L105–L116. doi: 10.1152/ajplung.00290.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu Y, Bubolz AH, Shi Y, Newman PJ, Newman DK, Gutterman DD. Peroxynitrite reduces the endothelium-derived hyperpolarizing factor component of coronary flow-mediated dilation in PECAM-1-knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R57–R65. doi: 10.1152/ajpregu.00424.2005. [DOI] [PubMed] [Google Scholar]
- 93.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 94.Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ. Res. 2003;93:354–363. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- 95.Rizzo V, Morton C, DePaola N, Schnitzer JE, Davies PF. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1720–H1729. doi: 10.1152/ajpheart.00344.2002. [DOI] [PubMed] [Google Scholar]
- 96.Zhou X, Bohlen HG, Miller SJ, Unthank JL. NAD(P)H oxidase-derived peroxide mediates elevated basal and impaired flow-induced NO production in SHR mesenteric arteries in vivo. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1008–H1016. doi: 10.1152/ajpheart.00114.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid. Redox Signaling. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu Y, Li H, Bubolz AH, Zhang DX, Gutterman DD. Endothelial cytoskeletal elements are critical for flow-mediated dilation in human coronary arterioles. Med. Biol. Eng. Comput. 2008;46:469–478. doi: 10.1007/s11517-008-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feletou M, Verbeuren TJ, Vanhoutte PM. Endothelium-dependent contractions in SHR: a tale of prostanoid TP and IP receptors. Br. J. Pharmacol. 2009;156:563–574. doi: 10.1111/j.1476-5381.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vanhoutte PM, Tang EH. Endothelium-dependent contractions: when a good guy turns bad! J. Physiol. 2008;586:5295–5304. doi: 10.1113/jphysiol.2008.161430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feletou M, Huang Y, Vanhoutte PM. Vasoconstrictor prostanoids. Pflugers Arch. 2010;459:941–950. doi: 10.1007/s00424-010-0812-6. [DOI] [PubMed] [Google Scholar]
- 102.Virdis A, Ghiadoni L, Taddei S. Human endothelial dysfunction: EDCFs. Pflugers Arch. 2010;459:1015–1023. doi: 10.1007/s00424-009-0783-7. [DOI] [PubMed] [Google Scholar]
- 103.Tang EH, Leung FP, Huang Y, Feletou M, So KF, Man RY, Vanhoutte PM. Calcium and reactive oxygen species increase in endothelial cells in response to releasers of endothelium-derived contracting factor. Br. J. Pharmacol. 2007;151:15–23. doi: 10.1038/sj.bjp.0707190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alvarez Y, Briones AM, Hernanz R, Perez-Giron JV, Alonso MJ, Salaices M. Role of NADPH oxidase and iNOS in vasoconstrictor responses of vessels from hypertensive and normotensive rats. Br. J. Pharmacol. 2008;153:926–935. doi: 10.1038/sj.bjp.0707575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vanhoutte PM, Feletou M, Taddei S. Endothelium-dependent contractions in hypertension. Br. J. Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iwatani Y, Kosugi K, Isobe-Oku S, Atagi S, Kitamura Y, Kawasaki H. Endothelium removal augments endothelium-independent vasodilatation in rat mesenteric vascular bed. Br. J. Pharmacol. 2008;154:32–40. doi: 10.1038/bjp.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wong SL, Leung FP, Lau CW, Au CL, Yung LM, Yao X, Chen ZY, Vanhoutte PM, Gollasch M, Huang Y. Cyclooxygenase-2-derived prostaglandin F2alpha mediates endothelium-dependent contractions in the aortae of hamsters with increased impact during aging. Circ. Res. 2009;104:228–235. doi: 10.1161/CIRCRESAHA.108.179770. [DOI] [PubMed] [Google Scholar]
- 108.Liu CQ, Wong SL, Leung FP, Tian XY, Lau CW, Lu L, Yao X, Chen ZY, Yao T, Huang Y. Prostanoid TP receptor-mediated impairment of cyclic AMP-dependent vasorelaxation is reversed by phosphodiesterase inhibitors. Eur. J. Pharmacol. 2010;632:45–51. doi: 10.1016/j.ejphar.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 109.Gomez E, Schwendemann C, Roger S, Simonet S, Paysant J, Courchay C, Verbeuren TJ, Feletou M. Aging and prostacyclin responses in aorta and platelets from WKY and SHR rats. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2198–H2211. doi: 10.1152/ajpheart.00507.2008. [DOI] [PubMed] [Google Scholar]
- 110.Gao YJ, Lee RM. Hydrogen peroxide is an endothelium-dependent contracting factor in rat renal artery. Br. J. Pharmacol. 2005;146:1061–1068. doi: 10.1038/sj.bjp.0706423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wilson SJ, Cavanagh CC, Lesher AM, Frey AJ, Russell SE, Smyth EM. Activation-dependent stabilization of the human thromboxane receptor: role of reactive oxygen species. J. Lipid Res. 2009;50:1047–1056. doi: 10.1194/jlr.M800447-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barton GM. A calculated response: control of inflammation by the innate immune system. J. Clin. Invest. 2008;118:413–420. doi: 10.1172/JCI34431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 114.Silva MT. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J. Leukocyte Biol. 2010;87:93–106. doi: 10.1189/jlb.0809549. [DOI] [PubMed] [Google Scholar]
- 115.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 116.Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J. Immunol. 2008;180:6439–6446. doi: 10.4049/jimmunol.180.10.6439. [DOI] [PubMed] [Google Scholar]
- 117.Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ. Res. 2007;101:234–247. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- 118.Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ. Res. 2009;105:223–230. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 120.Heib V, Becker M, Taube C, Stassen M. Advances in the understanding of mast cell function. Br. J. Haematol. 2008;142:683–694. doi: 10.1111/j.1365-2141.2008.07244.x. [DOI] [PubMed] [Google Scholar]
- 121.Dietrich N, Rohde M, Geffers R, Kroger A, Hauser H, Weiss S, Gekara NO. Mast cells elicit proinflammatory but not type I interferon responses upon activation of TLRs by bacteria. Proc. Natl. Acad. Sci. USA. 2010;107:8748–8753. doi: 10.1073/pnas.0912551107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Laskin DL. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem. Res. Toxicol. 2009;22:1376–1385. doi: 10.1021/tx900086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weissler A, Mekori YA, Mor A. The role of mast cells in non-allergic inflammation. Isr. Med. Assoc. J. 2008;10:843–845. [PubMed] [Google Scholar]
- 125.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gauley J, Pisetsky DS. The translocation of HMGB1 during cell activation and cell death. Autoimmunity. 2009;42:299–301. doi: 10.1080/08916930902831522. [DOI] [PubMed] [Google Scholar]
- 127.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol. Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin TJ, Garduno R, Boudreau RT, Issekutz AC. Pseudomonas aeruginosa activates human mast cells to induce neutrophil transendothelial migration via mast cell-derived IL-1 alpha and beta. J. Immunol. 2002;169:4522–4530. doi: 10.4049/jimmunol.169.8.4522. [DOI] [PubMed] [Google Scholar]
- 129.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 130.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr. Opin. Rheumatol. 2007;19:289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 131.Tapping RI. Innate immune sensing and activation of cell surface Toll-like receptors. Semin. Immunol. 2009;21:175–184. doi: 10.1016/j.smim.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 132.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat. Rev. Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baccala R, Gonzalez-Quintial R, Lawson BR, Stern ME, Kono DH, Beutler B, Theofilopoulos AN. Sensors of the innate immune system: their mode of action. Nat. Rev. Rheumatol. 2009;5:448–456. doi: 10.1038/nrrheum.2009.136. [DOI] [PubMed] [Google Scholar]
- 134.Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ. Res. 2010;106:842–853. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 136.Ward JR, Wilson HL, Francis SE, Crossman DC, Sabroe I. Translational mini-review series on immunology of vascular disease: inflammation, infections and Toll-like receptors in cardiovascular disease. Clin. Exp. Immunol. 2009;156:386–394. doi: 10.1111/j.1365-2249.2009.03886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11:91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 138.Verstrepen L, Bekaert T, Chau TL, Tavernier J, Chariot A, Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-kappaB: variations on a common theme. Cell. Mol. Life Sci. 2008;65:2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu Y, Chen GY, Zheng P. CD24-Siglec G/10 discriminates danger- from pathogen-associated molecular patterns. Trends Immunol. 2009;30:557–561. doi: 10.1016/j.it.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2007;4:444–454. doi: 10.1038/ncpcardio0938. [DOI] [PubMed] [Google Scholar]
- 141.Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 2010;48:1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lichtnekert J, Vielhauer V, Zecher D, Kulkarni OP, Clauss S, Segerer S, Hornung V, Mayadas TN, Beutler B, Akira S, Anders HJ. Trif is not required for immune complex glomerulonephritis: dying cells activate mesangial cells via Tlr2/Myd88 rather than Tlr3/Trif. Am. J. Physiol. Renal Physiol. 2009;296:F867–F874. doi: 10.1152/ajprenal.90213.2008. [DOI] [PubMed] [Google Scholar]
- 143.Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyocytes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc. Res. 2006;72:384–393. doi: 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 144.Xu H, Su Z, Wu J, Yang M, Penninger JM, Martin CM, Kvietys PR, Rui T. The alarmin cytokine, high mobility group box 1, is produced by viable cardiomyocytes and mediates the lipopolysaccharide-induced myocardial dysfunction via a TLR4/phosphatidylinositol 3-kinase gamma pathway. J. Immunol. 2010;184:1492–1498. doi: 10.4049/jimmunol.0902660. [DOI] [PubMed] [Google Scholar]
- 145.Zhou H, Andonegui G, Wong CH, Kubes P. Role of endothelial TLR4 for neutrophil recruitment into central nervous system microvessels in systemic inflammation. J. Immunol. 2009;183:5244–5250. doi: 10.4049/jimmunol.0901309. [DOI] [PubMed] [Google Scholar]
- 146.Park HS, Chun JN, Jung HY, Choi C, Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc. Res. 2006;72:447–455. doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 147.Chun J, Prince A. Ca2+ signaling in airway epithelial cells facilitates leukocyte recruitment and transepithelial migration. J. Leukocyte Biol. 2009;86:1135–1144. doi: 10.1189/jlb.0209072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 149.Qin YH, Dai SM, Tang GS, Zhang J, Ren D, Wang ZW, Shen Q. HMGB1 enhances the proinflammatory activity of lipopolysaccharide by promoting the phosphorylation of MAPK p38 through receptor for advanced glycation end products. J. Immunol. 2009;183:6244–6250. doi: 10.4049/jimmunol.0900390. [DOI] [PubMed] [Google Scholar]
- 150.Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H. Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin) J. Leukocyte Biol. 2007;81:49–58. doi: 10.1189/jlb.0306200. [DOI] [PubMed] [Google Scholar]
- 151.Bianchi ME. HMGB1 loves company. J. Leukocyte Biol. 2009;86:573–576. doi: 10.1189/jlb.1008585. [DOI] [PubMed] [Google Scholar]
- 152.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger-associated olecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Swindle EJ, Metcalfe DD. The role of reactive oxygen species and nitric oxide in mast cell-dependent inflammatory processes. Immunol. Rev. 2007;217:186–205. doi: 10.1111/j.1600-065X.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 154.Mortaz E, Redegeld FA, Sarir H, Karimi K, Raats D, Nijkamp FP, Folkerts G. Cigarette smoke stimulates the production of chemokines in mast cells. J. Leukocyte Biol. 2008;83:575–580. doi: 10.1189/jlb.0907625. [DOI] [PubMed] [Google Scholar]
- 155.Kim GY, Lee JW, Ryu HC, Wei JD, Seong CM, Kim JH. Proinflammatory cytokine IL-1beta stimulates IL-8 synthesis in mast cells via a leukotriene B4 receptor 2-linked pathway, contributing to angiogenesis. J. Immunol. 2010;184:3946–3954. doi: 10.4049/jimmunol.0901735. [DOI] [PubMed] [Google Scholar]
- 156.Gilchrist M, McCauley SD, Befus AD. Expression, localization, and regulation of NOS in human mast cell lines: effects on leukotriene production. Blood. 2004;104:462–469. doi: 10.1182/blood-2003-08-2990. [DOI] [PubMed] [Google Scholar]
- 157.Swindle EJ, Metcalfe DD, Coleman JW. Rodent and human mast cells produce functionally significant intracellular reactive oxygen species but not nitric oxide. J. Biol. Chem. 2004;279:48751–48759. doi: 10.1074/jbc.M409738200. [DOI] [PubMed] [Google Scholar]
- 158.Niu XF, Ibbotson G, Kubes P. A balance between nitric oxide and oxidants regulates mast cell-dependent neutrophil–endothelial cell interactions. Circ. Res. 1996;79:992–999. doi: 10.1161/01.res.79.5.992. [DOI] [PubMed] [Google Scholar]
- 159.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care Med. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 160.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 161.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 163.Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J. Biol. Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- 164.Check J, Byrd CL, Menio J, Rippe RA, Hines IN, Wheeler MD. Src kinase participates in LPS-induced activation of NADPH oxidase. Mol. Immunol. 2010;47:756–762. doi: 10.1016/j.molimm.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Maitra U, Singh N, Gan L, Ringwood L, Li L. IRAK-1 contributes to lipopolysaccharide-induced reactive oxygen species generation in macrophages by inducing NOX-1 transcription and Rac1 activation and suppressing the expression of antioxidative enzymes. J. Biol. Chem. 2009;284:35403–35411. doi: 10.1074/jbc.M109.059501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pacquelet S, Johnson JL, Ellis BA, Brzezinska AA, Lane WS, Munafo DB, Catz SD. Cross-talk between IRAK-4 and the NADPH oxidase. Biochem. J. 2007;403:451–461. doi: 10.1042/BJ20061184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ. Res. 2005;96:818–822. doi: 10.1161/01.RES.0000163631.07205.fb. [DOI] [PubMed] [Google Scholar]
- 168.Tang D, Shi Y, Kang R, Li T, Xiao W, Wang H, Xiao X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J. Leukocyte Biol. 2007;81:741–747. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Paul-Clark MJ, McMaster SK, Sorrentino R, Sriskandan S, Bailey LK, Moreno L, Ryffel B, Quesniaux VF, Mitchell JA. Toll-like receptor 2 is essential for the sensing of oxidants during inflammation. Am. J. Respir. Crit. Care Med. 2009;179:299–306. doi: 10.1164/rccm.200707-1019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang Z, Rui T, Yang M, Valiyeva F, Kvietys PR. Alveolar macrophages from septic mice promote polymorphonuclear leukocyte transendothelial migration via an endothelial cell Src kinase/NADPH oxidase pathway. J. Immunol. 2008;181:8735–8744. doi: 10.4049/jimmunol.181.12.8735. [DOI] [PubMed] [Google Scholar]
- 171.Song JD, Lee SK, Kim KM, Kim JW, Kim JM, Yoo YH, Park YC. Redox factor-1 mediates NF-kappaB nuclear translocation for LPS-induced iNOS expression in murine macrophage cell line RAW 264.7. Immunology. 2008;124:58–67. doi: 10.1111/j.1365-2567.2007.02736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Abbas K, Breton J, Planson AG, Bouton C, Bignon J, Seguin C, Riquier S, Toledano MB, Drapier JC. Nitric oxide activates an Nrf2/sulfiredoxin antioxidant pathway in macrophages. Free Radic. Biol. Med. 2011;51:107–114. doi: 10.1016/j.freeradbiomed.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 173.Pleskova M, Beck KF, Behrens MH, Huwiler A, Fichtlscherer B, Wingerter O, Brandes RP, Mulsch A, Pfeilschifter J. Nitric oxide down-regulates the expression of the catalytic NADPH oxidase subunit Nox1 in rat renal mesangial cells. FASEB J. 2006;20:139–141. doi: 10.1096/fj.05-3791fje. [DOI] [PubMed] [Google Scholar]
- 174.Selemidis S, Dusting GJ, Peshavariya H, Kemp-Harper BK, Drummond GR. Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc. Res. 2007;75:349–358. doi: 10.1016/j.cardiores.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 175.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lefer DJ, Jones SP, Girod WG, Baines A, Grisham MB, Cockrell AS, Huang PL, Scalia R. Leukocyte–endothelial cell interactions in nitric oxide synthase-deficient mice. Am. J. Physiol. 1999;276:H1943–H1950. doi: 10.1152/ajpheart.1999.276.6.H1943. [DOI] [PubMed] [Google Scholar]
- 177.Kubes P, Granger DN. Leukocyte–endothelial cell interactions evoked by mast cells. Cardiovasc. Res. 1996;32:699–708. [PubMed] [Google Scholar]
- 178.Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, van den Berg WB. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010;62:647–657. doi: 10.1002/art.27290. [DOI] [PubMed] [Google Scholar]
- 179.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukocyte Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sugimoto N, Rui T, Yang M, Bharwani S, Handa O, Yoshida N, Yoshikawa T, Kvietys PR. Points of control exerted along the macrophage–endothelial cell–polymorphonuclear neutrophil axis by PECAM-1 in the innate immune response of acute colonic inflammation. J. Immunol. 2008;181:2145–2154. doi: 10.4049/jimmunol.181.3.2145. [DOI] [PubMed] [Google Scholar]
- 181.Brechot N, Gomez E, Bignon M, Khallou-Laschet J, Dussiot M, Cazes A, Alanio-Brechot C, Durand M, Philippe J, Silvestre JS, Van Rooijen N, Corvol P, Nicoletti A, Chazaud B, Germain S. Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice. PLoS One. 2008;3:e3950. doi: 10.1371/journal.pone.0003950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr. Opin. Immunol. 2007;19:31–38. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 183.Farley KS, Wang LF, Razavi HM, Law C, Rohan M, McCormack DG, Mehta S. Effects of macrophage inducible nitric oxide synthase in murine septic lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L1164–L1172. doi: 10.1152/ajplung.00248.2005. [DOI] [PubMed] [Google Scholar]
- 184.Thorley AJ, Ford PA, Giembycz MA, Goldstraw P, Young A, Tetley TD. Differential regulation of cytokine release and leukocyte migration by lipopolysaccharide-stimulated primary human lung alveolar type II epithelial cells and macrophages. J. Immunol. 2007;178:463–473. doi: 10.4049/jimmunol.178.1.463. [DOI] [PubMed] [Google Scholar]
- 185.Madorin WS, Rui T, Sugimoto N, Handa O, Cepinskas G, Kvietys PR. Cardiac myocytes activated by septic plasma promote neutrophil transendothelial migration: role of platelet-activating factor and the chemokines LIX and KC. Circ. Res. 2004;94:944–951. doi: 10.1161/01.RES.0000124395.20249.AE. [DOI] [PubMed] [Google Scholar]
- 186.Rui T, Cepinskas G, Feng Q, Ho YS, Kvietys PR. Cardiac myocytes exposed to anoxia–reoxygenation promote neutrophil transendothelial migration. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H440–H447. doi: 10.1152/ajpheart.2001.281.1.H440. [DOI] [PubMed] [Google Scholar]
- 187.Lefebvre JS, Marleau S, Milot V, Levesque T, Picard S, Flamand N, Borgeat P. Toll-like receptor ligands induce polymorphonuclear leukocyte migration: key roles for leukotriene B4 and platelet-activating factor. FASEB J. 2010;24:637–647. doi: 10.1096/fj.09-135624. [DOI] [PubMed] [Google Scholar]
- 188.Cirino G, Fiorucci S, Sessa WC. Endothelial nitric oxide synthase: the Cinderella of inflammation? Trends Pharmacol. Sci. 2003;24:91–95. doi: 10.1016/S0165-6147(02)00049-4. [DOI] [PubMed] [Google Scholar]
- 189.Kurose I, Wolf R, Grisham MB, Aw TY, Specian RD, Granger DN. Micro-vascular responses to inhibition of nitric oxide production: role of active oxidants. Circ. Res. 1995;76:30–39. doi: 10.1161/01.res.76.1.30. [DOI] [PubMed] [Google Scholar]
- 190.Forstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat. Clin. Pract. Cardiovasc. Med. 2008;5:338–349. doi: 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- 191.Niu XF, Smith CW, Kubes P. Intracellular oxidative stress induced by nitric oxide synthesis inhibition increases endothelial cell adhesion to neutrophils. Circ. Res. 1994;74:1133–1140. doi: 10.1161/01.res.74.6.1133. [DOI] [PubMed] [Google Scholar]
- 192.Lazzerini G, Del Turco S, Basta G, O'Loghlen A, Zampolli A, Caterina RD. Prominent role of NF-kappaB in the induction of endothelial activation by endogenous nitric oxide inhibition. Nitric Oxide. 2009;21:184–191. doi: 10.1016/j.niox.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 193.Rui T, Feng Q, Lei M, Peng T, Zhang J, Xu M, Abel ED, Xenocostas A, Kvietys PR. Erythropoietin prevents the acute myocardial inflammatory response induced by ischemia/reperfusion via induction of AP-1. Cardiovasc. Res. 2005;65:719–727. doi: 10.1016/j.cardiores.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 194.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Granger DN, Kubes P. The microcirculation and inflammation: modulation of leukocyte–endothelial cell adhesion. J. Leukocyte Biol. 1994;55:662–675. [PubMed] [Google Scholar]
- 196.Kamei M, Carman CV. New observations on the trafficking and diapedesis of monocytes. Curr. Opin. Hematol. 2010;17:43–52. doi: 10.1097/MOH.0b013e3283333949. [DOI] [PubMed] [Google Scholar]
- 197.Scheiermann C, Kunisaki Y, Jang JE, Frenette PS. Neutrophil microdomains: linking heterocellular interactions with vascular injury. Curr. Opin. Hematol. 2010;17:25–30. doi: 10.1097/MOH.0b013e328333d2a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hyduk SJ, Cybulsky MI. Role of alpha4beta1 integrins in chemokine-induced monocyte arrest under conditions of shear stress. Microcirculation. 2009;16:17–30. doi: 10.1080/10739680802425195. [DOI] [PubMed] [Google Scholar]
- 199.Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc. Res. 2010;87:300–310. doi: 10.1093/cvr/cvq137. [DOI] [PubMed] [Google Scholar]
- 200.Shao JY, Ting-Beall HP, Hochmuth RM. Static and dynamic lengths of neutrophil microvilli. Proc. Natl. Acad. Sci. USA. 1998;95:6797–6802. doi: 10.1073/pnas.95.12.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Klinke A, Nussbaum C, Kubala L, Friedrichs K, Rudolph TK, Rudolph V, Paust HJ, Schroder C, Benten D, Lau D, Szocs K, Furtmuller PG, Heeringa P, Sydow K, Duchstein HJ, Ehmke H, Schumacher U, Meinertz T, Sperandio M, Baldus S. Myeloperoxidase attracts neutrophils by physical forces. Blood. 2011;117:1350–1358. doi: 10.1182/blood-2010-05-284513. [DOI] [PubMed] [Google Scholar]
- 202.Annecke T, Chappell D, Chen C, Jacob M, Welsch U, Sommerhoff CP, Rehm M, Conzen PF, Becker BF. Sevoflurane preserves the endothelial glycocalyx against ischaemia–reperfusion injury. Br. J. Anaesth. 2010;104:414–421. doi: 10.1093/bja/aeq019. [DOI] [PubMed] [Google Scholar]
- 203.Chappell D, Dorfler N, Jacob M, Rehm M, Welsch U, Conzen P, Becker BF. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock. 2010;34:133–139. doi: 10.1097/SHK.0b013e3181cdc363. [DOI] [PubMed] [Google Scholar]
- 204.Mulivor AW, Lipowsky HH. Inhibition of glycan shedding and leukocyte–endothelial adhesion in postcapillary venules by suppression of matrix–metalloprotease activity with doxycycline. Microcirculation. 2009;16:657–666. doi: 10.3109/10739680903133714. [DOI] [PubMed] [Google Scholar]
- 205.Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat. Immunol. 2005;6:902–910. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- 206.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 207.Fernandez-Borja M, van Buul JD, Hordijk PL. The regulation of leucocyte transendothelial migration by endothelial signalling events. Cardiovasc. Res. 2010;86:202–210. doi: 10.1093/cvr/cvq003. [DOI] [PubMed] [Google Scholar]
- 208.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell. Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 209.Utgaard JO, Jahnsen FL, Bakka A, Brandtzaeg P, Haraldsen G. Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. J. Exp. Med. 1998;188:1751–1756. doi: 10.1084/jem.188.9.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhu L, Luo Y, Chen T, Chen F, Wang T, Hu Q. Ca2+ oscillation frequency regulates agonist-stimulated gene expression in vascular endothelial cells. J. Cell Sci. 2008;121:2511–2518. doi: 10.1242/jcs.031997. [DOI] [PubMed] [Google Scholar]
- 211.Kokura S, Yoshida N, Yoshikawa T. Anoxia/reoxygenation-induced leukocyte–endothelial cell interactions. Free Radic. Biol. Med. 2002;33:427–432. doi: 10.1016/s0891-5849(02)00852-3. [DOI] [PubMed] [Google Scholar]
- 212.Ichikawa H, Flores S, Kvietys PR, Wolf RE, Yoshikawa T, Granger DN, Aw TY. Molecular mechanisms of anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Circ. Res. 1997;81:922–931. doi: 10.1161/01.res.81.6.922. [DOI] [PubMed] [Google Scholar]
- 213.Yoshida N, Granger DN, Anderson DC, Rothlein R, Lane C, Kvietys PR. Anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Am. J. Physiol. 1992;262:H1891–H1898. doi: 10.1152/ajpheart.1992.262.6.H1891. [DOI] [PubMed] [Google Scholar]
- 214.Victorino GP, Ramirez RM, Chong TJ, Curran B, Sadjadi J. Ischemia–reperfusion injury in rats affects hydraulic conductivity in two phases that are temporally and mechanistically separate. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2164–H2171. doi: 10.1152/ajpheart.00419.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Shen HM, Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2006;40:928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 216.Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc. Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 217.Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ. J. 2009;73:411–418. doi: 10.1253/circj.cj-08-1102. [DOI] [PubMed] [Google Scholar]
- 218.Ray R, Shah AM. NADPH oxidase and endothelial cell function. Clin. Sci. (London) 2005;109:217–226. doi: 10.1042/CS20050067. [DOI] [PubMed] [Google Scholar]
- 219.Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid. Redox Signaling. 2009;11:791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ushio-Fukai M. Vascular signaling through G protein-coupled receptors: new concepts. Curr. Opin. Nephrol. Hypertens. 2009;18:153–159. doi: 10.1097/MNH.0b013e3283252efe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zinkevich NS, Gutterman DD. ROS induced ROS release in vascular biology: redox–redox signaling. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H647–H653. doi: 10.1152/ajpheart.01271.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Miyoshi T, Yamashita K, Arai T, Yamamoto K, Mizugishi K, Uchiyama T. The role of endothelial interleukin-8/NADPH oxidase 1 axis in sepsis. Immunology. 2010;131:331–339. doi: 10.1111/j.1365-2567.2010.03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Takano M, Meneshian A, Sheikh E, Yamakawa Y, Wilkins KB, Hopkins EA, Bulkley GB. Rapid upregulation of endothelial P-selectin expression via reactive oxygen species generation. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H2054–H2061. doi: 10.1152/ajpheart.01001.2001. [DOI] [PubMed] [Google Scholar]
- 224.Simon F, Fernandez R. Early lipopolysaccharide-induced reactive oxygen species production evokes necrotic cell death in human umbilical vein endothelial cells. J. Hypertens. 2009;27:1202–1216. doi: 10.1097/HJH.0b013e328329e31c. [DOI] [PubMed] [Google Scholar]
- 225.Cepinskas G, Lush CW, Kvietys PR. Anoxia/reoxygenation-induced tolerance with respect to polymorphonuclear leukocyte adhesion to cultured endothelial cells: a nuclear factor-kappaB-mediated phenomenon. Circ. Res. 1999;84:103–112. doi: 10.1161/01.res.84.1.103. [DOI] [PubMed] [Google Scholar]
- 226.Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc. Res. 2005;65:16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 227.Kuhr F, Lowry J, Zhang Y, Brovkovych V, Skidgel RA. Differential regulation of inducible and endothelial nitric oxide synthase by kinin B1 and B2 receptors. Neuropeptides. 2010;44:145–154. doi: 10.1016/j.npep.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kuhlencordt PJ, Rosel E, Gerszten RE, Morales-Ruiz M, Dombkowski D, Atkinson WJ, Han F, Preffer F, Rosenzweig A, Sessa WC, Gimbrone MA, Jr, Ertl G, Huang PL. Role of endothelial nitric oxide synthase in endothelial activation: insights from eNOS knockout endothelial cells. Am. J. Physiol. Cell Physiol. 2004;286:C1195–C1202. doi: 10.1152/ajpcell.00546.2002. [DOI] [PubMed] [Google Scholar]
- 229.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 230.Sugiyama T, Levy BD, Michel T. Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells. J. Biol. Chem. 2009;284:12691–12700. doi: 10.1074/jbc.M809295200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wu F, Tyml K, Wilson JX. iNOS expression requires NADPH oxidase-dependent redox signaling in microvascular endothelial cells. J. Cell. Physiol. 2008;217:207–214. doi: 10.1002/jcp.21495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sun J, Druhan LJ, Zweier JL. Reactive oxygen and nitrogen species regulate inducible nitric oxide synthase function shifting the balance of nitric oxide and superoxide production. Arch. Biochem. Biophys. 2010;494:130–137. doi: 10.1016/j.abb.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rubio-Gayosso I, Platts SH, Duling BR. Reactive oxygen species mediate modification of glycocalyx during ischemia–reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2247–H2256. doi: 10.1152/ajpheart.00796.2005. [DOI] [PubMed] [Google Scholar]
- 234.Bruegger D, Rehm M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Conzen P, Becker BF. Exogenous nitric oxide requires an endothelial glycocalyx to prevent postischemic coronary vascular leak in guinea pig hearts. Crit. Care. 2008;12:R73. doi: 10.1186/cc6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kurzelewski M, Czarnowska E, Beresewicz A. Superoxide- and nitric oxide-derived species mediate endothelial dysfunction, endothelial glycocalyx disruption, and enhanced neutrophil adhesion in the post-ischemic guinea-pig heart. J. Physiol. Pharmacol. 2005;56:163–178. [PubMed] [Google Scholar]
- 236.Mamdouh Z, Mikhailov A, Muller WA. Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J. Exp. Med. 2009;206:2795–2808. doi: 10.1084/jem.20082745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Phillipson M, Kaur J, Colarusso P, Ballantyne CM, Kubes P. Endothelial domes encapsulate adherent neutrophils and minimize increases in vascular permeability in paracellular and transcellular emigration. PLoS One. 2008;3:e1649. doi: 10.1371/journal.pone.0001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Woodfin A, Voisin MB, Nourshargh S. Recent developments and complexities in neutrophil transmigration. Curr. Opin. Hematol. 2010;17:9–17. doi: 10.1097/MOH.0b013e3283333930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wittchen ES. Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front. Biosci. 2009;14:2522–2545. doi: 10.2741/3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. J. Leukocyte Biol. 2005;77:487–495. doi: 10.1189/jlb.0904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Cook-Mills JM. Hydrogen peroxide activation of endothelial cell-associated MMPs during VCAM-1-dependent leukocyte migration. Cell. Mol. Biol. (Noisy-le-grand) 2006;52:8–16. [PMC free article] [PubMed] [Google Scholar]
- 243.Carman CV, Springer TA. Trans-cellular migration: cell–cell contacts get intimate. Curr. Opin. Cell Biol. 2008;20:533–540. doi: 10.1016/j.ceb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Vestweber D, Broermann A, Schulte D. Control of endothelial barrier function by regulating vascular endothelial-cadherin. Curr. Opin. Hematol. 2010;17:230–236. doi: 10.1097/MOH.0b013e328338664b. [DOI] [PubMed] [Google Scholar]
- 245.Aghajanian A, Wittchen ES, Allingham MJ, Garrett TA, Burridge K. Endothelial cell junctions and the regulation of vascular permeability and leukocyte transmigration. J. Thromb. Haemostasis. 2008;6:1453–1460. doi: 10.1111/j.1538-7836.2008.03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sallee JL, Wittchen ES, Burridge K. Regulation of cell adhesion by protein-tyrosine phosphatases. II. Cell–cell adhesion. J. Biol. Chem. 2006;281:16189–16192. doi: 10.1074/jbc.R600003200. [DOI] [PubMed] [Google Scholar]
- 247.Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am. J. Pathol. 2008;172:521–533. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.van Buul JD, Anthony EC, Fernandez-Borja M, Burridge K, Hordijk PL. Proline-rich tyrosine kinase 2 (Pyk2) mediates vascular endothelial-cadherin-based cell–cell adhesion by regulating beta-catenin tyrosine phosphorylation. J. Biol. Chem. 2005;280:21129–21136. doi: 10.1074/jbc.M500898200. [DOI] [PubMed] [Google Scholar]
- 249.Deem TL, Cook-Mills JM. Vascular cell adhesion molecule 1 (VCAM-1) activation of endothelial cell matrix metalloproteinases: role of reactive oxygen species. Blood. 2004;104:2385–2393. doi: 10.1182/blood-2004-02-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Reijerkerk A, Kooij G, van der Pol SM, Khazen S, Dijkstra CD, de Vries HE. Diapedesis of monocytes is associated with MMP-mediated occludin disappearance in brain endothelial cells. FASEB J. 2006;20:2550–2552. doi: 10.1096/fj.06-6099fje. [DOI] [PubMed] [Google Scholar]
- 251.Schulz B, Pruessmeyer J, Maretzky T, Ludwig A, Blobel CP, Saftig P, Reiss K. ADAM10 regulates endothelial permeability and T-cell transmigration by proteolysis of vascular endothelial cadherin. Circ. Res. 2008;102:1192–1201. doi: 10.1161/CIRCRESAHA.107.169805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lischper M, Beuck S, Thanabalasundaram G, Pieper C, Galla HJ. Metalloproteinase mediated occludin cleavage in the cerebral microcapillary endothelium under pathological conditions. Brain Res. 2010;1326:114–127. doi: 10.1016/j.brainres.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 253.Schubert-Unkmeir A, Konrad C, Slanina H, Czapek F, Hebling S, Frosch M. Neisseria meningitidis induces brain microvascular endothelial cell detachment from the matrix and cleavage of occludin: a role for MMP-8. PLoS Pathog. 2010;6:e1000874. doi: 10.1371/journal.ppat.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wang Q, Doerschuk CM. Neutrophil-induced changes in the biomechanical properties of endothelial cells: roles of ICAM-1 and reactive oxygen species. J. Immunol. 2000;164:6487–6494. doi: 10.4049/jimmunol.164.12.6487. [DOI] [PubMed] [Google Scholar]
- 255.Martinelli R, Gegg M, Longbottom R, Adamson P, Turowski P, Greenwood J. ICAM-1-mediated endothelial nitric oxide synthase activation via calcium and AMP-activated protein kinase is required for transendothelial lymphocyte migration. Mol. Biol. Cell. 2009;20:995–1005. doi: 10.1091/mbc.E08-06-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E, Greenwood J. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J. Cell Sci. 2008;121:29–37. doi: 10.1242/jcs.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Abdala-Valencia H, Cook-Mills JM. VCAM-1 signals activate endothelial cell protein kinase Calpha via oxidation. J. Immunol. 2006;177:6379–6387. doi: 10.4049/jimmunol.177.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Brandt DT, Goerke A, Heuer M, Gimona M, Leitges M, Kremmer E, Lammers R, Haller H, Mischak H. Protein kinase C delta induces Src kinase activity via activation of the protein tyrosine phosphatase PTP alpha. J. Biol. Chem. 2003;278:34073–34078. doi: 10.1074/jbc.M211650200. [DOI] [PubMed] [Google Scholar]
- 259.Adam AP, Sharenko AL, Pumiglia K, Vincent PA. Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers. J. Biol. Chem. 2010;285:7045–7055. doi: 10.1074/jbc.M109.079277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Deem TL, Abdala-Valencia H, Cook-Mills JM. VCAM-1 activation of endothelial cell protein tyrosine phosphatase 1B. J. Immunol. 2007;178:3865–3873. doi: 10.4049/jimmunol.178.6.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Aird WC. Phenotypic heterogeneity of the endothelium. I. Structure, function, and mechanisms. Circ. Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 262.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu. Rev. Physiol. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 263.Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc. Res. 2010;87:320–330. doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tse D, Stan RV. Morphological heterogeneity of endothelium. Semin. Thromb. Hemostasis. 2010;36:236–245. doi: 10.1055/s-0030-1253447. [DOI] [PubMed] [Google Scholar]
- 265.Aberdeen GW, Wiegand SJ, Bonagura TW, Jr, Pepe GJ, Albrecht ED. Vascular endothelial growth factor mediates the estrogen-induced breakdown of tight junctions between and increase in proliferation of microvessel endothelial cells in the baboon endometrium. Endocrinology. 2008;149:6076–6083. doi: 10.1210/en.2008-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Karakotchian M, Fraser IS. An ultrastructural study of microvascular interendothelial tight junctions in normal endometrium. Micron. 2007;38:632–636. doi: 10.1016/j.micron.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 267.Cardoso FL, Brites D, Brito MA. Looking at the blood–brain barrier: molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010;64:328–363. doi: 10.1016/j.brainresrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 268.Forster C. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Walker DC, MacKenzie A, Hosford S. The structure of the tricellular region of endothelial tight junctions of pulmonary capillaries analyzed by freeze-fracture. Microvasc. Res. 1994;48:259–281. doi: 10.1006/mvre.1994.1054. [DOI] [PubMed] [Google Scholar]
- 270.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol. Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 271.Kvietys PR. The gastrointestinal circulation. In: Granger DN, Granger JP, editors. Integrated Systems Physiology: from Molecule to Function. Morgan & Claypool Life Sci; 2010. [PubMed] [Google Scholar]
- 272.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 273.Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010;20:142–149. doi: 10.1016/j.tcb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 274.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood–brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Birukova AA, Zebda N, Fu P, Poroyko V, Cokic I, Birukov KG. Association between adherens junctions and tight junctions via Rap1 promotes barrier protective effects of oxidized phospholipids. J. Cell Physiol. 2011;226:2052–2062. doi: 10.1002/jcp.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Walsh TG, Murphy RP, Fitzpatrick P, Rochfort KD, Guinan AF, Murphy A, Cummins PM. Stabilization of brain microvascular endothelial barrier function by shear stress involves VE-cadherin signaling leading to modulation of pTyr-occludin levels. J. Cell. Physiol. 2011;226:3053–3063. doi: 10.1002/jcp.22655. [DOI] [PubMed] [Google Scholar]
- 278.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat. Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 279.Gavard J, Gutkind JS. VE-cadherin and claudin-5: it takes two to tango. Nat. Cell Biol. 2008;10:883–885. doi: 10.1038/ncb0808-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Dvorak AM, Feng D. The vesiculo-vacuolar organelle (VVO): a new endothelial cell permeability organelle. J. Histochem. Cytochem. 2001;49:419–432. doi: 10.1177/002215540104900401. [DOI] [PubMed] [Google Scholar]
- 281.Lossinsky AS, Shivers RR. Structural pathways for macromolecular and cellular transport across the blood–brain barrier during inflammatory conditions. Histol. Histopathol. 2004;19:535–564. doi: 10.14670/HH-19.535. [DOI] [PubMed] [Google Scholar]
- 282.Aird A, Wrachtrup J, Schulten K, Tietz C. Possible pathway for ubiquinone shuttling in Rhodospirillum rubrum revealed by molecular dynamics simulation. Biophys. J. 2007;92:23–33. doi: 10.1529/biophysj.106.084715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol. Rev. 2008;88:451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 284.Satchell SC, Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am. J. Physiol. Renal Physiol. 2009;296:F947–F956. doi: 10.1152/ajprenal.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Apkarian RP. The fine structure of fenestrated adrenocortical capillaries revealed by in-lens field-emission scanning electron microscopy and scanning transmission electron microscopy. Scanning. 1997;19:361–367. doi: 10.1002/sca.4950190503. [DOI] [PubMed] [Google Scholar]
- 286.Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sun Y, Hu G, Zhang X, Minshall RD. Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ. Res. 2009;105:676–685. doi: 10.1161/CIRCRESAHA.109.201673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Muller WA. Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Rostgaard J, Qvortrup K. Electron microscopic demonstrations of filamentous molecular sieve plugs in capillary fenestrae. Microvasc. Res. 1997;53:1–13. doi: 10.1006/mvre.1996.1987. [DOI] [PubMed] [Google Scholar]
- 290.Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J. Am. Soc. Nephrol. 2007;18:2885–2893. doi: 10.1681/ASN.2007010119. [DOI] [PubMed] [Google Scholar]
- 291.Salmon AH, Neal CR, Sage LM, Glass CA, Harper SJ, Bates DO. Angiopoietin-1 alters microvascular permeability coefficients in vivo via modification of endothelial glycocalyx. Cardiovasc. Res. 2009;83:24–33. doi: 10.1093/cvr/cvp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Engelhardt B, Sorokin L. The blood–brain and the blood–cerebrospinal fluid barriers: function and dysfunction. Semin. Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 293.Bolton GR, Deen WM, Daniels BS. Assessment of the charge selectivity of glomerular basement membrane using Ficoll sulfate. Am. J. Physiol. 1998;274:F889–F896. doi: 10.1152/ajprenal.1998.274.5.F889. [DOI] [PubMed] [Google Scholar]
- 294.Kvietys PR, Granger DN. Endothelial cell monolayers as a tool for studying microvascular pathophysiology. Am. J. Physiol. 1997;273:G1189–G1199. doi: 10.1152/ajpgi.1997.273.6.G1189. [DOI] [PubMed] [Google Scholar]
- 295.Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc. Res. 2010;87:262–271. doi: 10.1093/cvr/cvq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Curry FR, Adamson RH. Vascular permeability modulation at the cell, microvessel, or whole organ level: towards closing gaps in our knowledge. Cardiovasc. Res. 2010;87:218–229. doi: 10.1093/cvr/cvq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Rodrigues SF, Granger DN. Role of blood cells in ischaemia–reperfusion induced endothelial barrier failure. Cardiovasc. Res. 2010;87:291–299. doi: 10.1093/cvr/cvq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Potter DR, Jiang J, Damiano ER. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ. Res. 2009;104:1318–1325. doi: 10.1161/CIRCRESAHA.108.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kubes P, Granger DN. Nitric oxide modulates microvascular permeability. Am. J. Physiol. 1992;262:H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- 300.Kurose I, Kubes P, Wolf R, Anderson DC, Paulson J, Miyasaka M, Granger DN. Inhibition of nitric oxide production: mechanisms of vascular albumin leakage. Circ. Res. 1993;73:164–171. doi: 10.1161/01.res.73.1.164. [DOI] [PubMed] [Google Scholar]
- 301.Rumbaut RE, Wang J, Huxley VH. Differential effects of L-NAME on rat venular hydraulic conductivity. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H2017–H2023. doi: 10.1152/ajpheart.2000.279.4.H2017. [DOI] [PubMed] [Google Scholar]
- 302.Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik AB. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L371–L381. doi: 10.1152/ajplung.00175.2004. [DOI] [PubMed] [Google Scholar]
- 303.Harris NR. Opposing effects of L-NAME on capillary filtration rate in the presence or absence of neutrophils. Am. J. Physiol. 1997;273:G1320–G1325. doi: 10.1152/ajpgi.1997.273.6.G1320. [DOI] [PubMed] [Google Scholar]
- 304.Baldwin AL, Thurston G, al Naemi H. nhibition of nitric oxide synthesis increases venular permeability and alters endothelial actin cytoskeleton. Am. J. Physiol. 1998;274:H1776–H1784. doi: 10.1152/ajpheart.1998.274.5.H1776. [DOI] [PubMed] [Google Scholar]
- 305.He P. Leucocyte/endothelium interactions and microvessel permeability: coupled or uncoupled? Cardiovasc. Res. 2010;87:281–290. doi: 10.1093/cvr/cvq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Sharma M, McCarthy ET, Savin VJ, Lianos EA. Nitric oxide preserves the glomerular protein permeability barrier by antagonizing superoxide. Kidney Int. 2005;68:2735–2744. doi: 10.1111/j.1523-1755.2005.00744.x. [DOI] [PubMed] [Google Scholar]
- 307.Sharma M, Zhou Z, Miura H, Papapetropoulos A, McCarthy ET, Sharma R, Savin VJ, Lianos EA. ADMA injures the glomerular filtration barrier: role of nitric oxide and superoxide. Am. J. Physiol. Renal Physiol. 2009;296:F1386–F1395. doi: 10.1152/ajprenal.90369.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Chen YH, Xu X, Sheng MJ, Zheng Z, Gu Q. Effects of asymmetric dimethylarginine on bovine retinal capillary endothelial cell proliferation, reactive oxygen species production, permeability, intercellular adhesion molecule-1, and occludin expression. Mol. Vision. 2011;17:332–340. [PMC free article] [PubMed] [Google Scholar]
- 309.May JM, Qu ZC. Nitric oxide mediates tightening of the endothelial barrier by ascorbic acid. Biochem. Biophys. Res. Commun. 2011;404:701–705. doi: 10.1016/j.bbrc.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Wojciak-Stothard B, Torondel B, Zhao L, Renne T, Leiper JM. Modulation of Rac1 activity by ADMA/DDAH regulates pulmonary endothelial barrier function. Mol. Biol. Cell. 2009;20:33–42. doi: 10.1091/mbc.E08-04-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Hatakeyama T, Pappas PJ, Hobson RW, 2nd, Boric MP, Sessa WC, Duran WN. Endothelial nitric oxide synthase regulates microvascular hyper-permeability in vivo. J. Physiol. 2006;574:275–281. doi: 10.1113/jphysiol.2006.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Pope AJ, Karuppiah K, Cardounel AJ. Role of the PRMT–DDAH–ADMA axis in the regulation of endothelial nitric oxide production. Pharmacol. Res. 2009;60:461–465. doi: 10.1016/j.phrs.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat. Med. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 314.Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- 315.Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, Lisanti MP. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice: treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J. Biol. Chem. 2002;277:40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- 316.Song L, Ge S, Pachter JS. Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood. 2007;109:1515–1523. doi: 10.1182/blood-2006-07-034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Nouvion AL, Oubaha M, Leblanc S, Davis EC, Jastrow H, Kammerer R, Breton V, Turbide C, Ergun S, Gratton JP, Beauchemin N. CEACAM1: a key regulator of vascular permeability. J. Cell Sci. 2010;123:4221–4230. doi: 10.1242/jcs.073635. [DOI] [PubMed] [Google Scholar]
- 318.Yuanz SY. New insights into eNOS signaling in microvascular permeability. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1029–H1031. doi: 10.1152/ajpheart.00509.2006. [DOI] [PubMed] [Google Scholar]
- 319.Rosengren BI, Rippe A, Rippe C, Venturoli D, Sward K, Rippe B. Transvascular protein transport in mice lacking endothelial caveolae. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1371–H1377. doi: 10.1152/ajpheart.01364.2005. [DOI] [PubMed] [Google Scholar]
- 320.Grande G, Rippe C, Rippe A, Rahman A, Sward K, Rippe B. Unaltered size selectivity of the glomerular filtration barrier in caveolin-1 knockout mice. Am. J. Physiol. Renal Physiol. 2009;297:F257–F262. doi: 10.1152/ajprenal.00075.2009. [DOI] [PubMed] [Google Scholar]
- 321.Boueiz A, Hassoun PM. Regulation of endothelial barrier function by reactive oxygen and nitrogen species. Microvasc. Res. 2009;77:26–34. doi: 10.1016/j.mvr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 322.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 323.Knezevic II, Predescu SA, Neamu RF, Gorovoy MS, Knezevic NM, Easington C, Malik AB, Predescu DN. Tiam1 and Rac1 are required for platelet-activating factor-induced endothelial junctional disassembly and increase in vascular permeability. J. Biol. Chem. 2009;284:5381–5394. doi: 10.1074/jbc.M808958200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Birukova AA, Arce FT, Moldobaeva N, Dudek SM, Garcia JG, Lal R, Birukov KG. Endothelial permeability is controlled by spatially defined cytoskeletal mechanics: atomic force microscopy force mapping of pulmonary endothelial monolayer. Nanomedicine. 2009;5:30–41. doi: 10.1016/j.nano.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Shen Q, Rigor RR, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc. Res. 2010;87:272–280. doi: 10.1093/cvr/cvq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Ito Y, Abril ER, Bethea NW, McCuskey MK, Cover C, Jaeschke H, McCuskey RS. Mechanisms and pathophysiological implications of sinusoidal endothelial cell gap formation following treatment with galactosamine/endotoxin in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G211–G218. doi: 10.1152/ajpgi.00312.2005. [DOI] [PubMed] [Google Scholar]
- 327.Feng D, Nagy JA, Pyne K, Hammel I, Dvorak HF, Dvorak AM. Pathways of macromolecular extravasation across microvascular endothelium in response to VPF/VEGF and other vasoactive mediators. Microcirculation. 1999;6:23–44. [PubMed] [Google Scholar]
- 328.Moos MP, Mewburn JD, Kan FW, Ishii S, Abe M, Sakimura K, Noguchi K, Shimizu T, Funk CD. Cysteinyl leukotriene 2 receptor-mediated vascular permeability via transendothelial vesicle transport. FASEB J. 2008;22:4352–4362. doi: 10.1096/fj.08-113274. [DOI] [PubMed] [Google Scholar]
- 329.Kurose I, Granger DN, Evans DJ, Jr, Evans DG, Graham DY, Miyasaka M, Anderson DC, Wolf RE, Cepinskas G, Kvietys PR. Helicobacter pylori-induced microvascular protein leakage in rats: role of neutrophils, mast cells, and platelets. Gastroenterology. 1994;107:70–79. doi: 10.1016/0016-5085(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 330.Kurose I, Pothoulakis C, LaMont JT, Anderson DC, Paulson JC, Miyasaka M, Wolf R, Granger DN. Clostridium difficile toxin A-induced microvascular dysfunction: role of histamine. J. Clin. Invest. 1994;94:1919–1926. doi: 10.1172/JCI117542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Granger DN, Korthuis RJ. Physiologic mechanisms of postischemic tissue injury. Annu. Rev. Physiol. 1995;57:311–332. doi: 10.1146/annurev.ph.57.030195.001523. [DOI] [PubMed] [Google Scholar]
- 332.Kurose I, Wolf R, Grisham MB, Granger DN. Modulation of ischemia/reperfusion-induced microvascular dysfunction by nitric oxide. Circ. Res. 1994;74:376–382. doi: 10.1161/01.res.74.3.376. [DOI] [PubMed] [Google Scholar]
- 333.Cara DC, Ebbert KV, McCafferty DM. Mast cell-independent mechanisms of immediate hypersensitivity: a role for platelets. J. Immunol. 2004;172:4964–4971. doi: 10.4049/jimmunol.172.8.4964. [DOI] [PubMed] [Google Scholar]
- 334.Gaboury JP, Niu XF, Kubes P. Nitric oxide inhibits numerous features of mast cell-induced inflammation. Circulation. 1996;93:318–326. doi: 10.1161/01.cir.93.2.318. [DOI] [PubMed] [Google Scholar]
- 335.Naidu BV, Krishnadasan B, Farivar AS, Woolley SM, Thomas R, Van Rooijen N, Verrier ED, Mulligan MS. Early activation of the alveolar macro-phage is critical to the development of lung ischemia–reperfusion injury. J. Thorac. Cardiovasc. Surg. 2003;126:200–207. doi: 10.1016/s0022-5223(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 336.Nakamura M, Fujishima S, Sawafuji M, Ishizaka A, Oguma T, Soejima K, Matsubara H, Tasaka S, Kikuchi K, Kobayashi K, Ikeda E, Sadick M, Hebert CA, Aikawa N, Kanazawa M, Yamaguchi K. Importance of interleukin-8 in the development of reexpansion lung injury in rabbits. Am. J. Respir. Crit. Care Med. 2000;161:1030–1036. doi: 10.1164/ajrccm.161.3.9906039. [DOI] [PubMed] [Google Scholar]
- 337.Cepinskas G, Noseworthy R, Kvietys PR. Transendothelial neutrophil migration: role of neutrophil-derived proteases and relationship to transendothelial protein movement. Circ. Res. 1997;81:618–626. doi: 10.1161/01.res.81.4.618. [DOI] [PubMed] [Google Scholar]
- 338.Hermant B, Bibert S, Concord E, Dublet B, Weidenhaupt M, Vernet T, Gulino-Debrac D. Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. J. Biol. Chem. 2003;278:14002–14012. doi: 10.1074/jbc.M300351200. [DOI] [PubMed] [Google Scholar]
- 339.Hu G, Vogel SM, Schwartz DE, Malik AB, Minshall RD. Intercellular adhesion molecule-1-dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ. Res. 2008;102:e120–e131. doi: 10.1161/CIRCRESAHA.107.167486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Hu G, Minshall RD. Regulation of transendothelial permeability by Src kinase. Microvasc. Res. 2009;77:21–25. doi: 10.1016/j.mvr.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 341.Sendo T, Sumimura T, Itoh Y, Goromaru T, Aki K, Yano T, Oike M, Ito Y, Mori S, Nishibori M, Oishi R. Involvement of proteinase-activated receptor-2 in mast cell tryptase-induced barrier dysfunction in bovine aortic endothelial cells. Cell Signalling. 2003;15:773–781. doi: 10.1016/s0898-6568(03)00014-7. [DOI] [PubMed] [Google Scholar]
- 342.Farley KS, Wang L, Mehta S. Septic pulmonary microvascular endothelial cell injury: role of alveolar macrophage NADPH oxidase. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L480–L488. doi: 10.1152/ajplung.90201.2008. [DOI] [PubMed] [Google Scholar]
- 343.Zhao M, Fernandez LG, Doctor A, Sharma AK, Zarbock A, Tribble CG, Kron IL, Laubach VE. Alveolar macrophage activation is a key initiation signal for acute lung ischemia–reperfusion injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L1018–L1026. doi: 10.1152/ajplung.00086.2006. [DOI] [PubMed] [Google Scholar]
- 344.Wong RK, Baldwin AL, Heimark RL. Cadherin-5 redistribution at sites of TNF-alpha and IFN-gamma-induced permeability in mesenteric venules. Am. J. Physiol. 1999;276:H736–H748. doi: 10.1152/ajpheart.1999.276.2.H736. [DOI] [PubMed] [Google Scholar]
- 345.Asako H, Kurose I, Wolf R, DeFrees S, Zheng ZL, Phillips ML, Paulson JC, Granger DN. Role of H1 receptors and P-selectin in histamine-induced leukocyte rolling and adhesion in postcapillary venules. J. Clin. Invest. 1994;93:1508–1515. doi: 10.1172/JCI117129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Yang Z, Sharma AK, Linden J, Kron IL, Laubach VE. CD4+ T lymphocytes mediate acute pulmonary ischemia–reperfusion injury. J. Thorac. Cardiovasc. Surg. 2009;137:695–702. doi: 10.1016/j.jtcvs.2008.10.044. discussion 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Shigematsu T, Wolf RE, Granger DN. T-lymphocytes modulate the microvascular and inflammatory responses to intestinal ischemia–reperfusion. Micro-circulation. 2002;9:99–109. doi: 10.1038/sj/mn/7800126. [DOI] [PubMed] [Google Scholar]
- 348.Osman M, Russell J, Granger DN. Lymphocyte-derived interferon-gamma mediates ischemia-reperfusion-induced leukocyte and platelet adhesion in intestinal microcirculation. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G659–G663. doi: 10.1152/ajpgi.90495.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Tennenberg SD, Weller JJ. Endotoxin-induced, neutrophil-mediated endothelial cytotoxicity is enhanced by T-lymphocytes. J. Surg. Res. 1997;69:11–13. doi: 10.1006/jsre.1996.4996. [DOI] [PubMed] [Google Scholar]
- 350.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet–neutrophil aggregation. J. Clin. Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Usatyuk PV, He D, Bindokas V, Gorshkova IA, Berdyshev EV, Garcia JG, Natarajan V. Photolysis of caged sphingosine-1-phosphate induces barrier enhancement and intracellular activation of lung endothelial cell signaling pathways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;300:L840–L850. doi: 10.1152/ajplung.00404.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Adamson RH, Sarai RK, Altangerel A, Thirkill TL, Clark JF, Curry FR. Sphingosine-1-phosphatemodulation of basal permeability and acute inflammatory responses in rat venular microvessels. Cardiovasc. Res. 2010;88:344–351. doi: 10.1093/cvr/cvq184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Lucke S, Levkau B. Endothelial functions of sphingosine-1-phosphate. Cell. Physiol. Biochem. 2010;26:87–96. doi: 10.1159/000315109. [DOI] [PubMed] [Google Scholar]
- 354.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Granger DN. Role of xanthine oxidase and granulocytes in ischemia–reperfusion injury. Am. J. Physiol. 1988;255:H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- 356.Kurose I, Wolf RE, Grisham MB, Granger DN. Hypercholesterolemia enhances oxidant production in mesenteric venules exposed to ischemia/reperfusion. Arterioscler. Thromb. Vasc. Biol. 1998;18:1583–1588. doi: 10.1161/01.atv.18.10.1583. [DOI] [PubMed] [Google Scholar]
- 357.Han JY, Horie Y, Fan JY, Sun K, Guo J, Miura S, Hibi T. Potential of 3,4-dihydroxy-phenyl lactic acid for ameliorating ischemia–reperfusion-induced microvascular disturbance in rat mesentery. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G36–G44. doi: 10.1152/ajpgi.90284.2008. [DOI] [PubMed] [Google Scholar]
- 358.Steiner DR, Gonzalez NC, Wood JG. Mast cells mediate the microvascular inflammatory response to systemic hypoxia. J. Appl. Physiol. 2003;94:325–334. doi: 10.1152/japplphysiol.00637.2002. [DOI] [PubMed] [Google Scholar]
- 359.Cuschieri J, Maier RV. Oxidative stress, lipid rafts, and macrophage reprogramming. Antioxid. Redox Signaling. 2007;9:1485–1497. doi: 10.1089/ars.2007.1670. [DOI] [PubMed] [Google Scholar]
- 360.Sumi N, Nishioku T, Takata F, Matsumoto J, Watanabe T, Shuto H, Yamauchi A, Dohgu S, Kataoka Y. Lipopolysaccharide-activated microglia induce dysfunction of the blood–brain barrier in rat microvascular endothelial cells co-cultured with microglia. Cell. Mol. Neurobiol. 2010;30:247–253. doi: 10.1007/s10571-009-9446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Granger DN. Ischemia–reperfusion: mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation. 1999;6:167–178. [PubMed] [Google Scholar]
- 362.Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, Liu QH, Malik AB. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice. J. Immunol. 2002;168:3974–3982. doi: 10.4049/jimmunol.168.8.3974. [DOI] [PubMed] [Google Scholar]
- 363.Inauen W, Payne DK, Kvietys PR, Granger DN. Hypoxia/reoxygenation increases the permeability of endothelial cell monolayers: role of oxygen radicals. Free Radic. Biol. Med. 1990;9:219–223. doi: 10.1016/0891-5849(90)90031-d. [DOI] [PubMed] [Google Scholar]
- 364.Han J, Shuvaev VV, Muzykantov VR. Catalase and superoxide dismutase conjugated with platelet-endothelial cell adhesion molecule antibody distinctly alleviate abnormal endothelial permeability caused by exogenous reactive oxygen species and vascular endothelial growth factor. J. Pharmacol. Exp. Ther. 2011;338:82–91. doi: 10.1124/jpet.111.180620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Hu Q, Yu ZX, Ferrans VJ, Takeda K, Irani K, Ziegelstein RC. Critical role of NADPH oxidase-derived reactive oxygen species in generating Ca2+ oscillations in human aortic endothelial cells stimulated by histamine. J. Biol. Chem. 2002;277:32546–32551. doi: 10.1074/jbc.M201550200. [DOI] [PubMed] [Google Scholar]
- 366.Djordjevic T, Pogrebniak A, BelAiba RS, Bonello S, Wotzlaw C, Acker H, Hess J, Gorlach A. The expression of the NADPH oxidase subunit p22phox is regulated by a redox-sensitive pathway in endothelial cells. Free Radic. Biol. Med. 2005;38:616–630. doi: 10.1016/j.freeradbiomed.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 367.Chen W, Pendyala S, Natarajan V, Garcia JG, Jacobson JR. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;295:L575–L583. doi: 10.1152/ajplung.00428.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J. Biol. Chem. 2009;284:25602–25611. doi: 10.1074/jbc.M109.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Duran WN, Breslin JW, Sanchez FA. The NO cascade, eNOS location, and microvascular permeability. Cardiovasc. Res. 2010;87:254–261. doi: 10.1093/cvr/cvq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Speyer CL, Neff TA, Warner RL, Guo RF, Sarma JV, Riedemann NC, Murphy ME, Murphy HS, Ward PA. Regulatory effects of iNOS on acute lung inflammatory responses in mice. Am. J. Pathol. 2003;163:2319–2328. doi: 10.1016/S0002-9440(10)63588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Farley KS, Wang LF, Law C, Mehta S. Alveolar macrophage inducible nitric oxide synthase-dependent pulmonary microvascular endothelial cell septic barrier dysfunction. Microvasc. Res. 2008;76:208–216. doi: 10.1016/j.mvr.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 372.Bucci M, Roviezzo F, Posadas I, Yu J, Parente L, Sessa WC, Ignarro LJ, Cirino G. Endothelial nitric oxide synthase activation is critical for vascular leakage during acute inflammation in vivo. Proc. Natl. Acad. Sci. USA. 2005;102:904–908. doi: 10.1073/pnas.0408906102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Zhou X, He P. Endothelial [Ca2+]i and caveolin-1 antagonistically regulate eNOS activity and microvessel permeability in rat venules. Cardiovasc. Res. 2010;87:340–347. doi: 10.1093/cvr/cvq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Di Lorenzo A, Fernandez-Hernando C, Cirino G, Sessa WC. Akt1 is critical for acute inflammation and histamine-mediated vascular leakage. Proc. Natl. Acad. Sci. USA. 2009;106:14552–14557. doi: 10.1073/pnas.0904073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Peng X, Abdulnour RE, Sammani S, Ma SF, Han EJ, Hasan EJ, Tuder R, Garcia JG, Hassoun PM. Inducible nitric oxide synthase contributes to ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 2005;172:470–479. doi: 10.1164/rccm.200411-1547OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Peng XQ, Damarla M, Skirball J, Nonas S, Wang XY, Han EJ, Hasan EJ, Cao X, Boueiz A, Damico R, Tuder RM, Sciuto AM, Anderson DR, Garcia JG, Kass DA, Hassoun PM, Zhang JT. Protective role of PI3-kinase/Akt/eNOS signaling in mechanical stress through inhibition of p38 mitogen-activated protein kinase in mouse lung. Acta Pharmacol. Sin. 2010;31:175–183. doi: 10.1038/aps.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Kuebler WM, Yang Y, Samapati R, Uhlig S. Vascular barrier regulation by PAF, ceramide, caveolae, and NO–an intricate signaling network with discrepant effects in the pulmonary and systemic vasculature. Cell. Physiol. Biochem. 2010;26:29–40. doi: 10.1159/000315103. [DOI] [PubMed] [Google Scholar]
- 378.Klabunde RE, Anderson DE. Role of nitric oxide and reactive oxygen species in platelet-activating factor-induced microvascular leakage. J. Vasc. Res. 2002;39:238–245. doi: 10.1159/000063689. [DOI] [PubMed] [Google Scholar]
- 379.Yang Y, Yin J, Baumgartner W, Samapati R, Solymosi EA, Reppien E, Kuebler WM, Uhlig S. Platelet-activating factor reduces endothelial nitric oxide production: role of acid sphingomyelinase. Eur. Respir. J. 2010;36:417–427. doi: 10.1183/09031936.00095609. [DOI] [PubMed] [Google Scholar]
- 380.McGown CC, Brown NJ, Hellewell PG, Reilly CS, Brookes ZL. Beneficial microvascular and anti-inflammatory effects of pravastatin during sepsis involve nitric oxide synthase III. Br. J. Anaesth. 2010;104:183–190. doi: 10.1093/bja/aep361. [DOI] [PubMed] [Google Scholar]
- 381.McGown CC, Brown NJ, Hellewell PG, Brookes ZL. ROCK induced inflammation of the microcirculation during endotoxemia mediated by nitric oxide synthase. Microvasc. Res. 2011;81:281–288. doi: 10.1016/j.mvr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 382.Kolluru GK, Tamilarasan KP, Rajkumar AS, Geetha Priya S, Rajaram M, Saleem NK, Majumder S, Jaffar Ali BM, Illavazagan G, Chatterjee S. Nitric oxide/cGMP protects endothelial cells from hypoxia-mediated leakiness. Eur. J. Cell Biol. 2008;87:147–161. doi: 10.1016/j.ejcb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 383.Irwin DC, McCord JM, Nozik-Grayck E, Beckly G, Foreman B, Sullivan T, White M, Crossno TJ, Bailey D, Flores SC, Majka S, Klemm D, van Patot MC. A potential role for reactive oxygen species and the HIF-1α–VEGF pathway in hypoxia-induced pulmonary vascular leak. Free Radic. Biol. Med. 2009;47:55–61. doi: 10.1016/j.freeradbiomed.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Wong D, Dorovini-Zis K, Vincent SR. Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood–brain barrier. Exp. Neurol. 2004;190:446–455. doi: 10.1016/j.expneurol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 385.Sanchez FA, Kim DD, Duran RG, Meininger CJ, Duran WN. Internalization of eNOS via caveolae regulates PAF-induced inflammatory hyperpermeability to macromolecules. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1642–H1648. doi: 10.1152/ajpheart.00629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Li B, Yao J, Morioka T, Oite T. Nitric oxide increases albumin permeability of isolated rat glomeruli via a phosphorylation-dependent mechanism. J. Am. Soc. Nephrol. 2001;12:2616–2624. doi: 10.1681/ASN.V12122616. [DOI] [PubMed] [Google Scholar]
- 387.Scatena R, Bottoni P, Pontoglio A, Giardina B. Pharmacological modulation of nitric oxide release: new pharmacological perspectives, potential benefits and risks. Curr. Med. Chem. 2010;17:61–73. doi: 10.2174/092986710789957841. [DOI] [PubMed] [Google Scholar]
- 388.Sanchez FA, Savalia NB, Duran RG, Lal BK, Boric MP, Duran WN. Functional significance of differential eNOS translocation. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1058–H1064. doi: 10.1152/ajpheart.00370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Surapisitchat J, Jeon KI, Yan C, Beavo JA. Differential regulation of endothelial cell permeability by cGMP via phosphodiesterases 2 and 3. Circ. Res. 2007;101:811–818. doi: 10.1161/CIRCRESAHA.107.154229. [DOI] [PubMed] [Google Scholar]
- 390.Al-Naemi H, Baldwin AL. Nitric oxide: role in venular permeability recovery after histamine challenge. Am. J. Physiol. 1999;277:H2010–H2016. doi: 10.1152/ajpheart.1999.277.5.H2010. [DOI] [PubMed] [Google Scholar]
- 391.Igarashi J, Michel T. S1P and eNOS regulation. Biochim. Biophys. Acta. 2008;1781:489–495. doi: 10.1016/j.bbalip.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 392.Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb. Haemostasis. 2010;103:40–55. doi: 10.1160/TH09-06-0403. [DOI] [PubMed] [Google Scholar]
- 393.Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc. Res. 2010;87:243–253. doi: 10.1093/cvr/cvq086. [DOI] [PubMed] [Google Scholar]
- 394.Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB, Mehta D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ. Res. 2008;103:1164–1172. doi: 10.1161/01.RES.0000338501.84810.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Stockton R, Reutershan J, Scott D, Sanders J, Ley K, Schwartz MA. Induction of vascular permeability: beta PIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by PAK. Mol. Biol. Cell. 2007;18:2346–2355. doi: 10.1091/mbc.E06-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.van Nieuw Amerongen GP, Beckers CM, Achekar ID, Zeeman S, Musters RJ, van Hinsbergh VW. Involvement of Rho kinase in endothelial barrier maintenance. Arterioscler. Thromb. Vasc. Biol. 2007;27:2332–2339. doi: 10.1161/ATVBAHA.107.152322. [DOI] [PubMed] [Google Scholar]
- 397.Sawada N, Li Y, Liao JK. Novel aspects of the roles of Rac1 GTPase in the cardiovascular system. Curr. Opin. Pharmacol. 2010;10:116–121. doi: 10.1016/j.coph.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.van Wetering S, van Buul JD, Quik S, Mul FP, Anthony EC, ten Klooster JP, Collard JG, Hordijk PL. Reactive oxygen species mediate Rac-induced loss of cell–cell adhesion in primary human endothelial cells. J. Cell Sci. 2002;115:1837–1846. doi: 10.1242/jcs.115.9.1837. [DOI] [PubMed] [Google Scholar]
- 399.McNally JS, Saxena A, Cai H, Dikalov S, Harrison DG. Regulation of xanthine oxidoreductase protein expression by hydrogen peroxide and calcium. Arterioscler. Thromb. Vasc. Biol. 2005;25:1623–1628. doi: 10.1161/01.ATV.0000170827.16296.6e. [DOI] [PubMed] [Google Scholar]
- 400.Sawada N, Kim HH, Moskowitz MA, Liao JK. Rac1 is a critical mediator of endothelium-derived neurotrophic activity. Sci. Signalling. 2009;2:ra10. doi: 10.1126/scisignal.2000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Kuhlmann CR, Tamaki R, Gamerdinger M, Lessmann V, Behl C, Kempski OS, Luhmann HJ. Inhibition of the myosin light chain kinase prevents hypoxia-induced blood–brain barrier disruption. J. Neurochem. 2007;102:501–507. doi: 10.1111/j.1471-4159.2007.04506.x. [DOI] [PubMed] [Google Scholar]
- 402.Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, Brandes RP. NADPH oxidase plays a central role in blood–brain barrier damage in experimental stroke. Stroke. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 403.Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ. Res. 2008;102:347–355. doi: 10.1161/CIRCRESAHA.107.160176. [DOI] [PubMed] [Google Scholar]
- 404.Cai H, Liu D, Garcia JG. CaM kinase II-dependent pathophysiological signalling in endothelial cells. Cardiovasc. Res. 2008;77:30–34. doi: 10.1093/cvr/cvm010. [DOI] [PubMed] [Google Scholar]
- 405.Dessy C, Feron O, Balligand JL. The regulation of endothelial nitric oxide synthase by caveolin: a paradigm validated in vivo and shared by the 'endothelium-derived hyperpolarizing factor'. Pflugers Arch. 2010;459:817–827. doi: 10.1007/s00424-010-0815-3. [DOI] [PubMed] [Google Scholar]
- 406.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann. N. Y. Acad. Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 407.van Hinsbergh VW, van Nieuw Amerongen GP. Intracellular signalling involved in modulating human endothelial barrier function. J. Anat. 2002;200:549–560. doi: 10.1046/j.1469-7580.2002.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Thibeault S, Rautureau Y, Oubaha M, Faubert D, Wilkes BC, Delisle C, Gratton JP. S-nitrosylation of beta-catenin by eNOS-derived NO promotes VEGF-induced endothelial cell permeability. Mol. Cell. 2010;39:468–476. doi: 10.1016/j.molcel.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 409.Gunduz D, Thom J, Hussain I, Lopez D, Hartel FV, Erdogan A, Grebe M, Sedding D, Piper HM, Tillmanns H, Noll T, Aslam M. Insulin stabilizes microvascular endothelial barrier function via phosphatidylinositol 3-kinase/Akt-mediated Rac1 activation. Arterioscler. Thromb. Vasc. Biol. 2010;30:1237–1245. doi: 10.1161/ATVBAHA.110.203901. [DOI] [PubMed] [Google Scholar]
- 410.Sawada N, Salomone S, Kim HH, Kwiatkowski DJ, Liao JK. Regulation of endothelial nitric oxide synthase and postnatal angiogenesis by Rac1. Circ. Res. 2008;103:360–368. doi: 10.1161/CIRCRESAHA.108.178897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J. Mol. Med. (Berlin) 2007;85:1295–1300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- 412.Coulon C, Georgiadou M, Roncal C, De Bock K, Langenberg T, Carmeliet P. From vessel sprouting to normalization: role of the prolyl hydroxylase domain protein/hypoxia-inducible factor oxygen-sensing machinery. Arterioscler. Thromb. Vasc. Biol. 2010;30:2331–2336. doi: 10.1161/ATVBAHA.110.214106. [DOI] [PubMed] [Google Scholar]
- 413.Pollard JW. Trophicmacrophages in development and disease. Nat. Rev. Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Dehne N, Brune B. HIF-1 in the inflammatory microenvironment. Exp. Cell Res. 2009;315:1791–1797. doi: 10.1016/j.yexcr.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 415.Fong GH. Regulation of angiogenesis by oxygen sensing mechanisms. J. Mol. Med. 2009;87:549–560. doi: 10.1007/s00109-009-0458-z. [DOI] [PubMed] [Google Scholar]
- 416.Taylor CT, Cummins EP. The role of NF-kappaB in hypoxia-induced gene expression. Ann. N. Y. Acad. Sci. 2009;1177:178–184. doi: 10.1111/j.1749-6632.2009.05024.x. [DOI] [PubMed] [Google Scholar]
- 417.Hellwig-Burgel T, Stiehl DP, Wagner AE, Metzen E, Jelkmann W. Hypoxia-inducible factor-1 (HIF-1): a novel transcription factor in immune reactions. J. Interferon Cytokine Res. 2005;25:297–310. doi: 10.1089/jir.2005.25.297. [DOI] [PubMed] [Google Scholar]
- 418.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 419.Brune B, Zhou J. Nitric oxide and superoxide: interference with hypoxic signaling. Cardiovasc. Res. 2007;75:275–282. doi: 10.1016/j.cardiores.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 420.Berchner-Pfannschmidt U, Tug S, Kirsch M, Fandrey J. Oxygen-sensing under the influence of nitric oxide. Cell Signalling. 2010;22:349–356. doi: 10.1016/j.cellsig.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 421.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc. Natl. Acad. Sci. USA. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J. Physiol. 2008;586:4055–4059. doi: 10.1113/jphysiol.2008.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Frede S, Berchner-Pfannschmidt U, Fandrey J. Regulation of hypoxia-inducible factors during inflammation. Methods Enzymol. 2007;435:405–419. doi: 10.1016/S0076-6879(07)35021-0. [DOI] [PubMed] [Google Scholar]
- 424.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N, Chilvers ER. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J. Exp. Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Gonsalves CS, Kalra VK. Hypoxia-mediated expression of 5-lipoxygenase-activating protein involves HIF-1alpha and NF-kappaB and microRNAs 135a and 199a-5p. J. Immunol. 2010;184:3878–3888. doi: 10.4049/jimmunol.0902594. [DOI] [PubMed] [Google Scholar]
- 427.Bosco MC, Puppo M, Blengio F, Fraone T, Cappello P, Giovarelli M, Varesio L. Monocytes and dendritic cells in a hypoxic environment: spotlights on chemotaxis and migration. Immunobiology. 2008;213:733–749. doi: 10.1016/j.imbio.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 428.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–5066. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 429.Signorelli S, Jennings P, Leonard MO, Pfaller W. Differential effects of hypoxic stress in alveolar epithelial cells and microvascular endothelial cells. Cell. Physiol. Biochem. 2010;25:135–144. doi: 10.1159/000272066. [DOI] [PubMed] [Google Scholar]
- 430.Sato W, Kosugi T, Zhang L, Roncal CA, Heinig M, Campbell-Thompson M, Yuzawa Y, Atkinson MA, Grant MB, Croker BP, Nakagawa T. The pivotal role of VEGF on glomerular macrophage infiltration in advanced diabetic nephropathy. Lab Invest. 2008;88:949–961. doi: 10.1038/labinvest.2008.60. [DOI] [PubMed] [Google Scholar]
- 431.Scharte M, Han X, Bertges DJ, Fink MP, Delude RL. Cytokines induce HIF-1 DNA binding and the expression of HIF-1-dependent genes in cultured rat enterocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G373–G384. doi: 10.1152/ajpgi.00076.2002. [DOI] [PubMed] [Google Scholar]
- 432.Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, De Smet F, Vinckier S, Aragones J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M, Ratcliffe P, Maxwell P, Carmeliet P. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, Zhang W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP5 (Ccl12) in astrocytes. J. Neuroinflammation. 2007;4:12. doi: 10.1186/1742-2094-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 435.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 436.Bonello S, Zahringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Gorlach A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler. Thromb. Vasc. Biol. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 437.Zhang L, Li L, Liu H, Prabhakaran K, Zhang X, Borowitz JL, Isom GE. HIF-1α activation by a redox-sensitive pathway mediates cyanide-induced BNIP3 upregulation and mitochondrial-dependent cell death. Free Radic. Biol. Med. 2007;43:117–127. doi: 10.1016/j.freeradbiomed.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Wang D, Malo D, Hekimi S. Elevated mitochondrial reactive oxygen species generation affects the immune response via hypoxia-inducible factor-1alpha in long-lived Mclk1+/− mouse mutants. J. Immunol. 2010;184:582–590. doi: 10.4049/jimmunol.0902352. [DOI] [PubMed] [Google Scholar]
- 439.Klimova T, Chandel NS. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 2008;15:660–666. doi: 10.1038/sj.cdd.4402307. [DOI] [PubMed] [Google Scholar]
- 440.Pan Y, Mansfield KD, Bertozzi CC, Rudenko V, Chan DA, Giaccia AJ, Simon MC. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Mol. Cell. Biol. 2007;27:912–925. doi: 10.1128/MCB.01223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Acker T, Fandrey J, Acker H. The good, the bad and the ugly in oxygen-sensing:. ROS, cytochromes and prolyl-hydroxylases. Cardiovasc. Res. 2006;71:195–207. doi: 10.1016/j.cardiores.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 442.Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2007;27:2524–2531. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- 443.Desouki MM, Kulawiec M, Bansal S, Das GM, Singh KK. Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol. Ther. 2005;4:1367–1373. doi: 10.4161/cbt.4.12.2233. [DOI] [PubMed] [Google Scholar]
- 444.Lee SB, Bae IH, Bae YS, Um HD. Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J. Biol. Chem. 2006;281:36228–36235. doi: 10.1074/jbc.M606702200. [DOI] [PubMed] [Google Scholar]
- 445.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS–PKCe signaling axis in pulmonary artery smooth muscle cells. Free Radic. Biol. Med. 2008;45:1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Martinez-Ruiz A, Lamas S. Two decades of new concepts in nitric oxide signaling: from the discovery of a gas messenger to the mediation of nonenzymatic posttranslational modifications. IUBMB Life. 2009;61:91–98. doi: 10.1002/iub.144. [DOI] [PubMed] [Google Scholar]
- 447.Martinez-Ruiz A, Lamas S. S-nitrosylation: a potential new paradigm in signal transduction. Cardiovasc Res. 2004;62:43–52. doi: 10.1016/j.cardiores.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 448.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 449.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ. Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Ckless K, van der Vliet A, Janssen-Heininger Y. Oxidative–nitrosative stress and post-translational protein modifications: implications to lung structure–function relations. Arginase modulates NF-kappaB activity via a nitric oxide-dependent mechanism. Am. J. Respir. Cell Mol. Biol. 2007;36:645–653. doi: 10.1165/rcmb.2006-0329SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Bosworth CA, Toledo JC, Jr, Zmijewski JW, Li Q, Lancaster JR., Jr Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc. Natl. Acad. Sci. USA. 2009;106:4671–4676. doi: 10.1073/pnas.0710416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Matthews JR, Botting CH, Panico M, Morris HR, Hay RT. Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res. 1996;24:2236–2242. doi: 10.1093/nar/24.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Grumbach IM, Chen W, Mertens SA, Harrison DG. A negative feedback mechanism involving nitric oxide and nuclear factor kappa-B modulates endothelial nitric oxide synthase transcription. J. Mol. Cell. Cardiol. 2005;39:595–603. doi: 10.1016/j.yjmcc.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 454.de la Torre A, Schroeder RA, Punzalan C, Kuo PC. Endotoxin-mediated S-nitrosylation of p50 alters NF-kappa B-dependent gene transcription in ANA-1 murine macrophages. J. Immunol. 1999;162:4101–4108. [PubMed] [Google Scholar]
- 455.Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc. Natl. Acad. Sci. USA. 2004;101:8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Park SW, Huq MD, Hu X, Wei LN. Tyrosine nitration on p65: a novel mechanism to rapidly inactivate nuclear factor-kappaB. Mol. Cell. Proteomics. 2005;4:300–309. doi: 10.1074/mcp.M400195-MCP200. [DOI] [PubMed] [Google Scholar]
- 457.Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, Li CY. Regulation of HIF-1alpha stability through S-nitrosylation. Mol. Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Sumbayev VV, Budde A, Zhou J, Brune B. HIF-1 alpha protein as a target for S-nitrosation. FEBS Lett. 2003;535:106–112. doi: 10.1016/s0014-5793(02)03887-5. [DOI] [PubMed] [Google Scholar]
- 459.Yasinska IM, Sumbayev VV. S-nitrosation of Cys-800 of HIF-1alpha protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS Lett. 2003;549:105–109. doi: 10.1016/s0014-5793(03)00807-x. [DOI] [PubMed] [Google Scholar]
- 460.Park YK, Ahn DR, Oh M, Lee T, Yang EG, Son M, Park H. Nitric oxide donor, (+/−)-S-nitroso-N-acetylpenicillamine, stabilizes transactive hypoxia-inducible factor-1alpha by inhibiting von Hippel-Lindau recruitment and asparagine hydroxylation. Mol. Pharmacol. 2008;74:236–245. doi: 10.1124/mol.108.045278. [DOI] [PubMed] [Google Scholar]
- 461.Lima B, Lam GK, Xie L, Diesen DL, Villamizar N, Nienaber J, Messina E, Bowles D, Kontos CD, Hare JM, Stamler JS, Rockman HA. Endogenous S-nitrosothiols protect against myocardial injury. Proc. Natl. Acad. Sci. USA. 2009;106:6297–6302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Taylor CT, Moncada S. Nitric oxide, cytochrome C oxidase, and the cellular response to hypoxia. Arterioscler. Thromb. Vasc. Biol. 2010;30:643–647. doi: 10.1161/ATVBAHA.108.181628. [DOI] [PubMed] [Google Scholar]
- 463.Berchner-Pfannschmidt U, Yamac H, Trinidad B, Fandrey J. Nitric oxide modulates oxygen sensing by hypoxia-inducible factor 1-dependent induction of prolyl hydroxylase 2. J. Biol. Chem. 2007;282:1788–1796. doi: 10.1074/jbc.M607065200. [DOI] [PubMed] [Google Scholar]
- 464.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 465.De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P. Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way. Arterioscler. Thromb. Vasc. Biol. 2009;29:639–649. doi: 10.1161/ATVBAHA.109.185165. [DOI] [PubMed] [Google Scholar]
- 466.Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial–mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 467.Carmeliet P. Angiogenesis in life, disease andmedicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 468.Karamysheva AF. Mechanisms of angiogenesis. Biochemistry (Moscow) 2008;73:751–762. doi: 10.1134/s0006297908070031. [DOI] [PubMed] [Google Scholar]
- 469.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 470.Jin SW, Patterson C. The opening act: vasculogenesis and the origins of circulation. Arterioscler. Thromb. Vasc. Biol. 2009;29:623–629. doi: 10.1161/ATVBAHA.107.161539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Holderfield MT, Hughes CC. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ. Res. 2008;102:637–652. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- 472.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 473.Sainson RC, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, Davis J, Conn E, Hughes CC. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008;111:4997–5007. doi: 10.1182/blood-2007-08-108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Carmi Y, Voronov E, Dotan S, Lahat N, Rahat MA, Fogel M, Huszar M, White MR, Dinarello CA, Apte RN. The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis. J. Immunol. 2009;183:4705–4714. doi: 10.4049/jimmunol.0901511. [DOI] [PubMed] [Google Scholar]
- 475.van Hinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc. Res. 2008;78:203–212. doi: 10.1093/cvr/cvm102. [DOI] [PubMed] [Google Scholar]
- 476.Yana I, Sagara H, Takaki S, Takatsu K, Nakamura K, Nakao K, Katsuki M, Taniguchi S, Aoki T, Sato H, Weiss SJ, Seiki M. Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J. Cell Sci. 2007;120:1607–1614. doi: 10.1242/jcs.000679. [DOI] [PubMed] [Google Scholar]
- 477.Varani J, Perone P, Warner RL, Dame MK, Kang S, Fisher GJ, Voorhees JJ. Vascular tube formation on matrix metalloproteinase-1-damaged collagen. Br. J. Cancer. 2008;98:1646–1652. doi: 10.1038/sj.bjc.6604357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Vestweber D, Winderlich M, Cagna G, Nottebaum AF. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol. 2009;19:8–15. doi: 10.1016/j.tcb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 480.Mukherjee S, Tessema M, Wandinger-Ness A. Vesicular trafficking of tyrosine kinase receptors and associated proteins in the regulation of signaling and vascular function. Circ. Res. 2006;98:743–756. doi: 10.1161/01.RES.0000214545.99387.e3. [DOI] [PubMed] [Google Scholar]
- 481.Mochizuki N. Vascular integrity mediated by vascular endothelial cadherin and regulated by sphingosine 1-phosphate and angiopoietin-1. Circ. J. 2009;73:2183–2191. doi: 10.1253/circj.cj-09-0666. [DOI] [PubMed] [Google Scholar]
- 482.Sawant DA, Tharakan B, Adekanbi A, Hunter FA, Smythe WR, Childs EW. Inhibition of VE-cadherin proteasomal degradation attenuates microvascular hyperpermeability. Microcirculation. 2011;18:46–55. doi: 10.1111/j.1549-8719.2010.00067.x. [DOI] [PubMed] [Google Scholar]
- 483.London NR, Whitehead KJ, Li DY. Endogenous endothelial cell signaling systems maintain vascular stability. Angiogenesis. 2009;12:149–158. doi: 10.1007/s10456-009-9130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Gavard J. Breaking the VE-cadherin bonds. FEBS Lett. 2009;583:1–6. doi: 10.1016/j.febslet.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 485.Semenza GL. Regulation of vascularization by hypoxia-inducible factor 1. Ann. N. Y. Acad. Sci. 2009;1177:2–8. doi: 10.1111/j.1749-6632.2009.05032.x. [DOI] [PubMed] [Google Scholar]
- 486.Tilki D, Hohn HP, Ergun B, Rafii S, Ergun S. Emerging biology of vascular wall progenitor cells in health and disease. Trends Mol. Med. 2009;15:501–509. doi: 10.1016/j.molmed.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 487.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Ceradini DJ, Gurtner GC. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc. Med. 2005;15:57–63. doi: 10.1016/j.tcm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 489.Ushio-Fukai M, Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266:37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc. Res. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 491.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ. Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 492.El-Remessy AB, Al-Shabrawey M, Platt DH, Bartoli M, Behzadian MA, Ghaly N, Tsai N, Motamed K, Caldwell RB. Peroxynitrite mediates VEGF's angiogenic signal and function via a nitration-independent mechanism in endothelial cells. FASEB J. 2007;21:2528–2539. doi: 10.1096/fj.06-7854com. [DOI] [PubMed] [Google Scholar]
- 493.Youn JY, Wang T, Cai H. An ezrin/calpain/PI3K/AMPK/eNOSs1179 signaling cascade mediating VEGF-dependent endothelial nitric oxide production. Circ. Res. 2009;104:50–59. doi: 10.1161/CIRCRESAHA.108.178467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. USA. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Stahmann N, Woods A, Spengler K, Heslegrave A, Bauer R, Krause S, Viollet B, Carling D, Heller R. Activation of AMP-activated protein kinase by vascular endothelial growth factor mediates endothelial angiogenesis independently of nitric-oxide synthase. J. Biol. Chem. 2010;285:10638–10652. doi: 10.1074/jbc.M110.108688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Selvakumar B, Hess DT, Goldschmidt-Clermont PJ, Stamler JS. Co-regulation of constitutive nitric oxide synthases and NADPH oxidase by the small GTPase Rac. FEBS Lett. 2008;582:2195–2202. doi: 10.1016/j.febslet.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Yang B, Rizzo V. TNF-alpha potentiates protein-tyrosine nitration through activation of NADPH oxidase and eNOS localized in membrane rafts and caveolae of bovine aortic endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H954–H962. doi: 10.1152/ajpheart.00758.2006. [DOI] [PubMed] [Google Scholar]
- 498.Galvez BG, Matias-Roman S, Yanez-Mo M, Vicente-Manzanares M, Sanchez-Madrid F, Arroyo AG. Caveolae are a novel pathway for membrane-type 1matrix metalloproteinase traffic in human endothelial cells. Mol. Biol. Cell. 2004;15:678–687. doi: 10.1091/mbc.E03-07-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Genis L, Gonzalo P, Tutor AS, Galvez BG, Martinez-Ruiz A, Zaragoza C, Lamas S, Tryggvason K, Apte SS, Arroyo AG. Functional interplay between endothelial nitric oxide synthase and membrane type 1 matrix metalloproteinase in migrating endothelial cells. Blood. 2007;110:2916–2923. doi: 10.1182/blood-2007-01-068080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Ridnour LA, Windhausen AN, Isenberg JS, Yeung N, Thomas DD, Vitek MP, Roberts DD, Wink DA. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA. 2007;104:16898–16903. doi: 10.1073/pnas.0702761104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci. STKE. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 502.Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA, Jr, Terada LS. Subcellular targeting of oxidants during endothelial cell migration. J. Cell Biol. 2005;171:893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Oubaha M, Gratton JP. Phosphorylation of endothelial nitric oxide synthase by atypical PKC zeta contributes to angiopoietin-1-dependent inhibition of VEGF-induced endothelial permeability in vitro. Blood. 2009;114:3343–3351. doi: 10.1182/blood-2008-12-196584. [DOI] [PubMed] [Google Scholar]
- 504.Sanchez FA, Rana R, Kim DD, Iwahashi T, Zheng R, Lal BK, Gordon DM, Meininger CJ, Duran WN. Internalization of eNOS and NO delivery to subcellular targets determine agonist-induced hyperpermeability. Proc. Natl. Acad. Sci. USA. 2009;106:6849–6853. doi: 10.1073/pnas.0812694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Morbidelli L, Pyriochou A, Filippi S, Vasileiadis I, Roussos C, Zhou Z, Loutrari H, Waltenberger J, Stossel A, Giannis A, Ziche M, Papapetropoulos A. The soluble guanylyl cyclase inhibitor NS-2028 reduces vascular endothelial growth factor-induced angiogenesis and permeability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R824–R832. doi: 10.1152/ajpregu.00222.2009. [DOI] [PubMed] [Google Scholar]
- 506.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ. Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 507.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp. Cell Res. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 508.Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, Olsen BR, Alitalo K. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell–cell and cell–matrix contacts. Nat. Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- 509.Thomas M, Augustin HG. The role of the angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12:125–137. doi: 10.1007/s10456-009-9147-3. [DOI] [PubMed] [Google Scholar]
- 510.Benest AV, Salmon AH, Wang W, Glover CP, Uney J, Harper SJ, Bates DO. VEGF and angiopoietin-1 stimulate different angiogenic phenotypes that combine to enhance functional neovascularization in adult tissue. Microcirculation. 2006;13:423–437. doi: 10.1080/10739680600775940. [DOI] [PubMed] [Google Scholar]
- 511.Takakura N, Kidoya H. Maturation of blood vessels by haematopoietic stem cells and progenitor cells: involvement of apelin/APJ and angiopoietin/Tie2 interactions in vessel caliber size regulation. Thromb. Haemostasis. 2009;101:999–1005. [PubMed] [Google Scholar]
- 512.Kidoya H, Ueno M, Yamada Y, Mochizuki N, Nakata M, Yano T, Fujii R, Takakura N. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 2008;27:522–534. doi: 10.1038/sj.emboj.7601982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Kidoya H, Naito H, Takakura N. Apelin induces enlarged and nonleaky blood vessels for functional recovery from ischemia. Blood. 2010;115:3166–3174. doi: 10.1182/blood-2009-07-232306. [DOI] [PubMed] [Google Scholar]
- 514.Antonelli-Orlidge A, Saunders KB, Smith SR, D'Amore PA. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc. Natl. Acad. Sci. USA. 1989;86:4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Sato Y, Tsuboi R, Lyons R, Moses H, Rifkin DB. Characterization of the activation of latent TGF-beta by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J. Cell Biol. 1990;111:757–763. doi: 10.1083/jcb.111.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Zhao YD, Ohkawara H, Rehman J, Wary KK, Vogel SM, Minshall RD, Zhao YY, Malik AB. Bone marrow progenitor cells induce endothelial adherens junction integrity by sphingosine-1-phosphate-mediated Rac1 and Cdc42 signaling. Circ. Res. 2009;105:696–704. doi: 10.1161/CIRCRESAHA.109.199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Lebman DA, Spiegel S. Cross-talk at the crossroads of sphingosine-1-phosphate, growth factors, and cytokine signaling. J. Lipid Res. 2008;49:1388–1394. doi: 10.1194/jlr.R800008-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Lee HM, Jeon BH, Won KJ, Lee CK, Park TK, Choi WS, Bae YM, Kim HS, Lee SK, Park SH, Irani K, Kim B. Gene transfer of redox factor-1 inhibits neointimal formation: involvement of platelet-derived growth factor-beta receptor signaling via the inhibition of the reactive oxygen species-mediated Syk pathway. Circ. Res. 2009;104:219–227. doi: 10.1161/CIRCRESAHA.108.178699. [DOI] [PubMed] [Google Scholar]
- 520.ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smoothmuscle cell chemotaxis, but not proliferation. Cardiovasc. Res. 2006;71:331–341. doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 521.Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS. An essential role for Rac1 in endothelial cell function and vascular development. FASEB J. 2008;22:1829–1838. doi: 10.1096/fj.07-096438. [DOI] [PubMed] [Google Scholar]
- 522.Yu J, de Muinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc. Natl. Acad. Sci. USA. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Benest AV, Stone OA, Miller WH, Glover CP, Uney JB, Baker AH, Harper SJ, Bates DO. Arteriolar genesis and angiogenesis induced by endothelial nitric oxide synthase overexpression results in a mature vasculature. Arterioscler. Thromb. Vasc. Biol. 2008;28:1462–1468. doi: 10.1161/ATVBAHA.108.169375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Chandra SM, Razavi H, Kim J, Agrawal R, Kundu RK, de Jesus Perez V, Zamanian RT, Quertermous T, Chun HJ. Disruption of the apelin–APJ system worsens hypoxia-induced pulmonary hypertension. Arterioscler. Thromb. Vasc. Biol. 2011;31:814–820. doi: 10.1161/ATVBAHA.110.219980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Filippin LI, Moreira AJ, Marroni NP, Xavier RM. Nitric oxide and repair of skeletal muscle injury. Nitric Oxide. 2009;21:157–163. doi: 10.1016/j.niox.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 526.Takuwa Y, Okamoto Y, Yoshioka K, Takuwa N. Sphingosine-1-phosphate signaling and biological activities in the cardiovascular system. Biochim. Biophys. Acta. 2008;1781:483–488. doi: 10.1016/j.bbalip.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 527.Esmon CT. The interactions between inflammation and coagulation. Br. J. Haematol. 2005;131:417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 528.Levi M, van der Poll T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc. Med. 2005;15:254–259. doi: 10.1016/j.tcm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 529.van der Poll T. Coagulation and inflammation. J. Endotoxin Res. 2001;7:301–304. [PubMed] [Google Scholar]
- 530.Egbrink MG, Van Gestel MA, Broeders MA, Tangelder GJ, Heemskerk JM, Reneman RS, Slaaf DW. Regulation of microvascular thromboembolism in vivo. Microcirculation. 2005;12:287–300. doi: 10.1080/10739680590925628. [DOI] [PubMed] [Google Scholar]
- 531.Broeders MA, Tangelder GJ, Slaaf DW, Reneman RS, oude Egbrink MG. Hypercholesterolemia enhances thromboembolism in arterioles but not venules: complete reversal by L-arginine. Arterioscler. Thromb. Vasc. Biol. 2002;22:680–685. doi: 10.1161/01.atv.0000013287.08141.74. [DOI] [PubMed] [Google Scholar]
- 532.Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: the clot thickens. Am. J. Gastroenterol. 2007;102:174–186. doi: 10.1111/j.1572-0241.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 533.Granger DN, Rodrigues SF, Yildirim A, Senchenkova EY. Microvascular responses to cardiovascular risk factors. Microcirculation. 2010;17:192–205. doi: 10.1111/j.1549-8719.2009.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Leopold JA, Loscalzo J. Oxidative risk for atherothrombotic cardiovascular disease. Free Radic. Biol. Med. 2009;47:1673–1706. doi: 10.1016/j.freeradbiomed.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Levi M, van der Poll T, ten Cate H, van Deventer SJ. The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur. J. Clin. Invest. 1997;27:3–9. doi: 10.1046/j.1365-2362.1997.570614.x. [DOI] [PubMed] [Google Scholar]
- 536.Remkova A, Remko M. The role of renin–angiotensin system in prothrombotic state in essential hypertension. Physiol. Res. 2010;59:13–23. doi: 10.33549/physiolres.931525. [DOI] [PubMed] [Google Scholar]
- 537.Yoshida H, Granger DN. Inflammatory bowel disease: a paradigm for the link between coagulation and inflammation. Inflammatory Bowel Dis. 2009;15:1245–1255. doi: 10.1002/ibd.20896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Gorlach A. Redox regulation of the coagulation cascade. Antioxid. Redox Signaling. 2005;7:1398–1404. doi: 10.1089/ars.2005.7.1398. [DOI] [PubMed] [Google Scholar]
- 539.Altieri D. Interface between inflammation and coagulation. In: Ley K, editor. Physiology of Inflammation. New York: Oxford Univ. Press; 2001. pp. 402–422. [Google Scholar]
- 540.Andrews RK, Berndt MC. Platelet physiology and thrombosis. Thromb. Res. 2004;114:447–453. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 541.Furie B, Furie BC. Mechanisms of thrombus formation. N. Engl. J. Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 542.Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signalling. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin. Thromb. Hemostasis. 2005;31:217–233. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- 544.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2007;27:1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 545.Herkert O, Djordjevic T, BelAiba RS, Gorlach A. Insights into the redox control of blood coagulation: role of vascular NADPH oxidase-derived reactive oxygen species in the thrombogenic cycle. Antioxid. Redox Signaling. 2004;6:765–776. doi: 10.1089/1523086041361695. [DOI] [PubMed] [Google Scholar]
- 546.Binder BR, Christ G, Gruber F, Grubic N, Hufnagl P, Krebs M, Mihaly J, Prager GW. Plasminogen activator inhibitor 1: physiological and pathophysiological roles. News Physiol. Sci. 2002;17:56–61. doi: 10.1152/nips.01369.2001. [DOI] [PubMed] [Google Scholar]
- 547.Esmon CT. The impact of the inflammatory response on coagulation. Thromb. Res. 2004;114:321–327. doi: 10.1016/j.thromres.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 548.Herkert O, Gorlach A. Redox control of tissue factor expression in smooth muscle cells and other vascular cells. Methods Enzymol. 2002;352:220–231. doi: 10.1016/s0076-6879(02)52021-8. [DOI] [PubMed] [Google Scholar]
- 549.Golino P, Ragni M, Cirillo P, Avvedimento VE, Feliciello A, Esposito N, Scognamiglio A, Trimarco B, Iaccarino G, Condorelli M, Chiariello M, Ambrosio G. Effects of tissue factor induced by oxygen free radicals on coronary flow during reperfusion. Nat. Med. 1996;2:35–40. doi: 10.1038/nm0196-35. [DOI] [PubMed] [Google Scholar]
- 550.Cadroy Y, Dupouy D, Boneu B, Plaisancie H. Polymorphonuclear leukocytes modulate tissue factor production by mononuclear cells: role of reactive oxygen species. J. Immunol. 2000;164:3822–3828. doi: 10.4049/jimmunol.164.7.3822. [DOI] [PubMed] [Google Scholar]
- 551.Golino PAG, Ragni M, Cirillo P, Scognamiglio A, Condorelli M, Chiariello M. xygen radicals induce a procoagulant state in cultured coronary endothelial cells by inducing tissue factor synthesis and by inhibiting tissue-factor pathway inhibitor. Circulation. 1995;92:I354. [Abstract] [Google Scholar]
- 552.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 553.Gorlach A, Brandes RP, Bassus S, Kronemann N, Kirchmaier CM, Busse R, Schini-Kerth VB. Oxidative stress and expression of p22phox are involved in the up-regulation of tissue factor in vascular smooth muscle cells in response to activated platelets. FASEB J. 2000;14:1518–1528. [PubMed] [Google Scholar]
- 554.Orthner CL, Rodgers GM, Fitzgerald LA. Pyrrolidine dithiocarbamate abrogates tissue factor (TF) expression by endothelial cells: evidence implicating nuclear factor-kappa B in TF induction by diverse agonists. Blood. 1995;86:436–443. [PubMed] [Google Scholar]
- 555.Slupsky JR, Kalbas M, Willuweit A, Henn V, Kroczek RA, Muller-Berghaus G. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb. Haemostasis. 1998;80:1008–1014. [PubMed] [Google Scholar]
- 556.Djordjevic T, Hess J, Herkert O, Gorlach A, BelAiba RS. Rac regulates thrombin-induced tissue factor expression in pulmonary artery smooth muscle cells involving the nuclear factor-kappaB pathway. Antioxid. Redox Signaling. 2004;6:713–720. doi: 10.1089/1523086041361703. [DOI] [PubMed] [Google Scholar]
- 557.Menschikowski M, Hagelgans A, Eisenhofer G, Siegert G. Regulation of endothelial protein C receptor shedding by cytokines is mediated through differential activation of MAP kinase signaling pathways. Exp. Cell Res. 2009;315:2673–2682. doi: 10.1016/j.yexcr.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 558.Nalian A, Iakhiaev AV. Possible mechanisms contributing to oxidative inactivation of activated protein C: molecular dynamics study. Thromb. Haemostasis. 2008;100:18–25. doi: 10.1160/TH07-12-0750. [DOI] [PubMed] [Google Scholar]
- 559.Glaser CB, Morser J, Clarke JH, Blasko E, McLean K, Kuhn I, Chang RJ, Lin JH, Vilander L, Andrews WH, et al. Oxidation of a specific methionine in thrombomodulin by activated neutrophil products blocks cofactor activity: a potential rapid mechanism for modulation of coagulation. J. Clin. Invest. 1992;90:2565–2573. doi: 10.1172/JCI116151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Yamaji K, Wang Y, Liu Y, Abeyama K, Hashiguchi T, Uchimura T, Krishna Biswas K, Iwamoto H, Maruyama I. Activated protein C, a natural anticoagulant protein, has antioxidant properties and inhibits lipid peroxidation and advanced glycation end products formation. Thromb. Res. 2005;115:319–325. doi: 10.1016/j.thromres.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 561.De Cristofaro R, Landolfi R. Oxidation of human alpha-thrombin by the myeloperoxidase–H2O2–chloride system: structural and functional effects. Thromb. Haemostasis. 2000;83:253–261. [PubMed] [Google Scholar]
- 562.Van Patten SM, Hanson E, Bernasconi R, Zhang K, Manavalan P, Cole ES, McPherson JM, Edmunds T. Oxidation of methionine residues in antithrombin: effects on biological activity and heparin binding. J. Biol. Chem. 1999;274:10268–10276. doi: 10.1074/jbc.274.15.10268. [DOI] [PubMed] [Google Scholar]
- 563.Gray E, Barrowcliffe TW. Inhibition of antithrombin III by lipid peroxides. Thromb. Res. 1985;37:241–250. doi: 10.1016/0049-3848(85)90012-x. [DOI] [PubMed] [Google Scholar]
- 564.Upchurch GR, Jr, Ramdev N, Walsh MT, Loscalzo J. Prothrombotic consequences of the oxidation of fibrinogen and their inhibition by aspirin. J. Thromb. Thrombolysis. 1998;5:9–14. doi: 10.1023/a:1008859729045. [DOI] [PubMed] [Google Scholar]
- 565.Lawrence DA, Loskutoff DJ. Inactivation of plasminogen activator inhibitor by oxidants. Biochemistry. 1986;25:6351–6355. doi: 10.1021/bi00369a001. [DOI] [PubMed] [Google Scholar]
- 566.Swiatkowska M, Szemraj J, Al-Nedawi KN, Pawlowska Z. Reactive oxygen species upregulate expression of PAI-1 in endothelial cells. Cell. Mol. Biol. Lett. 2002;7:1065–1071. [PubMed] [Google Scholar]
- 567.Dimova EY, Samoylenko A, Kietzmann T. Oxidative stress and hypoxia: implications for plasminogen activator inhibitor-1 expression. Antioxid. Redox Signaling. 2004;6:777–791. doi: 10.1089/1523086041361596. [DOI] [PubMed] [Google Scholar]
- 568.Gorlach A, Diebold I, Schini-Kerth VB, Berchner-Pfannschmidt U, Roth U, Brandes RP, Kietzmann T, Busse R. Thrombin activates the hypoxiainducible factor-1 signaling pathway in vascular smooth muscle cells: role of the p22(phox)-containing NADPH oxidase. Circ. Res. 2001;89:47–54. doi: 10.1161/hh1301.092678. [DOI] [PubMed] [Google Scholar]
- 569.Vulin AI, Stanley FM. Oxidative stress activates the plasminogen activator inhibitor type 1 (PAI-1) promoter through an AP-1 response element and cooperates with insulin for additive effects on PAI-1 transcription. J. Biol. Chem. 2004;279:25172–25178. doi: 10.1074/jbc.M403184200. [DOI] [PubMed] [Google Scholar]
- 570.Yang Y, Loscalzo J. Regulation of tissue factor expression in human microvascular endothelial cells by nitric oxide. Circulation. 2000;101:2144–2148. doi: 10.1161/01.cir.101.18.2144. [DOI] [PubMed] [Google Scholar]
- 571.Perez-Ruiz A, Montes R, Velasco F, Lopez-Pedrera C, Antonio Paramo J, Orbe J, Hermida J, Rocha E. Regulation by nitric oxide of endotoxin-induced tissue factor and plasminogen activator inhibitor-1 in endothelial cells. Thromb. Haemostasis. 2002;88:1060–1065. [PubMed] [Google Scholar]
- 572.Solovey A, Kollander R, Milbauer LC, Abdulla F, Chen Y, Kelm RJ, Jr, Hebbel RP. Endothelial nitric oxide synthase and nitric oxide regulate endothelial tissue factor expression in vivo in the sickle transgenic mouse. Am. J. Hematol. 2010;85:41–45. doi: 10.1002/ajh.21582. [DOI] [PubMed] [Google Scholar]
- 573.Jiang DJ, Cao Y, Xin HY, Li XH, Luo ZQ, Li YJ. Asymmetric dimethylarginine induces tissue factor expression in monocytes via NF-kappaB-dependent pathway: role in acute coronary syndromes. Atherosclerosis. 2009;205:554–560. doi: 10.1016/j.atherosclerosis.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 574.Adam JM, Ettelaie C, Naseem KM, James NJ, Bradley NJ, Bruckdorfer KR. Modification of tissue factor by peroxynitrite influences its procoagulant activity. FEBS Lett. 1998;429:347–350. doi: 10.1016/s0014-5793(98)00627-9. [DOI] [PubMed] [Google Scholar]
- 575.Nielsen VG, Crow JP, Mogal A, Zhou F, Parks DA. Peroxynitrite decreases hemostasis in human plasma in vitro. Anesth. Analg. 2004;99:21–26. doi: 10.1213/01.ANE.0000116962.93953.70. [DOI] [PubMed] [Google Scholar]
- 576.Bouchie JL, Hansen H, Feener EP. Natriuretic factors and nitric oxide suppress plasminogen activator inhibitor-1 expression in vascular smoothmuscle cells: role of cGMP in the regulation of the plasminogen system. Arterioscler. Thromb. Vasc. Biol. 1998;18:1771–1779. doi: 10.1161/01.atv.18.11.1771. [DOI] [PubMed] [Google Scholar]
- 577.Katoh M, Egashira K, Mitsui T, Chishima S, Takeshita A, Narita H. Angiotensin-converting enzyme inhibitor prevents plasminogen activator inhibitor-1 expression in a rat model with cardiovascular remodeling induced by chronic inhibition of nitric oxide synthesis. J. Mol. Cell. Cardiol. 2000;32:73–83. doi: 10.1006/jmcc.1999.1053. [DOI] [PubMed] [Google Scholar]
- 578.Kaikita K, Fogo AB, Ma L, Schoenhard JA, Brown NJ, Vaughan DE. Plasminogen activator inhibitor-1 deficiency prevents hypertension and vascular fibrosis in response to long-term nitric oxide synthase inhibition. Circulation. 2001;104:839–844. doi: 10.1161/hc3301.092803. [DOI] [PubMed] [Google Scholar]
- 579.Brown NJ, Muldowney JA, 3rd, Vaughan DE. Endogenous NO regulates plasminogen activator inhibitor-1 during angiotensin-converting enzyme inhibition. Hypertension. 2006;47:441–448. doi: 10.1161/01.HYP.0000202478.79587.1a. [DOI] [PubMed] [Google Scholar]
- 580.Iafrati MD, Vitseva O, Tanriverdi K, Blair P, Rex S, Chakrabarti S, Varghese S, Freedman JE. Compensatory mechanisms influence hemostasis in setting of eNOS deficiency. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1627–H1632. doi: 10.1152/ajpheart.00819.2004. [DOI] [PubMed] [Google Scholar]
- 581.Gugliucci A. Human plasminogen is highly susceptible to peroxynitrite inactivation. Clin. Chem. Lab. Med. 2003;41:1064–1068. doi: 10.1515/CCLM.2003.164. [DOI] [PubMed] [Google Scholar]
- 582.Nielsen VG, Crow JP, Zhou F, Parks DA. Peroxynitrite inactivates tissue plasminogen activator. Anesth. Analg. 2004;98:1312–1317. doi: 10.1213/01.ane.0000111105.38836.f6. [DOI] [PubMed] [Google Scholar]
- 583.Vadseth C, Souza JM, Thomson L, Seagraves A, Nagaswami C, Scheiner T, Torbet J, Vilaire G, Bennett JS, Murciano JC, Muzykantov V, Penn MS, Hazen SL, Weisel JW, Ischiropoulos H. Pro-thrombotic state induced by post-translational modification of fibrinogen by reactive nitrogen species. J. Biol. Chem. 2004;279:8820–8826. doi: 10.1074/jbc.M306101200. [DOI] [PubMed] [Google Scholar]
- 584.Bressler NM, Broekman MJ, Marcus AJ. Concurrent studies of oxygen consumption and aggregation in stimulated human platelets. Blood. 1979;53:167–178. [PubMed] [Google Scholar]
- 585.Burch JW, Burch PT. Glutathione disulfide production during arachidonic acid oxygenation in human platelets. Prostaglandins. 1990;39:123–134. doi: 10.1016/0090-6980(90)90069-8. [DOI] [PubMed] [Google Scholar]
- 586.Krotz F, Sohn HY, Pohl U. Reactive oxygen species: players in the platelet game. Arterioscler. Thromb. Vasc. Biol. 2004;24:1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- 587.Caccese D, Pratico D, Ghiselli A, Natoli S, Pignatelli P, Sanguigni V, Iuliano L, Violi F. Superoxide anion and hydroxyl radical release by collagen-induced platelet aggregation—role of arachidonic acid metabolism. Thromb. Haemostasis. 2000;83:485–490. [PubMed] [Google Scholar]
- 588.Finazzi-Agro A, Menichelli A, Persiani M, Biancini G, Del Principe D. Hydrogen peroxide release from human blood platelets. Biochim. Biophys. Acta. 1982;718:21–25. doi: 10.1016/0304-4165(82)90004-6. [DOI] [PubMed] [Google Scholar]
- 589.Wachowicz B, Olas B, Zbikowska HM, Buczynski A. Generation of reactive oxygen species in blood platelets. Platelets. 2002;13:175–182. doi: 10.1080/09533710022149395. [DOI] [PubMed] [Google Scholar]
- 590.Krotz F, Sohn HY, Gloe T, Zahler S, Riexinger T, Schiele TM, Becker BF, Theisen K, Klauss V, Pohl U. NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood. 2002;100:917–924. doi: 10.1182/blood.v100.3.917. [DOI] [PubMed] [Google Scholar]
- 591.Sohn HY, Keller M, Gloe T, Morawietz H, Rueckschloss U, Pohl U. The small G-protein Rac mediates depolarization-induced superoxide formation in human endothelial cells. J. Biol. Chem. 2000;275:18745–18750. doi: 10.1074/jbc.M000026200. [DOI] [PubMed] [Google Scholar]
- 592.Essex DW. Redox control of platelet function. Antioxid. Redox Signaling. 2009;11:1191–1225. doi: 10.1089/ars.2008.2322. [DOI] [PubMed] [Google Scholar]
- 593.Freedman JE. Oxidative stress and platelets. Arterioscler. Thromb. Vasc. Biol. 2008;28:s11–s16. doi: 10.1161/ATVBAHA.107.159178. [DOI] [PubMed] [Google Scholar]
- 594.Pignatelli P, Sanguigni V, Lenti L, Ferro D, Finocchi A, Rossi P, Violi F. gp91phox-dependent expression of platelet CD40 ligand. Circulation. 2004;110:1326–1329. doi: 10.1161/01.CIR.0000134963.77201.55. [DOI] [PubMed] [Google Scholar]
- 595.Seno T, Inoue N, Gao D, Okuda M, Sumi Y, Matsui K, Yamada S, Hirata KI, Kawashima S, Tawa R, Imajoh-Ohmi S, Sakurai H, Yokoyama M. Involvement of NADH/NADPH oxidase in human platelet ROS production. Thromb. Res. 2001;103:399–409. doi: 10.1016/s0049-3848(01)00341-3. [DOI] [PubMed] [Google Scholar]
- 596.Carnevale R, Pignatelli P, Lenti L, Buchetti B, Sanguigni V, Di Santo S, Violi F. LDL are oxidatively modified by platelets via GP91(phox) and accumulate in human monocytes. FASEB J. 2007;21:927–934. doi: 10.1096/fj.06-6908com. [DOI] [PubMed] [Google Scholar]
- 597.Clutton P, Miermont A, Freedman JE. Regulation of endogenous reactive oxygen species in platelets can reverse aggregation. Arterioscler. Thromb. Vasc. Biol. 2004;24:187–192. doi: 10.1161/01.ATV.0000105889.29687.CC. [DOI] [PubMed] [Google Scholar]
- 598.Begonja AJ, Gambaryan S, Geiger J, Aktas B, Pozgajova M, Nieswandt B, Walter U. Platelet NAD(P)H-oxidase-generated ROS production regulates alphaIIbbeta3-integrin activation independent of the NO/cGMP pathway. Blood. 2005;106:2757–2760. doi: 10.1182/blood-2005-03-1047. [DOI] [PubMed] [Google Scholar]
- 599.Gkaliagkousi E, Ritter J, Ferro A. Platelet-derived nitric oxide signaling and regulation. Circ. Res. 2007;101:654–662. doi: 10.1161/CIRCRESAHA.107.158410. [DOI] [PubMed] [Google Scholar]
- 600.Freedman JE, Li L, Sauter R, Keaney JJ. alpha-Tocopherol and protein kinase C inhibition enhance platelet-derived nitric oxide release. FASEB J. 2000;14:2377–2379. doi: 10.1096/fj.00-0360fje. [DOI] [PubMed] [Google Scholar]
- 601.Radomski MW, Palmer RM, Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl. Acad. Sci. USA. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Radomski MW, Palmer RM, Moncada S. Characterization of the L-arginine: nitric oxide pathway in human platelets. Br. J. Pharmacol. 1990;101:325–328. doi: 10.1111/j.1476-5381.1990.tb12709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Muruganandam A, Mutus B. Isolation of nitric oxide synthase from human platelets. Biochim. Biophys. Acta. 1994;1200:1–6. doi: 10.1016/0304-4165(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 604.Freedman JE, Sauter R, Battinelli EM, Ault K, Knowles C, Huang PL, Loscalzo J. Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the NOSIII gene. Circ. Res. 1999;84:1416–1421. doi: 10.1161/01.res.84.12.1416. [DOI] [PubMed] [Google Scholar]
- 605.Chakrabarti S, Varghese S, Vitseva O, Tanriverdi K, Freedman JE. CD40 ligand influences platelet release of reactive oxygen intermediates. Arterioscler. Thromb. Vasc. Biol. 2005;25:2428–2434. doi: 10.1161/01.ATV.0000184765.59207.f3. [DOI] [PubMed] [Google Scholar]
- 606.Krotz F, Sohn HY, Keller M, Gloe T, Bolz SS, Becker BF, Pohl U. Depolarization of endothelial cells enhances platelet aggregation through oxidative inactivation of endothelial NTPDase. Arterioscler. Thromb. Vasc. Biol. 2002;22:2003–2009. doi: 10.1161/01.atv.0000043454.08172.51. [DOI] [PubMed] [Google Scholar]
- 607.Salvemini D, de Nucci G, Sneddon JM, Vane JR. Superoxide anions enhance platelet adhesion and aggregation. Br. J. Pharmacol. 1989;97:1145–1150. doi: 10.1111/j.1476-5381.1989.tb12572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Pignatelli P, Sanguigni V, Lenti L, Loffredo L, Carnevale R, Sorge R, Violi F. Oxidative stress-mediated platelet CD40 ligand upregulation in patients with hypercholesterolemia: effect of atorvastatin. J. Thromb. Haemostasis. 2007;5:1170–1178. doi: 10.1111/j.1538-7836.2007.02533.x. [DOI] [PubMed] [Google Scholar]
- 609.Bakdash N, Williams MS. Spatially distinct production of reactive oxygen species regulates platelet activation. Free Radic. Biol. Med. 2008;45:158–166. doi: 10.1016/j.freeradbiomed.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 610.Handin RI, Karabin R, Boxer GJ. Enhancement of platelet function by superoxide anion. J. Clin. Invest. 1977;59:959–965. doi: 10.1172/JCI108718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Ambrosio G, Golino P, Pascucci I, Rosolowsky M, Campbell WB, DeClerck F, Tritto I, Chiariello M. Modulation of platelet function by reactive oxygen metabolites. Am. J. Physiol. 1994;267:H308–H318. doi: 10.1152/ajpheart.1994.267.1.H308. [DOI] [PubMed] [Google Scholar]
- 612.Ambrosio G, Tritto I, Golino P. Reactive oxygen metabolites and arterial thrombosis. Cardiovasc. Res. 1997;34:445–452. doi: 10.1016/s0008-6363(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 613.Salvemini D, de Nucci G, Vane JR. Superoxide dismutase cooperates with prostacyclin to inhibit platelet aggregation: a comparative study in washed platelets and platelet rich plasma. Thromb. Haemostasis. 1991;65:421–424. [PubMed] [Google Scholar]
- 614.Ambrosio G, Oriente A, Napoli C, Palumbo G, Chiariello P, Marone G, Condorelli M, Chiariello M, Triggiani M. Oxygen radicals inhibit human plasma acetylhydrolase, the enzyme that catabolizes platelet-activating factor. J. Clin. Invest. 1994;93:2408–2416. doi: 10.1172/JCI117248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Canoso RT, Rodvien R, Scoon K, Levine PH. Hydrogen peroxide and platelet function. Blood. 1974;43:645–656. [PubMed] [Google Scholar]
- 616.Levine PH, W R, Simon J, Scoon KL, Krinsky NI. Leucocyte–platelet interaction: release of hydrogen peroxide by granulocytes as a modulator of platelet reactions. J. Clin. Invest. 1976;57:955–963. doi: 10.1172/JCI108372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Stuart MJ, Holmsen H. Hydrogen peroxide, an inhibitor of platelet function: effect on adenine nucleotide metabolism, and the release reaction. Am. J. Hematol. 1977;2:53–63. doi: 10.1002/ajh.2830020108. [DOI] [PubMed] [Google Scholar]
- 618.Redondo PC, Salido GM, Rosado JA, Pariente JA. Effect of hydrogen peroxide on Ca2+ mobilisation in human platelets through sulphydryl oxidation dependent and independent mechanisms. Biochem. Pharmacol. 2004;67:491–502. doi: 10.1016/j.bcp.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 619.Coleman LG, Jr, Polanowska-Grabowska RK, Marcinkiewicz M, Gear AR. LDL oxidized by hypochlorous acid causes irreversible platelet aggregation when combined with low levels of ADP, thrombin, epinephrine, or macrophage-derived chemokine (CCL22) Blood. 2004;104:380–389. doi: 10.1182/blood-2003-08-2961. [DOI] [PubMed] [Google Scholar]
- 620.Radomski MW, Palmer RM, Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br. J. Pharmacol. 1987;92:639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Freedman JE, Loscalzo J, Barnard MR, Alpert C, Keaney JF, Michelson AD. Nitric oxide released from activated platelets inhibits platelet recruitment. J. Clin. Invest. 1997;100:350–356. doi: 10.1172/JCI119540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Chen LY, Mehta JL. Variable effects of L-arginine analogs on L-arginine–nitric oxide pathway in human neutrophils and platelets may relate to different nitric oxide synthase isoforms. J. Pharmacol. Exp. Ther. 1996;276:253–257. [PubMed] [Google Scholar]
- 623.Cerwinka WH, Cooper D, Krieglstein CF, Feelisch M, Granger DN. Nitric oxide modulates endotoxin-induced platelet–endothelial cell adhesion in intestinal venules. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1111–H1117. doi: 10.1152/ajpheart.00391.2001. [DOI] [PubMed] [Google Scholar]
- 624.Feil R, Lohmann SM, de Jonge H, Walter U, Hofmann F. Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ. Res. 2003;93:907–916. doi: 10.1161/01.RES.0000100390.68771.CC. [DOI] [PubMed] [Google Scholar]
- 625.Rex S, Freedman JE. Inhibition of platelet function by the endothelium. In: Michelson AD, editor. Platelets. New York: Academic Press; 2007. p. 251.p. 279. [Google Scholar]
- 626.Morrell CN, Matsushita K, Chiles K, Scharpf RB, Yamakuchi M, Mason RJ, Bergmeier W, Mankowski JL, Baldwin WM, 3rd, Faraday N, Lowenstein CJ. Regulation of platelet granule exocytosis by S-nitrosylation. Proc. Natl. Acad. Sci. USA. 2005;102:3782–3787. doi: 10.1073/pnas.0408310102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Peire MA, Puig-Parellada P. Oxygen free radicals and nitric oxide are involved in the thrombus growth produced by iontophoresis of ADP. Pharmacol. Res. 1998;38:353–356. doi: 10.1006/phrs.1998.0372. [DOI] [PubMed] [Google Scholar]
- 628.Yao SK, Ober JC, Gonenne A, Clubb FJ, Jr, Krishnaswami A, Ferguson JJ, Anderson HV, Gorecki M, Buja LM, Willerson JT. Active oxygen species play a role in mediating platelet aggregation and cyclic flow variations in severely stenosed and endothelium-injured coronary arteries. Circ. Res. 1993;73:952–967. doi: 10.1161/01.res.73.5.952. [DOI] [PubMed] [Google Scholar]
- 629.Meng YY, Trachtenburg J, Ryan US, Abendschein DR. Potentiation of endogenous nitric oxide with superoxide dismutase inhibits platelet-mediated thrombosis in injured and stenotic arteries. J. Am. Coll. Cardiol. 1995;25:269–275. doi: 10.1016/0735-1097(94)00349-u. [DOI] [PubMed] [Google Scholar]
- 630.Hashimoto I, Nakanishi H, Shono Y, Tanaka S. The effects of desferrioxamine on thrombus formation in injured microvessels of the rabbit ear. J. Med. Invest. 1999;46:200–204. [PubMed] [Google Scholar]
- 631.Day SM, Duquaine D, Mundada LV, Menon RG, Khan BV, Rajagopalan S, Fay WP. Chronic iron administration increases vascular oxidative stress and accelerates arterial thrombosis. Circulation. 2003;107:2601–2606. doi: 10.1161/01.CIR.0000066910.02844.D0. [DOI] [PubMed] [Google Scholar]
- 632.Kuwano K, Ikeda H, Oda T, Nakayama H, Koga Y, Toshima H, Imaizumi T. Xanthine oxidase mediates cyclic flow variations in a canine model of coronary arterial thrombosis. Am. J. Physiol. 1996;270:H1993–H1999. doi: 10.1152/ajpheart.1996.270.6.H1993. [DOI] [PubMed] [Google Scholar]
- 633.Ozuyaman B, Godecke A, Kusters S, Kirchhoff E, Scharf RE, Schrader J. Endothelial nitric oxide synthase plays a minor role in inhibition of arterial thrombus formation. Thromb. Haemostasis. 2005;93:1161–1167. doi: 10.1160/TH03-09-0588. [DOI] [PubMed] [Google Scholar]
- 634.Harbrecht BG, Billiar TR, Stadler J, Demetris AJ, Ochoa J, Curran RD, Simmons RL. Inhibition of nitric oxide synthesis during endotoxemia promotes intrahepatic thrombosis and an oxygen radical-mediated hepatic injury. J. Leukocyte Biol. 1992;52:390–394. doi: 10.1002/jlb.52.4.390. [DOI] [PubMed] [Google Scholar]
- 635.Broeders MA, Tangelder GJ, Slaaf DW, Reneman RS, oude Egbrink MG. Endogenous nitric oxide protects against thromboembolism in venules but not in arterioles. Arterioscler. Thromb. Vasc. Biol. 1998;18:139–145. doi: 10.1161/01.atv.18.1.139. [DOI] [PubMed] [Google Scholar]
- 636.Wilson KM, McCaw RB, Leo L, Arning E, Lhotak S, Bottiglieri T, Austin RC, Lentz SR. Prothrombotic effects of hyperhomocysteinemia and hypercholesterolemia in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2007;27:233–240. doi: 10.1161/01.ATV.0000251607.96118.af. [DOI] [PubMed] [Google Scholar]
- 637.Moore C, Tymvios C, Emerson M. Functional regulation of vascular and platelet activity during thrombosis by nitric oxide and endothelial nitric oxide synthase. Thromb. Haemostasis. 2010;104:342–349. doi: 10.1160/TH09-11-0764. [DOI] [PubMed] [Google Scholar]
- 638.Heeringa P, van Goor H, Itoh-Lindstrom Y, Maeda N, Falk RJ, Assmann KJ, Kallenberg CG, Jennette JC. Lack of endothelial nitric oxide synthase aggravates murine accelerated anti-glomerular basement membrane glomerulonephritis. Am. J. Pathol. 2000;156:879–888. doi: 10.1016/S0002-9440(10)64957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF., Jr NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Sartoretto JL, Kalwa H, Pluth MD, Lippard SJ, Michel T. Hydrogen peroxide differentially modulates cardiac myocyte nitric oxide synthesis. Proc. Natl. Acad. Sci. USA. 2011;108:15792–15797. doi: 10.1073/pnas.1111331108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Tian J, Hou Y, Lu Q, Wiseman DA, Vasconcelos Fonsesca F, Elms S, Fulton DJ, Black SM. A novel role for caveolin-1 in regulating endothelial nitric oxide synthase activation in response to H2O2 and shear stress. Free Radic. Biol. Med. 2010;49:159–170. doi: 10.1016/j.freeradbiomed.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Oakley FD, Abbott D, Li Q, Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid. Redox Signaling. 2009;11:1313–1333. doi: 10.1089/ars.2008.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Touyz RM, Briones AM, Sedeek M, Burger D, Montezano AC. NOX isoforms and reactive oxygen species in vascular health. Mol. Interv. 2011;11:27–35. doi: 10.1124/mi.11.1.5. [DOI] [PubMed] [Google Scholar]
- 644.Sanchez FA, Rana R, Gonzalez FG, Iwahashi T, Duran RG, Fulton DJ, Beuve AV, Kim DD, Duran WN. Functional significance of cytosolic endothelial nitric oxide synthase (eNOS): regulation of hyperpermeability. J. Biol. Chem. 2011;286:30409–30414. doi: 10.1074/jbc.M111.234294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Gehrmann W, Elsner M. A specific fluorescence probe for hydrogen peroxide detection in peroxisomes. Free Radic. Res. 2011;45:501–506. doi: 10.3109/10715762.2011.560148. [DOI] [PubMed] [Google Scholar]
- 646.Quinn SR, O'Neill LA. A trio of microRNAs that control Toll-like receptor signalling. Int. Immunol. 2011;23:421–425. doi: 10.1093/intimm/dxr034. [DOI] [PubMed] [Google Scholar]
- 647.Schroen B, Heymans S. Small but smart—microRNAs in the centre of inflammatory processes during cardiovascular diseases, the metabolic syndrome, and ageing. Cardiovasc. Res. 2011 doi: 10.1093/cvr/cvr268. (in press) [DOI] [PubMed] [Google Scholar]
- 648.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 649.Fu Y, Zhang Y, Wang Z, Wang L, Wei X, Zhang B, Wen Z, Fang H, Pang Q, Yi F. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am. J. Nephrol. 2010;32:581–589. doi: 10.1159/000322105. [DOI] [PubMed] [Google Scholar]
- 650.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 651.Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem. Biophys. Res. Commun. 2010;393:643–648. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.McCall CE, El Gazzar M, Liu T, Vachharajani V, Yoza B. Epigenetics, bioenergetics, and microRNA coordinate gene-specific reprogramming during acute systemic inflammation. J. Leukocyte Biol. 2011;90:439–446. doi: 10.1189/jlb.0211075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat. Immunol. 2011;12:5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]