Abstract
Objectives:
Juvenile myoclonic epilepsy (JME) is characterized by myoclonic jerks of the upper limbs, often triggered by cognitive stressors. Here we aim to reconcile this particular seizure phenotype with the known frontal lobe type neuropsychological profile, photosensitivity, hyperexcitable motor cortex, and recent advanced imaging studies that identified abnormal functional connectivity of the motor cortex and supplementary motor area (SMA).
Methods:
We acquired fMRI and diffusion tensor imaging (DTI) in a cohort of 29 patients with JME and 28 healthy control subjects. We used fMRI to determine functional connectivity and DTI-based region parcellation and voxel-wise comparison of probabilistic tractography data to assess the structural connectivity profiles of the mesial frontal lobe.
Results:
Patients with JME showed alterations of mesial frontal connectivity with increased structural connectivity between the prefrontal cognitive cortex and motor cortex. We found a positive correlation between DTI and fMRI-based measures of structural and functional connectivity: the greater the structural connectivity between these 2 regions, the greater the observed functional connectivity of corresponding areas. Furthermore, connectivity was reduced between prefrontal and frontopolar regions and increased between the occipital cortex and the SMA.
Conclusion:
The observed alterations in microstructural connectivity of the mesial frontal region may represent the anatomic basis for cognitive triggering of motor seizures in JME. Changes in the mesial frontal connectivity profile provide an explanatory framework for several other clinical observations in JME and may be the link between seizure semiology, neurophysiology, neuropsychology, and imaging findings in JME.
Juvenile myoclonic epilepsy (JME) is the most common idiopathic generalized epilepsy syndrome in adults, characterized by myoclonic jerks of the arms and generalized tonic-clonic seizures.1 Seizures are typically provoked by sleep deprivation, but also by reading, writing, or other cognitive activities.2
We recently described increased motor cortex activation in JME with increasing cognitive effort and increased functional connectivity to prefrontal cognitive networks.3 Impaired task-related deactivation of the supplementary motor area (SMA) suggested a role of the SMA as a relay, facilitating increased functional connectivity between prefrontal cognitive areas and the motor system.3 These findings link the motor cortex hyperexcitability hypothesis4 to neuropsychological activation studies, showing increased spike frequency in the central region during demanding cognitive activities.5
Subtle morphometric abnormalities of medial frontal gray matter have been repeatedly described in patients with JME6,7 and interpreted as microdysgenesis or regional neuronal loss. We recently reported reduced fractional anisotropy (FA) suggestive of regional neuronal loss that correlated with specific neuropsychological deficits.7
How can this finding of reduced FA be reconciled with the observation of increased functional connectivity in the same group of patients? Our objective was to analyze the microstructural connectivity of the medial frontal lobes in JME, using diffusion tensor imaging (DTI)−based regional parcellation and tractography, and to clarify the relationship between structural and functional connectivity of the motor system with prefrontal cognitive networks.
METHODS
Standard protocol approvals, registration, and patient consent.
This study was approved by the UCL Hospital Research Ethics Committee. Written informed consent was obtained from all patients and volunteers participating in this study.
We acquired brain fMRI and DTI data for 29 patients with JME (table e-1 on the Neurology® Web site at www.neurology.org) and 28 healthy control subjects, on a General Electric Excite HDx 3-T scanner. Details of the inclusion/exclusion criteria, fMRI acquisitions and analyses have been recently reported elsewhere.3 DTI data were acquired using a cardiac-gated echo-planar imaging sequence, as described previously8 and processed with FSL 4.1.5 software (http://www.fmrib.ox.ac.uk/fsl), using a two-fiber model accounting for crossing fibers.
Imaging analysis of the medial frontal region presents challenges, because of the ill-defined anatomic boundaries of the SMA. To overcome this, we have applied DTI-based voxel-wise connectivity profiling and clustering, which allows the delineation of the SMA (part of the motor system), and its separation from the pre-SMA (part of the frontal lobe cognitive network).9
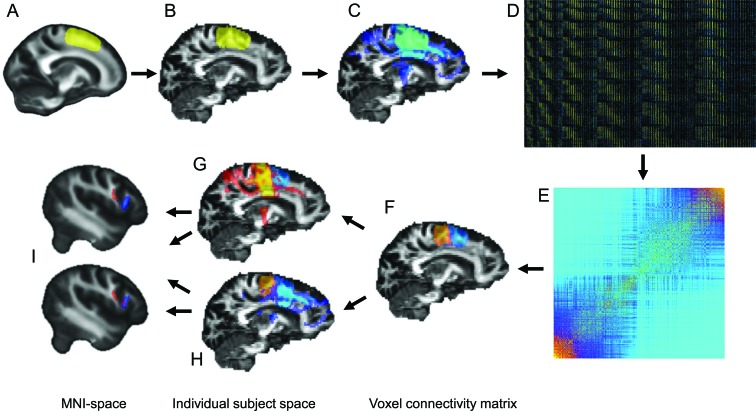
A left medial frontal seed region was defined in Montreal Neurological Institute (MNI) space (figure 1A) and back-normalized into each subject's individual DTI data space using SPM5 software (figure 1B). Probabilistic tractography was performed from every voxel within this seed region (figure 1C), and the metric of connectivity to a downsampled (5 mm) whole-brain mask was determined for every seed voxel. Following the method of Johansen-Berg et al.,9 the resulting connectivity matrices (figure 1D) were reordered with spectral clustering (figure 1E) to parcellate the seed region in 2 distinct clusters: the SMA and the pre-SMA (figure 1F), based solely on their DTI connectivity profile. Tractography was repeated, seeded separately from the SMA and from the pre-SMA clusters, providing connectivity maps for both regions for every subject (figure 1, G and H). The connectivity maps show, for every brain voxel, how many samples are passing through it from the seed cluster. Individual connectivity maps were normalized to MNI space in 1-mm resolution for voxel-wise group comparison (figure 1I) using a 2-sample t test.
Figure 1. Image processing pipeline.
A region of interest (ROI) was defined in the left medial frontal lobe in Montreal Neurological Institute (MNI) space (A) and back-normalized to each subject's individual diffusion tensor imaging (DTI) data space (B). Probabilistic tracking was performed, seeding from every voxel of the ROI (C). A voxel-wise connectivity map was created (D), representing the probability of every seed voxel (y axis) to be connected to a certain region of the whole brain (x axis), a representative selection from matrix is shown (total matrix size: ∼1,800 9,000). This connectivity matrix was reordered, using k means and spectral clustering techniques constrained to identify 2 clusters. The reordered matrix (E) shows 2 distinct clusters of seed voxels, representing the supplementary motor area (SMA) (orange) and pre-SMA regions (blue), separated solely by their DTI connectivity profile. Tracking was repeated from these 2 clusters (F), with the resulting tracts for the SMA cluster (G) and pre-SMA cluster (H) normalized to MNI space for voxel-wise group comparison (I).
RESULTS
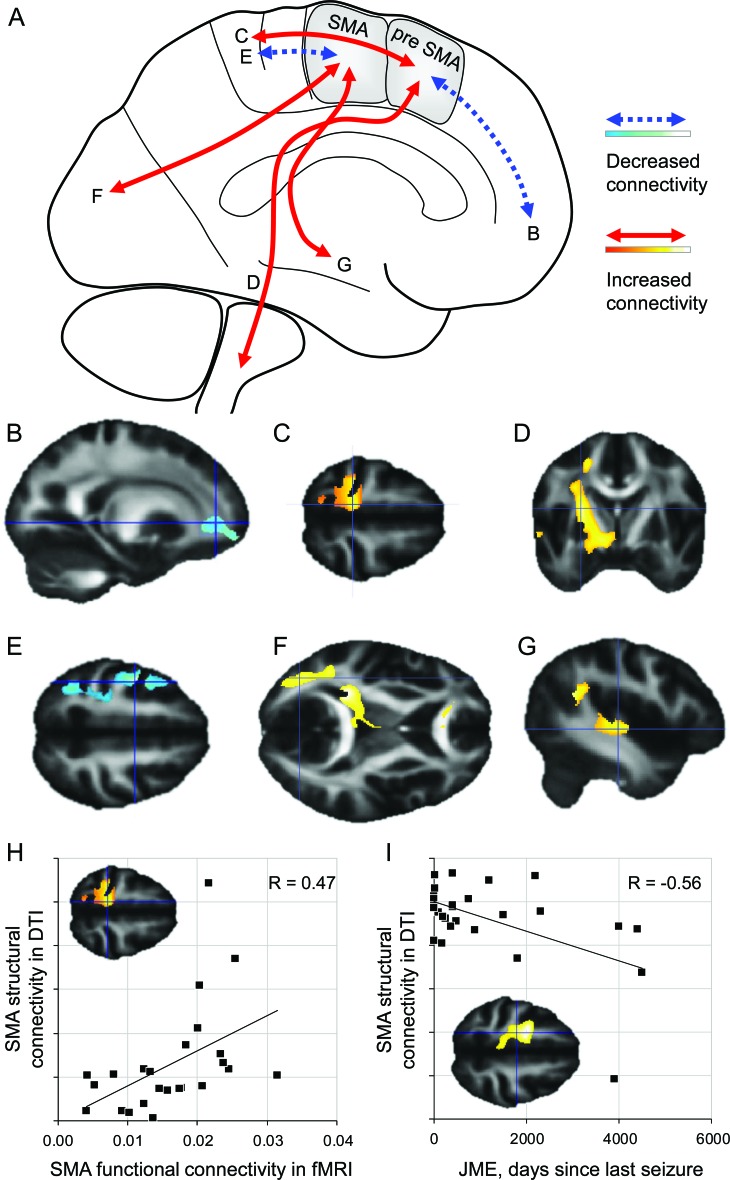
DTI seed region parcellation into SMA and pre-SMA was similar for patients with JME and control subjects with regard to cluster size, position, and cluster membership probability. We found differences for the voxel-wise comparison of both connectivity maps (figure 2A, table 1 ): in patients with JME compared with control subjects, the pre-SMA cluster showed reduced connectivity to the prefrontal and frontopolar areas (figure 2B) and increased connectivity to the central region (figure 2C) and descending motor pathways (figure 2D). The SMA cluster showed decreased connectivity to the primary motor cortex (figure 2E) and increased connectivity to the occipital lobe (figure 2F) and lateral temporal neocortex (figure 2G).
Figure 2. Alterations of structural connectivity in juvenile myoclonic epilepsy (JME).
(A) Summary of findings in JME patients. The anterior pre-supplementary motor area (SMA) cluster showed reduced connectivity to anterior frontopolar regions (B) and increased connectivity to the medial central region (C) and descending motor pathways (D). The posterior SMA cluster showed reduced connectivity to the central region (E) but increased connectivity to the temporal neocortex (F) and occipital cortex (G). (H) Functional connectivity between the prefrontal cortex and SMA found with fMRI, correlated significantly with the structural connectivity shown with diffusion tensor imaging (DTI). (I) Connectivity of the SMA also correlated with disease activity.
Table 1.
DTI connectivity results
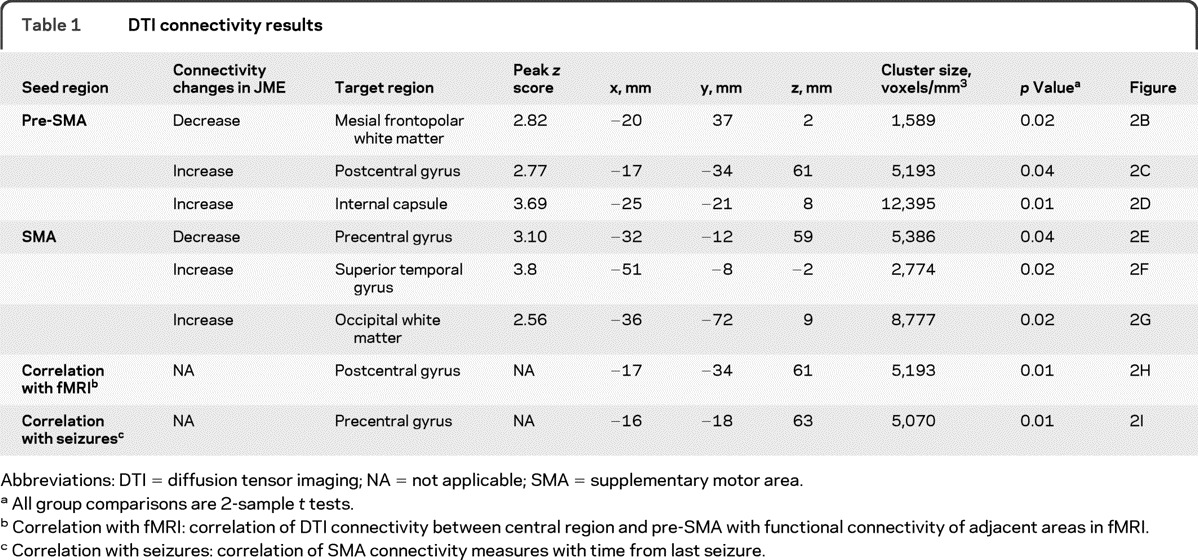
Abbreviations: DTI = diffusion tensor imaging; NA = not applicable; SMA = supplementary motor area.
All group comparisons are 2-sample t tests.
Correlation with fMRI: correlation of DTI connectivity between central region and pre-SMA with functional connectivity of adjacent areas in fMRI.
Correlation with seizures: correlation of SMA connectivity measures with time from last seizure.
After correction for multiple comparisons (false discovery rate), the peak voxels of all group differences were significant, at p < 0.05 (2-tailed t test).
In JME, the structural connectivity measure from the pre-SMA cluster to the medial central region correlated positively with the functional connectivity between the adjacent dorsolateral prefrontal cortex and the medial central region, as shown by fMRI (Pearson's correlation coefficient R = 0.47, p = 0.01) (figure 2H).
The shorter the interval since the last seizure, the higher the connectivity found between pre-SMA and central region correlation coefficient R = −0.56, p = 0.001) (figure 2I). There was no correlation between connectivity measures and cognitive performance. In patients with photosensitivity, connectivity between the SMA and the occipital cortex was stronger (n = 5, not statistically significant), and this subgroup alone did not show increased connectivity of the pre-SMA.
DISCUSSION
We showed an increased measure of microstructural connectivity between the prefrontal cognitive network (pre-SMA), and the motor system in JME, and this correlated positively with functional connectivity between these 2 systems, which was described recently.3 These findings suggest that structural alterations in the medial frontal region underlie the functional hyperconnectivity and might be crucial in the pathophysiology of JME. Although our patient numbers were too small for detailed subgroup analyses, this structural-functional connectivity profile of the medial frontal region provides a framework to explain several characteristic features of JME:
Increased connectivity between prefrontal cortex and the central region enables cognitive activity to elicit epileptiform discharges in the central region5 and trigger myoclonic jerks.2 The increased connectivity between temporal neocortex and SMA provides an additional pathway for cognitive effects on the motor system.
Reduced connectivity between the pre-SMA region and the frontopolar cortex may be the anatomic basis for impairment of frontal lobe function in JME.10 The absence of a linear correlation in our cohort may be a limitation of the relatively good performing patient group and could be more accessible in a population with a broader range of cognitive performance.
Increased connectivity between SMA and occipital cortex, which was stronger in photosensitive patients, may explain the provocative effect of photic stimulation to elicit frontocentral discharges and seizures.
DTI provides detailed information about degree and directionality of water diffusivity in white matter, in which the anatomy of axonal bundles restricts free water diffusivity across fibers. This can be summarized in condensed measures such as mean diffusivity or FA, but these measures do not fully use the available information within a DTI dataset. Voxel-wise diffusion tensor tractography and connectivity-based fingerprinting of brain regions are advanced DTI analysis techniques, which provide much more detailed information about the microstructural anatomy of brain regions. Furthermore, we can now explain our previous finding of reduced FA within the context of increased functional connectivity. Reductions in FA are usually interpreted as a loss of fiber density or integrity, representing neuronal loss or neuronal damage. However, FA is also reduced in areas in which intersecting neuronal bundles allow diffusion of water in multiple directions. In the SMA, the main afferent and efferent fibers run in a superior-inferior direction descending to motor pathways. Here we showed that patients with JME have a higher proportion of fibers traveling in an anterior-posterior direction from the pre-SMA to the central region, thereby crossing the descending fibers from the SMA. Such an increased proportion of crossing fibers in JME provides an explanation for reduced FA, even if all fibers are structurally and functionally intact and the number of fibers remains unchanged.
This finding links several clinical observations in JME, particularly the triggering of motor seizures through cognitive effort. Similar findings may come to light in other diseases as well, in which the truth behind reduced FA may be more complex than just neuronal loss.
Supplementary Material
GLOSSARY
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- JME
juvenile myoclonic epilepsy
- MNI
Montreal Neurological Institute
- SMA
supplementary motor area
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Vollmar: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis. Dr. O'Muircheartaigh: drafting/revising the manuscript. Dr. Symms: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, contribution of vital reagents/tools, study supervision, obtaining funding. Prof. Baker: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision. Dr. Thompson: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision. Prof. Kumari: drafting/revising the manuscript, study concept or design, obtaining funding. J. Stretton: study concept or design, acquisition of data. Prof. Duncan: drafting/revising the manuscript, analysis or interpretation of data, study supervision, obtaining funding. Prof. Richardson: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision, obtaining funding. Prof. Koepp: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding.
DISCLOSURE
Dr. Vollmar, Dr. O'Muircheartaigh, and Dr. Symms report no disclosures. Prof. Barker received honoraria for teaching for and serving on scientific advisory boards of General Electric Healthcare. Dr. Thompson, Prof. Kumari, and J. Stretton report no disclosures. Prof. Duncan serves on scientific advisory boards for and/or has received funding for travel from GE Healthcare. Prof. Richardson reports no disclosures. Prof. Koepp served on a scientific advisory board of GE. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Janz D, Christian W. Impulsive petit mal. Dtsch Z Nervenheilk 1957;176:348–386 [Google Scholar]
- 2. Inoue Y, Seino M, Kubota H, Yamakaku K, Tanaka M, Yagi K. Epilepsy with praxisinduced seizures. In: Wolf P. ed. Epileptic Seizures and Syndromes. London: John Libbey, 1994:81−91 [Google Scholar]
- 3. Vollmar C, O'Muircheartaigh J, Barker GJ, et al. Motor system hyperconnectivity in juvenile myoclonic epilepsy: a cognitive functional magnetic resonance imaging study. Brain 2011;134:1710–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Badawy RA, Curatolo JM, Newton M, Berkovic SF, Macdonell RA. Changes in cortical excitability differentiate generalized and focal epilepsy. Ann Neurol 2007;61:324–331 [DOI] [PubMed] [Google Scholar]
- 5. Matsuoka H, Takahashi T, Sasaki M, et al. Neuropsychological EEG activation in patients with epilepsy. Brain 2000;123:318–330 [DOI] [PubMed] [Google Scholar]
- 6. Woermann FG, Free SL, Koepp MJ, Sisodiya SM, Duncan JS. Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel-based analysis of MRI. Brain 1999;122:2101–2108 [DOI] [PubMed] [Google Scholar]
- 7. O'Muircheartaigh J, Vollmar C, Barker GJ, et al. Focal structural changes and cognitive dysfunction in juvenile myoclonic epilepsy. Neurology 2011;76:34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yogarajah M, Focke NK, Bonelli SB, et al. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain 2010;133:2348–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johansen-Berg H, Behrens TE, Robson MD, et al. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA 2004;101:13335–13340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wandschneider B, Kopp UA, Kliegel M, et al. Prospective memory in patients with juvenile myoclonic epilepsy and their healthy siblings. Neurology 2010;75:2161–2167 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.